Abstract
Purpose:
NOEMTR is a technique used to investigate brain lipids and macromolecules in greater detail than other techniques and benefits from increased contrast at 7T. However, this contrast can become degraded due to B1+ inhomogeneities present at ultra-high field strengths. High-permittivity dielectric pads (DP) have been used to correct for these inhomogeneities via displacement currents generating secondary magnetic fields. The purpose of this work is to demonstrate that dielectric pads can be used to mitigate B1+ inhomogeneities and improve NOEMTR contrast in the temporal lobes at 7T.
Methods:
Partial 3D NOEMTR contrast images and whole brain B1+ field maps were acquired on a 7T MRI across six healthy subjects. Calcium titanate DP, having a relative permittivity of 110, were placed next to the subject’s head near the temporal lobes. Pad corrected NOEMTR images had a separate post-processing linear correction applied.
Results:
DP provided supplemental B1+ to the temporal lobes while also reducing the B1+ magnitude across the posterior and superior regions of the brain. This resulted in a statistically significant increase in NOEMTR contrast in substructures of the temporal lobes both with and without linear correction. The padding also produced a convergence in NOEMTR contrast towards approximately equal mean values.
Conclusion:
NOEMTR images showed significant improvement in temporal lobe contrast when DP were used which resulted from an increase in B1+ homogeneity across the entire brain slab. DP-derived improvements in NOEMTR are expected to increase the robustness of the brain substructural measures both in healthy and pathological conditions.
Keywords: NOEMTR, Ultra-High Field, Dielectric Padding, B1+ Inhomogeneity Correction
Introduction
Nuclear Overhauser Effect magnetization transfer ratio (NOEMTR) is an emerging MRI technique that has been used to investigate macromolecules in the brain, such as lipids and proteins1. This imaging technique takes advantage of magnetization transfer via dipolar cross-relaxation between saturated (lipids and proteins) and unsaturated (bulk water) spin systems, to produce endogenous contrast. Previous studies have demonstrated the application of NOEMTR imaging in mapping the degraded protein content in high-grade brain tumors2, identifying regional brain damage due to acute ischemic strokes2, and investigating neurodegenerative disorders such as Parkinson’s3 and Alzheimer’s4. This gives NOEMTR imaging considerable clinical potential as a significant imaging biomarker for many pathologies especially at ultra-high field strengths (≥7T). However, images acquired at these ultra-high field strengths experience substantial B1+ field inhomogeneities due to the shortened radiofrequency (RF) wavelengths used5,6. As a result, NOEMTR suffers from significant signal loss in the resulting contrast images. This drop in NOEMTR signal is most dramatic in the inferior regions of the brain, specifically in the mesial temporal lobe slices.
While several methods exist that attempt to correct for these B1+ inhomogeneities at 7T, such as post-processing algorithms or parallel transmit (pTx) techniques, they have their own sets of drawbacks and limitations7. One correction method that has seen a degree of success has been the use of high-permittivity aqueous dielectric padding placed near the subject’s head to supplement regional low B1+ magnitude8–10. This supplemental magnetic field results from displacement currents being produced via tangential electric fields causing an increased polarization of the bulk high-permittivity materials and is described in Eq (1) below. In this expression total current density is composed of conduction (Jc) and displacement currents (JD).
| (1) |
The resulting spatial augmentation of the B1+ field has been described as a localized increase near the pads with a decrease across regions of the tissue farther away. This was suggested by Brink et al.11 who indirectly showed that B1+ drop near the temporal bone in the inner ear could be increased at the cost of global head B1+. Dielectric pad usage and development has been extensively targeted towards neuroimaging8,12–15, cardiac and vascular imaging16–18, and musculoskeletal imaging19–21 at 1.5T, 3T, and 7T field strengths.
Many metabolic imaging sequences benefit greatly from B1+ correction and therefore are good techniques for quantifying their influence via B1+ field mapping techniques. A recent study from our group showed aqueous calcium titanate padding was effective in reducing B1+ inhomogeneities present in glutamate-weighted CEST (GluCEST) maps at 7T located in inferior regions of the brain, such as the temporal lobes and hippocampus22. However, NOEMTR contrast relies primarily on a low magnitude highly homogenous B1+ field to generate adequate contrast, whereas GluCEST requires both high magnitude and high B1+ homogeneity. Therefore, the aims of this work are as follows: 1) To demonstrate the feasibility and initial reproducibility of using aqueous calcium titanate dielectric padding to correct primarily for volumetric NOEMTR B1+ inhomogeneities in a 3D hippocampal slab of the brain and improve resulting contrast. 2) To describe the spatial augmentation behavior of the B1+ field across the whole brain when influenced by dielectric padding.
Methods
Dielectric Pads
Two 18×18 cm2 dielectric pads were utilized in this work, with a composition of CaTiO3 suspended in D2O (7TNS, Multiwave Imaging, Marseille, France) having an estimated relative permittivity of 110 at 300MHz23.
