Abstract
Background and Objectives
Recent data suggest increasing global prevalence of multiple sclerosis (MS). Early diagnosis of MS reduces the burden of disability-adjusted life years and associated health care costs. Yet diagnostic delays persist in MS care and even within national health care systems with robust resources, comprehensive registries, and MS subspecialist referral networks. The global prevalence and characteristics of barriers to expedited MS diagnosis, particularly in resource-restricted regions, have not been extensively studied. Recent revisions to MS diagnostic criteria demonstrate potential to facilitate earlier diagnosis, but global implementation remains largely unknown.
Methods
The Multiple Sclerosis International Federation third edition of the Atlas of MS was a survey that assessed the current global state of diagnosis including adoption of MS diagnostic criteria; barriers to diagnosis with respect to the patient, health care provider, and health system; and existence of national guidelines or national standards for speed of MS diagnosis.
Results
Coordinators from 107 countries (representing approximately 82% of the world population), participated. Eighty-three percent reported at least 1 “major barrier” to early MS diagnosis. The most frequently reported barriers included the following: “lack of awareness of MS symptoms among general public” (68%), “lack of awareness of MS symptoms among health care professionals” (59%), and “lack of availability of health care professionals with knowledge to diagnose MS” (44%). One-third reported lack of “specialist medical equipment or diagnostic tests.” Thirty-four percent reported the use of only 2017 McDonald criteria (McD-C) for diagnosis, and 79% reported 2017 McD-C as the “most commonly used criteria.” Sixty-six percent reported at least 1 barrier to the adoption of 2017 McD-C, including “neurologists lack awareness or training” by 45%. There was no significant association between national guidelines pertaining to MS diagnosis or practice standards addressing the speed of diagnosis and presence of barriers to early MS diagnosis and implementation of 2017 McD-C.
Discussion
This study finds pervasive consistent global barriers to early diagnosis of MS. While these barriers reflected a lack of resources in many countries, data also suggest that interventions designed to develop and implement accessible education and training can provide cost-effective opportunities to improve access to early MS diagnosis.
Introduction
Recent data suggest an increasing global prevalence of multiple sclerosis (MS).1-6 Revisions to the MS diagnostic criteria7 over the past 2 decades have facilitated earlier diagnosis,8-10 by obviating the need to wait for a second clinical event to diagnose MS. For example, in a cohort of 785 persons with MS from 9 European centers, the median time to diagnosis was 58.5 months based on a second clinical event, 13.0 months based on the 2010 McDonald diagnostic criteria, and 3.2 months based on the 2017 McDonald criteria (McD-C).11 Expedited diagnosis consequentially has resulted in earlier treatment, even based on differences in the 2010 and 2017 criteria,8 which is associated with improved clinical outcomes for people with MS.12-15 Earlier diagnosis of MS carries the potential to ease the high global burden1 associated with this disease through a reduction in disability-adjusted life years and associated health care cost.
Despite the importance of rapid diagnosis, diagnostic delays persist in MS care16-19 and even within national health care systems with robust resources, comprehensive registries, and MS subspecialist referral networks.20-22 A variety of contextual factors related to the affected individual, the health system, and policy likely contribute to delays between symptom onset and confirmed diagnosis of MS. The Anderson model of total patient delay23 suggests that an individual needs to recognize bodily changes and appraise symptoms as warranting a need for care and then to obtain an appointment. That health care provider needs to appraise the individual and determine that investigations and referrals are needed. The specialist (neurologist) must be accessible and may need to complete additional investigations to confirm diagnosis.
The global prevalence and characteristics of barriers within this diagnostic pathway that prevent expedited MS diagnosis, particularly in resource-restricted regions, have not been extensively studied. Moreover, the success of global dissemination and implementation efforts surrounding revisions to MS diagnostic criteria particularly remains largely unknown. Scant “real-world” data limited to the United Kingdom and the United States continue to highlight miscomprehension and lack of implementation of contemporary criteria.24-26
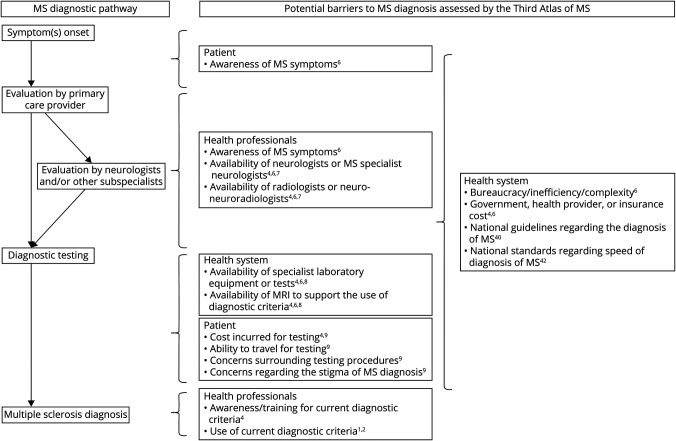
This study aimed to synthesize novel global data focused on MS diagnosis collected as part of the recently completed Multiple Sclerosis International Federation's (MSIF) third edition of the Atlas of MS—a worldwide study of the epidemiology of MS and the global availability and accessibility of diagnostic and clinical resources for people with MS. We reported data focused on barriers to MS diagnosis, including the implementation of contemporary MS diagnostic criteria, which had been incorporated into the Atlas of MS for the first time (Figure 1). We further analyzed the influence of World Bank (WB) category, World Health Organization (WHO) region, the presence of national standards surrounding MS diagnosis, and the presence of national guidelines focused on MS diagnosis on barriers to diagnosis. These data from 107 countries representing 82% of the world's population carries the potential to inform future clinical, educational, and health care policy—derived interventions aimed to improve early and accurate diagnosis of MS.
Figure 1. Potential Patient–Related, Health Professional–Related, and Health Care System–Related Barriers on the Diagnostic Pathway to MS Assessed by the Third Atlas of MS.
