Abstract
Intestinal schistosomiasis is hyperendemic in many sub-Saharan African countries. In Uganda, it is endemic at both Lake Albert (LA) and Lake Victoria (LV) and caused by S. mansoni that uses Biomphalaria snails as obligatory intermediate snail hosts. To shed light on local patterns of infection, we utilised two PCR-based methods to detect S. mansoni within Biomphalaria spp. as collected at the Ugandan shorelines of Lake Albert and Lake Victoria from 2009–2010. Overall, at our Lake Albert sites, the mean infection prevalence was 12.5% (15 of 120 snails), while at our Lake Victoria sites the prevalence was 5% (3 of 60 snails). At our Lake Albert sites, the highest infection prevalence of 13.3% (8 of 60 snails) was at Walukuba, while at our Lake Victoria sites, the highest infection prevalence of 10% (2 of 20 snails) was at Lwanika. Three species of Biomphalaria, B. pfeifferi, B. stanleyi and B. sudanica, were identified at our Lake Albert collection sites, while only a single species, B. choanomphala, was identified at our Lake Victoria collection sites. Biomphalaria stanleyi (2 of 20 snails; 15%) had the highest infection prevalence, followed by B. sudanica (5 of 60 snails; 13.3%), B. pfeifferi (4 of 40 snails; 10%) and B. choanomphala (3 of 60 snails; 5%). Of the Biomphalaria species identified, B. choanomphala had the highest haplotype (gene) diversity score, followed by B. stanleyi, B. sudanica and B. pfeifferi. Sites with a higher mean prevalence of S. mansoni infection had higher intra-species haplotype diversity scores than sites with a lower mean prevalence. The wet seasons (LA: 13.3%; LV: 8.7%) had a consistently higher mean infection prevalence of S. mansoni than the dry seasons (LA: 9.5%; LV: 5%) for all species and all sites tested at both Lake Albert (n = 480) and Lake Victoria (n = 320), though the difference was not statistically significant.
Author summary
Human schistosomiasis is a parasitic disease caused by the intravascular trematode genus Schistosoma. The disease is contracted through contact with contaminated freshwater sources infested with snails, which serve as the intermediate host for the parasite’s larval form. Schistosoma mansoni causes the intestinal form of the disease and utilises Biomphalaria as its intermediate snail host. To better understand the prevalence of S. mansoni infection, molecular detection methods can be used to monitor the levels and patterns of infection within Biomphalaria populations. In this study, the authors examined the prevalence of S. mansoni infection within Biomphalaria snails collected from six sites along the Ugandan shorelines of Lake Albert and Lake Victoria from 2009 to 2010. The study revealed that infection was more prevalent at Lake Albert compared to Lake Victoria. Different species of Biomphalaria snails were found at each lake, with some species having a higher infection prevalence than others. The study also investigated the impact of seasonality on infection prevalence, with the wet seasons having an overall higher prevalence of infection compared to the dry seasons, although the difference was not statistically significant. This research enhances our understanding of S. mansoni infection patterns among African Biomphalaria snails.
Introduction
Schistosomiasis is a parasitic disease caused by infection with digenetic trematodes of the genus Schistosoma. It is estimated that 133 million children and 108 million adults are infected with schistosomiasis worldwide, with over 700 million people being at risk of infection [1]. Schistosomiasis is most prevalent in sub-Saharan Africa, with approximately 93% of infections and up to 90% of individuals at risk of infection living within sub-Saharan African countries [2,3]. The disease manifests as either intestinal (caused by Schistosoma mansoni, S. intercalatum, S. japonicum or S. mekongi) or urogenital forms (caused by S. haematobium) [4]. Schistosoma mansoni is the leading global cause of intestinal schistosomiasis in humans and accounts for 33% of all schistosomiasis cases [5].
Schistosomiasis is particularly prevalent in East Africa, with Tanzania having the highest national prevalence with 51.5% (an estimated 23.2 million infected) [6], followed by Uganda with 25.6% (11 million infected) [7] and Kenya with 14.5% (6 million infected) [8,9]. The distribution of schistosomiasis is dependent on the ecological requirements of the intermediate snail host, with the availability of freshwater habitats limiting the spread of schistosomiasis [10,11]. East Africa has a high prevalence of schistosomiasis due to the abundance of diverse freshwater environments (lakes, ponds, streams, dams and irrigation canals) that intermediate snail hosts inhabit [12]. Combined with poor water hygiene and sanitation, this provides an optimal environment for the transmission of schistosomiasis [12]. Sousa-Figueiredo et al. 2010 reported intestinal schistosomiasis is high among Ugandan shoreline villages, with Lake Albert having a prevalence of 82.2% in mothers and 68.7% in children, while Lake Victoria had a lower prevalence of infection, with 66.7% of mothers and 58.6% of children being infected [13]. This disparity in prevalence has been suggested to be the result of different species of Biomphalaria being present at each lake, with Lake Albert having reports of B. pfeifferi, B. stanleyi and B. sudanica, while Lake Victoria has had reports of B. choanomphala, B. pfeifferi and B. sudanica [14,15].
The freshwater snail genus Biomphalaria acts as the intermediate host for S. mansoni, with the African Great Lakes, Lake Albert and Lake Victoria providing a favourable habitat for multiple species of Biomphalaria [10,11,16,17]. All African Biomphalaria species are capable of transmitting S. mansoni infection [14], though some species (e.g. B. pfeifferi) are considered more important than others [18,19]. The rate of schistosome infection within a Biomphalaria population has traditionally been measured by observing how many snails shed cercariae over a 35–42 day period [20]. Previous studies using this traditional cercarial shedding method have shown that snails at Lake Albert consistently have a higher infection rate than snails at Lake Victoria [15,21]. Of the Biomphalaria species found at the African Great Lakes, B. stanleyi is reported as consistently having a high prevalence of S. mansoni infection [12,15,21,22], while B. choanomphala is reported as consistently having a low infection prevalence [15,21,23]. A meta-analysis by Hailegebriel et al. (2020) estimated the pooled prevalence of S. mansoni infection in Biomphalaria snails across Africa was on average 5.6% [19]. However, of the 51 studies investigating schistosome infection within intermediate snail hosts, only seven used molecular detection methods, while the rest used the traditional cercarial shedding method [19]. Molecular detection methods (molecular xenomonitoring) have several advantages over traditional cercarial shedding methods, as they do not require live snail specimens, are considerably less time consuming, can specifically detect S. mansoni infection, and can detect infection in both prepatent and shedding snails [24–31]. However, not all prepatent snails go on to shed cercariae, which can lead to exaggerated levels of infection when using molecular detection methods. Therefore, the use of both detection methods would ultimately give the best representation of infection prevalence within a snail population.
The prevalence of S. mansoni infection within a Biomphalaria population is affected by multiple factors. For example, past studies have associated snail populations with low levels of genetic variability with a higher prevalence of S. mansoni [32,33]. In addition, environmental factors such as altitude, water conductivity, water depth, water pH, temperature, droughts and floods have been shown to affect the prevalence of schistosome infections in snail populations [34–39]. As a result, many have speculated that S. mansoni prevalence differs throughout the year due to changes in environmental conditions between the seasons. For example, Uganda has a bimodal climate with two wet seasons (from March to May and from September to November) and two dry seasons (from December to February and from June to August) that take place every year [40]. Adoka et al. (2014) [41] reported that people living at the shoreline of Lake Victoria believed that intestinal schistosomiasis was more prevalent in the wet seasons than the dry seasons. Rowel et al. (2015) [15] found evidence in support of this, with their results showing that the number of Biomphalaria shedding cercariae was higher during the wet seasons than the dry seasons. However, there are few studies which explore the effect seasonality has on schistosome prevalence within snail populations [42].
Here we use two PCR-based, molecular xenomonitoring detection methods to investigate the infection prevalence of S. mansoni in Biomphalaria species found at the Ugandan shorelines of three Lake Albert and three Lake Victoria collection sites. Additionally, we investigate the effect seasonality has on the prevalence of S. mansoni infection by comparing the number of infected snails for each of the four wet and four dry seasons that took place between 2009 and 2010. Lastly, we measured the extent of the intraspecies genetic diversity present in the Biomphalaria species identified at the sites investigated, in order to determine whether there is any relationship between the prevalence of infection and the amount of snail host diversity.
