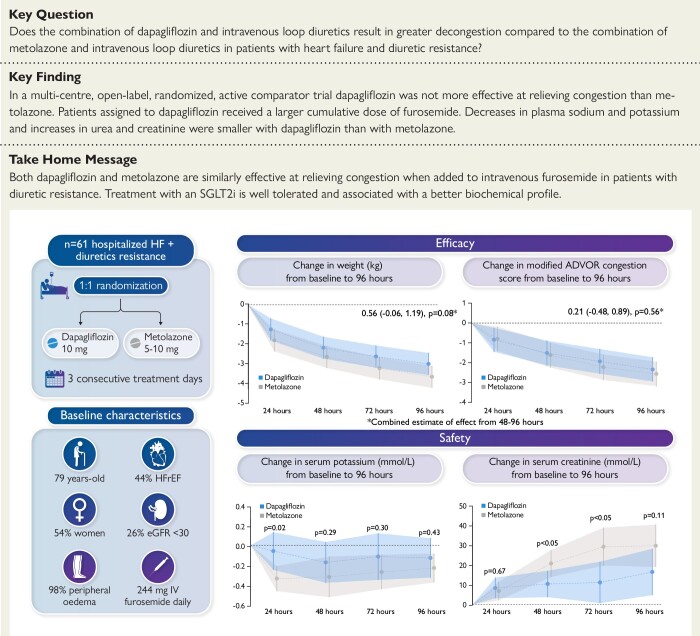
Abstract
Background and Aims
To examine the decongestive effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin compared to the thiazide-like diuretic metolazone in patients hospitalized for heart failure and resistant to treatment with intravenous furosemide.
Methods and results
A multi-centre, open-label, randomized, and active-comparator trial. Patients were randomized to dapagliflozin 10 mg once daily or metolazone 5–10 mg once daily for a 3-day treatment period, with follow-up for primary and secondary endpoints until day 5 (96 h). The primary endpoint was a diuretic effect, assessed by change in weight (kg). Secondary endpoints included a change in pulmonary congestion (lung ultrasound), loop diuretic efficiency (weight change per 40 mg of furosemide), and a volume assessment score. 61 patients were randomized. The mean (±standard deviation) cumulative dose of furosemide at 96 h was 977 (±492) mg in the dapagliflozin group and 704 (±428) mg in patients assigned to metolazone. The mean (±standard deviation) decrease in weight at 96 h was 3.0 (2.5) kg with dapagliflozin compared to 3.6 (2.0) kg with metolazone [mean difference 0.65, 95% confidence interval (CI) −0.12,1.41 kg; P = 0.11]. Loop diuretic efficiency was less with dapagliflozin than with metolazone [mean 0.15 (0.12) vs. 0.25 (0.19); difference −0.08, 95% CI −0.17,0.01 kg; P = 0.10]. Changes in pulmonary congestion and volume assessment score were similar between treatments. Decreases in plasma sodium and potassium and increases in urea and creatinine were smaller with dapagliflozin than with metolazone. Serious adverse events were similar between treatments.
Conclusion
In patients with heart failure and loop diuretic resistance, dapagliflozin was not more effective at relieving congestion than metolazone. Patients assigned to dapagliflozin received a larger cumulative dose of furosemide but experienced less biochemical upset than those assigned to metolazone.
Trial registration
ClinicalTrials.gov Identifier: NCT04860011
Keywords: Heart failure, Diuretic resistance, Metolazone, Thiazide, Dapagliflozin, Sodium-glucose cotransporter 2 inhibitor
Structured Graphical Abstract
Structured Graphical Abstract.
ADVOR, Acetazolamide in Decompensated Heart Failure with Volume Overload; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; eGFR, estimated glomerular filtration rate; IV, intravenous.
See the editorial comment for this article ‘Prevention and treatment of diuretic resistance in acute heart failure: when to use which combination of diuretics?’, by P. Martens et al ., https://doi.org/10.1093/eurheartj/ehad463.
Introduction
Patients with heart failure (HF) who do not achieve the therapeutically desired diuresis despite a high dose of a loop diuretic are said to have ‘diuretic resistance’ and this lack of response is associated with worse clinical outcomes including prolonged hospital stay, higher risk of readmission after hospital discharge, and greater symptom burden and mortality.1–6 The usual treatment for this problem is to add a different diuretic to simultaneously block sodium resorption in a separate segment of the nephron.7–18 The commonest approach is to add a thiazide (or thiazide-like) diuretic acting in the distal convoluted tubule, although this can cause worsening kidney function, hyponatraemia, and hypokalaemia.7,8,11–16,19,20 However, there has been recent interest in agents acting on the proximal tubule because most sodium is absorbed in this segment. One such treatment, acetazolamide, has been shown to enhance decongestion when added to an intravenous (IV) loop diuretic in a placebo-controlled trial, although this was associated with a small increase in creatinine.17 The sodium-glucose cotransporter type 2 (SGLT2) is also responsible for sodium absorption in the proximal tubule and SGLT2 inhibitors might also augment the natriuretic and aquaretic action of loop diuretics.21,22 These agents are of particular interest as they are not known to cause electrolyte disturbances, as they have been postulated to lead to a smaller reduction in blood volume, relative to interstitial fluid volume, compared to loop diuretics, and because they improve outcomes in patients with HF.23,24 If correct, the latter difference might lead to less kidney dysfunction with an SGLT2 inhibitor compared to a conventional diuretic.
To test whether an SGLT2 inhibitor might be an alternative to a thiazide-like diuretic in the treatment of patients with loop diuretic resistance, we compared the addition of dapagliflozin or metolazone to loop diuretic treatment in patients hospitalized with HF who remained congested despite treatment with a high dose of IV furosemide. Metolazone was chosen as the reference therapy because it is believed to be at least as potent as alternative thiazide diuretics, effective in patients with a low glomerular filtration rate, and is recommended in guidelines. We hypothesized that dapagliflozin would lead to greater decongestion than metolazone but cause less kidney dysfunction. The primary endpoint of this randomized trial was the diuretic effect, measured as the reduction in weight, over 5 days (96 h).
Methods
Study design
This was a multi-centre, open-label, randomized, active-comparator, controlled clinical trial designed, and conducted by the Heart Failure Research Group at the University of Glasgow, sponsored by NHS Greater Glasgow & Clyde and The University of Glasgow. The Clinical Trials Unit at the Robertson Centre for Biostatistics (RCB, University of Glasgow) was responsible for data management and statistical analysis. The study protocol and statistical analysis plan are included in the Supplementary data online, appendix. This study was performed according to the UK Policy Framework for Health and Social Care Research, The Medicines for Human Use (Clinical Trials) Regulations, and the Declaration of Helsinki, and was approved by the Research Ethics Committee (REC) and the Health Research Authority (HRA). All patients provided written informed consent. This trial is registered at ClinicalTrials.gov identifier: NCT04860011; EudraCT Number: 2020-004832-48.
