Abstract
Aims
The aims of this study were to assess prescription patterns, dosages, discontinuation rates, and association with prognosis of conventional heart failure medications in patients with transthyretin cardiac amyloidosis (ATTR-CA).
Methods and results
A retrospective analysis of all consecutive patients diagnosed with ATTR-CA at the National Amyloidosis Centre between 2000 and 2022 identified 2371 patients with ATTR-CA. Prescription of heart failure medications was greater among patients with a more severe cardiac phenotype, comprising beta-blockers in 55.4%, angiotensin-converting enzyme inhibitors (ACEis)/angiotensin II receptor blockers (ARBs) in 57.4%, and mineralocorticoid receptor antagonists (MRAs) in 39.0% of cases. During a median follow-up of 27.8 months (interquartile range 10.6–51.3), 21.7% had beta-blockers discontinued, and 32.9% had ACEi/ARBs discontinued. In contrast, only 7.5% had MRAs discontinued. A propensity score-matched analysis demonstrated that treatment with MRAs was independently associated with a reduced risk of mortality in the overall population [hazard ratio (HR) 0.77 (95% confidence interval (CI) 0.66–0.89), P < .001] and in a pre-specified subgroup of patients with a left ventricular ejection fraction (LVEF) >40% [HR 0.75 (95% CI 0.63–0.90), P = .002]; and treatment with low-dose beta-blockers was independently associated with a reduced risk of mortality in a pre-specified subgroup of patients with a LVEF ≤40% [HR 0.61 (95% CI 0.45–0.83), P = .002]. No convincing differences were found for treatment with ACEi/ARBs.
Conclusion
Conventional heart failure medications are currently not widely prescribed in ATTR-CA, and those that received medication had more severe cardiac disease. Beta-blockers and ACEi/ARBs were often discontinued, but low-dose beta-blockers were associated with reduced risk of mortality in patients with a LVEF ≤40%. In contrast, MRAs were rarely discontinued and were associated with reduced risk of mortality in the overall population; but these findings require confirmation in prospective randomized controlled trials.
Keywords: Cardiac ATTR amyloidosis, Heart failure, Heart failure medications, Beta-blockers, Mineralocorticoid receptor antagonists
Structured Graphical Abstract
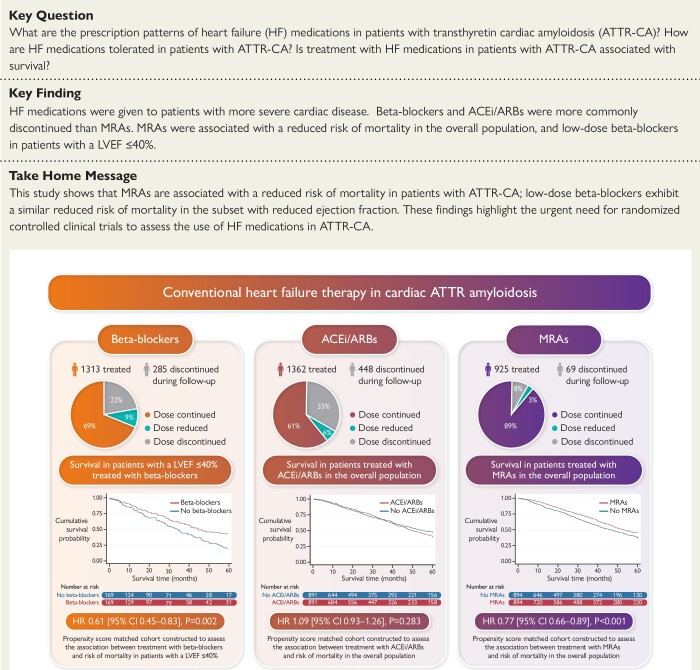
Structured Graphical Abstract.
Discontinuation rates of heart failure medications in patients with cardiac ATTR amyloidosis. Kaplan–Meier curves comparing survival in patients treated with heart failure medications to propensity score-matched patients not treated with heart failure medications, followed by a Cox proportional hazards regression analysis. ACEi, angiotensin-converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; MRAs, mineralocorticoid receptor antagonists; LVEF, left ventricular ejection fraction; HR, hazard ratio; CI, confidence interval.
See the editorial comment for this article ‘Neurohormonal blockade in transthyretin amyloidosis: perhaps one size does not fit all?’, by R.K. Cheng and S.A.M. Cuddy, https://doi.org/10.1093/eurheartj/ehad357.
Introduction
Transthyretin cardiac amyloidosis (ATTR-CA) causes progressive, fatal, heart failure (HF), due to misfolding of transthyretin (TTR), forming insoluble amyloid fibrils, which are deposited within the myocardial extracellular space.1,2 Until recently, ATTR-CA was considered a rare, untreatable disease. However, improvements in diagnostics coupled with emerging high-cost therapies are challenging these long-held beliefs. The ATTR-CA is far more common than previously suspected, and there is a potential for successful therapeutic intervention.3
The only drug proved to be associated with prognostic benefit in ATTR-CA is tafamidis, which is a highly specific drug that targets the circulating TTR protein and stabilizes the TTR tetramer to prevent dissociation into amyloidogenic monomers that deposit in the myocardium, causing an infiltrative and restrictive cardiomyopathy. Tafamidis was shown in a phase 3 placebo-controlled trial (ATTR-ACT) to reduce the combined primary endpoint of cardiovascular hospitalizations and mortality.4 However, unfortunately, the high cost associated with tafamidis has resulted in restricted use, and tafamidis has not been approved for the treatment of ATTR-CA in many countries.5
At present, it is unknown whether conventional HF medications that have substantial benefits in patients with HF of other aetiologies may also benefit those with ATTR-CA, as patients with known ATTR-CA have been excluded from previous HF trials.6–13 Hence, the value of conventional HF medications in patients with ATTR-CA is still debated. Small-scale studies have yielded contrasting results, with some suggesting that low doses of conventional HF medications are well tolerated,14,15 while others reported that not only are these medications poorly tolerated, but they may result in worse outcomes.16,17 The lack of large-scale clinical trials has resulted in a significant knowledge gap, although a position statement from the European Society of Cardiology (ESC) working group on myocardial and pericardial diseases regarding HF medications in ATTR-CA recommends stopping beta-blockers, and avoiding angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs), and are silent about mineralocorticoid receptor antagonists (MRAs).18
The aims of this study were to: (i) assess the prescription pattern of conventional HF medications in patients with ATTR-CA; (ii) assess the dosages and discontinuation rates of HF medications in patients with ATTR-CA; and (iii) assess the association between treatment with HF medications and survival in patients with ATTR-CA.
Methods
Consecutive patients in whom a diagnosis of ATTR-CA was confirmed at the National Amyloidosis Centre (NAC), between January 2000 and September 2022, were included. Patients with evidence of ATTR-polyneuropathy were excluded, as many have autonomic neuropathy and are not treated with HF medications due to concomitant postural hypotension.
Between 2000 and 2005 the diagnosis of ATTR-CA was established based on HF symptoms together with a characteristic CA echocardiogram and either direct endomyocardial biopsy proof of ATTR-amyloid or ATTR-amyloid in an extra-cardiac biopsy. From 2006 onwards cardiac magnetic resonance was added to the assessment if there was diagnostic doubt. From 2010 onwards, 99mTechnetium labelled 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scintigraphy was utilized, and diagnosis established based on ATTR-amyloid in an extra-cardiac biopsy with cardiac uptake on 99mTc-DPD scintigraphy; or grade 2–3 cardiac uptake on 99mTc-DPD scintigraphy in the absence of biochemical evidence of a plasma cell dyscrasia. All patients underwent genetic sequencing of the TTR gene and provided written consent for their data to be retrospectively analysed and published, in line with the Declaration of Helsinki and approval from the Royal Free Hospital ethics committee (REC 21/PR/0620).
All patients are enrolled into a protocolized follow-up program that consists of 6–12 monthly consultations. Data regarding whether HF medications were initiated, continued, or stopped, and medication dosages were all recorded. Medication classes were defined based on the ESC HF guidelines and comprised beta-blockers, ACEi/ARBs and MRAs. Target doses from the guidelines enabled comparisons by converting the daily dose to a percentage of the target dose. Medication classes were recorded regardless of whether the specific drug had been used in previous HF trials.19 Management decisions utilized a combined decision-making process involving local clinicians and the NAC team. Considering the knowledge gap, decisions concerning the initiation or discontinuation of HF medications were made following each clinical assessment on a case-by-case basis.
Statistical analysis
Statistical analysis was performed using Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). All continuous variables were tested for normality (Shapiro–Wilk test) and presented as mean ± standard deviation if the distribution was normal or median [interquartile range (IQR)] otherwise, other than N-terminal pro-B-type natriuretic peptide (NT-proBNP) which was log-transformed for bivariate testing. The independent sample t-test was used to compare means if the data were normally distributed in each treatment group, or its non-parametric equivalent was used to compare the distributions of the two treatment groups. One-way analysis of variance if the data were normally distributed in each treatment group was used to compare means in more than two groups; or its non-parametric equivalent was used to compare the distributions of multiple groups. A significant result was followed by post hoc Bonferroni corrected pairwise comparisons to establish where differences lay. Categorical data are presented as absolute numbers and frequencies (%) and compared using the χ2 test.
