Abstract
Aims
Stroke is an important problem in patients with heart failure (HF), but the intersection between the two conditions is poorly studied across the range of ejection fraction. The prevalence of history of stroke and related outcomes were investigated in patients with HF.
Methods and results
Individual patient meta-analysis of seven clinical trials enrolling patients with HF with reduced (HFrEF) and preserved ejection fraction (HFpEF). Of the 20 159 patients with HFrEF, 1683 (8.3%) had a history of stroke, and of the 13 252 patients with HFpEF, 1287 (9.7%) had a history of stroke. Regardless of ejection fraction, patients with a history of stroke had more vascular comorbidity and worse HF. Among those with HFrEF, the incidence of the composite of cardiovascular death, HF hospitalization, stroke, or myocardial infarction was 18.23 (16.81–19.77) per 100 person-years in those with prior stroke vs. 13.12 (12.77–13.48) in those without [hazard ratio 1.37 (1.26–1.49), P < 0.001]. The corresponding rates in patients with HFpEF were 14.16 (12.96–15.48) and 9.37 (9.06–9.70) [hazard ratio 1.49 (1.36–1.64), P < 0.001]. Each component of the composite was more frequent in patients with stroke history, and the risk of future stroke was doubled in patients with prior stroke. Among patients with prior stroke, 30% with concomitant atrial fibrillation were not anticoagulated, and 29% with arterial disease were not taking statins; 17% with HFrEF and 38% with HFpEF had uncontrolled systolic blood pressure (≥140 mmHg).
Conclusion
Heart failure patients with a history of stroke are at high risk of subsequent cardiovascular events, and targeting underutilization of guideline-recommended treatments might be a way to improve outcomes in this high-risk population.
Keywords: Heart failure, Stroke, Atrial fibrillation, Natriuretic peptides, Risk factors
Structured Graphical Abstract
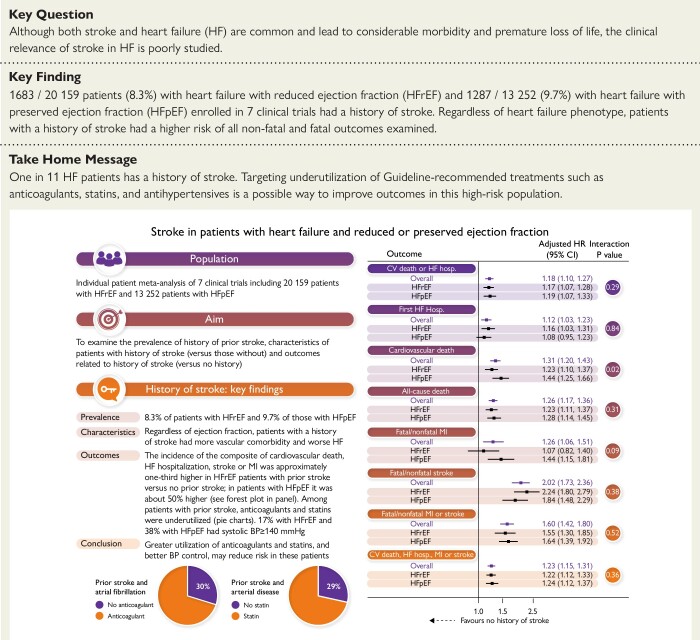
Structured Graphical Abstract.
Stroke is an important problem in patients with HF, and the intersection between the two conditions is poorly studied. In an individual patient meta-analysis of seven clinical trials including 20 159 patients with HFrEF and 13 252 patients with HFpEF, we found that ∼1 in 11 patients had a history of stroke. Regardless of ejection fraction, patients with a history of stroke had more vascular comorbidity, more severe HF, and worse outcomes. The incidence of the composite of cardiovascular death, HF hospitalization, stroke, or MI was approximately one-third higher in HFrEF patients with prior stroke vs. no prior stroke; in patients with HFpEF, it was ∼50% higher (see forest plot in panel). Among patients with prior stroke, there was underutilization of statins and anticoagulants and suboptimal blood pressure control, pointing to therapeutic avenues to reduce the high risk experienced by these patients. Panel (forest plot) shows HRs for the first occurrence of the clinical outcomes of interest according to history of stroke (vs. no history of stroke). HRs are provided for the overall pooled cohort and each HF phenotype separately. BP, blood pressure; CI, confidence interval; CV, cardiovascular; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hosp., hospitalization; HR, hazard ratio; MI, myocardial infarction.
See the editorial comment for this article ‘Redefining the role of heart failure in stroke’, by J. Witsch and S.E. Kasner, https://doi.org/10.1093/eurheartj/ehad360.
Introduction
Stroke is an important problem in patients with heart failure, but the intersection between the two conditions is poorly studied across the range of ejection fraction, even though each is common and results in considerable morbidity and premature loss of life.1–4 Patients with heart failure, including those in sinus rhythm, are at increased risk of stroke, probably because of underlying atherosclerosis and a heightened risk of thromboembolism. Concerning the latter, a role for Virchow’s triad has been postulated, especially in patients with reduced ejection fraction, where the left ventricular endocardium may be abnormal and blood stasis may occur, along with activation of blood coagulation; in this context, atrial myopathy, without atrial fibrillation, may also be relevant in patients with heart failure and preserved ejection fraction (HFpEF) as well as in those with reduced ejection fraction (HFrEF).2,5,6 Other factors such as hypotension and abnormal autoregulation of the cerebral circulation may also be important.7,8 Conversely, there is also evidence that stroke may induce cardiac dysfunction, as a consequence of ‘stroke–heart syndrome’, thought to result from stroke-induced autonomic dysfunction and inflammation.9–12 Information about the incidence of stroke is available from stroke prevention trials using anticoagulants in patients with HFrEF13,14 but not in patients with HFpEF. However, very little is known in either heart failure phenotype about the characteristics and outcomes of patients with prevalent stroke, despite a history of stroke being reported in up to 15% of ambulatory patients with heart failure, compared with 1%–3% of the general population.1–4 Because the overlap between heart failure and prevalent stroke has not been studied in detail, we examined the clinical characteristics of patients with HFrEF and HFpEF with prior stroke, compared with those without a history of stroke, and the clinical outcomes in patients with prior stroke compared with those without.15–17 Our aims included description of the potential impact of stroke on quality of life and non-atherothrombotic outcomes in patients with heart failure, across the spectrum of ejection fraction, as this has not been reported before. We also aimed to describe the use of therapies to improve atherothrombotic as well as heart failure outcomes as this may identify gaps in care and the opportunity to improve outcomes. We did this using patient-level data from three recent HFrEF trials (ATMOSPHERE, PARADIGM-HF, and DAPA-HF) and four HFpEF trials (CHARM-Preserved, I-Preserve, TOPCAT, and PARAGON-HF).18–24
Methods
Trials and patients included
In this post hoc analyses study, we pooled individual patient data from large HFrEF (ATMOSPHERE NCT00853658, PARADIGM-HF NCT01035255, and DAPA-HF NCT03036124) and HFpEF (CHARM-Preserved NCT00634712, I-Preserve NCT00095238, TOPCAT NCT00094302, and PARAGON-HF NCT01920711) trials (although originally described as HFpEF trials, these trials also included some patients with the recently defined HFrEF). The designs and results of these trials have been reported previously.18–24 The patients included in these trials had prevalent heart failure, and the inclusion and exclusion criteria for the two types of trials were broadly similar and are summarized in Supplementary data online, Table S1. The endpoint definitions used by the Clinical Endpoint Committee were also provided in Supplementary data online, Table S2. Each trial was approved by the ethics committee at participating centres, and all the patients provided written informed consent.
To ensure consistency in how HFpEF was defined, only patients in CHARM-Preserved with a left ventricular ejection fraction (LVEF) ≥ 45% were included (450 patients with LVEF <45% excluded), and because of concerns about the integrity of the TOPCAT trial, only patients enrolled in the Americas were included in the present analysis (1066 from Russia and 612 from Georgia excluded). A total of 12 patients had missing information about a history of stroke (2 from TOPCAT-Americas and 10 from PARAGON-HF). As a result, the numbers of patients, in the final analysis, were as follows: 7016 from ATMOSPHERE, 8399 from PARADIGM-HF, 4744 from DAPA-HF, 2573 from CHARM-Preserved, 4128 from I-Preserve, 1765 from TOPCAT, and 4786 from PARAGON-HF.
Clinical outcomes
The original primary outcomes for each trial included are shown in Supplementary data online, Table S1. For the present analyses, the time to the first occurrence of the composite of cardiovascular death or hospitalization for heart failure was examined, along with the components of this composite and death from any cause. We also analysed the occurrence of fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, and the composite of these two atherothrombotic outcomes. Finally, we examined a composite of all the major fatal and non-fatal adverse cardiovascular outcomes described above, i.e. time to first occurrence of cardiovascular death, heart failure hospitalization, non-fatal myocardial infarction, or non-fatal stroke. Each outcome had been adjudicated by endpoint committees as described in the original trial reports.
Statistical analysis
Baseline characteristics were expressed either as means with standard deviations or medians with interquartile ranges (IQRs) for continuous variables, while presented as counts and percentages for categorical variables. Baseline characteristics between two groups were compared by two-sample Student’s t-test or Mann–Whitney U test as appropriate for continuous variables and Pearson’s χ2 test for categorical variables.
