Abstract
Background
Antibiotics are frequently prescribed unnecessarily in outpatients with coronavirus disease 2019 (COVID-19). We sought to evaluate factors associated with antibiotic prescribing in outpatients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
We performed a population-wide cohort study of outpatients aged ≥66 years with polymerase chain reaction–confirmed SARS-CoV-2 from 1 January 2020 to 31 December 2021 in Ontario, Canada. We determined rates of antibiotic prescribing within 1 week before (prediagnosis) and 1 week after (postdiagnosis) reporting of the positive SARS-CoV-2 result, compared to a self-controlled period (baseline). We evaluated predictors of prescribing, including a primary-series COVID-19 vaccination, in univariate and multivariable analyses.
Results
We identified 13 529 eligible nursing home residents and 50 885 eligible community-dwelling adults with SARS-CoV-2 infection. Of the nursing home and community residents, 3020 (22%) and 6372 (13%), respectively, received at least 1 antibiotic prescription within 1 week of a SARS-CoV-2 positive result. Antibiotic prescribing in nursing home and community residents occurred, respectively, at 15.0 and 10.5 prescriptions per 1000 person-days prediagnosis and 20.9 and 9.8 per 1000 person-days postdiagnosis, higher than the baseline rates of 4.3 and 2.5 prescriptions per 1000 person-days. COVID-19 vaccination was associated with reduced prescribing in nursing home and community residents, with adjusted postdiagnosis incidence rate ratios (95% confidence interval) of 0.7 (0.4–1) and 0.3 (0.3–0.4), respectively.
Conclusions
Antibiotic prescribing was high and with little or no decline following SARS-CoV-2 diagnosis but was reduced in COVID-19–vaccinated individuals, highlighting the importance of vaccination and antibiotic stewardship in older adults with COVID-19.
Keywords: COVID-19, SARS-CoV-2, antibiotic use, inappropriate, antibiotic stewardship
Outpatient antibiotic use around the time of SARS-CoV-2 diagnosis is common. A completed primary COVID-19 (2-dose) vaccination series is associated with significantly reduced antibiotic use around the time of SARS-CoV-2 diagnosis.
Graphical Abstract
Graphical Abstract.
The coronavirus disease 2019 (COVID-19) pandemic has had dramatic effects on population health and healthcare delivery [1, 2]. Hundreds of millions of people worldwide have had documented infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent of COVID-19, and millions have died as a result [3].
During the course of the pandemic, treatment guidelines have evolved rapidly [4]. Throughout this time, a salient feature of published guidelines was that the use of antibiotic therapy for the treatment of concurrent bacterial infection was not recommended for outpatients with COVID-19 [4, 5]. The rationale behind these recommendations included: (1) COVID-19 infection was rarely associated with bacterial coinfection [6]; (2) accumulating evidence did not support antibiotic therapy either as a primary therapeutic or empiric treatment of coinfections [7, 8]; and (3) the use of antibiotic therapy has not generally been supported in similar viral respiratory infections, including coronaviruses, with the exception of possibly empirically in severely ill individuals [9].
Although guidelines do not support their use, antibiotics are frequently prescribed in both inpatients and outpatients with confirmed COVID-19 [10]. One rapid review and meta-analysis suggested that up to 75% of all individuals with known COVID-19 received antibiotics, including 60% of individuals from studies including outpatients [10]. A large retrospective study among Medicare beneficiaries aged ≥65 years in the United States found high rates of inappropriate antibiotic prescribing in outpatients with suspected COVID-19, ranging from 24% to 33% [6, 11]. While it is challenging to adjudicate appropriateness of antibiotic therapy in patients receiving antibiotics for respiratory infections, it is likely that the majority of these prescriptions in patients with COVID-19 are unnecessary [6, 10, 11]. Individual risks of antibiotic therapy include drug toxicities, Clostridioides difficile infection, and other flora-related complications [12, 13]. Population-level risks include the selection and transmission of antimicrobial-resistant organisms [14]. All of these potential consequences are of particular concern for older adults and vulnerable residents of nursing homes [15].
It is generally accepted that COVID-19 vaccination reduces the incidence and severity of COVID-19 infection [16, 17]. If antibiotic prescribing is dictated in part by the patient's disease severity, then it may be expected that COVID-19 vaccination would be associated with reduced antibiotic prescribing on both a population level [18] and for individuals with COVID-19. To date, the majority of data on antibiotic prescribing during the pandemic has been at aggregated population levels [19, 20], and studies using individual-level data are needed to better establish factors that may impact prescribing, such as COVID-19 vaccination.
In this study, we sought to evaluate how antibiotics are prescribed in patients with COVID-19, including the timing of prescribing, types of antibiotics prescribed, and associated risk factors including COVID-19 vaccination status.
METHODS
Study Design
We performed a population-wide retrospective study evaluating the relationship between timing of outpatient antibiotic prescription claims and the detection of SARS-CoV-2 in both residents of nursing homes and other community-dwelling older adults.
Population/Datasets
We evaluated antibiotic prescribing in individuals with a first laboratory-confirmed identification of SARS-CoV-2 (index event) among all residents aged ≥66 years, across the province of Ontario from 1 January 2020 until 31 December 2021 using ICES (formerly Institute for Clinical Evaluative Sciences) databases (see Supplementary Materials). The index event was the date the test was reported to the ordering clinician (not the date of collection) of a positive polymerase chain reaction (PCR) test for SARS-CoV-2. Projects that use data collected by ICES under section 45 of the personal health information protection act, and use no other data, are exempt from research ethics board review.
