Abstract
Retroviruses contain two plus-strand genomic RNAs, which are stably but noncovalently joined in their 5′ regions by a dimer linkage structure (DLS). Two models have been put forward to explain the mechanisms by which the RNAs dimerize; each model emphasizes the role of specific molecular determinants. The kissing-loop model implicates interactions between palindromic sequences in the DLS region. The second model proposes that purine-rich stretches in the region form purine quartet structures. Here, we present an examination of the in vitro dimerization of Moloney murine sarcoma virus (MuSV) RNA in the context of these two models. Dimers were found to form spontaneously in a temperature-, time-, concentration-, and salt-dependent manner. In contrast to earlier reports, we found that deletion of neither the palindrome nor the consensus purine motifs (PuGGAPuA) affected the level of dimer formation at low concentrations of RNA. Rather, different purine-rich sequences, i.e., consecutive stretches of guanines, were found to enhance both in vitro RNA dimerization and in vivo viral replication. Biochemical evidence further suggests that these guanine-rich (G-rich) stretches form guanine quartet structures. We also found that the palindromic sequences could support dimerization at significantly higher RNA concentrations. In addition, the G-rich stretches were as important as the palindromic sequence for maintaining efficient viral replication. Overall, our data support a model that entails contributions from both of the previously proposed mechanisms of retroviral RNA dimerization.
A unique feature of retroviruses is that they encapsidate a dimer of identical plus-strand genomic RNAs. Electron microscopy and sedimentation studies indicate that the two RNAs are held together noncovalently near their 5′ ends in a union referred to as the dimer linkage structure (DLS) (3, 21, 27, 35). This region of the genome also encodes major regulatory features important to the viral life cycle, including the primer binding site, the major splice donor, the gag start codon, and the cis-acting encapsidation (ψ) signal. Thus, the DLS directly or indirectly plays roles in reverse transcription of viral genomic RNA, viral RNA splicing specificity, translational control, and determination of the specificity of viral RNA encapsidation (40).
Although it has been recognized for some time that the DLS resides near the 5′ end of the genome, the mechanism of dimerization remains obscure. Currently, two models for dimerization have been put forward. The kissing-loop model for DLS formation proposes that palindromic sequences (Pal) in a hairpin loop interact through Watson-Crick base pairing. An RNA containing the human immunodeficiency virus type 1 (HIV-1) DLS was shown to dimerize in vitro through interactions of a 6-base Pal (GUGCAC) (23, 34, 38, 41, 46). Furthermore, studies with synthetic RNAs and antisense oligonucleotides implicated a 16-base, self-complementary sequence of the Moloney murine leukemia virus (MuLV) as part of a putative DLS region (16, 17, 42, 43, 49). However, mutations engineered to ablate Pal in MuLV did not affect the stability of genomic RNAs isolated from virions (4, 7, 18, 24, 45). Furthermore, the kissing-loop model cannot explain some unusual features of the retroviral RNA dimers, e.g., their resistance to denaturing conditions and the inability of antisense DLS RNA to form dimers (29, 34). Finally, the formation of heterodimers between the DLS sequences of HIV-1 and other retroviruses that lack homology between their Pal sequences suggests a role for other determinants of dimerization (29).
For the second model of dimerization, phylogenetic analyses of putative retroviral DLSs revealed a unique purine-rich motif (PuGGAPuA) that is conserved among retroviruses (29). It has been proposed that these consensus purine motifs may form purine-base tetrads, in part because they are stabilized by monovalent cations such as those observed in human telomeric DNA (53). A similar kind of structure that involves consecutive guanine stretches has also been proposed (1, 48). Structural probing and biochemical studies support the contention that the HIV-1 DLS is maintained by these guanine-rich (G-rich) sequences (1, 2, 29, 48). In addition, in vitro and in vivo studies of rat retrotransposon VL30 and Rous sarcoma virus DLS RNAs provide evidence that G-rich sequences can function in dimer pairing (12, 25, 50, 51).
Here, in an attempt to synthesize these apparently disparate findings, we characterize each of the signals that constitute the two different models of retroviral dimerization. Our results suggest that the G-rich and Pal sequences both play roles in dimerization while the consensus purine motifs do not. Although Pal was sufficient to allow dimerization of Moloney murine sarcoma virus (MuSV) RNA at high concentrations, it was dispensable at lower concentrations. By contrast, the G-rich regions were critical for establishing dimers at low concentrations of RNA. Overall, these observations support a novel model in which the initial steps of MuSV RNA dimerization are mediated by the G-rich sequences and in which this initial interaction then raises the local RNA concentration to a point at which interactions might occur between the Pal sequences. Finally, we present data, obtained with a viral vector system, that show that both the palindromic and the G-rich sequences play an important role in maintaining efficient viral replication.
MATERIALS AND METHODS
Plasmid constructions.
The pLNBS plasmid contains the MuSV DLS and a neomycin resistance gene. pLNBS is a modified version of the pLNL6-based pLN plasmid (32). The modification involved subcloning the retroviral sequences of pLN into a pBluescript II KS(+) vector to permit a higher vector yield during propagation in Escherichia coli (36). MuSV is a replication-defective virus that was derived from MuLV and in which most of the pol and env open reading frames have been replaced by that of the c-mos gene. The MuSV DLS sequence possesses greater than 95% homology to that of the MuLV (44). The nucleotides are numbered according to the MuSV sequence recently submitted to GenBank (accession no. AF033813) (Fig. 1A and B).
