Abstract
PURPOSE
Telegenetics services can expand access to guideline-recommended cancer genetic testing. However, access is often not distributed equitably to all races and ethnicities. We evaluated the impact of an on-site nurse-led cancer genetics service in a diverse Veterans Affairs Medical Center (VAMC) oncology clinic on likelihood of germline testing (GT) completion.
METHODS
We conducted an observational retrospective cohort study of patients who were referred for cancer genetics services at the Philadelphia VAMC between October 1, 2020, and February 28, 2022. We evaluated the association between genetics service (on-site v telegenetics) and likelihood of GT completion in a subcohort of new consults, excluding patients with prior consults and those referred for known history of germline mutations.
RESULTS
A total of 238 Veterans, including 108 (45%) seen on site, were identified for cancer genetics services during the study period, with the majority referred for a personal (65%) or family (26%) history of cancer. In the subcohort of new consults, 121 Veterans (54% self-identified race/ethnicity [SIRE]-Black), including 60 (50%) seen on site, were included in the analysis of germline genetic testing completion. In a univariate analysis, patients who were seen by the on-site genetics service had 3.2-fold higher likelihood of completing GT (relative risk, 3.22; 95% CI, 1.89 to 5.48) compared with the telegenetics service. In multivariable regression analysis, the on-site genetics service was associated with higher likelihood of GT completion, but this association was only statistically significant in SIRE-Black compared with SIRE-White Veterans (adjusted RR, 4.78; 95% CI, 1.53 to 14.96; P < .001; P-interaction of race × genetics service = .016).
CONCLUSION
An on-site nurse-led cancer genetics service embedded in a VAMC Oncology practice was associated with higher likelihood of germline genetic testing completion than a telegenetics service among self-identified Black Veterans.
APN-Led Genetics program improves cancer genetic testing rates in racially diverse Veteran population.
INTRODUCTION
The detection of hereditary syndromes plays a large role in the screening and management of several cancer subtypes, including breast, ovarian, prostate, pancreatic, colon, and others.1,2 Although there are a growing number of indications for germline genetic testing among patients with cancer or at risk for hereditary cancer syndromes, there are several multilevel barriers to germline testing (GT) completion. Barriers to testing include operational barriers, such as clinic workflow, time constraints, and lack of access to traditional medical or cancer genetic services.3-8 Additionally, there is a critical shortage of genetics service providers, both in and outside the Veterans Affairs Health System (VHA).7,9 GT completion barriers have been magnified among racial minorities, even in cases for which there is a clear indication for testing, or if the testing is provided free of cost.10,11 As a result, there are missed opportunities to identify patients with hereditary cancer syndromes, provide appropriate surveillance and risk reduction, diagnose cancer earlier, identify at risk family members, and offer precision oncology treatments.
CONTEXT
Key Objective
We sought to determine if an on-site cancer genetics service was associated with increased completion of germline genetic testing when compared with the standard centralized telegenetics service, and if this effect varied by self-identified race/ethnicity (SIRE). Although prospective randomized trials have shown that completion rates are similar between in-person and telegenetics, our study asks this question in real-world population at an urban Veteran Affairs Medical Center, and specifically evaluates whether there is a differential effect of the on-site genetics service by SIRE.
Knowledge Generated
We found that patients who were seen by an embedded on-site cancer genetics service in a Veterans' Affairs oncology clinic increased genetic testing rates compared with those who received centralized telegenetics referrals. The impact of the on-site cancer genetics service was seen in non-Hispanic African American Veterans but not in non-Hispanic White Veterans.
Relevance
An oncology clinic embedded, nurse-led genetic testing service increases genetic testing rates among self-identified Black Veterans compared to a centralized telegenetics service.
VHA provides health care to over nine million Veterans and is the largest integrated health system in the United States. Genetics care is provided in the VHA by either referral to a centralized team providing nationwide telegenetics care, traditional site- or region-specific teams, or referral to community non-VA care.7,12 Although telehealth is an emerging and important tool in expanding access to genetic services overall,13 there is also evidence that telehealth can widen racial and ethnic disparities with respect to genetic testing and evaluation, including within the VHA.12 Additionally, there is evidence that older individuals and some subgroups of individuals experiencing barriers such as homelessness—populations over-represented among Veterans—face greater challenges in using telehealth services.14,15
Genetics services are defined as a part of basic nursing practice in the published Scope and Standards of Clinical Genetics Nursing Practice, first published in 1998 by the American Nurses Association and the International Society of Nurses in Genetics (ISONG, updated in 2016).16 The essentials for graduate degree nurses outlines the role of advanced practice registered nurses and includes genetic risk assessment, counseling, testing, results interpretation, clinical management, as well as ethical, legal, and social implications. The success of APRN-led genetics clinics has previously been documented in the literature, including in the Veterans Health Administration5-7,17-21; however, it is unknown the effect of these services on non-White patients.
