Absolute risks of uterine perforation and expulsion associated with intrauterine devices are low but vary with breastfeeding, postpartum insertion timing, and heavy menstrual bleeding status.
Abstract
OBJECTIVE:
The APEX-IUD (Association of Perforation and Expulsion of Intrauterine Devices) study evaluated the association of postpartum timing of intrauterine device (IUD) insertion, breastfeeding, heavy menstrual bleeding, and IUD type (levonorgestrel-releasing vs copper) with risks of uterine perforation and IUD expulsion in usual clinical practice. We summarize the clinically important findings to inform counseling and shared decision making.
METHODS:
APEX-IUD was a real-world (using U.S. health care data) retrospective cohort study of individuals aged 50 years and younger with IUD insertions between 2001 and 2018 and with electronic health record data. Cumulative incidences of uterine perforation and IUD expulsion were calculated. Adjusted hazard ratios (aHRs) and 95% CIs were estimated from proportional hazards models with control of confounding.
RESULTS:
Among the study population of 326,658, absolute risk of uterine perforation was low overall (cumulative incidence, 0.21% [95% CI 0.19–0.23%] at 1 year and 0.61% [95% CI 0.56–0.66% at 5 years]) but was elevated for IUDs inserted during time intervals within 1 year postpartum, particularly among those between 4 days and 6 weeks postpartum (aHR 6.71, 95% CI 4.80–9.38), relative to nonpostpartum insertions. Among postpartum insertions, IUD expulsion risk was greatest for insertions in the immediate postpartum period (0–3 days after delivery) compared with nonpostpartum (aHR 5.34, 95% CI 4.47–6.39). Postpartum individuals who were breastfeeding had a slightly elevated risk of perforation and lowered risk of expulsion than those not breastfeeding. Among nonpostpartum individuals, those with a heavy menstrual bleeding diagnosis were at greater risk of expulsion than those without (aHR 2.84, 95% CI 2.66–3.03); heavy menstrual bleeding also was associated with a slightly elevated perforation risk. There was a slightly elevated perforation risk and slightly lower expulsion risk associated with levonorgestrel-releasing IUDs compared with copper IUDs.
CONCLUSION:
Absolute risk of adverse outcomes with IUD insertion is low. Clinicians should be aware of the differences in risks of uterine perforation and expulsion associated with IUD insertion during specific postpartum time periods and with a heavy menstrual bleeding diagnosis. This information should be incorporated into counseling and decision making for patients considering IUD insertion.
FUNDING SOURCE:
Bayer AG.
CLINICAL TRIAL REGISTRATION:
EU PAS register, EUPAS33461.
Intrauterine devices (IUDs) provide highly effective long-term contraception, with an estimated risk of unintended pregnancy of 0.9% across 3 years.1 Among women of reproductive age, an estimated 14% worldwide and 12% in the United States use an IUD.2,3 Potential adverse outcomes associated with IUD use include IUD expulsion and, rarely, IUD-related uterine perforation. The expulsion of an IUD may result in unintended pregnancy if undetected. An IUD-related uterine perforation, if complete, requires surgery to retrieve the device from the abdominal cavity and, if partial, may require hysteroscopy for removal. It is important for health care professionals to understand the magnitude of these risks and differences by patient characteristics and IUD insertion–related factors to contextualize and individualize patient counseling.
Large cohort studies have evaluated the risk of IUD-related uterine perforation in European and U.S. populations.4–8 The European Active Surveillance Study for Intrauterine Devices observed an increased risk of uterine perforation with IUD insertions within 36 weeks of delivery and with breastfeeding near the time of IUD insertion.4,5 In response to these findings, this retrospective cohort study, APEX-IUD (Association of Perforation and Expulsion of Intrauterine Devices), was initiated as a postmarketing requirement by the U.S. Food and Drug Administration to evaluate the incidence of IUD-related uterine perforation and IUD expulsion and their association with postpartum timing and breastfeeding status at IUD insertion among U.S. individuals. In the United States, compared with European Union countries, it has recently become more common to place IUDs in the immediate postpartum period, most commonly just after delivery of the placenta; thus, understanding the risk of uterine perforation in relation to the duration of time between a delivery and IUD placement was of particular interest.
The APEX-IUD study aimed to assess whether the timing of postpartum IUD insertion, breastfeeding, IUD type (levonorgestrel [LNG]-releasing vs copper IUD), or heavy menstrual bleeding was associated with a higher risk of uterine perforation and IUD expulsion in U.S. individuals. Prior publications have described the study methods, outcome validation, and results of these analyses in detail.6–11 A separate analysis, requested by the European Medicines Association, evaluated whether certain demographic, reproductive, and medical variables, including younger age at IUD insertion, race and ethnicity, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), parity, history of heavy menstrual bleeding, and history of dysmenorrhea, were independent risk factors for IUD expulsion among nonpostpartum individuals.12 The aim of this article is to summarize the clinically important findings from previous APEX-IUD publications to inform counseling, shared decision making, and consent for individuals who use or are considering IUD use (Box 1).
Box 1. Informed Consent and Counseling Points for Patients Considering an Intrauterine Device.