Superscript numbers indicate the Atlas question number that assessed each specific potential barrier to diagnosis. MS = multiple sclerosis.
Methods
This cross-sectional study conforms to CROSS reporting guidelines.
Setting
The aim of the Atlas was to provide a comprehensive understanding of the global burden of MS. These open-source data are intended as a tool to highlight disparities and inequalities, raise disease awareness, encourage improvements in surveillance systems and service provisions, inform and evidence advocacy efforts, and support the development of public policy to optimize the quality of life of people living with MS. The first edition of the Atlas of MS was published in 2008 in collaboration with the WHO. Data were updated by the MSIF in 2013 (second edition), and the third edition was launched in 2020–2022. Data were collected by questionnaire. The third edition surveyed key epidemiologic findings and barriers and inequalities in relation to diagnosis, access to disease-modifying therapies, and rehabilitation and symptom management.
Questionnaire Development
The Atlas questionnaire was designed through an iterative process by an Atlas working group composed of MSIF members (MS organizations from 12 countries) and a panel of 16 Atlas expert advisors from 15 countries. These 2 groups included representation from all 6 WHO regions,15 all 4 WB economy income categories,27 and specific expertise for the data collected. The MSIF International Working Group on Access and the MSIF International Medical and Scientific Board provided a further review of the questionnaire. These groups were composed of a range of stakeholders including clinicians, researchers, epidemiologists, volunteers/staff of MS organizations, and people affected by MS. Epidemiologists from McKing Consulting Corporation provided consultation regarding methodology, questionnaire design, data collection, and analyses.
Initially, important issues potentially affecting MS diagnosis and care in each region were identified by the Atlas working group. Second, the group reviewed questions from the second edition of the Atlas for relevance to these issues. Relevant questions were retained or modified to conform to the updated priorities of the third edition. Third, the questions, drafted in English, were reviewed for clarity by the MSIF staff and consultants, the Atlas Working Group, Atlas Expert Advisors, and the MSIF International Working Group for Access.
A finalized draft was converted to an online survey tool incorporating skip logic using Surveymonkey.com for pilot testing. The questionnaire was then pilot tested by country coordinators from 5 countries (Argentina, Australia, India, Singapore, and Zambia) representing different WHO regions, WB income groups, and health care systems. No changes were made because of the pilot evaluation.
In collaboration with Guildhawk, a language and technology company, the questionnaire was translated and reviewed by a qualified native-speaking linguist in French and Spanish. An independent linguist proofread the translated files for additional quality assurance, and an additional review by Guildhawk staff was performed to check for accuracy. Each translation provided by Guildhawk was subsequently independently verified by a native French and a native Spanish speaker drawn from the Atlas working group and its expert advisors. This resulted in minor changes to the translated text before finalization and distribution to country coordinators through an electronic Word document. During this process, the text was not translated back to English from French or Spanish before dissemination.
The final subsection of the questionnaire (eAppendix 1, links.lww.com/WNL/C901) relating to the data presented in this study included 13 questions that assessed the following: the current state of diagnosis including adoption of MS diagnostic criteria; barriers to diagnosis with respect to the patient, health care provider, and health system; and existence of national guidelines or national standards for MS diagnosis (including specifically the speed of diagnosis). A glossary of terms was included within each question. Sources of data reported were also queried.
Standard Protocol Approvals, Registrations, and Patient Consents
The Atlas of MS study methodology and questionnaire was not reviewed or approved by an institutional review board—this study surveyed health care professionals.
Questionnaire Distribution
International contacts were identified through MSIF's network of MS organizations, the MSIF International Medical and Scientific Board, International Working Group on Access, previous Atlas contacts, the World Federation of Neurology, the Atlas working group and expert advisors, and regional International Committees for the Treatment and Research in Multiple Sclerosis and from scientific literature. Country coordinators, typically representatives from MS organizations, neurologists, epidemiologists, or researchers, were identified in each country and subsequently asked to complete the questionnaire while making use of all possible sources of information available to them including collaborating with other experts in the country where possible or necessary. Country coordinators were identified for 138 countries and were queried regarding preferred questionnaire language; 123/138 (89.1%) requested English, 9 requested French, and 6 requested Spanish.
Data were collected between October 2019 and April 2020 through the online survey tool, and country coordinators were provided electronic PDF or Word documents to allow collaboration and verification of data with other experts.
Analysis
Descriptive statistics are reported. For analyses WB high-income and upper middle-income categories were merged to create a high/upper middle-income category, and lower middle-income and low-income were combined to create a lower middle/low-income category due to sample size limitations.
The independent variables of interest were as follows: (1) Use of 2017 McD-C (yes/no, not sure excluded); (2) most commonly use 2017 McD-C (yes/no, not sure was excluded); and (3) the presence of at least 1 major barrier to early diagnosis of MS (yes/no, not sure was excluded). The outcomes of interest were as follows: (1) any national standards or targets set relating to the diagnosis, treatment, or monitoring of patients with MS in the country (yes/no); (2) any national guidelines for diagnosis, treatment, or living with MS? (yes/no); and (3) speed of diagnosis as a standard (yes/no).
We conducted a Fisher exact test to test the association between the survey responses for these variables and WB income categories or WHO regions and the unadjusted association between 2 survey responses. We used a series of multivariable logistic regression models to test the associations between the independent variables and outcomes of interest while adjusting for WB income and WHO region. We reported the generalized variance inflation factor28 to test the multicollinearity of WB income and WHO regions, c-statistics to test discriminating ability, and p value of Hosmer-Lemeshow Goodness of Fit (GOF) test to test the model fit (eTable 1, links.lww.com/WNL/C903).29 A c-statistic of 0.5 indicates the model is no better than chance at predicting the outcome, and 1.0 indicates perfect classification. A p value for the GOF test of >0.05 indicates a good fit.