All mortality data were obtained via the UK Office of National Statistics, which is the formal government registry for all deaths throughout the UK. The mortality endpoint was defined as time to death from date of diagnosis for all deceased patients and time to censor date (25 October 2022) from date of diagnosis among the remainder. Follow-up was restricted to ≤60 months, after which patients were censored due to the majority of events occurring in the first 60 months, and a low number of patients at risk after 60 months. To account for amyloid-specific disease-modifying therapy or clinical trials, patients were censored at their start date.
Survival was evaluated using Cox proportional hazards regression analysis, providing estimated hazard ratios (HRs) with 95% confidence intervals (CIs). The proportional hazards assumption was checked and confirmed using weighted Schoenfeld residuals. With regard to the survival analysis, patients were classed as being treated with HF medications if they were treated continuously for at least 6 months following their initial assessment, or an event occurred within the first 6 months while patients were continuously treated. If the medication was stopped during the first 6 months, then patients were classed as not taking the medication. The initial survival analysis was performed on the whole study population using a multivariable Cox proportional hazards regression adjusting for covariates selected a priori based on clinical relevance, association with HF medication treatment and association with survival [age, sex, ischaemic heart disease (IHD), diabetes mellitus, hypertension, atrial fibrillation, NAC disease stage, wild-type or hereditary ATTR-CA, interventricular septal thickness in diastole (IVSd), longitudinal strain, beta-blocker, ACEi/ARBs, and MRAs].
Propensity score (PS) matching is widely used to reduce confounding biases in observational studies. The PS is a score between 0 and 1 that reflects the likelihood of the patient receiving one of the HF medications of interest conditional on a set of variables, so that those with similar PSs are independent of these variables. Prior to PS matching, missing data were replaced using single imputation, whereby missing values of numerical variables were replaced by the relevant median, and missing values of categorical variables were replaced by the relevant mode, to overcome potential bias introduced by excluding patients with missing data. In order to compare two particular HF medications, a PS for each individual was determined using all the aforementioned variables, apart from the HF medications being assessed. After finding the area of common support (in which the histograms of the PSs overlapped), the patients were then matched on the basis of their PSs in the two medication groups in a 1:1 ratio using the nearest neighbour approach without replacement and calliper width equal to 0.20 times the standard deviation of the logit of the PSs. The adequacy of matching was verified by ensuring that the standardized differences between groups were <0.10 for all variables used to create the PS. A Cox proportional hazards regression model was then applied using the matched groups to compare the effect on survival of the two medications of interest. Additional PS-matched analyses specified a priori were carried out in the subgroup of patients with a left ventricular ejection fraction (LVEF) ≤40% and the subgroup of patients with a LVEF >40% (based on the guideline definition for HF with reduced ejection fraction being a LVEF ≤40%).19 Kaplan–Meier curves were constructed with statistical significance being assessed with a log-rank test. Significant results were followed by sensitivity analyses to assess whether these results could be replicated; firstly using an ‘intention to treat’ approach whereby patients were classed as treated, or not treated based on their treatment status at diagnosis (rather than over the first 6 months), secondly without censoring patients for the start date of clinical trials or disease-modifying therapy, and lastly analysing the medication use as a time-varying exposure. Statistical significance was defined as P < .05.
Results
We identified 2371 patients diagnosed with ATTR-CA. The population compromised 1840 (77.6%) with wild-type ATTR-CA and 531 (22.4%) with hereditary ATTR-CA. The mean age of patients was 77.5 ± 7.3 years, and 90.0% were men. About two-thirds of patients were in New York Heart Association (NYHA) classes I and II, the median NT-proBNP was 2925 ng/L, and the mean LVEF was 48.2% [531 (22.4%) had a LVEF ≤40%). Most patients were in NAC stages 1 (45.8%) or 2 (36.0%). Approximately half of the patients had concomitant atrial fibrillation/flutter, and 54.2% had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Overall, 1955 patients (82.4%) were treated with a diuretic. In most cases (76.8% patients) a loop diuretic was prescribed either alone or in combination (Table 1). A total of 467 (19.7%) patients were enrolled into clinical trials, or treated with disease-modifying therapy (clinical trials, n = 377; tafamidis, n = 90). These patients were younger and had a milder cardiac phenotype at diagnosis than the rest of the study population (see Supplementary data online, Table S1).
Table 1.
Baseline characteristics and echocardiographic parameters for the overall population, and for patients separated into those with a LVEF >40% and a LVEF ≤40%
| Baseline characteristics | Overall study population (n = 2371) | Patients with a LVEF >40% (n = 1840) | Patients with a LVEF ≤40% (n = 531) | P-value |
|---|---|---|---|---|
| Age | 77.5 ± 7.3 | 77.6 ± 7.3 | 76.9 ± 7.3 | .035 |
| Sex (male) | 2110 (90.0%) | 1637 (89.0%) | 473 (89.1%) | .943 |
| Ethnicity | <.001 | |||
| Caucasian | 1893 (79.8%) | 1525 (82.9%)* | 368 (69.3%) | |
| Afro-Caribbean | 444 (18.7%) | 288 (15.7%)* | 156 (29.4%) | |
| Asian | 22 (0.9%) | 18 (1.0%) | 4 (0.8%) | |
| Other | 12 (0.5%) | 9 (0.5%) | 3 (0.6%) | |
| wtATTR | 1840 (77.6%) | 1487 (80.8%) | 353 (66.5%) | <.001 |
| hATTR | 531 (22.4%) | 353 (19.2%) | 178 (33.5%) | <.001 |
| AF/flutter | 1223 (51.6%) | 937 (50.9%) | 286 (53.9%) | .233 |
| IHD | 476 (20.1%) | 374 (20.3%) | 102 (19.2%) | .571 |
| Diabetes mellitus | 374 (15.8%) | 273 (14.8%) | 101 (19.0%) | .020 |
| Hypertension | 828 (34.9%) | 631 (34.3%) | 197 (37.1%) | .232 |
| Stroke/TIA | 109 (4.6%) | 182 (9.9%) | 76 (14.3%) | .004 |
| CKD stages 3–5 | 1288 (54.3%) | 953 (51.8%) | 335 (63.1%) | <.001 |
| Cardiac devices | ||||
| PPM | 214 (9.0%) | 173 (9.4%) | 41 (7.7%) | .234 |
| ICD | 46 (1.9%) | 29 (1.6%) | 17 (3.2%) | .017 |
| CRT-D | 23 (1.0%) | 14 (0.8%) | 9 (1.7%) | .053 |
| CRT-P | 37 (1.6%) | 24 (1.3%) | 13 (2.4%) | .061 |
| Heart failure severity | ||||
| NYHA class | <.001 | |||
| 1 | 317 (13.4%) | 280 (15.2%)* | 37 (7.0%) | |
| 2 | 1387 (58.5%) | 1093 (59.4%)* | 294 (55.4%) | |
| 3 | 435 (18.3%) | 279 (15.2%)* | 156 (29.4%) | |
| 4 | 30 (1.3%) | 19 (1.0%) | 11 (2.1%) | |
| Missing | 202 | 169 | 33 | |
| NAC stage | <.001 | |||
| 1 | 1086 (45.8%) | 926 (50.3%)* | 160 (30.1%) | |
| 2 | 853 (36.0%) | 613 (33.3%)* | 240 (45.2%) | |
| 3 | 395 (16.7%) | 266 (14.5%)* | 129 (24.3%) | |
| Missing | 37 | 35 | 2 | |
| NT-proBNP (ng/L) | 2925 (1530–5321) | 2597 (1394–4786) | 4123 (2484–7201) | <.001 |
| eGFR (mL/min/1.73 m²) | 58 (46–71) | 59 (47–72) | 54 (43–66) | <.001 |
| 6-Min walk test (meters) | 347 (247–430) | 354 (256–436) | 322 (216–407) | <.001 |
| 6-Min walk test (% predicted) | 71.2 ± 26.5 | 72.9 ± 25.7 | 64.7 ± 28.3 | <.001 |
| Systolic blood pressure (mmHg) | 125.1 ± 21.4 | 126.3 ± 22.1 | 121.3 ± 18.7 | <.001 |
| Diastolic blood pressure (mmHg) | 74.4 ± 12.7 | 73.9 ± 12.8 | 75.9 ± 12.4 | .020 |
| Heart rate (b.p.m.) | 72.2 ± 13.7 | 71.1 ± 13.2 | 75.8 ± 14.4 | <.001 |
| Echocardiographic parameters | ||||
| IVSd (mm) | 16.9 ± 2.4 | 16.9 ± 2.4 | 17.0 ± 2.4 | .321 |
| PWTd (mm) | 16.3 ± 2.5 | 16.3 ± 2.5 | 16.4 ± 2.6 | .220 |
| MWT (mm) | 17.1 ± 2.4 | 17.1 ± 2.4 | 17.2 ± 2.4 | .326 |
| Left atrial area (cm2) | 26.2 ± 5.5 | 26.1 ± 5.5 | 26.6 ± 6.4 | .069 |
| Right atrial area (cm2) | 24.5 ± 6.5 | 23.9 ± 6.4 | 26.3 ± 6.4 | <.001 |
| Stroke volume (mL) | 37.3 ± 13.9 | 39.9 ± 13.8 | 29.4 ± 10.9 | <.001 |
| Simpson’s biplane LVEF (%) | 48.2 ± 10.6 | 52.7 ± 7.2 | 33.6 ± 5.3 | <.001 |
| Longitudinal strain (%) | −10.8 ± 3.6 | −11.7 ± 3.5 | −8.1 ± 2.6 | <.001 |
| TAPSE (mm) | 15.1 ± 4.9 | 15.9 ± 4.9 | 12.6 ± 3.5 | <.001 |
| E/e′ | 16.8 ± 6.4 | 16.5 ± 6.2 | 17.8 ± 7.0 | <.001 |
| Medications | ||||
| Beta-blockers | 1313 (55.4%) | 971 (52.8%) | 342 (64.4%) | <.001 |
| ACEi/ARBs | 1362 (57.4%) | 1041 (56.6%) | 321 (60.5%) | .112 |
| MRAs | 925 (39.0%) | 673 (36.6%) | 252 (47.5%) | <.001 |
| Loop diuretics | 1808 (76.8%) | 1357 (74.3%) | 451 (85.3%) | <.001 |
Patients with hATTR-CA had the following variants: p.(Val142Ile) = 392, p.(Thr80Ala) = 93, p.(Ile127Val) = 12, p.(Ile88Leu) = 6, p.(Ser97Tyr) = 6, p.(Glu62Asp) = 4, p.(Glu109Lys) = 3, p.(Gly26Ser) = 3, p.(Val40Ile) = 2, p.(Val50Met) = 2, p.(Ala56Pro) = 1, p.(Asp58Tyr) = 1, p.(Asp58Val) = 1, p.(Asp59Val) = 1, p.(Glu74Gln) = 1, p.(Glu74Gly) = 1, p.(Glu74Leu) = 1, and p.(Phe64Leu) = 1.