Outcomes
Our outcomes of interest were the prescription of a systemic antibiotic in the “prediagnosis” and “postdiagnosis” windows, as determined by date of an antibiotic prescription claim in the Ontario Drug Benefit program database [21]. The prediagnosis window is the time period where an individual may be symptomatic but is not yet formally diagnosed with COVID-19, and we defined this as within 7 days prior to the report of SARS-CoV-2 detection. The postdiagnosis window is the period of time after a COVID-19 diagnosis has been reported to a clinician, and we defined this as the period on or within 7 days after formal reporting of SARS-CoV-2 detection. The term “peridiagnosis” refers to the combined pre- and postdiagnosis periods. To compare antibiotic use with baseline rates for a given individual, we also evaluated dispensed antibiotics during a 4-week self-controlled period spanning from 4 to 8 weeks prior to the index date.
Covariates
For both nursing home and community groups, we considered the following covariates (Supplementary Table 1 and Supplementary Methods) that might be associated with the likelihood of antibiotic prescribing: demographic characteristics, healthcare utilization measures, provider characteristics, and prior completed primary-series COVID-19 vaccination (defined as ≥2 COVID-19 vaccinations within Ontario prior to index event). For individuals in the nursing home group, we also considered facility characteristics, and measures of residents’ health and functional status captured by the Resident Assessment Instrument-Minimum Data Set 2.0 (RAI-MDS 2.0), including validated scales for activities of daily living [22], cognitive performance [23], depressive symptoms [24], aggressive behaviors, and frailty [25–27]. For individuals in the community group, we also considered area-level social determinants of health (neighborhood ethnic diversity quintile and neighborhood income quintile) [28].
Person-Time
In determining the outcome of interest, we did not capture person-time that occurred in a hospitalized setting given that we could not ascertain antibiotic prescribing in this environment. Person-time was counted while an individual was alive, and not after documented death. An individual could be counted in 1 period (eg, the prediagnosis window) even if they had died later on (eg, in the postdiagnosis window).
Statistical Analysis
We generated descriptive statistics of antibiotic prescribing, expressed as the incidence rate of new prescriptions per 1000 person-days. Incidence rate ratios (IRRs) were determined by comparing the incidence rates during the prediagnosis and postdiagnosis windows to the self-controlled period; 95% confidence intervals (CIs) were generated for IRRs. We stratified IRRs by antibiotic class, as well as selected covariates. We performed multivariable regression analysis with generalized linear models. All analyses were performed using SAS Enterprise Guide Version 7.1.
We generated plots of day-specific IRRs by group (nursing homes and community residents) across the peridiagnosis period. Individuals did not contribute to the outcome or person-time on days they were admitted to hospital. We stratified by COVID-19 vaccination.
See the Supplementary Materials for further details on methods.
RESULTS
Characteristics of Older Adults With SARS-CoV-2 in Nursing Homes and the Community
Between 1 January 2020 and 31 December 2021, we identified 70 974 adult individuals (≥66 years of age) with a PCR test positive for SARS-CoV-2 in Ontario. After applying our exclusion criteria (Supplementary Figure 1), 64 414 remained in the cohort. Of these, 13 529 individuals were residents of nursing homes and 50 885 were community-residing older adults. Of the nursing home residents, 3020 (22%) had at least 1 antibiotic prescription claim in the peridiagnosis period and 2622 of these residents receiving an antibiotic did not have documented obstructive lung disease or immunocompromise. Of the community-dwelling individuals, 6372 (13%) had at least 1 antibiotic prescription claim in the peridiagnosis period and 5327 of these residents receiving an antibiotic did not have documented obstructive lung disease or immunocompromised status. Baseline characteristics of nursing home and community residents by antibiotic exposure are shown in Table 1 and Supplementary Table 2. Of all of the eligible nursing home residents, the majority were female (69%), the mean age was 85 years, and 7% had 2 or more doses of COVID-19 vaccine at the time of SARS-CoV-2 detection. The majority of nursing home residents lived in large care facilities with >99 beds (85%), and most were located in an urban setting (96%). Of all the eligible community residents, just over half were female (52%), the mean age was 75 years, and 19% had 2 or more doses of COVID-19 vaccine at the time of SARS-CoV-2 detection. The majority of community residents lived in neighborhoods corresponding to the 2 highest neighborhood ethnic diversity quintiles (60%); however, residence was more evenly distributed among neighborhood income quintiles (range, 16%–23%). COVID-19 cases declined substantially from July to December 2021, following the rollout of vaccination efforts occurring in the prior periods.
Table 1.