Trial participants
Adult patients hospitalized for worsening HF (regardless of ejection fraction) with diuretic resistance defined as insufficient decongestion (decrease in weight <1 kg or negative fluid balance <1 L) over the prior 24 h despite treatment with high dose IV loop diuretic (equivalent to ≥160 mg IV furosemide in 24 h) were eligible.15 Additional inclusion criteria were plasma B-type natriuretic peptide (BNP) ≥ 100 pg/mL or plasma N-terminal proBNP (NT-proBNP) ≥ 400 pg/mL, persisting congestion (defined as any of pitting peripheral oedema, ascites, elevated jugular venous pressure, or radiographic or ultrasonic evidence of pulmonary congestion) and an expected hospital length of stay >3 days. Exclusion criteria included type 1 diabetes, an estimated glomerular filtration rate (eGFR) < 20 mL/min/1.73 m2, and receipt of an SGLT2 inhibitor, thiazide, or thiazide-like diuretic in the 48 h before randomization. A full list of inclusion and exclusion criteria is given in the protocol in the Supplementary data online, appendix.
Randomization and treatment allocation
Participants were randomized using an online web portal in a 1:1 ratio, to receive dapagliflozin or metolazone, employing a mixed minimization and randomization approach, designed to maintain a balance between treatment groups for left ventricular ejection fraction (LVEF) (≤40% and >40%), eGFR (≤30 mL/min/1.73 m2, > 30 mL/min/1.73 m2) and trial site. Participants had to be randomized within 24 h of screening, and the allocated study drug was administered within 1 h of randomization.
Patients were assigned to dapagliflozin 10 mg once daily or metolazone 5–10 mg once daily for up to three consecutive days. Treating physicians were permitted to select a dose of either 5 mg or 10 mg metolazone, according to their clinical judgement, as this reflects dosing with this agent in routine practice. Up-titration or down-titration of the dose of treatment was permitted at the discretion of the treating physician. The dose of dapagliflozin was fixed at 10 mg as this is the dose proven in HF trials and recommended in guidelines. Either of the randomized treatments could be stopped or continued (or the alternative treatment commenced), at the treating physician’s discretion after the 3-day trial period.
No dose of loop diuretic was specified.
Follow-up and endpoints
Study participants were followed-up daily for 5 days (96 h) for all clinical endpoints, reviewed at hospital discharge, and reassessed 90 days after discharge.
The primary endpoint was the diuretic effect, as assessed by mean change in weight, from randomization to 96 h. The secondary endpoints were the change in congestion, assessed using lung ultrasound (LUS), loop diuretic efficiency, and a volume assessment (‘congestion’) score, assessed over the same period.
Loop diuretic efficiency was defined as weight loss in kilograms divided by the equivalent of 40 mg of furosemide. LUS examinations were performed by trained investigators using a phased array transducer with a Philips Lumify handheld ultrasound machine and an eight-zone protocol (four zones on each hemithorax; 6 s video clips), in addition to an assessment of each hemidiaphragm, as described previously.25,26 LUS measures of congestion were: (1) the sum of B-lines in eight zones, and (2) pleural effusion size (the sum of pleural effusion scores from each hemidiaphragm), as described in the online appendix along with a description of the imputation procedures. LUS images were analysed in a core laboratory (www.ultrasoundcore.net) at the Brigham Women’s Hospital, Boston, USA, blinded to clinical characteristics, treatment assignment, and outcomes.
The volume assessment score was a modification of the score used in the Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR) trial and a detailed description of this is provided in the online appendix (Supplementary data online, Table S1).17
Change in NT-proBNP was an exploratory endpoint, measured in a core laboratory, using automated measurements (e411, Roche Diagnostics).
Safety assessments and adverse events
Safety endpoints included changes in kidney function, serum sodium, and potassium from randomization to 96 h. A clinically significant worsening in kidney function was defined as an increase in serum creatinine of >26.5 μmol/L (0.3 mg/dL) from baseline. Hypokalaemia and hyperkalaemia were defined as serum potassium ≤3.5 mmol/L and ≥5.5 mmol/L respectively, and hyponatraemia was defined as a serum sodium concentration ≤125 mmol/L.
The occurrence of adverse events was recorded daily from the date of randomization until the earliest of (a) 5 days post-completion of trial treatment, (b) the date of crossover to non-trial dapagliflozin or metolazone, or (c) the date of discharge. In addition, adverse events of interest were recorded at each study visit using a safety questionnaire.
Sample size calculation and statistical analysis
We estimated that 27 patients per treatment group (54 patients in total) would provide 90% power (α level = 0.05) to detect a clinically meaningful difference of 2 kg in mean weight change (≃2 L fluid) between the two groups at 96 h, assuming a standard deviation (SD) of 2.2 kg. A final sample size of approximately 60 participants was planned to account for potential dropouts.
The primary and secondary efficacy analyses were conducted according to the intention-to-treat principle (i.e. in all patients), and it was planned that safety analyses would be performed in patients taking at least one dose of randomized treatment (which, in the event, was also in all patients).
Baseline characteristics are summarized as mean SD or median (first and third quartile, Q1, Q3) for continuous variables and counts (percentages) for categorical variables. For the primary and secondary endpoint analyses, randomized groups were compared using a mixed effects linear regression model of endpoint measurements at all time points. The model included a random effect for participants. Fixed effects were included for time point, LVEF, eGFR, and trial site. To take account of possible differences in treatment time course, two models were fitted. In one, fixed effects were included for separate treatment effects at each post-baseline visit and, in the other, fixed effects were included for a treatment effect at 24 h, and a common treatment effect at 48, 72, and 96 h. Treatment effect estimates from both models are reported with 95% confidence intervals (CIs) and P-values. Model-predicted means from Model 2 at each time point are presented graphically with 95% CIs. For the safety outcome measures, Firsher’s exact test, t-test, and Wilcoxon–Mann–Whitney tests were used to test for differences between groups. All analyses were performed using R (version 4.0.0).
Results
Patients
Between 05 May 2021 to 03 January 2023, 1651 patients with HF who were receiving ≥160 mg IV furosemide daily were screened, the most common reason for exclusion was absence of diuretic resistance (Supplementary data online, appendix and Figure S1). 61 patients were randomized at seven sites across the UK. All participants were included in the intention-to-treat analysis. One patient was randomized but withdrew consent before receiving investigational treatment (Supplementary data online, appendix and Figure S1). The remaining 60 participants had data on the primary endpoint available at all assessment points. Data on vital status were available for all participants. No patients crossed over between treatment groups during the 3 days of study drug administration. Three patients in the metolazone arm were prescribed dapagliflozin between 72 and 96 h, and nine patients in the metolazone arm were prescribed dapagliflozin at discharge. Among the 30 patients initially assigned to dapagliflozin, two were prescribed metolazone between 72 and 96 h, and 4 prescribed metolazone at discharge.
