Implications.
In nature, insects are part of the natural diet of aquaculture species, poultry, and pigs.
Nutritional value of insect meals is comparable or higher than conventional protein sources in animal feed.
Large quantities and consistent quality and chemical composition of insect meals are required for use in animal feed.
Insect meals may improve animal health and welfare.
Insect frass is a good fertilizer with a lower environmental load than artificial fertilizers.
Introduction
Insects are great candidates to support the sustainable development of the feed industry. In the last decade, the global industrialized insect farming has increased, aiming to deliver the market with large amounts of insect-derived products for feed purposes (van Huis, 2022). The demand for insect protein will rise from 120,000 metric tons to 500,000 metric tons by 2030, according to an estimation by De Jong and Nikolik (2021) in a RABO Bank report. According to the report, the price of a metric ton of insect protein will decrease from EUR 3,500 to 5,500 during the scale-up phase (2020) to EUR 1,500 to 2,500 during the maturity phase (2030), making insect protein more competitive with conventional protein sources. During this transition period, the main market for insect protein, or so-called insect meal, is pet food and aquaculture but pets’ share of the total market will fall from 54% in 2020 to 30% in 2030, while aquafeed’s share will rise from 17 to 40%, resulting in 200,000 metric tons in 2030, which is still only 1% of the total aquafeed market. Currently, research is focusing on additional values beyond the nutritional value in the protein transition concept. Insect-derived specific compounds such as chitin, antimicrobial peptides, and medium-chain fatty acids (mainly lauric acid) have the ability for antibacterial and immunomodulating effects. Research is also focusing to make insects as feed more sustainable in a circular economy model. In EU insects can only be fed with materials of vegetal origin and some materials of animal origin such as milk, eggs and their products, honey, rendered fat, or blood products from non-ruminant animals (Reg.(EU) 2022/1104). Circularity and sustainability can be given a boost if slaughterhouse or rendering derived-products, catering waste, and unsold products from supermarkets or food industries containing meat or fish will be allowed to feed the insects, once proven to be safe. These waste sources cannot be fed directly to monogastric animals and this makes insects more essential in the food chain as insects convert these waste sources into highly valuable insect-derived products which can be applied in animal nutrition.
This review aims to describe the interest in applying insects in the circular economy, the production process, the nutritional and health properties of insect meals (in particular of the main insect species used for feed purposes), and the main performance results obtained with aquaculture and monogastric livestock species fed insect meals. A short overview of the interest in insect frass is also discussed. Finally, some challenges and main prospects are presented.
Circular economy, production process, and nutritional value
The most promising and used insect species for feed production are the black soldier fly (Hermetia illucens, HI) (Figure 1), the yellow mealworm (Tenebrio molitor, TM) and, to a lesser extent, the common housefly (Musca domestica, MD) (van Huis, 2022).
Figure 1.
Black soldier fly (Hermetia illucens) larvae (Photo: Umberto Diecinove 2022).
The sustainability of insects relies on their ability to bio-convert, with low environmental impact, not otherwise valorized low-value waste streams into nutrients. For these reasons, insects in the circular economy match several sustainable development goals (Figure 2). One factor that impairs the insect sustainability is the high energy costs needed to maintain the high temperatures for their growth. However, coupling insect factories with renewable energy sources could solve this issue.
Figure 2.
Circular Economy and Sustainable Development Goals related to insect farming.
At the end of the rearing process, the insect larvae are sieved from frass, cleaned and, if not used as live larvae mainly for poultry feeding purposes, devitalized by blanching, boiling, drying, cooling, freezing, or freeze drying (Ravi et al., 2020). The devitalization techniques affect the derived-product quality in terms of protein solubility and bio-availability, lipid oxidation, product color, and microbial load (Ravi et al., 2020). Insects can then be further processed to obtain two main fractions: insect-derived meals (with high levels of protein) and insect oils. As exhaustively described by Ravi et al. (2020), for HI two processing methods are used. In the dry method, dry larvae are pressed under cold (T = 25 °C) or hot (T > 60 °C) conditions, to obtain a defatted meal. The level of fat residue is dependent on the temperature and pressure used.
In the wet mode, fresh larvae are reduced into pulp, treated with enzymes to hydrolyze proteins and the different fractions are separated using a tricanter. The nutritional value, including amino acids, of full-fat and defatted insect meals for feed purposes largely varies depending on the species, the life stage, and the rearing substrate. Table 1 reports a small section of published data on the composition of full-fat and defatted HI and TM meals. Other values can be found in different publications. For MD, no data covering defatted meals are present in literature.
Table 1.