The incidence rate for each outcome was reported per 100 patient-years. Cox proportional hazard models were used to compute the hazard ratio (HR) with 95% confidence interval (CI) for the time-to-first occurrence of each endpoint. We reported crude and adjusted HRs from models including region, age, gender, heart rate, systolic blood pressure, body mass index, New York Heart Association (NYHA) functional class, LVEF, estimated glomerular filtration rate (eGFR), N-terminal pro B-type natriuretic peptide (NT-proBNP), atrial fibrillation, ischaemic aetiology, history of myocardial infarction, history of diabetes mellitus, treatment arm, and within-trial clustering. Multivariable analyses were conducted with multiple imputation using chained equations, in which 20 imputed data sets were created and the estimates of analysis per data set were integrated using Rubin’s rule. Additionally, two sensitivity analyses for the outcomes were undertaken: (i) excluding patients from I-Preserve because a history of stroke or transient ischaemic attack (TIA) was collected at baseline (rather than a history of stroke alone) and (ii) excluding patients from CHARM-Preserved because of the unavailability of NT-proBNP.
The occurrence of each endpoint according to history of stroke was compared with Kaplan–Meier curves and the log-rank test. The incidence rate for each individual and composite time-to-first outcome was evaluated across the spectrum of LVEF using a Poisson regression model, in which LVEF was examined using restricted cubic splines employing 5 knots (placed at 5th, 27.5th, 50th, 72.5th, and 95th percentiles).25
The analysis was completed using Stata/SE version 17.0 (Stata Corp, College Station, TX, USA), and a conventional two-tailed P < 0.05 was considered to be statistically significant. To calculate standardized mean difference (95% CI), we used stddiff package of R software version 4.1.3.
Results
Of the 20 159 patients with HFrEF, 1683 (8.3%) had a history of stroke, and of the 13 252 patients with HFpEF, 1287 (9.7%) had a history of stroke. This proportion ranged from 7.0%–9.8% in the HFrEF trials and 8.6%–10.6% in the HFpEF trials.
Baseline characteristics of patients with and without a history of stroke
The characteristics of patients according to a history of stroke are shown in Table 1 (HFrEF and HFpEF separately) and Supplementary data online, Table S3 (all heart failure patients combined).
Table 1.
Baseline of HF patients with and without stroke
| HFrEF (N = 20 159) | SMDa 95% (CI) | HFpEF (N = 13252) | SMDa 95% CI | |||
|---|---|---|---|---|---|---|
| No prior stroke (n = 18 476) | Prior stroke (n = 1683) | No prior stroke (n = 11 965) | Prior stroke (n = 1287) | |||
| Baseline characteristics | ||||||
| Age, years | 64.0 ± 11.6 | 66.8 ± 10.0 | 0.262 (0.212–0.312) | 71.0 ± 9.1 | 72.2 ± 8.4 | 0.140 (0.082–0.198) |
| Age groups, years | 0.276 (0.226–0.326) | 0.141 (0.083–0.198) | ||||
| ≤40 | 642 (3.5) | 18 (1.1) | 33 (0.3) | 0 (0.0) | ||
| 41–55 | 3413 (18.5) | 190 (11.3) | 588 (4.9) | 42 (3.3) | ||
| 56–70 | 8653 (46.8) | 837 (49.7) | 4826 (40.3) | 477 (37.1) | ||
| >70 | 5768 (31.2) | 638 (37.9) | 6518 (54.5) | 768 (59.7) | ||
| Sex | 0.049 (−0.001 to 0.099) | 0.042 (−0.016 to 0.099) | ||||
| Female | 4124 (22.3) | 342 (20.3) | 6284 (52.5) | 649 (50.4) | ||
| Male | 14 352 (77.7) | 1341 (79.7) | 5681 (47.5) | 638 (49.6) | ||
| Region | 0.206 (0.156–0.256) | 0.153 (0.096–0.211) | ||||
| North America | 1304 (7.1) | 152 (9.0) | 2999 (25.1) | 368 (28.6) | ||
| Latin Americab | 3118 (16.9) | 251 (14.9) | 1281 (10.7) | 95 (7.4) | ||
| Western Europe | 4068 (22.0) | 384 (22.8) | 3640 (30.4) | 381 (29.6) | ||
| Eastern Europec | 5749 (31.1) | 625 (37.1) | 3149 (26.3) | 319 (24.8) | ||
| Asia/Pacific and other | 4237 (22.9) | 271 (16.1) | 896 (7.5) | 124 (9.6) | ||
| Race | 0.167 (0.117–0.217) | 0.116 (0.058–0.173) | ||||
| White | 12 244 (66.3) | 1225 (72.8) | 10 385 (86.8) | 1109 (86.2) | ||
| Black | 690 (3.7) | 73 (4.3) | 526 (4.4) | 67 (5.2) | ||
| Asian | 4116 (22.3) | 273 (16.2) | 636 (5.3) | 86 (6.7) | ||
| Others | 1426 (7.7) | 112 (6.7) | 418 (3.5) | 25 (1.9) | ||
| SBP, mmHg | 122.2 ± 16.6 | 123.1 ± 16.6 | 0.056 (0.006–0.106) | 133.1 ± 16.4 | 133.5 ± 16.8 | 0.027 (−0.031 to 0.084) |
| SBP category | 0.075 (0.025–0.125) | 0.045 (−0.012 to 0.103) | ||||
| <110 | 4059 (22.0) | 359 (21.3) | 676 (5.7) | 83 (6.4) | ||
| 110–119 | 3936 (21.3) | 314 (18.7) | 1526 (12.8) | 151 (11.7) | ||
| 120–129 | 4232 (22.9) | 410 (24.4) | 2361 (19.7) | 249 (19.3) | ||
| 130–129 | 3263 (17.7) | 308 (18.3) | 2834 (23.7) | 310 (24.1) | ||
| ≥140 | 2984 (16.2) | 292 (17.3) | 4565 (38.2) | 494 (38.4) | ||
| DBP, mmHg | 74.0 ± 10.6 | 74.1 ± 10.4 | 0.010 (−0.040 to 0.060) | 76.0 ± 10.6 | 75.5 ± 10.8 | 0.053 (−0.004 to 0.111) |
| PP, mmHg | 48.2 ± 13.0 | 49.0 ± 13.0 | 0.063 (0.013–0.113) | 57.1 ± 14.4 | 58.1 ± 15.0 | 0.069 (0.012–0.127) |
| MAP, mmHg | 90.1 ± 11.4 | 90.4 ± 11.3 | 0.033 (−0.017 to 0.083) | 95.0 ± 10.9 | 94.8 ± 11.1 | 0.021 (−0.036 to 0.079) |
| HR, bpm | 72.0 ± 12.1 | 71.2 ± 12.0 | 0.064 (0.014–0.114) | 70.7 ± 11.7 | 71.4 ± 12.1 | 0.064 (0.006–0.122) |
| BMI, kg/m2 | 27.1 (24.0–31.0) | 27.1 (24.2–31.0) | 0.029 (−0.021 to 0.079) | 29.4 (26.2–33.7) | 29.4 (26.2–33.6) | 0.007 (−0.051 to 0.064) |
| Weight category | 0.069 (0.019–0.119) | 0.045 (−0.013 to 0.102) | ||||
| < 18.5 | 372 (2.0) | 22 (1.3) | 60 (0.5) | 3 (0.2) | ||
| 18.5–25.0 | 5363 (29.1) | 463 (27.6) | 1964 (16.5) | 215 (16.7) | ||
| 25.0–30 | 6929 (37.6) | 663 (39.5) | 4346 (36.4) | 469 (36.5) | ||
| ≥ 30.0 | 5784 (31.4) | 532 (31.7) | 5559 (46.6) | 599 (46.6) | ||
| CHA2DS2-VAScd | 3.0 (2.0–4.0) | 6.0 (5.0–7.0) | 1.784 (1.731–1.837) | 4.0 (4.0–5.0) | 7.0 (6.0–7.0) | 1.947 (1.872–2.022) |
| Comorbidities and personal habits | ||||||
| Atrial fibrillation (history) | 6501 (35.2) | 798 (47.4) | 0.250 (0.200–0.300) | 4657 (38.9) | 620 (48.2) | 0.188 (0.130–0.246) |
| Hypertension | 12 448 (67.4) | 1347 (80.0) | 0.291 (0.241–0.341) | 10 345 (86.5) | 1160 (90.1) | 0.114 (0.057–0.172) |