Selected Baseline Characteristics of Older Nursing Home and Community Residents With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection, by Receipt of Antibiotics in the Prediagnosis and/or Postdiagnosis Periods or No Receipt of Antibiotics
| Characteristic | Nursing Home Residents | Community Residents | ||||
|---|---|---|---|---|---|---|
| No Antibiotics | Prediagnosis | Postdiagnosis | No Antibiotics | Prediagnosis | Postdiagnosis | |
| Sample size, No. | 10 509 | 1275 | 2121 | 44 513 | 3419 | 3341 |
| Demographics | ||||||
| Age, y, mean (SD) | 85.3 (8.2) | 84.9 (8.2) | 85.1 (8.3) | 74.7 (7.7) | 75.7 (7.9) | 75.7 (8.1) |
| Female sex | 7338 (69.8) | 821 (64.4) | 1361 (64.2) | 22 980 (51.6) | 1807 (52.9) | 1804 (54.0) |
| COVID-19 vaccinated | 777 (7.4) | 83 (6.5) | 116 (5.5) | 8825 (19.8) | 428 (12.5) | 397 (11.9) |
| Healthcare utilization | ||||||
| Physician visits prior 12 mo, mean (SD) | 14.9 (8.6) | 16.1 (11.2) | 15.7 (10.5) | 10.2 (10.2) | 14.0 (11.4) | 13.1 (11.8) |
| Hospitalizations prior 12 mo, mean (SD) | 0.29 (0.68) | 0.39 (0.79) | 0.39 (0.82) | 0.17 (0.55) | 0.23 (0.64) | 0.23 (0.69) |
| Antibiotics prior 6 mo | 3281 (31.2) | 732 (57.4) | 1015 (47.9) | 9146 (20.5) | 1324 (38.7) | 1182 (35.4) |
| Comorbidities | ||||||
| Asthma | 79 (0.8) | 16 (1.3) | 15 (0.7) | 1551 (3.5) | 265 (7.8) | 222 (6.6) |
| Cancer | 640 (6.1) | 95 (7.5) | 152 (7.2) | 6221 (14.0) | 609 (17.8) | 506 (15.1) |
| Chronic kidney disease | 1698 (16.2) | 265 (20.8) | 444 (20.9) | 4866 (10.9) | 483 (14.1) | 450 (13.5) |
| COPD | 313 (3.0) | 68 (5.3) | 108 (5.1) | 1213 (2.7) | 232 (6.8) | 204 (6.1) |
| Coronary artery disease | 804 (7.7) | 108 (8.5) | 198 (9.3) | 2658 (6.0) | 242 (7.1) | 250 (7.5) |
| Congestive heart failure | 602 (5.7) | 116 (9.1) | 170 (8.0) | 2071 (4.7) | 216 (6.3) | 207 (6.2) |
| Dementia | 3621 (34.5) | 381 (29.9) | 691 (32.6) | 1511 (3.4) | 104 (3.0) | 148 (4.4) |
| Diabetes mellitus | 2639 (25.1) | 351 (27.5) | 620 (29.2) | 14 149 (31.8) | 1209 (35.4) | 1184 (35.4) |
| Hypertension | 1606 (15.3) | 244 (19.1) | 379 (17.9) | 14 863 (33.4) | 1288 (37.7) | 1236 (37.0) |
| Hypothyroidism | 1162 (11.1) | 164 (12.9) | 227 (10.7) | 5999 (13.5) | 539 (15.8) | 531 (15.9) |
| Ischemic stroke | 1269 (12.1) | 168 (13.2) | 260 (12.3) | 1209 (2.7) | 113 (3.3) | 117 (3.5) |
| Immunocompromised | 611 (5.8) | 126 (9.9) | 204 (9.6) | 3169 (7.1) | 433 (12.7) | 351 (10.5) |
| Liver disease | 233 (2.2) | 35 (2.7) | 58 (2.7) | 810 (1.8) | 72 (2.1) | 61 (1.8) |
| Time period | ||||||
| January–June 2020 | 3783 (36.0) | 563 (44.2) | 656 (30.9) | 2577 (5.8) | 336 (9.8) | 238 (7.1) |
| July–December 2020 | 3686 (35.1) | 373 (29.3) | 740 (34.9) | 9709 (21.8) | 844 (24.7) | 721 (21.6) |
| January–June 2021 | 2402 (22.9) | 272 (21.3) | 634 (29.9) | 21 863 (49.1) | 1620 (47.4) | 1773 (53.1) |
| July–December 2021 | 638 (6.1) | 67 (5.3) | 91 (4.3) | 10 364 (23.3) | 619 (18.1) | 609 (18.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SD, standard deviation.
Antibiotic Prescribing Rates Pre- and Postdiagnosis of SARS-CoV-2 Infection
Prescribing of any antibiotic in nursing home residents was frequent around the time of SARS-CoV-2 detection (Table 2), and occurred in 15.0 per 1000 person-days and 20.9 per 1000 person-days in the pre and postdiagnosis periods compared to 4.3 per 1000 person-days in the self-controlled period. The corresponding IRRs of antibiotic prescription were 3.5 (95% CI, 3.3–3.8) and 4.9 (95% CI, 4.6–5.3) in the pre- and postdiagnosis periods, respectively, compared to self-controlled periods. Antibiotic prescribing in community-residing older adults was also common around the time of SARS-CoV-2 detection (Table 3), and occurred in 10.5 per 1000 person-days and 9.8 per 1000 person-days in the pre and postdiagnosis periods compared to 2.5 per 1000 person-days in the self-controlled period. The IRRs of antibiotic prescription were 4.1 (95% CI, 3.9–4.3) and 3.8 (95% CI, 3.7–4.0) in the pre and postdiagnosis periods, respectively, compared to the self-controlled period. In a sensitivity analysis, shifting the index time by 24 hours later had minor impacts on IRRs of prescribing in either group. There were no major changes in findings in the nursing home cohort (prediagnosis IRR, 4.3; postdiagnosis IRR, 4.5), and in the community cohort there was a small decline from pre- to postdiagnosis (IRR, 4.9 to 3.2) though still markedly higher than the control period.
Table 2.
Antibiotic Prescribing Rates (per 1000 Person-Days) and Incidence Rate Ratio (Relative to Self-controlled periods) of Antibiotic Prescribing, for Older Residents of Nursing Homes With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2, Stratified by Selected Covariates and by Prediagnosis, Postdiagnosis, and Self-controlled Periods
| Variable | Prediagnosis | Postdiagnosis | Control Period | |||
|---|---|---|---|---|---|---|
| Rate | IRR (95% CI) | Rate | IRR (95% CI) | Rate | IRR | |
| All | 14.97 | 3.52 (3.28–3.77) | 20.92 | 4.92 (4.59–5.27) | 4.25 | Ref |
| Age, y | ||||||
| 65–74 | 15.92 | 3.41 (2.83–4.10) | 22.02 | 4.71 (3.89–5.70) | 4.67 | Ref |
| 75–84 | 15.76 | 3.57 (3.15–4.06) | 20.33 | 4.61 (4.05–5.24) | 4.41 | Ref |
| ≥85 | 14.37 | 3.52 (3.21–3.86) | 20.99 | 5.14 (4.70–5.63) | 4.08 | Ref |
| Sex | ||||||
| Female | 13.98 | 3.29 (3.03–3.58) | 19.06 | 4.49 (4.13–4.88) | 4.24 | Ref |
| Male | 17.13 | 4.01 (3.53–4.55) | 25.14 | 5.88 (5.20–6.65) | 4.27 | Ref |
| COVID-19 vaccine | ||||||
| <2 doses | 15.00 | 3.59 (3.34–3.85) | 21.43 | 5.12 (4.77–5.50) | 4.19 | Ref |
| ≥2 doses | 14.56 | 2.81 (2.18–3.63) | 14.40 | 2.78 (2.13–3.64) | 5.18 | Ref |
| Index period | ||||||
| January–June 2020 | 18.33 | 4.31 (3.86–4.83) | 18.71 | 4.40 (3.91–4.96) | 4.25 | Ref |
| July–December 2020 | 12.75 | 3.11 (2.75–3.51) | 20.58 | 5.02 (4.45–5.66) | 4.10 | Ref |
| January–June 2021 | 13.34 | 3.14 (2.71–3.63) | 26.54 | 6.24 (5.46–7.14) | 4.25 | Ref |
| July–December 2021 | 13.95 | 2.66 (2.03–3.50) | 13.17 | 2.52 (1.87–3.38) | 5.23 | Ref |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; IRR, incidence rate ratio; Ref, reference.