Nutritional value of full-fat and defatted insect meals
| Hermetia illucens | Tenebrio molitor | Musca domestica | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFa | min | max | DFb | min | max | FFc | min | max | DF d | min | max | FFe | min | max | |
| Nutrients (g/100 g DM, unless otherwise stated) | |||||||||||||||
| Dry matter | 93.0 | 88.1 | 97.9 | 93.5 | 86.8 | 96.8 | 95.6 | 95.6 | 95.6 | 95.0 | 91.8 | 97.9 | 92.0 | 92.0 | 92.0 |
| Crude protein | 43.2 | 42.7 | 43.8 | 50.5 | 38.5 | 71.2 | 47 | 47 | 47 | 69.9 | 63.0 | 75.4 | 53.9 | 50.4 | 57.9 |
| Crude fat | 31.4 | 29.2 | 33.6 | 15.1 | 6.8 | 29.9 | 29.6 | 29.6 | 29.6 | 8.2 | 5.7 | 10.7 | 20.4 | 18.9 | 22.1 |
| Phosphorus | 1.1 | 0.7 | 1.3 | 0.7 | 0.7 | 0.7 | 1.6 | 1.6 | 1.6 | ||||||
| Calcium | 1.2 | 1.0 | 1.6 | 0.1 | 0.1 | 0.1 | 0.5 | 0.5 | 0.5 | ||||||
| Magnesium | 0.2 | 0.1 | 0.5 | 0.3 | 0.3 | 0.3 | |||||||||
| Chlorine | 0.2 | 0.1 | 0.2 | ||||||||||||
| Sodium | 0.3 | 0.2 | 0.4 | 0.5 | 0.5 | 0.5 | |||||||||
| Ash | 9.5 | 6.6 | 12.4 | 9.5 | 9.0 | 10.3 | 2.6 | 2.6 | 2.6 | 4.9 | 4.8 | 5.0 | 7.9 | 6.5 | 10.1 |
| GE (MJ/kg DM) | 22.4 | 19.2 | 25.4 | 23.3 | 23.0 | 23.5 | 22.9 | 22.9 | 22.9 | ||||||
| Amino acids (g/100 g DM) | |||||||||||||||
| Methionine | 0.6 | 0.5 | 0.8 | 1.0 | 0.7 | 1.3 | 0.7 | 0.7 | 0.7 | 1.7 | 0.9 | 2.6 | 1.5 | 1.1 | 1.7 |
| Threonine | 1.6 | 1.3 | 2.0 | 2.2 | 1.8 | 2.5 | 2.5 | 2.5 | 2.5 | 2.9 | 2.8 | 3.0 | 2.9 | 1.8 | 3.6 |
| Valine | 2.2 | 1.7 | 2.8 | 3.2 | 2.3 | 3.9 | 3.3 | 3.3 | 3.3 | 4.0 | 3.5 | 4.6 | 2.6 | 2.0 | 2.9 |
| Isoleucine | 1.7 | 1.4 | 2.0 | 2.4 | 1.9 | 3.0 | 2.1 | 2.1 | 2.1 | 2.9 | 2.4 | 3.5 | 2.1 | 1.6 | 2.5 |
| Leucine | 2.8 | 2.3 | 3.3 | 3.9 | 3.1 | 5.3 | 3.8 | 3.8 | 3.8 | 5.2 | 4.7 | 6.0 | 3.6 | 2.7 | 4.2 |
| Phenylalanine | 1.8 | 1.4 | 2.1 | 2.3 | 1.9 | 3.1 | 1.9 | 1.9 | 1.9 | 2.4 | 2.2 | 2.7 | 3.4 | 2.3 | 4.1 |
| Histidine | 1.3 | 1.1 | 1.5 | 1.5 | 1.3 | 2.0 | 1.4 | 1.4 | 1.4 | 2.2 | 1.9 | 2.4 | 1.7 | 1.2 | 2.0 |
| Lysine | 2.6 | 2.2 | 3.1 | 3.3 | 2.4 | 4.1 | 2.7 | 2.7 | 2.7 | 4.0 | 3.9 | 4.0 | 4.6 | 3.1 | 4.9 |
| Arginine | 2.1 | 1.7 | 2.4 | 2.6 | 2.1 | 3.0 | 2.5 | 2.5 | 2.5 | 3.7 | 3.6 | 3.8 | 2.9 | 2.3 | 3.3 |
| Tryptophan | 0.5 | 0.3 | 0.7 | 0.7 | 0.3 | 1.0 | 0.5 | 0.5 | 0.5 | 0.8 | 0.8 | 0.8 | 3.1 | 0.8 | 4.5 |
| Cysteine | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | 0.4 | 0.6 | 1.3 | 0.4 | 1.9 |
| Aspartic acid | 3.8 | 2.8 | 4.7 | 5.0 | 3.9 | 6.1 | 3.8 | 3.8 | 3.8 | 5.6 | 5.3 | 6.0 | 5.6 | 3.8 | 6.8 |
| Serine | 1.7 | 1.4 | 2.0 | 2.3 | 1.9 | 3.0 | 2.3 | 2.3 | 2.3 | 3.4 | 3.3 | 3.7 | 1.7 | 1.6 | 1.8 |
| Glutamic acid | 5.2 | 4.5 | 5.8 | 6.0 | 4.9 | 8.0 | 6.0 | 6.0 | 6.0 | 8.4 | 8.2 | 8.7 | 7.8 | 5.9 | 9.2 |
| Proline | 2.3 | 2.2 | 2.4 | 3.3 | 2.57 | 5.1 | 3.1 | 3.1 | 3.1 | 5.2 | 4.5 | 6.0 | 2.2 | 1.7 | 2.6 |
| Glycine | 2.2 | 1.7 | 2.7 | 3.0 | 2.31 | 3.9 | 2.7 | 2.7 | 2.7 | 3.6 | 3.4 | 3.7 | 2.7 | 2.1 | 3.1 |
| Alanine | 2.7 | 2.6 | 2.9 | 3.8 | 3.13 | 4.9 | 4.2 | 4.2 | 4.2 | 5.5 | 4.9 | 5.9 | 3.4 | 2.9 | 3.8 |
| Tyrosine | 3.2 | 2.9 | 3.4 | 3.0 | 0.00 | 4.2 | 3.14 | 3.1 | 3.1 | 3.2 | 0.0 | 5.1 | 3.6 | 2.4 | 4.4 |
| Total amino acids | 38.6 | 32.2 | 45.1 | 49.2 | 42.14 | 55.3 | 47.2 | 47.2 | 47.2 | 64.47 | 62.3 | 68.4 | 56.2 | 39.8 | 67.0 |
FF, full-fat insect meals; DF, defatted insect meals.