| CHDe | 11 507 (62.3) | 1239 (73.6) | 0.245 (0.195–0.295) | 6340 (53.0) | 760 (59.1) | 0.122 (0.065–0.180) |
| Angina pectorisf | 4608 (24.9) | 527 (31.3) | 0.142 (0.092–0.192) | 3902 (32.6) | 449 (34.9) | 0.048 (−0.009 to 0.106) |
| MI | 7717 (41.8) | 856 (50.9) | 0.183 (0.133–0.233) | 3052 (25.5) | 411 (31.9) | 0.142 (0.085–0.200) |
| Prior PCI/CABG | 6092 (33.0) | 633 (37.6) | 0.097 (0.047–0.147) | 2900 (24.2) | 362 (28.1) | 0.089 (0.031–0.146) |
| PCI | 4378 (23.7) | 426 (25.3) | 0.038 (−0.012 to 0.087) | 1574 (19.1) | 187 (21.1) | 0.049 (−0.021 to 0.118) |
| CABG | 2752 (14.9) | 328 (19.5) | 0.122 (0.072–0.172) | 1272 (15.4) | 169 (19.0) | 0.095 (0.026–0.164) |
| Cerebral vascular disease | ||||||
| Subtypes of stroke | ||||||
| Ischaemicg | 1130 (94.9) | 402 (79.1) | ||||
| Haemorrhagicg | 56 (4.7) | 25 (4.9) | ||||
| Other/unknowng | - | 81 (15.9) | ||||
| Prior TIA | 494 (2.7) | 125 (7.4) | 0.218 (0.168–0.268) | 172 (4.0) | 54 (10.7) | 0.256 (0.164–0.349) |
| Carotid artery diseaseh | 641 (3.5) | 190 (11.3) | 0.303 (0.253–0.353) | 238 (5.6) | 55 (10.9) | 0.193 (0.101–0.285) |
| Carotid artery stenosis | 579 (3.1) | 184 (10.9) | 0.309 (0.259–0.359) | 191 (4.5) | 42 (8.3) | 0.156 (0.064–0.249) |
| Carotid artery revascularization | 183 (1.0) | 52 (3.1) | 0.149 (0.099–0.199) | 30 (0.7) | 8 (1.6) | 0.083 (−0.009 to 0.175) |
| Carotid artery endarterectomy | - | - | 41 (1.0) | 19 (3.7) | 0.184 (0.092–0.276) | |
| PADi | 1053 (5.7) | 171 (10.2) | 0.166 (0.116–0.216) | 393 (6.7) | 72 (10.8) | 0.147 (0.066–0.227) |
| Lower limb stenosis | 341 (2.4) | 53 (4.4) | 0.108 (0.050–0.167) | 90 (2.1) | 16 (3.2) | 0.065 (−0.027 to 0.157) |
| Lower limb revascularization | 350 (1.9) | 44 (2.6) | 0.049 (−0.001 to 0.098) | 102 (2.4) | 13 (2.6) | 0.011 (−0.081 to 0.103) |
| Intermittent claudication | 587 (4.1) | 87 (7.1) | 0.131 (0.072–0.189) | 131 (3.1) | 29 (5.7) | 0.129 (0.037–0.221) |
| PAOD | 273 (6.4) | 51 (10.9) | 0.163 (0.067–0.258) | - | - | |
| Renal artery stenosis | 55 (0.3) | 14 (0.8) | 0.071 (0.021–0.121) | 22 (0.5) | 10 (2.0) | 0.132 (0.040–0.224) |
| Abdominal aortic aneurism | 242 (1.3) | 43 (2.6) | 0.091 (0.041–0.140) | 54 (1.3) | 9 (1.8) | 0.042 (−0.050 to 0.134) |
| Non-cardiovascular systems | ||||||
| COPD/asthma | 2686 (14.5) | 269 (16.0) | 0.040 (−0.010 to 0.090) | 1513 (15.7) | 213 (20.0) | 0.112 (0.048–0.175) |
| Diabetes Mellitus | 6173 (33.4) | 661 (39.3) | 0.122 (0.072–0.172) | 4170 (34.9) | 541 (42.0) | 0.148 (0.091–0.206) |
| Anaemiaj | 4245 (23.4) | 367 (22.2) | 0.028 (−0.023 to 0.078) | 2296 (22.3) | 286 (25.2) | 0.068 (0.007–0.129) |
| Current smoker | 2559 (13.9) | 243 (14.4) | 0.017 (−0.033 to 0.067) | 1371 (11.5) | 155 (12.1) | 0.018 (−0.040 to 0.075) |
| HF characteristics and investigations | ||||||
| Ischaemic aetiologyk | 10 498 (56.8) | 1142 (67.9) | 0.229 (0.179–0.279) | 4417 (36.9) | 539 (41.9) | 0.102 (0.044–0.159) |
| Time since HF diagnosis | 0.277 (0.227–0.327) | 0.101 (0.040–0.163) | ||||
| ≤1 year | 5647 (30.6) | 335 (19.9) | 4610 .6) | 448 (39.8) | ||
| >1–5 years | 6978 (37.8) | 638 (37.9) | 3712 (35.9) | 431 (38.2) | ||
| >5 years | 5847 (31.7) | 710 (42.2) | 2011 (19.5) | 248 (22.0) | ||
| Previous hospitalization for HF | 10720 (58.0) | 993 (59.0) | 0.020 (−0.030 to 0.070) | 6223 (52.0) | 679 (52.8) | 0.015 (−0.043 to 0.072) |
| NYHA class III/IV | 5574 (30.2) | 623 (37.0) | 0.145 (0.095–0.195) | 5188 (43.4) | 619 (48.1) | 0.095 (0.038–0.153) |
| Quality of life scores | ||||||
| KCCQ clinical summary score | 74.7 ± 19.7 | 70.5 ± 20.9 | 0.205 (0.152–0.257) | 69.1 ± 20.9 | 65.9 ± 20.9 | 0.154 (0.073–0.235) |
| MLWHF | - | - | 42.0 (26.0–58.0) | 44.0 (28.0–61.0) | 0.101 (−0.005 to 0.206) | |
| Signs of congestion | ||||||
| Dyspnoea on effort | 12 110 (85.4) | 1057 (86.9) | 0.041 (−0.017 to 0.100) | 7812 (94.9) | 836 (94.1) | 0.035 (−0.035 to 0.104) |
| Dyspnoea at rest | 558 (3.9) | 54 (4.4) | 0.025 (−0.034 to 0.084) | 575 (7.0) | 63 (7.1) | 0.004 (−0.065 to 0.073) |
| Orthopnoea | 906 (6.4) | 65 (5.3) | 0.044 (−0.014 to 0.103) | 1732 (21.1) | 214 (24.1) | 0.072 (0.003–0.142) |
| PND | 699 (4.9) | 57 (4.7) | 0.011 (−0.047 to 0.070) | 431 (6.5) | 65 (8.9) | 0.090 (0.013–0.166) |
| Fatigue | 6998 (49.4) | 693 (57.0) | 0.153 (0.095–0.212) | 2122 (49.7) | 308 (60.6) | 0.221 (0.129–0.313) |
| Oedema | 2905 (20.5) | 287 (23.6) | 0.075 (0.017–0.134) | 5464 (45.7) | 649 (50.5) | 0.095 (0.038–0.153) |
| S3 gallop | 1301 (9.2) | 88 (7.2) | 0.071 (0.012–0.129) | 513 (5.0) | 49 (4.4) | 0.028 (−0.033 to 0.090) |
| JVD | 1351 (9.5) | 126 (10.4) | 0.028 (−0.031 to 0.086) | 1338 (11.3) | 153 (12.1) | 0.023 (−0.035 to 0.081) |
| Rales | 1254 (8.8) | 111 (9.1) | 0.010 (−0.049 to 0.068) | 1992 (16.7) | 216 (16.8) | 0.003 (−0.055 to 0.061) |
| ECG findings and NT-proBNP | ||||||
| Atrial fibrillation/flutter | 4377 (23.9) | 511 (30.5) | 0.150 (0.100–0.200) | 2743 (23.0) | 371 (28.8) | 0.133 (0.076–0.191) |
| LBBBl | 2649 (21.2) | 205 (19.7) | 0.035 (−0.028 to 0.099) | 564 (7.5) | 58 (6.9) | 0.024 (−0.047 to 0.096) |
| Paced rhythm | 2070 (11.3) | 242 (14.5) | 0.095 (0.045–0.145) | 767 (6.4) | 95 (7.4) | 0.037 (−0.020 to 0.095) |
| NT-proBNP, pg/mL | 1406 (782–2705) | 1572 (922–3118) | 0.153 (0.102–0.204) | 650 (314–1380) | 913 (383–1693) | 0.226 (0.156–0.297) |
| Atrial fibrillation/flutterm | 1845 (1128–3223) | 1995 (1162–3440) | 0.081 (−0.012 to 0.175 | 1496 (1053–2215) | 1620 (1046–2342) | 0.051 (−0.073 to 0.176) |
| No atrial fibrillation/flutterm | 1261 (711–2493) | 1429 (814–2879) | 0.143 (0.082–0.203) | 455 (227–885) | 565 (292–1227) | 0.212 (0.127–0.297) |
| LVEF and other laboratory investigations | ||||||
| LVEF, % | 29.4 ± 6.3 | 29.8 ± 6.3 | 0.065 (0.015–0.114) | 57.9 ± 8.5 | 57.9 ± 8.6 | 0.009 (−0.048 to 0.067) |
| Troponin T, ng/L | 18.0 (11.5–27.0) | 20.0 (13.1–30.2) | 0.202 (0.110–0.294) | 16.0 (11.0–24.0) | 18.0 (13.0–27.0) | 0.252 (0.074–0.430) |
| >99th centile | 2983 (64.0) | 361 (71.1) | 0.150 (0.059–0.242) | 634 (56.0) | 95 (69.3) | 0.263 (0.085–0.441) |
