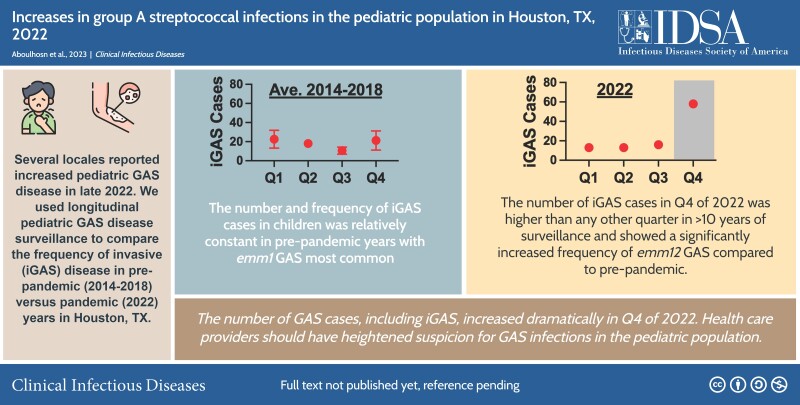
Abstract
Beginning in October 2022, we observed a substantial increase in the total number of cases of invasive group A Streptococcus (GAS) disease in the pediatric population in Houston, Texas. Emm12 GAS strains were disproportionately represented but the overall proportion of invasive GAS infections observed during the current spike was similar to prepandemic years.
Keywords: group A Streptococcus, invasive, outbreak, emm type, children
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/increases-in-group-a-streptococcal-infections-in-the-pediatric-population-in-houston-tx-2022-d4061766-9dc7-4850-80e5-792060811999
Streptococcus pyogenes (group A Streptococcus [GAS]) causes a variety of disease manifestations in children. The most common clinical presentations are benign, including pharyngitis and superficial skin infections [1]. However, invasive GAS infection (iGAS) also results in significant morbidity and mortality in this patient population. iGAS is defined as GAS invading a normally sterile site in the body [2]. Recently, reports of increases in iGAS cases relative to the numbers prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic have been reported in England [3], the Netherlands [4], and multiple other regions [5], including a number of pediatric deaths. These reports, along with growing concerns among United States (US) healthcare providers, led the Centers for Disease Control and Prevention (CDC) to publish a health advisory through the CDC Health Alert Network for possible increases in pediatric iGAS [6]. Due to the sudden increase in the number of iGAS cases in other locales, we queried our longitudinal pediatric GAS infection surveillance conducted over the past 10 years in the Houston metropolitan area for changes suggestive of a GAS outbreak.
METHODS
We used our ongoing active GAS prospective surveillance in the Texas Children's Hospital (TCH) system as previously described [7]. We grouped available GAS strain surveillance data into 2 separate periods: prepandemic (2014–2018) and pandemic (2022). The GAS isolates captured by the clinical microbiology laboratory at TCH were grown, stocked, and emm typed as previously described [7]. Demographic information and GAS disease types (invasive, skin and soft tissue infection [SSTI], and pharyngeal [PHG]) were determined using data derived from the electronic medical record. iGAS was defined using established criteria [2]. Aggregated prepandemic (2014–2018) GAS disease trends were compared to the pandemic year for which we have complete data (2022). We also compared emm type frequency between the 2 periods. Select strains were examined for resistance to tetracycline, erythromycin, and clindamycin using disk diffusion as previously described [8]. Categorical data were compared using χ2 test and continuous data were compared using Student t test. P values <.05 were considered significant following correction for multiple comparisons (Bonferroni).