Among the insect-derived proteins, defatted meals are of major interest because they are richer in crude protein (CP), lower in ether extract (EE) (Table 1) and more stable and easier to include in feed formulations than the full-fat ones. The nutritional value of the insect meals can vary both due to the rearing substrate and the defatting process, which could also affect the quality of the final products and—in turn—the nutrient digestibility and the animal growth performance. Therefore, particular care should be taken during processing. Finally, the nitrogen-to-protein conversion factor is an important parameter in formulating diets with insect-derived meals. An overestimation of insects’ CP can result in unbalanced diets and poor growth performance (Hua, 2021).
The important factors to be taken into account in feed formulation with insect-derived products have recently been reviewed (Gasco et al., 2023). These factors are insect species and composition, processing method, availability and consistency of supply, nutrient digestibility, anti-nutritional factors, physical pellet properties, palatability, stability, safety, costs, impact on product quality, and legislation.
Insect Meals in Animal Feed
Aquaculture
To allow the development of aquaculture production, alternative proteins to fishmeal (FM) have been extensively studied. Nowadays, the aquaculture industry uses plant-based ingredients as well as by-products of animal origin (Hua, 2021). Insect-derived products can play a major role in the development of a sustainable aquaculture and trials have been conducted to assess nutrient digestibility, growth performance, product quality, and the impact on fish and shrimp health (Hua 2021; Liland et al., 2021; Prakoso et al., 2022; Tran et al., 2022).
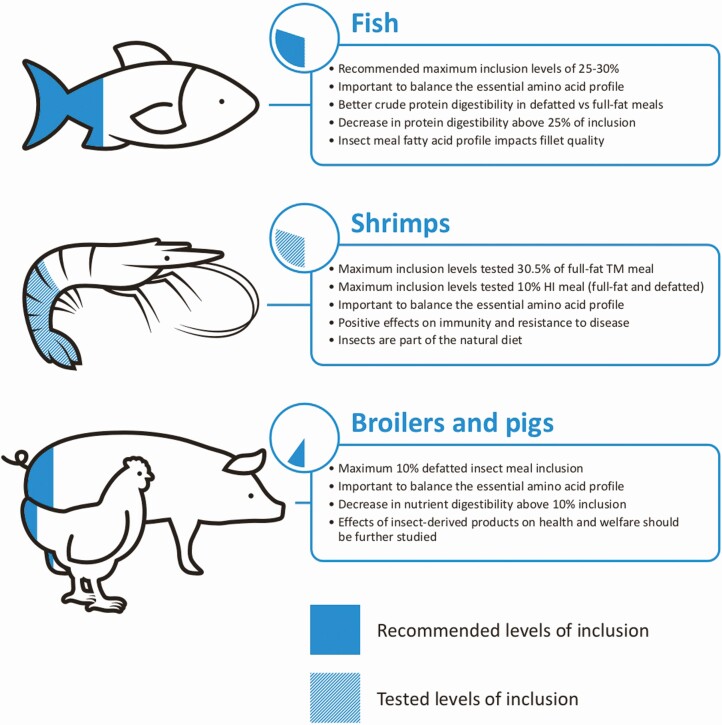
In carnivorous fish species, usually insect meals are included in substitution of FM. Based on recent meta-analyses, it is possible to state that inclusion levels of insect meals up to 25% to 30% support good growth performances (Hua, 2021; Liland, et al., 2021), but the optimal inclusion level seems to be dependent on the fish and the insect species (Hua, 2021). High HI inclusion levels (>30%) reduced the growth parameters, while TM meal seems more tolerated (Hua, 2021; Prakoso et al., 2022). Specifically, the use of TM meal in aquafeed (from 2.5% to 65% of inclusion) did not affect the growth performance (Hua, 2021). In salmons, rainbow trout, sea bass or seabream, the digestibility of CP starts to decrease at an insect meal inclusion level of 25% (Liland et al., 2021) and this was often correlated to the increase of chitin (above 2% to 3%) that worsens specific growth rate and feed conversion ratio (Tran et al., 2022). Chitin binds to digestive enzymes, which may impair the nutrient absorption in the proximal intestine. In addition, insect chitin is embedded in a matrix of other components including proteins, lipids, and minerals and this could impair the accessibility of digestive enzymes to these nutrients. Despite this, studies on the CP digestibility of insect meals (defatted HI and TM meals) in rainbow trout (Gasco et al., 2022) and sea bass (Basto et al., 2020) reported general high coefficients ranging from 80 to about 93%, also when the chitin content was ≥ 4%, supporting the high potential of these products in aquafeed. Authors indicated how differences are not only due to insect species but also to insect meal production techniques. As far as EE digestibility is concerned, values of defatted meals are always above 92%, while lower values are reported for full-fat insect meals.