| Neutrophils, 103 cells/µL | 4.2 (3.4–5.3) | 4.3 (3.5–5.4) | 0.052 (−0.007 to 0.112) | 4.2 (3.3–5.2) | 4.3 (3.5–5.3) | 0.096 (0.03–0.162) |
| Platelets, 109 cells/µL | 191.0 (159.0–229.0) | 187.0 (159.0–221.0) | 0.085 (0.025–0.145) | 218.0 (181.0–262.0) | 215.0 (180.0–256.0) | 0.056 (−0.005 to 0.118) |
| Haemoglobin, g/L | 138.0 (127.0–149.0) | 140.0 (128.0–150.0) | 0.078 (0.028–0.129) | 136.0 (125.0–146.0) | 134.0 (123.3–145.0) | 0.072 (0.011–0.134) |
| Sodium, mmol/L | 141.0 (139.0–142.0) | 141.0 (139.0–143.0) | 0.087 (0.037–0.137) | 140.0 (138.0–142.0) | 141.0 (139.0–143.0) | 0.067 (0.006–0.128) |
| Potassium, mmol/L | 4.5 (4.2–4.8) | 4.5 (4.2–4.8) | 0.019 (−0.032 to 0.069) | 4.4 (4.1–4.7) | 4.4 (4.1–4.7) | 0.078 (0.017–0.139) |
| ALT, U/L | 17.0 (13.0–24.0) | 17.0 (13.0–23.0) | 0.076 (0.025–0.126) | 18.0 (14.0–25.0) | 18.0 (13.0–25.0) | 0.067 (0.006–0.128) |
| Bilirubin, μmol/L | 10.0 (7.0–14.0) | 10.0 (8.0–14.0) | 0.003 (−0.047 to 0.053) | 9.0 (6.8–12.0) | 9.0 (6.8–12.0) | 0.055 (−0.006 to 0.116) |
| Albumin, g/L | 43.0 (41.0–45.0) | 43.0 (41.0–45.0) | 0.047 (−0.012 to 0.106) | 42.0 (38.0–45.0) | 42.0 (39.0–45.0) | 0.024 (−0.039 to 0.088) |
| BUN, mmol/L | 7.0 (5.5–8.9) | 7.5 (6.1–9.7) | 0.233 (0.183–0.283) | 7.1 (5.7–9.3) | 7.5 (6.0–10.0) | 0.110 (0.048–0.171) |
| Creatinine, μmol/L | 93.0 (79.0–111.0) | 99.9 (83.3–121.0) | 0.261 (0.211–0.311) | 88.4 (71.0–108.7) | 95.0 (79.0–118.0) | 0.194 (0.133–0.255) |
| eGFR, mL/min/1.73 m2 | 68.0 (55.0–82.0) | 63.0 (50.0–76.0) | 0.279 (0.23–0.329) | 65.2 (51.7–80.0) | 61.1 (47.7–76.5) | 0.191 (0.130–0.252) |
| eGFR <60, mL/min/1.73 m2 | 6112 (33.1) | 724 (43.0) | 0.206 (0.156–0.256) | 4192 (40.4) | 561 (49.0) | 0.172 (0.111–0.234) |
| HbA1c, % | 6.1 (5.7–6.8) | 6.1 (5.7–7.1) | 0.069 (−0.026 to 0.165) | 6.2 (5.8–7.0) | 6.3 (5.8–7.2) | 0.091 (0.007–0.176) |
| Medication and other interventions | ||||||
| Diuretics | 15 369 (83.2) | 1400 (83.2) | 0.000 (−0.050 to 0.050) | 10342 (86.4) | 1148 (89.2) | 0.084 (0.027–0.142) |
| Loop | 14 013 (75.8) | 1278 (75.9) | 0.002 (−0.048 to 0.052) | 6692 (64.6) | 800 (70.9) | 0.134 (0.072–0.195) |
| Thiazides | 1271 (6.9) | 120 (7.1) | 0.010 (−0.040 to 0.060) | 2386 (23.0) | 222 (19.7) | 0.082 (0.021–0.144) |
| Digitalis | 5216 (28.2) | 452 (26.9) | 0.031 (−0.019 to 0.081) | 1692 (14.1) | 210 (16.3) | 0.061 (0.003–0.118) |
| Beta-blocker | 17 233 (93.3) | 1568 (93.2) | 0.004 (−0.046 to 0.054) | 8156 (68.2) | 889 (69.1) | 0.019 (−0.038 to 0.077) |
| MRA n,o | 9747 (52.8) | 896 (53.2) | 0.010 (−0.040 to 0.060) | 1942 (18.8) | 230 (20.4) | 0.041 (−0.021 to 0.102) |
| ACEI/ARB/ARNIp | 18 213 (98.6) | 1644 (97.7) | 0.066 (0.016–0.116) | 5548 (94.3) | 632 (94.9) | 0.027 (−0.053 to 0.107) |
| CCB | 1698 (9.2) | 228 (13.5) | 0.138 (0.088–0.188) | 4294 (35.9) | 499 (38.8) | 0.060 (0.002–0.117) |
| Nitrates | 3137 (17.0) | 301 (17.9) | 0.024 (−0.026 to 0.074) | 2641 (22.1) | 309 (24.0) | 0.046 (−0.012 to 0.103) |
| Statins | 10 442 (56.5) | 1119 (66.5) | 0.206 (0.156–0.256) | 4775 (49.7) | 631 (59.2) | 0.193 (0.130–0.256) |
| In patients with arterial diseaseq | 8374 (71.7) | 933 (73.5) | 0.041 (−0.017 to 0.099) | 3011 (60.7) | 419 (65.8) | 0.105 (0.023–0.188) |
| Antiarrhythmics | 2077 (11.2) | 190 (11.3) | 0.002 (−0.048 to 0.051) | 1131 (9.5) | 135 (10.5) | 0.035 (−0.023 to 0.092) |
| Antiplatelet | 10 255 (55.5) | 940 (55.9) | 0.007 (−0.043 to 0.057) | 5102 (42.6) | 589 (45.8) | 0.063 (0.005–0.120) |
| Aspirin | 9388 (50.8) | 828 (49.2) | 0.032 (−0.018 to 0.082) | 6064 (50.7) | 613 (47.6) | 0.061 (0.004–0.119) |
| Anticoagulant | 5957 (32.2) | 815 (48.4) | 0.334 (0.284–0.384) | 3143 (26.3) | 460 (35.7) | 0.206 (0.148–0.263) |
| Atrial fibrillation/flutterm | 3240 (74.0) | 401 (78.5) | 0.105 (0.013–0.196) | 1828 (66.6) | 259 (69.8) | 0.068 (−0.040 to 0.177) |
| Atrial fibrillation history | 4509 (69.4) | 603 (75.6) | 0.139 (0.066–0.213) | 2744 (58.9) | 387 (62.4) | 0.072 (−0.012 to 0.155) |
| CHA2DS2-VASc score ≥2 | 5495 (32.6) | 815 (48.4) | 0.328 (0.277–0.378) | 2194 (28.7) | 313 (35.2) | 0.141 (0.071–0.210) |
| Alpha adrenoceptor blocker | 189 (1.0) | 23 (1.4) | 0.032 (−0.018 to 0.082) | 389 (6.6) | 51 (7.7) | 0.041 (−0.039 to 0.121) |
| Insulin of patients with diabetes | 1555 (25.2) | 182 (27.5) | 0.053 (−0.027 to 0.133) | 866 (30.8) | 126 (33.2) | 0.053 (−0.055 to 0.160) |
| Pacemaker | 2186 (11.8) | 278 (16.5) | 0.135 (0.085–0.185) | 1005 (8.4) | 135 (10.5) | 0.072 (0.014–0.129) |
| ICD | 3209 (17.4) | 324 (19.3) | 0.049 (−0.001 to 0.099) | 76 (0.6) | 13 (1.0) | 0.042 (−0.016 to 0.099) |
| CRT-P or CRT-D | 1176 (6.4) | 145 (8.6) | 0.086 (0.036–0.135) | - | - | |
Data are presented as mean ± SD, median (IQR) for continuous measures, and n (%) for categorical measures. Missing number of the variables in each trial can be found in Supplementary data online, Table S4.
Absolute SMD.
Including Central America.
including Central Europe and Russia.
CHA2DS2-VASc = congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), prior stroke (2 points), vascular disease (myocardial infarction or peripheral artery disease, 1 point), age 65–74 years (1 point), and gender (1 point for female sex).
CHD = angina, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, and ischaemic aetiology.
Defined as current angina in CHARM-Preserved.
As a percentage of all strokes.
Carotid artery disease = carotid artery stenosis, carotid artery revascularization, and carotid endarterectomy (defined with data available in each trial, also refer to Supplementary data online, Table S3).
PAD = history of peripheral artery disease, lower limb stenosis, lower limb revascularization, intermittent claudication, and peripheral arterial occlusive disease (defined with data available in each trial, also refer to Supplementary data online, Table S3).
Haemoglobin < 130 g/L for male and 120 g/L for female.
In TOPCAT-Americas, ischaemic aetiology = angina, myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting.
Patients with paced rhythm excluded.
Based on electrocardiogram.
TOPCAT-Americas excluded.
Spironolactone in I-Preserve and CHARM-Preserved.