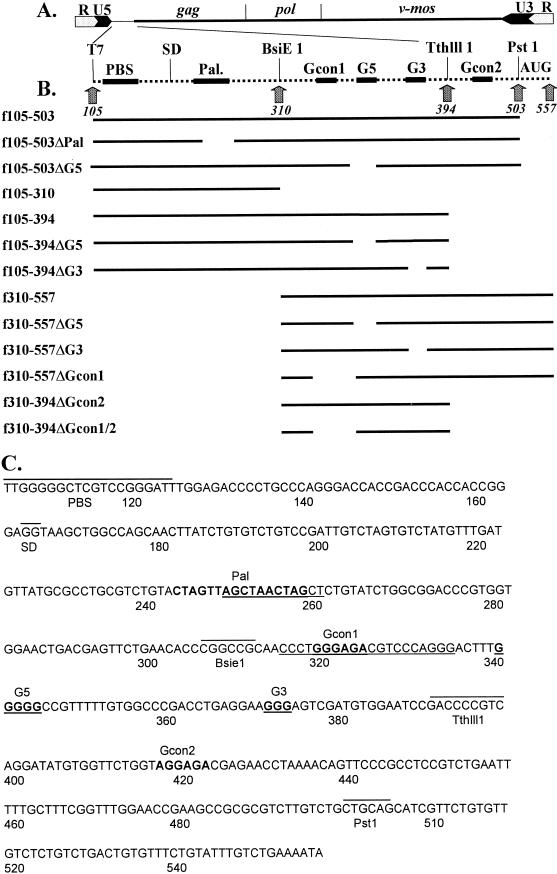
FIG. 1.
MuSV RNA sequences involved in dimerization. (A) Representation of the MuSV genome. Numbering is with respect to the viral mRNA, which begins at the transcriptional initiation site and ends at the polyadenylation site (i.e., 5′R to 3′R) (GenBank accession no. AF033813). Viral long terminal repeats, gag, pol, and v-mos genes, and restriction endonuclease cleavage sites are shown. (B) Diagram of the RNAs used in this study. Gcon1, first consensus purine motif; Gcon2, second consensus purine motif; G5 and G3, consecutive guanines; PBS, primer binding site; SD, splice donor site; AUG, gag initiation site. (C) Sequence of the DLS region of MuSV. The locations of restriction sites of interest, the primer binding site (PBS), and the splice donor site (SD) are indicated by lines above the sequence. Pal, the consecutive guanines (G5 and G3), and the consensus purine motifs (PuGGAPuA) are shown in boldface and are noted above the sequences. The deletions of the Pal, G-rich, and first consensus purine motif sequences are underlined.
By using PCR, DNA fragments were synthesized with the MuSV DLS region of pLNBS as the template, a forward primer identical to the primer binding site at nucleotide (nt) 105 and carrying a 5′ EcoRI site (5′GAATTCTGGGGGCTCGTCCGGGAT3′), and a reverse primer beginning at position 557 (5′TATTTTCAGACAAATACAGAAAC3′). The PCR products were then digested with EcoRI and PstI (position 503) and cloned into the vector pGEM3Zf(+) (Promega) behind its T7 promoter, which was used in transcribing the inserted sequences. In a similar fashion, several deletion mutations were constructed with primers that incorporated the desired deletions (Fig. 1C) (20). The sequences of all the constructions were confirmed by sequencing by the Sanger dideoxy termination method with Sequenase 2.0 (USB).
Preparation of RNA samples.
Prior to transcription, template DNAs (∼2.0 μg) were linearized with various restriction enzymes (PstI, BsiEI, and TthlllI) that subsequently define the 3′ ends of the products. The linearized DNAs were phenol-chloroform extracted and ethanol precipitated, and the pellets were resuspended in 2.0 μl of water. Transcription mixtures (20 μl) contained 1.0 μg of DNA template, 0.5 mM (each) unlabeled ATP, GTP, and UTP, 50 μM unlabeled CTP, and 2.5 μM [α-32P]CTP. The reaction was carried out with a T7 MAXIscript kit as suggested by the manufacturer (Ambion). All RNA transcripts starting at position 310 were the result of transcription of PCR products of pLNBS with the forward primer carrying the T7 promoter sequence and the reverse primer as above.
RNA transcripts were separated by electrophoresis on 5% polyacrylamide–8 M urea gels at 250 V for 2 h in 1× TBE buffer (0.09 M Tris-borate, 0.002 M EDTA). RNA samples were eluted from the gels with elution buffer as suggested by the manufacturer (Ambion). The purified RNAs were then ethanol precipitated, resuspended in 100 μl of diethylpyrocarbonate-treated water, and stored at −70°C. The products were quantitated by counting in a Beckman LS6500 scintillation counter.
RNA dimerization assay.
For the dimerization assay, 8-μl portions of ∼1.5 nM [α-32P]RNA were heated at 95°C for 3 min and chilled on ice for 3 min, and 2 μl of D5X buffer (500 mM NaCl, 250 mM Tris-HCl [pH 7]) was added. The samples were then incubated at the temperatures indicated in the text for 1 h. At the end of the incubation period, the samples were chilled and separated by electrophoresis on 5% nondenaturing polyacrylamide gels in the presence of 1× TBE buffer. The gels were dried and exposed directly on a PhosphorImager screen for 18 to 20 h. The amount of dimer formation was quantitated with a 445SI PhosphorImager system and ImageQuaNT image analysis software. The fraction of RNA in dimeric form was estimated by taking the ratio of the amount of dimeric RNA to the amount of the sum of both the monomeric and dimeric species. Concentration-dependent, time-dependent, and salt-dependent measurements were made in a similar manner, varying the appropriate parameters. In the salt-dependent dimerization assays of f587–985, the reaction mixtures were incubated for 30 min rather than 1 h. The time-dependent dimerization comparisons of f105–503, f105–503ΔPal, and f105–503ΔG5 were done in a manner similar to the standard dimerization reaction, except that f105–503ΔPal RNA was incubated at 50°C whereas the other RNAs were incubated at 60°C.