Materials and methods
Sample sites and sample selection
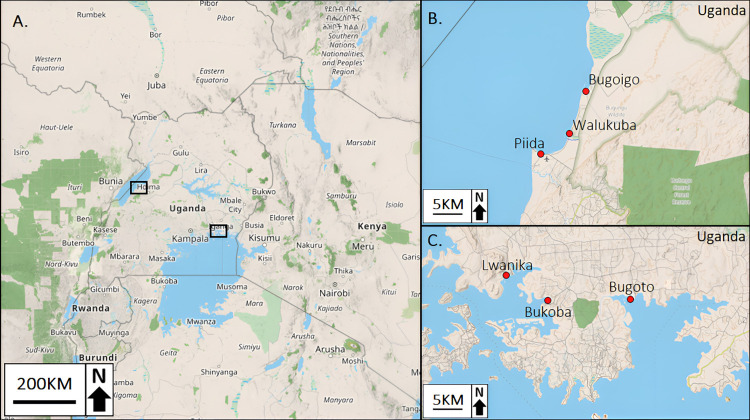
Biomphalaria snails used in this study were collected once a month for 29 consecutive months between 2009 and 2010 from three sites in the Buliisa district on the Ugandan shoreline of Lake Albert (Bugoigo: 1.908°N, 31.409°E; Piida: 1.819°N, 31.328°E; Walukuba: 1.842°N, 31.378°E) and three sites in the Mayuge district on the Ugandan shoreline of Lake Victoria (Bugoto: 0.319°N, 33.620°E; Bukoba: 0.312°N, 33.492°E; Lwanika: 0.351°N, 33.446°E), as part of the Wellcome Trust funded, Schistosomiasis In Mothers and Infants (SIMI) project [13,43,44,45]. (Fig 1 and see Rowel et al., 2015 [15] for further details about the collections). At each site, snails were collected from both the lake edge, which was often marshy shoreline, and the deeper waters of the lake (~1m depth). Approximately half of the snails collected were preserved in 70% ethanol and were held as a reference archival collection at the Liverpool School of Tropical Medicine, UK. Overall, 2,645 randomly selected snails were preserved from the original 6,183 collected at Lake Albert, and 6,382 randomly selected snails were preserved from the original 13,172 collected at Lake Victoria.
Fig 1.
(A) Map showing the collection site locations at Lake Albert and Lake Victoria in Uganda. (B) The three collection sites of Lake Albert (Bugoigo, Piida and Walukuba) and (C) the three collection sites of Lake Victoria (Bugoto, Bukoba and Lwanika; Created using OpenStreetMap, https://www.openstreetmap.org).
Snail identification and genetic diversity
All of the preserved Biomphalaria species collected over the two year period were initially identified to the species level using conchological identification methods [14]. At each site, twenty individuals of each species identified were selected for further molecular analysis. These selected individuals all came from the August 2010 collection, as this period had the highest number of viable specimens available. For each snail, DNA was extracted using a modified CTAB extraction method as described in Joof et al. (2020) [46], with extracted samples being resuspended in 100μl of TE, pH 8.0 (10mM Tris-HCl, 0.1mM EDTA) buffer. After extraction, DNA yields were measured using a NanoPhotometer N50 (Implen, München, Germany). The identification of each specimen was confirmed using 16S and COI genotyping. For the 16S gene, we used a modified version of the 16Sar/16Sbr primers designed by Palumbi et al. (1991) [47] (16Sarm: 5’-CTT CTC GAC TGT TTA TCA AAA ACA-‘3 and 16Sbrm: 5’-GCC GGT CTG AAC TCA GAT CAT-‘3). For COI, we used the universal COI primers designed by Folmer et al. (1994) [48] (LCO1490: 5’-GGT CAA ATC ATA AAG ATA TTG G-‘3 and HCO2198: 5’-TAA ACT TCA GGG TGA CCA AAA AAT CA-‘3). All PCR reactions were performed using Promega GoTaq G2 Master Mix buffer, with 1μl of DNA template added to 24μl of 1X Master Mix buffer (1U TAQ, 0.2μM primers, 200μMdNTPs, 3mM MgCl2). The PCR cycling conditions used for both the 16S and COI primer sets were identical, with an initial denaturation at 96°C for 1minute, followed by 34 cycles of 94°C for 1min, 50°C for 1min, 72°C for 1min and a final extension at 72°C for 10mins. All PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide and were observed under UV light. All 16S and COI PCR products were purified and sequenced using Macrogen’s EZ-Seq service.
All sequences were aligned using the Muscle algorithm in the program Seaview v5 [49], with misaligned sections of the 16S and the COI being fixed by hand and sites for tree building selected using the Gblocks program [50]. Samples were identified to the species-level using a concatenated 16S and COI phylogenetic tree incorporating GenBank references from Jørgensen et al. (2007) [51], Plam et al. (2008) [52], Standley et al. (2014) [53] and Zhang et al. (2018) [54] (S1 Table). Phylogenetic trees were constructed using the Maximum Likelihood method, using a General Time Reversible model incorporating gamma correction (GTR+Γ) in the program PhyML v3.1 [55], with bootstrap analysis undertaken using 1000 replicates. After confirming which species were present at our Lake Albert and Lake Victoria sites, we then measured the genetic variability of each species using DNASP v6 [56] to calculate Haplotype (gene) diversity (Hd) scores and nucleotide diversity (π) values [57]. MEGA-X [58] was used to calculate pairwise distances, with distances corrected using the Maximum Composite Likelihood (MCL) method. Genealogical relationships of the 16S and COI haplotypes were constructed using Median-Joining (MJ) networks [59] using the software NETWORK v5 (Fluxus Technology Ltd. www.Fluxus-engineering.com; S2 Table).
Infection detection
The prevalence of S. mansoni infection at Bugoigo, Piida, Walukuba, Bugoto, Bukoba and Lwanika was measured by initially testing twenty individuals of each species at a single time-point (August 2010). All of the DNA extracts were first tested using the LSU1iii/LSU3iii primers (LSU-1iii: 5′-TGC GAG AAT TAA TGT GAA TTG C-3′ and LSU-3iii: 5′- ACG GTA CTT GTC CGC TAT CG-3′) developed by Fontanilla et al. (2017) [60] to ensure that our DNA extracts were amplifiable. All PCR reactions were performed using Promega GoTaq G2 Master Mix buffer, with 1μl of DNA template added to 24μl of 1X Master Mix buffer (1U TAQ, 0.2μM primers, 200μM dNTPs, 3mM MgCl2). The PCR cycling conditions for the LSU-1iii/3iii primers was an initial denaturation at 96°C for 2min, followed by 35 cycles of 94°C for 30sec, 45°C for 1 min, 72°C for 2min and a final extension step at 72°C for 5 min.
After confirming the quality of our DNA extracts, we tested for S. mansoni infection using two different primer sets, firstly SmF/R (designed by Sandoval et al. 2006) [27] and then ND5 (designed by Lu et al. 2016) [30]. Only samples that tested positive with the SmF/R primer set were subjected to further testing using the ND5 primer set. This additional testing was carried out because the ND5 primer set possesses the ability to differentiate between human and non-human schistosome species based on the length of the diagnostic band [30]. All PCR reactions were performed using Promega GoTaq G2 Master Mix buffer, with 1μl of DNA template diluted to 50ng/μl. Alongside the Biomphalaria samples, two negative controls (water and uninfected B. glabrata DNA) and two positive controls (pure S. mansoni DNA and infected B. glabrata DNA) were also included. These controls were provided by Professor Mike Doenhoff, School of Biology, University of Nottingham. The PCR reaction mixture and cycling conditions for the SmF/R and ND5 primer sets were followed precisely as described by Sandoval et al. (2006) [27] and Lu et al. (2016) [30], respectively. Schistosoma mansoni infection was confirmed by running the PCR products on a 2% agarose gel containing ethidium bromide and observing whether a diagnostic band was present under UV light.