MR Image Acquisition
All images were acquired on a 7T MRI (Magnetom Terra, Siemens Healthcare, Erlangen, Germany) using a single channel transmit 32-channel receive phased array head coil (Nova Medical, Wilmington, MA, USA). Images were acquired on six healthy male subjects (ages: 23–44) after obtaining informed consent under an approved IRB protocol. Dielectric pads were placed near the temporal lobes on either side of the subject’s head and due to their physical size, they extended forward to partially cover the face. Volumetric NOEMTR data was acquired using a modified 3D turbo FLASH sequence, with a gradient echo readout TR = 3.5s, TE = 1.79ms, and shot TR = 6s. Each image was acquired in three shots (separate saturation/acquisition cycles) across a total scan time of approximately 10 minutes. The 3D slab was positioned in an oblique orientation in line with the hippocampus and had a thickness of 24mm (2mm per slice resolution), with an in-plane resolution of 1 × 1mm2 and a matrix size of 240 × 180. Magnetization preparation was achieved by using a B1, RMS pulse amplitude of 0.72μT, with a pulse length of 3s. Hanning-shaped pulses of 99 ms duration with 1ms inter-pulse delay were applied at offset frequencies from −5 to 5ppm in step sizes of 0.2ppm relative to water proton resonance frequency, along with 100ppm saturation offset. Additionally, whole brain B1+ field maps were acquired on a single subject under the same dielectric padding conditions with an isotropic resolution of 2mm. Subsequently, these whole brain B1 maps were then used to generate difference maps across the whole brain volume by subtracting the data taken with no pads from that taken with pads.
Analysis and Post-processing
The acquired −3.5ppm NOE signal was subtracted from and normalized to the 100ppm signal to account for the large confounding amide signal present at 3.5ppm. This can be seen in Eq (2) below where S0 is the intensity of the signal from the saturation pulse applied at 100ppm.
| (2) |
It should be noted that while this quantity can measure relative changes, it shows an aggregate effect of NOE, magnetization transfer (MT), and direct saturation (DS). The resulting NOEMTR images were corrected for B0 inhomogeneities using WASSR acquisition acquired at offsets frequencies ranging from 0 to ±1ppm in step sizes of 0.1ppm24 and B1 field maps were generated as in Volz et al.25. An additional linear B1+ post-processing correction step was also applied separately to the acquired NOEMTR images after B0 correction via local interpolation to a second order polynoimal26. To assess the efficacy of dielectric pads on specific brain regions, the NOEMTR contrast images were segmented between gray and white matter as well as various bilateral sub-cortical regions such as the thalamus, hippocampus, and amygdala. Linear mixed effect models were performed in the R statistical environment to estimate the effect of dielectric padding on the changes in relative B1+ and NOEMTR contrast. Image post-processing was performed using MATLAB (The Mathworks Inc., Natick, MA, USA) and SPM1227, cortical and sub-cortical tissue segmentation was performed using HD-BET28, FSL29, and ANTS30.
Results