I-Preserve and CHARM-Preserved excluded.
Arterial disease = coronary heart disease, peripheral artery disease, and carotid artery disease.
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; ARNI, angiotensin-converting enzyme inhibitors; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CCB, dihydropyridine calcium-channel blocker; CHARM-Preserved, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy with defifibrillator; CRT-P, cardiac resynchronization therapy with pacemaker; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; ICD, implantable cardioverter defibrillator; I-Preserve, Irbesartan in heart failure with Preserved ejection fraction trial; JVD, jugular venous distension; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MI, myocardial infarction; MLWHF, Minnesota Living With Heart Failure questionnaire; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; PAD, peripheral artery disease; PAOD, peripheral arterial occlusive disease; PCI, percutaneous coronary intervention; PND, paroxysmal nocturnal dyspnea; PP, pulse Pressure; SMD, standardized mean difference; TIA, transient ischaemic attack; TOPCAT-Americas, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial.
Demographics, smoking habits, and physiologic measures
Patients with a history of stroke were slightly older and more likely to come from North America but did not differ by sex. Blood pressure, pulse pressure, heart rate, body mass index, and history of current smoking did not differ meaningfully between patients with and without a history of stroke.
Comorbidities associated with stroke
A history of atrial fibrillation [and atrial fibrillation of the baseline electrocardiogram (ECG)], diabetes, hypertension, coronary artery disease, and peripheral artery disease was more frequent in patients with a history of stroke compared with those without. Mean eGFR was substantially lower in patients with a history of stroke, as was the proportion of patients with chronic kidney disease (CKD) (eGFR < 60 mL/min/1.73 m2).
Carotid artery disease and history of transient ischaemic attack
In the trials which documented history of carotid artery disease (including carotid endarterectomy) and TIA, each of these was more frequent in patients with a history of stroke compared with those without.
Heart failure history and characteristics
Patients with a history of stroke were more likely to have an ischaemic aetiology and longer-standing heart failure. They were also more likely to have worse NYHA functional class and patient-reported health-related quality of life. Each of the KCCQ domain scores was lower (worse) in patients with a history of stroke (see Supplementary data online, Figure S1). Regarding Minnesota Living with Heart Failure scores, the results for each domain and most individual questions were higher (worse) in patients with a history of stroke (see Supplementary data online, Figures S2 and S3). Similarly, among the subgroup of patients with data from the EQ-5D, those with a history of stroke reported more problems related to mobility, self-care, and usual activities (see Supplementary data online, Tables S5 and S6). History of stroke was associated with a substantially higher frequency of fatigue, more oedema, and a higher NT-proBNP concentration, even in patients without atrial fibrillation. However, mean LVEF did not differ between patients with and without a history of stroke.
Baseline treatment
Patients with a history of stroke were more likely to be treated with a calcium channel blocker, statin, and anticoagulant. However, the use of statins was relatively low in patients with arterial disease (74% in HFrEF and 66% in HFpEF), as was the use of anticoagulants in those with atrial fibrillation (76% in HFrEF and 62% in HFpEF). Patients with prior stroke were also more likely to have a pacemaker (and in those with HFrEF, cardiac resynchronization therapy).
Clinical outcomes according to history of stroke
Outcomes in patients with and without a history of stroke are shown in Table 2, Figure 1 (all heart failure patients combined), and the Structured Graphical Abstract.
Table 2.
Clinical outcomes of HF patients with and without prior stroke
| Total events | Events per 100 person-years (95% CI) | Stroke vs. nonstroke | ||||
|---|---|---|---|---|---|---|
| No prior stroke (n = 30 441) |
Prior stroke (n = 2970) |
No prior stroke (n = 30 441) |
Prior stroke (n = 2970) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
| CV death or HF hosp. | ||||||
| Overall | 7578 (24.9) | 934 (31.5) | 9.97 (9.75–10.20) | 13.54 (12.70–14.44) | 1.34 (1.26–1.44) | 1.18 (1.10–1.27) |
| HFrEF | 4756 (25.7) | 521 (31.0) | 11.89 (11.55–12.23) | 15.66 (14.37–17.06) | 1.30 (1.19–1.43) | 1.17 (1.07–1.28) |
| HFpEF | 2822 (23.6) | 413 (32.1) | 7.84 (7.56–8.14) | 11.57 (10.51–12.74) | 1.46 (1.32–1.62) | 1.19 (1.07–1.33) |
| First HF hosp. | ||||||
| Overall | 4806 (15.8) | 586 (19.7) | 6.32 (6.15–6.50) | 8.50 (7.84–9.21) | 1.32 (1.21–1.44) | 1.12 (1.03–1.23) |
| HFrEF | 2761(14.9) | 307 (18.2) | 6.90 (6.65–7.16) | 9.23 (8.25–10.32) | 1.31 (1.17–1.48) | 1.16 (1.03–1.31) |
| HFpEF | 2045 (17.1) | 279 (21.7) | 5.68 (5.44–5.93) | 7.82 (6.95–8.79) | 1.35 (1.19–1.53) | 1.08 (0.95–1.23) |
| Cardiovascular death | ||||||
| Overall | 4327 (14.2) | 576 (19.4) | 5.23 (5.08–5.39) | 7.49 (6.90–8.13) | 1.44 (1.32–1.57) | 1.31 (1.20–1.43) |
| HFrEF | 3024 (16.4) | 348 (20.7) | 6.98 (6.74–7.24) | 9.50 (8.55–10.55) | 1.37 (1.22–1.53) | 1.23 (1.10–1.37) |
| HFpEF | 1303 (10.9) | 228 (17.7) | 3.31 (3.13–3.49) | 5.67 (4.98–6.45) | 1.73 (1.50–1.99) | 1.44 (1.25–1.66) |
| All-cause death | ||||||
| Overall | 5672 (18.6) | 740 (24.9) | 6.86 (6.68–7.04) | 9.62 (8.95–10.34) | 1.41 (1.31–1.52) | 1.26 (1.17–1.36) |
| HFrEF | 3623 (19.6) | 423 (25.1) | 8.36 (8.10–8.64) | 11.54 (10.49–12.69) | 1.39 (1.26–1.54) | 1.23 (1.11–1.37) |
| HFpEF | 2049 (17.1) | 317 (24.6) | 5.20 (4.98–5.43) | 7.88 (7.06–8.79) | 1.53 (1.36–1.72) | 1.28 (1.14–1.45) |
| Fatal/nonfatal MI | ||||||
| Overall | 1032 (3.4) | 148 (5.0) | 1.27 (1.19–1.35) | 1.97 (1.68–2.32) | 1.54 (1.30–1.83) | 1.26 (1.06–1.51) |
| HFrEF | 534 (2.9) | 59 (3.5) | 1.25 (1.15–1.36) | 1.64 (1.27–2.12) | 1.31 (1.00–1.71) | 1.07 (0.82–1.40) |
| HFpEF | 498 (4.2) | 89 (6.9) | 1.29 (1.18–1.41) | 2.28 (1.85–2.80) | 1.75 (1.39–2.19) | 1.44 (1.15–1.81) |
| Fatal/nonfatal stroke | ||||||
| Overall | 963 (3.2) | 200 (6.7) | 1.18 (1.11–1.26) | 2.68 (2.34–3.08) | 2.27 (1.95–2.64) | 2.02 (1.73–2.36) |
| HFrEF | 491 (2.7) | 99 (5.9) | 1.15 (1.05–1.25) | 2.79 (2.29–3.40) | 2.44 (1.97–3.03) | 2.24 (1.80–2.79) |
| HFpEF | 472 (3.9) | 101 (7.9) | 1.22 (1.11–1.33) | 2.58 (2.13–3.14) | 2.12 (1.71–2.63) | 1.84 (1.48–2.29) |
| Fatal/nonfatal MI or stroke | ||||||
| Overall | 1939 (6.4) | 329 (11.1) | 2.41 (2.31–2.52) | 4.51 (4.05–5.02) | 1.86 (1.65–2.09) | 1.60 (1.42–1.80) |
| HFrEF | 998 (5.4) | 148 (8.8) | 2.36 (2.22–2.51) | 4.24 (3.61–4.98) | 1.79 (1.51–2.13) | 1.55 (1.30–1.85) |
| HFpEF | 941 (7.9) | 181 (14.1) | 2.47 (2.32–2.63) | 4.75 (4.11–5.50) | 1.91 (1.63–2.24) | 1.64 (1.39–1.92) |
| CV death, HF hosp., MI, or stroke | ||||||
| Overall | 8436 (27.7) | 1067 (35.9) | 11.36 (11.12–11.60) | 16.13 (15.19–17.13) | 1.40 (1.32–1.50) | 1.23 (1.15–1.31) |
| HFrEF | 5159 (27.9) | 583 (34.6) | 13.12 (12.77–13.48) | 18.23 (16.81–19.77) | 1.37 (1.26–1.49) | 1.22 (1.12–1.33) |
| HFpEF | 3277 (27.4) | 484 (37.6) | 9.37 (9.06–9.70) | 14.16 (12.96–15.48) | 1.49 (1.36–1.64) | 1.24 (1.12–1.37) |
The adjusted hazard ratio is from a model adjusted for region, age, gender, heart rate, SBP, BMI, NYHA functional class III/IV, LVEF, eGFR, NT-proBNP (log-transformed), atrial fibrillation, ischaemic aetiology, MI, diabetes mellitus, treatment arm, and within-trial clustering.
