Abstract
Background
Bacterial infections cause substantial pain and disability among people who inject drugs. We described time trends in hospital admissions for injecting-related infections in England.
Methods
We analyzed hospital admissions in England between January 2002 and December 2021. We included patients with infections commonly caused by drug injection, including cutaneous abscesses, cellulitis, endocarditis, or osteomyelitis, and a diagnosis of opioid use disorder. We used Poisson regression to estimate seasonal variation and changes associated with coronavirus disease 2019 (COVID-19) response.
Results
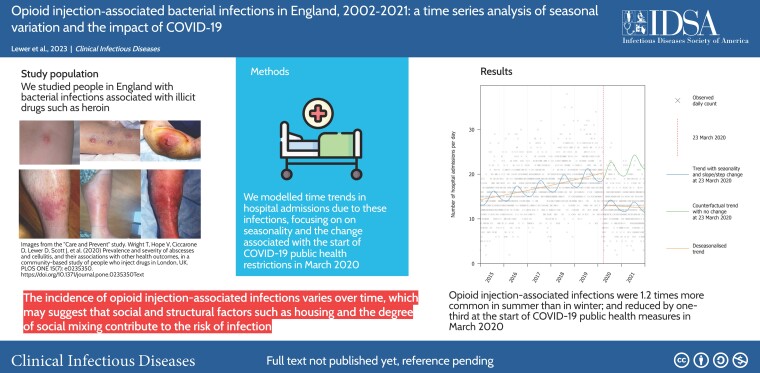
There were 92 303 hospital admissions for injection-associated infections between 2002 and 2021. Eighty-seven percent were skin, soft-tissue, or vascular infections; 72% of patients were male; and the median age increased from 31 years in 2002 to 42 years in 2021. The rate of admissions reduced from 13.97 per day (95% confidence interval [CI], 13.59–14.36) in 2003 to 8.94 (95% CI, 8.64–9.25) in 2011, then increased to 18.91 (95% CI, 18.46–19.36) in 2019. At the introduction of COVID-19 response in March 2020, the rate of injection-associated infections reduced by 35.3% (95% CI, 32.1–38.4). Injection-associated infections were also seasonal; the rate was 1.21 (95% CI, 1.18–1.24) times higher in July than in February.
Conclusions
This incidence of opioid injection-associated infections varies within years and reduced following COVID-19 response measures. This suggests that social and structural factors such as housing and the degree of social mixing may contribute to the risk of infection, supporting investment in improved social conditions for this population as a means to reduce the burden of injecting-related infections.
Keywords: substance use disorders, bacterial infections, injection drug use, time series analysis
After increasing for a decade, the incidence of opioid injection–associated bacterial infections in England reduced by one-third at the introduction of coronavirus disease 2019 pandemic response measures. Injection-associated infections are also seasonal, peaking in summer. These trends suggest structural determinants of risk.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/opioid-injection-associated-bacterial-infections-in-england-2002-2021-a-time-series-analysis-of-seasonal-variation-and-the-impact-of-covid-19-bc3f2abb-cb0a-46ac-8688-6d62e520582e
Bacterial infections cause substantial pain and disability among people who inject psychoactive drugs. Skin abscesses and cellulitis around injecting sites are the most frequent problems, with surveys of people who inject drugs finding lifetime prevalence ranging from 6% to 69% [1]. Invasive infections are also common; the rate of hospital admission for infective endocarditis among people injecting heroin in London is 44 times higher than in the general population [2]. In contrast to viral infections such as human immunodeficiency virus and hepatitis C, which are transmitted in blood via shared injecting equipment, injecting-associated bacterial infections are usually caused by patients’ own skin and mouth flora, typically gram-positive cocci [3–7]. Infections can also be caused by bacterial spores contaminating drugs, though this is rare [8].
Data from hospitals suggest that the number of injection-associated infections has increased since the early 2000s in many countries, including England [9], Norway [10], the United States [6, 11], Canada [12], Australia [13], and India [14]. In North America, this may be due to increases in prescription opioid dependence and subsequent increases in injection of illicit opioids [15]. In addition to the increasing prevalence of illicit opioid use, fentanyl has become more common in the illicit drug market and is associated with more frequent injection, which in turn leads to more reuse and sharing of equipment [16, 17]. However, the incidence of infections has also increased in England despite stable or decreasing prevalence of illicit opioid use [18, 19] and the continued rarity of synthetic opioids in the illicit drug supply [20]. One common factor between North America and England is the increased use of stimulants [21, 22], often in combination with opioids, which is associated with more frequent injection and risk of infections. However, the reasons for increasing incidence in different countries are not known.
Although many studies have described long-term trends in injection-associated infections, few have investigated seasonal and short-term trends. Many other infections have seasonal patterns that have informed public health responses, such as seasonal immunization campaigns and heightened hospital infection control measures in winter. Bacterial skin infections in the general population are more common in summer [23], and studies in New York in the 1990s and Stockholm between 2007 and 2018 suggest that the rate of injection-associated infections is also higher in summer [7].
The incidence of many infections was affected by the public health response to coronavirus disease 2019 (COVID-19). Nonpharmaceutical interventions included restrictions on movement, closure of public places, behavioral advice, and support for vulnerable groups including improved housing (more detail is provided in Supplementary Information). These interventions had a dramatic effect on seasonal respiratory infections but also reduced many nonrespiratory infections [24, 25], and may have affected injection-associated infections.