Transmit efficiency maps seen in Figure 1, show that when pads were present the values in the temporal lobes increased while posterior region values decreased compared to maps acquired without padding present, reflecting a net increase in homogeneity within the image slab. This change in B1+ between pad conditions is further emphasized by the corresponding transmit efficiency difference maps which show a clear and consistent increase in the temporal lobe regions and decrease in the posterior regions. However, results showed no statistically significant differences present in transmit efficiency values (p = 0.25) between padding conditions. It should be noted that this lack of significance most likely reflects the minimal change in mean B1+ while not considering the increase in overall homogeneity across the slab. These results are summarized in Table 1 showing mean and standard deviation values. These values show approximately the same mean transmit efficiency value, 0.048, for both pad conditions. However, a reduction in standard deviation with pads was seen, 0.011, compared to no pads present, 0.013.
Figure 1.
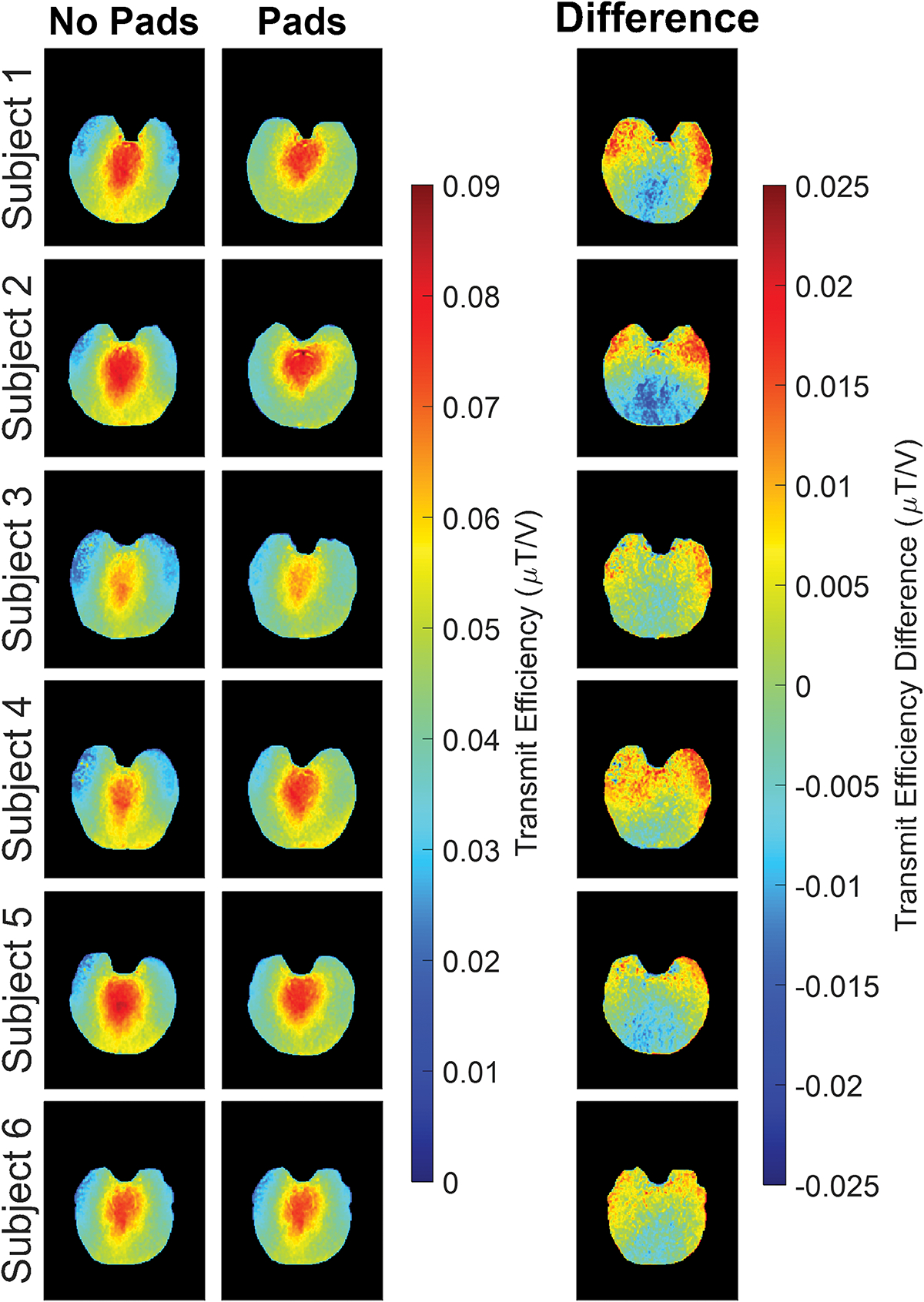
Acquired transmit efficiency maps shown for all six subjects with and without dielectric padding for a hippocampal slice. Data acquired with dielectric pads present shows an increase in homogeneity in the temporal lobes and posterior region of the brain compared to those acquired without. Generated transmit efficiency difference maps can also be seen for each subject emphasizing the differences.
Table 1.
Calculated mean and standard deviation values for transmit efficiency and NOEMTR contrast images with and without linear correction across all subjects and padding conditions. All values were calculated across the entire 3D slab excluding the first and last slices.
| Transmit Efficiency (μT/V) | NOEMTR Contrast (%) (No Linear Correction) | NOEMTR Contrast (%) (Linear Correction) | ||
|---|---|---|---|---|
| No Pads | Subject 1 | 0.049 (0.013) | 15.63 (8.55) | 22.54 (10.53) |
| Subject 2 | 0.051 (0.014) | 21.72 (8.42) | 29.39 (9.52) | |
| Subject 3 | 0.043 (0.012) | 16.37 (12.42) | 22.58 (14.18) | |
| Subject 4 | 0.048 (0.013) | 18.53 (8.26) | 27.02 (9.73) | |
| Subject 5 | 0.050 (0.014) | 23.54 (8.49) | 30.82 (7.85) | |
| Subject 6 | 0.048 (0.013) | 21.35 (8.82) | 29.37 (9.67) | |
| Total | 0.048 (0.013) | 19.52 (9.16) | 26.95 (10.38) | |
| Pads | Subject 1 | 0.050 (0.010) | 22.02 (8.54) | 32.15 (10.51) |
| Subject 2 | 0.048 (0.012) | 22.29 (6.99) | 31.44 (8.77) | |
| Subject 3 | 0.043 (0.009) | 22.60 (8.52) | 31.49 (10.33) | |
| Subject 4 | 0.048 (0.010) | 21.37 (6.86) | 30.36 (8.29) | |
| Subject 5 | 0.049 (0.011) | 23.18 (7.56) | 30.98 (7.91) | |
| Subject 6 | 0.048 (0.011) | 22.72 (7.00) | 30.84 (8.17) | |
| Total | 0.048 (0.011) | 22.36 (7.57) | 31.21 (8.99) | |
Corresponding uncorrected NOEMTR contrast images utilizing pads reflect this change in B1+ in the form of increased contrast in the temporal lobes and was consistently increased across all six subjects, as seen in Figure 2. The images without linear post-processing in the upper half of Figure 2 showed a statistically significant difference (p < 0.001) between padding conditions. A separate linear correction was applied to these same NOEMTR images, seen in the bottom half of Figure 2, and show the same contrast enhancement in the temporal lobe regions as well as additional overall enhancement in the posterior regions of the brain when pads were used. These linear corrected results also produced a statistically significant difference (p < 0.001) between padding conditions. This is further supported by box plots in Figure 3, showing the variability for each individual subject between pad and post-processing conditions averaged over all slices. Consistently seen across all subjects’, pads, and linear correction alone each generally increased NOEMTR contrast while also reducing the NOEMTR variability. The highest amount of contrast and lowest amount of variability was achieved when both methods were applied together. More general histograms showing contrast changes between experimental conditions can be viewed in the supporting information as figure S1.
Figure 2.
