Abstract
Herpes simplex virus (HSV) establishes latent infection in the sensory neuron and possibly in non-neuronal tissue, particularly the cornea. During latency only one region of the HSV genome is transcribed, producing RNAs known as latency associated transcripts (LAT). The gene for LAT overlaps with the HSV gene for the protein ICPO in the downstream regions of both genes. Latency can occur in the absence of LAT. This study reports the detection of ICPO/LAT and thymidine kinase (TK) gene fragments by the polymerase chain reaction in DNA extracted from the corneas and trigeminal ganglia of latently infected rabbits. Both genes were detected in four of four trigeminal ganglia tested and in three of five corneas tested. More importantly, this study reports the first detection of LAT in RNA extracted from 9% of corneas from latently infected rabbits (n = 22) by the polymerase chain reaction. LAT was detected in RNA from 100% of the corresponding trigeminal ganglia (n = 22). Although LAT is not essential for latency, it remains the only known molecular marker for latent HSV infections. Detection of LAT in these rabbit corneas suggests that HSV latency may occur in this non-neuronal tissue and that reactivation from non-neuronal tissue may occur at a low frequency in animals in which HSV latency has been established.
Full text
PDF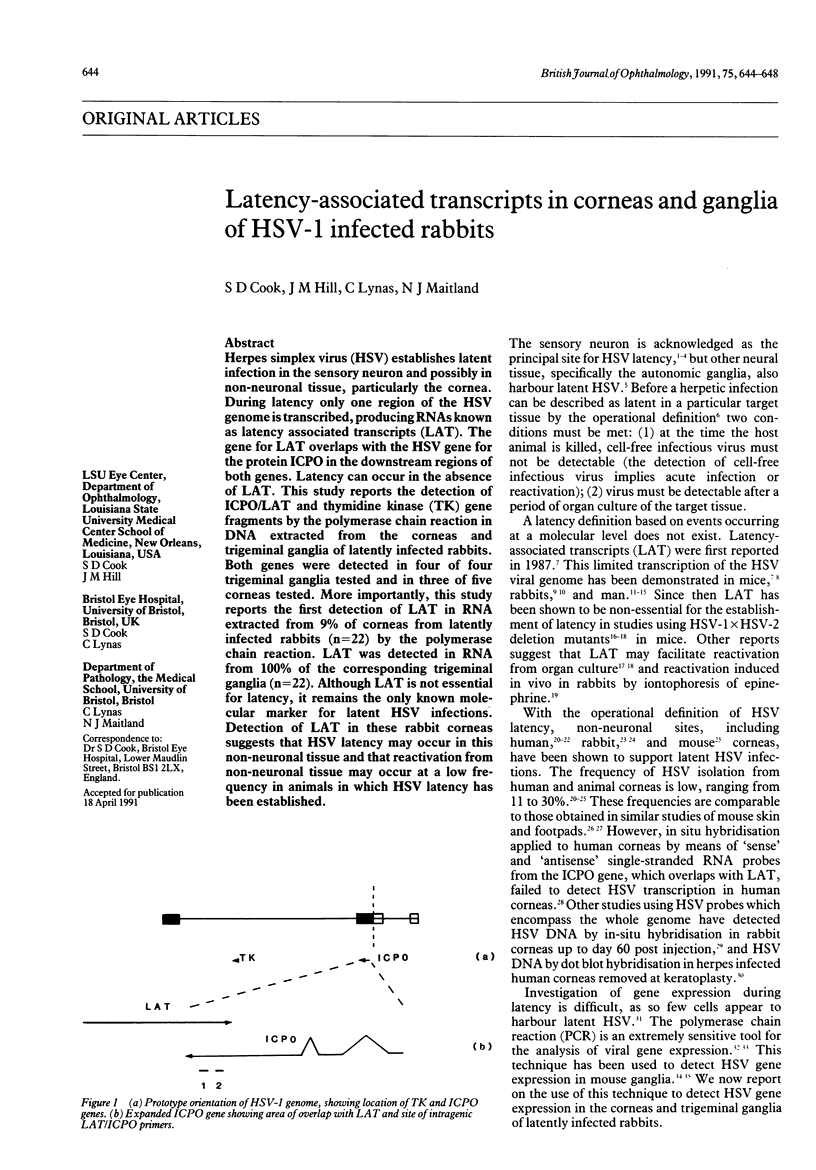



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abghari S. Z., Stulting R. D. Recovery of herpes simplex virus from ocular tissues of latently infected inbred mice. Invest Ophthalmol Vis Sci. 1988 Feb;29(2):239–243. [PubMed] [Google Scholar]
- Al-Saadi S. A., Clements G. B., Subak-Sharpe J. H. Viral genes modify herpes simplex virus latency both in mouse footpad and sensory ganglia. J Gen Virol. 1983 May;64(Pt 5):1175–1179. doi: 10.1099/0022-1317-64-5-1175. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. D., Batra S. K., Brown S. M. Recovery of herpes simplex virus from the corneas of experimentally infected rabbits. J Gen Virol. 1987 Jul;68(Pt 7):2013–2017. doi: 10.1099/0022-1317-68-7-2013. [DOI] [PubMed] [Google Scholar]
- Coupes D., Klapper P. E., Cleator G. M., Bailey A. S., Tullo A. B. Herpesvirus simplex in chronic human stromal keratitis. Curr Eye Res. 1986 Oct;5(10):735–738. doi: 10.3109/02713688609000013. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Sakuma T., Baba M., Pauwels R., Balzarini J., Rosenberg I., Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987 Dec;8(5-6):261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- Deshmane S. L., Fraser N. W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989 Feb;63(2):943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta Y., Rootman D. S., Xie L. X., Kiritoshi A., Hill J. M. Recurrent HSV-1 corneal lesions in rabbits induced by cyclophosphamide and dexamethasone. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):371–376. [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Harbour D. A., Blyth W. A. Isolation of herpes simplex virus from the skin of clinically normal mice during latent infection. J Gen Virol. 1980 Mar;47(1):205–207. doi: 10.1099/0022-1317-47-1-205. [DOI] [PubMed] [Google Scholar]