RESULTS
In 2022, a total of 318 individual GAS cases were identified. GAS strains derived from invasive disease (iGAS) accounted for 31.4% (n = 100), SSTI for 17.6% (n = 56), and PHG for 50.9% (n = 162). The median age of all GAS cases was 6.1 years with a slight male predominance (53.8%) (Supplementary Table 1). Examination of ages affected by GAS infections showed iGAS to be more frequently identified in children aged 0–4 years (45/101 [44.6%]) compared to SSTI (20/56 [35.1%]) or PHG (49/162 [30.2%]) but was not statistically significant (Supplementary Table 2). Among the 100 iGAS cases identified in the 2022 cohort, 25% (25/100) did not undergo viral testing and 34% (34/100) were only tested for presence of SARS-CoV-2 (data not shown). Of cases with known race or ethnicity (288/318 [90.5%]), the majority were White (222/288 [77.0%]) and 48.3% were Hispanic (139/288). No significant differences in demographic characteristics were observed between disease types (Supplementary Table 1).
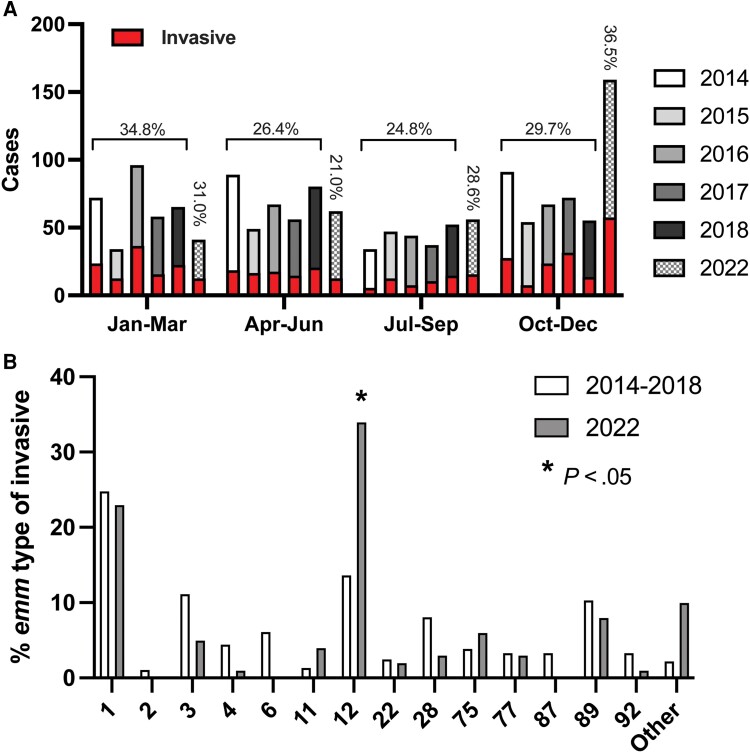
Given that increases in iGAS have been reported in other locales, we compared the total number and frequency of iGAS in the 2022 surveillance year to prepandemic years for which we have complete data (2014–2018). Figure 1A shows the total number of cases annually for 2014–2018 and the pandemic year 2022 by 3-month intervals (quarters). Total cases for 2022 exceeded prepandemic years only in the final quarter. The total number of cases in October through December 2022 exceeded any interval in our prepandemic surveillance. We also examined the proportion of cases defined as invasive for each interval. Prepandemic surveillance consistently showed a peak iGAS frequency in the first quarter (January–March). Interestingly, the proportion of iGAS in October–December 2022 was similar to prior peaks in January–March but not significantly different than the maximum observed prior to the pandemic (Figure 1A ).
Figure 1.
Comparison of pediatric group A streptococcal (GAS) infections in prepandemic and pandemic periods. A, Total pediatric GAS cases by 3-month period (quarter) in prepandemic (2014–2018) and pandemic (2022) years. Vertical bars represent total cases for the given year with red insets indicating total invasive GAS cases. Percentages for invasive GAS in the 2014–2018 period by quarter are given along with percentage of invasive GAS cases in 2022. B, Frequency of GAS emm types among invasive pediatric infections for prepandemic (2014–2018) and pandemic (2022) years. Only those emm types with >10 observations (combined) are shown. P value was determined by χ2 test following Bonferroni correction. Complete emm type data are provided in Supplementary Table 2.