Cation-dependent thermal dissociation.
The RNA sample f792–1040 (52.5 nM) was incubated for 3 h at 60°C in dimerization buffer containing a final concentration of 100 mM NaCl and 50 mM Tris-HCl (pH 7). At the end of the incubation, the RNA was precipitated by addition of 2 volumes of ethanol and 0.1 volume of 3 M sodium acetate (pH 5.2), and after centrifugation, the pellet was washed twice with 70% ethanol. After being dried, the RNA was resuspended in water. Equal amounts of RNA (9.0 nM) were then diluted into buffer to give final concentrations of 100 mM each monovalent salt (LiCl, NaCl, KCl, RbCl, and CsCl) in 50 mM Tris-HCl (pH 7). The samples were heated at temperatures ranging from 40 to 95°C, and aliquots were taken out at 10-min intervals and chilled on ice until needed for electrophoresis. For an untreated control, an equivalent sample of the RNA was taken prior to temperature treatment. All samples were separated on 5% nondenaturing polyacrylamide gels as described above.
Cell culture, transfections, and infections.
GP+E86 murine ecotropic packaging cells (28) and NIH 3T3 murine fibroblast cells (ATCC CRL 1658) were maintained in Dulbecco’s modified Eagle’s medium containing high glucose (4.5 g/liter) and either 10% newborn calf serum or 10% calf serum. Then 20 μg of wild-type or mutant pLNBS DNAs were transfected into 5.0 × 105 GP+E86 cells in a 60-mm polystyrene dish by calcium phosphate precipitation with a transfection kit (Gibco-BRL). The efficiency of transfection was monitored by a colorimetric assay of secretory alkaline phosphatase gene expression from the pCMV-SEAP (Tropix) vector, which was cotransfected (0.25 μg) with the pLNBS vector. Virus particles in the transfection supernatant collected 48 h posttransfection were normalized to the level of the alkaline phosphatase gene expression and used to infect 2.5 × 105 NIH 3T3 cells in the presence of 8 μg of Polybrene per ml. Viral titers were determined on these cells by measuring G418-resistant colony formation as described previously (37). The deletions of the palindromic and the G-rich stretches are outlined in Fig. 1C. ΔPsi was made by deleting part of the ψ sequence from the SpeI site (position 243) to the EcoRI site (41 nt upstream of the neo AUG site).
RESULTS
MuSV DLS dimerizes in a temperature-, concentration-, and salt-dependent manner.
Initial experiments were designed to evaluate the effects of reaction conditions on spontaneous dimerization of the DLS region of MuSV RNA (Fig. 1). Dimerization of a 398-nt fragment containing the Pal as well as purine-rich sequences (f105–503) was optimal at 60°C and was not supported by temperatures lower than 40°C or higher than 70°C (Fig. 2). In addition, we found that f105–503 dimer formation was RNA concentration, time, and salt dependent (data not shown).
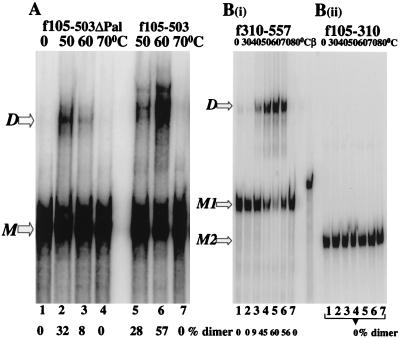
FIG. 2.
Pal is dispensable in dimer formation but is required to stabilize the dimeric structure. (A) An RNA fragment lacking 10 of the 16 nt of Pal (f105–503ΔPal) dimerizes less efficiently than the full-length fragment (f105–503). (B) The RNA fragment containing Pal alone (f105–310) does not form dimers [B(ii)], whereas [B(i)] one lacking Pal but carrying the downstream sequence (f310–557) forms dimers efficiently [B(i)]. M, monomeric RNA; D, dimeric RNA; M1, monomeric f310–557 RNA; M2, monomeric f105–310 RNA; β, an RNA marker of 330 nt was transcribed from the β-globin gene (Ambion).
Neither the Pal sequences nor the consensus purine motifs (PuGGAPuA) are required for dimerization.
RNAs lacking part or all of the self-complementary sequence were tested to determine whether the Pal sequence is required for dimer pairing (Fig. 2). The f105–503ΔPal fragment (Fig. 1A and C), which carries a deletion of 10 of the 16 nt of Pal, was still able to dimerize (Fig. 2A). The optimal temperature for dimer formation of this fragment was 50°C, 10°C lower than that of the full-length f105–503 fragment (compare lanes 2 and 6). Also, at optimal temperatures, f105–503ΔPal dimerized only 50% as efficiently as the full-length molecule did. These observations imply that the palindromic sequence may help stabilize dimeric RNA.
To examine whether the palindrome itself was sufficient for dimer formation, an RNA fragment (f105–310) spanning the Pal sequence was tested under conditions identical to those used for the full-length construct. Figure 2B(ii) shows that Pal alone was not sufficient for dimerization. In contrast, a downstream fragment (f310–557), lacking Pal altogether, dimerized in a manner similar to that seen for the full-length fragment [Fig. 2B(i)]. This region carries multiple purine-rich sequences (Fig. 1C).