Patients were randomized a median (Q1, Q3) of 6 (4, 11) days after admission. Their median age was 79 years, and 46% were men (Table 1). The median LVEF was 45% and the median NT-proBNP level was 4053 pg/mL. Overall, 44% of patients had an LVEF of ≤40%. Most patients had peripheral oedema (98%), pulmonary crepitations (93%), elevated jugular venous pressure (75%), and a third of patients had ascites (36%). The median (Q1, Q3) LUS B-line count was 12 (6, 18).
Table 1.
Baseline characteristics according to treatment allocation
| Characteristic | All (n = 61) | Dapagliflozin (n = 30) | Metolazone (n = 31) |
|---|---|---|---|
| Age (years) | 79(71–85) | 79 (73–86) | 79 (68–84) |
| Male sex—n (%) | 28 (46) | 13 (43) | 15 (48) |
| White race—n (%) | 59 (97) | 29 (97) | 30 (97) |
| BMI (kg/m2) | 33 (27–37) | 32 (27–36) | 33 (28–38) |
| SBP (mmHg) | 116 (106–128) | 115 (104–128) | 118 (109–127) |
| Heart rate (bpm) | 72 (66–83) | 71 (66–82) | 72 (67–85) |
| HF history | |||
| Ischaemic aetiology—n (%) | 20 (33) | 10 (33) | 10 (32) |
| Valvular HF—n (%) | 12 (20) | 5 (17) | 7 (23) |
| LVEF (%) | 45 (35–55) | 45 (35–55) | 45 (35–55) |
| LVEF ≤40%—n (%) | 27 (44) | 13 (43) | 14 (45) |
| Prior HF Hospitalization—n (%) | 35 (57) | 12 (40) | 23 (74) |
| Past medical history—n (%) | |||
| Type 2 diabetes | 28 (46) | 19 (63) | 9 (29) |
| Myocardial infarction | 21 (34) | 9 (30) | 12 (39) |
| Stroke | 5 (8) | 0 | 5 (16) |
| AF/flutter | 41 (67) | 18 (60) | 23 (74) |
| Peripheral arterial disease | 3 (5) | 2 (7) | 1 (3) |
| Chronic anaemiaa | 37 (61) | 19 (63) | 18 (58) |
| CKDb | 55 (90) | 28 (93) | 27 (87) |
| Physical examination | |||
| Elevated JVP (>4 cm)—n (%) | 46 (75) | 21 (70) | 25 (81) |
| Pulmonary crepitations—n (%) | 57 (93) | 27 (90) | 30 (97) |
| Peripheral oedema—n (%) | 60 (98) | 30 (100) | 30 (97) |
| Ascites—n (%) | 22 (36) | 7 (23) | 15 (48) |
| Modified ADVOR clinical congestion score | 6.0 (5.0–9.0) | 6.0 (5.5–8.0) | 7.0 (5.2–9.0) |
| Pleural effusionc—n (%) | 29 (48) | 13 (43) | 16 (52) |
| Pleural effusion size score | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (0.0–2.0) |
| B-lines (total number B-lines) | 12.0 (5.8–18.0) | 12.0 (6.2–18.2) | 12.5 (3.5–17.8) |
| Baseline blood tests | |||
| NT-proBNP (pg/mL) | 4053 (1768–6461) | 4855 (1792–9753) | 3806 (1228–6140) |
| eGFR (mL/min/1.73 m2) | 40.7 (32.4–54.4) | 40.7 (34.1–50.7) | 40.7 (29.2–59.1) |
| eGFR <30 mL/min/1.7 3 m2—n (%) | 14 (26) | 7 (25) | 7 (26) |
| Sodium (mmol/L) | 138 (135–140) | 138 (133–139) | 139 (137–141) |
| Potassium (mmol/L) | 4.0 (3.8–4.3) | 4.1 (3.8–4.4) | 4.0 (3.8–4.2) |
| Urea (mmol/L) | 12.4 (8.3–17.2) | 12.4 (9.6–15.9) | 12.2 (7.8–18.7) |
| Creatinine (μmol/L) | 130 (101–172) | 131 (101–168) | 130 (101–172) |
| HbA1c (mmol/mol) | 43.5 (37.0–51.2) | 44.5 (37.0–56.5) | 40.0 (37.8- 50.0) |
| Treatment before admission—n (%) | |||
| ACEi/ARB/ARNI | 19 (31) | 12 (40) | 7 (23) |
| Beta-blocker | 45 (74) | 25 (83) | 20 (65) |
| MRA | 22 (36) | 11 (37) | 11 (35) |
| Loop diuretic | 54 (89) | 26 (87) | 28 (90) |
| Thiazide or thiazide-like diuretic | 8 (13) | 3 (10) | 5 (16) |
| SGLT2i | 2 (3) | 2 (7) | 0 |
| ICD/CRT | 3 (5) | 1 (3) | 2 (6) |
| Treatment at randomization—n (%) | |||
| ACEi/ARB/ARNI | 14 (23) | 10 (33) | 4 (13) |
| Beta-blocker | 46 (75) | 24 (80) | 22 (71) |
| MRA | 22 (36) | 10 (33) | 12 (39) |
| Total daily loop diuretic dose at randomization (mg) | 244 (120) | 260 (139) | 229 (99) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ADVOR, Acetazolamide in Decompensated Heart Failure with Volume Overload; AF, atrial fibrillation; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; ICD, implantable cardioverter defibrillator; JVP, jugular venous pressure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; SBP, systolic blood pressure; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Values expressed as n (%) or median (quartile 1–quartile 3), or mean (SD).
Female Hb <120 g/L; Male Hb <130 g/L.
eGFR <60 mL/min/1.73 m2.
Assessed clinically.
Comorbidities were common, in particular atrial fibrillation/flutter (67%), anaemia (61%), and type 2 diabetes (46%). Most participants had chronic kidney disease (CKD) (90%). The median eGFR was 41 mL/min/1.73 m2 at baseline, and 26% of patients had Grade 4 CKD (eGFR <30 mL/min/1.73 m2).
Patient characteristics were largely balanced between treatment groups at baseline, except for a higher proportion with type 2 diabetes and a higher median NT-proBNP in the dapagliflozin arm and some more evidence of congestion in the metolazone arm.