In cancer genetics, acuity for consults can be high, particularly in the context of treatment-related decisions for many cancers with (US) Food and Drug Administration–approved therapeutic options that target specific germline carriers. We hypothesized that an on-site nurse-led genetics program could ameliorate challenges with telegenetics to improve cancer genetic testing and genetic care needs in a racially diverse, urban, academic-affiliated oncology practice.
METHODS
Cancer Genetics Service
Before October 1, 2020, genetics services were provided at the Corporal Michael J. Crescenz Veterans Affairs Medical Center (CMCVAMC) by a centralized telegenetics service located in Salt Lake City, UT. Veterans who receive telegenetics consults do so in the setting that is most convenient to them—typically their home—and there is no specifically designated space within the Veterans Affairs Medical Center for telemedicine appointments. On October 1, 2020, we initiated a cancer genetics service staffed with an experienced advanced practice nurse geneticist (L.B.A.) and cancer genetics–trained medical oncologist (K.N.M.) at the CMCVAMC (on-site genetics service). The nurse provided on-site genetic services and point-of-care testing, as well as telegenetics, as needed, because of COVID-19 pandemic restrictions and geographical distance of Veterans.
L.B.A. identified Veterans who would benefit from a genetic evaluation on the basis of diagnoses, age at diagnoses, and family history (Appendix Fig A1, online only). This was achieved via provider referral, tumor board referral, and review of prior genetic consults that were not completed. Veterans with prior identification of a hereditary cancer syndrome were also referred to the program for long-term follow-up and surveillance. Once identified, the nurse reached out to the Veteran, either by phone or face-to-face, to set up a genetic consultation. Consultation included intake of current medical and family history, review of the pros and cons of testing, discussion of Veteran values and preferences, and ascertainment of service history and potential exposures. After approval with experienced pathologists (J.P., D.J.), genetic testing was ordered on the basis of National Cancer Care Network (NCCN) guidelines.
Retrospective Cohort Study Design
This study was performed after approval by VA central and local human investigations committees and in accordance with an assurance filed with and approved by the Department of Health and Human Services (CMCVAMC IRB #1607692). We conducted an observational, retrospective cohort study of Veterans who were referred for genetics services between October 1, 2020, through data cutoff of February 28, 2022, to evaluate the association between genetics service (on-site v telegenetics) on the likelihood of GT completion. Patients were followed from the index date (time of consult) until the completion of GT, death before testing, or the data cutoff.
All patients were included in the descriptive analysis of the data (Figs 1A and 1D). Patients were included in the logistic regression analysis evaluating the association between genetics service and GT completion if they met the following inclusion criteria: (1) new active consult for genetics service during the study period with no prior consults placed within VHA; (2) no prior genetic testing; (3) consult indication of personal/family history of cancer or colonic polyposis; and (4) self-identified race/ethnicity (SIRE) non-Hispanic Black or SIRE non-Hispanic White, as only 5.5% of patients identified as Hispanic ethnicity, and each of the other racial groups consisted of no more than six patients (Table 1).
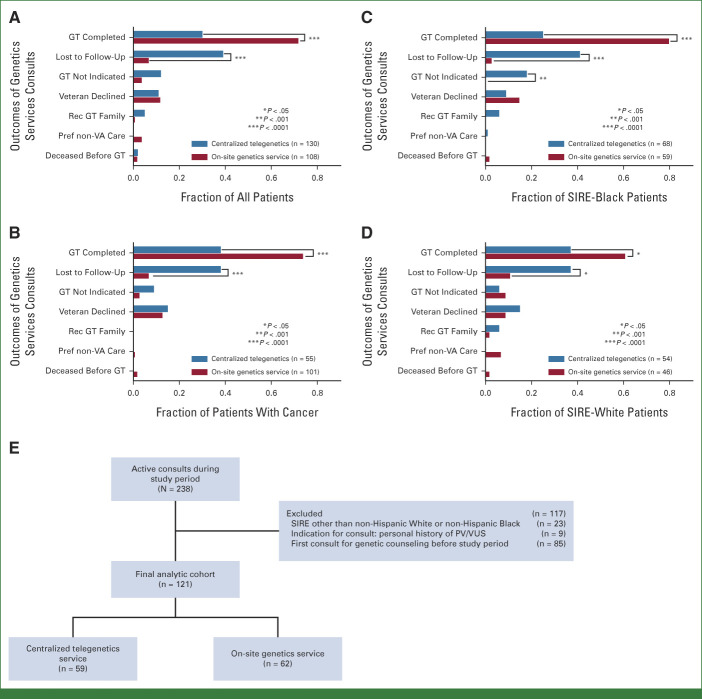
FIG 1.