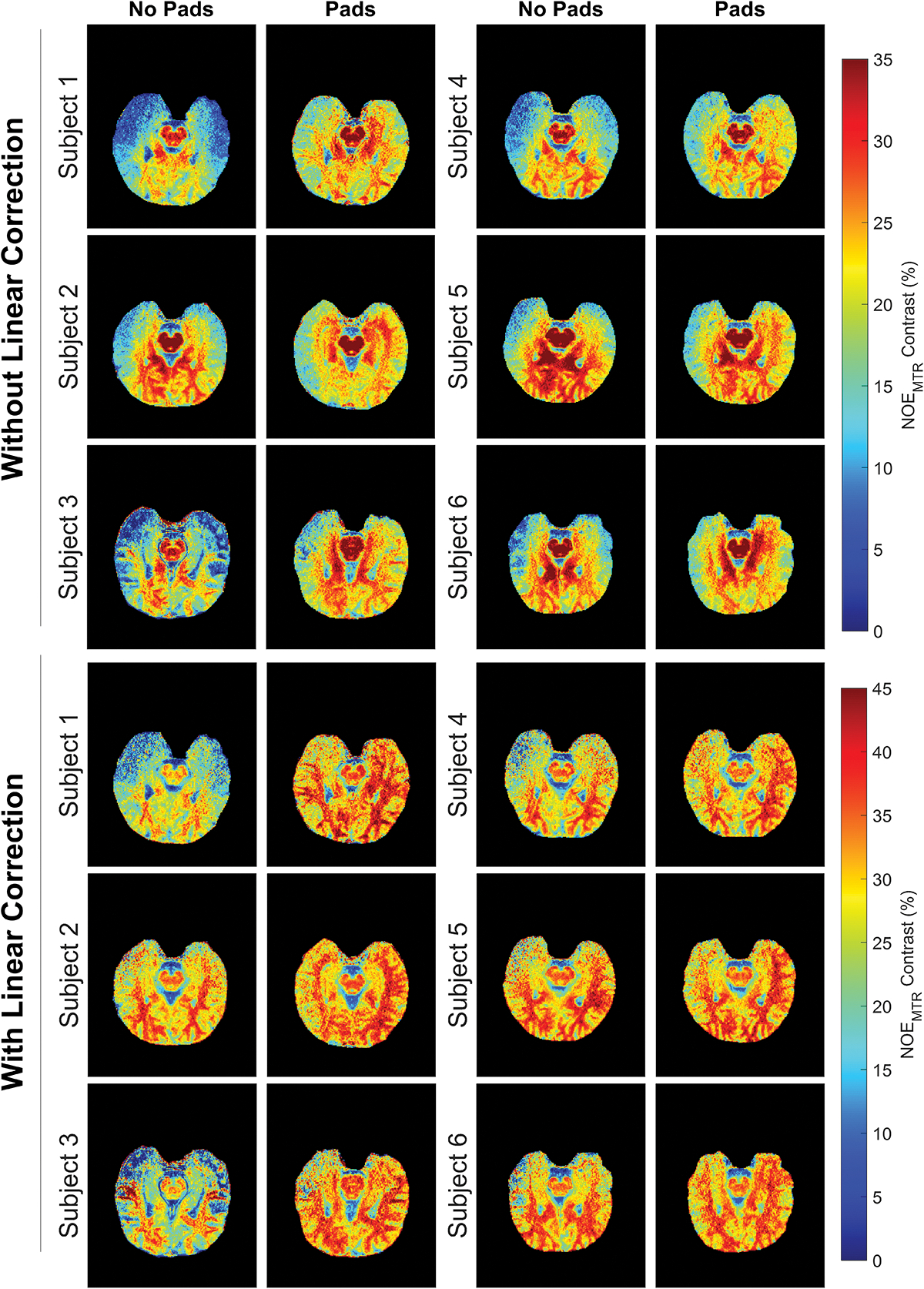
NOEMTR images acquired across all six subjects with and without dielectric pads, between post-processing conditions. The images seen in the upper half have not had post-processing applied to them while the images in the bottom half have had this correction applied. Images acquired with only dielectric padding show a substantial increase in contrast in the temporal lobe and posterior regions compared to those acquired without padding. When linear correction is incorporated the amount of contrast enhancement is further increased.
Figure 3.