All statistical analyses were conducted in R version 4.0.2. Data are available on request to the MSIF.
Results
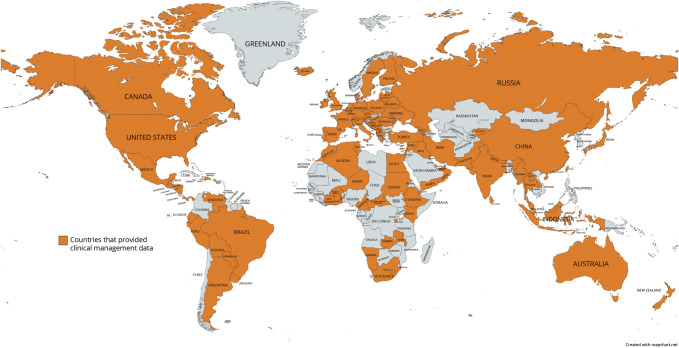
Coordinators representing 107 countries (approximately 82% of the world population) participated in the section of the Atlas questionnaire focused on MS diagnosis (Figure 2, and eTables 2 and 3, links.lww.com/WNL/C904 and links.lww.com/WNL/C905). Descriptive open-source data are available from the MSIF (atlasofms.org). Data generated by each country coordinator completing the survey comprise the reported results from each country.
Figure 2. Countries That Provided Clinical Management Data for the Atlas of MS Third Edition.
MS = multiple sclerosis.
Current State of MS Diagnosis
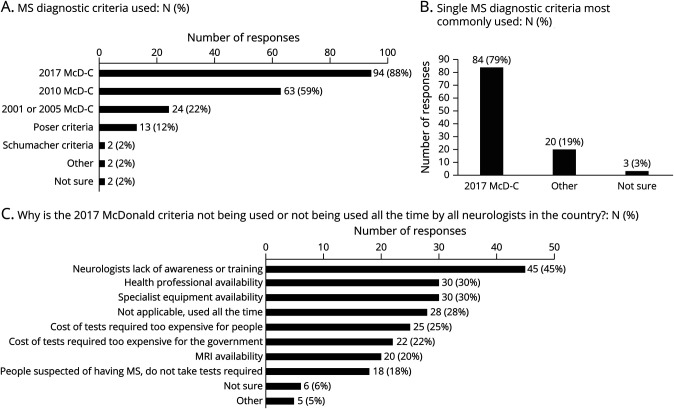
The type of source consulted by country coordinators to provide information on diagnostic criteria included a published academic paper or poster: 51/107 (48%), patient data: 54/107 (50%), personal opinion: 72/107 (67%), opinion of others: 45/107 (42%), and other: 3/107 (3%). Figure 3 details responses to the 3 questions surrounding MS diagnostic criteria. Schumacher (1965), Poser (1983), and McD-C and its revisions (2001, 2005, 2011, 2017) all currently remained in use. Thirty-six (34%) countries reported using only 2017 McD-C for diagnosis. WB high/upper middle-income countries were more likely (n = 31, 42%) than lower middle/low-income countries (n = 5, 16%) (p = 0.007) to report using only 2017 McD-C for diagnosis compared with any McD-C, while there was no difference by WHO region (p = 0.13). Two countries (Burundi, Morocco) reported not using any McD-C criteria.
Figure 3. Use of MS Diagnostic Criteria.
(A) Responses to the query for all MS diagnostic criteria currently used by providers (may select more than 1 response). (B) Responses to a second question querying the single “most commonly” used MS diagnostic criteria by providers, demonstrating the proportion indicating 2017 McDonald criteria. (C) Responses regarding why 2017 McDonald criteria is not being used or not being used all the time by providers in the country (may select more than 1 response). MS = multiple sclerosis.
The 2017 McD-C were reported as the “most commonly used criteria” to diagnose MS in 84 (79%) countries, and this was more likely in WB high/upper middle-income countries than WB lower middle/low-income countries (66 [90%] vs 18 [58%], p < 0.001) but did not differ by WHO region (p = 0.31).
Procedures reported as used for the diagnosis of MS included neurologic examination by 106/106 (100%), MRI by 103/106 (97%), spinal tap by 96/106 (91%), evoked potentials by 69/106 (65%), optical coherence tomography by 40/106 (38%), and other by 5/106 (5%). MRI was not reported as a procedure used to diagnose MS in 3 (3%) countries (Brundi, Central African Republic, and Malawi).
Barriers to Diagnosis
Sources consulted by country coordinators surrounding barriers to diagnosis included published academic articles or posters: 30/106 (82%), patient data: 57/106 (54%), personal opinion: 81/106 (76%), opinion of others: 54/106 (51%), and other: 3/106 (3%).
