Abstract
Purpose
Retinal ischemia is a common cause of a variety of eye diseases, such as retinopathy of prematurity, diabetic retinopathy, and vein occlusion. Protein kinase RNA-activated-like endoplasmic reticulum (ER) kinase (PERK), one of the main ER stress sensor proteins, has been involved in many diseases. In this study, we investigated the role of PERK in ischemia-induced retinopathy using a mouse model of oxygen-induced retinopathy (OIR).
Methods
OIR was induced by subjecting neonatal pups to 70% oxygen at postnatal day 7 (P7) followed by returning to room air at P12. GSK2606414, a selective PERK inhibitor, was orally administrated to pups right after they were returned to room air once daily until 1 day before sample collection. Western blot, immunostaining, and quantitative PCR were used to assess PERK phosphorylation, retinal changes, and signaling pathways in relation to PERK inhibition.
Results
PERK phosphorylation was prominently increased in OIR retinas, which was inhibited by GSK2606414. Concomitantly, PERK inhibition significantly reduced retinal neovascularization (NV) and retinal ganglion cell (RGC) loss, restored astrocyte network, and promoted revascularization. Furthermore, PERK inhibition downregulated the recruitment/proliferation of mononuclear phagocytes but did not affect OIR-upregulated canonical angiogenic pathways.
Conclusions
Our results demonstrate that PERK is involved in ischemia-induced retinopathy and its inhibition using GSK2606414 could offer an effective therapeutic intervention aimed at alleviating retinal NV while preventing neuron loss during retinal ischemia.
Keywords: ischemia-induced retinopathy, neovascularization, protein kinase RNA-activated-like ER kinase (PERK), endoplasmic reticulum (ER) stress, retina
Retinal ischemia is a common cause of a wide range of retinal pathologies, including retinopathy of prematurity, diabetic retinopathy, and retinal vein occlusion, which are characterized by pathological neovascularization (NV) due to vessel degeneration, occlusion, or retarded growth.1–3 Surgical vitrectomy, laser photocoagulation, and intravitreal injection of anti-VEGF agents are conventional therapies for retinal NV.4,5 But due to the invasive nature and partial efficacy of current treatment options, the rate of recurrent NV remains high. Therefore, it is critical to identify novel targets for retinal NV to improve therapeutic outcomes.
The endoplasmic reticulum (ER) is a membrane-bound organelle where synthesis, folding, and trafficking of protein occurs. When ER homeostasis is disrupted by a number of insults, unfolded or misfolded proteins are accumulated in the ER and saturate the capacity of the ER to fold proteins, which causes ER stress and activates intracellular signal transduction pathways, collectively known as the unfolded protein response (UPR).6–8 It has been reported that ER stress is implicated in various pathological conditions, including ischemia, and UPR is involved in physiological or pathological angiogenesis by regulating angiogenic factors,9,10 endothelial function,11 inflammation,12 oxidative stress,13 and other cellular events.14–16 Protein kinase RNA-activated-like ER kinase (PERK) is considered as an ER stress sensor protein and plays a key role in the UPR pathway.17 PERK activation can reduce the load of misfolded proteins in the ER by phosphorylating the α subunit of eukaryotic initiation factor 2 (eIF2α) to attenuate the synthesis of general proteins and thereby decrease their entry into the ER lumen.18,19 Although this mechanism is important for the re-establishment of ER homeostasis during ER stress, chronic PERK activation causes tissue injury and can lead to various diseases, such as Alzheimer's disease, Parkinson's disease, Marinesco-Sjögren syndrome, amyotrophic lateral sclerosis, and light-induced photoreceptor injury.20–22 Although ischemia-induced retinopathy is a prevalent eye disorder, little is known about the role of PERK in ischemia-induced retinopathy.
In this study, using a well-established mouse model for ischemia-induced retinopathy (oxygen-induced retinopathy [OIR]), we investigated the potential use of putative compound for the treatment of ischemia-induced retinal NV. We demonstrated that PERK phosphorylation was prominently increased in OIR retinas, and GSK2606414, a selective PERK inhibitor explored for the treatment of various diseases, attenuated PERK phosphorylation, and significantly reduced retinal pathology in OIR.
Methods
Animals and OIR Model
C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and bred in the animal resources center at the University of Texas Medical Branch. All experimental procedures were conducted in accordance with the Association of Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic Vision and Research and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. In OIR model, pups and their mothers were subjected to hyperoxia (70% oxygen) at postnatal day 7 (P7) and then returned to room air (21% oxygen) at P12.23 The litter size was kept at six pups for each dam. Pups were weighed at P7 and only the litter with mean body weight around 4 g was placed into oxygen chamber for further experiment. Age-matched pups were raised in room air (RA) serving as control. Both female and male pups were used.
GSK2606414 Treatment
GSK2606414 was provided by GlaxoSmithKline. It was dissolved at 5 mg/mL in water containing 0.5% hydroxypropyl methyl cellulose (HPMC) and 0.1% Tween-80 (pH 4.0). Pups in each litter were weighted at P12 and divided into two subgroups according to body weight (3 pups/group/time). One group of pups were orally treated with vehicle (water containing 0.5% HPMC and 0.1% Tween-80, pH 4.0), whereas the other group of pups were treated with GSK2606414 (50 mg/kg) using an oral gavage feeding tube once daily from P12 to P16 for sample collection at P17 (n = 42/group totally, which were used for morphological, protein, and mRNA analysis) or from P12 to P18 for sample collection at P19 (n = 12/group for morphological analysis).