Single and composite clinical outcomes (rate per 100 person-years) in patients with and without prior stroke.
BMI, body mass index; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; hosp. hospitalization; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
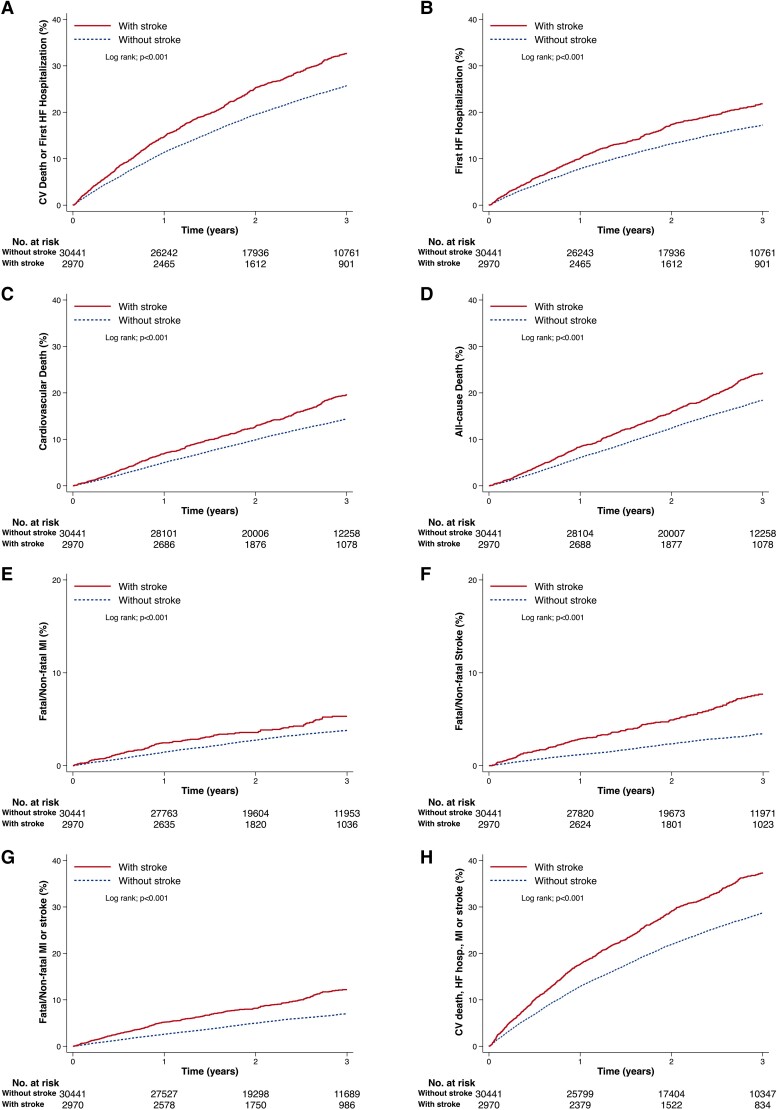
Figure 1.
Kaplan–Meier curves for clinical outcomes in heart failure patients with and without prior stroke. (A) Cardiovascular death or first hospitalization for heart failure; (B) hospitalization for heart failure; (C) cardiovascular death; (D) all-cause death; (E) fatal/non-fatal myocardial infarction; (F) fatal/non-fatal stroke; (G) fatal/non-fatal myocardial infarction or stroke; (H) cardiovascular death, hospitalization for heart failure, myocardial infarction, or stroke. The number at risk for the event of interest of heart failure patients with and without prior stroke was shown below each graph. CV, cardiovascular; HF, heart failure; hosp., hospitalization; MI, myocardial infarction.
Regardless of heart failure phenotype, patients with a history of stroke had a higher risk of all non-fatal and fatal outcomes examined. However, when HFrEF and HFpEF were examined separately, there was a significant interaction between heart failure phenotype and risk of death from cardiovascular causes, with the risk of this outcome being greater in patients with HFpEF (HR 1.73, 95% CI 1.50–1.99) compared with patients with HFrEF (HR 1.37, 95% CI 1.22–1.53). This led to a similar trend for the composite of the time-to-first occurrence of cardiovascular death or hospitalization for HF, driven by the mortality component. The risk of heart failure hospitalization was also significantly higher in patients with a history of stroke, compared with patients without such a history, although the relative elevation in risk was similar in patients with HFrEF and HFpEF.
The greatest excess risk in patients with a history of stroke, in relative terms, was for fatal or non-fatal stroke (i.e. recurrent stroke), which was doubled, and patients with a history of stroke also had a higher risk of myocardial infarction, compared with those with no history of stroke.
Although the elevation of risk of atherothrombotic events was sizeable in relative terms, the absolute excess risk was modest because such events were infrequent by comparison with heart failure hospitalization or death. However, when the broadest composite outcome (time-to-first occurrence of cardiovascular death, hospitalization for HF, non-fatal stroke, or non-fatal myocardial infarction) was examined, the absolute excess risk in patients with a history of stroke was substantial at ∼5/100 person-years, and this absolute excess was similar in HFrEF and HFpEF.
Examination of overall death and death by category confirmed a higher risk of death overall in patients with a history of stroke, and the proportion of deaths due to cardiovascular causes was slightly greater because of a few per cent more deaths from stroke and myocardial infarction (see Supplementary data online, Figure S4).
Although most individual HRs were attenuated by adjustment for recognized prognostic variables, the overall picture was broadly unaltered (Table 2).
Sensitivity analyses excluding I-Preserve (because a history of stroke was not differentiated from a history of TIA) and CHARM-Preserved (because of the unavailability of NT-proBNP for adjustment) gave essentially identical results (see Supplementary data online, Table S7). Surprisingly, we also found a similar pattern of events/risk related to TIA as seen for stroke, although there was a somewhat weaker relationship with non-atherothrombotic events in patients with HFpEF (see Supplementary data online, Table S8).
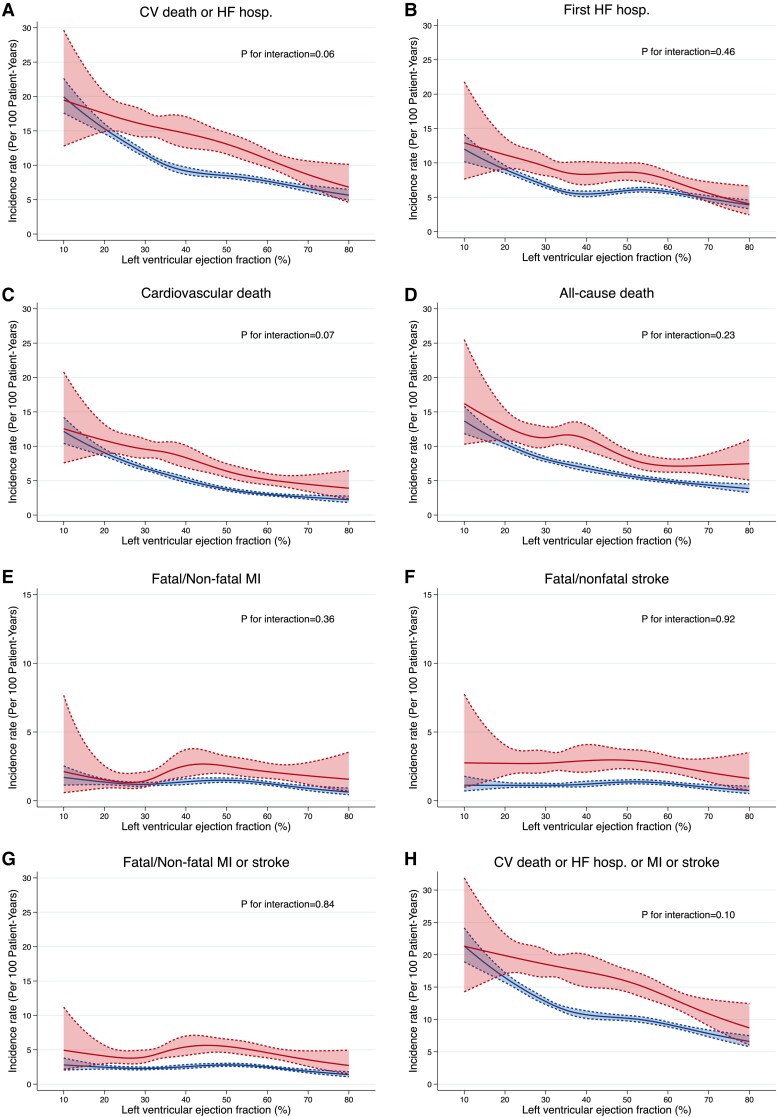
Clinical outcomes across the spectrum of LVEF according to history of stroke
Figure 2 shows the incidence rate per 100 person-years for the outcomes of interest across the range of LVEF, shown as a continuous variable. The risk of heart failure hospitalization and death (whether from any cause or cardiovascular causes) increased as LVEF decreased. By contrast, the incidence rate of atherothrombotic outcomes (myocardial infarction and stroke) remained constant across the range of LVEF. The risk of all outcomes was higher in patients with a history of stroke; inspection of Figure 2 showed that, essentially, the lines relating each outcome to LVEF in patients with a history of stroke ran in parallel to those in patients without a history of stroke but were shifted upwards.