(A) Outcomes of genetics services consults among all patients with an active consult during the study period. (B) Outcomes of genetics services consults among all patients with cancer. Outcomes of all genetics services consults among all (C) SIRE-Black and (D) SIRE-White patients. (E) Flowchart depicting the analysis of predictors of germline testing completion. GT, germline testing; Pref, preferred; PV, pathogenic variant; Rec, recommended; SIRE, self-identified race/ethnicity; VUS, variant of uncertain significance.
TABLE 1.
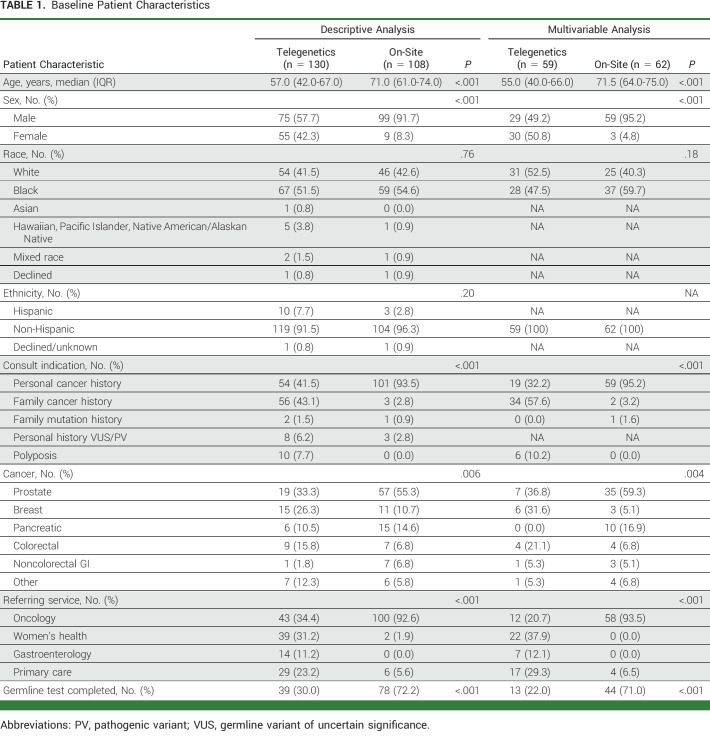
Baseline Patient Characteristics
Data Collection
Data for all patients, regardless of genetic service, were collected via manual chart abstraction (L.B.A./K.N.M.). Baseline patient characteristics and potential confounders included sex assigned at birth, age at the time of consult, SIRE, indication for genetic testing, referring clinician, and cancer diagnosis (if active cancer was the reason for consult). The primary outcome of the study was germline genetic testing completion, also determined by manual chart review. Genetic testing outcome for patients was defined in categories as (1) genetic testing completed, (2) genetic testing not indicated by NCCN/American College of Medical Genetics guidelines, (3) patient declined testing, (4) genetic testing of family member advised, (5) patient had preference for non-VA care, or (6) patient was deceased before testing. All other patients for whom a genetic testing outcome could not be categorized as such were classified as lost to follow-up.
Statistical Analysis
All statistical analyses were performed using Stata 17.0 (StataCorp LLC., College Station, TX, 2021). Descriptive statistics were used to examine and compare baseline patient characteristics between the two groups. Continuous variables were assessed for normality using the Shapiro-Wilk test and visual inspection of the distribution of the data. Differences in baseline continuous variables were tested between the two groups using the t-test for normally distributed variables and Wilcoxon rank-sum test for non-normally distributed variables. We used the Pearson's chi-squared test—or Fisher's exact test in cases when expected cell values were <5—to compare baseline categorical variables between the two groups.