Immunostaining of Retinal Whole Mounts
The eyeballs were collected and fixed, and then the retinas were dissected, washed, blocked, and permeabilized, as previously described.24 The retinas were incubated with Alexa Fluor 594-conjugated Isolectin B4 (1:200; ThermoFisher Scientific, Waltham, MA, USA) or primary antibodies against GFAP (1:500, Z033401-2; Agilent Technologies, Santa Clara, CA, USA), CD206 (1:200, 141710; BioLegend, San Diego, CA, USA), Iba1 (1:200, 019-19741; FUJIFILM Wako Chemicals, Richmond, VA, USA), and RBPMS (1:200, ABN1362; MilliporeSigma, Burlington, MA, USA) at 4°C overnight. After rinsing, the retinas were incubated with appropriate Alexa Fluor 488-conjugated secondary antibodies (1:400; ThermoFisher Scientific) for 4 hours at 4°C. Last, the retinas were mounted and images were obtained using a confocal microscope (LSM 800; Carl Zeiss, Inc., Thornwood, NY, USA). To quantify central avascular area and neovascular tufts of the retina, the surface areas of the total retina, central avascular zone, and neovascularization were measured using ImageJ software (Bethesda, MD, USA), as previously described.25,26 The avascular area and neovascularization were expressed as a percentage of the total retinal surface area. For cell counting, eight images were taken at the peripheral region of each retinal flatmounts, and cells were manually counted and averaged for each sample.
Immunostaining of Retinal Sections
Retinal cryosections (10 µm) were prepared after embedding in optimal cutting temperature compound. Immunostaining of the sections was performed as described previously27 with primary antibody p-PERK (SAB4301310; MilliporeSigma) and secondary antibody conjugated to Alexa Fluor 594. Then sections were counterstained with DAPI (blue) for the nuclei to identify the retinal nuclear layers. Fluorescent images were taken by confocal microscopy.
Western Blot Analysis
Retinal protein was extracted with RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, and 1 mM EDTA), supplemented with Complete Protease and Phosphatase Inhibitors (Roche Applied Science, Indianapolis, IN, USA). Protein concentration was measured using BCA Protein Detection Kit (Pierce, Rockford, IL, USA). Then, 10 µg of protein per sample was run on SDS-polyacrylamide gels and transferred to PVDF membranes. The primary antibodies included PERK (3192; Cell Signaling, Danvers, MA, USA), p-PERK (3179; Cell Signaling; and SAB4301310; MilliporeSigma), and α-Tubulin (T9026; MilliporeSigma). Last, proteins were detected with Bio-Rad ChemiDoc XRS+ (Bio-Rad Laboratories, Hercules, CA, USA). The band intensities were quantified and normalized against PERK. Relative protein expression changes were expressed as x-fold change in relation to control retinas.
Real-Time Quantitative RT-PCR
Retinal RNA was extracted with RNAqueous-4PCR kit (ThermoFisher Scientific) and converted to cDNA using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific). Quantitative PCR was performed using SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA) on a StepOnePlus PCR system (Applied Biosystems). Primer sequences for mouse transcripts were as follows: VEGF For-5′-TAC CTC CAC CAT GCC AAG TG-3′; VEGF Rev-5′-TCA TGG GAC TTC TGC TCT CCT T-3′; Angiopoietin2 For-5′-ACA CCG AGA AGA TGG CAG TGT-3′; Angiopoietin2 Rev-5′-CTC CCG AAG CCC TCT TTG TA-3′; EPO For-5′-CCC CCA CGC CTC ATC TG-3′; EPO Rev-5′-TGC CTC CTT GGC CTC TAA GA-3′; Dll4 For-5′-GAC CTG CGG CCA GAG ACT T-3′; Dll4 Rev-5′- GAG CCT TGG ATG ATG ATT TGG-3′; FGF2 For-5′-TGG TAT GTG GCA CTG AAA CGA-3′; FGF2 Rev-5′-TCC AGG TCC CGT TTT GGA T-3′; CD11b For-5′- AAA CCA CAG TCC CGC AGA GA-3′; CD11b Rev-5′-CGT GTT CAC CAG CTG GCT TA-3′; CD86 For-5′-TGG GCT TGG CAA TCC TTA TC-3′; CD86 Rev-5′-TCC ACG GAA ACA GCA TCT GA-3′; CD206 For-5′-CCC AAG GGC TCT TCT AAA GCA-3′; CD206 Rev-5′-CGC CGG CAC CTA TCA CA-3′; CX3CR1 For-5′-TCG GTC TGG TGG GAA ATC TG-3′; CX3CR1 Rev-5′-GGC TTC CGG CTG TTG GT-3′; Hprt For-5′-GAA AGA CTT GCT CGA GAT GTC ATG-3′; and Hprt Rev-5′-CAC ACA GAG GGC CAC AAT GT-3′. Data were normalized to the internal control Hprt. The ΔΔCT method was used to calculate the fold difference in different transcripts.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism program (GraphPad Software, Inc., La Jolla, CA, USA). Results were expressed as mean ± standard error of the mean (SEM). The P values were calculated by Student's t-test or one-way ANOVA, and P < 0.05 indicates that the difference is statistically significant.