To examine the seasonal prevalence of infection at each site and of each species, we tested twenty individuals at both Lake Albert and Lake Victoria for each of the four wet (March to May and September to November) and four dry (December to February and June to August) seasons that occurred within the two year collection period (January 2009 to December 2010; rainfall data for Uganda is provided in S1 Fig). However, due to the limited number of samples available at Piida and Bukoba, only two of the three Lake Albert (Bugoigo Walukuba) and Lake Victoria (Bugoto and Lwanika) sites could be tested. Likewise, due to the limited number of B. stanleyi samples available, only B. choanomphala (Bukoba and Lwanika), B. pfeifferi (Walukuba) and B. sudanica (Walukuba and Bugoigo) could be tested. SPSS v26 (IBM, Armonk, USA) [61] was used to perform a Pearson’s chi-squared (X2) test with Yates’ correction to compare the prevalence of infection. The summary of the samples tested for infection can be found in S3 Table.
GenBank accessions
GenBank accession numbers for the Biomphalaria 16S and COI sequences used from Jørgensen et al. (2007) [51], Plam et al., 2008 [52], Standley et al. (2014) [53] and Zhang et al. (2018) [54] can be found in S1 Table. The DNA sequences generated in this study are available in GenBank accession numbers OQ924749-OQ924929 for the 16S gene and OQ849817-OQ849997 for the COI gene (further information can be found in S1 and S2 Tables).
Results
Infection prevalence at the African great Lakes
Of the sites tested, Lake Albert had the highest infection prevalence of S. mansoni, with an overall prevalence of 12.5% (15 PCR positive snails out of 120). Conversely, our Lake Victoria sites had a lower prevalence of only 5% (3/60). When partitioned by site, the Lake Albert sites had a higher mean prevalence of infection than the Lake Victoria sites (Table 1). Walukuba had the highest prevalence of infection of the Lake Albert sites with 13.3% (8/60), followed by Bugoigo with 12.5% (5/40) and Piida with 10% (2/20) (Table 1). Of the Lake Victoria sites, Lwanika had the highest prevalence with 10% (2/20), followed by Bugoto and Bukoba with 5% (1/20) for both sites (Table 1). All of our SmF/R positive Biomphalaria samples were confirmed to be infected with S. mansoni as every positive sample gave a diagnostic band length of ~302bp when tested with the ND5 primer set.
Table 1. Mean prevalence of S. mansoni infection and the number of unique 16S/COI haplotypes (No.), haplotype diversity scores (Hd) and nucleotide diversity values (π) of each Biomphalaria species genotyped at our Lake Albert and Lake Victoria collection sites (August 2010 collection).
| Lake Albert | |||||||||
| Species | No. Infected | Site Infection | 16S | COI | |||||
| No. | Hd | π | No. | Hd | π | ||||
| Bugoigo | B. sudanica (n = 20) | 3 | 12.5% | 7 | 0.784 | 0.000 | 3 | 0.532 | 0.002 |
| B. pfeifferi (n = 20) | 2 | 2 | 0.337 | 0.000 | 4 | 0.489 | 0.001 | ||
| Piida | B. sudanica (n = 20) | 2 | 10% | 6 | 0.716 | 0.000 | 2 | 0.521 | 0.002 |
| Walukuba | B. stanleyi (n = 20) | 3 | 13.3% | 10 | 0.884 | 0.002 | 10 | 0.815 | 0.003 |
| B. sudanica (n = 20) | 3 | 10 | 0.884 | 0.001 | 4 | 0.553 | 0.002 | ||
| B. pfeifferi (n = 20) | 2 | 3 | 0.468 | 0.001 | 6 | 0.832 | 0.002 | ||
| Lake Victoria | |||||||||
| Species | No. Infected | Site Infection | 16S | COI | |||||
| No. | Hd | π | No. | Hd | π | ||||
| Bugoto | B. choanomphala (n = 20) | 1 | 5% | 11 | 0.884 | 0.008 | 5 | 0.774 | 0.004 |
| Bukoba | B. choanomphala (n = 20) | 1 | 5% | 15 | 0.958 | 0.007 | 10 | 0.89 | 0.005 |
| Lwanika | B. choanomphala (n = 20) | 2 | 10% | 16 | 0.963 | 0.008 | 9 | 0.826 | 0.005 |
Note: Schistosoma mansoni infection was determined based on whether snails had a diagnostic band for both SmF/R (~350bp) and ND5 (~302bp).
Of the sites tested, we found three Biomphalaria species, B. pfeifferi, B. stanleyi and B. sudanica, at Lake Albert and one species, B. choanomphala, at Lake Victoria (S2 and S3 Figs). Of the four species identified, B. stanleyi had the highest prevalence of S. mansoni infection with 15% (3/20), followed by B. sudanica with 13.3% (8/60), B. pfeifferi with 10% (4/40), and B. choanomphala with 5% (3/60) (Table 1). Biomphalaria choanomphala exhibited two different shell morphologies, with 45 of the 60 snails exhibiting a lacustrine shell morphology (S2 Fig); all of the infected B. choanomphala snails at our Lake Victoria sites exhibited the lacustrine shell morphology. In addition to the four Biomphalaria species, we identified an Asian Gyraulus species at both Lake Albert and Lake Victoria (S2 Fig). There have been no published reports of Schistosoma infection in Gyraulus, and we detected no cases of S. mansoni infection in the Asian Gyraulus species found at Lake Albert (0/10) or Lake Victoria (0/10).
Genetic diversity of the Biomphalaria species at the African great Lakes
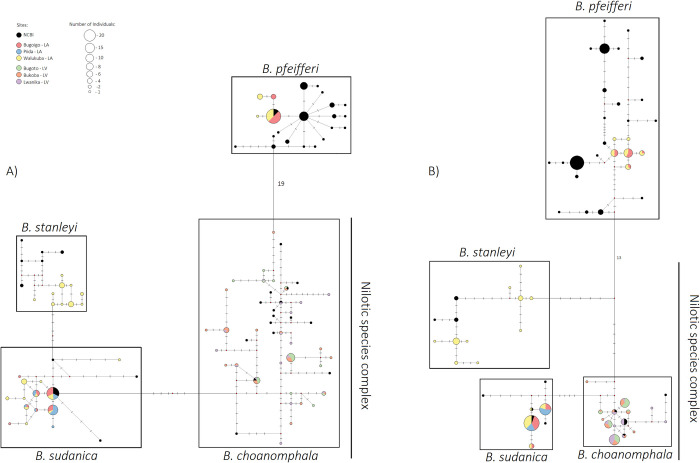
Of the Biomphalaria species found at our collection sites, B. choanomphala (n = 60) had 31 haplotypes for the 16S gene fragment, followed by B. sudanica (n = 60) with 14, B. stanleyi (n = 20) with 10 and B. pfeifferi (n = 40) with four (Table 1 and Fig 2A). For the COI gene fragment, B. choanomphala had 14 haplotypes, followed by B. stanleyi with 10, B. pfeifferi with six and B. sudanica with four (Table 1 and Fig 2B). Of the B. choanomphala snails sequenced, the lacustrine specimens had 21 unique 16S haplotypes and 12 unique COI haplotypes, while the non-lacustrine specimens had 14 unique 16S haplotypes and 8 unique COI haplotypes. Several of the 16S and COI haplotypes were shared between lacustrine and non-lacustrine individuals (S4 Fig). The haplotype diversity (Hd) scores for the 16S gene were highest for B. choanomphala with 0.945, followed by B. sudanica with 0.833, B. stanleyi with 0.884 and B. pfeifferi with 0.422. For the COI gene, haplotype diversity (Hd) scores were highest for B. choanomphala with 0.842, followed by B. stanleyi with 0.815, B. pfeifferi with 0.618 and B. sudanica with 0.553. Overall, the haplotypes were not highly divergent for both the 16S and COI. The nucleotide diversity values were highest for the B. choanomphala populations at Lake Victoria for both the 16S (0.007–0.008) and COI (0.004–0.005), while all of the Biomphalaria species at Lake Albert had very low nucleotide diversity values for both the 16S (0.000–0.002) and COI (0.001–0.003; Table 1). The intra-species pairwise distances of the 16S was the highest for B. choanomphala (0.0–1.8%), followed by B. stanleyi (0.0–0.8%), B. sudanica (0.0–0.8%) and B. pfeifferi (0.0–0.1%). Conversely, the intra-species pairwise distances of the COI was the highest for B. pfeifferi (0.0–1.4%), followed by B. stanleyi (0.0–1.3%), B. choanomphala (0.0–1.2%) and B. sudanica (0.0–0.4%).