We performed a regression analysis using a generalized linear model to obtain relative risk (RR) for the variables included in the model and to measure the effect of the on-site genetics service on the likelihood of completion of germline genetic testing.22 Because of failure of the generalized linear model to converge with a binary outcome distribution, a normal distribution was specified for the generalized linear model, as has been previously described.22 To address confounding, we included the following variables in the regression model that were deemed to be potential confounders a priori: age (continuous), biological sex (binary), SIRE (binary), and indication for consult (personal history of cancer v family history of cancer v polyposis). Referral service was not included because of overlap with indication for consult. We assessed for an interaction between SIRE and the type of genetics service (telegenetics v on-site) and evaluated for statistical significance of the interaction using the likelihood-ratio test as part of our primary model. We included this interaction term because we hypothesized that the relationship between genetics service and GT outcome may vary on the basis of SIRE.12 We performed a sensitivity analysis—also known as the E-value—to determine the degree of unmeasured confounding that would be necessary to move the point estimate of our observed findings to the null.23-25 We also performed an exploratory analysis comparing the results of GT between SIRE-non-Hispanic White and non-Hispanic-Black individuals.
RESULTS
Descriptive Analysis of Cancer Genetics Referrals
Between October 1, 2020, and February 28, 2021, 238 total consults were placed from CMCVAMC for cancer genetic testing, 130 to the centralized telegenetics service and 108 to the on-site genetics service. Baseline patient characteristics are shown in Table 1. Veterans who were seen by the on-site genetics service were older (median age 71 v 57 years) and were more likely to be male (92% v 58%), have a personal history of cancer as the indication for consult (94% v 42%), and be referred by the oncology service (93% v 34%) when compared with those referred to the telegenetics service. Among Veterans with a personal history of cancer, those seen by the on-site genetics service were more likely to have prostate cancer (55% v 33%) and were less likely to have a history of breast cancer (11% v 26%). Over 50% of the consults were for patients with self-identified non-Hispanic Black race/ethnicity (SIRE-Black).
In the overall descriptive cohort, Veterans who were seen by the on-site genetics had higher rates of genetic testing completion and lower rates of being lost to follow-up (Figs 1A-1D). This was true among all Veterans with any active cancer genetics related consult during the study period (Fig 1A), among those with a personal cancer history and an active consult during the study period (Fig 1B), and for both SIRE-White and SIRE-Black patients (Figs 1C and 1D).
Spectrum of Genetic Testing Results
Of the full descriptive cohort of 238 Veterans, a total of 117 underwent GT after consultation with genetics services, and one Veteran had sample failure. Fourteen Veterans tested positive for a pathogenic variant (PV; 12%; 95% CI, 6.7 to 19.3), and 27 Veterans were found to have a variant of uncertain significance (VUS; 23%; 95% CI, 15.8 to 31.8). The most prevalent PV was ATM (N = 4), followed by MSH2 (N = 3). The most prevalent VUS was MSH6 (N = 6). There was not enough evidence to conclude a difference in PV rate (18.4% v 7.8%; P = .091) or VUS rate (20.4% v 25.0%; P = .57) between SIRE non-Hispanic White Veterans and non-Hispanic Black Veterans.
Predictors of Genetic Testing Completion
To study the association between type of genetics service (telegenetics v on-site) with genetic testing completion, a multivariable logistic regression analysis was performed in a restricted cohort. Patients were excluded if they were reconsulted after a prior failed consult, consulted for a known PV, or were not of non-Hispanic White or non-Hispanic Black SIRE. One hundred twenty-one patients were included in final analysis (Fig 1E, Table 1). Patients who were seen by the on-site genetics consult service had higher age, were more likely to be male, were more likely to be referred because of a personal cancer history, and were more likely to be referred by an oncology service (medical or radiation oncology; Table 1).
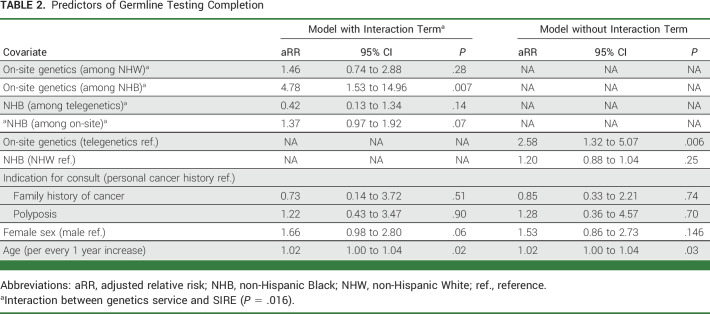
In an unadjusted analysis, patients who were seen by the on-site genetics service had 3.22-fold higher likelihood of completing GT compared with those who were seen by a centralized genetics service (unadjusted RR, 3.22; 95% CI, 1.89 to 5.48). After adjusting for covariates in a multivariable model (Table 2), there remained an association between the on-site genetics service and likelihood of GT (adjusted RR [aRR], 2.58; 95% CI, 1.32 to 5.07; P = .006). We assessed for an interaction between SIRE and genetics service on the outcome of GT completion, which was significant (P = .016). Being seen by the on-site genetics service was associated with 1.46-fold higher likelihood of genetic testing completion when compared with the centralized genetics service among non-Hispanic White Veterans (aRR, 1.46; 95% CI, 0.74 to 2.88; P = .276). For non-Hispanic Black Veterans, being seen by the on-site genetics service was associated with a 4.78-fold higher likelihood of GT completion when compared with Veterans who were seen by the centralized telegenetics service (aRR, 4.78; 95% CI, 1.53 to 14.96; P = .007).