Results
PERK is Activated in the Retinas of OIR Mice
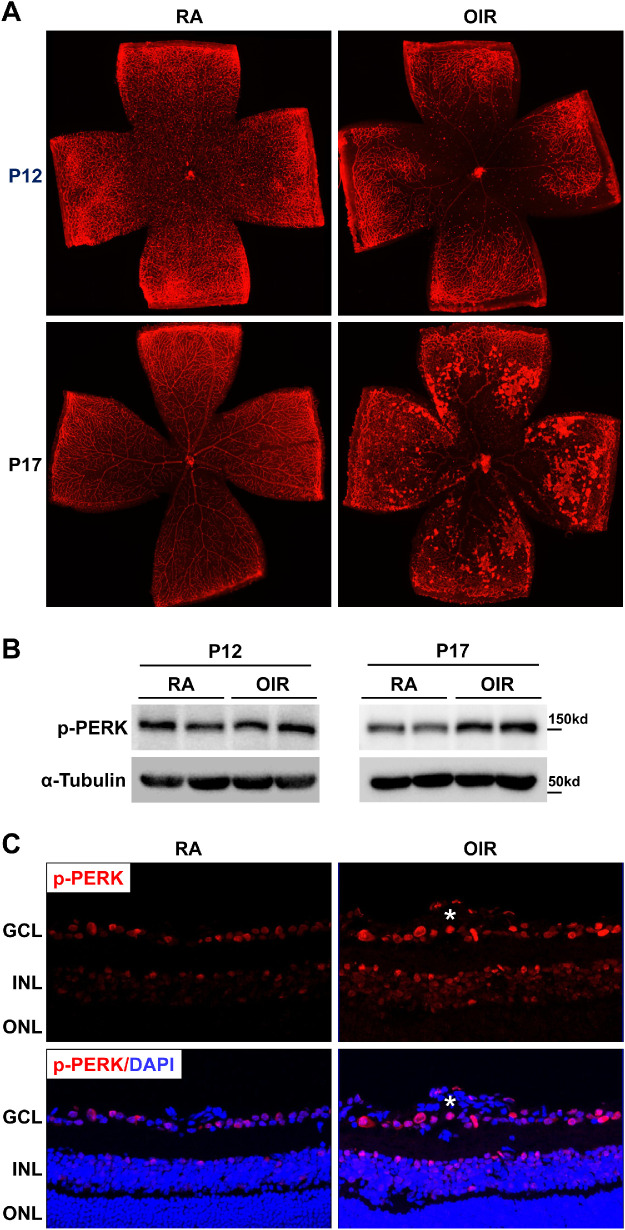
A mouse model of OIR was used to investigate the role of PERK in retinal NV. In this model, P7 pups were subjected to 70% oxygen (hyperoxia) until P12,23,28–31 which induces vascular regression and obliteration in the central retina at P12 (Fig. 1A, upper panel). After returning mice to RA, relative hypoxia promoted aberrant vessel growth and NV reached its maximum at P17 (Fig. 1A, lower panel). To determine whether PERK was activated during OIR, the level of PERK phosphorylation at Thr980, which serves as a marker for PERK activation,32,33 was evaluated by Western blot analysis. We observed that p-PERK expression was not altered at P12 but increased at P17 in the retinas of OIR mice compared with that of RA controls (Fig. 1B). Consistently, immunostaining of p-PERK in retinal sections demonstrated that p-PERK was mainly increased in the ganglion cell layer (GCL), inner nuclear layer (INL), and neovessels in OIR retinas at P17 (Fig. 1C). Together, these data indicate that PERK was activated in the retinas of OIR mice.
Figure 1.
The phosphorylation level of PERK is increased in the retinas of OIR. WT mice were subjected to OIR or maintained in room air (RA) as control. Eyeballs or retinas were collected at P12 and P17. (A) Representative images of retinal flatmounts with Isolectin B4 staining for retinal vasculature at P12 and P17. (B) Phosphorylated PERK in the retina at P12 and P17 was evaluated by Western blot. The α-tubulin was used as the internal loading control. (C) Representative images of phosphorylated PERK in retinal sections at P17 were shown. DAPI (blue) was used to counterstain nuclei and identify the retinal nuclear layers as indicated by the labels. The white asterisk denotes the neovessel (n = 4). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
PERK Inhibitor GSK2606414 Attenuates Retinal NV and Avascular Area in OIR
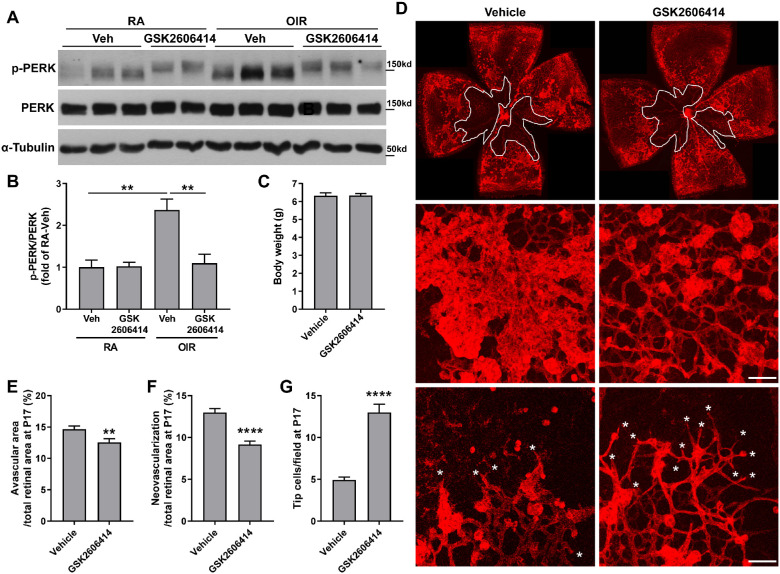
GSK2606414 has been identified as a selective PERK inhibitor and explored in various diseases.34–36 To determine whether PERK activation had a role in retinal NV, vehicle or PERK inhibitor GSK2606414 (50 mg/kg) was administered by oral gavage once daily to OIR pups from P12 to P16. We measured the phosphorylation level of PERK in the retinas at P17 and observed that OIR-induced upregulation of p-PERK was significantly abolished by the treatment of GSK2606414 (Figs. 2A, 2B), suggesting that oral administration of GSK2606414 can inhibit PERK activation in the retinas during OIR. In addition, PERK inhibition did not affect the body weight of OIR mice (Fig. 2C). Next, we analyzed retinal NV and avascular area in vehicle- or GSK2606414-treated OIR mice at the peak of NV (P17). Retinal NV and vessel obliteration were observed in the retinas of the OIR + vehicle treatment group. In comparison, the avascular region and NV was significantly decreased by 14.3% and 29.5%, respectively, accompanied with increased tip cells (2.64-fold) in the OIR + GSK2606414 treatment group (Figs. 2D–2G).