Figure 2.
Incidence rates of outcomes across LVEF in heart failure patients with (upper lines/red) and without (lower lines/blue) prior stroke. (A) Cardiovascular death or first hospitalization for heart failure; (B) hospitalization for heart failure; (C) cardiovascular death; (D) all-cause death; (E) fatal/non-fatal myocardial infarction; (F) fatal/non-fatal stroke; (G) fatal/non-fatal myocardial infarction, or stroke; (H) cardiovascular death, hosp. for heart failure, myocardial infarction, or stroke. All the incidence rates are expressed as per 100 patient-years. CV, cardiovascular; HF, heart failure; hosp., hospitalization; MI, myocardial infarction.
Discussion
To the best of our knowledge, this is the first large individual patient analysis of the clinical characteristics of heart failure patients with and without a history of stroke.17,26–28 The COACH trial reported higher rates of hospitalization and death in 105 heart failure patients with a history of stroke, compared with 918 patients without stroke in the Netherlands, but did not differentiate by ejection fraction phenotype or report atherothrombotic outcomes, including recurrent stroke.26 Approximately 1 in 11 patients with heart failure had a prior stroke. We provided a detailed description of a wide range of outcomes in these individuals. Our analysis included patients with both of the main LVEF phenotypes, and we examined outcomes across the spectrum of LVEF.
A history of stroke added substantially to cardiovascular risk in patients with heart failure, whether HFrEF or HFpEF. Specifically, looking at the broadest composite endpoint, reflecting all major adverse cardiovascular outcomes faced by patients with heart failure (cardiovascular death, hospitalization for heart failure, non-fatal stroke, or non-fatal myocardial infarction), patients had a 40% higher risk in relative terms and an excess absolute risk of ∼5/100 person-years of follow-up. Although, as might be anticipated, a history of stroke was a particularly powerful predictor of atherothrombotic events (risk of myocardial infarction 50% higher and risk of stroke doubled), the risk of heart failure hospitalization was also elevated (∼30% higher), and the risk of cardiovascular death was also considerably higher (∼40% higher). The former likely reflects the finding that patients with a history of stroke had evidence of more severe heart failure and the excess risk was attenuated by adjustment for variables known to predict outcomes in heart failure. However, the elevation in risk of cardiovascular death was not as attenuated by adjustment, presumably reflecting the direct contribution of stroke (and myocardial infarction) to cardiovascular death in heart failure.
While, as expected, the incidence rates of heart failure and death were higher in the lower range of LVEF, no such pattern was seen for stroke (or myocardial infarction), and the relatively low incidence of these atherothrombotic events was similar across the range of LVEF studied and, therefore, similar in patients with HFrEF and HFpEF. This argues against the oft-repeated suggestion that low LVEF is associated with an increased risk of stroke, and in a recent analysis of incident stroke in HFrEF, LVEF was not a predictor.29,30 A history of hypertension was more common in patients with a prior stroke although patients with and without a history of stroke had similar average blood pressure and the incidence of stroke was similar across the range of systolic blood pressure at baseline. However, a substantial minority of participants with and without stroke had uncontrolled systolic blood pressure (≥140 mmHg), especially among those with HFpEF (38%) compared with HFrEF (17%).
Patients with a history of stroke were considerably more likely to have concomitant atrial fibrillation and also had more evidence of atherosclerosis, including coronary artery disease, peripheral artery disease, and carotid artery disease. These findings are obviously relevant to the potential causation of stroke and point to potentially beneficial therapeutic interventions. Surprisingly, only 70% of patients with atrial fibrillation had been prescribed an anticoagulant, and only 71% of patients with arterial disease were treated with a statin at baseline, suggesting scope for improving secondary prevention. There may also be a role for anticoagulation in selected patients in sinus rhythm but this needs to be tested in prospective randomized trials.30,31
Interestingly, one of the biggest differences between patients with and without a history of stroke was in eGFR and the proportion of participants with chronic kidney disease. There is a great deal of debate about whether low eGFR per se is a risk factor for stroke or merely a biomarker reflecting the effects of hypertension, diabetes, and perhaps other diseases on the kidney.32,33 We are not aware of this association having been identified in heart failure previously.
Patients with a history of stroke also reported worse health-related quality of life than those without a prior stroke, although it is not clear whether this reflected more severe heart failure or the residual effects of stroke. However, our analysis of the EQ-5D showed that patients with a prior stroke reported much more limitations in their mobility and ability to undertake ordinary activities. It was also notable that fatigue was considerably more common in patients with a history of stroke compared to those without. Post-stroke fatigue is a well-recognized and sometimes debilitating consequence of stroke.34
Limitations
Our study has several limitations. The patients analysed were enrolled in clinical trials, i.e. were relatively selected and may not be representative of patients in ordinary clinical practice. Ejection fraction could be measured by different methods in the included trials. Medical history was based on answers to questions in the trial case report forms, and completion of these may have varied by trial and by country. Patients with severe strokes are unlikely to have been enrolled, and we did not have any measure of stroke severity. Similarly, a major limitation was that we did not have information on the type of stroke. Future studies with thorough imaging-based adjudication will be important to help understand the most important causes of stroke in patients with heart failure, for example, embolism or small vessel disease, as this aetiological information could suggest therapeutic strategies. Patients with clinically significant uncorrected primary valvular disease were excluded from the trials analysed, as were patients with uncontrolled arrhythmias. This may have resulted in underestimation of the true prevalence of stroke in a broad real-world population of heart failure due to any cause. Some data were not available in specific trials as described in Supplementary data online, Table S4.
Conclusions
About 1 in 11 patients in this pooled heart failure trial database had a history of stroke. Their annualized rate of cardiovascular death, hospitalization for heart failure, non-fatal stroke, or non-fatal myocardial infarction was ∼18% (compared with 13% in those without a history of stroke); the corresponding rates in patients with HFpEF were 14% and 9%, respectively, and their risk of further stroke was twice as high as in patients without a history of stroke. The relatively low rates of use of anticoagulants in patients with atrial fibrillation and statins in patients with arterial disease, along with poor blood pressure control (especially in patients with HFpEF) point to potential therapeutic opportunities to reduce this risk.
Supplementary Material
Contributor Information
Mingming Yang, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK; Department of Cardiology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
Toru Kondo, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK; Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Jawad H Butt, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK; Department of Cardiology, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark.
William T Abraham, Division of Cardiovascular Medicine, The Ohio State University, OH, USA.
Inder S Anand, VA Medical Center, Minneapolis, MN, USA; University of Minnesota, Minneapolis, MN, USA.
Akshay S Desai, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA, USA.
Lars Køber, Department of Cardiology, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark.
Milton Packer, Baylor Heart and Vascular Institute, Baylor University Medical Center, Dallas, TX, USA.
Marc A Pfeffer, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA, USA.
Jean L Rouleau, Institut de Cardiologie de Montréal, Université de Montréal, Montréal, Canada.
Marc S Sabatine, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Scott D Solomon, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA, USA.
Karl Swedberg, Department of Molecular and Clinical Medicine, University of Gothenburg, Gothenburg, Sweden; National Heart and Lung Institute, Imperial College London, London, UK.
Michael R Zile, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA.
Pardeep S Jhund, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
M.Y. reports travel grants from AstraZeneca. T.K. reports speaker fees from Abbott, Ono Pharma, Otsuka Pharma, Novartis, AstraZeneca, Bristol-Myers Squibb, and Abiomed. J.H.B. reports advisory board honoraria from Bayer. W.T.A. has received personal fees from Abbott; has received consulting fees from Boehringer Ingelheim, Impulse Dynamics, and Respicardia; has received salary support from V-Wave Medical; and has received research support from the NHLBI, all for studies performed within the heart failure arena. I.S.A. reports receiving fees for serving on a steering committee from AstraZeneca, ARCA biopharma, Amgen, and LivaNova; fees for serving as chair of a data and safety monitoring board from Boston Scientific; fees for serving on an end point committee from Boehringer Ingelheim; and fees for serving on an advisory board from Zensun. A.S.D. reports consulting fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, DalCor Pharmaceuticals, and Regeneron, grant support (paid to Brigham and Women’s Hospital) and consulting fees from Alnylam Pharmaceuticals and Novartis, and advisory board fees from Corvidia and Relypsa. L.K. reports other support from AstraZeneca and personal fees from Novartis and Boehringer as a speaker. M.P. reports consulting fees from AbbVie, Akcea, Actavis, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, Novo Nordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance. M.A.P. reports research grant support through Brigham and Women’s Hospital from Novartis; and consulting fees from AstraZeneca, Boehringer Ingelheim and Eli Lilly Alliance, Corvidia, DalCor, GlaxoSmithKline, National Heart, Lung, and Blood Institute (NHLBI) CONNECTs (Master Protocol Committee), Novartis, Novo Nordisk, Peerbridge, and Sanofi; and has equity in DalCor. J.L.R. reports grants and consulting fees from Novartis and consulting fees from Abbott, AstraZeneca, MyoKardia, and Sanofi. K.S. reports honoraria from AstraZeneca, Boehringer Ingelheim and Novartis. M.S.S. reports grants from Bayer, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda; grants and personal fees from Amgen, AstraZeneca, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, and Novartis; and personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, DalCor, Dyrnamix, Esperion, IFM Therapeutics, and Ionis. M.S.S. is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from Abbott, Aralez, Roche, and Zora Biosciences. S.D.S. reports grants from AstraZeneca, Bellerophon, Celladon, Ionis, Lone Star Heart, Mesoblast, National Institutes of Health/National Heart, Lung, and Blood Institute, Sanofi Pasteur, and Eidos; grants and personal fees from Alnylam, Amgen, AstraZeneca, BMS, Gilead, GSK, MyoKardia, Novartis, Theracos, Bayer, and Cytokinetics; and personal fees from Akros, Corvia, Ironwood, Merck, Roche, Takeda, Quantum Genomics, AoBiome, Janssen, Cardiac Dimensions, Tenaya, and Daichi-Sankyo. K.S. reports honoraria from AstraZeneca, Boehringer Ingelheim and Novartis. M.R.Z. reports research funding from Novartis and has been a consultant for Novartis, Abbott, Boston Scientific, CVRx, EBR, Endotronics, Ironwood, Merck, Medtronic, and Myokardia V Wave. P.S.J. reports other from AstraZeneca, personal fees from Novartis and Cytokinetics, and grants from Boehringer Ingelheim. J.J.V.M. reports payments through Glasgow University from work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos Personal lecture fees: the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Global Clinical Trial Partners (GCTP).