The role of external influences such as seasons and the COVID-19 response could inform our understanding of infection dynamics and determinants of risk, and therefore how infections could be prevented. We used hospital data from England to describe long-term trends, seasonal patterns, and the impact of COVID-19 public health responses.
METHODS
We did a descriptive time series analysis of trends in opioid injection–associated bacterial infections.
Data Source
We used the Hospital Episode Statistics database, which includes clinical information about patients admitted to all National Health Service hospitals in England [26]. We included emergency admissions in which the admission date was between 1 January 2002 and 31 December 2021, and the patient was aged between 15 and 64 years at admission.
Hospital Episode Statistics is a database of episodes of care led by a single doctor or medical team; therefore, a hospital admission can include multiple records. To avoid double counting, we only included the first episode in each admission.
We included patients with a primary diagnosis of cutaneous abscess, cellulitis, other skin and soft tissue infections, phlebitis, or thrombophlebitis (grouped as “localized infections”), endocarditis, septicemia, osteomyelitis or septic arthritis, or necrotizing fasciitis (grouped as “invasive infections”). International Classification of Diseases, 10th revision (ICD-10), codes for these diagnoses are listed in Supplementary Material. We defined “opioid injection-associated infections” as those with a secondary diagnosis of opioid use disorder.
A cohort study of community-recruited people who use illicit opioids found that 53% of participants admitted to hospital because of the infections listed here had a secondary diagnosis of opioid use disorder [27], and this proportion was stable over time. This suggests that our method captures approximately half of opioid injection-associated infections and produces valid time trends.
Variables
For each patient, we extracted the primary diagnosis, age at admission, sex, ethnicity, duration of admission, mode of discharge (standard, self-discharge, died, or other), Index of Multiple Deprivation (a measure of the socioeconomic status of the patient's neighborhood [28]), whether the patient was known to be homeless (defined as having “no fixed abode” or the ICD-10 code Z59.0; “homelessness”), whether the patient had an unplanned hospital admission within 28 days following discharge, and whether an operative procedure was performed during the admission.
Statistical Analysis
We described the demographic and clinical characteristics of patients. To investigate seasonality and the change in rate associated with COVID-19 response, we focused on hospital admissions on or after 1 January 2015 and aggregated the data into daily counts. We used a Poisson model with a dependent variable of the count of admissions per day. Independent variables were a linear term for time defined as the number of days after 1 January 2015, an interaction term between time and a binary indicator for before/after 23 March 2020 (the date when strict COVID-19 restrictions were introduced in England), and a harmonic term over the calendar year with 2 sine and cosine pairs. The interaction term estimates a step-change and a change of slope, and the harmonic term estimates seasonality [29]. We reported the day of year when the peak and low predicted admissions occurred, and estimated magnitude of seasonality as the ratio between the peak and low, with confidence intervals (CI) estimated using a Monte-Carlo method.
We repeated these procedures for invasive infections in isolation and 2 other patient groups: (1) staphylococcal and streptococcal skin infections that did not have a secondary diagnosis of opioid use disorder (reflective of infections in the general population, presumed unrelated to drug use) and (2) noninfectious complications of drug use, using 2 of the UK government's measures for tracking drug-related health harms [30]: “admissions for drug-related mental and behavioral disorders”, and “admissions for poisoning by drug misuse.” The purpose of these comparisons was to help us interpret time trends in opioid injection–associated infections; in particular, whether time trends are driven by changes in drug use behaviors or by microbiological factors that may also affect the wider population.
We also did a supplementary analysis to test if COVID-19 was associated with a change in the clinical severity of patients admitted to hospital with opioid injection–associated infections. We compared admissions in the 12 months before and after 23 March 2020, in terms of the duration of admissions, the proportion that died, and the proportion of infections that were invasive. If COVID-19 was associated with reduced accessibility of hospitals, we expected an increased severity of admissions.
Analysis was done using SQL and R version 4.2.1. Analysis code is available at https://www.github.com/danlewer/irid_trends.
Approvals
This analysis was done as part of the UK Health Security Agency's surveillance of opioid injection–associated infections, and therefore research ethics was not required. This was a secondary analysis of anonymized data.