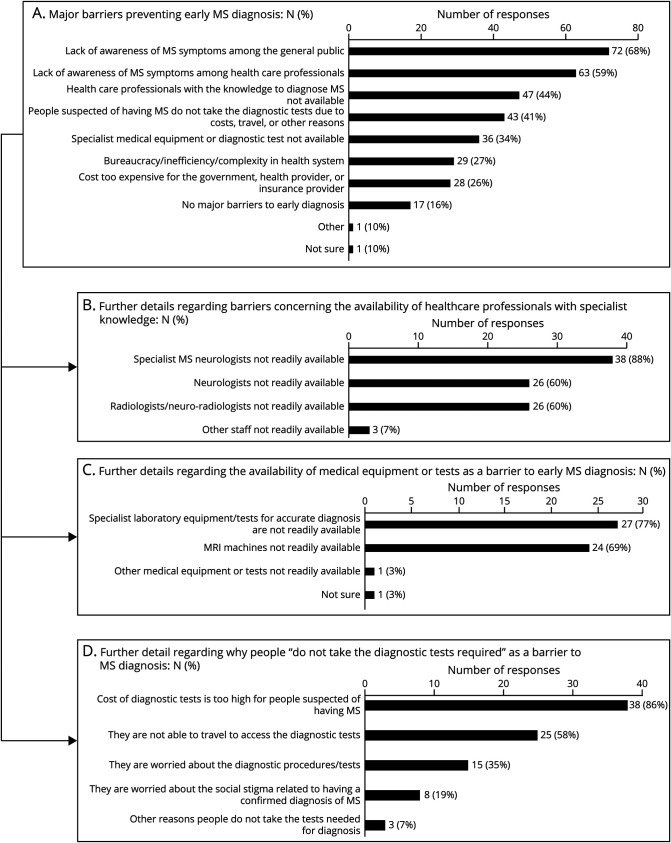
More than 80% (n = 88) of countries reported at least 1 “major barrier” to early MS diagnosis (Figure 4). From the patient perspective (of symptom appraisal and care seeking), the most frequently reported barrier was lack of awareness of MS symptoms among “general public” by 72 (68%). From the health care provider perspective, the most commonly reported barrier was “lack of awareness of MS symptoms among health care professionals” (n = 63, 59%). This barrier was closely related to a lack of availability of “health care professionals with knowledge to diagnose MS” (n = 47, 44%). Of the countries reporting a lack of knowledgeable health professionals, 38 (88%) reported specialist MS neurologists are “not readily available” and 26 (60%) reported neurologists are “not readily available.” This problem was compounded by a lack of “specialist medical equipment or diagnostic tests” in one-third of countries (n = 36). Of those countries, 24 (69%) reported MRI machines, and 27 (77%) reported “specialist laboratory equipment/tests for accurate diagnosis” are “not readily available.”
Figure 4. Major Barriers to Early MS Diagnosis.
(A) Responses to query regarding major barriers that prevent early diagnosis of MS (may indicate more than 1), and further responses obtained in a follow-up question if barriers involving the (B) availability of “health care professionals with subspecialist knowledge,” (C) “medical equipment or tests,” or (D) “people do not take the diagnostic tests,” were selected. MS = multiple sclerosis.
WB lower middle/low-income countries reported at least 1 major barrier preventing early MS diagnosis more frequently than WB high/upper middle-income countries (31 [94%] vs 57 [78%], p = 0.05), while there were no significant differences between WHO regions (p = 0.09).
Sixty-six (66%) countries reported at least 1 barrier to the adoption of 2017 McD-C, most often that “neurologists lack awareness or training” (n = 45, 45%). WB lower middle/low-income countries reported at least 1 barrier to the adoption of 2017 McD-C more often than WB high/upper middle-income countries (29 [88%] vs 37 [51%], p < 0.001). Europe (19 [46%]) and Eastern Mediterranean (9 [50%]) WHO regions were less likely to report at least 1 barrier to the adoption of 2017 McD-C compared with all other regions (Africa: 13 [87%], America: 13 [76%], Western Pacific: 7 [78%], South East Asia: 5 [83%]) (p < 0.001).
Guidelines or National Standards
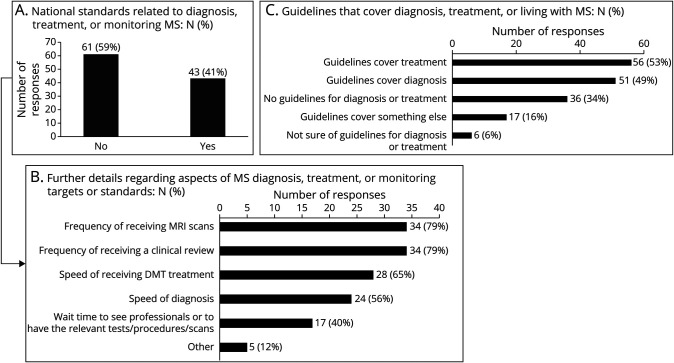
Fifty-one (49%) countries reported having national guidelines that cover the diagnosis of MS (Figure 5). WB high/upper middle-income countries reported national guidelines covering the diagnosis of MS more often than WB lower middle/low-income countries (45 [63%] vs 6 [18%], p < 0.001). Europe and Eastern Mediterranean WHO regions were more likely to report national guidelines covering the diagnosis of MS than all other WHO regions (33 [56%] vs 18 [36%], p < 0.001).
Figure 5. National Standards or Guidelines Relating to MS.
Proportion of responses indicating (A) national standards related to MS diagnosis, (B) national standards focused on the speed of diagnosis of MS, or (C) the presence of national guidelines that cover diagnosis of MS. MS = multiple sclerosis.
Forty-three (41%) countries reported national standards pertaining to MS care, and of those, 24 (56%) countries reported standards for “speed of diagnosis.” WB high/upper middle-income countries also reported national standards more frequently than WB lower middle/low-income countries (36/73 [49%] vs 7/31 [18%], p = 0.016). National standards pertaining to MS was also associated with WHO region (p < 0.001). Europe and Eastern Mediterranean WHO regions were more likely to report such national standards compared with all other WHO regions (35 [59%] vs 8 [18%], p < 0.001)
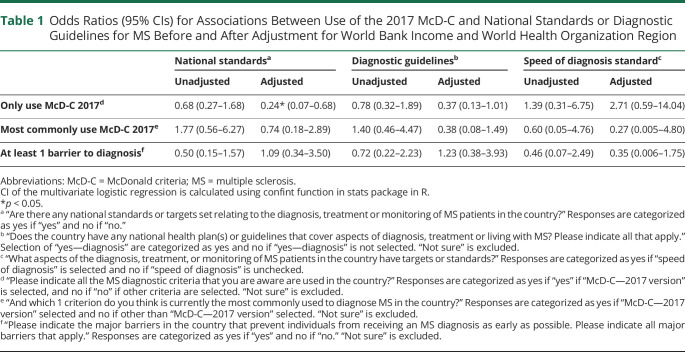
Countries reporting “only using McD-C 2017” had 77% reduced odds of having “national standards” (odds ratio [OR] 0.23, 95% CI 0.07–0.68, c-statistic = 0.75, Hosmer-Lemeshow GOF p-value = 0.56) and 63% reduced odds of having “guidelines that cover diagnosis” (OR 0.37, 95% CI 0.13–1.01), only after adjusting for WB income category and WHO region (Table 1 and eFigure 1, links.lww.com/WNL/C902). There was no significant association between countries reporting 2017 McD-C as the “most commonly” used criteria and the presence of diagnostic guidelines, before or after adjusting for WB income category and WHO region.