AF, atrial fibrillation; IHD, ischaemic heart disease; TIA, transient ischaemic attack; CKD, chronic kidney disease; PPM, permanent pacemaker; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; NYHA, New York Heart Association; NAC, National Amyloidosis Centre; NT-proBNP, N-terminal pro B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; IVSd, interventricular septum in diastole; PWTd, posterior wall thickness in diastole; MWT, maximal wall thickness; LVEF, left ventricular ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
P < .05.
Prescription pattern of heart failure medications
Beta-blockers
A total of 1313 (55.4%) patients were treated with beta-blockers (64.4% in patients with a LVEF ≤40%) at diagnosis. Those treated with beta-blockers had a higher prevalence of IHD, diabetes mellitus, and atrial fibrillation compared to patients not receiving this type of treatment. Those treated with beta-blockers had a more severe cardiac phenotype, with a worse functional capacity as evidenced by NYHA class and 6-min walk test (6MWT), and a higher NAC disease stage [a greater proportion of patients had stage 3 (severe) disease]. The median NT-proBNP among patients treated with beta-blockers was significantly higher, while median eGFR was significantly lower than patients not receiving beta-blockers. Patients treated with beta-blockers had a larger bi-atrial size, lower LVEF, lower tricuspid annular plane systolic excursion (TAPSE), and worse longitudinal strain than those not receiving this type of treatment.
Renin-angiotensin system blockers
A total of 1362 (57.4%) patients were treated with an ACEi or ARB (60.5% in patients with a LVEF ≤40%) at diagnosis. As for beta-blockers, those treated with ACEi/ARBs had a higher prevalence of IHD, diabetes mellitus, and atrial fibrillation compared to patients not receiving this type of treatment. In addition, patients treated with an ACEi/ARB were more likely to have hypertension than patients not receiving this type of treatment. Those treated with ACEi/ARBs had a severe cardiac phenotype, with a higher NYHA class and NAC disease stage, and a higher proportion of patients having chronic kidney disease stages 3–5 than patients not receiving ACEi/ARBs. Patients treated with ACEi/ARBs had a larger bi-atrial size, lower LVEF, and worse longitudinal strain than those not receiving this type of treatment.
Mineralocorticoid receptor antagonists
A total of 925 (39.0%) patients were treated with an MRA (47.5% in patients with a LVEF ≤40%) at diagnosis. Those treated with MRAs had a higher prevalence of diabetes mellitus and atrial fibrillation but, unlike beta-blocker and ACEi/ARB treatment, patients treated with an MRA did not have more IHD. Those treated with MRAs had a more severe cardiac phenotype, with a worse functional capacity as evidenced by NYHA class and 6MWT, and a higher NAC disease. The median NT-proBNP among patients treated with MRAs was significantly higher, while median eGFR was significantly lower than patients not receiving MRAs. Patients treated with MRAs had a larger right atrial area, lower stroke volume, lower LVEF, lower TAPSE, higher E/e′, and worse longitudinal strain than those not receiving this type of treatment (Table 2).
Table 2.
Baseline characteristics and echocardiographic parameters for patients treated with heart failure medications compared to patients not treated with heart failure medications
| Variables | Patients with cardiac ATTR amyloidosis split by treatment with beta-blockers | Patients with cardiac ATTR amyloidosis split by treatment with ACEi/ARBs | Patients with cardiac ATTR amyloidosis split by treatment with MRAs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients treated with beta-blockers (n = 1313, 55.4%) | Patients not treated with beta-blockers (n = 1058, 44.6%) | P-value | Patients treated with ACEi/ARB (n = 1362, 57.4%) | Patients not treated with ACEi/ARB (n = 1009, 42.6%) | P-value | Patients treated with MRAs (n = 925, 39.0%) | Patients not treated with MRAs (n = 1446, 61.0%) | P-value | |
| Baseline characteristics | |||||||||
| Age | 77.4 ± 6.9 | 77.6 ± 7.7 | .456 | 77.4 ± 6.7 | 77.6 ± 8.0 | .546 | 76.9 ± 6.9 | 77.9 ± 7.5 | .001 |
| Sex (male) | 1172 (89.3%) | 938 (88.7%) | .641 | 1224 (89.9%) | 886 (87.8%) | .113 | 824 (89.1%) | 1286 (88.9%) | .912 |
| Ethnicity | .016 | .040 | <.001 | ||||||
| Caucasian | 1030 (78.4%) | 863 (81.6%) | 1069 (78.5%) | 824 (81.7%) | 695 (75.1%)* | 1198 (82.8%) | |||
| Afro-Caribbean | 269 (20.5%)* | 175 (16.5%) | 278 (20.4%)* | 166 (16.5%) | 215 (23.2%)* | 229 (15.8%) | |||
| Asian | 11 (0.8%) | 11 (1.0%) | 9 (0.7%) | 13 (1.3%) | 9 (1.0%) | 13 (0.9%) | |||
| Other | 3 (0.2%)* | 9 (0.9%) | 6 (0.4%) | 6 (0.6%) | 6 (0.6%) | 6 (0.4%) | |||
| wtATTR | 1021 (77.8%) | 819 (77.4%) | .839 | 1067 (78.3%) | 773 (76.6%) | .318 | 686 (74.2%) | 1154 (79.8%) | <.001 |
| hATTR | 292 (22.2%) | 239 (22.6%) | .839 | 295 (21.7%) | 236 (23.4%) | .318 | 239 (25.8%) | 292 (20.2%) | <.001 |
| AF/flutter | 755 (57.5%) | 468 (44.2%) | <.001 | 728 (53.5%) | 495 (49.1%) | .034 | 515 (55.7%) | 708 (49.0%) | .001 |
| IHD | 300 (22.8%) | 176 (16.6%) | <.001 | 301 (22.1%) | 175 (17.3%) | .004 | 198 (21.4%) | 278 (19.2%) | .196 |
| Diabetes mellitus | 241 (18.4%) | 133 (12.6%) | <.001 | 249 (18.2%) | 125 (12.4%) | <.001 | 168 (18.2%) | 206 (14.2%) | .011 |
| Hypertension | 479 (36.5%) | 349 (33.0%) | .079 | 549 (40.3%) | 279 (27.7%) | <.001 | 335 (36.2%) | 493 (34.1%) | .290 |
| Stroke/TIA | 137 (10.4%) | 121 (11.4%) | .436 | 143 (10.5%) | 115 (11.4%) | .487 | 97 (10.5%) | 161 (11.1%) | .670 |