IUD INSERTION BY POSTPARTUM STATUS
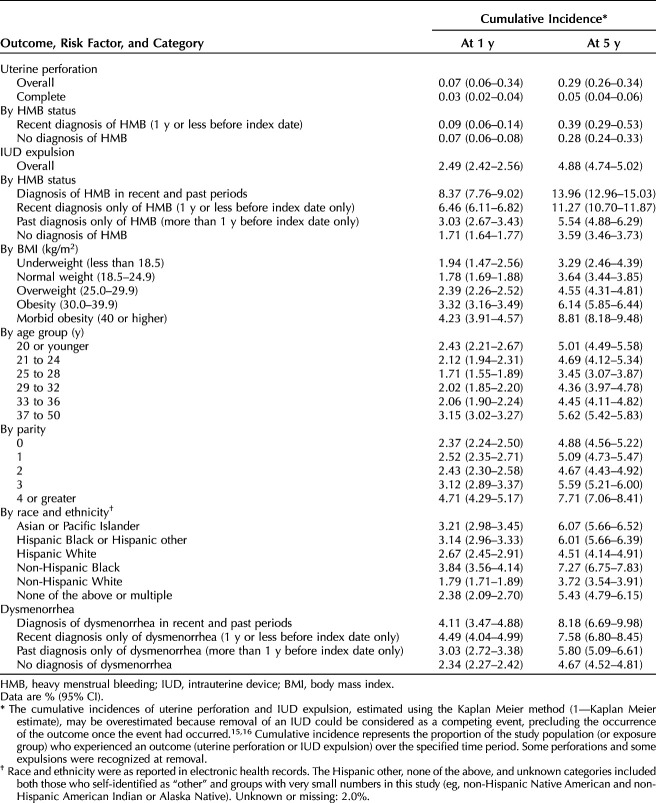
• Nulliparous individuals and those beyond 52 weeks postpartum at insertion can be reassured that the absolute risk of complete uterine perforation requiring surgical intervention is extremely low, with a cumulative incidence of 0.03% at 1 year and 0.05% at 5 years.
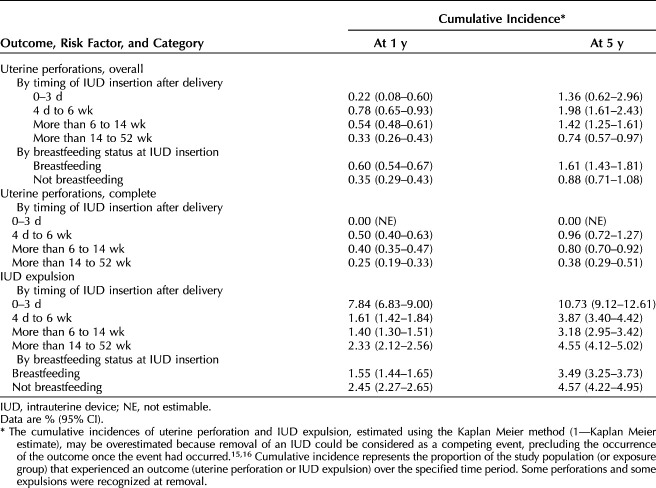
• Uterine perforation is slightly greater within 52 weeks postpartum and is greatest between 4 days and 6 weeks postpartum.
• For individuals considering immediate postpartum IUD insertion (within 3 days), partial uterine perforation is rare (cumulative incidence of 0.22% at 1 year and 1.36% at 5 years), and there were no complete perforations; however, this low risk must be considered in the context of known incidence of expulsion of approximately 8% at 1 year and 11% at 5 years and the potential need for immediate and effective contraception should expulsion occur. These findings should be interpreted with caution due to smaller numbers of insertions in this group (n=2,788).
• Risk of adverse outcomes with postpartum IUD insertion should be discussed antenatally to ensure informed decision making about the optimal timing of insertion.
USE OF IUDS IN BREASTFEEDING PATIENTS
• The modestly higher risk of uterine perforation and lower risk of IUD expulsion associated with breastfeeding should be considered alongside the known benefits of breastfeeding in shared decision-making discussions with patients who are considering IUD use during the postpartum period.
USE OF IUDS IN PATIENTS WITH HMB
• The risk of IUD-related uterine perforation is slightly higher in individuals with HMB.
• Although the risk of expulsion is 3 times higher in patients with recent HMB than in patients without recent HMB, the potential benefit of treating HMB with an LNG-IUD may outweigh the low absolute risk. Patients should be aware of the increased risk of expulsion with HMB, and follow-up may be individualized.
• Hormonal IUDs were used more often than copper IUDs in patients with recent HMB (96% vs 2%),7 highlighting the known clinical benefit of this method to treat HMB.
RISKS BY IUD TYPE
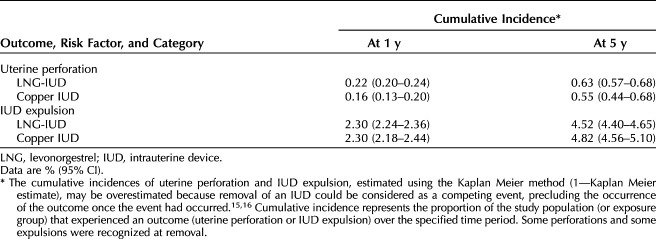
• Although LNG-IUDs were associated with a slightly elevated risk of IUD-related uterine perforation and a slightly lower risk of IUD expulsion relative to copper IUDs, our results do not suggest clinically significant differences in perforation or expulsion risk with LNG-IUDs vs copper IUDs, as the differences in cumulative incidence for these outcomes are exceedingly low.