Research performed using insects in shrimp nutrition is limited and recently reviewed by Sánchez‐Muros et al. (2020). No differences in Litopenaeus vannamei performances are reported up to 30.5% of full-fat TM meal inclusion fully substituting FM as far as a correct balance of essential amino acids is ensured. In a non-balanced formula, a percentage including up to 7% of defatted HI meal negatively affected the growth of L. vannamei. Compared to a dietary treatment containing FM, the inclusion of 10% of a full-fat HI in partial substitution of FM resulted in an improvement of the performances of L. vannamei juveniles. However, no differences emerged when full-fat TM was used even if both insect meals treatments reported high digestibility coefficients (ADC) for crude protein (ADCCP: 84% and 85%, respectively) and ether extract (ADCEE: 95% and 97%, respectively; Shin and Lee, 2021). Richardson et al. (2021) confirmed the positive effect of HI meal (defatted) on the growth performance of L. vannamei. The main considerations on the use of insect meals in fish and shrimps are reported in Figure 3.
Figure 3.
General aspects on the use of insect meals in diets for fish, shrimps, broilers and pigs.
Poultry
Refining and innovating along the poultry value chain are crucial to further improve the sustainability of this sector. The relatively high demand for protein-rich feed ingredients in this sector makes particularly interesting to exchange conventional protein sources by insect protein sources. A recent review reported that insect protein derived from HI and MD can substitute conventional protein to a certain extent without adversely affecting production performance (Dörper et al., 2021). In general, it can be concluded that, balancing the essential amino acids profile, insect meals can be included up to 10% in diets for broilers and laying hens without affecting nutrient digestibility, growth performance, and product quality (Dörper et al., 2021). Dietary inclusion levels of insect meal over 10% resulted in inconsistent results among studies (Dörper et al., 2021). The insects can be used live (fresh), dried, and as processed forms in poultry diets. Being part of the natural diet of poultry, live larvae can improve birds’ welfare. In broilers, provision of live larvae (Figure 4) at the highest frequency (four times per day) and 10% of the estimated dietary dry matter (DM) intake determined the most prominent increase in activity and better leg health without affecting broiler performance (Ipema et al., 2020).
Figure 4.
Poultry feeding live Hermetia illucens larvae.
Pigs
In global compound feed production, pig feed took the second place in 2021 (24.1% of share). Regular pig feed is switching to functional and premium variants to improve the immunity of the animals as well as to reduce the risk of metabolic disorders, acidosis, injuries, and infections. A shift towards more sustainable feed ingredients is foreseen to improve the sustainability of the entire pig production (Figure 5). Insects are one of the novel protein sources to substitute conventional ones and have been evaluated in a recent review which concluded that the nutrient digestibility of insect proteins was comparable to conventional protein sources (Veldkamp and Vernooij, 2021). However, nutrient digestibility of insect-based diets as well as the related effects on growth performance in pigs fed insect-based diets were different among studies (Veldkamp and Vernooij, 2021).
Figure 5.
Live Hermetia illucens larvae can also be provided to piglets.
The variability in nutrient digestibility of insect-based diets and related effects on growth performance were due to changes in diet ingredients and nutrient composition when insect products were included but also due to different insect species and life stages, processing techniques, palatability, and age of the pigs used in the studies. Globally, when balancing the amino acids profile of the diet, insect products can be included up to 10% to partly replace conventional protein-rich feed ingredients in pig diets without affecting growth performance, product quality and health. Also, live HI larvae can be provided to pigs. In a study, larvae were provided to piglets (25 d old) twice a day (75 g [day 1 to 4] or 150 g [day 5 to 11]) and it was concluded that post-weaning live HI larvae provisioning had beneficial effects on piglet behavior, by facilitating exploration behaviors and reducing the need to orally manipulate objects and pen mates. Neophobic responses towards a novel object were also reduced and performance of piglets that consumed a small amount of larvae was maintained (Ipema et al., 2021). Figure 3 reports the main aspects on the use of insect meals in poultry and pig diets.