Fig 2.
Median-Joining haplotype network of the Biomphalaria species found at Lake Albert (B. pfeifferi n = 40; B. stanleyi n = 20; B. sudanica n = 60) and Lake Victoria (B. choanomphala n = 60) using (A) 16S rRNA gene fragment (395bp) and (B) Cytochrome Oxidase Subunit I gene fragment (520bp). This network was generated using the software NETWORK v5. Circles represent each haplotype and circle size represents the numbers of individuals sharing a haplotype. Diamonds represent intermediate haplotypes, while hatch marks between points represent the number of nucleotide substitutions (substitutions more than five are indicated by numbers). Gaps were included in the 16S and COI alignments. Reference sequence information for the 16S and COI networks can be found in S1 and S2 Tables, respectively.
Seasonality of infection prevalence
At Lake Albert we examined the seasonal changes in infection prevalence at two sites (Bugoigo and Walukuba). One species (B. sudanica) was tested at Bugoigo, while two species (B. pfeifferi and B. sudanica) were tested at Walukuba. Piida and B. stanleyi were not tested due to a lack of samples. At Bugoigo, the wet seasons had a mean infection prevalence of 12.5% (10/80), while the dry seasons had a mean infection prevalence of 10% (8/80) (Table 2). At Walukuba, the wet seasons had a mean infection prevalence of 13.8% (22/160), while the dry seasons had a mean infection prevalence of 9.4% (15/160) (Table 2).
Table 2. Mean prevalence of infection of the wet and dry seasons at Lake Albert and Lake Victoria between 2009–2010.
| Lake Albert | ||||||
| Site | Species | First Dry (n = 40) | First Wet (n = 40) | Second Dry (n = 40) | Second Wet (n = 40) | Overall Infection (n = 160) |
| Walukuba | B. pfeifferi | 4 (10%) | 6 (15%) | 4 (10%) | 6 (15%) | 20 (12.5%) |
| B. sudanica | 3 (7.5%) | 5 (12.5%) | 4 (10%) | 5 (12.5%) | 17 (10.6%) | |
| Bugoigo | B. sudanica | 4 (10%) | 5 (12.5%) | 4 (10%) | 5 (12.5%) | 18 (11.3%) |
| Lake Victoria | ||||||
| Site | Species | First Dry (n = 40) | First Wet (n = 40) | Second Dry (n = 40) | Second Wet (n = 40) | Overall Infection (n = 160) |
| Lwanika | B. choanomphala | 2 (5%) | 3 (7.5%) | 3 (7.5%) | 4 (10%) | 12 (7.5%) |
| Bugoto | B. choanomphala | 1 (2.5%) | 3 (7.5%) | 2 (5%) | 4 (10%) | 10 (6.3%) |
Note: First Dry: Dec-Feb; First Wet: Mar-May; Second Dry: Jun-Aug; Second Wet: Sep-Nov.
At Lake Victoria, we examined the seasonal changes in prevalence of infection among Biomphalaria populations (B. choanomphala) at two sites (Bugoto and Lwanika). Bukoba was not tested due to a lack of samples. At Lwanika, the wet seasons had a mean infection prevalence of 8.8% (7/80), while the dry seasons had a mean infection prevalence of 6.3% (5/80) (Table 2). Bugoto had a mean infection prevalence of 8.8% (7/80) for the wet seasons and 3.8% (3/80) for the dry seasons (Table 2).
Overall, the prevalence of S. mansoni infection was consistently higher in the wet seasons than the dry seasons for both Lake Albert and Lake Victoria (Table 2 and S1 Fig). The overall mean prevalence of infection at Lake Albert for the four wet seasons was 13.3% (32/240), while the four dry seasons was 9.5% (23/240) (Table 2). Similarly, the overall mean prevalence of infection at Lake Victoria was 8.7% (14/160) for the wet seasons and 5% (8/160) for the dry seasons (Table 2). Nevertheless, a chi-square (X2) analysis found there was no significant difference in the prevalence of infection between the wet and dry seasons (p = 0.252 for Lake Albert and p = 0.269 for Lake Victoria). When comparing the prevalence of infection for the first and second wet season we found no difference for the Lake Albert sites. Likewise, there was no difference in infection prevalence for the first and second dry season. For Lake Victoria, we found that the first wet season had a lower mean prevalence of infection than the second wet season. Similarly, the first dry season also had a lower prevalence of infection than the second dry season (Table 2).
In order to test consistency in our infection prevalence estimates, we compared the prevalence of infection measured in our seasonality dataset against our single time point (August 2010) dataset. The single time point dataset found a mean infection prevalence of 12.5% (15/120) for Lake Albert, while the seasonality dataset found a mean infection prevalence of 11.5% (55/480). Lake Victoria had an infection prevalence of 5% (3/60) for the single time point dataset, while the seasonality dataset had an infection prevalence of 7.2% (23/320). Of the species tested, B. sudanica had an infection prevalence of 13.3% for the single time point dataset and an infection prevalence of 10.9% for the seasonality dataset. The mean infection prevalence of the B. pfeifferi snails was 10% for the single time point dataset and 12.5% for the seasonality dataset. Lastly, the B. choanomphala snails had a mean infection prevalence of 5% for the single time point dataset and 6.9% for the seasonality dataset. A chi-square (X2) analysis found there was no significant (P> 0.05) difference in the prevalence of S. mansoni infection in Biomphalaria snails between the two datasets. The overall averages for both datasets can be found in S3 Table.
Discussion
Of the six sites investigated which formed the surveillance area for the SIMI project, we found that Lake Albert (12.5%) had a higher prevalence of infected Biomphalaria snails than Lake Victoria (5%). Similarly, Rowel et al. (2015) [15] also reported that Lake Albert had a higher prevalence of shedding Biomphalaria snails (8.9%) compared to the Biomphalaria snails found at Lake Victoria (2.1%). When partitioned by site, we found Walukuba (13.8%) had the highest prevalence of infection of our Lake Albert sites, while Lwanika (10%) had the highest prevalence of infection of the Lake Victoria sites. Likewise, Rowel et al. (2015) [15] found that Walukuba (12.3%) had the highest prevalence of shedding Biomphalaria snails of the Lake Albert sites and Lwanika (3.8%) had the highest prevalence of shedding Biomphalaria snails of the Lake Victoria sites. Our result of Lake Albert having a higher prevalence of S. mansoni infection than Lake Victoria is consistent with previous findings [15,21,22,62]. The Vector Control Division (VCD) of the Ugandan Ministry of Health have had concerns about this issue, as despite the similar transmission rates of schistosomiasis and comparable mass drug administration programs present at both of the Great African Lakes, Lake Albert consistently has higher levels of severe morbidity compared to Lake Victoria. The Uganda Schistosomiasis Multidisciplinary Research Centre (U-SMRC) suggests several hypotheses as to why there is a higher prevalence of schistosomiasis at the Ugandan shoreline of Lake Albert when compared to the Ugandan shoreline of Lake Victoria: (I) variations in the immune systems of the local people (e.g. differences in microbiome, nutrition and lifestyle); (II) the genetic makeup of the parasite populations (e.g. differences in immunogenic/ immunoregulatory antigens expressed by the parasite and varying levels of praziquantel resistance); (III) the abundance and number of snail species found near human activity (e.g. differences in susceptibility of the snail host and the intensity of exposure to the parasite) [63].
Infection prevalence of the Biomphalaria species found at the African great Lakes
When partitioned by species, we found that B. stanleyi (15%) had the highest prevalence of infection at our Lake Albert sites, followed by B. sudanica (13.3%) and B. pfeifferi (10%). Our results are similar to Kazibwe et al. (2006) [12] and Rowel et al. (2015) [15], who similarly reported B. stanleyi snails as having the highest prevalence of infection at Lake Albert. At our Lake Victoria sites, B. choanomphala had an infection prevalence of 5%, with all of the infected individuals having a lacustrine shell morphology. Our results are similar to Mutuku et al. (2021) [64], who reported that S. mansoni infection and cercarial production was significantly higher in the lacustrine form of B. choanomphala than the non-lacustrine form, regardless of miracidium dosage or whether the eggs came from allopatric or sympatric sources. However, Rowel et al. (2015) [15] and Gouvras et al. (2017) [65] found the opposite trend, with the non-lacustrine form of B. choanomphala having a higher S. mansoni infection rate than the lacustrine form.