Table 3.
Antibiotic Prescribing Rates (per 1000 Person-Days) and Incidence Rate Ratios (Relative to Self-controlled Period) of Antibiotic Prescribing, for Community-Residing Older Adults With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection, Stratified by Selected Covariates and by Prediagnosis, Postdiagnosis, and Self-controlled Periods
| Variable | Prediagnosis | Postdiagnosis | Control Period | |||
|---|---|---|---|---|---|---|
| Rate | IRR (95% CI) | Rate | IRR (95% CI) | Rate | IRR | |
| All | 10.47 | 4.12 (3.92–4.33) | 9.78 | 3.84 (3.65–4.04) | 2.54 | Ref |
| Age, y | ||||||
| 65–74 | 9.38 | 4.68 (4.37–5.01) | 8.60 | 4.29 (4.00–4.60) | 2.00 | Ref |
| 75–84 | 11.73 | 4.30 (3.92–4.71) | 10.62 | 3.89 (3.54–4.28) | 2.73 | Ref |
| ≥85 | 12.68 | 2.80 (2.50–3.14) | 13.79 | 3.05 (2.73–3.41) | 4.52 | Ref |
| Sex | ||||||
| Female | 10.69 | 3.91 (3.66–4.19) | 10.08 | 3.69 (3.45–3.95) | 2.73 | Ref |
| Male | 10.23 | 4.37 (4.06–4.70) | 9.45 | 4.04 (3.74–4.35) | 2.34 | Ref |
| COVID-19 vaccine | ||||||
| <2 doses | 11.34 | 4.48 (4.24–4.73) | 10.82 | 4.27 (4.04–4.52) | 2.53 | Ref |
| ≥2 doses | 6.74 | 2.60 (2.30–2.93) | 5.64 | 2.17 (1.92–2.46) | 2.60 | Ref |
| Index period | ||||||
| January–June 2020 | 18.38 | 4.53 (3.84–5.33) | 13.15 | 3.24 (2.70–3.89) | 4.06 | Ref |
| July–December 2020 | 11.90 | 4.67 (4.19–5.19) | 9.62 | 3.77 (3.37–4.21) | 2.55 | Ref |
| January–June 2021 | 9.93 | 4.15 (3.86–4.46) | 10.60 | 4.43 (4.12–4.76) | 2.39 | Ref |
| July–December 2021 | 8.17 | 3.31 (2.97–3.69) | 7.44 | 3.02 (2.71–3.36) | 2.47 | Ref |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; IRR, incidence rate ratio; Ref, reference.
Antibiotic prescribing rates across strata of interest in the 2 SARS-CoV-2–infected cohorts are summarized in Tables 2 and 3. In nursing home residents with SARS-CoV-2, IRRs of postdiagnosis antibiotic prescribing were similar across the 3 age groups evaluated (Table 2) and appeared to be higher in men (5.9 [95% CI, 5.2–6.7]) compared to women (4.5 [95% CI, 4.1–4.9]). IRRs of postdiagnosis antibiotic prescribing in nursing home residents was lower in those with completed primary-series COVID-19 vaccination compared with those without, at 2.8 (95% CI, 2.1–3.6) and 5.1 (95% CI, 4.8–5.5), respectively. In community residents with SARS-CoV-2, IRRs of postdiagnosis antibiotic prescribing showed a slight decline with increasing age (Table 3) and appeared to be higher in men (4.0 [95% CI, 3.7–4.4]) compared to women (3.7 [95% CI, 3.4–4.0]). The IRR of postdiagnosis antibiotic prescribing in community residents was lower in those with completed primary-series COVID-19 vaccination compared to those without, at 2.2 (95% CI, 1.9–2.5) and 4.3 (95% CI, 4.0–4.5), respectively. While IRRs of antibiotic prescribing in nursing homes over the last 6-month period of July–December 2021 (2.5 [95% CI, 1.9–3.4]) were lower than the initial period of January–June 2020 (4.4 [95% CI, 3.9–5.0]), there were no major prescribing differences for the community-residing older adults, with IRRs of 3.0 (95% CI, 2.7–3.4) and 3.2 (95% CI, 2.7–3.9) in the first and last 6-month time periods, respectively.
We also looked specifically at respiratory and nonrespiratory antibiotics (Supplementary Tables 3 and 4). Respiratory antibiotics were highly prescribed, with IRRs of 6.7 (95% CI, 6.0–7.5) and 11.7 (95% CI, 10.7–12.9) for the pre- and postdiagnosis periods in nursing home residents, and IRRs of 7.4 (95% CI, 7.0–8.0) and 7.1 (95% CI, 6.7–7.6) for the pre- and postdiagnosis periods for community residents. Nonrespiratory antibiotics, however, showed minimal or no significant differences in prescribing, with IRRs of 1.7 (95% CI, 1.5–1.8) and 1.0 (95% CI, .9–1.1) for the pre- and postdiagnosis periods in nursing home residents, and IRRs of 1.5 (95% CI, 1.4–1.6) and 1.2 (95% CI, 1.1–1.4) for the pre- and postdiagnosis periods for community residents.