| CKD stages 3–5 | 797 (60.7%) | 491 (46.4%) | <.001 | 774 (56.8%) | 514 (50.9%) | .004 | 573 (61.9%) | 715 (49.4%) | <.001 |
| Cardiac devices | |||||||||
| PPM | 118 (9.0%) | 96 (9.1%) | .942 | 138 (10.1%) | 76 (7.5%) | .029 | 95 (10.3%) | 119 (8.2%) | .091 |
| ICD | 32 (2.4%) | 14 (1.3%) | .051 | 28 (2.1%) | 18 (1.8%) | .635 | 22 (2.4%) | 24 (1.7%) | .216 |
| CRT-D | 15 (1.1%) | 8 (0.8%) | .340 | 17 (1.2%) | 6 (0.6%) | .108 | 13 (1.4%) | 10 (0.7%) | .084 |
| CRT-P | 21 (1.6%) | 16 (1.5%) | .865 | 23 (1.7%) | 14 (1.4%) | .559 | 21 (2.3%) | 16 (1.1%) | .026 |
| Heart failure severity | |||||||||
| NYHA class | <.001 | <.001 | <.001 | ||||||
| 1 | 135 (10.3%)* | 182 (17.2%) | 143 (10.5%)* | 174 (17.2%) | 87 (9.4%)* | 230 (15.9%) | |||
| 2 | 755 (57.5%) | 632 (59.7%) | 795 (56.2%) | 592 (58.7%) | 555 (60.0%) | 832 (57.5%) | |||
| 3 | 286 (21.8%)* | 149 (14.1%) | 275 (20.2%)* | 160 (15.9%) | 219 (23.7%)* | 216 (14.9%) | |||
| 4 | 18 (1.4%) | 12 (1.1%) | 20 (1.5%) | 10 (1.0%) | 15 (1.6%) | 15 (1.0%) | |||
| Missing | 119 | 83 | 129 | 73 | 49 | 153 | |||
| NAC stage | <.001 | .047 | <.001 | ||||||
| 1 | 524 (40.0%)* | 562 (52.5%) | 607 (44.6%) | 479 (47.4%) | 379 (41.0%)* | 707 (48.9%) | |||
| 2 | 522 (39.8%)* | 331 (31.1%) | 519 (38.1%)* | 334 (33.1%) | 369 (39.9%)* | 484 (33.5%) | |||
| 3 | 254 (19.3%)* | 141 (13.3%) | 217 (15.9%) | 178 (17.6%) | 167 (18.1%) | 228 (15.8%) | |||
| Missing | 13 | 24 | 19 | 18 | 10 | 27 | |||
| NT-proBNP (ng/L) | 3369 (1886–5912) | 2391 (1285–4540) | <.001 | 2999 (1591–5274) | 2850 (1479–5381) | .095 | 3136 (1806–5420) | 2732 (1433–5248) | <.001 |
| eGFR (mL/min/1.73 m²) | 56 (45–69) | 62 (48–75) | <.001 | 58 (46–70) | 60 (46–73) | .139 | 55 (45–68) | 60 (47–74) | <.001 |
| 6-Min walk test (meters) | 343 (230–422) | 358 (268–442) | .001 | 349 (242–428) | 335 (253–433) | .985 | 336 (230–424) | 358 (266–437) | .004 |
| 6-Min walk test (% predicted) | 68.0 ± 26.6 | 75.7 ± 25.7 | <.001 | 71.3 ± 26.4 | 71.0 ± 26.6 | .853 | 67.9 ± 26.4 | 74.0 ± 26.3 | <.001 |
| Systolic blood pressure (mmHg) | 123.7 ± 20.2 | 127.0 ± 22.9 | <.001 | 124.8 ± 20.8 | 125.6 ± 22.3 | .028 | 121.8 ± 19.2 | 127.3 ± 22.6 | <.001 |
| Diastolic blood pressure (mmHg) | 74.0 ± 12.5 | 74.9 ± 13.0 | <.001 | 74.0 ± 13.3 | 75.0 ± 13.5 | .004 | 72.8 ± 11.5 | 75.5 ± 14.1 | <.001 |
| Heart rate (b.p.m.) | 71.2 ± 14.0 | 73.5 ± 13.1 | <.001 | 71.9 ± 13.5 | 72.7 ± 13.9 | .230 | 72.0 ± 14.1 | 72.4 ± 13.4 | .471 |
| Echocardiographic parameters | |||||||||
| IVSd (mm) | 17.00 ± 2.4 | 16.9 ± 2.5 | .672 | 17.0 ± 2.5 | 16.8 ± 2.4 | .051 | 17.1 ± 2.4 | 16.8 ± 2.5 | .015 |
| PWTd (mm) | 16.4 ± 2.5 | 16.3 ± 2.6 | .761 | 16.4 ± 2.5 | 16.3 ± 2.5 | .412 | 16.5 ± 2.5 | 16.3 ± 2.5 | .014 |
| MWT (mm) | 17.1 ± 2.4 | 17.1 ± 2.5 | .677 | 17.2 ± 2.4 | 17.0 ± 2.4 | .061 | 17.3 ± 2.3 | 17.0 ± 2.5 | .020 |
| Left atrial area (cm2) | 26.7 ± 5.4 | 25.6 ± 6.4 | <.001 | 26.54 ± 5.6 | 25.78 ± 5.4 | .008 | 26.5 ± 5.4 | 26.0 ± 5.7 | .113 |
| Right atrial area (cm2) | 25.1 ± 6.4 | 23.5 ± 6.4 | <.001 | 24.9 ± 6.6 | 23.8 ± 6.6 | <.001 | 25.1 ± 5.4 | 24.0 ± 6.4 | <.001 |
| Stroke volume (mL) | 36.6 ± 13.9 | 38.3 ± 14.0 | .023 | 37.7 ± 14.5 | 36.6 ± 13.0 | .126 | 35.9 ± 13.0 | 38.5 ± 14.6 | <.001 |
| LVEF (%) | 47.1 ± 10.7 | 49.5 ± 10.4 | <.001 | 47.7 ± 10.6 | 48.8 ± 10.7 | .013 | 46.4 ± 10.6 | 49.3 ± 10.5 | <.001 |
| LVEF ≤40% | 342 (26.0%) | 189 (17.9%) | <.001 | 321 (23.6%) | 210 (20.8%) | .112 | 252 (27.2%) | 279 (19.3%) | <.001 |
| Longitudinal strain (%) | −10.6 ± 3.5 | −11.1 ± 3.8 | <.001 | −10.6 ± 3.5 | −11.0 ± 3.7 | .014 | −10.2 ± 3.3 | −11.2 ± 3.8 | <.001 |
| TAPSE (mm) | 14.7 ± 4.8 | 15.5 ± 5.0 | .002 | 15.0 ± 4.7 | 15.2 ± 5.2 | .342 | 14.6 ± 4.8 | 15.5 ± 5.0 | <.001 |
| E/e′ | 16.7 ± 6.4 | 16.7 ± 6.5 | .567 | 16.9 ± 6.2 | 16.7 ± 6.7 | .640 | 17.2 ± 6.5 | 16.3 ± 6.3 | .036 |
BSA, body surface area; AF, atrial fibrillation; IHD, ischaemic heart disease; TIA, transient ischaemic attack; CKD, chronic kidney disease; PPM, permanent pacemaker; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; NYHA, New York Heart Association; NAC, National Amyloidosis Centre; NT-proBNP, N-terminal pro B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; IVSd, interventricular septum in diastole; PWTd, posterior wall thickness in diastole; MWT, maximal wall thickness; LVEF, left ventricular ejection fraction.
P < .05.
Combination heart failure therapy
A total of 417 (17.6%) patients were treated with all three classes of HF medications (beta-blocker, ACEi/ARB, and MRA) at diagnosis, 804 (33.9%) were treated with a combination of two classes of HF medications, 741 (31.3%) were treated with one of the three classes of HF medications, and 409 (17.2%) were not treated with any prognostic HF medications. The most frequent combination of two HF medications was a beta-blocker and ACEi/ARB in 454 (56.5%) patients, followed by a beta-blocker and MRA in 180 (22.4%) patients, and an ACEi/ARB and MRA in 170 (21.1%) patients. Those treated with more HF medications had a higher prevalence of IHD, diabetes mellitus, and atrial fibrillation. They had more severe HF, with a worse functional status, as evidenced by NYHA class and 6MWT, and a higher NAC disease stage, and a higher proportion of patients having chronic kidney disease stages 3–5. Patients treated with more HF medications had a larger left ventricular wall thickness, larger bi-atrial size, and worse biventricular systolic function (reflected in a lower TAPSE, LVEF, and worse longitudinal strain), and there was a greater use of HF medications in patients with a LVEF ≤40%(Table 3).
Table 3.