The rate of prescription of a renin-angiotensin system inhibitor was low (23%) although more patients were prescribed a beta-blocker (75%) and a mineralocorticoid receptor antagonist (MRA) (36%).
Loop diuretic use after randomization
The mean (SD) cumulative dose of furosemide administered over the 96 h after randomization was 977 (492) mg in the dapagliflozin group and 704 (428) mg in patients assigned to metolazone and (P = 0.02). The mean (SD) daily dose of furosemide was 255 (120) mg in the dapagliflozin group and 185 (115) mg in the metolazone group. The mean dose of furosemide per day is shown in Supplementary data online, Table S3. The mean (SD) daily dose of dapagliflozin and metolazone used over the three days of study treatment was 10 (0) mg and 5.4 (1.3) mg, respectively.
Primary efficacy endpoint
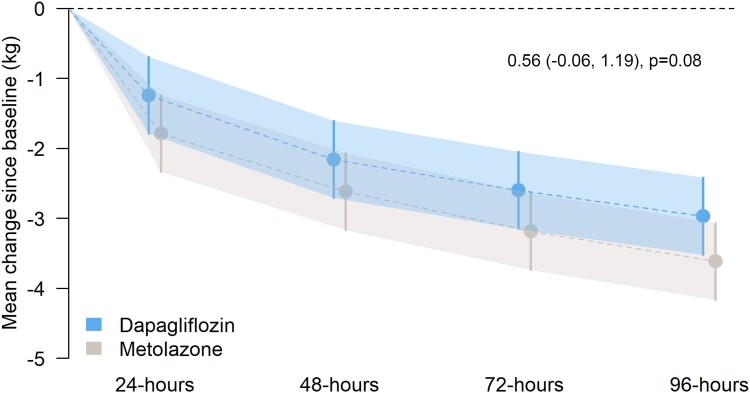
Weight loss was numerically but not statistically significantly smaller in patients treated with dapagliflozin compared with metolazone (Table 2 and Figure 1).
Table 2.
Primary and secondary efficacy endpoints
| Dapagliflozin (n = 30) | Metolazone (n = 31) | Between-group difference (95% CI)a | P-value | |||
|---|---|---|---|---|---|---|
| Primary endpoint | n = | n = | ||||
| Weight at baseline (kg) | 30 | 87.6 (20.2) | 31 | 91.7 (23.1) | ||
| Change from baseline (kg) | ||||||
| 24 h | 30 | −1.2 (1.2) | 30 | −1.8 (1.1) | 0.55 (−0.22, 1.31) | 0.17 |
| 48 h | 30 | −2.2 (1.3) | 30 | −2.6 (1.5) | 0.46 (−0.31, 1.22) | 0.25 |
| 72 h | 30 | −2.6 (1.8) | 30 | −3.2 (1.8) | 0.59 (−0.18, 1.35) | 0.14 |
| 96 h | 30 | −3.0 (2.5) | 30 | −3.6 (2.0) | 0.65 (−0.12, 1.41) | 0.11 |
| 48–96 h | — | — | — | — | 0.56 (−0.06, 1.19) | 0.08 |
| Secondary endpoints | ||||||
| Sum of B-lines on LUS (eight zones) at baseline | 26 | 12.0 (5.8, 18.0) | 30 | 12.5 (3.5, 17.8) | — | — |
| Change from baseline | ||||||
| 24 h | 23 | −2.0 (−3.5, 0.5) | 29 | −2.0 (−5.0, 2.0) | 0.54 (−1.56, 2.64) | 0.62 |
| 48 h | 25 | −3.0 (−5.0, 2.0) | 25 | −3.0 (−6.0, 0.0) | 0.28 (−1.85, 2.41) | 0.80 |
| 72 h | 25 | −3.0 (−5.0, 0.0) | 27 | −1.0 (−6.5, 1.5) | −0.24 (−2.35, 1.87) | 0.83 |
| 96 h | 24 | −3.0 (−5.2, -0.8) | 29 | −1.0 (−6.0, 1.0) | −1.16 (−3.27, 0.94) | 0.29 |
| 48–96 h | — | — | — | — | −0.38 (−2.09, 1.32) | 0.67 |
| Total pleural effusion score at baseline | 30 | 2.5 (1.6, 3.5) | 31 | 1.9 (1.0, 2.7) | — | — |
| Change from baseline | ||||||
| 24 h | 29 | −0.6 (−1.2, −0.2) | 30 | −0.1 (−0.7, 0.5) | −0.39 (−1.05, 0.26) | 0.24 |
| 48 h | 29 | −0.8 (−1.4, −0.2) | 30 | −0.5 (−1.0, −0.1) | −0.24 (−0.93, 0.46) | 0.50 |
| 72 h | 29 | −1.0 (−1.7, −0.3) | 30 | −0.7 (−1.3, −0.1) | −0.19 (−0.98, 0.60) | 0.63 |
| 96 h | 29 | −1.1 (−1.9, −0.3) | 30 | −0.7 (−1.4, −0.1) | −0.26 (−1.04, 0.52) | 0.50 |
| 48–96 h | — | — | — | — | −0.23 (−0.88, 0.42) | 0.48 |
| Loop diuretic efficiency | ||||||
| 24 h | 30 | 0.23 (0.22) | 29 | 0.34 (0.24) | −0.11 (−0.20, −0.01) | 0.03 |
| 48 h | 30 | 0.19 (0.13) | 30 | 0.30 (0.23) | −0.09 (−0.18, 0.00) | 0.07 |
| 72 h | 30 | 0.17 (0.13) | 30 | 0.27 (0.22) | −0.08 (−0.17, 0.01) | 0.10 |
| 96 h | 30 | 0.15 (0.12) | 30 | 0.25 (0.19) | −0.08 (−0.17, 0.01) | 0.10 |
| 48–96 h | — | — | — | — | −0.08 (−0.17, 0.00) | 0.07 |
| Modified ADVOR score at baseline | 30 | 5.8 (5.0, 6.6) | 31 | 6.3 (5.4, 7.2) | — | — |
| Change from baseline | ||||||
| 24 h | 29 | −0.8 (−1.4, −0.2) | 30 | −0.9 (−1.5, −0.2) | −0.04 (−0.85, 0.77) | 0.92 |
| 48 h | 29 | −1.4 (−2.1, −0.8) | 30 | −1.7 (−2.3, −1.0) | 0.10 (−0.75, 0.96) | 0.81 |
| 72 h | 29 | −1.9 (−2.6, −1.1) | 30 | −2.3 (−3.0, −1.5) | 0.29 (−0.57, 1.15) | 0.51 |
| 96 h | 29 | −2.2 (−3.0, −1.5) | 30 | −2.6 (−3.3, −1.9) | 0.22 (−0.63, 1.08) | 0.60 |
| 48–96 h | — | — | — | — | 0.21 (−0.48, 0.89) | 0.56 |
ADVOR, acetazolamide in decompensated heart failure with volume overload; LUS, lung ultrasound; CI, confidence interval.