Health effects
Insect-derived products seem able to exert positive health effect due to the presence of bioactive compounds. Indeed, as a natural defense mechanism toward the challenging environment they grew, insects develop antimicrobial peptides (AMP) that can stimulate fish, shrimps and livestock immunity. Insects contain chitin which has been mentioned as an anti-nutritional factor adversely affecting the nutrient digestion and absorption at high inclusion levels. On the other hand, chitin can stimulate the innate immune system, modulate the microbiota, and exert antioxidant and anti-inflammatory effects with positive outcomes on animal health (Veldkamp et al., 2022). Next to AMP and chitin, also medium-chain fatty acids (lauric acid) may exert antibacterial effects (Gasco et al., 2021; Veldkamp et al., 2022).
Insect-derived products can therefore sustain animal health and increase their resistance to diseases and may be used to reduce the use of antibiotics. These features can create benefits to insect-derived products and support their use in animal feed as additives (low inclusion levels) (Gasco et al., 2021; Veldkamp et al., 2022) and need to be studied in more detail. As reviewed by Gasco et al. (2021), insect meals seem to modulate and/or promote microbial diversity and—for this reason—could have a positive effect on livestock, fish, and shrimp health. A rich gut microbiota increases the competition, in terms of nutrient and colonization site, with pathogens and, consequently, may improve the disease resistance. In general, insect-derived products had not negative effect on the fish microbiota while, in poultry, their positive effect is observed when the inclusion level is lower than or equal to 10%. Finally, based on the scarce literature available to date, insect-derived products seem to improve also the health of pigs and crustaceans. Authors hypothesized antibacterial and probiotic effects of insect meals on piglets and finisher pigs, respectively (Gasco et al., 2021), while HI meal positively influenced antioxidant enzyme activity and non-specific immune responses in shrimp (Shin and Lee, 2021).
Insect frass
Insect frass is what is left at the end of the insect rearing and is defined as “a mixture of excrements derived from farmed insects, the feeding substrate, parts of farmed insects, dead eggs and with a content of dead farmed insects of not more than 5% in volume and not more than 3% in weight” (Commission Regulation (EU) 2021/1925). Depending on their initial DM and nutrient composition, the mass substrate reduction performed by HI ranges from 30% to 80%, with 200 to 693 kg (DM) frass production per ton of waste (Lopes et al., 2022). Frass contains good amounts of carbon (C), nitrogen (N), phosphorus (P), and potassium (K) and represents a valuable fertilizer able to substitute mineral sources and introduce organic material into the soil (Schmitt and de Vries, 2020). Differences in frass composition are reported, reflecting the rearing substrate nutritional value and the insect capacity in uploading nutrients. Moreover, the C and N contents of HI frass seem more stable (about 35% and 3%) than P and K values (from 0.3% to 5.2% and from 0.2% to 4.1%) (Schmitt and de Vries, 2020; Lopes et al., 2022). Recent research reported that the improvement in plant performances is not only due to frass nutrient composition, but also to the presence of bioactive compounds (among them, chitin) and microorganisms that seem to improve nutrient utilization from plants, to promote root and plant growth, to stimulate seed germination, and to increase plant drought and stress tolerance (Schmitt and de Vries, 2020; Barragán-Fonseca et al., 2022; Lopes et al., 2022). Compared to inorganic fertilizers, frass has a lower environmental impact in some categories (minus 0.265 g SO2 equivalents and minus 0.064 g SO2 equivalents per kg of frass used in substitution of mineral fertilizers for terrestrial acidification and aquatic acidification, respectively) (Schmitt and de Vries, 2020). For these reasons, frass closes the loop of the circular economy applied by insects.
Issues and further directions to consider for the future
Availability of adequate quantities and composition consistency of supplied insect meals are crucial parameters to allow the feed industry to adopt insect meals in animal formulation. Indeed, new formulations need not only research, but also marketing actions, and both require investments.
Due to low produced quantities and high production costs, the price of insect-based proteins is still high and in Europe not competitive when compared to FM or soybean meal. However, waste bioconversion, the decrease in dependency on less sustainable ingredients (FM, soybean meal), or the health benefits associated with the use of insect-based products, in combination with proper marketing of the final product, can justify relatively high prices.
The quality of insect-derived products highly depends on insect species and composition, development stage at harvest, and method of devitalization and processing. Therefore, the optimal transformation process, in terms of nutrient availability and, as a consequence, of growth performance, for each insect meal should be deeply investigated.
Safety is the most important parameter for a sector at its infant stage. Insect-derived products should be safe to be used as animal feed ingredient. Growing insects on biowaste sources that cannot be fed directly to aquaculture or livestock is one of the biggest challenges for the future to make insects the missing link in the food chain and this will give sustainability a boost.
Bioactive compounds contained in insects represent a promising natural alternative to antimicrobial agents and a possible solution to remediate antimicrobial resistance. Moreover, the possibility of stimulating the large-scale production of insect bioactive peptides represents a promising biotech business.