Baseline characteristics and echocardiographic parameters for the overall population, separated by the number of heart failure medications patients were treated with
| Baseline characteristics | Not treated with HF medications (n = 409, 17.2%) | Treated with one HF medication (n = 741, 31.3%) | Treated with two HF medications (n = 804, 33.9%) | Treated with three HF medications (n = 417, 17.6%) | P-value |
|---|---|---|---|---|---|
| Age | 76.8 ± 9.0* | 78.5 ± 6.9***** | 77.6 ± 6.6****** | 76.1 ± 6.9 | <.001 |
| Sex (male) | 359 (87.8%) | 653 (88.1%) | 727 (90.4%) | 371 (89.0%) | .412 |
| Ethnicity | <.001 | ||||
| Caucasian | 336 (82.2%)*** | 615 (83.0%)***** | 647 (80.5%)****** | 295 (70.7%) | |
| Afro-Caribbean | 66 (16.1%)*** | 113 (15.2%)***** | 146 (18.2%)****** | 119 (28.5%) | |
| Asian | 3 (0.7%) | 10 (1.3%) | 8 (1.0%) | 1 (0.2%) | |
| Other | 4 (1.0%) | 3 (0.4%) | 3 (0.4%) | 2 (0.5%) | |
| wtATTR | 298 (72.9%)*,*** | 607 (81.9%)***** | 638 (79.4%)****** | 297 (71.2%) | <.001 |
| hATTR | 111 (27.1%)*,*** | 134 (18.1%)***** | 166 (20.6%)****** | 120 (28.8%) | <.001 |
| AF/flutter | 145 (35.5%)*,**,*** | 389 (52.5%) | 458 (57.0%) | 231 (55.4%) | <.001 |
| IHD | 54 (13.2%)**,*** | 142 (19.2%) | 183 (22.8%) | 97 (23.3%) | <.001 |
| Diabetes mellitus | 41 (10.0%)**,*** | 104 (14.0%)***** | 133 (16.5%) | 96 (15.8%) | <.001 |
| Hypertension | 92 (22.5%)*,**,*** | 276 (37.2%) | 293 (36.4%) | 167 (40.0%) | <.001 |
| Stroke/TIA | 50 (12.2%) | 78 (10.5%) | 91 (11.3%) | 39 (9.4%) | .568 |
| CKD stages 3–5 | 151 (36.9%)*,**,*** | 394 (53.2%)***** | 479 (59.6%) | 264 (63.3%) | <.001 |
| Cardiac devices | |||||
| PPM | 30 (7.4%) | 55 (7.4%) | 91 (11.3%) | 378 (9.1%) | .031 |
| ICD | 6 (1.5%) | 11 (1.5%) | 16 (2.0%) | 13 (3.1%) | .227 |
| CRT-D | 1 (0.2%) | 6 (0.8%) | 9 (1.1%) | 7 (1.7%) | .187 |
| CRT-P | 1 (0.2%) | 13 (1.8%) | 17 (2.1%) | 6 (1.4%) | .092 |
| Heart failure severity | |||||
| NYHA class | <.001 | ||||
| 1 | 105 (25.7%)*,**,*** | 92 (12.4%)***** | 87 (10.8%) | 33 (7.9%) | |
| 2 | 251 (61.4%) | 412 (55.6%) | 479 (59.6%)****** | 245 (58.8%) | |
| 3 | 50 (12.2%)*** | 120 (16.2%)***** | 135 (16.8%)****** | 130 (31.2%) | |
| 4 | 3 (0.7%) | 9 (1.2%) | 10 (1.2%) | 8 (1.9%) | |
| Missing | 0 | 108 | 93 | 1 | |
| NAC stage | <.001 | ||||
| 1 | 214 (52.3%)*,**,*** | 346 (46.7%) | 333 (41.4%) | 166 (39.8%) | |
| 2 | 133 (32.5%)**,*** | 247 (33.3%)***** | 316 (39.3%) | 177 (42.4%) | |
| 3 | 44 (10.8%)*,** | 135 (18.2%) | 145 (18.0%) | 71 (17.0%) | |
| Missing | 18 | 13 | 10 | 3 | |
| NT-proBNP (ng/L) | 2142 (1038–4224)*,**,*** | 2899 (1517–5259)**** | 3254 (1705–5785) | 3201 (1958–5454) | <.001 |
| eGFR (mL/min/1.73 m²) | 66 (52–79)*,**,*** | 59 (45–71) | 56 (45–70) | 55 (46–66) | <.001 |
| 6-Min walk test (meters) | 368 (276–447)*** | 350 (264–437) | 345 (241–431) | 332 (221–414) | .015 |
| 6-Min walk test (% predicted) | 75.8 ± 26.8*** | 74.0 ± 26.0***** | 70.7 ± 25.9 | 65.4 ± 27.0 | <.001 |
| Systolic blood pressure (mmHg) | 127.6 ± 25.6**,*** | 128.0 ± 20.5****,***** | 123.2 ± 20.9 | 121.9 ± 18.8 | <.001 |
| Diastolic blood pressure (mmHg) | 75.3 ± 14.6**,*** | 76.0 ± 12.5****,***** | 73.6 ± 12.6 | 72.5 ± 11.4 | <.001 |
| Heart rate (b.p.m.) | 73.8 ± 13.0** | 72.7 ± 13.3 | 71.5 ± 14.2 | 71.3 ± 13.6 | .008 |
| Echocardiographic parameters | |||||
| IVSd (mm) | 16.6 ± 2.5** | 16.9 ± 2.5 | 17.1 ± 2.5 | 16.9 ± 2.2 | .011 |
| PWTd (mm) | 16.0 ± 2.6** | 16.4 ± 2.4 | 16.5 ± 2.5 | 16.2 ± 2.6 | .007 |
| MWT (mm) | 16.8 ± 2.5** | 17.1 ± 2.4 | 17.3 ± 2.5 | 17.1 ± 2.2 | .008 |
| Left atrial area (cm2) | 25.1 ± 5.5**,*** | 25.9 ± 5.7 | 26.6 ± 5.4 | 26.8 ± 5.4 | <.001 |
| Right atrial area (cm2) | 22.6 ± 6.2**,*** | 23.7 ± 6.5****,***** | 25.3 ± 6.1 | 25.4 ± 6.7 | <.001 |
| Stroke volume (mL) | 38.4 ± 13.8 | 38.2 ± 14.2 | 36.8 ± 13.8 | 36.3 ± 13.8 | .158 |
| LVEF (%) | 50.4 ± 10.2**,*** | 49.0 ± 10.5***** | 48.0 ± 10.8****** | 45.1 ± 10.2 | <.001 |
| LVEF ≤40% | 62 (15.2%)**,*** | 155 (20.9%) | 182 (22.6%)****** | 132 (31.7%) | <.001 |
| Longitudinal strain (%) | −11.6 ± 4.0**,*** | −11.2 ± 3.7**** | −10.4 ± 3.4 | −10.1 ± 3.3 | <.001 |
| TAPSE (mm) | 15.9 ± 5.1**,*** | 15.4 ± 5.0 | 14.7 ± 5.0 | 14.6 ± 4.4 | .001 |
| E/e′ | 16.5 ± 6.9 | 16.8 ± 6.4 | 16.8 ± 6.0 | 17.1 ± 6.6 | .707 |
HF, heart failure; AF, atrial fibrillation; IHD, ischaemic heart disease; TIA, transient ischaemic attack; CKD, chronic kidney disease; PPM, permanent pacemaker; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; NYHA, New York Heart Association; NAC, National Amyloidosis Centre; NT-proBNP, N-terminal pro B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; IVSd, interventricular septum in diastole; PWTd, posterior wall thickness in diastole; MWT, maximal wall thickness; LVEF, left ventricular ejection fraction.
P < .05 for no HF medications vs. one HF medication.
P < .05 for no HF medications vs. two HF medications.
P < .05 for no HF medications vs. three HF medications.
P < .05 for one HF medication vs. two HF medications.
P < .05 for one HF medication vs. three HF medications.
P < .05 for two HF medications vs. three HF medications.
Doses of heart failure medications and discontinuation rates
Beta-blockers
Of the 1313 patients treated with beta-blockers, over half were treated with ≤25% of the target dose for HF (n = 829, 63.1%).19 The most commonly prescribed beta-blocker was bisoprolol (n = 1164, 88.7%), with the majority of patients treated with ≤2.5 mg per day (n = 721, 61.9%). Only 75 (5.7%) patients had the target beta-blocker dose prescribed, most of which had atrial fibrillation (n = 58, 77.3%). The overwhelming majority of the study population (n = 1266, 96.4%) and all patients with a LVEF ≤40% (n = 342, 100.0%) were treated with beta-blockers approved for HF with reduced ejection fraction. During follow-up 285 (21.7%) patients had their beta-blocker discontinued [median duration to discontinuation: 14.1 (6.8–28.9) months], and 117 (8.9%) had their beta-blocker dose reduced [median duration to reduction: 15.7 (7.4–34.5) months]. Patients who discontinued beta-blocker treatment had a lower blood pressure and heart rate than those who continued treatment. Only 63 (4.8%) patients had their beta-blocker dose increased, of which only 8 patients eventually had the target dose prescribed. During follow-up, 55 patients were initiated on beta-blockers, and the majority were treated with ≤25% of the target dose (n = 44, 80.0%), of which 4 (7.2%) had their beta-blocker subsequently discontinued.