We next determined and compared the distribution of emm types among the prepandemic and pandemic GAS strain cohorts. Emm1 GAS was the dominant emm type in the prepandemic period, comprising 21.7% of the total and 24.9% of iGAS cases (Figure 1B , Supplementary Table 2). In contrast, the marked increase in GAS cases at the end of 2022 was driven primarily by emm12 GAS. In fact, the proportion of total and iGAS cases caused by emm12 was significantly greater in 2022 than the mean emm12 frequency in the prior interval (2014–2018) combined (Figure 1B , Supplementary Table 2). Inasmuch as multidrug resistance in emm12 GAS strains has been associated with clonal emergence [9], we examined a subset of emm12 GAS from 2022 (n = 24 [20.9%]) for antimicrobial resistance but did not identify strains with resistance to multiple antibiotics indicative of previous emm12 outbreaks (data not shown). Concomitant decreases in other emm types causing iGAS were observed in 2022, including emm3 and emm6, but were not statistically significant (Supplementary Table 2).
DISCUSSION
Our data show a marked increase in overall GAS disease beginning in October 2022 in Houston, Texas. The number of cases from all disease types exceeds that for any other observed period in nearly 10 years of surveillance. However, the proportion of cases identified as invasive was within previous years’ surveillance albeit occurring in a different quarter (October–December) compared to prepandemic (January–March). Previously, we reported a significant decline in invasive bacterial infections caused by GAS [10]. Most experts speculate that nonpharmaceutical interventions such as masking and social distancing contributed to the declines in incidence of many infectious diseases observed early in the coronavirus disease 2019 (COVID-19) pandemic (honeymoon effect). Unfortunately, the reduction or elimination of COVID-19 control measures had the unintended consequence of large outbreaks of non–COVID-19 infections due to exposure of a greater number of susceptible individuals (so-called divorce effect) [11]. Not surprisingly, the large outbreaks observed for respiratory syncytial virus and now iGAS are in infectious diseases for which we lack effective immunization—likely leading to amplification of the outbreak magnitude than would be otherwise expected [11]. Heightened awareness among public health officials, infectious disease experts, and other medical providers is essential as we navigate the current increase in GAS infections for the emergence of other infectious disease outbreaks.
We observed a significant increase in the number of infections caused by emm12 GAS strains compared to the prepandemic surveillance, in contrast to reports on the European continent [3–6]. The epidemiology of GAS infections in humans has been punctuated by the emergence of new, more successful emm-specific clones. Thus, one alternative explanation for the current increase in pediatric GAS infections is recent emergence of a novel GAS strain leading to more disease. Previously, emergence of scarlet fever in Hong Kong had been associated with emm12 GAS clones conferring resistance to multiple antibiotics carried on mobile genetic elements [9]. Similar clones have been identified in our own surveillance although without evidence of epidemic spread [12]. We did not identify a pattern of multidrug resistance among emm12 GAS strains isolated within the October–December 2022 peak (data not shown). However, it is possible that other genetic features of emm12 GAS strains in the current outbreak are contributing to the increase in overall GAS infections locally. Further investigation including whole genome sequencing of emm12 GAS may be warranted for evidence of clonal emergence.
Our study has several limitations. The retrospective design precludes examination of several factors for association with the increased GAS disease. For example, prior or concurrent viral infection is known to occur with GAS disease, but we observed inconsistent testing in our cohort. Thus, we are unable to assess the contribution of viral coinfection to GAS disease phenotype (invasive vs SSTI or PHG). We also acknowledge that our study design likely overestimates the prevalence of iGAS in our population. Compared to children with iGAS who are likely to require hospitalization and be captured by our surveillance, it is just as likely that those with noninvasive disease (ie, pharyngitis and SSTI) are more likely to be cared for in the community. This is supported by prior work demonstrating a much higher incidence of GAS pharyngitis (19.1/1000 outpatient visits) in the US compared to invasive disease (∼7.6/100 000 population) [13]. Additionally, our center and many others perform point-of-care antigen testing for GAS pharyngitis and if positive do not perform culture; such a practice likely also contributed to underestimation of noninvasive disease. In addition, our analysis does not include 2019–2021. However, the 5 years of surveillance leading up to the COVID-19 pandemic do not suggest prior iGAS trends as a major contributor to the current outbreak. Ongoing surveillance will be essential to assess for critical changes in the evolving GAS outbreak.