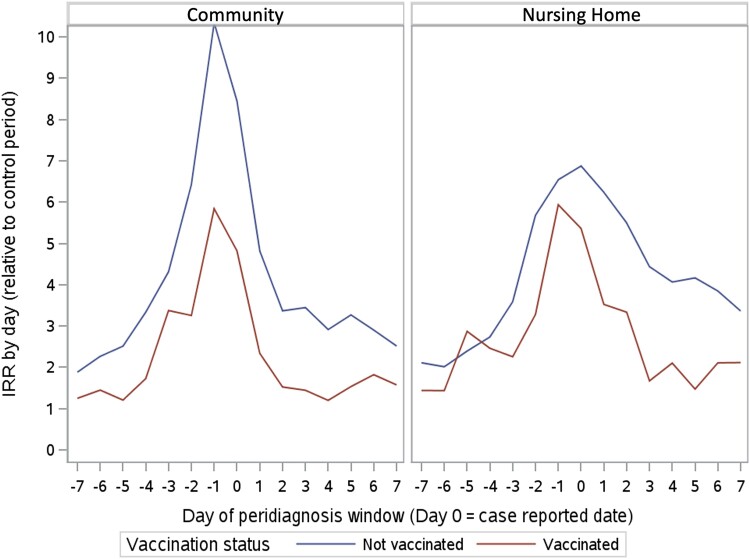
Incidence Rate Ratio Curves in the Peridiagnosis Period
The IRR curves of antibiotic prescription by day for both nursing home and community residents, with stratification by completed primary-series COVID-19 vaccination, are shown in Figure 1. For both the nursing home and community populations, the subgroups with completed primary-series COVID-19 vaccination showed lower IRRs throughout the peridiagnosis period.
Figure 1.
Incidence rate ratios (IRRs) (relative to the self-controlled period) of antibiotic prescriptions in nursing homes and community residents, stratified by coronavirus disease 2019 vaccination status.
Resident Characteristics Associated With Antibiotic Prescribing
A number of factors were significantly associated with incidence rates of antibiotic prescribing (Table 4 and Supplementary Table 5). Among nursing home residents, completed primary-series COVID-19 vaccination and female sex were associated with reductions in prescribing rates, with postdiagnosis adjusted IRRs of 0.7 (95% CI, .4–1.0) and 0.7 (95% CI, .7–.8), respectively. Other relevant variables in nursing home residents included the presence of chronic kidney disease and immunocompromised status, which were associated with increased prescribing, with postdiagnosis adjusted IRRs of 1.2 (95% CI, 1.1–1.4) and 1.3 (95% CI, 1.1–1.5), respectively. Among community residents, completed primary-series COVID-19 vaccination was associated with reductions in antibiotic prescribing rates, with a postdiagnosis adjusted IRR of 0.3 (95% CI, .3–.4). Other relevant variables in community residents included the presence of asthma and chronic obstructive lung disease, which were associated with increased prescribing with postdiagnosis adjusted IRRs of 1.5 (95% CI, 1.3–1.7) and 1.8 (95% CI, 1.5–2.1), respectively. The last 6-month time period (July–December 2021) was not associated with declines in postdiagnosis IRRs of prescribing compared to the initial period (January–June 2020) for nursing home residents (0.97 [95% CI, .6–1.7]) or community residents (1.6 [95% CI, 1.3–1.9]). There were no consistent associations with social determinants of health including income quintile or ethnic diversity quintile, and pre- or postdiagnosis prescribing rates, among community residents.
Table 4.
Selected Adjusted Incidence Rate Ratios From Multivariable Regression Models of Antibiotic Prescribing Rates in the Prediagnosis, Postdiagnosis, and Self-controlled Periods
| Variable | Nursing Home Residents | Community Residents | ||||
|---|---|---|---|---|---|---|
| Prediagnosis | Postdiagnosis | Control Period | Prediagnosis | Postdiagnosis | Control Period | |
| Demographics | ||||||
| Age | 0.99 (.99–1.00) | 1.00 (1.00–1.01) | 1.00 (.99–1.01) | 1.01 (1.01–1.02) | 1.02 (1.01–1.02) | 1.02 (1.01–1.02) |
| Female sex | 0.84 (.75–.95) | 0.74 (.67–.82) | 1.02 (.91–1.15) | 1.00 (.93–1.07) | 0.99 (.92–1.07) | 1.07 (.98–1.16) |
| COVID-19 vaccinated (≥2 doses) | 1.18 (.78–1.78) | 0.66 (.44–.99) | 1.09 (.67–1.76) | 0.43 (.37–.51) | 0.31 (.26–.37) | 1.18 (.95–1.46) |
| Healthcare utilization | ||||||
| Physician visits prior 12 mo | 1.01 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.01–1.02) | 1.01 (1.01–1.01) | 1.02 (1.02–1.03) |
| Hospitalizations prior 12 mo | 0.94 (.86–1.02) | 1.02 (.95–1.09) | 1.24 (1.16–1.33) | 0.92 (.87–.98) | 0.99 (.93–1.05) | 1.26 (1.20–1.34) |
| Receipt of antibiotics prior 6 mo | 2.23 (1.94–2.57) | 1.41 (1.26–1.57) | 3.49 (3.06–3.97) | 1.89 (1.76–2.03) | 1.71 (1.59–1.85) | 4.28 (3.94–4.64) |
| Comorbidities | ||||||
| Asthma | 1.32 (.79–2.23) | 0.70 (.42–1.15) | 0.72 (.45–1.15) | 1.54 (1.35–1.75) | 1.45 (1.25–1.69) | 1.09 (.92–1.30) |
| Cancer | 1.04 (.83–1.29) | 1.05 (.87–1.28) | 1.16 (.96–1.41) | 1.08 (.99–1.19) | 0.95 (.85–1.05) | 1.16 (1.04–1.28) |
| Chronic kidney disease | 1.02 (.88–1.17) | 1.21 (1.08–1.36) | 1.20 (1.04–1.39) | 1.03 (.93–1.15) | 1.09 (.97–1.22) | 1.02 (.91–1.15) |
| COPD | 1.17 (.94–1.46) | 1.26 (1.00–1.59) | 0.92 (.71–1.20) | 1.65 (1.43–1.91) | 1.75 (1.49–2.05) | 1.47 (1.24–1.75) |
| Coronary artery disease | 0.98 (.82–1.18) | 1.09 (.91–1.30) | 1.01 (.83–1.23) | 1.05 (.92–1.21) | 0.91 (.79–1.05) | 0.88 (.76–1.02) |