RESULTS
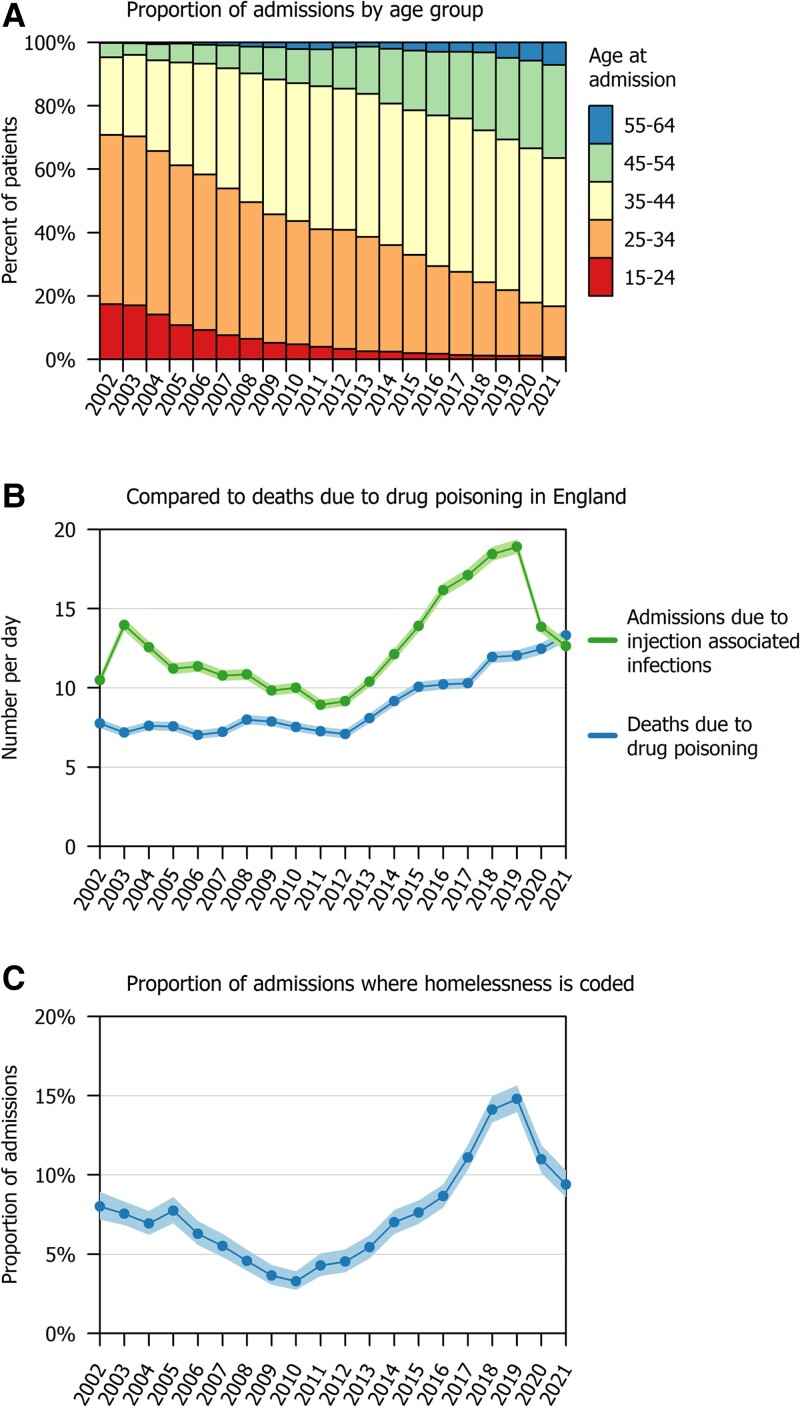
We identified 92 303 admissions between 2002 and 2021. The median age was 37 years (interquartile range [IQR], 31–42) and 66 359/92 303 (71.9%) were male. A total of 79 890/92 303 (86.6%) patients had localized infections and 12 413/92 303 (13.4%) had invasive infections (Table 1). The median age increased from 31 years (IQR, 26–36) in 2002 to 42 years (IQR, 37–48) in 2021, and in recent years there were very few patients aged under 25 (Figure 1A ). The proportion coded as experiencing homelessness reduced from 307/3827 (8.0%) in 2002 to 120/3653 (3.3%) in 2010, then increased to 1021/6902 (14.8%) in 2019, then reduced again to 433/4612 (9.4%) in 2021 (Figure 1, panel C). Other patient characteristics were consistent over time.
Table 1.
Characteristics of Patients Admitted to Hospitals in England With Opioid Injection–associated Bacterial Infections, 2002–2021
| Variable | Level | Number (%) |
|---|---|---|
| Total | 92 303 (100.0) | |
| Age | 15–24 | 4898 (5.3) |
| 25–34 | 32 382 (35.1) | |
| 35–44 | 38 511 (41.7) | |
| 45–54 | 14 234 (15.4) | |
| 55–64 | 2278 (2.5) | |
| Median [IQR] | 37 [31–42] | |
| Mean [SD] | 37.0 [8.2] | |
| Sex | Female | 25 944 (28.1) |
| Male | 66 359 (71.9) | |
| Ethnicitya | White British or White Other | 85 809 (93.0) |
| Black/African/Caribbean/Black British | 853 (0.9) | |
| Asian/Asian British | 703 (0.8) | |
| Mixed/Multiple ethnic groups | 899 (1.0) | |
| Other ethnicity or unknown | 4039 (4.4) | |
| Index of Multiple Deprivationa | 1—most deprived | 52 657 (57.0) |
| 2 | 20 403 (22.1) | |
| 4 | 10 024 (10.9) | |
| 3 | 5325 (5.8) | |
| 5—least deprived | 2746 (3.0) | |
| Missing | 1148 (1.2) | |
| Record of homelessness | 7602 (8.2) | |
| Discharge method | With clinical advice | 73 598 (79.7) |
| Before medically advised | 14 899 (16.1) | |
| Died | 732 (0.8) | |
| Other | 3074 (3.3) | |
| Duration (d) | Median [IQR] | 3 [1–5] |
| Mean [SD] | 4.8 [6.9] | |
| 28-d readmission | 17 366 (18.8) | |
| Operative procedure | 31 283 (33.9) | |
| Primary diagnosis | Cutaneous abscess | 29 634 (32.1) |
| Cellulitis | 20 046 (21.7) | |
| Other skin and soft-tissue infections | 8377 (9.1) | |
| Phlebitis and thrombophlebitis | 21 833 (23.7) | |
| Endocarditis | 2152 (2.3) | |
| Septicemia | 6087 (6.6) | |
| Osteomyelitis or septic arthritis | 3905 (4.2) | |
| Necrotizing fasciitis | 269 (0.3) |
Abbreviations: IQR = interquartile range; SD = standard deviation.