When compared to the original Rowel et al (2015) [15] study, our results observed a higher prevalence of S. mansoni infection at both the Lake Albert and Lake Victoria sites. Likely, this is a result of using molecular detection methods, which typically show higher levels of infection when compared to the traditional cercarial shedding method [30,46,66]. This is due to infected Biomphalaria snails not always producing cercariae during the usual 35–49 day incubation period. For example, colder temperatures can lead to delays in sporocyst development and shedding [34]. Similarly, delays to sporocyst development and shedding can arise due to the immune response to infection. The snail’s immunological response does not guarantee the complete eradication of all sporocysts and some sporocysts can release cercariae up to ten months post infection [67,68]. Ultimately these prepatent snails will be undetectable by the cercarial shedding method but are still detectable by molecular methods [30,46]. However, molecular methods can also overestimate the number of snails that present a risk. Lu et al. (2016) [30] found that not all PCR positive Biomphalaria snails went on to shed cercariae; some snails were able to successfully encapsulate and degrade the sporocysts during the prepatent period, which resulted in the infection failing. The chance of this happening was shown to be dependent on species, with the majority of PCR positive B. pfeifferi snails (60%) going on to shed cercariae, while only a minority of PCR positive B. sudanica snails (10%) went on to shed cercariae. It seems whether an infection is successful or not is dependent on schistosome-snail compatibility, with compatible schistosomes being able to successfully evade the host’s immune defences [69,70,71]. This means that a snail that is PCR positive for infection may not necessarily be capable of spreading that infection on to humans.
Moreover, Rowel et al. (2015) [15] reported that of the snails shedding cercariae, only 15.8% at Lake Albert and 13.9% at Lake Victoria were shedding S. mansoni cercariae (identified using general anatomical appearance) [72] as opposed to shedding cercariae of trematode species with no medical importance. Likely this difference in S. mansoni prevalence is the result of snails being co-infected with both S. mansoni and non-S. mansoni sporocysts simultaneously [66,73], which makes it more difficult to reliably identify the presence of S. mansoni cercariae since these S. mansoni cercariae can be obscured by other non-medically important cercariae and therefore missed. Molecular detection methods are able to detect more reliably whether or not S. mansoni is present, while ignoring the non-S. mansoni sporocysts.
Infection prevalence and Host-Snail genetic diversity
We found that the Biomphalaria species found at our Lake Victoria sites (B. choanomphala) had higher intraspecies genetic diversity than the Biomphalaria species (B. pfeifferi, B. stanleyi and B. sudanica) found at our Lake Albert sites. Furthermore, our Lake Victoria sites had a lower prevalence of infection than our Lake Albert sites. This is consistent with previous studies that have reported higher levels of intra-species genetic variation in host snails being linked to a lower prevalence of infection [32,33]. However, when we examined each of the sites individually, we found that sites which had a higher prevalence of infection also had Biomphalaria populations with higher levels of intraspecific genetic diversity (Table 1). For example, when we compared the haplotype diversity scores of the 16S and COI genes for the B. pfeifferi snails found at Walukuba with the B. pfeifferi snails found at Bugoigo, we found Walukuba had both a higher amount of genetic diversity (16S Hd: 0.468; COI Hd: 0.832) and a higher prevalence of infection (13.3%) than Bugoigo (16S Hd: 0.337; COI Hd: 0.489; infection prevalence: 12.5%) (Table 1). Similarly, we also found this trend for both B. sudanica and B. choanomphala snails (Table 1). B. sudanica snails at Walukuba had both a higher amount of genetic diversity (16S Hd: 0.884; COI Hd: 0.553) and prevalence of infection (13.3%) than B. sudanica snails found at Bugoigo (16S Hd: 0.784; COI Hd: 0.532; infection prevalence: 12.5%) and Piida (16S Hd: 0.716; COI Hd: 0.521; infection prevalence: 10%) (Table 1). Likewise, the B. choanomphala snails at Lwanika had higher amounts of genetic diversity (16S Hd: 0.963; COI Hd: 0.826) and prevalence of infection (10%) than the B. choanomphala snails at Bugoto (16S Hd: 0.884; COI Hd: 0.774; infection prevalence: 5%) (Table 1).
Biomphalaria snails within a population have shown variability in their susceptibility to S. mansoni infection, with some individuals being successfully infected and others remaining resistant, resulting in a phenomenon known as "compatibility polymorphism". The underlying reason of why this occurs is not yet fully understood, but two hypotheses have been suggested to explain this phenomenon, the “resistance hypothesis” and the “matching hypothesis” [74]. The former suggests that the snail host’s resistance and susceptibility status play a significant role in determining whether infection is successful, as vulnerable individuals lack the ability to recognise the parasite upon entry or produce an effective immunological response in time [75]. Previous research has shown differences in immune-related genes between compatible and incompatible snails, supporting this hypothesis [76]. Conversely, the latter hypothesis proposes that the success or failure of an infection is not determined by the susceptibility or resistance of an individual, but rather by the level of compatibility between the host and parasite phenotypes, suggesting all snails are potentially susceptible to infection if they encounter a schistosome with a matching phenotype [77,78]. Previous experimental treatments have supported this hypothesis, by showing infection rates increase when the phenotypic diversity of miracidia increases [76]. Of the two hypotheses suggested, our results support the assertions proposed by the matching hypothesis, as we found the prevalence of infection increased alongside snail host genetic diversity. This possibly suggests that sites with more diverse snail host populations have a higher probability of the parasite encountering a compatible host, while sites with lower levels of snail host genetic diversity have a lower probability of the parasite finding a suitable host due to fewer possible combinations being available.
Infection prevalence and seasonality
At our Lake Albert sites, we found that the wet seasons (March to May and September to November) had a higher prevalence of infection (13.3%) than the dry seasons (December to February and June to August) (9.6%). This was also the case at our Lake Victoria sites, with the wet seasons having a higher prevalence of infection (8.7%) than the dry seasons (5%). Rowel et al. (2015) [15] also observed a higher number of shedding Biomphalaria snails during the wet seasons at Lake Albert and Lake Victoria. Moreover, Kazibwe et al. (2006) [12] found the highest rates of cercarial shedding in B. stanleyi and B. sudanica snails at Lake Albert took place during the wet seasons. Similarly, Wolmarans et al. (2002) [79] found that South African B. pfeifferi snails collected during the wet season (January to April) had a higher cercarial shedding rate than B. pfeifferi snails collected during either the cold (May to August) or the warm (September to December) dry seasons. However, depending on where the parasitological survey is undertaken can lead to contradictory results as studies undertaken in Ethiopia [80], Nigeria [81], Tanzania [82] and Sudan [83] have found the opposite trend, with the dry seasons having a higher rate of S. mansoni infected snails than the wet seasons. Moreover, our chi-square (X2) analysis found that the prevalence of infection during the wet seasons was not significantly higher than the prevalence of infection during the dry seasons for both Lake Albert and Lake Victoria.
Supporting information
Rainfall data was collected by weather stations located near Lake Albert (Erusi Forest, Ihungu, and Kiryanga Gombolola) between 1904 and 2001, and by weather stations near Lake Victoria (Gayaza Isingiro, Ntusi, and Entebbe) between 1900 and 2005. Data and figures were adapted from the Nile basin water resources atlas (Nile Basin Initiative, 2017) [40].
(TIF)
Biomphalaria pfeifferi, B. stanleyi and B. sudanica were present at Lake Albert, while the two morphotypes (non-lacustrine and lacustrine) of B. choanomphala were present at Lake Victoria. In addition, an invasive, unidentified Asian Gyraulus species was present at Lake Albert and Lake Victoria. The shells are viewed from the apertural (left) and umbilical (right) shell angles.
(TIF)
This tree was generated using PhyML v3.1 using a GTR+Γ model and is rooted on Biomphalaria glabrata. Numbers on branches indicate the bootstrap percentages for 1000 replicates (bootstrap support values under 50% are not shown). The scale bar represents sequence divergence. Samples labelled ‘cf.’ had shell morphologies’ that looked like a specific species but were identified by the original authors as another species using molecular methods.