| Congestive heart failure | 1.16 (.96–1.40) | 0.99 (.80–1.23) | 1.30 (1.09–1.56) | 0.93 (.80–1.08) | 1.03 (.88–1.21) | 1.01 (.86–1.18) |
| Dementia | 0.86 (.75–.98) | 1.04 (.93–1.16) | 0.87 (.77–.98) | 0.76 (.62–.92) | 1.15 (.96–1.38) | 1.28 (1.07–1.54) |
| Diabetes mellitus | 0.98 (.87–1.10) | 1.13 (1.01–1.25) | 1.06 (.93–1.21) | 1.06 (.99–1.14) | 1.13 (1.05–1.22) | 1.15 (1.06–1.26) |
| Hypertension | 1.02 (.89–1.19) | 1.00 (.87–1.15) | 0.96 (.82–1.12) | 1.01 (.94–1.09) | 1.03 (.96–1.11) | 0.87 (.80–0.96) |
| Hypothyroidism | 1.04 (.89–1.21) | 0.86 (.73–1.02) | 1.02 (.87–1.21) | 1.04 (.95–1.14) | 1.10 (1.00–1.21) | 1.08 (.97–1.21) |
| Ischemic stroke | 1.01 (.86–1.18) | 0.92 (.79–1.07) | 1.00 (.84–1.18) | 1.07 (.89–1.29) | 1.16 (.95–1.41) | 1.00 (.81–1.24) |
| Immunocompromised | 1.38 (1.15–1.66) | 1.31 (1.13–1.52) | 1.15 (.96–1.39) | 1.49 (1.34–1.65) | 1.32 (1.17–1.49) | 1.35 (1.20–1.53) |
| Liver disease | 1.06 (.78–1.45) | 0.94 (.68–1.30) | 1.28 (.96–1.69) | 1.04 (.82–1.31) | 0.87 (.66–1.13) | 0.84 (.64–1.10) |
| Time period | ||||||
| January–June 2020 | Ref | Ref | Ref | Ref | Ref | Ref |
| July–December 2020 | 0.82 (.66–1.01) | 1.20 (.89–1.61) | 1.08 (.90–1.30) | 0.72 (.63–.82) | 0.83 (.71–.96) | 0.89 (.76–1.05) |
| January–June 2021 | 0.81 (.63–1.04) | 1.48 (1.10–2.00) | 1.02 (.85–1.22) | 0.60 (.53–.67) | 0.92 (.80–1.06) | 0.86 (.74–1.00) |
| July–December 2021 | 0.66 (.41–1.05) | 0.97 (.56–1.68) | 1.11 (.63–1.97) | 0.97 (.81–1.16) | 1.59 (1.31–1.92) | 0.79 (.62–1.01) |
Data are presented as incidence rate ratios (95% confidence intervals). Fully adjusted model includes all covariates noted in the Methods (see Supplementary Table 5).
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; Ref, reference.
DISCUSSION
In this large cohort study of outpatients with PCR-confirmed SARS-CoV-2 during the first 2 years of the COVID-19 pandemic, we found that antibiotic prescribing was high and largely sustained over time. Moreover, we found that the reporting of the presence of SARS-CoV-2 virus did not consistently reduce prescribing rates. Last, we found that a completed COVID-19 vaccination primary series was associated with a substantial reduction in rates of antibiotic prescribing in patients with confirmed SARS-CoV-2, reducing adjusted outpatient antibiotic prescribing rates up to 3-fold (69% reduction) compared to those in unvaccinated outpatients with SARS-CoV-2.
Previous literature has demonstrated high rates of antibiotic prescribing among those with COVID-19, ranging from 25% to 60% in populations with outpatients [10, 11]. This is in direct contrast to lack of strong evidence for bacterial coinfection in the outpatient setting [6] and multiple international guidelines, which generally do not recommend antibiotic therapy in outpatients with COVID-19 [4, 5]. While it is possible that some initial early antibiotic prescribing may have intended to provide direct antiviral effect (ie, azithromycin), these therapies were never broadly incorporated into guidelines, and evidence rapidly mounted against their use [29, 30]. Studies evaluating antibiotic prescribing in COVID-19 typically have not delineated the timing of prescribing or whether prescriptions were initiated before or after laboratory-confirmed diagnosis [11]. We hypothesized that antibiotic prescribing rates would substantially decline after a known diagnosis of COVID-19, given that prescribers would have concrete evidence of SARS-CoV-2 viral infection [31]. However, antibiotic prescribing rates after identification of SARS-CoV-2 were largely similar, or in some instances higher, than in the time periods immediately predating reporting of results. Perhaps this is due to prescribers treating patients based on severity of illness, and concern that bacterial infection may underlie the symptoms. This theory is compatible with the multitude of contextual factors that influence antibiotic prescribing [32]. As a sensitivity analysis, shifting index date later did result in a slight decline in postdiagnosis prescribing in the community cohort, though still substantially elevated from baseline. Overall, our findings support that viral testing itself is likely a poor antibiotic stewardship intervention, and this is consistent with the literature [33, 34].