Data Availability
P.S.J. takes responsibility for the integrity of the data and the accuracy of the data analysis. Please contact the corresponding author to discuss access to the data used in these analyses.
Funding
J.J.V.M. and P.S.J. are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217 and the Vera Melrose Heart Failure Research Fund. M.Y. is Funded by the China Scholarship Council.
Ethical Approval
Ethical Approval was not required.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Seol H, Kim JS. Prevalence, mechanisms, and management of ischemic stroke in heart failure patients. Semin Neurol 2021;41:340–347. 10.1055/s-0041-1726329 [DOI] [PubMed] [Google Scholar]
- 2. Barkhudaryan A, Doehner W, Scherbakov N. Ischemic stroke and heart failure: facts and numbers. An update. J Clin Med 2021;10:1146. 10.3390/jcm10051146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schumacher K, Kornej J, Shantsila E, Lip GYH. Heart failure and stroke. Curr Heart Fail Rep 2018;15:287–296. 10.1007/s11897-018-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim W, Kim EJ. Heart failure as a risk factor for stroke. J Stroke 2018;20:33–45. 10.5853/jos.2017.02810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sajeev JK, Kalman JM, Dewey H, Cooke JC, Teh AW. The atrium and embolic stroke: myopathy not atrial fibrillation as the requisite determinant? JACC Clin Electrophysiol 2020;6:251–261. 10.1016/j.jacep.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 6. Ronsoni RM, Saffi MAL, Gonçalves MVM, Nakayama IH, Luz Leiria TL. A new vision at the interface of atrial fibrillation and stroke. Front Cardiovasc Med 2021;8:689313. 10.3389/fcvm.2021.689313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caldas JR, Panerai RB, Salinet AM, Seng-Shu E, Ferreira GSR, Camara L, et al. . Dynamic cerebral autoregulation is impaired during submaximal isometric handgrip in patients with heart failure. Am J Physiol Heart Circ Physiol 2018;315:H254–h261. 10.1152/ajpheart.00727.2017 [DOI] [PubMed] [Google Scholar]
- 8. Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, et al. . Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke 2009;40:3706–3710. 10.1161/strokeaha.109.561670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheitz JF, Sposato LA, Schulz-Menger J, Nolte CH, Backs J, Endres M. Stroke–heart syndrome: recent advances and challenges. J Am Heart Assoc 2022;11:e026528. 10.1161/jaha.122.026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckley BJR, Harrison SL, Hill A, Underhill P, Lane DA, Lip GYH. Stroke–heart syndrome: incidence and clinical outcomes of cardiac complications following stroke. Stroke 2022;53:1759–1763. 10.1161/strokeaha.121.037316 [DOI] [PubMed] [Google Scholar]
- 11. Sposato LA, Hilz MJ, Aspberg S, Murthy SB, Bahit MC, Hsieh CY, et al. . Post-Stroke cardiovascular complications and neurogenic cardiac injury: jACC state-of-the-art review. J Am Coll Cardiol 2020;76:2768–2785. 10.1016/j.jacc.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 12. Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke–heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 2018;17:1109–1120. 10.1016/s1474-4422(18)30336-3 [DOI] [PubMed] [Google Scholar]
- 13. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. . Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012;366:1859–1869. 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehra MR, Vaduganathan M, Fu M, Ferreira JP, Anker SD, Cleland JGF, et al. . A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J 2019;40:3593–3602. 10.1093/eurheartj/ehz427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hebert K, Kaif M, Tamariz L, Gogichaishvili I, Nozadze N, Delgado MC, et al. . Prevalence of stroke in systolic heart failure. J Card Fail 2011;17:76–81. 10.1016/j.cardfail.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 16. Hebert K, Marzouka G, Arcement L, Julian E, Cortazar F, Dias A, et al. . Prevalence of vaccination rates in systolic heart failure: a prospective study of 549 patients by age, race, ethnicity, and sex in a heart failure disease management program. Congest Heart Fail 2010;16:278–283. 10.1111/j.1751-7133.2010.00190.x [DOI] [PubMed] [Google Scholar]
- 17. Kozdağ G, Yaymacı M, Işeri P, Ertaş G, Emre E, Bildirici U, et al. . Ischemic stroke history predicts increased cardiovascular mortality in chronic heart failure. Anadolu Kardiyol Derg 2011;11:421–427. 10.5152/akd.2011.109 [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, et al. . Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med 2016;374:1521–1532. 10.1056/NEJMoa1514859 [DOI] [PubMed] [Google Scholar]
- 20. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777–781. 10.1016/s0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 21. Carson P, Massie BM, McKelvie R, McMurray J, Komajda M, Zile M, et al. . The irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial: rationale and design. J Card Fail 2005;11:576–585. 10.1016/j.cardfail.2005.06.432 [DOI] [PubMed] [Google Scholar]
- 22. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, et al. . Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966–972.e910. 10.1016/j.ahj.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 24. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. . Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail 2017;5:471–482. 10.1016/j.jchf.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 25. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.: Springer; 2001. [Google Scholar]
- 26. Ski CF, van der Wal MHL, Le Grande M, van Veldhuisen DJ, Lesman-Leegte I, Thompson DR, et al. . Patients with heart failure with and without a history of stroke in the Netherlands: a secondary analysis of psychosocial, behavioural and clinical outcomes up to three years from the COACH trial. BMJ Open 2019;9:e025525. 10.1136/bmjopen-2018-025525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khafaji HA, Sulaiman K, Singh R, AlHabib KF, Asaad N, Alsheikh-Ali A, et al. . Clinical characteristics, precipitating factors, management and outcome of patients with prior stroke hospitalised with heart failure: an observational report from the Middle East. BMJ Open 2015;5:e007148. 10.1136/bmjopen-2014-007148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Yang MX, Tu Q, Tao LY, Liu G, An H, et al. . Impact of prior ischemic stroke on outcomes in patients with heart failure—a propensity-matched study. Circ J 2020;84:1797–1806. 10.1253/circj.CJ-20-0210 [DOI] [PubMed] [Google Scholar]
- 29. Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol 1999;33:1424–1426. 10.1016/s0735-1097(99)00033-9 [DOI] [PubMed] [Google Scholar]
- 30. Kondo T, Abdul-Rahim AH, Talebi A, Abraham WT, Desai AS, Dickstein K, et al. . Predicting stroke in heart failure and reduced ejection fraction without atrial fibrillation. Eur Heart J 2022;43:4469–4479. 10.1093/eurheartj/ehac487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merkler AE, Pearce LA, Kasner SE, Shoamanesh A, Birnbaum LA, Kamel H, et al. . Left ventricular dysfunction among patients with embolic stroke of undetermined source and the effect of rivaroxaban vs aspirin: a subgroup analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol 2021;78:1454–1460. 10.1001/jamaneurol.2021.3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dawod J, Coull BM. Chronic kidney disease is A biomarker rather than a risk factor for stroke. J Stroke Cerebrovasc Dis 2021;30:105869. 10.1016/j.jstrokecerebrovasdis.2021.105869 [DOI] [PubMed] [Google Scholar]
- 33. Wyld M, Webster AC. Chronic kidney disease is a risk factor for stroke. J Stroke Cerebrovasc Dis 2021;30:105730. 10.1016/j.jstrokecerebrovasdis.2021.105730 [DOI] [PubMed] [Google Scholar]
- 34. Alghamdi I, Ariti C, Williams A, Wood E, Hewitt J. Prevalence of fatigue after stroke: a systematic review and meta-analysis. Eur Stroke J 2021;6:319–332. 10.1177/23969873211047681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
P.S.J. takes responsibility for the integrity of the data and the accuracy of the data analysis. Please contact the corresponding author to discuss access to the data used in these analyses.