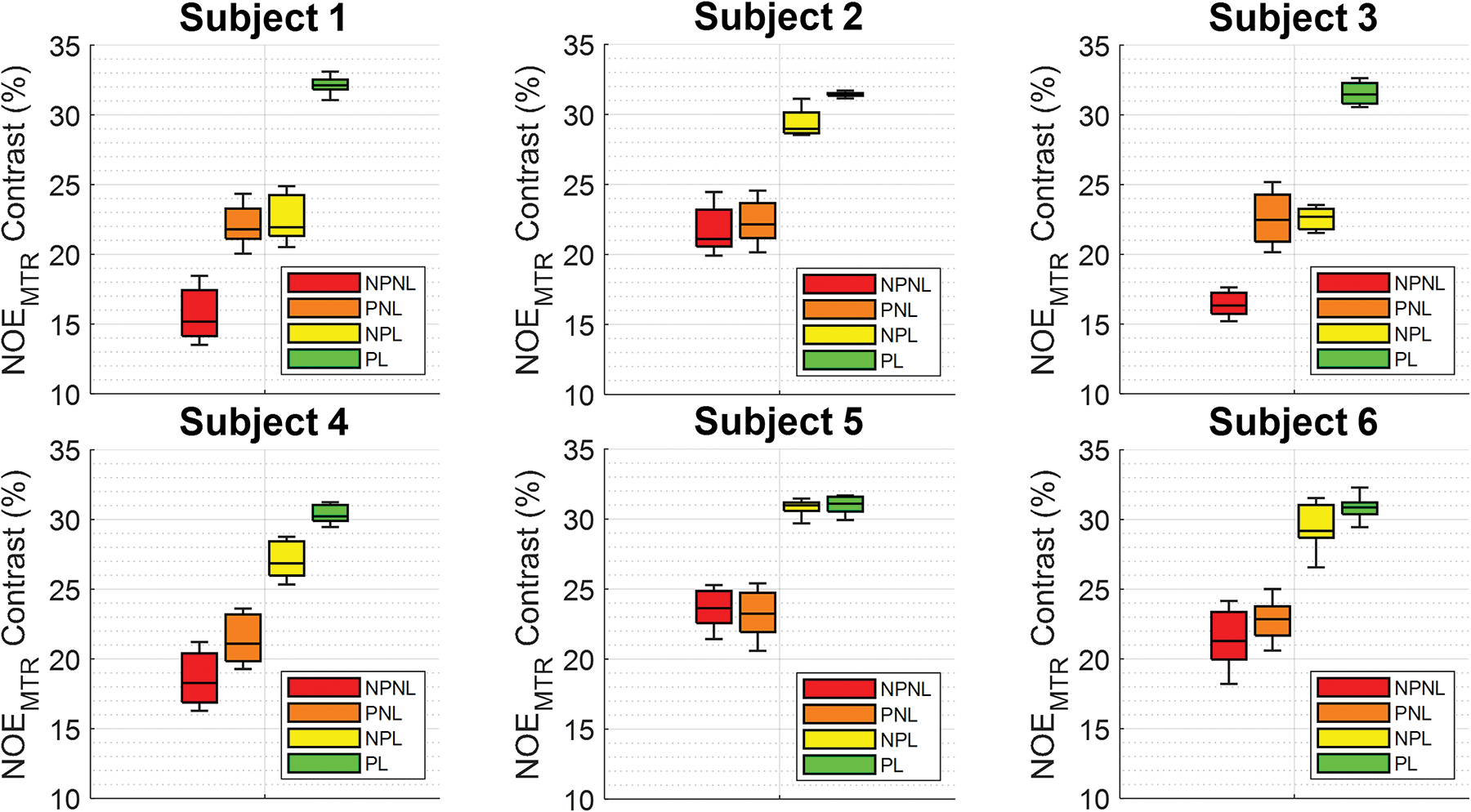
Boxplots showing summary for each subject across the experimental conditions. The labelled conditions seen here are as follows: No pads without linear correction (NPNL), pads without linear correction (PNL), no pads with linear correction (NPL), and pads with linear correction (PL). Pads and linear correction alone generally increase NOEMTR contrast while reducing variability, while applying both together achieve the highest contrast and lowest contrast variability.
The NOEMTR data shown in Table 1 here shows a highly consistent convergence in NOEMTR contrast values across all subjects towards approximately 22.3% mean for the non-linear corrected pad data compared to 19.5% with no pads. Similarly, a reduction in standard deviation was also seen with pads, 7.5%, compared to no pads present, 9.1%. This same trend was also seen for linear corrected data where the same convergence in NOEMTR values was seen towards approximately 31.2% with pads compared to 26.9% with no pads. Standard deviations saw the same trend with a reduction observed with pads, 8.9%, compared to no pads, 10.3%.
Figure 4 shows a full linear corrected NOEMTR acquisition from one subject (except for the first and last slices due to phasing artifacts being present) showing, in detail, the differences in contrast between pad conditions across the entire 3D slab. In these images the pads had a substantial corrective effect not only in the temporal lobe regions but also in the posterior regions of the brain as well. In addition, the effect of the pads can be consistently seen across all slices with a slight diminishment in the inferior slices. In the segmented subcortical regions, an increase in mean linear corrected NOEMTR contrast values were consistently observed when pads were used. These segmented regions and their corresponding histograms can be viewed in the supporting information as figures S2 and S3.
Figure 4.
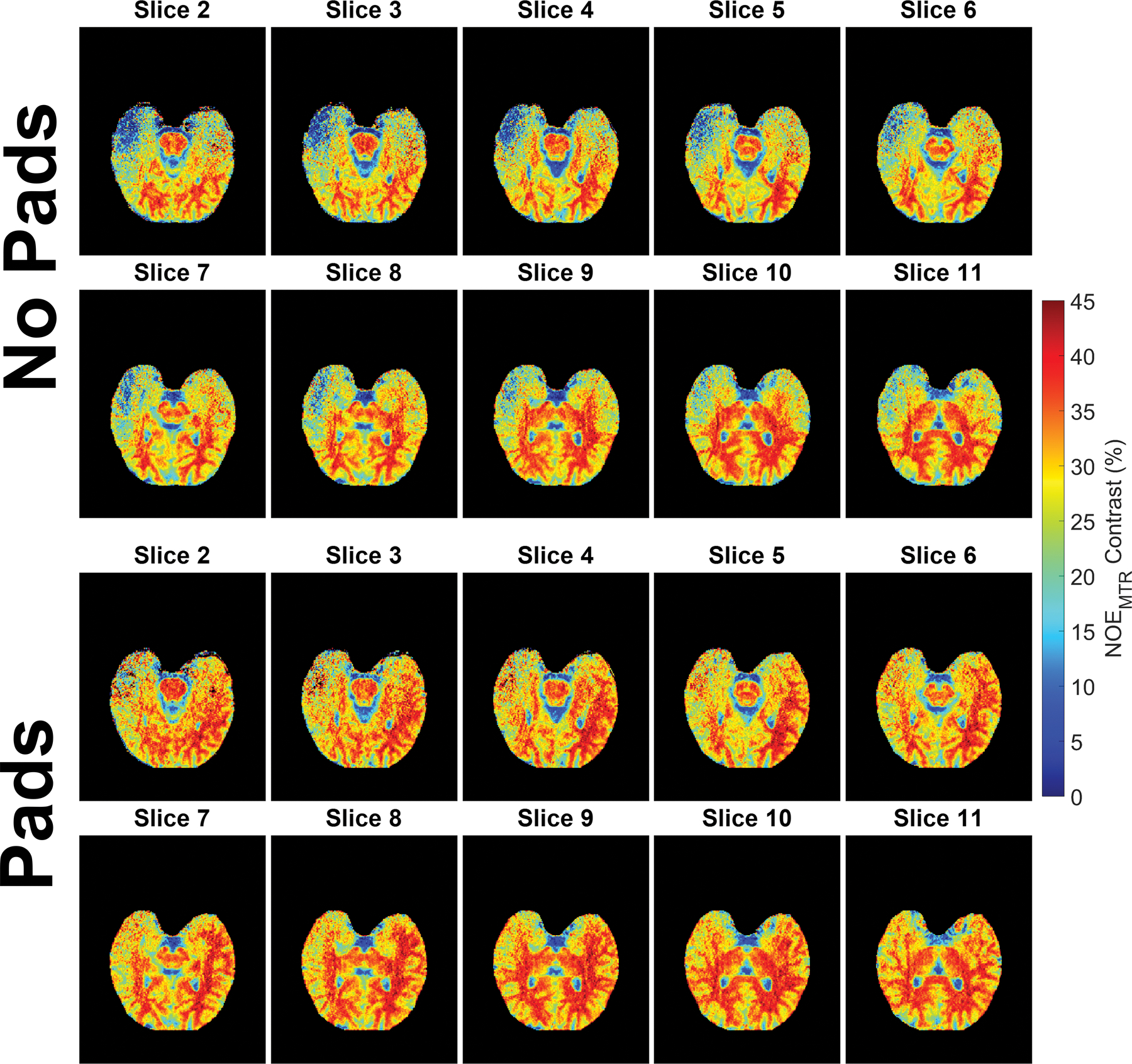
A same subject whole slab comparison of linear corrected NOEMTR images between padding conditions. In this data, slice 2 corresponds to more inferior regions of the slab while slice 11 is 20mm higher. Dielectric padding substantially increased NOEMTR contrast not only in the temporal lobe region but also in the posterior regions of the brain as well. The first and last slices were omitted due to the presence of phasing artifacts.
Discussion