TABLE 2.
Predictors of Germline Testing Completion
The results from our sensitivity analysis demonstrated that an unmeasured confounder—beyond the adjustments made for measured confounders included in the multivariable model—would have to have a strong and equal association (RR 9.03) with both the exposure and outcome to explain away our results in the non-Hispanic Black group (Appendix Fig A2, online only). Using the more conservative lower 95% confidence level of 1.53, the unmeasured confounder would have to have an equal association with both the exposure and outcome by risk ratios of 2.43 to explain away our findings in the non-Hispanic Black group.
DISCUSSION
In a single-center retrospective cohort study, we found that Veterans who were seen by an on-site nurse-led genetics service embedded in an oncology clinic had 3.2-fold higher likelihood of completing germline cancer genetic testing than Veterans who were seen by the centralized telegenetics service. After adjusting for potential confounders in a multivariable analysis, this association remained statistically significant in SIRE-Black Veterans only, who had 4.8-fold higher likelihood of completing cancer genetic GT if they were seen by the on-site genetics service (P-interaction race = .016). Prior evidence has demonstrated that racial disparities in genetics care persist in the telehealth setting within the VA.12 Importantly, our findings suggest that the presence of an on-site genetics service can potentially mitigate these disparities, while effectively increasing the proportion of completed GT for patients regardless of racial or ethnic background.
There are multiple possible explanations for differences in germline genetics completion on the basis of the type of genetics service used by Veterans. The embedded on-site genetics nurse has flexibility to see patients in the same physical space as their oncology follow-up or treatment visits. This facilitated better attendance at genetics appointments, as demonstrated by the decreased loss to follow-up with the on-site genetics nurse, even when restricted to oncology patients. Similarly, the availability of point-of-care testing in the on-site genetics program may also facilitate increased testing. In the on-site nurse-led genetics program, point-of-care testing is completed at the time of consultation, whereas in the telegenetics model, testing kits are mailed to the Veteran's mailing address, where the testing is to be completed later. This time delay allows for multiple intervening factors that could affect the completion of genetic testing. Thus, the difference in the timing of testing may represent a significant barrier, particularly for Veterans with unstable housing. Finally, another important explanation is that patients and Veterans want to hear about genetic testing from providers they trust,26 and this may be affected by the type of genetics service delivery. Although prospective randomized trials have demonstrated that outcomes related to patient satisfaction, distress, and decision conflict are similar between patients who receive telegenetics and in-person counseling, these studies were limited to those who consented for prospective trials and were amenable to random assignment to either usual care or telegenetics.27,28 In a real-world setting, telemedicine may affect the provider-patient interaction when discussing topics with the psychosocial complexity of genetic testing.
There are several limitations and potential sources of bias in this study. One potential source of bias is misclassification of outcome, as genetic testing completed outside of the VA would be classified as genetic testing noncompletion, which could bias toward or away from the null. Given that the majority of these patients were receiving either primary care or primary oncologic care within the VA, it is unlikely that non-VA genetic testing was prevalent among this population, and additionally, Veterans are less likely to complete genetic consultations outside of the VA.12 Since this is a retrospective analysis, there is the risk of unmeasured confounding, which is evidenced by the differences in patient populations between the two cohorts. This could bias toward or away from the null. Finally, our findings may not be generalizable to other health care models within the United States, as the VA does eliminate some barriers to GT, notably out-of-pocket cost, that might persist despite on-site genetics services.
Our study has several strengths. The cohort included in the study is a contemporary cohort during a relatively short study period, so there is less external influence from changing clinical guidelines or the COVID-19 pandemic. The use of self-identified race and ethnicity is another strength that greatly decreases the risk of misclassification of race in the study.