Figure 2.
PERK inhibition attenuates neovascularization and promotes revascularization in the retinas of OIR. Mice were subjected to OIR or maintained in RA as control, and they were treated with PERK inhibitor GSK2606414 (50 mg/kg) or vehicle from P12 to P16. Retinas or eyeballs were collected at P17. (A, B) Phosphorylated and total PERK was evaluated by Western blot. The α-tubulin was used as the internal loading control for normalization (n = 3-4; **P < 0.01). (C) Body weight of OIR mice treated with vehicle or GSK2606414 (n = 12). (D–G) Retinal flatmounts from vehicle- or GSK2606414-treated OIR mice at P17 were stained with Isolectin B4 and representative images were shown (upper panel). High magnification images were shown for neovascular tufts (middle panel) and tip cells (lower panel, white asterisks). Scale bar = 50 µm. Graphs represent the quantification of avascular and neovascularization area (n = 23–25) and the number of tip cells (n = 6). **P < 0.01 and ****P < 0.0001 compared with vehicle.
PERK Inhibition Reduces NV and Promotes Revascularization in OIR Retinas at P19
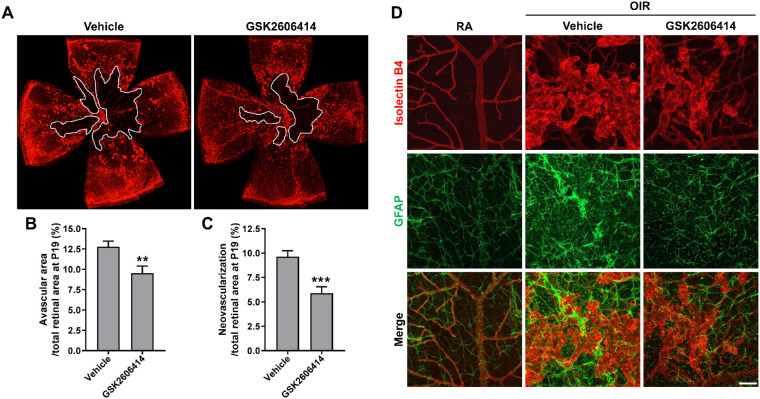
Because the reduction of avascular area at P17 (see Figs. 2D, 2E) indicates enhanced vascular repair after PERK inhibition, we further treated OIR mice for an additional 2 days (from P12 to P18) and collected samples at P19 to test if this observation would be further enhanced. At P19, the OIR + vehicle treatment group continued to exhibit high levels of retinal NV and avascular area. In contrast, avascular region and NV were reduced by 25.3% and 38.9%, respectively, in the retinas of the OIR + GSK2606414 treatment group at this time point (Figs. 3A–3C). Astrocytes guide the development of retinal vasculature and modulate NV during OIR. To evaluate if astrocyte network is altered after PERK inhibition in OIR, we stained the retinas with antibody against GFAP to identify astrocytes. In the retinas of RA control mice, astrocytes showed stellate/dendritic morphology and were closely correlated with normal retinal vasculature. In the OIR + vehicle treatment group, stronger GFAP immunoreactivity was observed in astrocytes which were disordered around the distorted and expanded neovasculature. Moreover, we observed spotted GFAP reactivity from the activated Müller cell end-feet within the superficial vascular plexus. In contrast, in the OIR + GSK2606414 treatment group, astrocytes were more orderly around the blood vessel. Although the activation of astrocytes and Müller cell end-feet was still observed in the OIR + GSK2606414 mice, it occurred to a lesser extent (Fig. 3D).
Figure 3.
Blockade of PERK attenuates vasculopathy and preserves astrocyte network in OIR at P19. OIR mice were treated with GSK2606414 (50 mg/kg) or vehicle from P12 to P18. Eyeballs were collected at P19 and dissected for retinal flatmounts. (A–C) Retinal flatmounts were stained with Isolectin B4 for retinal vasculature. Graphs represent the quantification of avascular area and neovascularization area at P19 (n = 12). **P < 0.01 and ***P < 0.001 compared with vehicle. (D) Astrocytes were stained with anti-GFAP antibody (green) and retinal vessels were stained with Isolectin B4 (red). Scale bar = 50 µm.
PERK Inhibition Does not Affect the Upregulation of Canonical Angiogenic Pathways in OIR
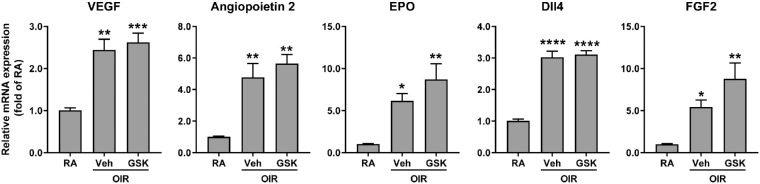
There are several important angiogenic factors that regulate pathological angiogenesis, such as VEGF, angiopoietin2, EPO, Dll4, and FGF2. Their gene transcripts, quantified by quantitative PCR, were upregulated in OIR retinas compared with RA controls (Fig. 4). However, PERK inhibition with GSK2606414 did not attenuate their upregulation, suggesting that PERK inhibition-abolished NV is not dependent on the alterations of canonical angiogenic signaling pathways.