In this work, calcium titanate dielectric pads were utilized in improving B1+ inhomogeneities present in 7T NOEMTR images and improving resulting contrast. Feasibility and initial reproducibility were shown by acquiring 3D hippocampal slab data across six healthy subjects with and without pads placed on either side of the head near the temporal lobes. The transmit efficiency maps acquired across the hippocampal slab show a substantial change not only in the temporal lobes but also in the posterior regions. This matches initial hypotheses on pad function by Teeuwisse et al.23 who described when using pads, a local B1+ increase is expected while globally it will decrease.
When viewing the NOEMTR images, dielectric pads showed a high degree of contrast enhancement across all six subjects with and without linear post-processing correction. The pads were not only able to improve the mean NOEMTR values within a single subject but also equalized the means between subjects towards a nominal value of approximately 22% without linear correction and 30% with linear correction. The linear correction applied to the no pad data provided some corrective effect but was unable to substantially improve the NOEMTR contrast values in the temporal lobes and additionally amplified the noise in those low B1+ regions.
The variability of these correction methods for all subjects individually, where data absent of pads or linear correction, showed the largest amount of NOEMTR variability along with the lowest mean value. Incorporating the pads or linear post-processing separately showed a relatively consistent improvement in both areas by increasing the mean contrast and decreasing the variability. However, incorporating both to each subject’s data showed a greater improvement than either alone could achieve, illustrated in the consistently high NOEMTR values and low variability. This supports the notion that a multi-corrective approach is needed to adequately improve inhomogeneities and resulting contrast without leading to worse quality within low B1+ regions. With the inferior slices of the acquired slab experiencing more inhomogeneities than the superior ones, evaluating the corrective effect on the whole slab on a per slice basis was also of interest. This is illustrated by showing that slice 2 saw almost just as much contrast enhancement compared to slice 11 in the complete subject acquisition presented.
Tissue specific analysis also showed that both gray and white matter saw an increase in NOEMTR with gray matter seeing the larger increase. With a larger fraction of gray matter being positioned physically closer to the pads during acquisition, this increase in contrast is expected. All sub-cortical linear corrected NOEMTR values also saw an increase when pads were used versus absent. This reflects that not only does superficial gray matter benefit from pad usage but also that deep gray matter structures (such as the thalamus, hippocampus, and amygdala) also can see a substantial amount of enhancement.