RISK FACTORS FOR EXPULSION
• IUDs are an effective long-term contraceptive. IUD expulsion is uncommon, but patient counseling should include signs and symptoms of expulsion and what to do in the event of IUD expulsion, in particular in patients at higher risk, including those with chronic and persistent HMB, those with BMIs higher than 25, those with parity of 4 or greater, and those younger than age 24 years.
CLINICAL SUMMARY
• The cumulative incidence of IUD-related uterine perforation is approximately 0.2% at 1 year and 0.6% at 5 years, and approximately half of the perforations are complete.
• Overall risk of IUD expulsion is nearly 5% at 5 years.
• Where an individual's risk factors suggest a potentially higher risk of uterine perforation, ultrasound guidance at insertion may be considered in an attempt to optimize placement and reduce perforation risk. Patients should be counseled about signs of and what to do in the event of device expulsion. Individual risk factors for IUD perforation and expulsion should be considered at the time of consent for an IUD insertion procedure and risks put into perspective. Risks associated with IUD insertion may be outweighed by the risks associated with unintended pregnancy.
IUD, intrauterine device; HMB, heavy menstrual bleeding; LNG, levonorgestrel.
METHODS
The APEX-IUD study was a retrospective cohort study that used data from electronic health records; it was conducted within three health care systems (Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington) and a research site using data from a health care information exchange in Indiana (Regenstrief Institute). The study design and validation have been described in detail previously.9,11 Each research site received institutional review board approval or an exemption for the conduct of this study, which qualified for a waiver of informed consent requirements. Kaiser Permanente Southern California also received approval from California state agencies for the use of vital statistics data.
The demographically diverse study population included individuals identified as female who were aged 50 years or younger at the time of the IUD insertion and were in the health care system for at least 52 weeks before insertion. Patients were followed from IUD insertion until the occurrence of an outcome (IUD-related uterine perforation or IUD expulsion) or a censoring event (IUD removal, reinsertion, or expiration; pregnancy; hysterectomy or other sterilization procedure; death; disenrollment from the health care system; or end of the study period, June 30, 2018), whichever occurred first.
In the primary analyses, potential risk factors of interest included postpartum status at IUD insertion (0–3 days, 4 days to 6 weeks, more than 6 to 14 weeks, or more than 14 to 52 weeks after a delivery, or nonpostpartum [more than 52 weeks postpartum or nulliparous]); breastfeeding status at the time of IUD insertion (yes or no, among those with a delivery in the previous 52 weeks); diagnosis of heavy menstrual bleeding in the 52 weeks before IUD insertion (identified using International Classification of Diseases, Ninth Revision and Tenth Revision, Clinical Modification codes); and type of IUD inserted (levonorgestrel-releasing vs copper). For the heavy menstrual bleeding risk factor analysis, only those without a delivery in the 52 weeks before insertion were included. An additional analysis evaluated other potential risk factors for expulsion in nonpostpartum individuals, including age, race and ethnicity, BMI, parity, experience with heavy menstrual bleeding (recent diagnosis [1 year or less before index date only], past diagnosis [more than 1 year before index date only], or both), and diagnosis of dysmenorrhea. Race and ethnicity were collected in this study as a potential risk factor for expulsion.
The outcomes of interest were IUD-related uterine perforation and IUD expulsion, both identified by the date on which the outcome was documented in the records. A perforation could have been recognized at the time of insertion or later; a diagnosis at more than 1 year after insertion in asymptomatic individuals is not uncommon. Perforation was defined as complete (IUD presence in the pelvis or abdominal cavity) or partial (IUD embedded in the myometrium as visualized on imaging or hysteroscopy with subsequent IUD removal, or partial perforation noted by a clinician at the time of removal). Expulsion was defined as complete (IUD located in the vagina, or not present in the uterus or abdomen on imaging, or the patient reported that the IUD was expelled or “fell out”) or partial (any portion of IUD in the cervix on imaging or visualized by the clinician, or IUD malpositioned on imaging and removed by the clinician).