Renin-angiotensin system blockers
Of the 1362 patients treated with ACEi/ARBs, over half were treated with ≤37.5% of the target dose (n = 728, 53.5%).19 The most commonly prescribed ACEi/ARB was ramipril (n = 701, 51.4%), with the majority of patients treated with ≤2.5 mg per day (n = 354, 50.5%). Only 158 (11.6%) patients were treated with the target ACEi/ARB dose. During follow-up 448 (32.9%) patients had their ACEi/ARB discontinued [median duration to discontinuation: 14.4 (6.9–26.8) months], and 77 (5.7%) had their ACEi/ARB dose reduced [median duration to reduction: 14.2 (7.4–26.6) months]. Patients who discontinued ACEi/ARB treatment had a lower blood pressure than those who continued treatment (see Supplementary data online, Table S2). Only 35 (2.6%) patients had their ACEi/ARB dose increased, of which only 3 patients were treated with the target dose. During follow-up, 41 patients were initiated on ACEi/ARBs, and the majority were treated with ≤37.5% of the target dose (n = 26, 63.4%) of which 8 (19.5%) had their ACEi/ARB subsequently discontinued.
Mineralocorticoid receptor antagonists
Of the 925 patients treated with MRAs, 742 (80.2%) were treated with spironolactone, and 183 (19.3%) were treated with eplerenone. The most commonly prescribed dose of both drugs was 25 mg (n = 657, 71.0%), followed by 50 mg (n = 79, 8.5%). During follow-up 69 (7.5%) patients had their MRAs discontinued [median duration to discontinuation: 12.5 (7.9–24.9) months], and 31 (3.4%) had their MRA dose reduced [median duration to reduction: 14.1 (7.9–24.9) months]. Only 77 (8.3%) patients had the dose of their MRA increased, of which 53 were prescribed with 50 mg. During follow-up, 158 patients were initiated on MRAs, and the majority were treated with ≥25 mg (n = 129, 81.6%), of which only 5 (3.2%) had their MRA subsequently discontinued.
Association between heart failure medication classes and survival
In the overall population, median follow-up was 27.8 months (IQR: 10.6–51.3), and the death rate was 14.9 deaths per 100 patient-years (95% CI 13.9–15.9). There were 1274 patients classed as being treated with beta-blockers for the survival analysis, and the death rate was 14.8 deaths per 100 patient-years (95% CI 13.5–16.2). There were 1306 patients classed as being treated with ACEi/ARBs for the survival analysis, and the death rate was 15.0 deaths per 100 patient-years (95% CI 13.8–16.4). There were 915 patients classed as being treated with MRAs for the survival analysis, and the death rate was 14.6 deaths per 100 patient-years (95% CI 13.1–16.1).
Multivariable Cox regression model
In a multivariable Cox regression analysis with covariates age, sex, IHD, diabetes mellitus, hypertension, atrial fibrillation, NAC disease stage, wild-type or hereditary ATTR-CA, IVSd, longitudinal strain, beta-blocker, ACEi/ARB, and MRA, only 4 covariates (age, hATTR-CA, higher NAC disease stage, and worse longitudinal strain) were associated with a higher risk of mortality; and only one treatment [MRA: HR 0.82 (95% CI 0.71–0.94), P = .004] was convincingly associated with a lower risk of mortality (see Supplementary data online, Table S3).
Propensity score-matched analyses
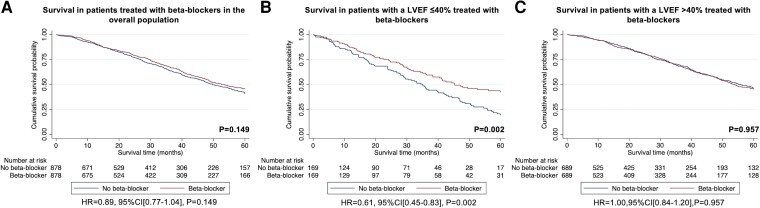
To minimize the potential selection bias inherent with the baseline treatment of HF medications we also performed PS-matched cohort analyses to assess the association between treatment with each HF medication and survival. Missing data were imputed for NAC stage in 37 patients, IVSd in 115 patients, and longitudinal strain in 296 patients. The remaining variables did not have any missing data. The PS-matched cohort constructed to assess the association between treatment with beta-blockers and risk of mortality comprised of 1756 patients (878 treated with beta-blockers vs. 878 not treated with beta-blockers) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 0.89 (95% CI 0.77–1.04), P = .149], although the 95% CI of the estimate was wide and did not exclude clinically important effects (see Supplementary data online, Table S4). A second PS-matched cohort was constructed to assess the association between treatment with beta-blockers and risk of mortality in patients with a LVEF ≤40%. This comprised of 338 patients (169 treated with beta-blockers vs. 169 not treated with beta-blockers), and demonstrated a 39% lower risk of mortality in patients treated with beta-blockers [HR 0.61 (95% CI 0.45–0.83), P = .002] (see Supplementary data online, Table S5). These findings were confirmed with sensitivity analysis, utilizing an ‘intention to treat’ approach [HR 0.58 (95% CI 0.42–0.81), P = .001], and whereby patients were no longer censored for the start date of clinical trials and disease modifying therapy [HR 0.63 (95% CI 0.47–0.85), P = .003], and where beta-blocker treatment was analysed as a time-varying exposure [HR 0.51 (95% CI 0.37–0.71), P < .001]. Following exclusion of patients with coexistent IHD and their corresponding pairs, repeat analysis confirmed a lower risk of mortality in patients with a LVEF ≤40% treated with beta-blockers [HR 0.56 (95% CI 0.38–0.83), P = .003]. A third PS-matched cohort was constructed to assess the association between treatment with beta-blockers and risk of mortality in patients with a LVEF >40%. This comprised of 1378 patients (689 treated with beta-blockers vs. 689 not treated with beta-blockers) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 1.00 (95% CI 0.84–1.20), P = .957], although the estimate was imprecise (Figure 1 and Supplementary data online, Table S6).
Figure 1.
Kaplan–Meier curves comparing survival in patients treated with beta-blockers to patients not treated with beta-blockers followed by a Cox proportional hazards regression analysis: (A) treatment with beta-blockers vs. no treatment with beta-blockers in the overall population, (B) treatment with beta-blockers vs. no treatment with beta-blockers in patients with a LVEF ≤40%, (C) treatment with beta-blockers vs. no treatment with beta-blockers in patients with a LVEF >40%
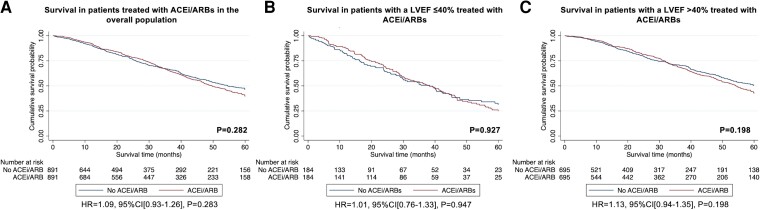
The PS-matched cohort constructed to assess the association between treatment with ACEi/ARBs and the risk of mortality comprised of 1782 patients (891 treated with ACEi/ARBs vs. 891 not treated with ACEi/ARBs) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 1.09 (95% CI 0.93–1.26), P = .283] (see Supplementary data online, Table S7). A second PS-matched analysis was constructed to assess the association between treatment with ACEi/ARBs and the risk of mortality in patients with a LVEF ≤40%. This comprised of 368 patients (184 treated with ACEi/ARBs vs. 184 not treated with ACEi/ARBs) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 1.01 (95% CI 0.76–1.33), P = .947], although the estimates were imprecise (Supplementary data online, Table S8). A third PS-matched analysis was constructed to assess the association between treatment with ACEi/ARBs and the risk of mortality in patients with a LVEF >40%. This comprised of 1390 patients (695 treated with ACEi/ARBs vs. 695 not treated with ACEi/ARBs) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 1.13 (95% CI 0.94–1.35), P = .198] (Figure 2 and Supplementary data online, Table S9).
Figure 2.