Figure 4.
Blockade of PERK does not affect canonical angiogenic pathway. OIR mice were treated with GSK2606414 (50 mg/kg) or vehicle from P12 to P16. Retinas were harvested at P17. The gene expressions of angiogenic factors were quantified by qPCR (n = 3-4). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with RA controls.
PERK Inhibition Downregulates the Recruitment/Proliferation of Mononuclear Phagocytes in the OIR Model
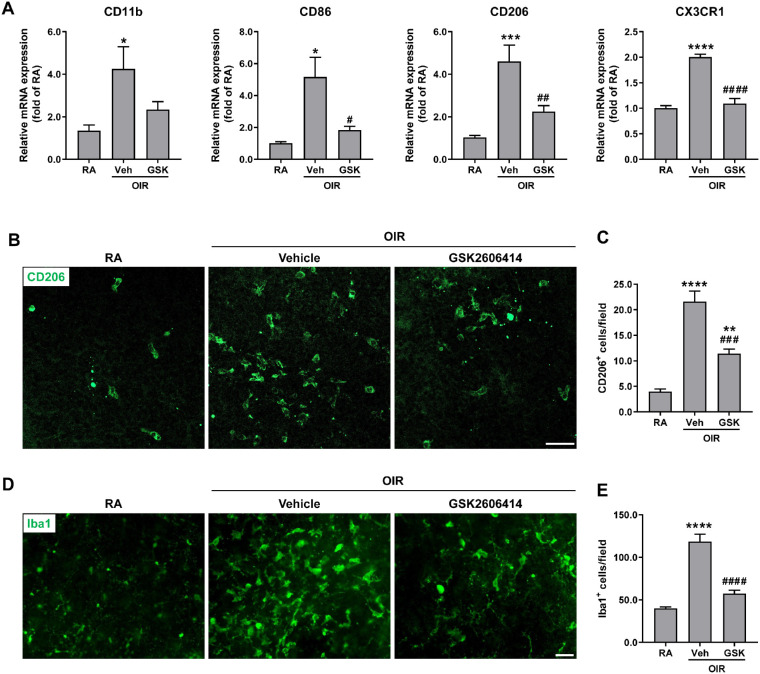
Mononuclear phagocytes including monocytes, macrophages, and microglia, have been shown to critically contribute to NV in OIR.37,38 Therefore, we examined the gene expressions of CD11b (a pan-myeloid marker), CD86 (a marker for M1 macrophages), CD206 (a marker for M2 macrophages), and CX3CR1 (a marker predominantly found on microglia within the central nervous system including the retina). We found that the expressions of CD11b, CD86, CD206, and CX3CR1 were dramatically increased in the OIR + vehicle group compared with RA controls, but they were significantly decreased after GSK2606414 treatment (Fig. 5A). Moreover, we stained retinal flatmounts with antibodies against CD206 and pan-microglia and macrophage marker Iba1. Consistently, CD206+ and Iba1+ cells in retinal flatmounts were markedly increased in OIR retinas, which were significantly decreased by PERK inhibition with GSK2606414 (Figs. 5B–5E).
Figure 5.
Blockade of PERK attenuates the recruitment/proliferation of mononuclear phagocytes in OIR. The OIR mice were treated with GSK2606414 (50 mg/kg) or vehicle from P12 to P16. Retinas or eyeballs were harvested at P17. (A) Marker genes for different mononuclear phagocytes were quantified by qPCR (n = 3–7). (B–E) Representative images of CD206 and Iba1 staining in retinal flatmounts from vehicle- or GSK2606414-treated OIR mice at P17 were shown. Scale bar = 50 µm. Graphs represent the quantification of the number of CD206+ cells and Iba1+ cells (n = 5-6). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with RA control; #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 compared with vehicle-treated OIR.
PERK Inhibition Attenuates Retinal Ganglion Cell Loss in the OIR Model
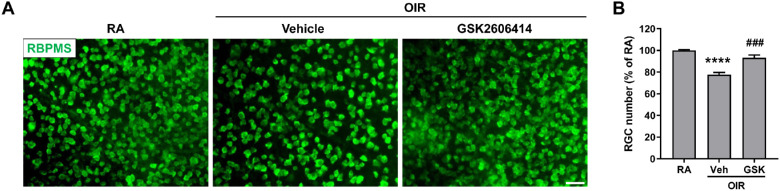
In addition to vasculopathy, neuronal injury including RGC loss is another feature in ischemia-induced retinopathies.39,40 To evaluate the effect of PERK on retinal neuronal cell survival in OIR, we assessed the loss of RGCs by quantifying the RGC number in the retina after labeling with anti-RBPMS antibody. Our data showed a reduction in the number of RGCs in the OIR + vehicle treatment group compared to the RA control group, which was significantly prevented by GSK2606414 treatment (22.46% RGC loss in the OIR + vehicle group versus 6.73% loss in the OIR + GSK2606414 group; Fig. 6). These data indicate that PERK inhibition with GSK2606414 prevents RGC loss in OIR.
Figure 6.