Characteristics of the study population were analyzed descriptively, including frequencies and percentages for categorical variables and mean, SD, median, and range for continuous variables. For each risk factor and outcome pairing, unadjusted 1-year and 5-year cumulative incidences and adjusted hazard ratios (aHRs) with 95% CIs were calculated. Cumulative incidence was calculated using the Kaplan Meier method. Hazard ratios (see Fig. 1) were estimated from proportional hazards models, with control of confounding through the use of propensity scores and overlap weighting in the hazard ratio models,13 on the basis of the values of covariates before or at the time of IUD insertion. Covariates for the propensity score models were chosen a priori or based on their association with the study outcome and confounding effects (see Anthony et al).11
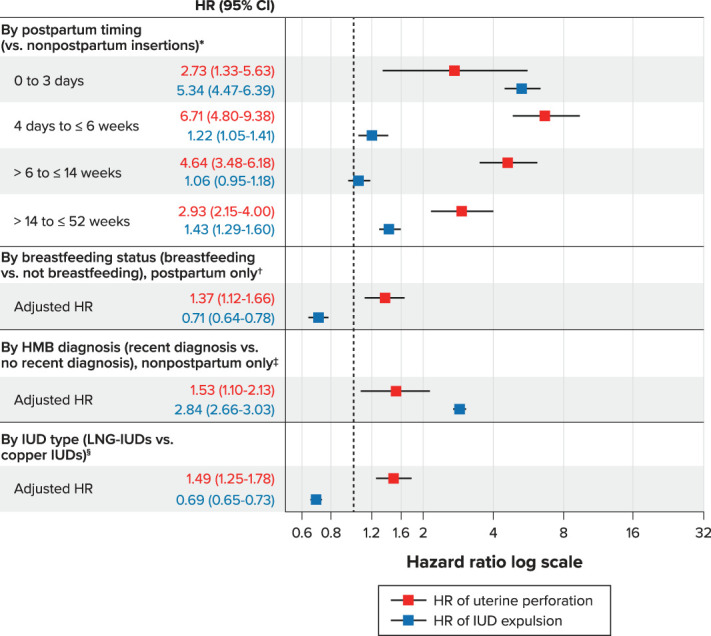
Fig. 1. Adjusted hazard ratios (HRs) for uterine perforation and intrauterine device (IUD) expulsion, by risk factor. Adjusted HRs were calculated using Cox models weighted with propensity score overlap weights. *For the postpartum timing risk factor, the following variables were included in the propensity score models for adjustment: IUD type, menorrhagia, age (tertiles), race and ethnicity, recent smoker (uterine perforation only), duration of look-back period (quartiles, uterine perforation only), calendar year of IUD insertion, body mass index (categorical), dysmenorrhea, uterine leiomyomas, parity, concomitant gynecologic procedure, difficult insertion, health care professional experience, site, calendar year×site, parity×site, age (tertiles)×site, and recent smoker×site (uterine perforation only). HMB, heavy menstrual bleeding; LNG, levonorgestrel. †For the breastfeeding risk factor, the following variables were included in the propensity score models for adjustment: postpartum timing, IUD type, menorrhagia, age (tertiles), race/ethnicity, recent smoker (uterine perforation only), duration of look-back period (quartiles, uterine perforation only), calendar year of IUD insertion, BMI (categorical), dysmenorrhea, uterine fibroids, parity, cesarean delivery any time before the index date (uterine perforation only), cesarean delivery for the most recent delivery (uterine perforation only), concomitant gynecologic procedures, difficult insertion, healthcare professional experience, live birth in 52 weeks before IUD insertion, site, and postpartum×site interaction. ‡For the HMB risk factor, the following variables were included in the propensity score models for adjustment: postpartum status (4 categories), breastfeeding status, IUD type, age (continuous for perforation, tertiles for expulsion), race/ethnicity, recent smoker (uterine perforation only), duration of look-back period (quartiles, uterine perforation only), calendar year of IUD insertion, BMI (categorical), dysmenorrhea, uterine fibroids, parity, cesarean delivery any time before the index date (only for perforation), cesarean delivery for the most recent delivery, live birth for the most recent delivery, concomitant gynecologic procedure, indicator of difficult IUD insertion, healthcare professional experience (quartiles), research site, and age (continuous for perforation and tertile for expulsion)×site interaction. §For the IUD type risk factor, the following variables were included in the propensity score models for adjustment: postpartum status (4 categories), breastfeeding status, menorrhagia diagnosis in the last 52 weeks, age (tertiles), race/ethnicity, recent smoker (uterine perforation only), duration of look-back period (quartiles, uterine perforation only), calendar year of IUD insertion (categorical), BMI (categorical), dysmenorrhea, uterine fibroids, parity, cesarean delivery at any time before the index date, cesarean delivery for the most recent delivery (uterine perforation only), live birth for the most recent delivery, concomitant gynecologic procedure, indicator of difficult IUD insertion, healthcare professional experience (quartiles), live birth in 52 weeks before IUD insertion, research site, and postpartum×site interaction (IUD expulsion only).