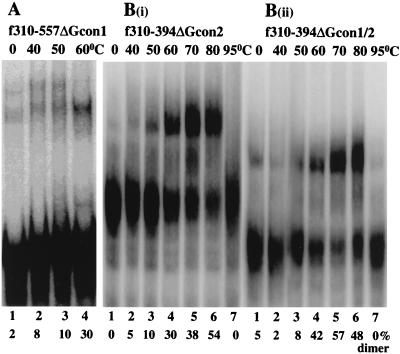
A close examination of the f310–557 sequence reveals two stretches of the consensus purine motif (GGGAGA [Gcon1] at positions 319 to 324 and AGGAGA [Gcon2] at positions 417 to 422) (Fig. 1C). Deletion of either one or both Gcon motifs did not inhibit dimer formation (Fig. 3), suggesting that these phylogenetically conserved sequences do not function in dimerization.
FIG. 3.
The consensus purine motifs (Gcon) are dispensable in MuSV dimerization. Temperature-dependent dimerization of RNA fragments carrying deletions of either one [(A) and B(i)] or both [B(ii)] motifs was carried out as described in Materials and Methods, except that f310–557ΔGcon1 was incubated for 30 min rather than 60 min.
At different concentrations of RNA, both guanine-rich and Pal sequences promote dimerization.
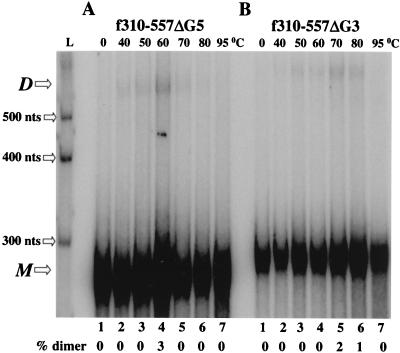
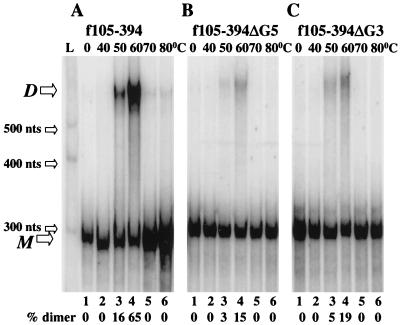
The f310–557 sequence also carries stretches of consecutive guanines at positions 340 to 344 (G5) and 371 to 373 (G3) (Fig. 1C). Since similar G-rich sequences are involved in initiating dimerization of HIV-1 DLS RNA (2, 48), the influence of these G-rich sequences was tested with MuSV RNA. As shown in Fig. 4, the deletion of either of these sequences virtually eliminated dimer formation. By contrast, extending the RNA fragment carrying only Pal (f105–310), which by itself did not dimerize [Fig. 2B(ii)], to include these two G-rich sequences restored the ability of the fragment to dimerize (f105–394) (Fig. 5A). No obvious structural rearrangement was predicted for f105–310 compared to f105–394 as calculated with MFold, a program to predict RNA secondary structures based on free-energy minimization (data not shown) (52, 55). Moreover, when G5 or G3 was individually deleted from f105–394, dimerization was severely diminished (Fig. 5B and C). The percentage of dimeric RNA at optimal temperature was reduced from 65% to as low as 15%. These data suggest that the G-rich stretches play an important role in dimer formation.
FIG. 4.
The consecutive guanine stretches (G-rich) are essential in MuSV dimerization. Dimerization of an RNA fragments carrying a deletion of the five consecutive guanines (f310–557ΔG5) (A) and the three consecutive guanines (f310–557ΔG3) (B) is severely inhibited. L, the RNA marker of 300, 400, and 500 nt was transcribed from the Century-Plus DNA templates (Ambion); M, monomeric RNA; D, dimeric RNA.
FIG. 5.
The G-rich sequences downstream from Pal rescue the dimerization defect of f105–310, and the deletions of the G-rich sequences greatly diminish dimer formation. (A) An extended molecule (f105–394) including Pal and downstream purine-rich sequences dimerizes efficiently. (B and C) Dimers of f310–557ΔG5 (B) and of f310–557ΔG3 (C) which lack the G-rich sequences are greatly reduced. All samples were assayed under the same conditions and separated on the same gel. L, the RNA marker of 300, 400, and 500 nt was transcribed from Century-Plus DNA templates (Ambion); M, monomeric RNA; D, dimeric RNA.
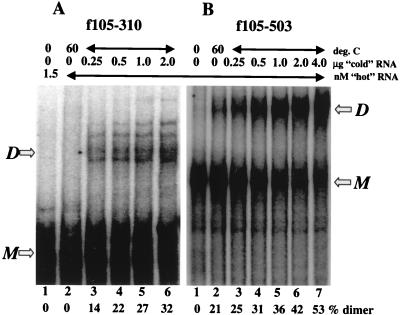
The observation that Pal is dispensable for RNA dimerization is at odds with results obtained by others (9, 10, 16, 23, 41, 49). Since we used an assay system with 103-fold less viral RNA than was used previously, we decided to examine the effects of RNA concentration on the role played by the different determinants. In these experiments, increasing amounts (0.25 to 2.0 μg, equivalent to 0.2 to 1.5 μM) of the identical but unlabeled RNA were added to the standard assay mixture containing a fixed amount of [α-32P]RNA (1.5 nM). Figure 6A shows that although the fragment carrying Pal alone, f105–310, was not able to form dimers at 1.5 nM (lane 2), it did dimerize at higher concentrations. A similar experiment was carried out with the full-length f105–503 fragment (Fig. 6B). The result mirrors the concentration dependence of the shorter fragment, except that a small amount of dimer was observed even at the lowest RNA concentration (Fig. 6B, lane 2). These data emphasize that dimerization of Pal is concentration dependent across the range of concentrations tested. Also, the observation that f105–310 RNA at 1.5 μM dimerized 10% less efficiently than did the full-length f105–503 suggests that the dimeric structure of this short fragment of RNA is less stable than that of the full-length molecule (Fig. 6A and B, lanes 6).
