Abstract
Background
Cerebral embolic events (CEEs) are common complications of infective endocarditis (IE), and their presence can modify diagnosis and therapeutic plans. The aim of the present study was to assess the role of cerebral imaging (Cer-Im) on diagnosis and management of patients with suspected IE.
Methods
This study was conducted at the Lausanne University Hospital, Lausanne, Switzerland, from January 2014 to June 2022. CEEs and IE were defined according to modified Duke criteria of the European Society of Cardiology (ESC) guidelines.
Results
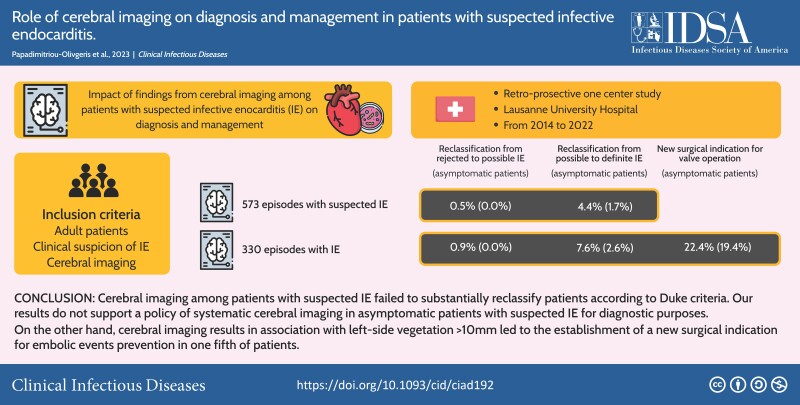
Among 573 patients with IE suspicion and Cer-Im, 239 (42%) patients had neurological symptoms. At least 1 CEE was found in 254 (44%) episodes. Based on Cer-Im findings, episodes were reclassified from rejected to possible or from possible to definite IE in 3 (1%) and 25 (4%) patients, respectively (0% and 2% in asymptomatic patients, respectively). Among the 330 patients with possible or definite IE, at least 1 CEE was found in 187 (57%) episodes. A new surgical indication (in association with left-side vegetation >10 mm) was established in 74/330 (22%) IE patients and 30/155 (19%) asymptomatic IE patients, respectively.
Conclusions
Cer-Im in asymptomatic patients with IE suspicion showed limited potential for improving the diagnosis of IE. In contrast, performing Cer-Im in asymptomatic patients with IE may be useful for decision making, because Cer-Im findings led to the establishment of new operative indication for valvular surgery in one fifth of patients according to ESC guidelines.
Keywords: infective endocarditis, MRI, embolization, ischemic lesions, valve surgery
Cerebral imaging among patients suspected of infective endocarditis failed to substantially reclassify patients according to Duke criteria. On the other hand, it led to the establishment of a new surgical indication for embolic events prevention in one fifth of patients.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/role-of-cerebral-imaging-on-diagnosis-and-management-in-patients-with-suspected-infective-endocarditis-39f43dba-0494-4dc6-bc42-58a3e79befef
Despite advances in diagnostic imaging and more aggressive treatment strategies, infective endocarditis (IE) remains a life-threatening condition associated with high in-hospital mortality (15%–30%) [1]. One of the most common complications contributing to the increased mortality is embolization, especially to the central nervous system, which is the most common site associated with embolic events (EEs) [2, 3]. Cerebral EEs (CEEs) have previously been associated with large vegetations and mitral valve involvement [4–6].
The most common CEEs are ischemic lesions, followed by hemorrhagic strokes, mycotic aneurysms and cerebral abscesses [4, 6–9]. Although many EEs to the central nervous system are symptomatic (neurologic deficit, confusion, seizures, headache), studies with systematic cerebral imaging studies (Cer-Im) found that the majority of CEEs are asymptomatic [2, 10–14]. Identification of CEEs can contribute to make the diagnosis of IE, since vascular phenomena are part of the minor Duke criteria [15]. Additionally, the presence of CEEs in patients with left-side valvular vegetations >10 mm is an indication for urgent valve surgery according to the 2015 European Society of Cardiology (ESC) guidelines in order to prevent subsequent embolism [15]. In a metanalysis of studies, where cerebral MRI was systematically performed, Cer-Im findings led to a change of treatment strategy in 13% and contributed to valvular surgery planification in 14% of patients [16].