The balance between antinutritional effects and health-promoting effects of insect-derived products needs to be studied in more detail as also the mode of action to set the most optimal inclusion level of these products in aquaculture and livestock nutrition.
More research is recommended to set the optimal inclusion level of insect-derived products for growth performance, health, and welfare. Further studies on the mode of action on beneficial health and welfare effects of inclusion of insect-derived products may give an additional value for the application of these products in animal diets.
Frass includes bioactive compounds and microorganisms which may improve nutrients utilization by plants, promote root and plant growth, stimulate seed germination, increase plant drought and stress tolerance and has a lower environmental load than artificial fertilizers.
About the Authors

Laura Gasco is a Research Full Professor at the Department of Agricultural, Forest and Food Sciences of the University of Turin (Italy). Laura focuses her research on fish, rabbit, and poultry farming and nutrition assessing performances and the impact of alternative ingredients on product quality. Since 2012 her research is focused on the use of insect meals as innovative ingredients in animal nutrition and on the optimisation of insect rearing for the production of high-quality raw materials. In particular, her research focuses on the black soldier fly (Hermetia illucens) and on the yellow mealworm (Tenebrio molitor). She is involved in projects related to insects as feed. Laura is president of the Study Commission Insects of the European Federation of Animal Science and a member of the editorial board of the Journal of Insects as Food and Feed.

Manuela Renna is an Associate Professor of Animal Nutrition at the Department of Veterinary Sciences of the University of Turin (Italy). She obtained her PhD in Agricultural, Forest and Food Sciences from the Department of Agricultural, Forest and Food Sciences of the University of Turin. Her main research interests are focused on the effects of animal diet on animal-derived food products quality. In the last years, she has focused her work on the use of innovative feed ingredients in farmed animal nutrition (both monogastrics and ruminants), with a special focus on agro-industrial by-products and insects.

Sara Bellezza Oddon is a Post Doc researcher at the Department of Agricultural, Forest and Food Sciences of the University of Turin (DISAFA - Italy). She obtained her PhD in Agricultural, Forest and Food Sciences from DISAFA on the thesis: “Advanced insect rearing: a new opportunity for feed production.” Her research topics are the rearing of insects (Hermetia illucens and Tenebrio molitor) and the maximization of the larvae growth through the determination of their nutritional requirements. She is working also on the use of insect meal as feed ingredient in rainbow trout and poultry, and the use of live larvae as environmental enrichment in broiler chickens and laying hens.

Somaya Naser El Deen is an entomologist researcher/ insects as feed and food in the Animal Nutrition department of Wageningen Livestock Research. Dr. Naser El Deen joined Wageningen Livestock Research in 2022. In 2021 she obtained her Ph.D. from the university of Naples “Parthenope” in Italy in “Environment, Resources and Sustainable Development” on the thesis “Analysis of the growth performance of Tenebrio molitor (Coleoptera: Tenebrionidae) reared for food and feed on by-products based diets.” Now she is working on insect production research on black soldier fly (Hermetia illucens) and yellow mealworm (Tenebrio molitor) as a novel protein source for food and feed. The main research topics are insect rearing, biowaste conversion by insects, new feeding substrates for larvae, larval nutritional requirements, application of insect-derived products in animal nutrition mainly for poultry and aquaculture, and the safety of these products. Dr. Naser E. Deen is involved in several national and international projects related to insects as feed, food and non-food.

Arya Rezaei Far is a researcher in Poultry Nutrition at the Animal Nutrition Department of Wageningen Livestock Research (WLR). He graduated with a Doctorate degree in Veterinary Science in Poultry Diseases at the University of Tehran in 2016 and a Master’s degree in Animal Nutrition at Wageningen University in 2020 with a thesis on circular diets in broilers. His main research topic is circularity in poultry nutrition, alternative and functional ingredients and their effects on the health and resilience of poultry flocks. Over the past 2 years at WLR, Arya has been involved in several national and international projects on insects as feed and circularity in poultry nutrition.

Teun Veldkamp is a senior researcher animal nutrition / insects as feed in the Animal Nutrition department of Wageningen Livestock Research. Dr. Veldkamp joined Wageningen Livestock Research in 1989. In 2002 he obtained his Ph.D. on the thesis “Heat stress and diet utilization in male turkeys – The role of dietary energy and amino acids.” Now he is working on poultry nutrition research in broilers, laying hens and turkeys. The main research topics in poultry nutrition are feed evaluation, amino acid requirements, feed additives: efficacy and tolerance trials for registration purposes and since 2012 Dr. Veldkamp is involved in many projects related to insects as feed, food and non-food. In these projects, he is focusing on biowaste conversion by use of insects, insect rearing, and application of insect-derived products in animal nutrition. Dr. Veldkamp is the coordinator of the H2020 project SUSINCHAIN (Sustainable Insect Chain) and is the former president of the Study Commission Insects of the European Federation of Animal Science (2016–2022). Furthermore, he is a member of the editorial board of the Journal of Insects as Food and Feed.