FIG. 6.
f105–310 dimerizes in a concentration-dependent manner. [α-32P]RNA (1.5 nM) was incubated with increasing amounts of unlabeled RNA at 60°C for 1 h before being separated on a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. (A) f105–310 fragment. For this experiment, a 0.25-μg addition in a 10-μl reaction mixture represents 3.8 × 104 nM RNA. (B) f105–503 fragment. All samples were assayed under the same conditions and separated on the same gel. M, monomeric RNA; D, dimeric RNA.
Dimers of the guanine-rich sequences are preferentially stabilized by monovalent cations with the smallest radius.
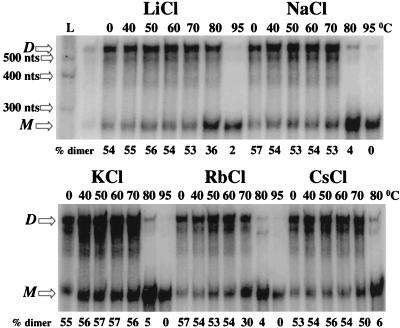
DNA or RNA containing runs of consecutive guanines may form four-stranded structures (G tetrads) by the formation of Hoogsteen base pairs. These structures are markedly stabilized by specific monovalent cations, apparently by reducing the electrostatic repulsion of the bases across the central tube of the molecules (6, 15, 19, 22, 54). Depending on their primary sequence, dimers containing quartet structures dissociate at various temperatures and also are stabilized to various degrees in different monovalent cations. These considerations prompted us to define the melting temperature of the G-rich f310–557 RNA in the presence of different monovalent cations. This RNA shows a preference for the cation with the smallest radius (Li+) (Fig. 7). The melting temperature of f310–557 in the presence of Li+ was higher than 80°C, about 10°C higher than observed in the presence of cations with larger radii. This preference for a small cation suggests that DLS RNA forms an ordered structure which contains G tetrads.
FIG. 7.
The f310–557 dimeric structure is preferentially stabilized by lithium cations. Samples of f310–557 RNA were allowed to dimerize, and the dimers were precipitated, washed with ethanol, and resuspended in water as described in Materials and Methods. Aliquots were then incubated at 40 to 95°C in the presence of the indicated salts. M, monomeric RNA; D, dimeric RNA; L, the RNA marker of 300, 400, and 500 nt was transcribed from the Century-Plus DNA templates (Ambion).
Viral replication is reduced by mutations in the Pal and G-rich sequences.
Finally, we examined the relative roles played by the Pal and G-rich elements of MuSV DLS in the context of a single round of virus replication. To do this, sequences containing various mutations were placed into the 5′ leader region of pLNBS, an MuLV-MuSV-derived retroviral vector containing a neo gene. The effect of the mutations on vector production following transfection into the GP+E86 retroviral packaging cell line was determined. The results obtained from three independent experiments for each of the mutants indicate the replication ability of each of the viral constructs (Fig. 1C) relative to the wild type (Table 1). While deletion of either the Pal sequence (ΔPal) or the G-rich stretches (ΔG5 or ΔG3) alone slightly reduced the titer of the virus, deletion of both signals (ΔPal/ΔG5 or ΔPal/ΔG3) produced up to a sevenfold-reduction in titer. Also, there was an additive effect of viral reduction when both signals were deleted. These data demonstrate that both the Pal and G-rich sequences play important roles in the MuSV viral life cycle.
TABLE 1.
Relative titers of mutant vectors
| Vectors | Titera (CFU/ml) | Relative titerb | Relative fold reduction |
|---|---|---|---|
| Wild type | 3.59 × 105 | 1.000 | |
| ΔPal | 1.38 × 105 | 0.479 ± 0.420 | 1.40–2.90 |
| ΔG5 | 9.10 × 104 | 0.441 ± 0.434 | 1.50–3.50 |
| ΔG3 | 1.44 × 105 | 0.468 ± 0.096 | 1.30–2.30 |
| ΔPal/ΔG5 | 6.60 × 104 | 0.198 ± 0.104 | 4.70–6.80 |
| ΔPal/ΔG3 | 8.10 × 104 | 0.266 ± 0.256 | 2.80–7.20 |
| ΔPsi | 1.60 × 103 | 0.005 ± 0.005 | 210–266 |
Titers of the vectors from one representative experiment.
Titers normalized to the wild type. Data were taken from three independent assays. Relative titers are given as mean ± standard deviation.
DISCUSSION
Several lines of observations have been made that support a two-stage model of MuSV-RNA dimerization. First, G-rich sequences, and not the phylogenetically conserved purine-rich motifs, drive the initial dimer formation, perhaps via the formation of an ordered quartet structure. Second, kissing-loop interactions are highly concentration dependent and their formation stabilizes RNA dimers. Finally, our data suggest that the G-rich stretches are as important as the Pal sequence in viral replication.