Baseline data are presented as mean (SD) or median (Q1, Q3).
Change from baseline data are presented as mean (SD) or median (Q1, Q3).
The between group differences in change from baseline data are presented as means with 95% confidence intervals from a mixed effects linear regression model measured at all visit time points including a random effect for the subject and fixed effects for the visit time point, baseline LVEF, baseline eGFR, and study site.
Between-group difference presented as mean difference (95% CI).
Figure 1.
Mean change in weight (kg) from randomization to 48, 72, and 96 h in dapagliflozin vs. metolazone groups. Model-predicted mean change in weight from baseline with 95% confidence intervals at each time point. The treatment effect estimate displayed in the text represents the between-group difference (dapagliflozin vs. metolazone) in the common effect estimate between 48 and 96 h.
The mean (SD) decrease in weight with dapagliflozin at 24, 48, 72, and 96 h with dapagliflozin was −1.2 (1.2) kg, −2.2 (1.3) kg, −2.6 (1.8) kg, and −3.0 (2.5), respectively compared to −1.8 (1.1) kg, −2.6 (1.5) Kg, −3.2 (1.8) kg, and −3.6 (2.0) kg, respectively, with metolazone. The modelled mean (95% CI) differences in change in weight at 24, 48, 72, and 96 h were 0.55 (−0.22, 1.31) kg (P = 0.17), 0.46 (−0.31, 1.22) kg (P = 0.25), 0.59 (−0.18, 1.35) kg (P = 0.14), and 0.65 (−0.12, 1.41) kg (P = 0.11), respectively.
In the alternative model, the estimated mean (95% CI) difference in change in weight was 0.55 (−0.22, 1.31) kg at 24 h (P = 0.17) and 0.56 (−0.06, 1.19) kg over 48–96 h (P = 0.08).
In a post hoc sensitivity analysis, we also adjusted the treatment effect for type 2 diabetes (yes/no), baseline NT-proBNP level, and as cities (yes/no). This did not meaningfully change the results (see Supplementary data online, Table S2 and Figure S5).
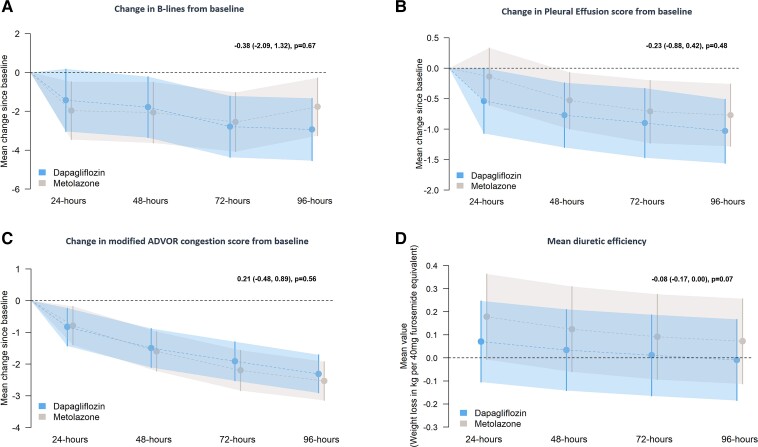
Secondary efficacy endpoints
The mean decrease in B-line count over 96 h was similar in patients assigned to dapagliflozin and metolazone (Table 2 and Figure 2A).
Figure 2.
Secondary endpoints—mean change in B-lines (panel A), pleural effusion score (panel B), and congestion score (panel C), from randomization to 48, 72, and 96 h. Mean diuretic efficiency (panel D) was calculated at 24, 48, 72, and 96 h. Model-predicted mean change from baseline with 95% confidence intervals at each time point. The treatment effect estimate displayed in the text represents the between-group difference (dapagliflozin vs. metolazone) in the common effect estimate between 48 and 96 h.
Overall, 17 patients assigned to dapagliflozin and 11 assigned to metolazone had a pleural effusion at baseline. Effusion score decreased similarly in the two treatment groups (Table 2 and Figure 2B).
The mean (95% CI) change in modified ADVOR volume assessment score at 24, 48, 72, and 96 h after randomization was also similar between treatment groups (Table 2 and Figure 2C).
Loop diuretic efficiency, defined as the change in weight (kg) per 40 mg of furosemide administered, was smaller with dapagliflozin than with metolazone at each time point after randomization although the difference was only significant at 24 h (Table 2 and Figure 2D).
Exploratory efficacy endpoints
The median (Q1, Q3) decreases in NT-proBNP in the dapagliflozin group at 24, 48, 72, and 96 h were 27 (−770, 429), −91 (−1676, 184), −361 (−1308, −52), and −436 (−1758, 76) pg/mL, respectively. The corresponding decreases in the metolazone group were 138 (−232, 1347) P = 0.19, 16 (−442, 1240) P = 0.23, −223 (−854, 826) P = 0.18, and −341 (−819, 481) P = 0.26 pg/mL. Urinary spot sodium was greater at all time points in the metolazone group (see Supplementary data online, Figure S2). Daily urine output and cumulative net fluid balance were similar between groups (see Supplementary data online, Figures S3 and S4).
Safety endpoints and adverse events
The prespecified laboratory safety assessments and adverse events of interest are shown in Table 3.
Table 3.