Although many previous studies already evaluated the impact of Cer-Im on IE diagnosis and modification of the therapeutic strategy, most of them only included limited number of patients [16]. Furthermore, all but one studies exclusively included patients with a final diagnosis of IE (definite or possible) [2, 3, 13, 16], and the only study assessing the role of Cer-Im in suspected IE only included 60 patients [5]. Therefore, we aimed to describe the frequency of symptomatic and asymptomatic CEEs by Cer-Im in a large cohort of patients with suspected or confirmed IE and to determine the impact of such findings on diagnosis and management.
METHODS
Study Design
This study was conducted at the Lausanne University Hospital, Lausanne, Switzerland, a 1100-bed primary and tertiary care hospital from January 2014 to June 2022 (2014–17: retrospective cohort; 2018 onward: prospective cohort). The study was approved by the ethics committee of the Canton of Vaud (CER-VD 2017–02137).
Patients
For the prospective cohort, the inclusion criteria were adult patients (≥18 years old) with clinical suspicion of IE (patients who had blood cultures drawn and an echocardiography performed specifically for the research of IE), Cer-Im realization (computed tomography [CT] scan or magnetic resonance imaging [MRI]) and written consent. For the retrospective cohort, the inclusion criteria were adult patients (≥18 years old) with possible or definite IE, Cer-Im realization, and absence of refusal of the use of their clinical data.
Data regarding demographics (age, sex), comorbidities, cardiac predisposing factors [15], microbiologic etiology, systemic symptoms, fever, acute heart failure, sepsis or septic shock, heart murmur, immunological phenomena [15], site of cardiac involvement and type of lesion (according to cardiac imaging modalities, macroscopic lesions on surgery or autopsy), cardiac surgery (timing), results of thoracoabdominal and cerebral imaging studies (timing, results) and embolic events (type, timing, symptoms) were retrieved from patients’ electronic health records.
Management of IE
According to internal guidelines, an infectious diseases consultation with a thorough physical examination was performed on a mandatory basis for all patients with a suspicion of IE. Thoracoabdominal and Cer-Im were performed in all patients with local symptoms; their realization in asymptomatic patients was at the discretion of the treating physician and infectious diseases consultant. An endocarditis-team was established on January 2018, comprising of infectious diseases specialists, cardiologists, cardiac surgeons, which reviewed all patients with IE suspicion during weekly meetings. Furthermore, in our center, valve surgery was proposed to patients with a vegetation >10 mm and EEs even if the EEs occurred before 5 days of appropriate antimicrobial treatment; the decision was validated for each individual patient by the endocarditis team.
Definitions
IE was defined according to the modified Duke criteria [15]. IE was characterized as community, healthcare, or nosocomial according to Friedman et al [17]. EEs were defined as septic lung emboli, renal or splenic emboli, mycotic aneurysm, intracranial ischemia or bleeding, cerebral abscess, conjunctival bleeding, retinal emboli, chorioretinitis, Janeway lesions or nail bed bleeding, and peripheral major vascular emboli.
Cerebral Imaging Results
A patient was considered symptomatic in the presence of neurologic deficit, confusion, coma, headache or seizures; the absence of all aforementioned symptoms categorized the patient as asymptomatic. CEEs detected by Cer-Im included ischemic brain lesions, intracranial bleeding, cerebral mycotic aneurysm, or abscess.
Impact of Cerebral Imaging
IE was classified according to the modified Duke criteria assessed at day 60, based on clinical, microbiological, imaging, surgical data or autopsy results (final diagnosis). A separate Duke classification was established blinded to the results of Cer-Im. Based on these 2 assessments, the rate of diagnostic reclassification (from rejected to possible and from possible to definite IE) was calculated for the whole cohort and in the subgroup of patients with a final diagnosis of possible or definite IE.
The changes in management due to Cer-Im were calculated among patients with possible or definite IE. The impact on management was defined as the establishment of a new indication for valve surgery and, subsequently, the effective performance of valvular surgery. Other interventions related to CEE included intravenous thrombolysis or endovascular thrombectomy for ischemic lesions, drainage of abscess or specific treatment of hemorrhagic lesions or mycotic aneurysms. Intravenous thrombolysis for ischemic lesions is contraindicated in IE patients [15]; it was performed in few patients because the suspicion of IE was substantiated only later.
Analysis
SPSS version 26.0 (SPSS, Chicago, Illinois, USA) software was used for data analysis. Categorical variables were analyzed using the χ2 or Fisher exact test and continuous variables with Mann-Whitney U test. Bivariate and multivariable logistic regression analyses were performed with dependent variable being CEEs found on Cer-Im in patients with IE suspicion and those with IE. Variables with P < .1 that did not contribute to multicollinearity (variance inflation factor assessment) were used in multivariable analyses. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. All statistic tests were 2-tailed, and P < .05 was considered statistically significant.