Kaplan–Meier curves comparing survival in patients treated with ACEi/ARBs to patients not treated with ACEi/ARBs followed by a Cox proportional hazards regression analysis: (A) treatment with ACEi/ARBs vs. no treatment with ACEi/ARBs in the overall population, (B) treatment with ACEi/ARBs vs. no treatment with ACEi/ARBs in patients with a LVEF ≤40%, (C) treatment with ACEi/ARBs vs. no treatment with ACEi/ARBs in patients with a LVEF >40%
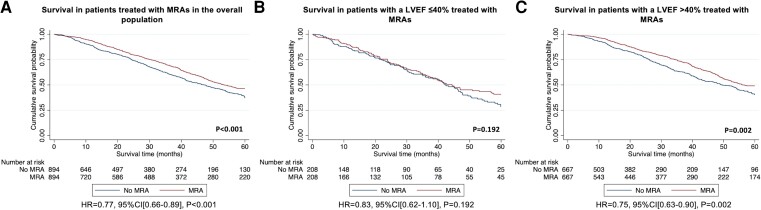
The PS-matched cohort constructed to assess the association between treatment with MRAs and the risk of mortality comprised of 1788 patients (894 patients treated with MRAs vs. 894 patients not treated with MRAs) and demonstrated that there was a 23% lower risk of mortality in patients treated with MRAs [HR 0.77 (95% CI 0.66–0.89), P < .001] (see Supplementary data online, Table S10). These findings were confirmed with sensitivity analysis, utilizing an ‘intention to treat’ approach [HR 0.81 (95% CI 0.69–0.94), P = .006]; and whereby patients were no longer censored for the start date of clinical trials and disease modifying therapy [HR 0.78 (95% CI 0.67–0.90), P < .001], and where MRA treatment was analysed as a time-varying exposure [HR 0.81 (95% CI 0.69–0.94), P = .004]. A second PS-matched analysis was constructed to assess the association between treatment with MRAs and the risk of mortality in patients with a LVEF ≤40%. This comprised of 416 patients (208 patients treated with MRAs vs. 208 patients not treated with MRAs) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 0.83 (95% CI 0.62–1.10), P = .192], although the 95% CI of the estimate was wide and did not exclude clinically important effects (see Supplementary data online, Table S11). A third PS-matched analysis was constructed to assess the association between treatment with MRAs and the risk of mortality in patients with a LVEF >40%. This comprised of 1334 patients (667 treated with MRAs vs. 667 not treated with MRAs) and demonstrated that there was a 25% lower risk of mortality in patients treated with MRAs [HR 0.75 (95% CI 0.63–0.90), P = .002] (Figure 3 and Supplementary data online, Table S12). These findings were confirmed with sensitivity analysis, utilizing an ‘intention to treat’ approach [HR 0.78 (95% CI 0.65–0.94), P = .008]; and whereby patients were no longer censored for the start date of clinical trials and disease modifying therapy [HR 0.79 (95% CI 0.66–0.94), P = .009], and where MRA treatment was analysed as a time-varying exposure [HR 0.77 (95% CI 0.65–0.93), P = .005].
Figure 3.
Kaplan–Meier curves comparing survival in patients treated with MRAs to patients not treated with MRAs followed by a Cox proportional hazards regression analysis: (A) treatment with MRAs vs. no treatment with MRAs in the overall population, (B) treatment with MRAs vs. no treatment with MRAs in patients with a LVEF ≤40%, (C) treatment with MRAs vs. no treatment with MRAs in patients with a LVEF >40%
Propensity score-matched analyses for combination therapy
In the overall population, a PS-matched cohort was constructed to compare the association with risk of mortality, between treatment with all three classes of HF medications (beta-blockers, ACEi/ARBs, and MRAs) and treatment with two classes of HF medications (beta-blockers and ACEi/ARBs). This comprised of 680 patients (340 treated with all three HF medications vs. 340 treated with beta-blockers and ACEi/ARBs) and demonstrated that there was a 37% lower risk of mortality in patients treated with all three HF medications [HR 0.63 (95% CI 0.49–0.80), P < .001] (see Supplementary data online, Table S13). These findings were confirmed with sensitivity analysis, utilizing an ‘intention to treat’ approach [HR 0.64 (95% CI 0.50–0.83), P < .001]; and whereby patients were no longer censored for the start date of clinical trials and disease modifying therapy [HR 0.65 (95% CI 0.51–0.82), P < .001], and where treatment was analysed as a time-varying exposure [HR 0.56 (95% CI 0.0.41–0.78), P = .001].
A PS-matched cohort was used to compare the association with risk of mortality, between treatment with two classes of HF medications (beta-blockers and ACEi/ARBs) and treatment with just ACEi/ARBs. This comprised of 558 patients (279 treated with beta-blockers and ACEi/ARBs vs. 279 treated with just ACEi/ARBs) and did not provide convincing evidence for a difference in the risk of mortality between the two groups [HR 1.06 (95% CI 0.81–1.39), P = .677], although the estimates were imprecise (see Supplementary data online, Table S14 and Supplementary data online, Figure S1). Data on reasons for medication discontinuation and the association between survival and both medication dosage and medication discontinuation are presented in Supplementary data online, Appendix S1.
Discussion
In this study we comprehensively evaluated the prescription pattern and discontinuation rates of HF medications in >2000 patients with ATTR-CA, and assessed the association between treatment with HF medications and the risk of mortality in these individuals. Our study demonstrated that: (i) patients with ATTR-CA and a severe cardiac phenotype were more commonly treated with HF medications; (ii) beta-blockers and ACEi/ARBs were generally prescribed in low doses and often discontinued, whereas in contrast, MRAs were rarely discontinued; and (iii) MRAs were independently associated with a lower risk of mortality in the overall population, and in patients with LVEF >40%; and low-dose beta-blockers were independently associated with a lower risk of mortality in patients with a LVEF ≤40% (Structured Graphical Abstract).
In the overall population of patients with ATTR-CA, a relatively low proportion were treated with beta-blockers (55.4%), ACEi/ARBs (57.4%), and MRAs (39.0%). Treatment with HF medications in patients with ATTR-CA appears to be driven by the presence of comorbidities and the severity of their cardiac disease. Heart failure medications were more commonly prescribed in patients with atrial fibrillation, diabetes mellitus, and chronic kidney disease. Beta-blockers and ACEi/ARBs are also more commonly prescribed in patients with IHD.15,20 Patients treated with conventional HF medications had more advanced cardiac disease as evidenced by worse functional capacity, a more severe NAC disease stage and lower indices of systolic function. Radial systolic impairment occurs in advanced ATTR-CA, and since the main evidence base for conventional HF medications is in patients with a LVEF ≤40%, the development of systolic impairment is likely to have contributed to greater use of HF medications in those with advanced cardiac disease.6–12
Beta-blockers and ACEi/ARBs were commonly discontinued, with over one-fifth of patients having their beta-blocker discontinued, and nearly one-third having their ACEi/ARB discontinued during follow-up. Beta-blocker intolerance may be exacerbated the underlying pathophysiology of ATTR-CA. In the context of a fixed stroke volume, caused by restrictive physiology, a higher heart rate is required to maintain cardiac output. The inability to augment stroke volume in response to the vasodilation may also contribute to the intolerance of ACEi/ARBs.15,21 In contrast, MRAs were rarely discontinued, with less than one-tenth having their MRA discontinued. This is probably related to the limited effect on blood pressure, compared with beta-blockers and ACEi/ARB, and their possible diuretic effect. The mainstay of symptom management in ATTR-CA has long been meticulous volume control, facilitated by high-dose loop diuretics. The MRAs may have a synergistic effect when utilized alongside loop diuretics and also increase potassium reabsorption, which is often needed when high doses of loop diuretics are utilized.22
In the current study, which represents the largest analysis of HF medications in patients with ATTR-CA to date, both regression-based and PS-matched analyses demonstrated that treatment with MRAs was independently associated with a lower risk of mortality in the overall ATTR-CA population; and PS-matched analysis demonstrated that low-dose beta-blockers were associated with a lower risk of mortality in patients with a LVEF ≤40%. MRAs were associated with a lower risk of mortality in patients with a LVEF >40%, but not in patients with a LVEF ≤40%. The point estimates for these analyses were similar, hence a greater sample size may have increased power sufficiently to demonstrate a benefit in patients with a LVEF ≤40%. Another possibility is that the benefit derived from MRAs is greater earlier in the disease process, and therefore increased survival benefit occurs in patients with a LVEF >40%. The reduced risk of mortality associated with low-dose beta-blockers in patients with a LVEF ≤40% was maintained when excluding patients with concomitant IHD, suggesting that the benefit is related to treating ATTR-CA rather than treating comorbidities, and this is consistent with previous HF trials that demonstrated that improved outcomes were confined to patients with a reduced ejection fraction.6,7,12
It has been well established that patients with ATTR-CA have a similar and possibly greater neurohormonal activation than is observed in patients with HF of different aetiologies. Furthermore, elevated neurohormone levels (specifically NT-proBNP and aldosterone) have been associated with a worse prognosis.23 It is therefore plausible that patients with ATTR-CA would derive prognostic benefit from neurohormonal modulation. However, a recent position statement by the ESC on the treatment of ATTR-CA recommended the withdrawal of beta-blockers, avoiding ACEi/ARBs, and did not discuss the use of MRAs in patients with ATTR-CA, reflecting the perceived poor tolerability of these agents and lack of trial evidence to support their use (and lack of differentiation between AL and ATTR-CA, the former having greater intolerance).18 Several small observational studies have contributed to these recommendations. However, differences in methodology and patient selection could explain our contrasting results. Previous studies have not matched patients, and therefore the worse outcomes in patients treated with HF medications were confounded by disease severity. Our study excluded patients with concomitant polyneuropathy, who often have autonomic disease and hypotension, resulting in a poor tolerance of HF medications.16,17 Importantly, our results are supported by a retrospective analysis of the TOPCAT trial, whereby an enriched cohort with echocardiographic characteristics of CA derived benefit from MRA therapy.22 This analysis is featured in a recent American College of Cardiology (ACC) consensus document that recommends MRA therapy alongside loop diuretics to augment diuresis.24 Our study is the first to sub-categorize ATTR-CA patients by LVEF. The majority of HF patients with a LVEF ≤40% experience chronic adrenergic overstimulation, and higher serum noradrenaline levels than their counterparts with preserved systolic function. A similar pathophysiological mechanism may exist in ATTR-CA, and therefore patients with a LVEF ≤40% could derive benefit from beta-blockade.23 Lastly, the majority were treated with bisoprolol (a cardio-selective beta-blocker), which potentially has a different haemodynamic profile to beta-blockers used in previous studies, while still providing neurohormonal modulation, and therefore, the observed benefit could potentially be confined to cardio-selective beta-blockers.