While corrective effects were seen consistently across the 20mm slab of the main NOEMTR acquisition region, dielectric pads augment the B1+ fields across the entirety of the brain. An additional aim of this work also sought to show the augmentation of the spatial B1+ field distribution across the whole brain. Volumetric difference maps show regions of both increasing and decreasing relative B1+ strength across the entire brain when dielectric pads were placed on either side of the head. Rupprecht et al.31 previously performed numerical calculations to show that in the presence of a high permittivity material, an increased amount of coil flux density inside the bulk material caused a decrease in magnitude outside the vicinity of the material. Furthermore, this flux “focusing” effect was observed to increase with the increasing volume of material. The full volumetric B1+ field data render can show this more clearly as it would be expected that the most B1+ flux is focused through the pad material onto the temporal lobes where the pads were placed. Moving posteriorly and superiorly away from the maximum B1+ enhancement location, the positive increase begins to fall off and is exchanged for a decrease in B1+, as would be expected considering there would be less flux traveling through those regions. An example of this whole brain volumetric rendering can be viewed in the supporting information as figure S4.
In this work, NOEMTR images were only acquired across a limited 20mm slab and while the change in whole brain B1+ could be investigated, change in whole brain NOEMTR could not be due to current sequence limitations. With further sequence optimization, future work should aim to incorporate acquisitions across the entire brain to characterize pad behavior not only on B1+ maps but also on resulting contrast images of interest. Additionally, this work did not track changes in SAR with and without dielectric pads. Previous studies have found that pad usage not only improved resulting image contrast but also lead to decreases in SAR levels16,21. Future work should also incorporate standard quantification of these changes especially for the benefit of SAR-limited sequences such as GluCEST.
In recent years, 7T has seen massive advancements in technical developments and clinical utility, especially in neurodegenerative disease diagnostics, with one of the biggest limitations, however, still being B1+ inhomogeneity. The most popular avenue of correcting NOEMTR images (and many others) is offline post-processing, However, these correction techniques rely on B1 dependent contrast data which may or may not be available at the B1-powers used in vivo, particularly in certain CEST-based techniques due to SAR limitations. Furthermore, it was also seen in this work and previous work22,32 that post-processing does not fully correct image regions that have particularly low B1+ values. Conversely, dielectric pads can bring regions of considerably low contrast into a more correctable regime. Therefore, multifaceted correction techniques which combine post-processing and pad usage and, even in the future, pTx, and artificial intelligence (AI) should be considered. Consideration should also be given to using dielectric pads and other methods to correct for the same B1+ inhomogeneities present in similar sequences at 7T such as creatine CEST (CrCEST)33 and transient NOE (tNOE)34.
Conclusion
The work described here demonstrates that high permittivity dielectric padding can be used to improve 7T NOEMTR contrast, with and without linear correction, particularly in the temporal lobes and in several subcortical regions. With dielectric pads being historically used in correcting for B1+ inhomogeneities associated with anatomical images, this work represents the first use of dielectric pads with the specific goal of improving NOEMTR contrast images. Acquired whole brain B1+ maps were also able to not only show the expected B1+ increases in the temporal lobes but also decreases in the posterior and superior regions of the brain, which contributed significantly to the NOEMTR contrast enhancement.
Supplementary Material
Acknowledgments
Research reported in this work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number P41EB029460 and by the National Institute of Aging of the National Institutes of Health under Award Numbers R01AG071725, R01AG063896, and R56AG062665.
References:
- 1.Liu GS, Song XL, Chan KWY, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. Nmr in Biomedicine. Jul 2013;26(7):810–828. doi: 10.1002/nbm.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones CK, Huang A, Xu JD, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7 T. Neuroimage. Aug 15 2013;77:114–124. doi: 10.1016/j.neuroimage.2013.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mennecke A, Khakzar KM, German A, et al. 7 tricks for 7 T CEST: Improving the reproducibility of multipool evaluation provides insights into the effects of age and the early stages of Parkinson’s disease. Nmr in Biomedicine. Mar 17 2022;doi:ARTN e4717 10.1002/nbm.4717 [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Wei ZL, Chan KWY, et al. Protein aggregation linked to Alzheimer’s disease revealed by saturation transfer MRI. Neuroimage. Mar 2019;188:380–390. doi: 10.1016/j.neuroimage.2018.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutland JW, Delman BN, Gill CM, Zhu C, Shrivastava RK, Balchandani P. Emerging use of ultra-high-field 7T MRI in the study of intracranial vascularity: State of the field and future directions. American Journal of Neuroradiology 2020. p. 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. Dec 2018;109:1–50. doi: 10.1016/j.pnmrs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 7.Padormo F, Beqiri A, Hajnal JV, Malik SJ. Parallel transmission for ultrahigh-field imaging. NMR Biomed. Sep 2016;29(9):1145–61. doi: 10.1002/nbm.3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines K, Smith NB, Webb AG. New high dielectric constant materials for tailoring the B1+ distribution at high magnetic fields. J Magn Reson. Apr 2010;203(2):323–7. doi: 10.1016/j.jmr.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Webb AG. Dielectric materials in magnetic resonance. Concepts in Magnetic Resonance Part A. 2011;38A(4):148–184. doi: 10.1002/cmr.a.20219 [DOI] [Google Scholar]