RESULTS
Study Population
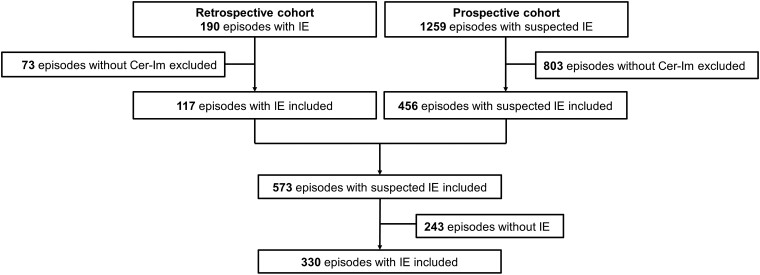
Among the 1259 episodes of the prospective cohort, 456 had a Cer-Im and were thus included (Figure 1). Out of the 190 episodes of the retrospective cohort, 117 had a Cer-Im and were included. In total, 573 episodes with suspected IE were included, and 330 (58%) episodes had possible of definite IE (definite IE: 278, possible: 52). For the remaining 243 (42%) episodes, the final diagnosis included other type of infection (175; 72%), stroke (24; 10%), and other diagnoses (44; 18%).
Figure 1.
Study flowchart. Abbreviations: Cer-Im, cerebral imaging; IE, infective endocarditis.
Cerebral Imaging in Patients With Suspected Infective Endocarditis
Among 573 episodes, 343 (60%) benefited from CT scan (265; 77% with contrast media) and 343 (60%) from MRI; 113 (20%) benefited from both CT and MRI (63 among them within 24 hours). The median time from IE suspicion to Cer-Im was 2 days (Q1–Q3: 1–6). Most of the episodes with Cer-Im were symptomatic (334; 58%) with the most frequently observed symptoms and signs being confusion or coma (178; 53%) and neurologic deficit (143; 43%).
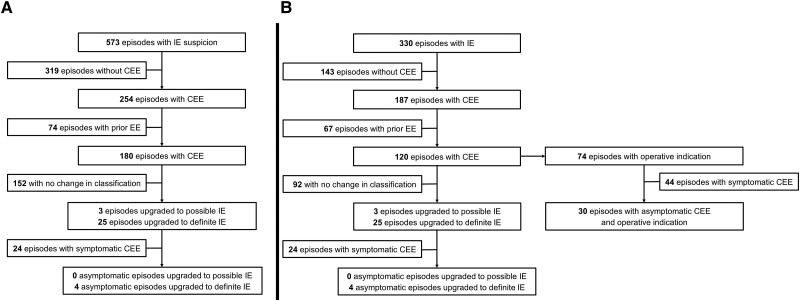
At least 1 CEE was found on Cer-Im in 254 (44%) episodes (Table 1). Ischemic lesions were the most commonly identified lesions (n = 251; 99%), followed by hemorrhagic lesions (n = 40; 14%). The comparison between cerebral CT and MRI for the detection of specific CEEs is shown in Supplementary Table 1. For 74 (13%) episodes, the Duke's vascular criterion was already fulfilled prior to the realization of the Cer-Im. Thus, 180 (31%) episodes met the vascular criterion due to the Cer-Im; 3 (1%) episodes were reclassified from rejected to possible IE and 25 (4%) from possible to definite IE according to modified Duke criteria (Figure 2). Among the 239 asymptomatic episodes, the vascular criterion was fulfilled by the Cer-Im in 39 (16%) episodes; no episode was reclassified from rejected to possible and 4 (2%) episodes from possible to definite IE. In 33 (6%) out of 573 episodes an intervention was directed toward the CEEs found on Cer-Im, none among 239 asymptomatic episodes (Table 1).
Table 1.