Genetics consults for patients with cancer or for patients at risk for hereditary cancer syndromes can be complex encounters, and for patients with active cancer, they occur during a stressful period of their care. Factors that should be considered and discussed with patients before testing include the benefits and limitations of testing, different types of test results, and the risk of psychological impact of test results.29 Although telegenetics has greatly expanded access to genetics evaluations, it is possible that a face-to-face interaction with a provider on site may be a better method for delivery of genetics consultations, given the inherent complexity in these encounters, particularly in the Veteran population. It is imperative to optimize these interactions and facilitate genetics services follow-up, as the ultimate results from testing—if indicated—have profound implications on matters that are important to patients: standard-of-care treatment and clinical trial options for patients as well as screening practices for family members.26 Therefore, while the findings in this study are not definitive and are only hypothesis-generating, they should be confirmed in a cluster randomized trial to increase applicability to the real world.
The indications for referral for cancer susceptibility genetic testing have increased exponentially in recent years because of advancement in understanding of the benefits cancer screening for at-risk individuals and the development of therapies for precision treatment of inherited cancers. As such, health care systems must adapt to increased cancer genetic testing needs without widening existing racial disparities in genetic testing. We have shown that an on-site nurse-led cancer genetics program in a racially diverse VA oncology clinic significantly increases genetic testing completion rates compared with telegenetics, especially among Veterans of self-identified Black race.
ACKNOWLEDGMENT
The authors are grateful to Ciana Anthony for critical reading of the manuscript during development and Douglas Ball MD for concept development.
APPENDIX
FIG A1.

Flowchart illustrating the workflow of an on-site, nurse-led genetics service. MD, medical doctor; NCCN, National Cancer Care Network.
FIG A2.

On-site, nurse-led genetics service process. Graph of the E-value sensitivity analysis, which illustrates how an unmeasured confounder would have to be strongly associated with the exposure (type of genetics service) and outcome (germline testing completion) among Black Veterans, given the observed adjusted RR to explain away the observed association (RR 4.78). The upper right portion of the graph represents all combinations of exposure-confounder relationships and confounder-outcome relationships that would be necessary for an unmeasured confounder to move the point estimate of the RR (4.78) or the lower 95% CI (1.53) to 1.0. The inflection points represent the points at which the unmeasured confounder is equally associated with both the exposure and outcome. RR, relative risk.
Jeffrey W. Shevach
Honoraria: MJH Life Sciences
Travel, Accommodations, Expenses: DAVA Oncology
Julie A. Lynch
Research Funding: Genomic Health (Inst), AstraZeneca (Inst), Myriad Genetics (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), CardioDx (Inst), Janssen (Inst)
Michael J. Kelley
Research Funding: Novartis (Inst), Bristol Myers Squibb (Inst), Regeneron (Inst), Genentech (Inst), EQRx, Mirati Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/827136
Maren T. Scheuner
Patents, Royalties, Other Intellectual Property: May 31, 2011, US 7,951,078 B2. Maren Theresa Scheuner, Method and apparatus for determining familial risk of disease, May 6, 2014, US 8,719,045. Paula W. Yoon, Maren T. Scheuner, Cynthia Jorgensen, Muin J. Khoury. Personal assessment including familial risk analysis for personalized disease prevention plan, October 2, 2001, US 6,297,014. Kent D Taylor, Maren T. Scheuner, Jerome I. Rotter, Huiying Yang. Genetic test to determine non-responsiveness to statin drug treatment (Inst)
Robert Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
Nevena Damjanov
Research Funding: Merck (Inst), Basilea (Inst)
Yu-Ning Wong
Employment: Janssen Scientific Affairs
Research Funding: AstraZeneca, Janssen Oncology (Inst)
Ravi B. Parikh
Stock and Other Ownership Interests: Merck, Google, GNS Healthcare, Onc.AI, Thyme Care
Consulting or Advisory Role: Thyme Care, Humana, NanOlogy, Merck
Research Funding: Humana
Patents, Royalties, Other Intellectual Property: Technology to integrate patient-reported outcomes into electronic health record algorithms
Travel, Accommodations, Expenses: The Oncology Institute of Hope and Innovation
Open Payments Link: https://openpaymentsdata.cms.gov/physician/701967
No other potential conflicts of interest were reported.
DISCLAIMER
This publication does not represent the views of the Department of Veterans Affairs or the US Government.