(TIF)
Each of the B. choanomphala snails shown are colour-coded to indicate whether they exhibited a non- lacustrine (black) or lacustrine (white) shell morphology. This network was generated using the software NETWORK v5. Circles represent each haplotype and circle size represents the numbers of individuals sharing a haplotype. Diamonds represent intermediate haplotypes, while hatch marks between points represent the number of nucleotide substitutions (substitutions more than five are indicated by numbers). Gaps were included in the 16S and COI alignments.
(TIF)
Seasonal prevalence of Schistosoma mansoni infection at our Lake Albert (A-C) and Lake Victoria (D-E) sites over the course of two years (2009–2010). Biomphalaria sudanica (n = 320) was tested at two sites in Lake Albert (A: Bugoigo & B: Walukuba), while B. pfeifferi (n = 160) was tested at one site (C: Walukuba). Biomphalaria choanomphala (n = 320) was tested at two sites at Lake Victoria (D: Bugoto & E: Lwanika). Black bars indicate the percentage of infected individuals (n = 20). (Dry 1: January-February 2009; Wet 1: March-May 2009; Dry 2: June-August 2009; Wet 2: September-November 2009; Dry 3: December 2009-February 2010; Wet 3: March-May 2010; Dry 4: June-August 2010; Wet 4: September-November 2010).
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Candia Rowel and Fred Besigye, for their efforts in collecting the specimens used in this study. We would also like to thank Dr. Narcis B. Kabatereine at the Vector Control Division of the Ugandan Ministry of Health, for his efforts as the co-investigator of the SIMI project.
Data Availability
All data is given within the manuscript itself. Sequence data is provided in GenBank accession numbers OQ924749-OQ924929 and OQ849817-OQ849997 (see S1 and S2 Tables for further details).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Current estimated total number of individuals with morbidity and mortality due to Schistosomiasis haematobium and S. Mansoni infection in Sub-Saharan Africa. World Health Organization. 2016;23. [Google Scholar]
- 2.Boko PM, Ibikounle M, Onzo-Aboki A, Tougoue JJ, Sissinto Y, Batcho W, et al. Schistosomiasis and soil transmitted helminths distribution in Benin: a baseline prevalence survey in 30 districts. PLoS One. 2016;11(9):e0162798. doi: 10.1371/journal.pone.0162798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onasanya A, Bengtson M, Oladepo O, Van Engelen J, Diehl JC. Rethinking the top-down approach to schistosomiasis control and elimination in sub-Saharan Africa. Frontiers in Public Health. 2021;9:622809. doi: 10.3389/fpubh.2021.622809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite immunology. 2014; (8):347–357. doi: 10.1111/pim.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schistosomiasis WHO. Soil transmitted helminthiases: progress report, 2020. Wkly Epidemiol Rec. 2021;96(48):585–95. [Google Scholar]
- 6.Mazigo HD, Nuwaha F, Kinung’hi SM, Morona D, de Moira AP, Wilson S, et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasites & vectors. 2012;5:1–20. doi: 10.1186/1756-3305-5-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exum NG, Kibira SP, Ssenyonga R, Nobili J, Shannon AK, Ssempebwa JC, et al. The prevalence of schistosomiasis in Uganda: A nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS neglected tropical diseases. 2019;13(8):e0007617. doi: 10.1371/journal.pntd.0007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuenté LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta tropica. 2013;128(2):423–440. doi: 10.1016/j.actatropica.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, et al. Community knowledge, attitudes and practices on schistosomiasis in western Kenya-the SCORE Project. The American journal of tropical medicine and hygiene. 2014;90(4):646. doi: 10.4269/ajtmh.13-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturrock RF. The schistosomes and their intermediate hosts. In Schistosomiasis. 2001; 7–83. [Google Scholar]
- 11.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. The Lancet infectious diseases. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 12.Kazibwe F, Makanga B, Rubaire-Akiiki C, Ouma J, Kariuki C, Kabatereine NB, et al. Ecology of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert, Western Uganda: snail distributions, infection with schistosomes and temporal associations with environmental dynamics. Hydrobiologia. 2006;568:433–444. [Google Scholar]
- 13.Sousa-Figueiredo JC, Pleasant J, Day M, Betson M, Rollinson D, Montresor A, et al. Treatment of intestinal schistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquantel dosing pole. International Health. 2010;2(2):103–113. doi: 10.1016/j.inhe.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DS. Freshwater snails of Africa and their medical importance. CRC press; 2002. [Google Scholar]
- 15.Rowel C, Fred B, Betson M, Sousa-Figueiredo JC, Kabatereine NB, Stothard JR. Environmental epidemiology of intestinal schistosomiasis in Uganda: population dynamics of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert and Lake Victoria with observations on natural infections with digenetic trematodes. BioMed research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crompton DW. How much human helminthiasis is there in the world?. The Journal of parasitology. 1999:397–403. [PubMed] [Google Scholar]
- 17.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Disease control priorities in developing countries. 2006. [PubMed] [Google Scholar]
- 18.Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123(7):211–28. doi: 10.1017/s0031182001007703 [DOI] [PubMed] [Google Scholar]
- 19.Hailegebriel T, Nibret E, Munshea A. Prevalence of Schistosoma mansoni and S. haematobium in snail intermediate hosts in Africa: a systematic review and meta-analysis. Journal of tropical medicine. 2020;2020. doi: 10.1155/2020/8850840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webbe G. Transmission of bilharziasis: 2. Production of cercariae. Bulletin of the World Health Organization. 1965;33(2):155. [PMC free article] [PubMed] [Google Scholar]
- 21.Adriko M, Standley CJ, Tinkitina B, Mwesigwa G, Kristensen TK, Stothard JR, Kabatereine NB. Compatibility of Ugandan Schistosoma mansoni isolates with Biomphalaria snail species from Lake Albert and Lake Victoria. Acta Tropica. 2013;128(2):303–308. doi: 10.1016/j.actatropica.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 22.Kazibwe F, Makanga B, Rubaire-Akiiki C, Ouma J, Kariuki C, Kabatereine NB, et al. Transmission studies of intestinal schistosomiasis in Lake Albert, Uganda and experimental compatibility of local Biomphalaria spp. Parasitology international. 2010;59(1):49–53. doi: 10.1016/j.parint.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Odongo-Aginya EI, Kironde FK, Kabatereine NB, Kategere P, Kazibwe F. Effect Of Seasonal Rainfall And Other Environmental Changes, On Snail Density And Infection Rates With Schistosoma mansoni Fifteen Years After The Last Snails\’Study In Kigungu, Entebbe, Uganda. East African medical journal. 2008;85(11):556–563. doi: 10.4314/eamj.v85i11.9675 [DOI] [PubMed] [Google Scholar]
- 24.Jannotti-Passos K, Vidigal TH, Dias-Neto E, Pena SD, Simpson AJ, Dutra WO, Souza CP, Carvalho-Parra JF. PCR amplification of the mitochondrial DNA minisatellite region to detect Schistosoma mansoni infection in Biomphalaria glabrata snails. Journal of Parasitology. 1997;83(3):395–399. [PubMed] [Google Scholar]
- 25.Hamburger J, Xin XY, Ramzy RM, Jourdane J, Ruppel AN. A polymerase chain reaction assay for detecting snails infected with bilharzia parasites (Schistosoma mansoni) from very early prepatency. The American journal of tropical medicine and hygiene. 1998;59(6):872–876. doi: 10.4269/ajtmh.1998.59.872 [DOI] [PubMed] [Google Scholar]
- 26.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandoval N, Siles-Lucas M, Perez-Arellano JL, Carranza C, Puente S, Lopez-Aban J, et al. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology. 2006;133(5):581–587. doi: 10.1017/S0031182006000898 [DOI] [PubMed] [Google Scholar]