We identified incomplete COVID-19 primary-series vaccination (0 or 1 dose) as a strong predictor of increased antibiotic prescribing. This was independent of time periods dominated by either wild-type, Alpha, or Delta variants of SARS-CoV-2 [35]. This may be due to increased severity of illness in those with incomplete COVID-19 primary-series vaccination and providers prescribing antibiotics over concern for alternative underlying infection. Interestingly, other strong predictors of antibiotic prescribing identified in this study were factors also related to increased severity of disease, and included preexisting respiratory diseases and sex [36, 37]. Prevalence of COVID-19 vaccination in these populations of individuals with COVID-19 is relatively low. This is primarily due to a greater density of cases (from 2020 to 2021) occurring early in the pandemic.
Notably, we did not identify strong associations between antibiotic prescribing and neighborhood-level social determinants of health such as income or ethnic diversity among community residents, in contrast to previous findings of associations between social determinants of health and other COVID-19–related management and outcome measures [36, 38]. While there was a signal for a possible decline in prescribing in nursing home populations in the last 6 months of the study period, this was not evident in the multivariable analyses, and overall there is not strong support for secular reductions in antibiotic prescribing.
This study is not without its limitations. First, our study was undertaken in the local pandemic and healthcare context of Ontario, Canada, which may limit generalizability [39]. Similarly, these findings were derived from observations from the initial 2 years of the pandemic and do not necessarily reflect the current interplay between vaccination and antibiotic prescribing. Second, we are unable to definitively identify whether a patient was asymptomatically infected versus symptomatic. However, our use of microbiologic confirmation of SARS-CoV-2 is likely a strong indicator of COVID-19 [40]. While we did not evaluate the use of diagnosis and/or billing codes, these may offer insight into mechanisms in future studies. Last, our dataset is limited in both the variables we can collect and to the time period we have analyzed. However, one of the benefits of restricting to the first 2 years of the pandemic is that SARS-CoV-2 testing was performed almost entirely via PCR and results were accessible via provincial laboratory information systems, reducing possible selection bias that may be introduced by nonreportable rapid antigen test positives. As we could not collect hospital-based antibiotic prescribing with our dataset, it is possible that individuals who were severely ill could have been hospitalized and received an antibiotic in that setting.
In summary, antibiotic prescribing around the time of identification of SARS-CoV-2 in outpatients was high and did not significantly decline throughout the pandemic. Moreover, the reported presence of SARS-CoV-2 did not seem to lead to substantial reductions in prescribing rates. COVID-19 vaccination appeared to be a significant factor in reducing antibiotic prescribing rates in patients with SARS-CoV-2, suggesting that the vaccine may play a role in broader antibiotic stewardship efforts. Future studies should seek to evaluate the population-level impacts of COVID-19 vaccination on antibiotic use.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Derek R MacFadden, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada; ICES, Toronto, Canada; Division of Infectious Diseases, The Ottawa Hospital, Ottawa, Canada.
Colleen Maxwell, ICES, Toronto, Canada; Schools of Pharmacy and Public Health Sciences, University of Waterloo, Waterloo, Canada.
Dawn Bowdish, Faculty of Health Sciences, McMaster University, Hamilton, Canada.
Susan Bronskill, ICES, Toronto, Canada.
James Brooks, Division of Infectious Diseases, The Ottawa Hospital, Ottawa, Canada.
Kevin Brown, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Public Health Ontario, Toronto, Canada.
Lori L Burrows, Faculty of Health Sciences, McMaster University, Hamilton, Canada.
Anna Clarke, ICES, Toronto, Canada.
Bradley Langford, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Public Health Ontario, Toronto, Canada.
Elizabeth Leung, Unity Health Toronto, Toronto, Canada; Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Canada.
Valerie Leung, Public Health Ontario, Toronto, Canada; Michael Garron Hospital, Toronto East Health Network, Toronto, Canada.
Doug Manuel, ICES, Toronto, Canada.
Allison McGeer, Sinai Health System, Toronto, Canada.
Sharmistha Mishra, ICES, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Department of Medicine, University of Toronto, Toronto, Canada; MAP Centre for Urban Health Solutions, St. Michael's Hospital, Unity Health Toronto, Toronto, Canada; Institute of Medical Science, University of Toronto, Toronto, Canada.
Andrew M Morris, Sinai Health System, Toronto, Canada.
Caroline Nott, Division of Infectious Diseases, The Ottawa Hospital, Ottawa, Canada.
Sumit Raybardhan, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Pharmacy Department, North York General Hospital, Toronto, Canada.
Mia Sapin, School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada.
Kevin L Schwartz, ICES, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Public Health Ontario, Toronto, Canada; Unity Health Toronto, Toronto, Canada.
Miranda So, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Canada; Toronto General Hospital Research Institute, Toronto, Canada.
Jean-Paul R Soucy, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Nick Daneman, ICES, Toronto, Canada; Division of Infectious Diseases, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada.
Notes
Author Contributions. All authors contributed to the study concept and design. D. R. M., A. C., and N. D. performed the data analysis. D. M. and N. D. drafted the manuscript. All authors contributed to the critical review of the manuscript.
Acknowledgments. Parts of these materials are based on data and information compiled and provided by the Ontario Ministry of Health (MOH) and the Canadian Institute for Health Information (CIHI). We thank IQVIA Solutions Canada Inc for use of its Drug Information File. This document used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from the Canada Post Corporation, and/or data adapted from the Ontario MOH Postal Code Conversion File, which contains data copied under license from the Canada Post Corporation and Statistics Canada. We thank the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Disclaimer. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement by ICES, the MOH, the Ministry of Long-Term Care (MLTC), or CIHI is intended or should be inferred. No endorsement by the OHDP, its partners, or the Province of Ontario is intended or should be inferred.