SUPPORT
Supported by the NCI (T32 CA009679, T32 HG009495 to J.W.S.; K08CA215312 to K.N.M.), Burroughs Wellcome Fund (#1017184 to K.N.M.), Basser Center for BRCA (J.W.S. and K.N.M.), Prostate Cancer Foundation (#20PILO01 and #20YOUN02 to K.N.M.), The Jonathan and Plum Simons Precision Oncology Center of Excellence (Y.-N.W., K.N.M., and K.R.), and the Department of Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI; VA HSR RES 13-457, J.A.L.).
J.W.S. and L.B.A. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey W. Shevach, Lisa B. Aiello, Julie A. Lynch, Jeffrey Petersen, Lori Hoffman-Hogg, Darshana Jhala, Ravi B. Parikh, Kara N. Maxwell
Financial support: Jeffrey W. Shevach, Ravi B. Parikh, Kara N. Maxwell
Administrative support: Julie A. Lynch, Ravi B. Parikh, Kara N. Maxwell
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Jeffrey W. Shevach, Lisa B. Aiello, Julie A. Lynch, Deborah Hartzfeld, Michael J. Kelley, Maren T. Scheuner, Robert Montgomery, Nevena Damjanov, Kyle Robinson, Yu-Ning Wong, Ravi B. Parikh, Kara N. Maxwell
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
On-Site Nurse-Led Cancer Genetics Program Increases Cancer Genetic Testing Completion in Black Veterans
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeffrey W. Shevach
Honoraria: MJH Life Sciences
Travel, Accommodations, Expenses: DAVA Oncology
Julie A. Lynch
Research Funding: Genomic Health (Inst), AstraZeneca (Inst), Myriad Genetics (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), CardioDx (Inst), Janssen (Inst)
Michael J. Kelley
Research Funding: Novartis (Inst), Bristol Myers Squibb (Inst), Regeneron (Inst), Genentech (Inst), EQRx, Mirati Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/827136
Maren T. Scheuner
Patents, Royalties, Other Intellectual Property: May 31, 2011, US 7,951,078 B2. Maren Theresa Scheuner, Method and apparatus for determining familial risk of disease, May 6, 2014, US 8,719,045. Paula W. Yoon, Maren T. Scheuner, Cynthia Jorgensen, Muin J. Khoury. Personal assessment including familial risk analysis for personalized disease prevention plan, October 2, 2001, US 6,297,014. Kent D Taylor, Maren T. Scheuner, Jerome I. Rotter, Huiying Yang. Genetic test to determine non-responsiveness to statin drug treatment (Inst)
Robert Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
Nevena Damjanov
Research Funding: Merck (Inst), Basilea (Inst)
Yu-Ning Wong
Employment: Janssen Scientific Affairs
Research Funding: AstraZeneca, Janssen Oncology (Inst)
Ravi B. Parikh
Stock and Other Ownership Interests: Merck, Google, GNS Healthcare, Onc.AI, Thyme Care
Consulting or Advisory Role: Thyme Care, Humana, NanOlogy, Merck
Research Funding: Humana
Patents, Royalties, Other Intellectual Property: Technology to integrate patient-reported outcomes into electronic health record algorithms
Travel, Accommodations, Expenses: The Oncology Institute of Hope and Innovation
Open Payments Link: https://openpaymentsdata.cms.gov/physician/701967
No other potential conflicts of interest were reported.
REFERENCES
- 1. Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 2. Weiss JM, Gupta S, Burke CA, et al. NCCN Guidelines® Insights: Genetic/familial high-risk assessment: Colorectal, version 1.2021. J Natl Compr Canc Netw. 2021;19:1122–1132. doi: 10.1164/jnccn.2021.0048. [DOI] [PubMed] [Google Scholar]
- 3. Paller CJ, Antonarakis ES, Beer TM, et al. Germline genetic testing in advanced prostate cancer; practices and barriers: Survey results from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. Clin Genitourinary Cancer. 2019;17:275–282.e1. doi: 10.1016/j.clgc.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeb S, Li R, Sanchez Nolasco T, et al. Barriers and facilitators of germline genetic evaluation for prostate cancer. The Prostate. 2021;81:754–764. doi: 10.1002/pros.24172. [DOI] [PubMed] [Google Scholar]
- 5. King EM, Smith EC. Diversification of nurse practitioner practice: Genetic cancer risk assessment. J Nurse Pract. 2020;16:447–452. [Google Scholar]
- 6. Laws A, Mulvey TM. Implementation of a high-risk breast clinic for comprehensive care of women with elevated breast cancer risk identified by risk assessment models in the community. JCO Oncol Pract. 2021;17:e217–e225. doi: 10.1200/OP.20.00256. [DOI] [PubMed] [Google Scholar]