Where ethnicity or Index of Multiple Deprivation data were missing, we used the most recent nonmissing value from other hospital admissions for that individual. Ethnicity categories are based on the UK Office for National Statistics approach.
Figure 1.
Annual trends in opioid injection-associated infections in England, 2002–2021. Shaded areas in panels B and C represent 95% confidence intervals.
A total of 14 899/92 303 (16.1%) patients left before medically advised, and 732 (0.8%) died in hospital. The median duration of admissions was 3 days (IQR, 1–5). For patients with localized infections the median duration was 2 days (IQR, 1–5) and 95/79 890 (0.1%) died in hospital, whereas for patients with invasive infections the median duration was 6 days (IQR, 3–14) and 637/12 413 (5.1%) died. Therefore, 637/732 (87%) of deaths occurred among patients with invasive infections.
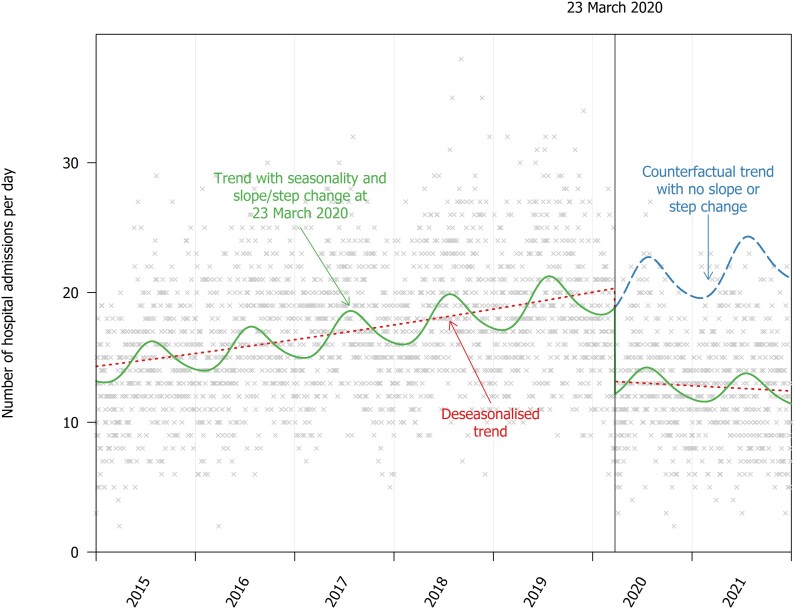
The rate of admissions reduced from 13.97 per day (95% CI, 13.59–14.36) in 2003 to 8.94 (95% CI, 8.64–9.25) in 2011, and then increased to 18.91 (95% CI ,18.46–19.36) in 2019 (Figure 2). This pattern was parallel to the rate of drug-related deaths in England, which also increased between 2011 and 2019. The rate of opioid injection–associated infections reduced in 2020 to 13.84 per day (95% CI, 13.46–14.23), whereas the rate of drug-related deaths continued to increase (Figure 1B ).
Figure 2.
Seasonality in hospital admissions because of injection-associated infections and the step-change at the introduction of COVID-19 public health responses.
The reduction in injecting-related infections in 2020 appeared to coincide with the start of COVID-19 public health measures. The time series model suggested that the rate reduced by 35.3% (95% CI, 32.1–38.4) following 23 March 2020, and this reduction was sustained throughout 2020 and 2021 (Figure 2).
Hospital admissions for opioid injection-associated infections were seasonal and the rate was 1.21 (95% CI, 1.18–1.24) times higher in July than in February.
The trend was similar for staphylococcal and streptococcal skin infections that were not related to illicit opioids. Hospital admissions for these infections also reduced at the start of COVID-19 response and peaked in summer. For hospital admissions related to noninfectious complications of illicit drug use, there was not a large step-change at the start of COVID-19, though the rate of these events reduced gradually after 23 March 2020. These events also peaked in summer. Seasonal variation and the step-change associated with COVID-19 are summarized in Table 2 with figures in Supplementary Information.
Table 2.