Contributor Information
Laura Gasco, Department of Agricultural, Forest and Food Sciences, University of Torino, Largo P. Braccini 2, 10095 Grugliasco, Italy.
Manuela Renna, Department of Veterinary Sciences, University of Torino, Largo P. Braccini 2, 10095 Grugliasco, Italy.
Sara Bellezza Oddon, Department of Agricultural, Forest and Food Sciences, University of Torino, Largo P. Braccini 2, 10095 Grugliasco, Italy.
Arya Rezaei Far, Wageningen University & Research, Wageningen Livestock Research, De Elst 1, 6700 AH Wageningen, the Netherlands.
Somaya Naser El Deen, Wageningen University & Research, Wageningen Livestock Research, De Elst 1, 6700 AH Wageningen, the Netherlands.
Teun Veldkamp, Wageningen University & Research, Wageningen Livestock Research, De Elst 1, 6700 AH Wageningen, the Netherlands.
Funding
Contributions to this paper have received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement nº 861976. This document reflects only the author’s view and the Commission is not responsible for any use that may be made of the information it contains.
Conflict of interest statement. None declared.
Literature Cited
- Barragán-Fonseca, K.Y., Nurfikari A., van de Zande E.M., Wantulla M., van Loon J.J.A., de Boer W., and Dicke M... 2022. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 27:646–654. doi: 10.1016/j.tplants.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Basto, A., Matos E., and Valente L.M... 2020. Nutritional value of different insect larvae meals as protein sources for European sea bass (Dicentrarchus labrax) juveniles. Aquaculture. 521:735085. doi: 10.1016/j.aquaculture.2020.735085. [DOI] [Google Scholar]
- Benzertiha, A., Kierończyk B., Kołodziejski P., Pruszyńska–Oszmałek E., Rawski M., Józefiak D., and Józefiak A... 2020. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poultry Science 99:196–206. doi: 10.3382/ps/pez450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong, B., and Nikolik G... 2021. No longer crawling: Insect protein to come of age in the 2020s. The Netherlands: RaboResearch, Rabobank, Utrecht. [Google Scholar]
- Dörper, A., Veldkamp T., and Dicke M... 2021. Use of black soldier fly and house fly in feed to promote sustainable poultry production. J. Insects Food Feed 7:761–780. doi: 10.3920/jiff2020.0064. [DOI] [Google Scholar]
- Gasco, L., Bellezza Oddon S., Vandenberg G.W., Veldkamp T., and Biasato I... 2023. Factors affecting the decision-making process of using insect-based products in animal feed formulations. J. Insects Food Feed. doi: 10.3920/JIFF2022.0164. [DOI] [Google Scholar]
- Gasco, L., Caimi C., Trocino A., Lussiana C., Oddon S.B., Malfatto V., Anedda R., Serra G., Biasato I., Schiavone A.,. et al. 2022. Digestibility of defatted insect meals for rainbow trout aquafeeds. J. Insects Food Feed 8:1385–1399. doi: 10.3920/jiff2021.0160. [DOI] [Google Scholar]
- Gasco, L., Józefiak A., and Henry M... 2021. Beyond the protein concept: health aspects of using edible insects on animals. J. Insects Food Feed 7:715–741. doi: 10.3920/jiff2020.0077. [DOI] [Google Scholar]
- Hall, H.N., Masey O’Neill H.V., Scholey D., Burton E., Dickinson M., and Fitches E.C... 2018. Amino acid digestibility of larval meal (Musca domestica) for broiler chickens. Poult. Sci. 97:1290–1297. doi: 10.3382/ps/pex433. [DOI] [PubMed] [Google Scholar]
- Heide, M.E.V., Nørgaard J.V., and Engberg R.M... 2021. Performance, nutrient digestibility and selected gut health parameters of broilers fed with black soldier fly, lesser mealworm and yellow mealworm. J. Insects Food Feed 7:1011–1022. doi: 10.3920/jiff2020.0150. [DOI] [Google Scholar]
- Heuel, M., Kreuzer M., Sandrock C., Leiber F., Mathys A., Guggenbühl B., Gangnat I.D.M., and Terranova M... 2022. Feeding value of black soldier fly larvae compared to soybean in methionine- and lysine-deficient laying hen diets. J. Insects Food Feed 8:989–999. doi: 10.3920/jiff2021.0178. [DOI] [Google Scholar]
- Hua, K. 2021. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture. 530:735732. doi: 10.1016/j.aquaculture.2020.735732. [DOI] [Google Scholar]