Blockade of PERK prevents RGC loss in OIR. The OIR mice were treated with GSK2606414 (50 mg/kg) or vehicle from P12 to P16. (A) Eyeballs were harvested at P17 and RGCs were stained with anti-RBPMS antibody (green) in retinal flatmounts. Scale bar = 50 µm. (B) Quantification of RGC number (n = 6). ****P < 0.0001 compared with RA control; ###P < 0.001 compared with vehicle-treated OIR.
Discussion
As one of the major sensor proteins for ER stress, PERK plays a key role in cell adaptive response during ER stress by reducing the load of misfolded proteins. As a result, although mice with PERK deletion are viable at birth, they have congenital skeletal dysplasias, exhibit postnatal growth retardation, and develop diabetes after P22.41,42 Nonetheless, overactive or chronic PERK activation causes tissue injury and leads to diseases. As a result, inhibition of PERK activation with GSK2606414 is beneficial in preventing or treating various pathological conditions. These include the prevention of neurodegeneration in a model of Parkinson's disease,43 the attenuation of light-induced photoreceptor injury,20 delaying Purkinje cell degeneration and the onset of motor deficits in a mouse model of Marinesco-Sjögren syndrome,22 the prevention of neuronal death, and improvement of neurological deficits after subarachnoid hemorrhage,44 as well as the protection against neuronal loss in tauopathy.45
In cultured retinal cells, PERK inhibition has been shown to attenuate high glucose-induced endothelial cell (EC) death in EC-retinal pigment epithelial (RPE) cell co-culture,46 block ER stress inducer thapsigargin-induced CXCL10 and CCL2 expression in a retinal photoreceptor cell line,47 and inhibit RPE cell proliferation.36 However, very few studies have been conducted to directly assess the role of PERK in the ocular pathology in in vivo models of eye diseases with the exception of studying the role of ATF4 and CHOP, two of downstream targets of PERK pathway, that have been found to cause the elevation of intraocular pressure and glaucomatous neurodegeneration by promoting trabecular meshwork dysfunction and cell death,48 trigger photoreceptor cell death in retinitis pigmentosa,49 induce VEGF upregulation and retinal inflammation in diabetic retinopathy,50 and be involved in RGC loss induced by NMDA, retinal ischemia-reperfusion, and optic nerve crush.51–53 Our data demonstrate that PERK plays a novel role in pathological angiogenesis during ischemia-induced retinopathy. We found that PERK phosphorylation was markedly increased in P17 OIR retinas and PERK inhibitor GSK2606414 not only effectively inhibited PERK phosphorylation but also significantly reduced pathological angiogenesis. Unlike the anti-VEGF agent that blocks physiological angiogenesis,54 PERK inhibition indeed promoted vascular repair, as demonstrated by increased normal vascular area (less avascular area) in OIR retinas. This feature is clinically desired because it will reduce retinal ischemia and therefore could fundamentally alleviate the cause of pathological angiogenesis. Moreover, we found PERK inhibition was neuroprotective because GSK2606414 treatment significantly attenuated RGC loss in OIR retinas. This is another clinically desired feature, as currently available treatments for ischemia-induced retinopathy, including surgical vitrectomy, laser photocoagulation, cryotherapy, and anti-VEGF agents, do not exhibit neuroprotective benefits.
The specific mechanism of how PERK promotes retinal NV in ischemia-induced retinopathy remains to be further studied. Both ATF4 and CHOP were reported to induce VEGF production in oxidative or hypoxic conditions.55–57 However, PERK inhibition by oral administration of GSK2606414 did not influence the upregulation of VEGF as well as other canonical angiogenic genes, such as Angiopoietin 2, EPO, Dll4, and FGF2 but significantly reduced retinal NV in OIR. This data suggests that other mechanisms are involved in PERK-regulated retinal NV. Recent studies indicate that mononuclear phagocytes, including microglia and infiltrated monocytes/macrophages, play a key role in the regulation of retinal NV.58 Mononuclear phagocytes can secrete soluble angiogenic and inflammatory factors that promote angiogenesis,59–61 and eliminating monocytes/macrophages or regulating microglial activation attenuates NV in OIR.37,38 To further explore the mechanisms of PERK-induced retinal NV, we assessed a series of mononuclear phagocytes markers, such as CD11b, CD86, CD206, CX3CR1, and Iba1, and found these markers were prominently increased in OIR retinas but were significantly attenuated by GSK2606414 treatment. Moreover, analysis of mononuclear phagocytes in retinal flatmount revealed that compared to retinas from vehicle-treated OIR group, those from GSK2606414-treatment mice exhibited fewer mononuclear phagocytes. Innate immunity is a crucial component of the immune response as innate immune cells are very sensitive and respond quickly to various pathological stimuli, during which ER stress drives many inflammatory transcription factors and genes’ responses. PERK-eIF2α pathway is found to inhibit the synthesis of IKβ and promote the nuclear translocation of NFκB in response to ER stress.62 Additionally, PERK can directly bind JAK1 to influence the activity of STAT3, followed by enhanced IL-6, CCL2, and CCL20 production.63 Our data suggest that PERK works as a modulator of mononuclear phagocytes recruitment/proliferation, and therefore promotes retinal NV in ischemia-induced retinopathy. Considering that PERK participates in retinal NV independent of canonical angiogenic molecules, including VEGF, the combination of PERK inhibitor with existing anti-VEGF therapy may offer better outcomes for pathological NV in ischemia-induced retinopathy.