Seasonality in Hospital Admissions Resulting From Infections, and the Step-change at the Introduction of COVID-19 Public Health Responses
| Event | Modeled Low | Modeled Peak | Peak-to-low Ratio (95% CI) | Percent Change at the Start of COVID-19 Restrictions (95% CI)a |
|---|---|---|---|---|
| Opioid injection–associated infections | 11 Feb | 21 Jul | 1.21 (1.18–1.24) | 35.3 (32.1–38.4) |
| Invasive opioid injection–associated infections | 16 Feb | 20 Jul | 1.37 (1.29–1.46) | 44.4 (38.1–50.2) |
| Nonopioid-associated skin infections | 31 Jan | 23 Jul | 1.13 (1.10–1.15) | 25.1 (22.7–27.4) |
| Poisoning from illicit drugs | 24 Jan | 2 Jul | 1.43 (1.39–1.47) | −7.3 (−12.1 to –2.6) |
| Drug-related mental and behavioral disorders | 27 Dec | 7 Jul | 1.28 (1.26–1.29) | 3.9 (2.1–5.8) |
Abbreviations: 95% CI, 95% confidence interval; COVID-19, coronavirus disease 2019.
Values represent the percentage reduction in rate on 23 March 2020 (eg, a value of 100 would represent a reduction to 0). A negative value represents an increase.
The severity of hospital admissions was similar in the 12 months before and after the introduction of strict COVID-19 restrictions (Table 3). The duration of admissions reduced slightly, whereas there was no evidence of a difference in the proportion of patients who died or had invasive infections.
Table 3.
Severity of Admissions for Injection-associated Infections, Comparing the 12 Months Before and After the Introduction of COVID-19 Public Health Responses
| Variable | Before COVID-19: 23 March 2019–22 March 2020 | During COVID-19: 23 March 2020–22 March 2021 | P | |
|---|---|---|---|---|
| Number of admissions, n (%) | 6834 (100.0) | 4714 (100.0) | ||
| Duration of admission (d) | Mean (SD) | 4.8 (6.8) | 4.1 (5.5) | <.001a |
| Median (IQR) | 3 (1–5) | 2 (1–5) | <.001b | |
| Died, n (%) | 80 (1.2) | 54 (1.1) | .97c | |
| Invasive infections, n (%) | 1326 (19.4) | 936 (19.9) | .563c |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range, SD, standard deviation.
a t test.
bWilcoxon signed-rank test.
cχ2 test.
DISCUSSION
Key Findings
The rate of hospital admissions for opioid injection–associated bacterial infections increased from 2011 to 2019, then decreased sharply at the introduction of COVID-19 public health measures in March 2020 and remained at this lower level throughout 2020 and 2021. This sustained reduction in injecting-related infections happened over a period when injection of illicit opioids likely did not reduce. The seasonality of infections is parallel to skin infections in the general population, which may suggest that environmental and microbiological factors such as circulation of bacteria affect the risk of infection among people who inject drugs.
Comparison With Other Studies
Studies in various countries have found that the incidence of bacterial infections among people who inject drugs increased before COVID-19 [6, 9–14], though the reasons for this general increase are unclear. In the United Kingdom, few people started injecting in the past 10 years [19] and the population size appears stable [18]; therefore, the increase is likely because of increasing risk for individuals.
We considered 4 potential explanations for increasing injection-associated infections in England between 2011 and 2019. First, an increase in injection of crack cocaine, which is associated with more frequent injecting and higher risk of infections. Cross-sectional surveys of people who inject drugs in England show that 36% (95% CI, 33–38) recently injected crack cocaine in 2012, compared with 57% (95% CI, 55–60) in 2019 [31]. Second, increasing homelessness, which is associated with bacterial infections because of lack of a clean area to inject and poor access to injecting equipment [32]. Homelessness in England increased over this period; for example, the number of rough sleepers identified by street outreach teams in London increased by 71% between 2012 and 2020 [33]. The prevalence of homelessness in our sample follows the same pattern. Third, increasing average age and duration of injecting, which may be associated with vascular damage and injecting intramuscularly or in more colonized parts of the body such as the groin. The median time since people first injected psychoactive drugs in England increased from 7 years in 2002 to 18 years in 2019 [19]. However, this demographic change occurred gradually over the whole period in the present study, whereas the rate of injection-associated infections reduced between 2002 and 2011 and then increased. Fourth, cuts to needle and syringe programs and wound care provided within community health services that treat opioid use disorder. Funding cuts since 2013 have meant these community services have lost clinical skills and now have limited scope for holistic care [34].
We observed a sudden reduction in injection-associated infections at the start of COVID-19 restrictions. We considered 3 potential explanations. First, COVID-19 may have led to reduced healthcare access and a lower probability that an individual with an infection would be admitted to the hospital. However, most evidence suggests that reduced hospital access was short-term. Admissions because of noncommunicable diseases such as self-harm [35–37] and acute cardiovascular events [38, 39] reduced in the weeks after public health measures were introduced in March 2020, but typically returned to prepandemic trends by September 2020. In our data, there was no large step-change in March 2020 in admissions for noninfectious complications of opioid use, suggesting that the accessibility of hospitals did not reduce suddenly for people who use illicit drugs. Further supporting this, we did not observe an increase in the clinical severity of patients admitted with infections during COVID-19, and invasive infections reduced to a similar degree as localized infections. Second, the reduction in infections may be due to reduced drug use. However, opioid-related deaths continued to increase in the United Kingdom during the COVID-19 pandemic [20], and qualitative evidence suggests that many people continued to inject drugs [40]. Third, the nonpharmaceutical interventions designed to reduce COVID-19 transmission may have reduced transmission of bacteria such as Staphylococci and Streptococci among people who inject drugs. This was the case for many infections in the general population. In contrast to noninfectious causes of hospital admissions, which “rebounded” in 2020, there were sustained reductions throughout 2020 and 2021 in healthcare use related to many viral and bacterial infections [24, 25, 41]. Our results show that this sustained reduction was evident for injection-associated infections.