Financial support. This work was supported by ICES, which is funded by an annual grant from the Ontario MOH and the MLTC. This study also received funding from the Canadian Institutes of Health Research (CIHR) and by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to COVID-19 and its related impacts.
References
- 1. COVID-19—implications for the health care system. N Engl J Med 2020; 383:1698. [DOI] [PubMed] [Google Scholar]
- 2. Maire J, Sattar A, Henry R, et al. How different COVID-19 recovery paths affect human health, environmental sustainability, and food affordability: a modelling study. Lancet Planet Health 2022; 6:e565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int. Accessed 2 October 2022.
- 4. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 [manuscript published online ahead of print 27 April 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO) . Therapeutics and COVID-19: living guideline. Geneva, Switzerland: WHO, 2022. [PubMed] [Google Scholar]
- 6. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler CC, Yu L-M, Dorward J, et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med 2021; 9:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. N Engl J Med 2017; 376:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021; 27:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsay SV, Bartoces M, Gouin K, Kabbani S, Hicks LA. Antibiotic prescriptions associated with COVID-19 outpatient visits among Medicare beneficiaries, April 2020 to April 2021. JAMA 2022; 327:2018–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olesen SW, MacFadden D, Grad YH. Cumulative probability of receiving an antibiotic prescription over time. N Engl J Med 2019; 380:1872–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daneman N, Bronskill SE, Gruneir A, et al. Variability in antibiotic use across nursing homes and the risk of antibiotic-related adverse outcomes for individual residents. JAMA Intern Med 2015; 175:1331–9. [DOI] [PubMed] [Google Scholar]
- 14. Goossens H, Ferech M, Vander Stichele R, Elseviers M; Project Group ESAC . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365:579–87. [DOI] [PubMed] [Google Scholar]
- 15. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging 2018; 13:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol 2022; 7:379–85. [DOI] [PubMed] [Google Scholar]
- 18. Buckley BS, Henschke N, Bergman H, et al. Impact of vaccination on antibiotic usage: a systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:1213–25. [DOI] [PubMed] [Google Scholar]
- 19. Knight BD, Shurgold J, Smith G, et al. The impact of COVID-19 on community antibiotic use in Canada: an ecological study. Clin Microbiol Infect 2022; 28:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King LM, Lovegrove MC, Shehab N, et al. Trends in US outpatient antibiotic prescriptions during the coronavirus disease 2019 pandemic. Clin Infect Dis 2021; 73:e652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003; 10:67–71. [PubMed] [Google Scholar]
- 22. Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci 1999; 54:M546–53. [DOI] [PubMed] [Google Scholar]
- 23. Hartmaier SL, Sloane PD, Guess HA, Koch GG, Mitchell CM, Phillips CD. Validation of the Minimum Data Set Cognitive Performance Scale: agreement with the Mini-Mental State Examination. J Gerontol A Biol Sci Med Sci 1995; 50:M128–33. [DOI] [PubMed] [Google Scholar]
- 24. Burrows AB, Morris JN, Simon SE, Hirdes JP, Phillips C.. Development of a minimum data set-based depression rating scale for use in nursing homes. Age Ageing 2000; 29:165–72. [DOI] [PubMed] [Google Scholar]
- 25. Maclagan LC, Maxwell CJ, Harris DA, et al. Sex differences in antipsychotic and benzodiazepine prescribing patterns: a cohort study of newly admitted nursing home residents with dementia in Ontario, Canada. Drugs Aging 2020; 37:817–27. [DOI] [PubMed] [Google Scholar]
- 26. Perlman CM, Hirdes JP. The Aggressive Behavior Scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc 2008; 56:2298–303. [DOI] [PubMed] [Google Scholar]
- 27. Maclagan LC, Maxwell CJ, Gandhi S, et al. Frailty and potentially inappropriate medication use at nursing home transition. J Am Geriatr Soc 2017; 65:2205–12. [DOI] [PubMed] [Google Scholar]
- 28. van Ingen T, Matheson FI. The 2011 and 2016 iterations of the Ontario Marginalization Index: updates, consistency and a cross-sectional study of health outcome associations. Can J Public Health 2011; 2022:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial. JAMA 2021; 326:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med 2020; 383:2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaughn VM, Gandhi TN, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis 2021; 72:e533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Azzawi R, Halvorsen PA, Risør T. Context and general practitioner decision-making—a scoping review of contextual influence on antibiotic prescribing. BMC Fam Pract 2021; 22:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao S, Lamb MM, Moss A, et al. Effect of rapid respiratory virus testing on antibiotic prescribing among children presenting to the emergency department with acute respiratory illness: a randomized clinical trial. JAMA Netw Open 2021; 4:e2111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tonkin-Crine SK, Tan PS, van Hecke O, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev 2017; 9:CD012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Public Health Ontario . COVID-19 variants of concern (VOCs). Available at: https://www.publichealthontario.ca/en/Diseases-and-Conditions/Infectious-Diseases/Respiratory-Diseases/Novel-Coronavirus/Variants. Accessed 3 October 2022.
- 36. Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open 2021; 4:e2116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID-19 infection. Clin Infect Dis 2020; 71:1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mishra S, Ma H, Moloney G, et al. Increasing concentration of COVID-19 by socioeconomic determinants and geography in Toronto, Canada: an observational study. Ann Epidemiol 2022; 65:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perroud JM, Soldano S, Avanceña ALV, Wagner A. Adult vaccination uptake strategies in low- and middle-income countries: a systematic review. Vaccine 2022; 40:5313–21. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt AS, McElrath EE, Claggett BL, et al. Accuracy of ICD-10 diagnostic codes to identify COVID-19 among hospitalized patients. J Gen Intern Med 2021; 36:2532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.