CONCLUSIONS
The SARS-CoV-2 pandemic has greatly impacted the epidemiology of multiple pediatric infections. Our study demonstrates a surge in pediatric GAS infections beginning in the last months of 2022. A heightened index of suspicion for GAS infection by healthcare providers and vigilance of public health professionals are warranted as the outbreak continues to evolve. Further examination of GAS strain (eg, genomic epidemiology, antimicrobial resistance patterns) and host (eg, age, comorbidities) characteristics are urgently needed as predictive tools for control of the current and future outbreaks of GAS disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Aya Aboulhosn, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston and Children's Memorial Hermann Hospital, Houston, Texas, USA.
Misu A Sanson, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston and Children's Memorial Hermann Hospital, Houston, Texas, USA.
Luis Alberto Vega, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston and Children's Memorial Hermann Hospital, Houston, Texas, USA.
Maria G Segura, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston and Children's Memorial Hermann Hospital, Houston, Texas, USA.
Sommer, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Marritta Joseph, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
J Chase McNeil, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Anthony R Flores, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston and Children's Memorial Hermann Hospital, Houston, Texas, USA.
Notes
Acknowledgments. The authors thank the staff of the clinical microbiology laboratory at Texas Children's Hospital for assistance in collecting the group A Streptococcus isolates used in this study.
Financial support. A. R. F. is supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers R21AI153663 and R21AI159059).
References
- 1. Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5:685–94. [DOI] [PubMed] [Google Scholar]
- 2. Flores AR, Chase McNeil J. Capsule-negative emm types are an increasing cause of pediatric group A streptococcal infections at a large pediatric hospital in Texas. J Pediatric Infect Dis Soc 2019; 8:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. United Kingdom Health Security Agency . Group A streptococcal infections: report on seasonal activity in England, 2022 to 2023.2023. Available at: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-report-on-seasonal-activity-in-england-2022-to-2023. Accessed 10 February 2023.
- 4. de Gier B, Marchal N, de Beer-Schuurman I, et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill 2023; 28:2200941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Increased incidence of scarlet fever and invasive group A Streptococcus infection—multi-country. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429. Accessed 10 February 2023.
- 6. Centers for Disease Control and Prevention . Increase in pediatric invasive group A streptococcal infections. Available at: https://emergency.cdc.gov/han/2022/han00484.asp. Accessed 10 February 2023.
- 7. Flores AR, McNeil JC, Shah B, et al. Capsule-negative emm types are an increasing cause of pediatric group A streptococcal infections at a large pediatric hospital in Texas. J Pediatric Infect Dis Soc 2018; 8:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanson MA, Macias OR, Shah BJ, et al. Unexpected relationships between frequency of antimicrobial resistance, disease phenotype and emm type in group A Streptococcus. Microb Genom 2019; 5:e000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies MR, Holden MT, Coupland P, et al. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat Genet 2015; 47:84–7. [DOI] [PubMed] [Google Scholar]
- 10. McNeil JC, Flores AR, Kaplan SL, et al. The indirect impact of the SARS-CoV-2 pandemic on invasive group A Streptococcus, Streptococcus pneumoniae and Staphylococcus aureus infections in Houston area children. Pediatr Infect Dis J 2021; 40:e313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollingsworth B, Okamoto KW, Lloyd AL. After the honeymoon, the divorce: unexpected outcomes of disease control measures against endemic infections. PLoS Comput Biol 2020; 16:e1008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cubria MB, Delgado J, Shah BJ, et al. Identification of epidemic scarlet fever group A Streptococcus strains in the paediatric population of Houston, TX, USA. Access Microbiol 2021; 3:000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewnard JA, King LM, Fleming-Dutra KE, et al. Incidence of pharyngitis, sinusitis, acute otitis media, and outpatient antibiotic prescribing preventable by vaccination against group A Streptococcus in the United States. Clin Infect Dis 2021; 73:e47–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.