Table 1.
Odds Ratios (95% CIs) for Associations Between Use of the 2017 McD-C and National Standards or Diagnostic Guidelines for MS Before and After Adjustment for World Bank Income and World Health Organization Region
No statistically significant association was identified between countries reporting a “speed of diagnosis” standard and reporting only the use of 2017 McD-C, before or after adjusting for WB income and WHO region (Table 1). There was also no association between countries reporting a “speed of diagnosis” standard and reporting 2017 McD-C as the “most commonly used” criteria, before or after adjusting for WB income and WHO region.
There was no significant association between countries with at least 1 barrier to early diagnosis and guidelines that cover diagnosis, before or after adjusting for WB income and WHO region. There was no statistically significant association between countries with at least 1 barrier to early diagnosis and the presence of national standards, before or after adjusting for WB income and WHO region (Table 1).
Discussion
We investigated the presence and characteristics of health care system barriers to MS diagnosis in 107 countries. Eight in 10 participating countries reported at least 1 “major barrier” to MS diagnosis and 6 in 10 reported at least 1 barrier to the adoption of 2017 McD-C and frequent concurrent use of prior less sensitive diagnostic criteria. We found that there were barriers to timely diagnosis that were pervasive throughout the entire patient journey—ranging from lack of patient awareness, to lack of health care provider awareness and knowledge/training, to lack of personnel and infrastructure to implement recommendations around diagnosis even if the knowledge and awareness were available.
Barriers to timely MS diagnosis reflected a lack of resources in many countries. This most often included a lack of health care professionals, including neurologists and subspecialty MS neurologists, in particular, and medical equipment or diagnostic tests such as MRI machines and other laboratory equipment, particularly in lower-income settings. In addition, people suspected of having MS often did not complete recommended testing due to cost and/or related required travel, indicating that health care disparities likely play an important role in delayed diagnoses. Compared with 94% of WB high/upper middle-income countries, only 58% of lower middle/low-income countries reported 2017 McD-C as the most commonly used diagnostic criteria, which may reflect a lack of trained health care professionals or other barriers to implementation at the health system level. WB lower middle/low-income countries were more affected by a lack of resources and were more likely to report at least 1 major barrier preventing early MS diagnosis, with almost every country (94%) reporting such. By contrast, these differences were not found with respect to WHO region. Improving the availability and accessibility of resources in these regions is likely to be complex and challenging, requiring country-specific interventions. However, efforts such as the development of laboratory testing using dried blood spots30 and portable low-field MRI sensitive to MS lesions31 show promise to reduce cost and travel-associated barriers to timely evaluation of possible MS diagnoses in resource-limited regions.
Of importance, data also suggest that interventions designed to develop and implement accessible education and training may provide cost-effective opportunities to improve access to early MS diagnosis. Lack of health care provider awareness or training were frequently reported barriers to early MS diagnosis and to the adoption of contemporary McD-C. Interventions leveraging resources already in place may improve access to early diagnosis. For instance, telemedicine may provide accessible and cost-effective opportunities for education, training, and direct care in resource-limited regions. A recent study demonstrated that teleneurology visits were feasible and acceptable for adult patients attending an outpatient neurology clinic in Zambia, while reducing expense and time associated with care.32 Telemedicine may also be implemented to train regional clinicians in MS diagnosis and care,33 who can subsequently serve as experts leading educational outreach efforts while providing subspecialist-level telemedicine consultations for local providers in their region. Once such efforts are successful, outreach to increase awareness among primary care providers and other non-neurologist physicians who may first encounter patients with symptoms suggestive of MS (e.g., ophthalmologists) along with the development of efficient referral networks is necessary. In spite of accessible and available care for early MS diagnosis in some regions, a lack of awareness of MS symptoms among the general public was also frequently reported—a barrier also amenable to educational efforts where models from other chronic disease interventions such as engaging mass media, faith-based institutions, and advocacy services may be informative.34
Although data support earlier diagnosis and improved outcomes in patients with MS because of revisions to MS diagnostic criteria over the past 20 years, there is scant literature concerning global implementation.24,25 This study highlights delay in widespread adoption of revised 2017 McD-C approximately 21–27 months after publication. Although most countries reported that 2017 McD-C was the most commonly used criteria, many also reported continued use of prior criteria as part of routine care, suggesting heterogeneous practice in these regions. Persistent utilization of earlier criteria may unnecessarily delay diagnosis in contrast to comparably more sensitive contemporary criteria in a small but significant group of patients.8 Approximately half WB high/upper middle-income countries reported at least 1 barrier to the adoption of 2017 McD-C, including 10% who did not report that 2017 McD-C was the most commonly used diagnostic criteria. This suggests that resource limitations were not the sole cause of delayed adoption of 2017 McD-C—improved dissemination and parallel education efforts should accompany any future revisions to MS diagnostic criteria to improve global implementation.