- Ipema, A.F., Bokkers E.A.M., Gerrits W.J.J., Kemp B., and Bolhuis J.E... 2021. Providing live black soldier fly larvae (Hermetia illucens) improves welfare while maintaining performance of piglets post-weaning. Sci. Rep. 11:7371. doi: 10.1038/s41598-021-86765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipema, A.F., Gerrits W.J.J., Bokkers E.A.M., Kemp B., and Bolhuis J.E... 2020. Provisioning of live black soldier fly larvae (Hermetia illucens) benefits broiler activity and leg health in a frequency- and dose-dependent manner. Appl. Anim. Behav. Sci. 230:105082. doi: 10.1016/j.applanim.2020.105082. [DOI] [Google Scholar]
- Leeper, A., Benhaïm D., Smárason B. Ö., Knobloch S., Òmarsson K. L., Bonnafoux T., Pipan M., Koppe W., Björnsdóttir R., and Øverland M... 2022. Feeding black soldier fly larvae (Hermetia illucens) reared on organic rest streams alters gut characteristics of Atlantic salmon (Salmo salar). Journal of Insects as Food and Feed 8:1355–1372. doi: 10.3920/jiff2021.0105 [DOI] [Google Scholar]
- Liland, N.S., Araujo P., Xu X.X., Lock E.-J., Radhakrishnan G., Prabhu A.J.P., and Belghit I... 2021. A meta-analysis on the nutritional value of insects in aquafeeds. J. Insects Food Feed 7:743–759. doi: 10.3920/jiff2020.0147. [DOI] [Google Scholar]
- Lopes, I.G., Yong J.W.H., and Lalander C... 2022. Frass derived from black soldier fly larvae treatment of biodegradable wastes. a critical review and future perspectives. Waste Manage. 142:65–76. doi: 10.1016/j.wasman.2022.02.007. [DOI] [PubMed] [Google Scholar]
- Makkar, H.P.S., Tran G., Heuzé V., and Ankers P... 2014. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- Prakoso, V.A., Irawan A., Iswantari A., Maulana F., Samsudin R., and Jayanegara A... 2022. Evaluation of dietary inclusion of black soldier fly (Hermetia illucens) larvae on fish production performance: a meta-analysis. J. Insects Food Feed 8:1373–1384. doi: 10.3920/jiff2021.0159. [DOI] [Google Scholar]
- Ravi, H.K., Degrou A., Costil J., Trespeuch C., Chemat F., and Vian M.A... 2020. Larvae mediated valorization of industrial, agriculture and food wastes: biorefinery concept through bioconversion, processes, procedures, and products. Process. 8:857. doi: 10.3390/pr8070857. [DOI] [Google Scholar]
- Richardson, A., Dantas-Lima J., Lefranc M., and Walraven M... 2021. Effect of a black soldier fly ingredient on the growth performance and disease resistance of juvenile pacific white shrimp (Litopenaeus vannamei). Animals. 11:1450. doi: 10.3390/ani11051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Muros, M.J., Renteria P., Vizcaino A., and Barroso F.G... 2020. Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Reviews in Aquaculture. 12:186–203. doi: 10.1111/raq.12312. [DOI] [Google Scholar]
- Schmitt, E., and de Vries W... 2020. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustainable Chem. 25:100335. doi: 10.1016/j.cogsc.2020.03.005. [DOI] [Google Scholar]
- Shin, J., and Lee K.-J... 2021. Digestibility of insect meals for Pacific white shrimp (Litopenaeus vannamei) and their performance for growth, feed utilization and immune responses. PLoS One 16:e0260305. doi: 10.1371/journal.pone.0260305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, M.S., Matos R., Araujo P., Lock E.J., Gopika R., Prabhu P.A.J., and Belghit I... 2022. In vitro assessment of protein digestibility and mineral solubility of black soldier fly larvae meals for monogastric animals. J. Insects Food Feed 8:953–966. doi: 10.3920/jiff2021.0197. [DOI] [Google Scholar]
- Tran, H.Q., Nguyen T.T., Prokešová M., Gebauer T., Doan H.V., and Stejskal V... 2022. Systematic review and meta-analysis of production performance of aquaculture species fed dietary insect meals. Reviews in Aquaculture. 14:1637–1655. doi: 10.1111/raq.12666. [DOI] [Google Scholar]
- van Huis, A. 2022. Edible insects: challenges and prospects. Entomol. Res. 52:161–177. doi: 10.1111/1748-5967.12582. [DOI] [Google Scholar]
- Veldkamp, T., Dong L., Paul A., and Govers C... 2022. Bioactive properties of insect products for monogastric animals – a review. J. Insects Food Feed 8:1027–1040. doi: 10.3920/jiff2021.0031. [DOI] [Google Scholar]
- Veldkamp, T., and Vernooij A.G... 2021. Use of insect products in pig diets. J. Insects Food Feed 7:781–793. doi: 10.3920/jiff2020.0091. [DOI] [Google Scholar]
- Weththasinghe, P., Hansen J., Rawski M., Józefiak D., Ghimire S., and Øverland M... 2021. Insects in Atlantic salmon (Salmo salar) diets – comparison between full-fat, defatted, and de-chitinised meals, and oil and exoskeleton fractions. J. Insects Food Feed 8:1235–1247. doi: 10.3920/jiff2021.0094. [DOI] [Google Scholar]