Fassett. Uterine Perforation and Expulsion Risks With IUDs. Obstet Gynecol 2023.
RESULTS
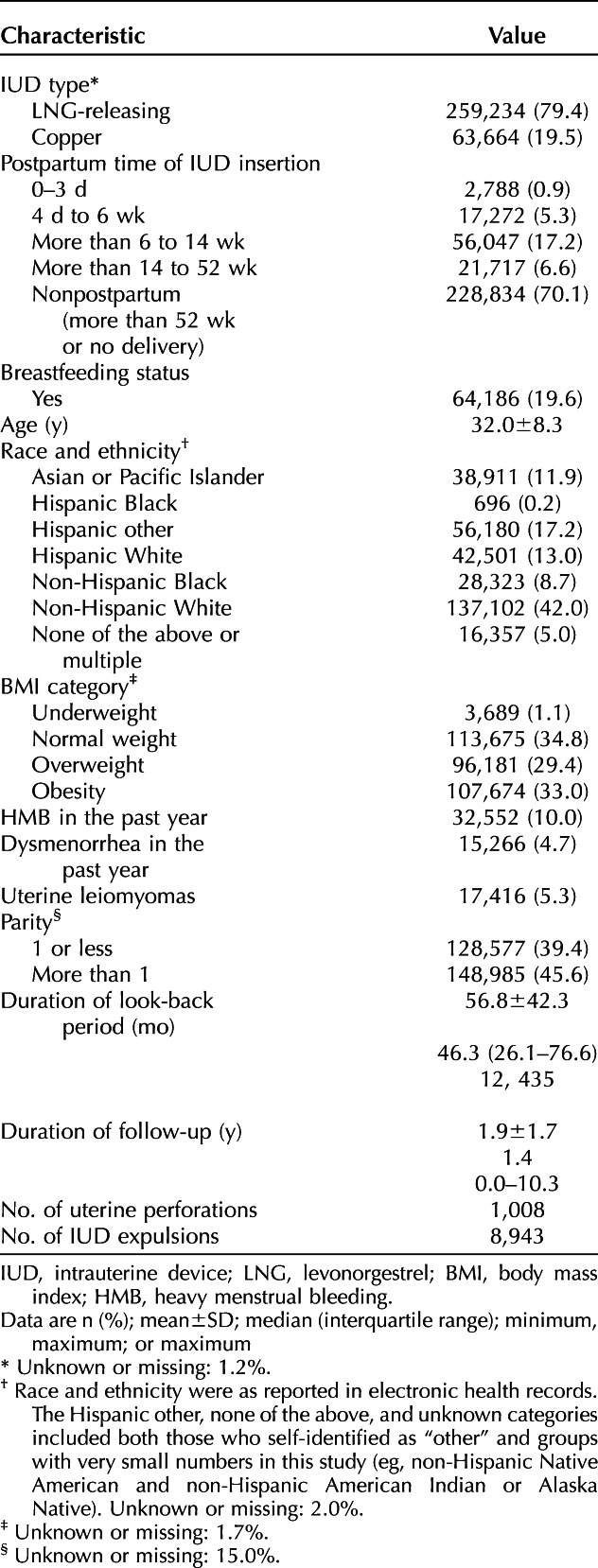
Among the racially and ethnically diverse study population of 326,658 individuals, 259,234 (79.4%) had an LNG-releasing IUD, and 63,664 (19.5%) had a copper IUD (Table 1) at IUD insertion. The mean±SD age at IUD insertion was 32.0±8.3 years. Most were nonpostpartum (n=228,834; 70.1%). Among postpartum individuals (n=97,824), most insertions occurred at more than 6 weeks to 14 weeks after delivery (n=56,047; 57.3%), followed by more than 14 weeks to 52 weeks after delivery (n=21,717; 22.2%) and then 4 days to 6 weeks after delivery (n=17,272; 17.7%); 2,788 insertions (2.9%) occurred 0–3 days postpartum (more than 98% of these insertions occurred on day 0). The median duration of follow-up from IUD insertion to an outcome or censoring event was 1.4 years (range 0.0–10.3 years).
Table 1.
Characteristics of the Study Population at the Time of Intrauterine Device Insertion (N=326,658)
A total of 1,008 uterine perforations were recognized (516 [51.2%] complete, 488 [48.4%] partial, and four [0.4%] of undetermined type). Overall cumulative 1-year and 5-year incidences of uterine perforation were 0.21% (95% CI 0.19–0.23%) and 0.61% (95% CI 0.56–0.66%), respectively (data not shown). A total of 8,943 IUD expulsions were recognized. Overall cumulative 1-year and 5-year incidences of expulsion were 2.29% (95% CI 2.24–2.35%) and 4.57% (95% CI 4.45–4.68%), respectively (data not shown).
Nonpostpartum individuals had the lowest 5-year cumulative incidence of uterine perforation (0.29% [95% CI 0.26–0.34%]) (Table 2). The 5-year cumulative incidence of uterine perforation was higher in those with postpartum IUD insertions across all postpartum timing intervals, compared with nonpostpartum individuals and was highest with insertions at 4 days to 6 weeks after a delivery (5-year cumulative incidence, 1.98% [95% CI 1.61–2.43%]) (Table 3). Although the absolute cumulative incidence of uterine perforation was low, perforation risk (based on aHRs) was consistently elevated among those with postpartum IUD insertions (0–3 days, 4 days to 6 weeks, more than 6 weeks to 14 weeks, or more than 14 weeks to 52 weeks) compared with nonpostpartum individuals (Fig. 1). The risk of perforation in individuals with insertions 4 days to 6 weeks postpartum was estimated to be 6.7 times that in nonpostpartum women (aHR 6.71; 95% CI 4.80–9.38); postpartum individuals with an insertion within 3 days of delivery had the lowest risk among those with insertions within 1 year postpartum (2.7 times the risk of perforation, all were partial) compared with those who were nonpostpartum (both partial and complete) (aHR 2.73; 95% CI 1.33–5.63).
Table 2.
Cumulative Incidence of Uterine Perforation and Intrauterine Device Expulsion, Nonpostpartum Individuals (More Than 52 Weeks Postpartum or Nulliparous) (n=228,834)
Table 3.
Cumulative Incidence of Uterine Perforation and Intrauterine Device Expulsion Within 52 Weeks Postpartum (n=97,824)
Among nonpostpartum insertions, 25.6% (79 of 309) of uterine perforations were complete perforations. The 5-year cumulative incidence of complete perforation in nonpostpartum individuals was 0.05% (95% CI 0.04–0.06%) (Table 2). Among postpartum individuals, complete perforations had the highest 5-year cumulative incidence in individuals with an IUD insertion 4 days to 6 weeks postpartum (0.96%; 95% CI 0.72–1.27%), and none were diagnosed in individuals with an insertion 0–3 days postpartum (Table 3).