This study has limitations. Few peer-reviewed publications exist on the availability of and access to MS diagnosis in many countries, and as a result, this study relied on expert opinion in the absence of such data. These data may therefore also not capture heterogeneity and unmeasured contextual factors that may influence MS diagnosis in each country. Future studies in these regions might pursue evaluation of administrative or direct clinical data to verify and better characterize these findings where possible. However, to improve confidence in these data, collaborations between country coordinators and other experts were requested and the enumeration of data sources consulted if available. Most participating countries referenced the independent evidence consulted (academic papers or patient data such as surveys or registries) rather than reliance on opinion alone. A future Atlas effort may consider directly collecting and assessing the evidence used to inform responses and any barriers to its availability experienced by country coordinators. Funding to support for the development of MS registries in resource-limited regions may expand global representation and increase quantitative country-specific data for future studies evaluating barriers to MS diagnosis. Engagement with regional clinicians, MS advocacy groups, and public awareness campaigns would be needed to aid development and ensure the success of such initiatives. However, efforts toward population surveillance of other diseases35 in low-income to middle-income countries suggest that single region or country-specific registries alone may be inadequate and that optimal approaches to comprehensive surveillance of MS will likely require leveraging multiple sources of regional health data and international collaboration.
In this study, there was underrepresentation of countries from the African WHO region and countries classified as WB low-income (eTables 2 and 3, links.lww.com/WNL/C904 and links.lww.com/WNL/C905), and these data therefore likely underestimate barriers to early MS diagnosis across these regions. This partly reflects the consequences of a severe lack of neurologists. For example, the number of neurologists in WHO Africa region is an estimated median of 0.04 per 100,000 people (compared with 6.6 in Europe).36 Many countries in Africa have 0 or 1–2 neurologists providing care. The resulting burden of patient care, teaching, and administrative responsibilities may make such providers less able to participate in initiatives such as the Atlas of MS. Furthermore, such countries have few if any neurologists with subspecialty MS expertise or capability to focus on MS, given neurologic care necessitated more broadly and by more prevalent neurologic conditions. In regions with few providers of neurologic care, knowledge for neurology is often further limited among non-neurologists, making recognition of symptoms of MS and referral to a neurologist even less likely. As a result, there are scant data concerning MS prevalence and care in Africa.37
Methodology used for the Atlas provides an overall estimate of barriers to early MS diagnosis in these regions but limits detailed assessments or conclusions regarding accurate or early diagnosis within or between regions. For instance, as recent data in the United States have highlighted, adoption of McD-C does not necessarily ensure accurate comprehension and application of its key elements.26 Similarly, despite few diagnostic barriers reported by some countries, social determinants of health and other factors may still create inequalities influencing health care access to facilitate early MS diagnosis for some patients.38 Further country-specific quantitative data concerning MS diagnosis are needed to better understand the experience of patients in these regions. Of importance, data collection was completed in April 2020 and, as a result, would not have adequately captured any impact of the coronavirus disease 2019 pandemic on MS diagnosis.
This study finds pervasive consistent global barriers to early diagnosis of MS. International expert consensus efforts have repeatedly affirmed optimal diagnostic approaches and the importance of early diagnosis to improve clinical outcomes in MS.7,39,40 The challenges of dissemination of research findings, practice guidelines, and diagnostic criteria, particularly in resource-limited regions, are well-documented.41-43 Indeed, this study found little association between national guidelines or standards for MS care and fewer barriers to early diagnosis. The development and improvement of much needed health care system resources required to ameliorate global barriers to MS diagnosis will necessitate difficult policy initiatives. Despite these issues, these data present a key opportunity to improve and expedite diagnosis of MS in many worldwide regions through provider and patient-focused educational interventions. Such approaches, incorporated in policy initiatives such as the recently WHO adopted Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders 2022–2031,44 optimize clinical resources that are already in place. Models implemented by organizations such as the World Federation of Neurology45 and others33,46-49 to assess and develop worldwide neurology-focused educational resources and programs, particularly in resource-limited regions, should be considered.
Glossary
- GOF
Goodness of Fit
- McD-C
McDonald criteria
- MS
multiple sclerosis
- MSIF
Multiple Sclerosis International Federation
- OR
odds ratio
- WB
World Bank
- WHO
World Health Organization
Appendix. Authors
Footnotes
Editorial, page 245
Study Funding
The Multiple Sclerosis International Federation (MSIF) is an alliance of national multiple sclerosis organizations. The MSIF receives income from a wide range of sources, including health care and other companies, individuals, member organizations, campaigns, foundations, and trusts. Over the past 5 years, the MSIF received funding from the following companies: Biogen, Bristol Myers Squibb, Janssen, Sanofi, Merck, Mylan, Novartis, and Roche—all of which is publicly disclosed.
Disclosure
A.J. Solomon participates in contracted research with Sanofi, Biogen, Novartis, Actelion, and Genentech/Roche, receives research support from Bristol Myers Squibb, personal compensation for consulting for Genentech, Biogen, Alexion, Celgene, Greenwich Biosciences, Horizon Therapeutics, TG Therapeutics, and Octave Bioscience, personal compensation nonpromotional speaking for EMD Serono, and provides expert witness testimony. R.A. Marrie receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn's and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and US Department of Defense. She is a coinvestigator on a study partially funded by Biogen Idec and Roche (no funds to her or her institution). She is supported by the Waugh Family Chair in Multiple Sclerosis. S. Viswanathan participates in contracted research with Alexion, Novartis, Sanofi, and Roche. J. Correale has received financial compensation for academic presentations, assistance to advisory boards, financial support for clinical and basic research, and travel assistance to congresses from the following companies: Biogen, Merck, Novartis, Roche, Bayer, Sanofi-Genzyme, Gador, Raffo, Bristol Myers Squibb, and Janssen. M. Magyari has served in scientific advisory board for Sanofi, Novartis, and Merck and has received honoraria for lecturing from Biogen, Merck, Novartis, Roche, Genzyme, and Bristol Myers Squibb. N. Robertson has received honoraria and/or support to attend educational meetings from Biogen, Novartis, Janssen, Genzyme, and Roche. His institution has also received research support from Biogen, Novartis, and Sanofi. D. Saylor has received funding from the National Multiple Sclerosis Society. W.E. Kaye receives funding from the Agency for Toxic Substances and Disease Registry, the National Multiple Sclerosis Society, the Association for the Accreditation of Human Research Protection Programs, and Rockefeller University. L. Rechtman and E. Bae have no disclosures to report. R.T. Shinohara receives consulting income from Octave Bioscience and compensation for scientific reviewing from the American Medical Association, the NIH, the Department of Defense, and the Emerson Collective. R. King, J. Laurson-Doube, and A. Helme have no disclosures to report. Go to Neurology.org/N for full disclosures.