Girard et al. demonstrated that the rate constant for MuLV RNA dimerization is between 2 × 102 and 7 × 103 M−1 s−1, significantly lower than that expected for annealing of complementary oligonucleotides (16). This observation suggests that the Watson-Crick base pairing implicit in the kissing-loop model may not be the only factor responsible for dimer formation. Recently, Tapia et al. indicated that although Pal sequences are important in promoting dimerization of MuLV RNA, this interaction occurs slowly and is kinetically enhanced by downstream sequences (49). It is worth noting that the consecutive guanine stretches are located downstream of the Pal sequence and may serve to enhance dimer formation.
The enhanced stability of the MuSV dimers in the presence of Li+ supports a role for the guanines in quartet-structure formation (Fig. 7). Also, Torrent et al. found that the VL30 dimeric RNA was stabilized in the presence of LiCl (50), and similar results were obtained with RSV RNA (25). In contrast, the proposed HIV-1 quartet structure is stabilized by KCl and is thought to involve the phylogenetically conserved purine-rich motifs (PuGGAPuA) (1, 48). These differences in the stabilizing cation, Li+ versus K+, may reflect structural differences: deletion of the MuSV consensus purine-rich motifs did not affect dimerization (Fig. 3). Similarly, the deletion of all five consensus purine motifs in the HIV-2 DLS did not alter the level of dimerization (5).
An important finding of this study is the demonstration that the influence of the Pal and the G-rich regions on dimerization varies with concentration. While RNA fragments carrying the G-rich regions effectively dimerized at low, possibly more physiologically relevant, concentrations, RNA fragments carrying the Pal sequence alone paired at only very high concentrations [Fig. 2B(ii) and 6A]. The observation of MuSV Pal sequence dimerization at high concentrations corroborates the features of MuLV Pal sequence dimerization observed by others (42, 43). In addition, it appears that the kissing loop serves as an important factor in determining dimer stability (Fig. 2A). Our findings are also consistent with the observation that uncharacterized sequences downstream from the kissing loop in MuLV play a role in dimer stability (49).
Viral replication assays with a vector system carrying the MuSV ψ region show that removal of either the Pal or the G-rich sequence affects viral replication. Of note, deletions of both signals (Pal and G-rich sequences) reduced viral production to a much greater extent than did deletion of either sequence alone (Table 1). These data corroborate recent observations indicating a dispensable but nonetheless significant role of the Pal sequence in several other viral systems. It was previously noted that Pal mutations affect steps other than dimerization in the viral life cycle (40). For instance, mutations in the palindrome of HIV-1 decreased viral infectivity (8, 24, 26, 39) and slowed viral replication (4, 18, 39). Most characteristically, the palindromic mutations greatly affected RNA encapsidation (4, 7, 8, 24, 30, 31, 39) and the synthesis of proviral DNA (39). However, no effect of these mutations on the synthesis of viral proteins (4) or the genomic RNA dimers isolated from the virions (7, 24, 45) was observed. Similarly, Mougel et al. found that a deletion spanning the Pal sequence in an MuLV vector moderately reduced viral RNA encapsidation and reverse transcription of the genomic RNA (33). Recently, Doria-Rose and Vogt observed that the Pal sequence is preferred but not required to maintain stable viral replication in RSV (11).
Overall, our data support a role for both the previously identified Pal region as well as two stretches of downstream guanines in retroviral dimerization. Mutational analysis points to the G-rich sequences as important determinants of dimer formation. The results presented here further indicate that the Pal sequences also act in the dimerization process, most probably by stabilizing the structure (Fig. 2A). It appears that the roles played by these determinants differ at different concentrations of RNA. At low concentrations of RNA, the G-rich regions appeared to be necessary for dimerization and the Pal sequence dispensable; fragments containing only the Pal regions could support dimerization at higher concentrations of RNA (Fig. 6A). These data suggest a two-stage model for MuSV dimerization in which the initial, low-concentration interaction between RNA molecules is mediated by the G-rich regions. This interaction may act to increase the local concentration of RNA, thereby facilitating base pairing of the Pal sequences to stabilize the dimer linkage structure. Similar strategies may be used by other viral systems to induce stable dimer formation (13, 14, 47).
Our viral infectivity data provide, for the first time, direct evidence that in addition to Pal, the guanine stretches play important functional roles in dimerization and/or other steps of the viral life cycle. Although we have identified the importance of both molecular determinants in efficient MuSV replication, the mechanisms through which the mutations exert their effect remain unknown. We are currently examining in more detail the precise biological and molecular roles of these elements.
ACKNOWLEDGMENTS
This work was supported by NIH grant K11 AI01107 to A.H.K. and a seed grant from the UCLA AIDS Institute to D.P.N. H.L. was supported in part by the UCLA Presidential Research Fellowship, the College Honors Naumburg Research Scholarship, and the AAP Drown Foundation Research Scholarship.
We thank John Sechelski, Nan N. Lee, Amy T. A. Tran, Liza Kim, Yanli Yang, and James Matsunaga for their help. We also thank Steve Pettit for his critical reading of the manuscript.