- Javier R. T., Stevens J. G., Dissette V. B., Wagner E. K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988 Sep;166(1):254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Kennedy P. G., Al-Saadi S. A., Clements G. B. Reactivation of latent herpes simplex virus from dissociated identified dorsal root ganglion cells in culture. J Gen Virol. 1983 Jul;64(Pt 7):1629–1635. doi: 10.1099/0022-1317-64-7-1629. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynas C., Cook S. D., Laycock K. A., Bradfield J. W., Maitland N. J. Detection of latent virus mRNA in tissues using the polymerase chain reaction. J Pathol. 1989 Apr;157(4):285–289. doi: 10.1002/path.1711570404. [DOI] [PubMed] [Google Scholar]
- Lynas C., Laycock K. A., Cook S. D., Hill T. J., Blyth W. A., Maitland N. J. Detection of herpes simplex virus type 1 gene expression in latently and productively infected mouse ganglia using the polymerase chain reaction. J Gen Virol. 1989 Sep;70(Pt 9):2345–2355. doi: 10.1099/0022-1317-70-9-2345. [DOI] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Mellerick D. M., Fraser N. W. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987 Jun;158(2):265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- O'Brien W. J., Taylor J. L. The isolation of herpes simplex virus from rabbit corneas during latency. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):357–364. [PubMed] [Google Scholar]
- Pavan-Langston D., Rong B. L., Dunkel E. C. Extraneuronal herpetic latency: animal and human corneal studies. Acta Ophthalmol Suppl. 1989;192:135–141. doi: 10.1111/j.1755-3768.1989.tb07104.x. [DOI] [PubMed] [Google Scholar]
- Pepose J. S. Herpes simplex keratitis: role of viral infection versus immune response. Surv Ophthalmol. 1991 Mar-Apr;35(5):345–352. doi: 10.1016/0039-6257(91)90184-h. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbaga E. M., Pavan-Langston D., Bean K. M., Dunkel E. C. Detection of HSV nucleic acid sequences in the cornea during acute and latent ocular disease. Exp Eye Res. 1988 Oct;47(4):545–553. doi: 10.1016/0014-4835(88)90093-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sedarati F., Izumi K. M., Wagner E. K., Stevens J. G. Herpes simplex virus type 1 latency-associated transcription plays no role in establishment or maintenance of a latent infection in murine sensory neurons. J Virol. 1989 Oct;63(10):4455–4458. doi: 10.1128/jvi.63.10.4455-4458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C., Tullo A. B., Easty D. L., Thomsitt J. Isolation of herpes simplex virus from the cornea in chronic stromal keratitis. Br J Ophthalmol. 1982 Oct;66(10):643–647. doi: 10.1136/bjo.66.10.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., O'Boyle D. R., 2nd, Lavi E., Fraser N. W. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J Virol. 1988 Sep;62(9):3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Haarr L., Porter D. D., Cook M. L., Wagner E. K. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988 Jul;158(1):117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Tullo A. B., Easty D. L., Shimeld C., Stirling P. E., Darville J. M. Isolation of herpes simplex virus from corneal discs of patients with chronic stromal keratitis. Trans Ophthalmol Soc U K. 1985;104(Pt 2):159–165. [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz M. A., Yamamoto H., Notkins A. L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976 Dec 9;264(5586):554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S., Ghiasi H. Fine mapping of the major latency-related RNA of herpes simplex virus type 1 in humans. J Gen Virol. 1988 Dec;69(Pt 12):3101–3106. doi: 10.1099/0022-1317-69-12-3101. [DOI] [PubMed] [Google Scholar]