References
- 1.GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285. doi: 10.1016/S1474-4422(18)30443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi: 10.1212/wnl.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almasi-Hashiani A, Sahraian MA, Eskandarieh S. Evidence of an increased prevalence of multiple sclerosis: a population-based study of Tehran registry during 1999-2018. BMC Neurol. 2020;20(1):169. doi: 10.1186/s12883-020-01747-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouhari A, Taheri G, Salari M, Moosazadeh M, Etemadifar M. Multiple sclerosis epidemiology in Asia and Oceania; A systematic review and meta-analysis. Mult Scler Relat Disord. 2021;54:103119. doi: 10.1016/j.msard.2021.103119 [DOI] [PubMed] [Google Scholar]
- 6.Ribbons K, Lea R, Tiedeman C, Mackenzie L, Lechner-Scott J. Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: a 50-year study. Mult Scler. 2017;23(8):1063-1071. doi: 10.1177/1352458516671819 [DOI] [PubMed] [Google Scholar]
- 7.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 8.Tintore M, Cobo-Calvo A, Carbonell P, et al. Effect of changes in MS diagnostic criteria over 25 years on time to treatment and prognosis in patients with clinically isolated syndrome. Neurology. 2021;97(17):e1641-e1652. doi: 10.1212/wnl.0000000000012726 [DOI] [PubMed] [Google Scholar]
- 9.McNicholas N, Lockhart A, Yap SM, et al. New versus old: implications of evolving diagnostic criteria for relapsing-remitting multiple sclerosis. Mult Scler. 2019;25(6):867-870. doi: 10.1177/1352458518770088 [DOI] [PubMed] [Google Scholar]
- 10.van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, et al. Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol. 2018;75(11):1392. doi: 10.1001/jamaneurol.2018.2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippi M, Preziosa P, Meani A, et al. Performance of the 2017 and 2010 revised McDonald criteria in predicting MS diagnosis after a clinically isolated syndrome: a MAGNIMS study. Neurology. 2022;98(1):e1-e14. doi: 10.1212/wnl.0000000000013016 [DOI] [PubMed] [Google Scholar]
- 12.Kavaliunas A, Manouchehrinia A, Stawiarz L, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. 2017;23(9):1233-1240. doi: 10.1177/1352458516675039 [DOI] [PubMed] [Google Scholar]
- 13.Chalmer TA, Baggesen LM, Nørgaard M, Koch-Henriksen N, Magyari M, Sorensen PS. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol. 2018;25(10):1262-e110. doi: 10.1111/ene.13692 [DOI] [PubMed] [Google Scholar]
- 14.Iaffaldano P, Lucisano G, Butzkueven H, et al. Early treatment delays long-term disability accrual in RRMS: results from the BMSD network. Mult Scler. 2021;27(10):1543-1555. doi: 10.1177/13524585211010128 [DOI] [PubMed] [Google Scholar]
- 15.Magyari M, Joensen H, Kopp TI, Pontieri L, Koch-Henriksen N. Changes in prognosis of the Danish multiple sclerosis population over time. Mult Scler. 2022;28(14):2190-2201. doi: 10.1177/13524585221110582 [DOI] [PubMed] [Google Scholar]
- 16.Mobasheri F, Jaberi AR, Hasanzadeh J, Fararouei M. Multiple sclerosis diagnosis delay and its associated factors among Iranian patients. Clin Neurol Neurosurg. 2020;199:106278. doi: 10.1016/j.clineuro.2020.106278 [DOI] [PubMed] [Google Scholar]
- 17.Cárdenas-Robledo S, Lopez-Reyes L, Arenas-Vargas LE, Carvajal-Parra MS, Guío-Sánchez C. Delayed diagnosis of multiple sclerosis in a low prevalence country. Neurol Res. 2021;43(7):521-527. doi: 10.1080/01616412.2020.1866374 [DOI] [PubMed] [Google Scholar]
- 18.Patti F, Chisari CG, Arena S, et al. Factors driving delayed time to multiple sclerosis diagnosis: results from a population-based study. Mult Scler Relat Disord. 2022;57:103361. doi: 10.1016/j.msard.2021.103361 [DOI] [PubMed] [Google Scholar]
- 19.Aires A, Barros A, Machado C, et al. Diagnostic delay of multiple sclerosis in a Portuguese population. Acta Med Port. 2019;32(4):289-294. doi: 10.20344/amp.11187 [DOI] [PubMed] [Google Scholar]
- 20.Willumsen JS, Aarseth JH, Myhr KM, Midgard R. High incidence and prevalence of MS in Møre and Romsdal County, Norway, 1950-2018. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e713. doi: 10.1212/nxi.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barin L, Kamm CP, Salmen A, et al. How do patients enter the healthcare system after the first onset of multiple sclerosis symptoms? The influence of setting and physician specialty on speed of diagnosis. Mult Scler. 2020;26(4):489-500. doi: 10.1177/1352458518823955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon AJ, Ascherio A. Early diagnosis of multiple sclerosis: further evidence for missed opportunity. Neurology. 2021;96(24):1111-1112. doi: 10.1212/wnl.0000000000012087 [DOI] [PubMed] [Google Scholar]
- 23.Andersen BL, Cacioppo JT, Roberts DC. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol. 1995;34(1):33-52. doi: 10.1111/j.2044-8309.1995.tb01047.x [DOI] [PubMed] [Google Scholar]
- 24.Hawkes CH, Giovannoni G. The McDonald criteria for multiple sclerosis: time for clarification. Mult Scler. 2010;16(5):566-575. doi: 10.1177/1352458510362441 [DOI] [PubMed] [Google Scholar]