REFERENCES
- 1.Awang G, Sen D. Mode of dimerization of HIV-1 genomic RNA. Biochemistry. 1993;32:11453–11457. doi: 10.1021/bi00093a024. [DOI] [PubMed] [Google Scholar]
- 2.Baudin F, Marquet R, Isel C, Darlix J-L, Ehresmann B, Ehresmann C. Functional sites in the 5′ region of human immunodeficiency virus type 1 RNA from defined structural domains. J Mol Biol. 1993;229:382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- 3.Bender W, Chien Y-H, Chattopadhyay S, Vogt P K, Gardner M B, Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978;25:888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhout B, van Wamel J L. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J Virol. 1996;70:6723–6732. doi: 10.1128/jvi.70.10.6723-6732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkhout B, Oude Essink B B, Schneveld I. In vitro dimerization of HIV-2 leader RNA in the absence of PuGGAPuA motifs. FASEB J. 1993;7:181–187. doi: 10.1096/fasebj.7.1.8422965. [DOI] [PubMed] [Google Scholar]
- 6.Cheong C, Moore P B. Solution structure of an unusually stable RNA tetraplex containing G- and U-quartet structures. Biochemistry. 1992;31:8406–8414. doi: 10.1021/bi00151a003. [DOI] [PubMed] [Google Scholar]
- 7.Clever J L, Wong M L, Parslow T G. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J Virol. 1996;70:5902–5908. doi: 10.1128/jvi.70.9.5902-5908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clever J L, Parslow T G. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J Virol. 1997;71:3407–3414. doi: 10.1128/jvi.71.5.3407-3414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darlix J-L. Cis elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990;64:774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlix J-L, Gabus C, Nugeyre M-T, Clavel F, Barre-Sinoussi F. cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- 11.Doria-Rose N, Vogt V M. In vivo selection of Rous sarcoma virus mutants with randomized sequences in the packaging signal. J Virol. 1998;72:8073–8082. doi: 10.1128/jvi.72.10.8073-8082.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y X, Fu W, Winter A J, Levin J G, Rein A. Multiple regions of Harvey sarcoma virus RNA can dimerize in vitro. J Virol. 1995;69:2486–2490. doi: 10.1128/jvi.69.4.2486-2490.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu W, Rein A. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J Virol. 1993;67:5443–5449. doi: 10.1128/jvi.67.9.5443-5449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu W, Gorelick R J, Rein A. Characterization of human immunodeficiency virus type 1 dimeric RNA from wild-type and protease-defective virions. J Virol. 1994;68:5013–5018. doi: 10.1128/jvi.68.8.5013-5018.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gellert M, Lipsett M N, Davies D R. Helix formation by guanylic acid. Proc Natl Acad Sci USA. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard P M, Bonnet-Mathoniere B, Muriaux D, Paoletti J. A short autocomplementary sequence in the 5′ leader region is responsible for dimerization of MoMuLV genomic RNA. Biochemistry. 1995;34:9785–9794. doi: 10.1021/bi00030a016. [DOI] [PubMed] [Google Scholar]
- 17.Girard P M, de Rocquigny H, Roques B P, Paoletti J. A model of psi dimerization: destabilization of the C278-G303 stem-loop by the nucleocapsid protein (NCp10) of MoMuLV. Biochemistry. 1996;35:8705–8714. doi: 10.1021/bi952454s. [DOI] [PubMed] [Google Scholar]
- 18.Haddrick M, Lear A L, Cann A J, Heaphy S. Evidence that a kissing loop structure facilitates genomic RNA dimerization in HIV-1. J Mol Biol. 1996;259:58–68. doi: 10.1006/jmbi.1996.0301. [DOI] [PubMed] [Google Scholar]
- 19.Hud N V, Smith F W, Anet F A, Feigon J. The selectivity for K+ versus Na+ in DNA quadruplexes is dominated by relative free energies of hydration: a thermodynamic analysis by 1H NMR. Biochemistry. 1996;35:15383–15390. doi: 10.1021/bi9620565. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel T A, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 21.Kung H J, Hu S, Bender W, Bailey J M, Davidson N, Nicolson M O, McAllister R M. RD-114, baboon, and wolly monkey viral RNAs compared in size and structure. Cell. 1976;7:609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- 22.Laughlan G, Murchie A I, Norman D G, Moore M H, Moody P C, Lilley D M, Luisi B. The high-resolution crystal structure of a parallel-stranded guanine tetraplex. Science. 1994;265:520–524. doi: 10.1126/science.8036494. [DOI] [PubMed] [Google Scholar]
- 23.Laughrea M, Jette L. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry. 1994;33:13464–13474. doi: 10.1021/bi00249a035. [DOI] [PubMed] [Google Scholar]
- 24.Laughrea M, Jette L, Mak J, Kleiman L, Liang C, Wainberg M A. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J Virol. 1997;71:3397–3406. doi: 10.1128/jvi.71.5.3397-3406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lear A L, Haddrick M, Heaphy S. A study of the dimerization of Rous sarcoma virus RNA in vitro and in vivo. Virology. 1995;212:47–57. doi: 10.1006/viro.1995.1452. [DOI] [PubMed] [Google Scholar]
- 26.Louis D C, Gotte D, Sanders-Buell E, Ritchey D W, Salminen M O, Carr J K, McCutchan F E. Infectious molecular clones with the nonhomologous dimer initiation sequences found in different subtypes of human immunodeficiency virus type 1 can recombine and initiate a spreading infection in vitro. J Virol. 1998;72:3991–3998. doi: 10.1128/jvi.72.5.3991-3998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisel J, Bender W, Hu S, Duesberg P H, Davidson N. Structure of 50 to 70S RNA from Moloney sarcoma viruses. J Virol. 1978;25:384–394. doi: 10.1128/jvi.25.1.384-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markowitz D, Goff S, Bank A. A safe packaging cell line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquet R, Baudin F, Gabus C, Darlix J-L, Mougel M, Ehresmann C, Ehresmann B. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res. 1991;91:2349–2357. doi: 10.1093/nar/19.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride M S, Schwartz M D, Panganiban A T. Efficient encapsidation of human immunodeficiency virus type 1 vectors and further characterization of cis elements required for encapsidation. J Virol. 1997;71:4544–4554. doi: 10.1128/jvi.71.6.4544-4554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adam M A, Miller D. Identification of a singnal in a murine retrovirus that is sufficient for packagaing of nonretroviral RNA into virions. J Virol. 1988;62:3802–3806. doi: 10.1128/jvi.62.10.3802-3806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mougel M, Zhang Y, Barklis E. cis-active structural motifs involved in specific encapsidation of Moloney murine leukemia virus RNA. J Virol. 1996;70:5043–5050. doi: 10.1128/jvi.70.8.5043-5050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muriaux D, Girard P-M, Bonnet-Mathoniere B, Paoletti J. Dimerization of HIV-1Lai RNA at low ionic strength: an autocomplementary sequence in the 5′ leader region is evidenced by an antisense oligonucleotide. J Biol Chem. 1995;270:8209–8216. doi: 10.1074/jbc.270.14.8209. [DOI] [PubMed] [Google Scholar]
- 35.Murti K G, Bondurant M, Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981;37:411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen, J. C. Unpublished data.