In summary, our data provide compelling evidence that PERK plays a key role in ischemia-induced retinopathy. Inhibition of PERK by oral administration of GSK2606414 effectively remitted the dysregulation of astrocyte network, relieved pathological angiogenesis, and enhanced the survival of RGCs likely through inhibiting recruitment/proliferation of mononuclear phagocytes. Because pathological angiogenesis can result from a variety of diseases, such as retinopathy of prematurity, diabetic retinopathy, retinal vein occlusion, wet age-related macular degeneration, and neovascular glaucoma, our data warrant further exploration of PERK inhibition in different NV models to assess whether it is an effective therapeutic strategy for these vision-threatening diseases.
Acknowledgments
Supported in part by National Institutes of Health grants EY026629, EY022694, and EY034376, Retina Research Foundation, and UT System Faculty STARs Award (to W.Z.); and National Institutes of Health grants EY031054 (to H.L).
Disclosure: S. Shi, None; C. Ding, None; S. Zhu, None; F. Xia, None; S.E. Buscho, None; S. Li, None; M. Motamedi, None; H. Liu, None; W. Zhang, None
References
- 1. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015; 49: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kermorvant-Duchemin E, Sapieha P, Sirinyan M, et al.. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. 2010; 120(1): 51–60. [DOI] [PubMed] [Google Scholar]
- 3. Fortmann SD, Grant MB.. Molecular mechanisms of retinal ischemia. Curr Opin Physiol. 2019; 7: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012; 181(2): 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012; 87(1): 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011; 334(6059): 1081–1086. [DOI] [PubMed] [Google Scholar]
- 7. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13(2): 89–102. [DOI] [PubMed] [Google Scholar]
- 8. Hetz C, Zhang K, Kaufman RJ.. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020; 21(8): 421–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Wang G, Kunte M, Shinde V, Gorbatyuk M.. Modulation of angiogenesis by genetic manipulation of ATF4 in mouse model of oxygen-induced retinopathy [corrected]. Invest Ophthalmol Vis Sci. 2013; 54(9): 5995–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu L, Qi X, Chen Z, et al.. Targeting the IRE1alpha/XBP1 and ATF6 arms of the unfolded protein response enhances VEGF blockade to prevent retinal and choroidal neovascularization. Am J Pathol. 2013; 182(4): 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lenna S, Han R, Trojanowska M.. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life. 2014; 66(8): 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Wang JJ, Yu Q, Wang M, Zhang SX.. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009; 583(9): 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma SH, Suresh Kumar J, Madhavan S, Nagarajan S. Morin supplementation modulates PERK branch of UPR and mitigates 1,2-dimethylhydrazine-induced angiogenesis and oxidative stress in the colon of experimental rats. Toxicol Mech Methods. 2020; 30(4): 306–315. [DOI] [PubMed] [Google Scholar]
- 14. Zhang SX, Sanders E, Fliesler SJ, Wang JJ.. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp Eye Res. 2014; 125: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorbatyuk MS, Starr CR, Gorbatyuk OS.. Endoplasmic reticulum stress: new insights into the pathogenesis and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2020; 79(100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K.. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010; 16(11-12): 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ron D, Walter P.. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8(7): 519–529. [DOI] [PubMed] [Google Scholar]
- 18. Harding HP ZY, Bertolotti A, Zeng H, Ron D.. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5(5): 897–904. [DOI] [PubMed] [Google Scholar]
- 19. Cui W, Li J, Ron D, Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr. 2011; 67(Pt 5): 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song JY, Fan B, Che L, et al.. Suppressing endoplasmic reticulum stress-related autophagy attenuates retinal light injury. Aging (Albany NY). 2020; 12(16): 16579–16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shacham T, Patel C, Lederkremer GZ.. PERK pathway and neurodegenerative disease: to inhibit or to activate? Biomolecules. 2021; 11(3): 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grande V, Ornaghi F, Comerio L, et al.. PERK inhibition delays neurodegeneration and improves motor function in a mouse model of Marinesco-Sjogren syndrome. Hum Mol Genet. 2018; 27(14): 2477–2489. [DOI] [PubMed] [Google Scholar]
- 23. Liu H, Mei FC, Yang W, et al.. Epac1 inhibition ameliorates pathological angiogenesis through coordinated activation of Notch and suppression of VEGF signaling. Sci Adv. 2020; 6(1): eaay3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu W, Xia F, Ha Y, et al.. Neuroprotective effects of HSF1 in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2019; 60(4): 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ameri H, Liu H, Liu R, et al.. TWEAK/Fn14 pathway is a novel mediator of retinal neovascularization. Invest Ophthalmol Vis Sci. 2014; 55(2): 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stahl A, Connor KM, Sapieha P, et al.. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009; 12(3): 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu W, Ha Y, Xia F, et al.. Neuronal Epac1 mediates retinal neurodegeneration in mouse models of ocular hypertension. J Exp Med. 2020; 217(4): e20190930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narayanan SP, Xu Z, Putluri N, et al.. Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. Cell Death Dis. 2014; 5(2): e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narayanan SP, Suwanpradid J, Saul A, et al.. Arginase 2 deletion reduces neuro-glial injury and improves retinal function in a model of retinopathy of prematurity. PLoS One. 2011; 6(7): e22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gersztenkorn D, Coletta C, Zhu S, et al.. Hydrogen sulfide contributes to retinal neovascularization in ischemia-induced retinopathy. Invest Ophthalmol Vis Sci. 2016; 57(7): 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B.. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010; 107(7): 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atkins C, Liu Q, Minthorn E, et al.. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013; 73(6): 1993–2002. [DOI] [PubMed] [Google Scholar]