We also found that injection-associated infections were more common in summer. To our knowledge, this pattern has only been observed in 2 small studies [7, 42]. This pattern has been observed for Staphylococcal infections generally [23], which may be due to sweating and proliferation of colonized bacteria, insect bites, or close contact for example via contact sports. An alternative theory to seasonality in colonization or transmission of bacteria is that that drug use is more common in summer. This may be supported by the higher rate of noninfectious complications of opioid use in summer; however, other data suggest that opioid-related deaths do not follow this seasonal pattern [43].
Implications for Policy and Practice
Most interventions to reduce the risk of injection-associated bacterial infections have focused on individual behaviors such as skin cleaning and using sterile and sharp needles [44–46]. These behaviors are effective but interventions promoting their use have mixed outcomes and have not reduced the population incidence of infections. In contrast, we observed that the public health interventions to control COVID-19 had a dramatic effect on injection-associated infections. In the United Kingdom, the most important intervention in relation to people who inject drugs may be closure of congregate homeless settings (such as night shelters) and an offer of hotel-based accommodation for people experiencing homelessness during the pandemic [47]. This intervention meant that many people previously injecting drugs in public places now had their own bathroom and a more hygienic place to prepare drugs. Other relevant interventions included reduced social mixing throughout the population and promotion of hand hygiene. The results suggest that interventions that improve accommodation and living standards are likely to have a greater effect on the rate of infections than behavioral advice.
Strengths and Limitations
We used a national database capturing all hospital admissions at National Health Service hospitals in England, which used the diagnostic coding system ICD-10 throughout the 20-year study. The database excludes private hospitals where the patient pays, though these hospitals account for a small minority of hospital activity and are typically used by wealthier people for elective procedures.
There are 4 key limitations. First, COVID-19 was associated with multiple policy and social changes, and we cannot determine which of these changes caused the reduction in injection-associated infections. Our comparisons with skin infections in the general population and noninfectious complications of opioid use provide clues that nonpharmaceutical interventions may have improved the risk environment (rather than reduced drug use), but this is not a definitive answer. Second, by using hospital inpatient data, we only captured severe infections. It is possible that the threshold for hospital admission changed over the study period and particularly during COVID-19 responses, either because of hospital policies or patient behaviors such as fear of contracting COVID-19 in hospital and the desire not to burden health services. However, we did not find that COVID-19 was associated with an increase in the clinical severity of patients admitted with injection-associated infections. Third, our definition of injection-associated infections may capture some patients who use opioids, but the infection was not caused by injection, for example the entry point may have been an insect bite or viral lesion. This is difficult to quantify, but in a cohort of people who inject heroin in London, the rate of bacterial infections was 50 times greater than in the general population, suggesting that most bacterial infections in this population are associated with injecting [2]. Fourth, coding practices may change over time, and our results would be particularly sensitive to a change in the recording of opioid use disorder as a secondary diagnosis. We used a cohort of people who use illicit opioids to show that the recording of this diagnosis appears stable over time [27].
Conclusion
The risk of bacterial infections among people who inject drugs changes over both the long and short term, including a substantial reduction associated with COVID-19 public health measures. This may suggest that social and structural factors such as housing and the degree of social mixing contribute to the risk of infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Dan Lewer, Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency, London, United Kingdom; Department of Epidemiology and Public Health, UCL, London, United Kingdom; Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, United Kingdom.
Thomas D Brothers, Department of Epidemiology and Public Health, UCL, London, United Kingdom; Department of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada.
Sara Croxford, Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency, London, United Kingdom.
Monica Desai, Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency, London, United Kingdom.
Eva Emanuel, Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency, London, United Kingdom.
Magdalena Harris, Department of Public Health, Environments and Society, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Vivian D Hope, Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency, London, United Kingdom; Public Health Institute, Liverpool John Moores University, Liverpool, United Kingdom.
Notes
Author contributions. Conceptualization: D. L., T. D. B. Data curation: D. L. Formal analysis: D. L. Investigation: D. L., T. D. B., S. C., M. D., E. E., M. H., V. H. Methodology: D. L., T. D. B. Supervision: M. D. Visualization: D. L. Writing—original draft: D. L. Writing—review and editing: D. L., T. D. B., S. C., M. D., E. E., M. H., V. H.
Acknowledgments. The authors acknowledge all patients and all participating centers for their efforts during the trial. They thank the Department of Medical Microbiology and Infectious Diseases of Erasmus MC for technical support and performing the majority of the AsperGenius polymerase chain reaction.
Financial support. D. L. was funded by the National Institute for Health and Care Research (NIHR; Doctoral Research Fellowship DRF-2018-11-ST2-016). T. D. B. is supported by the Dalhousie University Internal Medicine Research Foundation Fellowship, a Canadian Institutes of Health Research Fellowship (CIHR-FRN #171259), and through the Research in Addiction Medicine Scholars Program (National Institutes of Health [NIH])/National Institute on Drug Abuse; R25DA033211). M. H. is funded by NIHR (HSDR 133022; PHR 133188). V. H. was funded by NIHR (HSDR 133022; PHR 133188, 202988, RP-PG-0616-20008). The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, NHS, or the UK Department of Health and Social Care.