Safety assessments and adverse events of interest
| n= | Dapagliflozin (n = 30) | n= | Metolazone (n = 31) | P-value | |
|---|---|---|---|---|---|
| Change in serum urea from baseline, mmol/L | |||||
| 24 h | 30 | −0.0 (1.4) | 29 | 0.6 (1.5) | 0.26 |
| 48 h | 30 | −0.0 (1.9) | 29 | 1.9 (2.7) | <0.01 |
| 72 h | 28 | 0.1 (3.0) | 29 | 3.7 (3.9) | <0.01 |
| 96 h | 30 | −0.0 (3.7) | 29 | 4.4 (5.0) | <0.01 |
| Change in eGFR from baseline, mL/min/1.73 m2 | |||||
| 24 h | 30 | −3.0 (−5.8, −0.9) | 30 | −2.5 (−4.6, 0.3) | 0.83 |
| 48 h | 30 | −3.0 (−6.2, −0.1) | 30 | −5.2 (−9.9, −2.5) | 0.02 |
| 72 h | 28 | −3.7 (−7.5, 1.5) | 30 | −8.9 (−13.6, −3.4) | 0.01 |
| 96 h | 30 | −5.9 (−9.4, −0.8) | 30 | −7.3 (−12.3, −4.9) | 0.09 |
| Change in serum creatinine from baseline, µmol/L | |||||
| 24 h | 30 | 8.4 (14.6) | 30 | 6.9 (13.1) | 0.67 |
| 48 h | 30 | 10.4 (18.7) | 30 | 20.8 (18.9) | 0.04 |
| 72 h | 28 | 11.2 (28.2) | 30 | 29.3 (26.9) | 0.02 |
| 96 h | 30 | 16.5 (32.5) | 30 | 29.7 (29.7) | 0.11 |
| Impaired renal functiona | |||||
| Increase in serum creatinine concentration of >26.5 μmol/L | 30 | 14 (47) | 30 | 15 (50) | 1.00 |
| eGFR decrease > 50% | 30 | 2 (7) | 30 | 0 | 0.49 |
| Change in serum potassium from baseline, mmol/L | |||||
| 24 h | 29 | 0.0 (−0.4, 0.2) | 28 | 0.3 (−0.5, −0.1) | 0.02 |
| 48 h | 29 | −0.2 (−0.4, 0.0) | 28 | −0.3 (−0.6, 0.0) | 0.29 |
| 72 h | 28 | 0.0 (−0.6, 0.4) | 29 | −0.3 (−0.5, −0.1) | 0.30 |
| 96 h | 29 | −0.1 (−0.4, 0.2) | 28 | −0.3 (−0.4, 0.0) | 0.43 |
| Hypokalemia/hyperkalemiaa | |||||
| Serum potassium ≤3.0 mmol/L | 30 | 1 (3) | 30 | 3 (10) | 0.61 |
| Serum potassium ≤3.5 mmol/L | 30 | 15 (50) | 30 | 19 (63) | 0.44 |
| Serum potassium ≥5.5 mmol/L | 30 | 1 (3) | 30 | 0 | 1.00 |
| Change in serum sodium from baseline, mmol/L | |||||
| 24 h | 30 | 1.0 (−1.0, 2.8) | 30 | −1.0 (−2.0, 0.0) | <0.01 |
| 48 h | 30 | 1.0 (−1.0, 2.0) | 30 | −2.0 (−3.0, 0.0) | <0.01 |
| 72 h | 28 | 1.0 (−2.0, 2.2) | 30 | −2.0 (−5.0, −1.0) | <0.01 |
| 96 h | 30 | 0.5 (−1.0, 2.0) | 30 | −3.0 (−4.8, −1.2) | <0.01 |
| Hyponatraemiaa | |||||
| Serum sodium ≤125 mmol/L | 30 | 1 (3) | 30 | 0 | 1.00 |
| Serum sodium ≤130 mmol/L | 30 | 5 (17) | 30 | 4 (13) | 1.00 |
| AE of special interest— | 30 | — | 30 | — | — |
| Symptoms of hypotension/volume depletion | — | 0 | — | 4 (13) | 0.11 |
| Urinary tract infections | 0 | 1 (3) | 1.00 | ||
| Genital infections | 0 | 0 | n/a | ||
| Ketoacidosis | 0 | 0 | n/a | ||
| Hepatic injury | 0 | 0 | n/a | ||
| Clinically meaningful escalation of loop diuretic therapyb | 0 | 0 | n/a | ||
| New utilization/escalation of vasoactive therapy | 0 | 1 (3) | 1.00 | ||
| Renal replacement therapy | 0 | 0 | n/a | ||
| Worsening HF | 0 | 1 (3) | 1.00 | ||
AE, adverse event; eGFR, estimated glomerular filtration rate; n/a, not applicable.
At any time point between baseline and 96 h assessment.
Defined as >50% increase in daily dose.
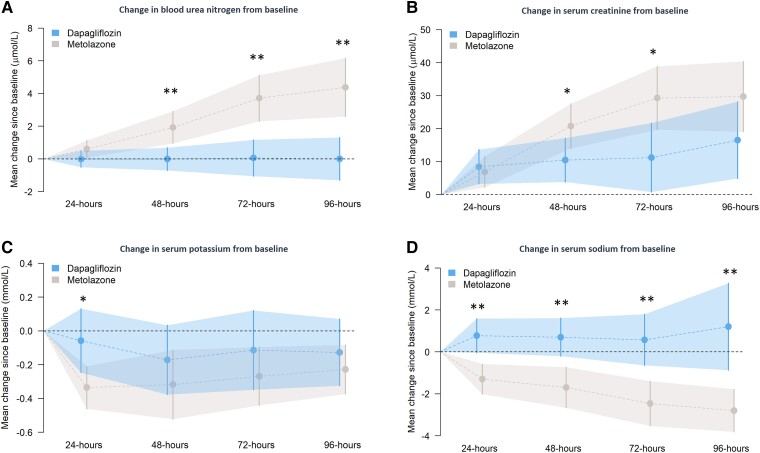
Serum sodium and potassium decreased more and urea and creatinine increased more, with metolazone compared to dapagliflozin, although only differences in urea and sodium were significant (Table 3 and Figure 3). However, there was no difference between treatments in the proportion of patients crossing the predefined thresholds for worsening kidney function, hyponatraemia, or hypokalaemia.
Figure 3.
Safety endpoints—mean change in blood urea nitrogen (panel A), creatinine (panel B), serum potassium (panel C), and serum sodium (panel D) from baseline with 95% confidence intervals at each time point. *P < 0.05; **P < 0.01.
There was no significant difference in adverse events of interest between metolazone and dapagliflozin although a higher proportion of patients (13%) treated with metolazone experienced symptoms of hypotension/volume depletion compared to those treated with dapagliflozin (0%) (P = 0.11).
Median (Q1, Q3) length of stay was similar between dapagliflozin and metolazone groups, at 20 (13, 32) and 19 (12, 26) days (P = 0.41), respectively. Mortality was similar between groups at all time points (see Supplementary data online, Figure S6), with two (7%) in-hospital deaths in the dapagliflozin group compared to 4 (13%) in the metolazone group. By 90 days, five patients (17%) in the dapagliflozin group and seven (23%) patients in the metolazone group had died. Time to first HF hospitalization and time to first HF hospitalization/ all cause mortality were similar between treatment groups (Supplementary data online, Figures S7 and S8).