- 25.Lumley R, Davenport R, Williams A. Most Scottish neurologists do not apply the 2010 McDonald criteria when diagnosing multiple sclerosis. J R Coll Physicians Edinb. 2015;45(1):23-26. doi: 10.4997/jrcpe.2015.106 [DOI] [PubMed] [Google Scholar]
- 26.Solomon AJ, Kaisey M, Krieger SC, et al. Multiple sclerosis diagnosis: knowledge gaps and opportunities for educational intervention in neurologists in the United States. Mult Scler. 2022;28(8):1248-1256. doi: 10.1177/13524585211048401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fantom N, Serajuddin U. The World Bank's Classification of Countries by Income. The World Bank; 2016. [Google Scholar]
- 28.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178-183. doi: 10.1080/01621459.1992.10475190 [DOI] [Google Scholar]
- 29.Hosmer DW, Lemeshow SJNY. Applied Logistic Regression. John Wiley & Sons; 2000. [Google Scholar]
- 30.Lim MD. Dried blood spots for global health diagnostics and surveillance: opportunities and challenges. Am J Trop Med Hyg. 2018;99(2):256-265. doi: 10.4269/ajtmh.17-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold TC, Tu D, Okar SV, et al. Sensitivity of portable low-field magnetic resonance imaging for multiple sclerosis lesions. Neuroimage Clin. 2022;35:103101. doi: 10.1016/j.nicl.2022.103101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asukile M, Chishimba L, Chomba M, et al. Implementation of a teleneurology clinic in Zambia during the COVID‐19 pandemic. Ann Neurol. 2022;91(4):445-454. doi: 10.1002/ana.26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaddumukasa M, Katabira E, Salata RA, et al. Global medical education partnerships to expand specialty expertise: a case report on building neurology clinical and research capacity. Hum Resour Health. 2014;12(1):75. doi: 10.1186/1478-4491-12-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de-Graft Aikins A, Boynton P, Atanga LL. Developing effective chronic disease interventions in Africa: insights from Ghana and Cameroon. Global Health. 2010;6:6. doi: 10.1186/1744-8603-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Echouffo-Tcheugui JB, Yaya S, Joshi R, Venkat Narayan KM, Kengne AP. Population surveillance of cardiovascular diseases in low-income to middle-income countries should leverage existing international collaborations. BMJ Global Health. 2018;3(5):e000866. doi: 10.1136/bmjgh-2018-000866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atlas: Country Resources for Neurological Disorders, 2nd ed. World Health Organization; 2017. [Google Scholar]
- 37.Heine M, Maartens D, Hanekom S, Derman W. Multiple Sclerosis in sub-Saharan Africa: a scoping review. Mult Scler Relat Disord. 2020;42:102133. doi: 10.1016/j.msard.2020.102133 [DOI] [PubMed] [Google Scholar]
- 38.Amezcua L, Rivera VM, Vazquez TC, Baezconde-Garbanati L, Langer-Gould A. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: a review. JAMA Neurol. 2021;78(12):1515-1524. doi: 10.1001/jamaneurol.2021.3416 [DOI] [PubMed] [Google Scholar]
- 39.Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(suppl 1):S5-S48. doi: 10.1016/j.msard.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 40.Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi: 10.1016/s1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 41.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225-1230. doi: 10.1016/s0140-6736(03)14546-1 [DOI] [PubMed] [Google Scholar]
- 42.Siddiqi K, Newell J, Robinson M. Getting evidence into practice: what works in developing countries? Int J Qual Health Care. 2005;17(5):447-454. doi: 10.1093/intqhc/mzi051 [DOI] [PubMed] [Google Scholar]
- 43.Chaudoir SR, Dugan AG, Barr CH. Measuring factors affecting implementation of health innovations: a systematic review of structural, organizational, provider, patient, and innovation level measures. Implement Sci. 2013;8(1):22. doi: 10.1186/1748-5908-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Lancet N WHO launches its Global Action Plan for brain health. Lancet Neurol. 2022;21(8):671. doi: 10.1016/s1474-4422(22)00266-6 [DOI] [PubMed] [Google Scholar]
- 45.Munsat T, Aarli J, Medina M, Birbeck G, Weiss A. International issues: educational programs of the World Federation of Neurology. Neurology. 2009;72(10):e46-e49. doi: 10.1212/01.wnl.0000344183.62422.b2 [DOI] [PubMed] [Google Scholar]
- 46.Cortez MM, Wold JJ, Renner DR. International issues: expanding neurologic education to resource-poor countries: lessons from Moi Teaching Hospital. Neurology. 2014;82(3):e18-e20. doi: 10.1212/wnl.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 47.Alemán MJ, Nematollahi S, Berkowitz AL. Expanding global access to neurology education. JAMA Neurol. 2021;78(10):1173-1174. doi: 10.1001/jamaneurol.2021.2685 [DOI] [PubMed] [Google Scholar]
- 48.DiBiase RM, Salas RME, Gamaldo CE, et al. Training in neurology: implementation and evaluation of an objective structured clinical-examination tool for neurology postgraduate trainees in Lusaka, Zambia. Neurology. 2021;97(7):e750-e754. doi: 10.1212/wnl.0000000000012134 [DOI] [PubMed] [Google Scholar]
- 49.Mateen FJ, Clark SJ, Borzello M, Kabore J, Seidi O. Neurology training in sub-Saharan Africa: a survey of people in training from 19 countries. Ann Neurol. 2016;79(6):871-881. doi: 10.1002/ana.24649 [DOI] [PubMed] [Google Scholar]