- 28.Abbasi I, King CH, Muchiri EM, Hamburger J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency. The American journal of tropical medicine and hygiene. 2010;83(2):427. doi: 10.4269/ajtmh.2010.09-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamburger J, Abbasi I, Kariuki C, Wanjala A, Mzungu E, Mungai P, et al. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. The American journal of tropical medicine and hygiene. 2013;88(2):344. doi: 10.4269/ajtmh.2012.12-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L, Zhang SM, Mutuku MW, Mkoji GM, Loker ES. Relative compatibility of Schistosoma mansoni with Biomphalaria sudanica and B. pfeifferi from Kenya as assessed by PCR amplification of the S. mansoni ND5 gene in conjunction with traditional methods. Parasites & Vectors. 2016;9(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldeira RL, Jannotti-Passos LK, Dos Santos Carvalho O. Use of molecular methods for the rapid mass detection of Schistosoma mansoni (Platyhelminthes: Trematoda) in Biomphalaria spp.(Gastropoda: Planorbidae). Journal of tropical medicine. 2017;2017. doi: 10.1155/2017/8628971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarne P, Théron A. Genetic structure in natural populations of flukes and snails: a practical approach and review. Parasitology. 2001;123(7):27–40. doi: 10.1017/s0031182001007715 [DOI] [PubMed] [Google Scholar]
- 33.Campbell G, Noble LR, Rollinson D, Southgate VR, Webster JP, Jones CS. Low genetic diversity in a snail intermediate host (Biomphalaria pfeifferi Krass, 1848) and schistosomiasis transmission in the Senegal River Basin. Molecular Ecology. 2010;19(2):241–256. doi: 10.1111/j.1365-294X.2009.04463.x [DOI] [PubMed] [Google Scholar]
- 34.Shiff CJ, Evans A, Yiannakis C, Eardley M. Seasonal influence on the production of Schistosoma haematobium and S. mansoni cercariae in Rhodesia. International Journal for Parasitology. 1975;5(1):119–123. [DOI] [PubMed] [Google Scholar]
- 35.Sturrock RF, Diaw OT, Talla I, Niang M, Piau JP, Capron A. Seasonality in the transmission of schistosomiasis and in populations of its snail intermediate hosts in and around a sugar irrigation scheme at Richard Toll, Senegal. Parasitology. 2001;123(7):77–89. [DOI] [PubMed] [Google Scholar]
- 36.Kabatereine NB, Brooker S, Tukahebwa EM, Kazibwe F, Onapa AW. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Medicine & International Health. 2004;9(3):372–380. doi: 10.1046/j.1365-3156.2003.01176.x [DOI] [PubMed] [Google Scholar]
- 37.John R, Ezekiel M, Philbert C, Andrew A. Schistosomiasis transmission at high altitude crater lakes in Western Uganda. BMC infectious Diseases. 2008;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Saez J, Mande T, Ceperley N, Bertuzzo E, Mari L, Gatto M, et al. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proceedings of the National Academy of Sciences. 2016;113(23):6427–6432. doi: 10.1073/pnas.1602251113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabo Z, Neubauer TA, Tumwebaze I, Stelbrink B, Breuer L, Hammoud C, et al. Factors Controlling the Distribution of Intermediate Host Snails of Schistosoma in Crater Lakes in Uganda: A Machine Learning Approach. Frontiers in Environmental Science. 2022;10:871735. [Google Scholar]
- 40.Nile Basin Initiative. Nile basin water resources Atlas [Internet]. 2017. Available from: http://atlas.nilebasin.org/start.
- 41.Adoka SO, Anyona DN, Abuom PO, Dida GO, Karanja D, Vulule JM, et al. Community perceptions of schistosomiasis transmission, prevalence and control in relation to aquatic habitats in the Lake Victoria basin of Kenya. East African medical journal. 2014;91(7):232–44. [PubMed] [Google Scholar]
- 42.Nwoko OE, Kalinda C, Chimbari MJ. Systematic review and meta-analysis on the infection rates of schistosome transmitting snails in Southern Africa. Tropical Medicine and Infectious Disease. 2022;7(5):72. doi: 10.3390/tropicalmed7050072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betson M, Sousa-Figueiredo JC, Rowell C, Kabatereine NB, Stothard JR. Intestinal schistosomiasis in mothers and young children in Uganda: investigation of field-applicable markers of bowel morbidity. The American journal of tropical medicine and hygiene. 2010;83(5):1048. doi: 10.4269/ajtmh.2010.10-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stothard JR, Sousa-Figueiredo JC, Betson M, Green HK, Seto EY, Garba A, et al. Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology. 2011;138(12):1593–1606. doi: 10.1017/S0031182011001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa-Figueiredo JC, Betson M, Atuhaire A, Arinaitwe M, Navaratnam AM, Kabatereine NB, et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joof E, Andrus PS, Sowunmi K, Onyango VM, Wade CM. Comparing PCR techniques against conventional cercarial shedding methods for detecting Schistosoma mansoni infection in Biomphalaria snails. Acta Tropica. 2020;212:105716. doi: 10.1016/j.actatropica.2020.105716 [DOI] [PubMed] [Google Scholar]
- 47.Palumbi SR, Martin A, Romano S, McMillan WO, Stice L, Grabowski G. The simple fool’s guide to PCR, version 2.0. University of Hawaii, Honolulu. 1991;45:26–28. [Google Scholar]
- 48.Folmer O, Hoeh WR, Black MB, Vrijenhoek RC. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Molecular Marine Biology and Biotechnology. 1994;3(5):294–299. [PubMed] [Google Scholar]
- 49.Gouy M, Tannier E, Comte N, Parsons DP. Seaview version 5: a multiplatform software for multiple sequence alignment, molecular phylogenetic analyses, and tree reconciliation. Multiple sequence alignment: methods and protocols. 2021:241–260. doi: 10.1007/978-1-0716-1036-7_15 [DOI] [PubMed] [Google Scholar]
- 50.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular biology and evolution. 2000;17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 51.Jørgensen A, Kristensen TK, Stothard JR. Phylogeny and biogeography of African Biomphalaria (Gastropoda: Planorbidae), with emphasis on endemic species of the great East African lakes. Zoological Journal of the Linnean Society. 2007;151(2):337–349. [Google Scholar]
- 52.Plam M, Jørgensen A, Kristensen TK, Madsen H. Sympatric Biomphalaria species (Gastropoda: Planorbidae) in Lake Albert, Uganda, show homoplasies in shell morphology. African Zoology. 2008;43(1):34–44. [Google Scholar]
- 53.Standley CJ, Goodacre SL, Wade CM, Stothard JR. The population genetic structure of Biomphalaria choanomphala in Lake Victoria, East Africa: implications for schistosomiasis transmission. Parasites & vectors. 2014;7:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang SM, Bu L, Laidemitt MR, Lu L, Mutuku MW, Mkoji GM, et al. Complete mitochondrial and rDNA complex sequences of important vector species of Biomphalaria, obligatory hosts of the human-infecting blood fluke, Schistosoma mansoni. Scientific reports. 2018;8(1):7341. doi: 10.1038/s41598-018-25463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 56.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular biology and evolution. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248 [DOI] [PubMed] [Google Scholar]
- 57.Nei M. Molecular evolutionary genetics. Columbia university press; 1987. [Google Scholar]
- 58.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular biology and evolution. 2018;35(6):1547. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular biology and evolution. 1999;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036 [DOI] [PubMed] [Google Scholar]
- 60.Fontanilla IK, Naggs F, Wade CM. Molecular phylogeny of the achatinoidea (mollusca: Gastropoda). Molecular Phylogenetics and Evolution. 2017;114:382–385. doi: 10.1016/j.ympev.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 61.SPSS. Statistics for Windows (Version 26.0) [Computer Software]. IBM Corp, Armonk, NY. 2019. [Google Scholar]
- 62.Levitz S, Standley CJ, Adriko M, Kabatereine NB, Stothard JR. Environmental epidemiology of intestinal schistosomiasis and genetic diversity of Schistosoma mansoni infections in snails at Bugoigo village, Lake Albert. Acta tropica. 2013;128(2):284–91. doi: 10.1016/j.actatropica.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 63.Tropical Medicine Research Centers. Uganda schistosomiasis multidisciplinary research centre (U-SMRC) [Internet]. 2023. Available from: https://tmrc-network.org/research-centers/uganda.