- 7. Scheuner MT, Myrie K, Peredo J, et al. Integrating germline genetics into precision oncology practice in the Veterans Health Administration: Challenges and opportunities. Fed Pract. 2020;37(suppl 4):S82–s88. doi: 10.12788/fp.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schooley BL, Horan TA, Lee PW, et al. Rural veteran access to healthcare services: Investigating the role of information and communication technologies in overcoming spatial barriers. Perspect Health Inf Manag. 2010;7:1f. [PMC free article] [PubMed] [Google Scholar]
- 9. Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet. 2018;178:24–37. doi: 10.1002/ajmg.c.31602. [DOI] [PubMed] [Google Scholar]
- 10. Kassem N, Althouse SK, Monahan P, et al. Racial disparities in family variant testing for cancer predisposition genes. Cancer Epidemiol Biomarkers Prev. 2022;31:1511. [Google Scholar]
- 11. Dharwadkar P, Greenan G, Stoffel EM, et al. Racial and ethnic disparities in germline genetic testing of patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. 2022;20:353–361.e3. doi: 10.1016/j.cgh.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheuner MT, Huynh AK, Chanfreau-Coffinier C, et al. Demographic differences among US Department of Veterans Affairs patients referred for genetic consultation to a centralized VA Telehealth Program, VA Medical Centers, or the community. JAMA Netw Open. 2022;5:e226687. doi: 10.1001/jamanetworkopen.2022.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cacioppo CN, Egleston BL, Fetzer D, et al. Randomized study of remote telehealth genetic services versus usual care in oncology practices without genetic counselors. Cancer Med. 2021;10:4532–4541. doi: 10.1002/cam4.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalicki AV, Moody KA, Franzosa E, et al. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69:2404–2411. doi: 10.1111/jgs.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garvin LA, Hu J, Slightam C, et al. Use of video telehealth tablets to increase access for Veterans experiencing homelessness. J Gen Intern Med. 2021;36:2274–2282. doi: 10.1007/s11606-021-06900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Nurses Association (ANA) and International Society of Nurses in Genetics (ISONG): Genetics/Genomics Nursing: Scope and Standards of Practice. ed 2. Silver Spring, MD American Nurses Association; 2016. [Google Scholar]
- 17. Ranallo L, Nye LE, Williams M, et al. Point of care genetic testing in a breast cancer survivorship clinic. J Clin Oncol. 2021;39 suppl 15; abstr 10580. [Google Scholar]
- 18. Scott N, O'Sullivan J, Asgeirsson K, et al. Changing practice: Moving to a specialist nurse-led service for BRCA gene testing. Br J Nurs. 2020;29:S6–s13. doi: 10.12968/bjon.2020.29.10.S6. [DOI] [PubMed] [Google Scholar]
- 19. Senter L, Hatfield R. Nurse practitioners & genetic counselors: Collaborative roles in a complex system. Nurse Pract. 2016;41:43–49. doi: 10.1097/01.NPR.0000470355.00838.a2. [DOI] [PubMed] [Google Scholar]
- 20. Thompson CA, Tiedt J, Beqiri M, et al. A retrospective evaluation of a nurse practitioner-led cancer genetics program. J Nurse Pract. 2022;18:276–284. [Google Scholar]
- 21. Yoes M-V, Thomas L. Hereditary cancer genetic risk assessment, testing, and counseling: A nurse practitioner–led program in a community setting. J Nurse Pract. 2020;16:660–665. [Google Scholar]
- 22. Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9:175–196. [Google Scholar]
- 23. Linden A, Mathur MB, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: The evalue package. Stata J. 2020;20:162–175. [Google Scholar]
- 24.Linden A, Mathur MB, VanderWeele TJ. evalue: Stata Module for Conducting Sensitivity Analyses for Unmeasured Confounding in Observational Studies. Statistical Software Components S458592. 2019. Department of Economics, Boston College. http://ideas.repec.org/c/boc/bocode/s458592.html. [Google Scholar]
- 25. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 26. Greenberg S, Slager S, Neil BO, et al. What men want: Qualitative analysis of what men with prostate cancer (PCa) want to learn regarding genetic referral, counseling, and testing. Prostate. 2020;80:441–450. doi: 10.1002/pros.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buchanan AH, Datta SK, Skinner CS, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: Cost, patient satisfaction and attendance. J Genet Couns. 2015;24:961–970. doi: 10.1007/s10897-015-9836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russo J, Giri VN. Germline testing and genetic counselling in prostate cancer. Nat Rev Urol. 2022;19:331–343. doi: 10.1038/s41585-022-00580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]