- 33. Hughes DT, Halliday M, Smith HL, et al.. Targeting the kinase insert loop of PERK selectively modulates PERK signaling without systemic toxicity in mice. Sci Signal. 2020; 13(644): eabb4749. [DOI] [PubMed] [Google Scholar]
- 34. Axten JM, Medina JR, Feng Y, et al.. Discovery of 7-methyl-5-(1-[3-(trifluoromethyl)phenyl]acetyl-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012; 55(16): 7193–7207. [DOI] [PubMed] [Google Scholar]
- 35. McLaughlin M, Pedersen M, Roulstone V, et al.. The PERK inhibitor GSK2606414 enhances reovirus infection in head and neck squamous cell carcinoma via an ATF4-dependent mechanism. Mol Ther Oncolytics. 2020; 16: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang X, Wei Y, Zhang T, et al.. Effects of GSK2606414 on cell proliferation and endoplasmic reticulum stress‑associated gene expression in retinal pigment epithelial cells. Mol Med Rep. 2017; 15(5): 3105–3110. [DOI] [PubMed] [Google Scholar]
- 37. Zhou L, Xu Z, Oh Y, et al.. Myeloid cell modulation by a GLP-1 receptor agonist regulates retinal angiogenesis in ischemic retinopathy. JCI Insight. 2021; 6(23): e93382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun X, Ma L, Li X, Wang J, Li Y, Huang Z.. Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-kappaB axis. Front Immunol. 2022; 13: 976729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dailey WA, Drenser KA, Wong SC, et al.. Norrin treatment improves ganglion cell survival in an oxygen-induced retinopathy model of retinal ischemia. Exp Eye Res. 2017; 164: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang C, Lafleur J, Mwaikambo BR, et al.. The role of lysophosphatidic acid receptor (LPA1) in the oxygen-induced retinal ganglion cell degeneration. Invest Ophthalmol Vis Sci. 2009; 50(3): 1290–1298. [DOI] [PubMed] [Google Scholar]
- 41. Zhang P, McGrath B, Li S, et al.. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002; 22(11): 3864–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001; 7(6): 1153–1163. [DOI] [PubMed] [Google Scholar]
- 43. Mercado G, Castillo V, Soto P, et al.. Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson's disease. Neurobiol Dis. 2018; 112: 136–148. [DOI] [PubMed] [Google Scholar]
- 44. Yan F, Cao S, Li J, et al.. Pharmacological inhibition of PERK attenuates early brain injury after subarachnoid hemorrhage in rats through the activation of Akt. Mol Neurobiol. 2017; 54(3): 1808–1817. [DOI] [PubMed] [Google Scholar]
- 45. Radford H, Moreno JA, Verity N, Halliday M, Mallucci GR.. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 2015; 130(5): 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, Fu Y, Xu X, et al.. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide. 2014; 40: 10–16. [DOI] [PubMed] [Google Scholar]
- 47. Zhu S, Liu H, Sha H, Qi L, Gao DS, Zhang W.. PERK and XBP1 differentially regulate CXCL10 and CCL2 production. Exp Eye Res. 2017; 155: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kasetti RB, Patel PD, Maddineni P, et al.. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat Commun. 2020; 11(1): 5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhootada Y, Kotla P, Zolotukhin S, et al.. Limited ATF4 expression in degenerating retinas with ongoing ER stress promotes photoreceptor survival in a mouse model of autosomal dominant retinitis pigmentosa. PLoS One. 2016; 11(5): e0154779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX.. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012; 61(2): 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sato K, Sato T, Ohno-Oishi M, et al.. CHOP deletion and anti-neuroinflammation treatment with hesperidin synergistically attenuate NMDA retinal injury in mice. Exp Eye Res. 2021; 213(108826. [DOI] [PubMed] [Google Scholar]
- 52. Nashine S, Liu Y, Kim BJ, Clark AF, Pang IH.. Role of C/EBP homologous protein in retinal ganglion cell death after ischemia/reperfusion injury. Invest Ophthalmol Vis Sci. 2014; 56(1): 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu Y, Park KK, Yang L, et al.. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012; 73(3): 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang W, Yokota H, Xu Z, et al.. Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model. Invest Ophthalmol Vis Sci. 2011; 52(9): 6384–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005; 280(21): 20331–20339. [DOI] [PubMed] [Google Scholar]
- 56. Hu Y, Lu X, Xu Y, et al.. Salubrinal attenuated retinal neovascularization by inhibiting CHOP-HIF1alpha-VEGF pathways. Oncotarget. 2017; 8(44): 77219–77232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pollreisz A, Afonyushkin T, Oskolkova OV, Gruber F, Bochkov VN, Schmidt-Erfurth U.. Retinal pigment epithelium cells produce VEGF in response to oxidized phospholipids through mechanisms involving ATF4 and protein kinase CK2. Exp Eye Res. 2013; 116: 177–184. [DOI] [PubMed] [Google Scholar]
- 58. Liu Z, Shi H, Xu J, et al.. Single-cell transcriptome analyses reveal microglia types associated with proliferative retinopathy. JCI Insight. 2022; 7(23): e160940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao C, Liu Y, Meng J, et al.. LGALS3BP in microglia promotes retinal angiogenesis through PI3K/AKT pathway during hypoxia. Invest Ophthalmol Vis Sci. 2022; 63(8): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang T, Tsirukis DI, Sun Y.. Targeting neuroinflammation in neovascular retinal diseases. Front Pharmacol. 2020; 11: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marneros AG. Role of inflammasome activation in neovascular age-related macular degeneration. FEBS J. 2023; 290(1): 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jiang HY, Wek SA, McGrath BC, et al.. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003; 23(16): 5651–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meares GP, Liu Y, Rajbhandari R, et al.. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol. 2014; 34(20): 3911–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]