Data availability. The authors used anonymous patient-level data from the Hospital Episode Statistics for England database. Patient-level data cannot be made publicly available to protect patient confidentiality. Researchers with appropriate permissions can use Hospital Episode Statistics by applying to NHS Digital: https://digital.nhs.uk/services/data-access-request-service-dars. We have published our analysis code and summary tables: https://github.com/danlewer/irid_trends.
References
- 1. Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend 2017; 171:39–49. [DOI] [PubMed] [Google Scholar]
- 2. Lewer D, Hope VD, Harris M, et al. . Incidence and treatment costs of severe bacterial infections among people who inject heroin: a cohort study in South London, England. Drug Alcohol Depend 2020; 212:108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langham FJ, Curtis SJ, Tang MJ, et al. . Acute injection-related infections requiring hospitalisation among people who inject drugs: clinical features, microbiology and management. Drug Alcohol Rev 2022; 41:1543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. London A, Lin D, Schade M. Microbiology of musculoskeletal infections in people who inject drugs at a rural tertiary care center. Infect Dis Clin Pract 2022; 30:e1198. [Google Scholar]
- 5. McCaughan H, Russell CD, O'Shea DT. Infected deep vein thrombophlebitis in people who inject drugs: missed opportunities and potential for alternative antimicrobial approaches. Infection 2022; 50:507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy NL, Baggs J, See I, et al. . Bacterial infections associated with substance use disorders, large cohort of United States hospitals, 2012–2017. Clin Infect Dis 2020; 71:e37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Damlin A, Westling K. Patients with infective endocarditis and history of injection drug use in a Swedish referral hospital during 10 years. BMC Infect Dis 2021; 21:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trayner KMA, Weir A, McAuley A, et al. . A pragmatic harm reduction approach to manage a large outbreak of wound botulism in people who inject drugs, Scotland 2015. Harm Reduct J 2018; 15:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewer D, Harris M, Hope V. Opiate injection–associated skin, soft tissue, and vascular infections, England, UK, 1997–2016. Emerg Infect Dis 2017; 23:1400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordal S, Kittang BR, Salminen P-R, et al. . Infective endocarditis in Western Norway: a 20-year retrospective survey. Infect Dis 2018; 50:757–63. [DOI] [PubMed] [Google Scholar]
- 11. Ciccarone D, Unick GJ, Cohen JK, Mars SG, Rosenblum D. Nationwide increase in hospitalizations for heroin-related soft tissue infections: associations with structural market conditions. Drug Alcohol Depend 2016; 163:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes T, Kitchen SA, Tailor L, et al. . Trends in hospitalizations for serious infections among people with opioid use disorder in Ontario, Canada. J Addict Med 2022; 16:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colledge-Frisby S, Jones N, Larney S, et al. . The impact of opioid agonist treatment on hospitalisations for injecting-related diseases among an opioid dependent population: a retrospective data linkage study. Drug Alcohol Depend 2022; 236:109494. [DOI] [PubMed] [Google Scholar]
- 14. Arora N, Panda PK, Cr P, et al. . Changing spectrum of infective endocarditis in India: an 11-year experience from an academic hospital in North India. Indian Heart J 2021; 73:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy 2019; 71:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenny KS, Kolla G, Greig S, et al. . Association of illicit fentanyl use with injection risk practices among people who inject drugs [manuscript published online ahead of print 18 November 2022]. AIDS Behav 2022; doi: 10.1007/s10461-022-03908-x. [DOI] [PubMed] [Google Scholar]
- 17. Lambdin BH, Bluthenthal RN, Zibbell JE, Wenger L, Simpson K, Kral AH. Associations between perceived illicit fentanyl use and infectious disease risks among people who inject drugs. Int J Drug Policy 2019; 74:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hay G, Rael dos Santos A, Reed H, Hope V. Estimates of the prevalence of opiate use and/or crack cocaine use, 2016/17: Sweep 13 report. Available at: https://phi.ljmu.ac.uk/wp-content/uploads/2019/03/Estimates-of-the-Prevalence-of-Opiate-Use-and-or-Crack-Cocaine-Use-2016-17-Sweep-13-report.pdf. Accessed 7 May 2019.
- 19. Lewer D, Croxford S, Desai M, et al. . The characteristics of people who inject drugs in the United Kingdom: changes in age, duration, and incidence of injecting, 1980–2019, using evidence from repeated cross-sectional surveys. Addiction 2022; 117:2471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Office for National Statistics . Deaths related to drug poisoning in England and Wales: 2021 registrations. 2022; Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2021registrations. Accessed 10 August 2022.
- 21. Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry 2021; 34:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. UK Health Security Agency . Shooting up: infections and other injecting-related harms among people who inject drugs in the UK, 2020. An update December 2021. 2022; Available at: https://www.gov.uk/government/publications/shooting-up-infections-among-people-who-inject-drugs-in-the-uk. Accessed 24 November 2022.