Nonpostpartum individuals had a 5-year cumulative incidence of IUD expulsion of 4.88% (95% CI 4.74–5.02%). Among postpartum individuals, those with insertions at more than 6 weeks to 14 weeks postpartum had the lowest 5-year cumulative incidence of IUD expulsion (3.18% [95% CI 2.95–3.42%]), and those with insertions 0–3 days postpartum had the highest 5-year cumulative incidence (10.73% [95% CI 9.12–12.61%]) (Table 3). After adjustment, the risk of IUD expulsion was elevated in those with IUD insertions 0–3 days postpartum (five times higher than in nonpostpartum individuals [aHR 5.34, 95% CI 4.47–6.39]), in those with insertions 4 days to 6 weeks postpartum relative to nonpostpartum insertions (aHR 1.22; 95% CI 1.05–1.41), and in those with insertions 14 weeks to at least 52 weeks postpartum relative to nonpostpartum insertions (aHR 1.43; 95% CI 1.29–1.60) (Fig. 1).
Among those with an IUD insertion within 52 weeks of delivery, the 5-year cumulative incidence of perforation was almost two times higher in breastfeeding individuals than in nonbreastfeeding individuals (1.61% [95% CI 1.43–1.81%] vs 0.88% [95% CI 0.71–1.08%]) (Table 3). After adjustment, postpartum individuals who were breastfeeding near the time of IUD insertion had an estimated risk of uterine perforation that was 1.4 times that of postpartum individuals who were not breastfeeding (aHR 1.37; 95% CI 1.12–1.66) (Fig. 1).
Among breastfeeding individuals, 340 of 526 uterine perforations (64.6%) were complete, and, among nonbreastfeeding individuals, 80 of 147 perforations (54.4%) were complete—both proportionately greater than partial perforations (data not shown).
Among those with an IUD insertion within 52 weeks of delivery, the 5-year cumulative incidence of IUD expulsion was lower in breastfeeding individuals than in nonbreastfeeding individuals (3.49% [95% CI 3.25–3.73%] vs 4.57% [95% CI 4.22–4.95%]) (Table 3). After adjustment, postpartum individuals who were breastfeeding near the time of IUD insertion had 0.7 times the risk of IUD expulsion relative to postpartum individuals who were not breastfeeding (aHR 0.71; 95% CI 0.64–0.78) (Fig. 1).
Among nonpostpartum individuals, those with a recent diagnosis of heavy menstrual bleeding (n=31,600) had a 5-year cumulative incidence of uterine perforation of 0.39% (95% CI 0.29–0.53%); those without a recent diagnosis of heavy menstrual bleeding (n=197,234) had a 5-year cumulative incidence of 0.28% (95% CI 0.24–0.33%) (Table 2). After adjustment, the risk of uterine perforation in those diagnosed with heavy menstrual bleeding was 1.5 times that in those without such a diagnosis (aHR 1.53; 95% CI 1.10–2.13) (Fig. 1).
Nonpostpartum individuals with a heavy menstrual bleeding diagnosis in the recent period (at least 1 year before insertion), past period (more than 1 year before insertion), or both periods had a higher 5-year cumulative incidence of IUD expulsion than those without a heavy menstrual bleeding diagnosis (Table 2). Those with a heavy menstrual bleeding diagnosis in the recent and past periods had the highest 5-year cumulative incidence of IUD expulsion (13.96%; 95% CI 12.96–15.03%). After adjustment, the risk of IUD expulsion was approximately three times higher in those with a diagnosis of heavy menstrual bleeding in the previous 52 weeks than in those without such a diagnosis (aHR 2.84; 95% CI 2.66–3.03) (Fig. 1).
Individuals with LNG-IUDs had a 5-year cumulative incidence of uterine perforation of 0.63% (95% CI 0.57–0.68%), and those with copper IUDs had a 5-year cumulative incidence of 0.55% (95% CI 0.44–0.68%) (Table 4). After adjustment, those with LNG-IUDs had an estimated risk of uterine perforation that was 1.5 times that of those with copper IUDs (aHR 1.49; 95% CI 1.25–1.78) (Fig. 1).
Table 4.
Cumulative Incidence of Uterine Perforation and Intrauterine Device Expulsion, by Intrauterine Device Type, Among Both Postpartum and Nonpostpartum Individuals With Known Intrauterine Device Type (n=322,898)
Five-year cumulative incidence of IUD expulsion was similar for LNG-IUDs and copper IUDs (4.52% [95% CI 4.40–4.65%] and 4.82% [95% CI 4.56–5.10%], respectively) (Table 4). After adjustment, the risk of IUD expulsion in individuals with LNG-IUDs was 0.7 times that of those with copper IUDs (aHR 0.69; 95% CI 0.65–0.73) (Fig. 1).
Of the six potential risk factors for IUD expulsion that were evaluated (age, race and ethnicity, BMI, parity, experience with heavy menstrual bleeding, and experience with dysmenorrhea), after mutual adjustment for other risk factors and covariates, heavy menstrual bleeding was associated with the highest expulsion risk, particularly in those with chronic and persistent heavy menstrual bleeding (5-year cumulative incidence, 13.96%; 95% CI 12.96–15.03%). Higher BMI; younger age at IUD insertion; four or more previous births; and being a member of non-Hispanic Black, Hispanic Black, Hispanic White, Asian or Pacific Islander, and multiple racial and ethnic groups (compared with non-Hispanic White) were all associated with a higher risk of IUD expulsion when accounting for other potential confounders; however, the 5-year cumulative incidence was modest at less than 10%. After adjustment for other risk factors and covariates, dysmenorrhea was not meaningfully associated with an elevated risk of expulsion.