Discussion
Some patients admitted to the hospital with worsening HF and congestion do not respond adequately to an IV loop diuretic. Guidelines recommend concomitant administration of another diuretic acting at a different site in the nephron to overcome this resistance and relieve persisting congestion. Usually, a thiazide diuretic or metolazone is recommended although there has also been recent interest in the use of acetazolamide. Like acetazolamide, SGLT2 inhibitors act in the proximal tubule and may augment the action of a loop diuretic.23,27,28 Because most sodium absorption takes place in the proximal tubule, we hypothesized that an agent acting in this segment of the nephron would lead to greater decongestion than one acting distally. However, the primary outcome of weight loss, a measure of decongestion, was not significantly different between patients randomly assigned to the SGLT2 inhibitor dapagliflozin compared to metolazone: mean (SD) decrease in weight at 96 h −3.0 (2.5) kg vs. −3.6 (2.0) kg, respectively, mean (95% CI) difference between groups 0.65 (−0.12, 1.41) (P = 0.11). The prespecified secondary outcomes which also reflected congestion, including the number of B-lines and size of pleural effusions on LUS, and the modified ADVOR volume assessment score, also decreased to a similar extent in each treatment group. Although these data collectively suggested equivalent decongestion in the two randomized treatment groups, this required a higher total dose of furosemide in the dapagliflozin group, with a mean total cumulative dose of 977 mg at 96 h, compared to 704 mg in the metolazone group. As a result, diuretic efficiency (kilogram of weight loss per 40 mg of furosemide), the final secondary endpoint, was lower in the dapagliflozin group compared to the metolazone group, suggesting a more modest natriuretic action of SGLT2 inhibitors than anticipated. However, despite the use of more furosemide, decongestion in the dapagliflozin group was achieved with smaller decreases in plasma sodium and potassium, and smaller increases in urea (blood urea nitrogen) and creatinine than in the metolazone group, in keeping with our hypothesis that SGLT2 inhibition would cause less kidney dysfunction and electrolyte disturbance than metolazone (Structured Graphical Abstract).
The present findings can be compared to those from other recent trials of combination diuretic therapy in patients hospitalized with worsening HF, albeit not specifically with diuretic resistance. In the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) trial, the median (interquartile range) weight loss over 72 h in patients randomly assigned to placebo in addition to IV furosemide was 1.5 (0.0–3.2) kg and 2.0 (2.1–4.6) kg in those assigned to hydrochlorothiazide, giving an adjusted placebo-corrected difference of 1.14 (0.42–1.84) kg.13 The total mean dose of furosemide administered from enrolment to 72 h was 375 mg in the placebo group and 340 mg in the hydrochlorothiazide group (compared with 756 mg in the dapagliflozin group and 566 mg in the metolazone group in the present trial). The greater diuretic effect of hydrochlorothiazide was achieved at the expense of worse renal function and more hypokalaemia.
Perhaps of more interest, is the ADVOR trial,17,29 given the proximity of site of action of both dapagliflozin and acetazolamide in the proximal tubule although neither directly inhibits sodium-hydrogen exchanger 3 which accounts for most sodium reabsorbtion in this segment of the nephron. In addition, acetazolamide appears to have relatively more effect on sodium compared to water excretion than SGLT2 inhibitors. The estimated mean decrease in weight in the placebo group by day 3 was 1.64 kg compared to 3.31 kg on acetazolamide, giving a placebo-corrected difference of approximately 1.68 kg. In the current trial, the mean (SD) decrease in weight at 72 h was 3.2 (1.8) kg in patients randomized to metolazone, consistent with the greater weight loss observed with combination diuretic therapy in ADVOR (and CLOROTIC). By comparison, the mean weight loss in patients assigned to dapagliflozin was 2.6 (1.8) kg which was not significantly different from the decrease in weight with metolazone. Although acetazolamide has not been compared directly to metolazone or a thiazide diuretic, its use in ADVOR led to a small but significant increase in creatinine, like that seen in previous studies with metolazone and thiazide diuretics. Potassium appeared to be lower with acetazolamide compared to placebo in ADVOR and acetazolamide has been reported to cause a reduction in potassium in other studies. Compared to values at admission (day 3 vs. day 0), serum potassium levels declined by 0.4 ± 0.3 mmol/L in the acetazolamide arm and 0.2 ± 0.2 mmol/L in the placebo arm (P = 0.022).30 However, on day 3, mild hypokalaemia (3–3.5 mmol/L) was not significantly more frequent with acetazolamide (P = 0.061).
Collectively, these trials show that each of a thiazide/thiazide-like diuretic, acetazolamide, and an SGLT2 inhibitor augments decongestion in patients already receiving IV loop diuretic. Because the patients studied in each trial were different, the treatments were not compared directly, and the dose of loop diuretic varied between treatment groups, it is not possible to draw firm conclusions about the relative efficacy of each therapy (or strategy). Moreover, in some countries, the selective vasopressin receptor 2 antagonist tolvaptan is another agent that can be used to augment diuresis.14
There is now irrefutable evidence of the benefit of SGLT2 inhibitors in HF, and guidelines recommend their initiation in the hospital, but, as with other therapies, once patients are ‘stabilized’. The present data suggest that SGLT2 inhibitors can be started earlier, if needed, to facilitate decongestion. More research into the treatment of diuretic resistance is needed and future investigation should focus on the safety and efficacy of adding a thiazide/thiazide-like diuretic or acetazolamide, and perhaps tolvaptan, in patients with persisting congestion despite treatment with a loop diuretic and SGLT2 inhibitor (and in patients with HF with reduced/mildly reduced ejection fraction, an MRA).
Limitations and strengths
The present trial was unblinded which may have led to bias. This was a pragmatic trial in which the clinicians responsible for the care of the participating patients were free to adjust the dose of furosemide as they thought appropriate. We did not attempt to mandate usual care and we do not believe that there is any universally agreed and routinely used furosemide-dosing protocol. Effectively, the comparison was of two decongestion strategies- one using furosemide plus metolazone and another using furosemide plus dapagliflozin. The latter resulted in the use of more furosemide than the former but, as we found, with less biochemical disturbance. The sample size was small but a post hoc power calculation showed sufficient power to detect a difference between treatments of 1 kg in weight. Nevertheless, in a larger trial, some of the differences between treatments, such as in diuretic efficiency, may have become statistically significant. There were some imbalances in patient characteristics between the treatment groups at baseline. Strengths of this trial include the use of LUS to assess congestion and the relatively large proportion of women included.
Conclusions
In hospitalized patients with HF and loop diuretic resistance, we did not prove that dapagliflozin was more effective at relieving congestion than metolazone. Patients assigned to dapagliflozin received a larger cumulative dose of furosemide but experienced less biochemical upset than those assigned to metolazone.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank the support given to the trial by the NHS Greater Glasgow and Clyde & University of Glasgow Sponsor teams.