While the observational analyses reported here have limitations in their ability to provide causal estimates of treatments in individuals with ATTR-CA, they do raise the question as to whether there could be benefit from some neurohumoral therapies in such patients and support testing this hypothesis in prospective randomized controlled trials.22 While clinical trials are clearly needed, we believe that the data presented in this study call into question the consensus recommendations to discontinue beta-blockers and that neglect to mention MRAs.18
Limitations
There is an unavoidable prescription bias, with comorbid patients with more advanced cardiac disease being treated with more HF medications; but it is also possible that clinicians may have avoided using HF medications in some higher risk patients. Treatment decisions were made on a case-by-case basis, and therefore clinical decisions must factor in each individual’s tolerance of HF medications. It is possible that patients may have discontinued HF medications prior to their first NAC assessment, and this could not be factored into the analysis. Although we performed multivariable adjustment and PS matching to account for confounders known to impact mortality in ATTR-CA, we cannot exclude the possibility of residual confounding. The present study should be considered hypothesis-generating and highlights the urgent need for randomized controlled trials. Some of the estimated HRs generated following pre-specified subgroup analysis were imprecise, and is likely to reflect the unavoidably small sample size. Lastly, a small minority were treated with angiotensin receptor-neprilysin inhibitors or sodium-glucose cotransporter 2 inhibitors and had a short duration of follow-up. Therefore, they were not included in the present study, and further studies will be required to assess these medications in patients with ATTR-CA.
Conclusions
In summary, in this large cohort of patients with ATTR-CA, a relatively low proportion were treated with conventional HF medications, and those that had a more severe cardiac phenotype were more commonly treated with HF medications. Beta-blockers and ACEi/ARBs were often prescribed at a low dose, and frequently discontinued; in contrast to MRAs which were rarely discontinued. Both regression and PS-matched analyses demonstrated that treatment with a MRA was independently associated with a lower risk of mortality in the overall ATTR-CA population; and PS-matched analysis demonstrated that treatment with a low-dose beta-blocker was independently associated with a lower risk of mortality in patients with a LVEF ≤40%, but these findings require confirmation in prospective randomized controlled trials.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Supplementary Material
Contributor Information
Adam Ioannou, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Paolo Massa, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Rishi K Patel, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Yousuf Razvi, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Aldostefano Porcari, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK; Center for Diagnosis and Treatment of Cardiomyopathies, Cardiovascular Department, Azienda Sanitaria Universitaria Giuliano-Isontina (ASUGI), University of Trieste, Via Giacomo Puccini, Trieste 34100, Italy.
Muhammad U Rauf, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Anita Jiang, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Giacomo Cabras, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Stefano Filisetti, Cardiology University Department, IRCCS Policlinico San Donato, Piazza Edmondo Malan, Milan 20097, Italy.
Roos E Bolhuis, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Francesco Bandera, Cardiology University Department, IRCCS Policlinico San Donato, Piazza Edmondo Malan, Milan 20097, Italy.
Lucia Venneri, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Ana Martinez-Naharro, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Steven Law, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Tushar Kotecha, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Ruta Virsinskaite, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Daniel S Knight, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Michele Emdin, Health Science Interdisciplinary Center, Scuola Superiore Sant’Anna, Via Giuseppe Moruzzi, Pisa 56127, Italy; Cardiovascular Department, Fondazione Toscana Gabriele Monasterio, Via Giuseppe Moruzzi, Pisa 56124, Italy.
Aviva Petrie, Biostatistics Unit, University College London Eastman Dental Institute, 256 Grays Inn Road, London WC1X 8LD, UK.
Helen Lachmann, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Ashutosh Wechelakar, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Mark Petrie, BHF Cardiovascular Research Centre, University of Glasgow, 126 University Pl, Glasgow G12 8TA, UK.
Alun Hughes, Institute of Cardiovascular Science, University College London, 1–19 Torrington Place, London WC1E 7HB, UK.
Nick Freemantle, University College London, London, UK.
Philip N Hawkins, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Carol Whelan, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
John J V McMurray, BHF Cardiovascular Research Centre, University of Glasgow, 126 University Pl, Glasgow G12 8TA, UK.
Julian D Gillmore, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Marianna Fontana, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, London NW3 2PF, UK.
Data Availability
The data underlying this article cannot be shared due to restrictions from the institutional ethics committee to protect patient privacy.
Funding
All authors declare no funding for this contribution.
Ethical Approval
Ethical Approval was not required.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–2891. 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ioannou A, Patel RK, Razvi Y, Porcari A, Knight D, Martinez-Naharro A, et al. . Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. JACC Cardiovasc Imaging 2022;16:464–477. 10.1016/j.jcmg.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 3. Ioannou A, Patel RK, Razvi Y, Porcari A, Sinagra G, Venneri L, et al. . Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation 2022;146:1657–1670. 10.1161/CIRCULATIONAHA.122.060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 5. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, et al. . Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation 2020;141:1214–1224. 10.1161/CIRCULATIONAHA.119.045093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. . Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001–2007. 10.1016/S0140-6736(99)04440-210376614 [DOI] [Google Scholar]
- 7. Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. 10.1056/NEJM200105313442201 [DOI] [PubMed] [Google Scholar]
- 8. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJV, et al. . Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2004;110:2180–2183. 10.1161/01.CIR.0000144474.65922.AA [DOI] [PubMed] [Google Scholar]
- 10. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999;341:709–717. 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 11. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 12. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, et al. . Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018;39:26–35. 10.1093/eurheartj/ehx564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, et al. . Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. 10.1056/NEJMoa1609758 [DOI] [PubMed] [Google Scholar]
- 14. Aimo A, Vergaro G, Castiglione V, Rapezzi C, Emdin M. Safety and tolerability of neurohormonal antagonism in cardiac amyloidosis. Eur J Intern Med 2020;80:66–72. 10.1016/j.ejim.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 15. Tini G, Cappelli F, Biagini E, Musumeci B, Merlo M, Crotti L, et al. . Current patterns of beta-blocker prescription in cardiac amyloidosis: an Italian nationwide survey. ESC Heart Fail 2021;8:3369–3374. 10.1002/ehf2.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aus dem Siepen F, Hein S, Bauer R, Katus HA, Kristen AV. Standard heart failure medication in cardiac transthyretin amyloidosis: useful or harmful? Amyloid 2017;24:132–133. 10.1080/13506129.2016.1272453 [DOI] [PubMed] [Google Scholar]
- 17. Cheng RK, Vasbinder A, Levy WC, Goyal P, Griffin JM, Leedy DJ, et al. . Lack of association between neurohormonal blockade and survival in transthyretin cardiac amyloidosis. J Am Heart Assoc 2021;10:e022859. 10.1161/JAHA.121.022859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. . Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology working group on myocardial and pericardial diseases. Eur J Heart Fail 2021;23:512–526. 10.1002/ejhf.2140 [DOI] [PubMed] [Google Scholar]
- 19. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–3726.34447992 [Google Scholar]
- 20. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. . Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351–366. 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 21. Barge-Caballero G, Barge-Caballero E, López-Pérez M, Bilbao-Quesada R, González-Babarro E, Gómez-Otero Iet al. . Beta-blocker exposure and survival in patients with transthyretin amyloid cardiomyopathy. Mayo Clin Proc 2022;97:261–273. 10.1016/j.mayocp.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 22. Sperry BW, Hanna M, Shah SJ, Jaber WA, Spertus JA. Spironolactone in patients with an echocardiographic HFpEF phenotype suggestive of cardiac amyloidosis: results from TOPCAT. JACC Heart Fail 2021;9:795–802. 10.1016/j.jchf.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 23. Vergaro G, Aimo A, Campora A, Castiglione V, Prontera C, Masotti S, et al. . Patients with cardiac amyloidosis have a greater neurohormonal activation than those with non-amyloidotic heart failure. Amyloid 2021;28:252–258. 10.1080/13506129.2021.1966624 [DOI] [PubMed] [Google Scholar]
- 24. Writing Committee; Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, et al. . 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol 2023;81:1076–1126. 10.1016/j.jacc.2022.11.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to restrictions from the institutional ethics committee to protect patient privacy.