- 10.Webb A, Shchelokova A, Slobozhanyuk A, Zivkovic I, Schmidt R. Novel materials in magnetic resonance imaging: high permittivity ceramics, metamaterials, metasurfaces and artificial dielectrics. Magn Reson Mater Phy. Dec 2022;35(6):875–894. doi: 10.1007/s10334-022-01007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brink WM, van der Jagt AM, Versluis MJ, Verbist BM, Webb AG. High permittivity dielectric pads improve high spatial resolution magnetic resonance imaging of the inner ear at 7 T. Invest Radiol. May 2014;49(5):271–7. doi: 10.1097/RLI.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 12.Alsop DC, Connick TJ, Mizsei G. A spiral volume coil for improved RF field homogeneity at high static magnetic field strength. Magn Reson Med. Jul 1998;40(1):49–54. doi: 10.1002/mrm.1910400107 [DOI] [PubMed] [Google Scholar]
- 13.Yang QX, Mao W, Wang J, et al. Manipulation of image intensity distribution at 7.0 T: passive RF shimming and focusing with dielectric materials. J Magn Reson Imaging. Jul 2006;24(1):197–202. doi: 10.1002/jmri.20603 [DOI] [PubMed] [Google Scholar]
- 14.Sica CT, Rupprecht S, Hou RJ, et al. Toward whole-cortex enhancement with an ultrahigh dielectric constant helmet at 3T. Magn Reson Med. Mar 2020;83(3):1123–1134. doi: 10.1002/mrm.27962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Reilly TPA, Webb AG, Brink WM. Practical improvements in the design of high permittivity pads for dielectric shimming in neuroimaging at 7T. J Magn Reson. Sep 2016;270:108–114. doi: 10.1016/j.jmr.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Brink WM, Webb AG. High permittivity pads reduce specific absorption rate, improve B1 homogeneity, and increase contrast-to-noise ratio for functional cardiac MRI at 3 T. Magn Reson Med. Apr 2014;71(4):1632–40. doi: 10.1002/mrm.24778 [DOI] [PubMed] [Google Scholar]
- 17.de Heer P, Bizino MB, Versluis MJ, Webb AG, Lamb HJ. Improved Cardiac Proton Magnetic Resonance Spectroscopy at 3 T Using High Permittivity Pads. Invest Radiol. Feb 2016;51(2):134–8. doi: 10.1097/RLI.0000000000000214 [DOI] [PubMed] [Google Scholar]
- 18.Lindley MD, Kim D, Morrell G, et al. High-permittivity thin dielectric padding improves fresh blood imaging of femoral arteries at 3 T. Invest Radiol. Feb 2015;50(2):101–7. doi: 10.1097/RLI.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zivkovic I, Teeuwisse W, Slobozhanyuk A, Nenasheva E, Webb A. High permittivity ceramics improve the transmit field and receive efficiency of a commercial extremity coil at 1.5 Tesla. Journal of Magnetic Resonance: The Authors; 2019. p. 59–65. [DOI] [PubMed] [Google Scholar]
- 20.Fagan AJ, Welker KM, Amrami KK, et al. Image Artifact Management for Clinical Magnetic Resonance Imaging on a 7 T Scanner Using Single-Channel Radiofrequency Transmit Mode. Invest Radiol. Dec 2019;54(12):781–791. doi: 10.1097/RLI.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 21.Koolstra K, Börnert P, Brink W, Webb A. Improved image quality and reduced power deposition in the spine at 3 T using extremely high permittivity materials. Magnetic Resonance in Medicine 2018. p. 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs PS, Benyard B, Cember A, et al. Repeatability of B-1(+) inhomogeneity correction of volumetric (3D) glutamate CEST via High-permittivity dielectric padding at 7T. Magnetic Resonance in Medicine. Aug 15 2022;doi: 10.1002/mrm.29409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magn Reson Med. May 2012;67(5):1285–93. doi: 10.1002/mrm.23108 [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. Jun 2009;61(6):1441–50. doi: 10.1002/mrm.21873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volz S, Noth U, Rotarska-Jagiela A, Deichmann R. A fast B1-mapping method for the correction and normalization of magnetization transfer ratio maps at 3 T. Neuroimage. Feb 15 2010;49(4):3015–26. doi: 10.1016/j.neuroimage.2009.11.054 [DOI] [PubMed] [Google Scholar]
- 26.Windschuh J, Zaiss M, Meissner JE, et al. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7T. Nmr in Biomedicine. May 2015;28(5):529–537. doi: 10.1002/nbm.3283 [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ. Statistical parametric mapping : the analysis of funtional brain images. 1st ed. Elsevier/Academic Press; 2007:vii, 647 p. [Google Scholar]
- 28.Isensee F, Schell M, Pflueger I, et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum Brain Mapp. Dec 1 2019;40(17):4952–4964. doi: 10.1002/hbm.24750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. Aug 15 2012;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 30.Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight j. 2009;2(365):1–35. [Google Scholar]
- 31.Rupprecht S, Sica CT, Chen W, Lanagan MT, Yang QX. Improvements of transmit efficiency and receive sensitivity with ultrahigh dielectric constant (uHDC) ceramics at 1.5 T and 3 T. Magnetic Resonance in Medicine. May 2018;79(5):2842–2851. doi: 10.1002/mrm.26943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cember ATJ, Hariharan H, Kumar D, Nanga RPR, Reddy R. Improved method for post-processing correction of B1 inhomogeneity in glutamate-weighted CEST images of the human brain. NMR Biomed. Jun 2021;34(6):e4503. doi: 10.1002/nbm.4503 [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Debnath A, Cai K, et al. Evaluating the feasibility of creatine-weighted CEST MRI in human brain at 7 T using a Z-spectral fitting approach. NMR Biomed. Dec 2019;32(12):e4176. doi: 10.1002/nbm.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar D, Benyard B, Soni ND, Swain A, Wilson N, Reddy R. Feasibility of transient nuclear Overhauser effect imaging in brain at 7 T. Magn Reson Med. Apr 2023;89(4):1357–1367. doi: 10.1002/mrm.29519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