- 23. Leekha S, Diekema DJ, Perencevich EN. Seasonality of staphylococcal infections. Clin Microbiol Infect 2012; 18:927–33. [DOI] [PubMed] [Google Scholar]
- 24. Groves HE, Papenburg J, Mehta K, et al. . The effect of the COVID-19 pandemic on influenza-related hospitalization, intensive care admission and mortality in children in Canada: a population-based study. Lancet Reg Health Am 2022; 7:100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cassell K, Zipfel CM, Bansal S, Weinberger DM. Trends in non-COVID-19 hospitalizations prior to and during the COVID-19 pandemic period, United States, 2017–2021. Nat Commun 2022; 13:5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017; 46:1093–1093i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewer D, Padmanathan P, Qummer ul Arfeen M, et al. . Healthcare use by people who use illicit opioids (HUPIO): development of a cohort based on electronic primary care records in England. Wellcome Open Res 2020; 5:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ministry of Housing, Communities & Local Government . English indices of deprivation 2019. 2019; Available at: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019. Accessed 8 July 2022.
- 29. Christiansen CF, Pedersen L, Sørensen HT, Rothman KJ. Methods to assess seasonal effects in epidemiological studies of infectious diseases—exemplified by application to the occurrence of meningococcal disease. Clin Microbiol Infect 2012; 18:963–9. [DOI] [PubMed] [Google Scholar]
- 30. NHS Digital . Statistics on drug misuse, England 2020. 2021; Available at: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-drug-misuse/2020/part-1-hospital-admissions-related-to-drug-misuse. Accessed 7 November 2022.
- 31. UK Health Security Agency . People who inject drugs: HIV and viral hepatitis unlinked anonymous monitoring survey tables (psychoactive): 2022 update. 2022; Available at: https://www.gov.uk/government/publications/people-who-inject-drugs-hiv-and-viral-hepatitis-monitoring. Accessed 17 November 2022.
- 32. Arum C, Fraser H, Artenie AA, et al. . Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Public Health 2021; 6:e309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greater London Authority . CHAIN annual report, Greater London: April 2021–March 2022. 2022; Available at: https://data.london.gov.uk/dataset/chain-reports. Accessed 17 November 2002.
- 34. Black C. Review of drugs: phase one report. 2020; Available at: https://www.gov.uk/government/publications/review-of-drugs-phase-one-report. Accessed 12 May 2020.
- 35. Steeg S, Bojanić L, Tilston G, et al. . Temporal trends in primary care-recorded self-harm during and beyond the first year of the COVID-19 pandemic: time series analysis of electronic healthcare records for 2.8 million patients in the Greater Manchester Care Record. eClinicalMedicine 2021; 41:101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steeg S, John A, Gunnell DJ, et al. . The impact of the COVID-19 pandemic on presentations to health services following self-harm: systematic review. Br J Psychiatry 2022; 221:603–12. [DOI] [PubMed] [Google Scholar]
- 37. Carr MJ, Steeg S, Webb RT, et al. . Effects of the COVID-19 pandemic on primary care-recorded mental illness and self-harm episodes in the UK: a population-based cohort study. Lancet Public Health 2021; 6:e124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solomon MD, Nguyen-Huynh M, Leong TK, et al. . Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID-19 surges. JAMA 2021; 326:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mafham MM, Spata E, Goldacre R, et al. . COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020; 396:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kesten JM, Holland A, Linton M-J, et al. . Living Under Coronavirus and Injecting Drugs in Bristol (LUCID-B): a qualitative study of experiences of COVID-19 among people who inject drugs. Int J Drug Policy 2021; 98:103391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kadambari S, Goldacre R, Morris E, Goldacre MJ, Pollard AJ. Indirect effects of the COVID-19 pandemic on childhood infection in England: population based observational study. BMJ 2022; 376:e067519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooper HLF, Wypij D, Krieger N. Police drug crackdowns and hospitalisation rates for illicit-injection-related infections in New York City. Int J Drug Policy 2005; 16:150–60. [Google Scholar]
- 43. Lewer D, Brothers T, Gasparrini A, Strang J. Seasonal, weekly, and other cyclical patterns in deaths due to drug poisoning in England and Wales. Addiction 2023; doi: 10.1111/add.16175. [DOI] [PubMed] [Google Scholar]
- 44. Roux P, Donadille C, Magen C, et al. . Implementation and evaluation of an educational intervention for safer injection in people who inject drugs in Europe: a multi-country mixed-methods study. Int J Drug Policy 2021; 87:102992. [DOI] [PubMed] [Google Scholar]
- 45. Mezaache S, Briand-Madrid L, Rahni L, et al. . A two-component intervention to improve hand hygiene practices and promote alcohol-based hand rub use among people who inject drugs: a mixed-methods evaluation. BMC Infect Dis 2021; 21:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phillips KT, Stewart C, Anderson BJ, Liebschutz JM, Herman DS, Stein MD. A randomized controlled trial of a brief behavioral intervention to reduce skin and soft tissue infections among people who inject drugs. Drug Alcohol Depend 2021; 221:108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Department for Levelling Up, Housing & Communities . Annex A: Support for people sleeping rough in England, 2021. 2022; Available at: https://www.gov.uk/government/statistics/rough-sleeping-snapshot-in-england-autumn-2021/annex-a-support-for-people-sleeping-rough-in-england-2021-not-official-statistics. Accessed 18 November 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.