- 64.Mutuku MW, Laidemitt MR, Spaan JM, Mwangi IN, Ochanda H, Steinauer ML, et al. Comparative vectorial competence of Biomphalaria sudanica and Biomphalaria choanomphala, snail hosts of Schistosoma mansoni, from transmission hotspots in Lake Victoria, Western Kenya. The Journal of Parasitology. 2021;107(2):349–57. doi: 10.1645/20-138 [DOI] [PubMed] [Google Scholar]
- 65.Gouvras AN, Allan F, Kinung’hi S, Rabone M, Emery A, Angelo T, et al. Longitudinal survey on the distribution of Biomphalaria sudanica and B. choanomophala in Mwanza region, on the shores of Lake Victoria, Tanzania: implications for schistosomiasis transmission and control. Parasites & Vectors. 2017;10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Born-Torrijos A, Poulin R, Raga JA, Holzer AS. Estimating trematode prevalence in snail hosts using a single-step duplex PCR: how badly does cercarial shedding underestimate infection rates?. Parasites & Vectors. 2014;7(1):1–1. doi: 10.1186/1756-3305-7-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borges CM, Souza CP, Andrade ZA. Histopathologic features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Memórias do Instituto Oswaldo Cruz. 1998;93:117–21. doi: 10.1590/s0074-02761998000700016 [DOI] [PubMed] [Google Scholar]
- 68.Lemos QT, Andrade ZA. Sequential histological changes in Biomphalaria glabrata during the course of Schistosoma mansoni infection. Memórias do Instituto Oswaldo Cruz. 2001;96:719–21. doi: 10.1590/s0074-02762001000500025 [DOI] [PubMed] [Google Scholar]
- 69.Théron A, Pages JR, Rognon A. Schistosoma mansoni: Distribution Patterns of Miracidia amongBiomphalaria glabrataSnail as Related to Host Susceptibility and Sporocyst Regulatory Processes. Experimental parasitology. 1997;85(1):1–9. doi: 10.1006/expr.1996.4106 [DOI] [PubMed] [Google Scholar]
- 70.Mitta G, Adema CM, Gourbal B, Loker ES, Theron A. Compatibility polymorphism in snail/schistosome interactions: From field to theory to molecular mechanisms. Developmental & Comparative Immunology. 2012;37(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Théron A, Rognon A, Gourbal B, Mitta G. Multi-parasite host susceptibility and multi-host parasite infectivity: a new approach of the Biomphalaria glabrata/Schistosoma mansoni compatibility polymorphism. Infection, Genetics and Evolution. 2014;26:80–8. doi: 10.1016/j.meegid.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 72.Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta tropica. 1984;41(2):181–202. [PubMed] [Google Scholar]
- 73.Outa JO, Sattmann H, Köhsler M, Walochnik J, Jirsa F. Diversity of digenean trematode larvae in snails from Lake Victoria, Kenya: First reports and bioindicative aspects. Acta tropica. 2020;206:105437. doi: 10.1016/j.actatropica.2020.105437 [DOI] [PubMed] [Google Scholar]
- 74.Mitta G, Gourbal B, Grunau C, Knight M, Bridger JM, Théron A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: an increasingly complex puzzle. Advances in parasitology. 2017;97:111–45. doi: 10.1016/bs.apar.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 75.Webster JP, Davies CM. Coevolution and compatibility in the snail–schistosome system. Parasitology. 2001;123(7):41–56. doi: 10.1017/s0031182001008071 [DOI] [PubMed] [Google Scholar]
- 76.Au MF, Williams GA, Hui JH. Status Quo and Future Perspectives of Molecular and Genomic Studies on the Genus Biomphalaria—The Intermediate Snail Host of Schistosoma mansoni. International Journal of Molecular Sciences. 2023;24(5):4895. doi: 10.3390/ijms24054895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theron A, Coustau C. Are Biomphalaria snails resistant to Schistosoma mansoni?. Journal of helminthology. 2005;79(3):187–91. doi: 10.1079/joh2005299 [DOI] [PubMed] [Google Scholar]
- 78.Theron A, Coustau C, Rognon A, Gourbiere S, Blouin MS. Effects of laboratory culture on compatibility between snails and schistosomes. Parasitology. 2008;135(10):1179–88. doi: 10.1017/S0031182008004745 [DOI] [PubMed] [Google Scholar]
- 79.Wolmarans CT, De Kock KN, Strauss HD, Bornman M. Daily emergence of Schistosoma mansoni and S. haematobium cercariae from naturally infected snails under field conditions. Journal of helminthology. 2002;76(3):273–7. doi: 10.1079/JOH2002122 [DOI] [PubMed] [Google Scholar]
- 80.Nwoko OE, Manyangadze T, Chimbari MJ. Spatial and seasonal distribution of human schistosomiasis intermediate host snails and their interactions with other freshwater snails in 7 districts of KwaZulu-Natal province, South Africa. Scientific Reports. 2023;13(1):7845. doi: 10.1038/s41598-023-34122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okeke OC, Ubachukwu PO. Trematode infections of the freshwater snail Biomphalaria pfeifferi from a south-east Nigerian community with emphasis on cercariae of Schistosoma. Journal of helminthology. 2017;91(3):295–301. doi: 10.1017/S0022149X16000353 [DOI] [PubMed] [Google Scholar]
- 82.Nzalawahe J. Trematode Infections in Freshwater Snails and Seasonal Variations in Iringa and Arumeru Districts, Tanzania. Tanzania Veterinary Journal. 2021;36(1). [Google Scholar]
- 83.Ismail HA, Ahmed AE, Cha S, Jin Y. The Life Histories of Intermediate Hosts and Parasites of Schistosoma haematobium and Schistosoma mansoni in the White Nile River, Sudan. International Journal of Environmental Research and Public Health. 2022;19(3):1508. doi: 10.3390/ijerph19031508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rainfall data was collected by weather stations located near Lake Albert (Erusi Forest, Ihungu, and Kiryanga Gombolola) between 1904 and 2001, and by weather stations near Lake Victoria (Gayaza Isingiro, Ntusi, and Entebbe) between 1900 and 2005. Data and figures were adapted from the Nile basin water resources atlas (Nile Basin Initiative, 2017) [40].
(TIF)
Biomphalaria pfeifferi, B. stanleyi and B. sudanica were present at Lake Albert, while the two morphotypes (non-lacustrine and lacustrine) of B. choanomphala were present at Lake Victoria. In addition, an invasive, unidentified Asian Gyraulus species was present at Lake Albert and Lake Victoria. The shells are viewed from the apertural (left) and umbilical (right) shell angles.
(TIF)
This tree was generated using PhyML v3.1 using a GTR+Γ model and is rooted on Biomphalaria glabrata. Numbers on branches indicate the bootstrap percentages for 1000 replicates (bootstrap support values under 50% are not shown). The scale bar represents sequence divergence. Samples labelled ‘cf.’ had shell morphologies’ that looked like a specific species but were identified by the original authors as another species using molecular methods.
(TIF)
Each of the B. choanomphala snails shown are colour-coded to indicate whether they exhibited a non- lacustrine (black) or lacustrine (white) shell morphology. This network was generated using the software NETWORK v5. Circles represent each haplotype and circle size represents the numbers of individuals sharing a haplotype. Diamonds represent intermediate haplotypes, while hatch marks between points represent the number of nucleotide substitutions (substitutions more than five are indicated by numbers). Gaps were included in the 16S and COI alignments.
(TIF)
Seasonal prevalence of Schistosoma mansoni infection at our Lake Albert (A-C) and Lake Victoria (D-E) sites over the course of two years (2009–2010). Biomphalaria sudanica (n = 320) was tested at two sites in Lake Albert (A: Bugoigo & B: Walukuba), while B. pfeifferi (n = 160) was tested at one site (C: Walukuba). Biomphalaria choanomphala (n = 320) was tested at two sites at Lake Victoria (D: Bugoto & E: Lwanika). Black bars indicate the percentage of infected individuals (n = 20). (Dry 1: January-February 2009; Wet 1: March-May 2009; Dry 2: June-August 2009; Wet 2: September-November 2009; Dry 3: December 2009-February 2010; Wet 3: March-May 2010; Dry 4: June-August 2010; Wet 4: September-November 2010).
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data is given within the manuscript itself. Sequence data is provided in GenBank accession numbers OQ924749-OQ924929 and OQ849817-OQ849997 (see S1 and S2 Tables for further details).