Contributor Information
Su Ern Yeoh, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Joanna Osmanska, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Mark C Petrie, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Katriona J M Brooksbank, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Andrew L Clark, Department of Cardiology, Hull University Teaching Hospitals NHS Trust, Castle Hill Hospital, Cottingham HU3 2JZ, UK.
Kieran F Docherty, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Paul W X Foley, Department of Cardiology, The Great Western Hospital, Swindon SN3 6BB, UK.
Kaushik Guha, Department of Cardiology, Portsmouth Hospitals University NHS Trust, Portsmouth PO6 3LY, UK.
Crawford A Halliday, Department of Cardiology, Royal Alexandria Hospital, NHS Greater Glasgow and Clyde, Paisley, UK.
Pardeep S Jhund, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Paul R Kalra, Department of Cardiology, Portsmouth Hospitals University NHS Trust, Portsmouth PO6 3LY, UK; Faculty of Science and Health, University of Portsmouth, Portsmouth PO1 2DT, UK.
Gemma McKinley, Robertson Centre for Biostatistics, School of Health and Wellbeing, University of Glasgow, Glasgow G12 8TB, UK.
Ninian N Lang, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Matthew M Y Lee, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Alex McConnachie, Robertson Centre for Biostatistics, School of Health and Wellbeing, University of Glasgow, Glasgow G12 8TB, UK.
James J McDermott, Biopharmaceuticals, Medical Affairs, AstraZeneca, Wilmington, DE 19803, USA.
Elke Platz, Cardiovascular Division, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.
Peter Sartipy, Cardiovascular, Renal and Metabolism, AstraZeneca, BioPharmaceuticals R&D, Gothenburg 431 83, Sweden.
Alison Seed, Lancashire Cardiac Centre, Blackpool Teaching Hospitals NHS Trust, Blackpool FY3 8NP, UK.
Bethany Stanley, Robertson Centre for Biostatistics, School of Health and Wellbeing, University of Glasgow, Glasgow G12 8TB, UK.
Robin A P Weir, Cardiology Department, University Hospital Hairmyres, Lanarkshire G75 8RG, UK.
Paul Welsh, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
John J V McMurray, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Ross T Campbell, BHF Glasgow Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
Trial data will be shared on a reasonable request to the corresponding author.
Funding
The DAPA-RESIST trial was funded by an investigator-initiated grant from AstraZeneca. J.J.V.M. is supported by a Centre of Research Excellence Grant from the British Heart Foundation (RE/18/6/34217).
References
- 1. Wilcox CS, Testani JM, Pitt B. Pathophysiology of diuretic resistance and its implications for the management of chronic heart failure. Hypertension 2020;76:1045–1054. 10.1161/HYPERTENSIONAHA.120.15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoorn EJ, Ellison DH. Diuretic resistance. Am J Kidney Dis 2017;69:136–142. 10.1053/j.ajkd.2016.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox ZL, Testani JM. Loop diuretic resistance complicating acute heart failure. Heart Fail Rev 2020;25:133–145. 10.1007/s10741-019-09851-9 [DOI] [PubMed] [Google Scholar]
- 4. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:1178–1195. 10.1016/j.jacc.2019.12.059 [DOI] [PubMed] [Google Scholar]
- 5. Gupta R, Testani J, Collins S. Diuretic resistance in heart failure. Curr Heart Fail Rep 2019;16:57–66. 10.1007/s11897-019-0424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015;12:184–192. 10.1038/nrcardio.2014.215 [DOI] [PubMed] [Google Scholar]
- 7. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion - a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail 2019;21:137–155. 10.1002/ejhf.1369 [DOI] [PubMed] [Google Scholar]
- 8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 9. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–2304. 10.1056/NEJMoa1210357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–683. 10.1016/j.jacc.2006.07.073 [DOI] [PubMed] [Google Scholar]
- 11. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–2543. 10.1001/jama.2013.282190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trullas JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sanchez-Marteles M, Conde-Martel A, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J 2023;44:411–421. 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 14. Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail 2020;8:157–168. 10.1016/j.jchf.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–1331. 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 16. Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, et al. Short-Term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol 2017;69:1409–1419. 10.1016/j.jacc.2016.12.035 [DOI] [PubMed] [Google Scholar]
- 17. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide In acute decompensated heart failure with volume overload. N Engl J Med 2022;387:1185–1195. 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 18. Greene SJ, Felker GM, Giczewska A, Kalogeropoulos AP, Ambrosy AP, Chakraborty H, et al. Spironolactone in acute heart failure patients with renal dysfunction and risk factors for diuretic resistance: from the ATHENA-HF trial. Can J Cardiol 2019;35:1097–1105. 10.1016/j.cjca.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emmens JE, Ter Maaten JM, Matsue Y, Figarska SM, Sama IE, Cotter G, et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail 2022;24:365–374. 10.1002/ejhf.2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012;5:54–62. 10.1161/CIRCHEARTFAILURE.111.963413 [DOI] [PubMed] [Google Scholar]
- 21. Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 2015;467:1881–1898. 10.1007/s00424-014-1619-7 [DOI] [PubMed] [Google Scholar]
- 22. Wilcox CS. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors. Hypertension 2020;75:894–901. 10.1161/HYPERTENSIONAHA.119.11684 [DOI] [PubMed] [Google Scholar]
- 23. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018;20:479–487. 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 24. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022;400:757–767. 10.1016/S0140-6736(22)01429-5 [DOI] [PubMed] [Google Scholar]
- 25. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 2019;7:849–858. 10.1016/j.jchf.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindner M, Thomas R, Claggett BL, Lewis EF, Groarke J, Merz AA, et al. Quantification of pleural effusions on thoracic ultrasound in acute heart failure. Eur Heart J Acute Cardiovasc Care 2020;9:513–521. 10.1177/2048872620901835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail 2020;22:713–722. 10.1002/ejhf.1713 [DOI] [PubMed] [Google Scholar]
- 28. Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, et al. Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J 2023;44:41–50. 10.1093/eurheartj/ehac530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martens P, Dauw J, Verbrugge FH, Nijst P, Meekers E Jr, Augusto SN, et al. Decongestion with Acetazolamide in acute decompensated heart failure across the Spectrum of left ventricular ejection fraction: a prespecified analysis from the ADVOR trial. Circulation 2023;147:201–211. 10.1161/CIRCULATIONAHA.122.062486 [DOI] [PubMed] [Google Scholar]
- 30. Dhont S, Martens P, Meekers E, Dauw J, Verbrugge FH, Nijst P, et al. Sodium and potassium changes during decongestion with Acetazolamide—a prespecified analysis from the ADVOR trial. Eur J Heart Fail 2023. 10.1002/ejhf.2863. Online ahead of print [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial data will be shared on a reasonable request to the corresponding author.