- 37.Olsen J C, Sechelski J. Use of sodium butyrate to enhance production of retroviral vectors expressing CFTR cDNA. Hum Gene Ther. 1995;6:1195–1202. doi: 10.1089/hum.1995.6.9-1195. [DOI] [PubMed] [Google Scholar]
- 38.Paillart J C, Marquet R, Skripkin E, Ehresmann B, Ehresmann C. Mutational analysis of the bipartite dimer linkage structure of human immunodeficiency virus type 1 genomic RNA. J Biol Chem. 1994;269:27486–27493. [PubMed] [Google Scholar]
- 39.Paillart J C, Berthoux L, Ottmann M, Darlix J L, Marquet R, Ehresmann B, Ehresmann C. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol. 1996;70:8348–8354. doi: 10.1128/jvi.70.12.8348-8354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paillart J C, Marquet R, Skripkin E, Ehresmann C, Ehresmann B. Dimerization of retroviral genomic RNAs: structural and functional implications. Biochimie. 1996;78:639–653. doi: 10.1016/s0300-9084(96)80010-1. [DOI] [PubMed] [Google Scholar]
- 41.Paillart J C, Skripkin E, Ehresmann B, Ehresmann C, Marquet R. A loop-loop “kissing” complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc Natl Acad Sci USA. 1996;93:5572–5577. doi: 10.1073/pnas.93.11.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti J, Mougel M, Tounekti N, Girard P M, Ehresmann C, Ehresmann B. Spontaneous dimerization of retroviral MoMuLV RNA. Biochimie. 1993;75:681–686. doi: 10.1016/0300-9084(93)90099-e. [DOI] [PubMed] [Google Scholar]
- 43.Prats A-C, Roy C, Wan P, Erard M, Housset V, Gabus C, Paoletti C, Darlix J L. cis-elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990;64:774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy P E, Smith M J, Aaroson S A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981;214:445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- 45.Sakuragi J-I, Panganiban A T. Human immunodeficiency virus type 1 RNA outside the primary encapsidation and dimer linkage region affects RNA dimer stability in vivo. J Virol. 1997;71:3250–3254. doi: 10.1128/jvi.71.4.3250-3254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skripkin E, Paillart J C, Marquet R, Ehresmann B, Ehresmann C. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc Natl Acad Sci USA. 1994;91:4945–4949. doi: 10.1073/pnas.91.11.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoltzfus C M, Snyder P N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975;16:1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundquist W I, Heaphy S. Evidence for interstrand quadruplex formation in the dimerization of human immunodeficiency virus type 1 genomic RNA. Proc Natl Acad Sci USA. 1993;90:3393–3397. doi: 10.1073/pnas.90.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapia M D, Metzler V, Mougel M, Ehresmann B, Ehresmann C. Dimerization of MoMuLV genomic RNA: redefinition of the role of the plaindromic stem-loop H1 (278–303) and new roles for stem-loops H2 (310–352) and H3 (355–374) Biochemistry. 1998;37:6077–6085. doi: 10.1021/bi9800303. [DOI] [PubMed] [Google Scholar]
- 50.Torrent C, Bordet T, Darlix J L. Analytical study of rat retrotransposon VL30 RNA dimerization in vitro and packaging in murine leukemia virus. J Mol Biol. 1994;240:434–444. doi: 10.1006/jmbi.1994.1459. [DOI] [PubMed] [Google Scholar]
- 51.Torrent C, Gabus C, Darlix J L. A small and efficient dimerization/packaging signal of rat VL30 RNA and its use in murine leukemia virus-VL30-derived vectors for gene transfer. J Virol. 1994;68:661–667. doi: 10.1128/jvi.68.2.661-667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tounekti N, Mougel M, Roy C, Marquet R, Darlix J-L, Paoletti J, Ehresmann B, Ehresmann C. Effect of dimerization on the conformation of the encapsidation Psi domain of Moloney murine leukemia virus RNA. J Mol Biol. 1992;223:205–220. doi: 10.1016/0022-2836(92)90726-z. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Patel D J. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure. 1993;1:263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
- 54.Williamson J R, Raghuraman M K, Cech T R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989;59:871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 55.Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]