DISCUSSION
The APEX-IUD study demonstrated low overall incidences of IUD-associated uterine perforation and IUD expulsion. The study findings expand on existing evidence demonstrating IUDs to be safe, with rare instances of adverse outcomes.
Clinicians should be aware of the small but relatively higher risks of uterine perforation associated with IUD insertion within 1 year postpartum compared with nonpostpartum insertion. Uterine perforation risk appeared to be greatest for those with IUDs placed from 4 days to 6 weeks postpartum, with a cumulative incidence of 1.98% at 5 years—more than six times that of nonpostpartum insertions, the cumulative incidence of which was 0.29% at 5 years. Nonetheless, uterine perforation was rare overall regardless of postpartum status and timing. Approximately half (51%) of uterine perforations were complete; clinical management for complete perforation requires intra-abdominal surgery by laparoscopy or laparotomy. The 5-year cumulative incidence of perforations that were determined to be complete was lower with nonpostpartum IUD insertions (0.05%) than with most postpartum insertion categories (4 days–6 weeks, 0.96%; 6–14 weeks, 0.80%; 14–52 weeks, 0.38%), except for the group with insertion at 0–3 days postpartum (0%). Timing of the insertion of an IUD should be a shared decision between the patient and the health care professional. Although the risk of IUD complications between 4 days and 6 weeks postpartum is increased, the absolute increased risk remains low.
Intrauterine device expulsion was also uncommon in APEX-IUD, albeit more frequent than uterine perforation, and was more frequent with immediate postpartum placement (within 3 days after delivery)—a practice that has become increasingly common since the study was completed—than with placement during later postpartum periods or in nonpostpartum individuals. Those with a delivery in the past year were at higher risk of uterine perforation when breastfeeding, particularly in the later postpartum periods, compared with those who were not, but breastfeeding individuals were at a lower risk of expulsion. Among nonpostpartum individuals, those diagnosed with heavy menstrual bleeding were at increased risk of expulsion and had a low absolute risk of perforation that was higher than that in nonpostpartum individuals without heavy menstrual bleeding. Although LNG-IUDs appeared to be associated with a higher risk of uterine perforation and a lower risk of expulsion than copper IUDs, the clinical relevance of the differences in both outcomes by IUD type is debatable.
The information presented here can assist the clinician in counseling patients about individual risks during preprocedure counseling and facilitate shared decision making. Timing of IUD placement and methods to reduce the risk of uterine perforation can be considered. Ultrasound guidance may be helpful for IUD placement in postpartum and breastfeeding individuals but has not been evaluated nor demonstrated to mitigate risk; additional studies are needed to make evidence-based recommendations. Health care professional experience is an important factor that may moderate outcomes14; we controlled for health care professional experience in these analyses. Patients should be counseled about signs that may suggest potential perforation (eg, pelvic pain, severe cramps, strings unable to be palpated). Under conditions in which IUD expulsion rates are relatively higher (immediate postpartum IUD placement, heavy menstrual bleeding), having a low threshold for scheduling a follow-up visit to ensure the IUD is in place may also be considered. To prevent unplanned pregnancies with unrecognized expulsion, patients should be counseled about the risk of IUD expulsion and informed of signs of expulsion (eg, device felt in the vagina, change in cramping or bleeding pattern, strings unable to be palpated) and the need for IUD replacement or alternate form of contraception in the event of an expulsion. Health care professionals and patients should weigh the elevated risks in the postpartum period against known contraceptive benefits and the convenience of early insertion.
Strengths of the findings from APEX-IUD include the largest and most demographically diverse cohort with more than 1 year of follow-up reported to date, minimal missing data, and control for more than 20 confounding factors. Some limitations are noted, however. Given the use of retrospective data and the observational design, there was no protocol-specified plan for the timing of follow-up; the timing of IUD-related perforation and IUD expulsion reflect the dates of diagnosis and not necessarily the time of the outcomes themselves, and some perforation and expulsion outcomes were recognized only at the time of IUD removal. In particular, outcomes for patients who are undergoing an IUD insertion within 1 year postpartum may be subject to surveillance bias, because perforation or expulsion outcomes may have been recognized because of more frequent contact with health care professionals during this time. The study data were subject to potential misclassification of the outcomes and risk factors, unmeasured confounding, and loss to follow-up. There were relatively small numbers in the 0-to-3-day postpartum group, diminishing the precision of estimates.
Intrauterine devices are a highly effective and patient-friendly form of long-term contraception. Early postpartum insertions gained heightened importance in light of recent COVID-related constraints on access to routine medical care and current state restrictions on abortion, emphasizing the need to reduce barriers to obtaining highly effective contraception. With appropriate counseling and monitoring for potential risks of uterine perforation and IUD expulsion for IUD insertions known to have slightly higher risks (during the first year postpartum, among breastfeeding individuals, and among those with heavy menstrual bleeding), IUDs can provide reliable, safe, long-term protection against unintended pregnancy.
Footnotes
RTI Health Solutions (RTI-HS) led the design of the study, analysis, reporting, and interpretation of the results in collaboration with study team members from Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC), Kaiser Permanente Washington (KPWA), Regenstrief Institute (RI), and Bayer. KPNC, KPSC, KPWA, and RI extracted the data, reviewed and confirmed a portion of the outcomes, compiled the data sets, and transferred the data to RTI-HS. All co-authors had access to aggregated data and participated in the development of the protocol, statistical analysis plan, and study report. The contracts between Bayer AG and each of the other organizations (KPNC, KPSC, KPWA, RI, RTI-HS) include independent publication rights. Bayer AG was provided the opportunity to review the manuscript before submission, and comments were advisory only.
Financial Disclosure Susan Reed has received funding from the National Institutes of Health (NIH) and Bayer, as well as royalties from UpToDate on chapters related to endometrial hyperplasia. Jeffrey F. Peipert receives research support from Bayer, Merck, and CooperSurgical, and has served on advisory boards for Bayer, CooperSurgical, and OCON. Darios Getahun receives research support from NIH, NIEH, DHHS, NICHD, Patient-Centered Outcomes Research Institute, Garfield Memorial Fund, Bayer AG, and HOLOGIC, Inc. Jennifer Gatz disclosed money was paid to her institution from Cook, Lily, and Janssen. Michael J. Fassett receives research support from Garfield Memorial Fund, Bayer AG, and HOLOGIC, Inc. Federica Pisa, Juliane Schoendorf, and Yesmean Wahdan are employees of Bayer, the marketing authorization holder for 3 IUD brands, among others, that were included in this study. Jinyi Wang and Mary S. Anthony are employed at RTI Health Solutions, and Mary E. Ritchey was employed at RTI Health Solutions at the time the analysis was conducted. This organization worked under contract with Bayer to conduct the analyses. No payment or support of any kind was received for drafting or review of this manuscript. Tina Raine-Bennett, an employee of KPNC during the conduct of the study, is currently an employee of Medicines360, a marketing authorization holder for one of the IUD brands included in this study. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D281.
The APEX-IUD study team thanks Kaiser Permanente and Regenstrief Institute members who contributed electronic health information to this study. Kate Lothman of RTI-HS provided medical writing services, which were funded by RTI-HS.
Figure.

No available caption
REFERENCES
- 1.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855 [DOI] [PubMed] [Google Scholar]
- 2.Buhling KJ, Zite NB, Lotke P, Black K. INTRA Writing Group. Worldwide use of intrauterine contraception: a review. Contraception 2014;89:162–73. doi: 10.1016/j.contraception.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 3.Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception 2018;98:467–70. doi: 10.1016/j.contraception.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 4.Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception 2015;91:274–9. doi: 10.1016/j.contraception.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Barnett C, Moehner S, Do Minh T, Heinemann K. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contraception Reprod Health Care 2017;22:424–8. doi: 10.1080/13625187.2017.1412427 [DOI] [PubMed] [Google Scholar]
- 6.Reed SD, Zhou X, Ichikawa L, Gatz JL, Peipert JF, Armstrong MA, et al. Intrauterine device-related uterine perforation incidence and risk (APEX-IUD): a large multisite cohort study. The Lancet 2022;399:2103–12. doi: 10.1016/S0140-6736(22)00015-0 [DOI] [PubMed] [Google Scholar]
- 7.Getahun D, Fassett MJ, Gatz J, Armstrong MA, Peipert JF, Raine-Bennett T, et al. Association between menorrhagia and risk of intrauterine device–related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol 2022;227:59.e1–9. doi: 10.1016/j.ajog.2022.03.025 [DOI] [PubMed] [Google Scholar]
- 8.Gatz JL, Armstrong MA, Postlethwaite D, Raine-Bennett T, Chillemi G, Alabaster A, et al. Association between intrauterine device type and risk of perforation and device expulsion: results from the Association of Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol 2022;227:57.e1–13. doi: 10.1016/j.ajog.2022.03.062 [DOI] [PubMed] [Google Scholar]
- 9.Anthony MS, Armstrong MA, Getahun D, Scholes D, Gatz J, Schulze-Rath R, et al. Identification and validation of uterine perforation, intrauterine device expulsion, and breastfeeding in four health care systems with electronic health records. Clin Epidemiol 2019;11:635–43. doi: 10.2147/CLEP.S201044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong MA, Raine-Bennett T, Reed SD, Gatz J, Getahun D, Schoendorf J, et al. Association of the timing of postpartum intrauterine device insertion and breastfeeding with risks of intrauterine device expulsion. JAMA Netw Open 2022;5:e2148474. doi: 10.1001/jamanetworkopen.2021.48474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony MS, Reed SD, Armstrong MA, Getahun D, Gatz JL, Saltus CW, et al. Design of the Association of Uterine Perforation and Expulsion of Intrauterine Device Study: a multisite retrospective cohort study. Am J Obstet Gynecol 2021;224:599. e1–599.e18. doi: 10.1016/j.ajog.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 12.Anthony MS, Zhou X, Schoendorf J, Reed SD, Getahun D, Armstrong MA, et al. Demographic, reproductive, and medical risk factors for intrauterine device expulsion. Obstet Gynecol 2022;140:1017–30. doi: 10.1097/AOG.0000000000005000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc 2018;113:390–400. doi: 10.1080/01621459.2016.1260466. [DOI] [Google Scholar]
- 14.Stumpf PG, Lenker RM. Insertion technique, not design, affects expulsion rates of postpartum intrauterine device. Contraception 1984;30:327–30. doi: 10.1016/s0010-7824(84)80024-4 [DOI] [PubMed] [Google Scholar]
- 15.Andersen PK, Geskus RB, De Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–70. doi: 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]