Type of Cerebral Embolic Events and Their Impact on Diagnosis and Management in Patients With Suspected Infective Endocarditis
| Total (n = 573) | Asymptomatic (n = 239) | Symptomatic (n = 334) | P* | ||||
|---|---|---|---|---|---|---|---|
| MRI | 343 | 60% | 124 | 52% | 219 | 66% | .001 |
| No cerebral embolic event | 319 | 56% | 172 | 72% | 147 | 44% | |
| Cerebral embolic event | 254 | 44% | 67 | 28% | 187 | 56% | <.001 |
| Ischemic lesions | 251 | 44% | 66 | 28% | 185 | 55% | .001 |
| Intravenous thrombolysisa | 12 | 4% | 0 | 0% | 12 | 4% | .002 |
| Endovascular thrombectomy | 17 | 3% | 0 | 0% | 17 | 5% | <.001 |
| Hemorrhagic lesions | 40 | 7% | 7 | 3% | 33 | 10% | .001 |
| Treatment of hemorrhagic lesions | 1 | <0.5% | 0 | <0.5% | 1 | <0.5% | 1.000 |
| Mycotic aneurysm | 18 | 3% | 7 | 3% | 11 | 3% | 1.000 |
| Treatment of mycotic aneurysm | 3 | 1% | 0 | 0% | 3 | 1% | .269 |
| Cerebral abscess | 12 | 2% | 2 | 1% | 10 | 3% | .084 |
| Drainage of abscess | 6 | 1% | 0 | 0% | 6 | 2% | .044 |
| Treatment of any cerebral embolic event | 33 | 6% | 0 | 0% | 33 | 10% | <.001 |
| New cerebral embolic eventb | 180 | 31% | 39 | 16% | 141 | 42% | <.001 |
| Reclassification from rejected to possible endocarditis | 3 | 1% | 0 | 0% | 3 | 1% | .269 |
| Reclassification from possible to definite endocarditis | 25 | 4% | 4 | 2% | 21 | 6% | .007 |
Data are depicted as number/percentage.
Abbreviation: MRI, magnetic resonance imaging.
*Comparison between symptomatic and asymptomatic patients.
Thrombolysis was performed in patients for which the infective endocarditis (IE) suspicion was not substantiated the moment of the decision.
Not prior cutaneous, ocular, or thoracoabdominal embolic event.
Figure 2.
Changes in diagnostic classification management due to cerebral imaging results in patients suspected (A) and those with infective endocarditis (B). Abbreviations: CEE, cerebral embolic event; Cer-Im, cerebral imaging; EE, embolic event; IE, infective endocarditis.
The comparison of episodes with and without EE on Cer-Im is shown on Table 2. In the multivariable analysis (Supplementary Table 2), the factors associated with the presence of CEE on Cer-Im included final IE diagnosis (P < .001; aOR 12.4, 95% CI: 6.64–23.2) and presence of neurologic symptoms (P < .001; aOR 5.02, 95% CI: 3.27–7.71), whereas presence of ocular EE prior to Cer-Im (P < .072; aOR 2.47, 95% CI: .92–6.62) and positive imaging Duke criterion (P < .113; aOR 1.59, 95% CI: .90–2.81) had no significant association with CEE discovery on Cer-Im.
Table 2.
Predictors of Embolic Events Detected by Cerebral Imaging Among Patients With Suspected Infected Endocarditis
| Without CEE (n = 319) | With CEE (n = 254) | P | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex | 226 | 71% | 174 | 69% | .583 |
| Age (y) | 67 | 49–75 | 67 | 53–76 | .885 |
| Comorbidities | |||||
| Atrial fibrillation | 71 | 22% | 47 | 19% | .299 |
| Congestive heart failure | 36 | 11% | 16 | 6% | .041 |
| Chronic obstructive pulmonary disease | 50 | 16% | 31 | 12% | .278 |
| Cirrhosis | 32 | 10% | 14 | 6% | .062 |
| Diabetes mellitus | 82 | 26% | 52 | 20% | .162 |
| Chronic kidney disease (moderate or severe) | 54 | 17% | 36 | 14% | .419 |
| Malignancy (solid organ or hematologic) | 61 | 19% | 27 | 11% | .005 |
| Obesity | 85 | 27% | 54 | 21% | .142 |
| Immunosuppression | 52 | 16% | 24 | 9% | .018 |
| Antithrombotic or anticoagulation treatment | 147 | 46% | 124 | 49% | .556 |
| Antithrombotic treatment | 86 | 27% | 71 | 28% | .851 |
| Anticoagulation treatment | 80 | 25% | 64 | 25% | 1.000 |
| Cardiac predisposing factors | 97 | 30% | 103 | 41% | .013 |
| Prosthetic valve | 47 | 15% | 61 | 24% | .005 |
| Bacteremia | 256 | 80% | 183 | 72% | .023 |
| Staphylococcus aureus | 128 | 41% | 81 | 34% | .112 |
| Coagulase negative staphylococci | 18 | 6% | 6 | 3% | .091 |
| Streptococci | 56 | 18% | 61 | 26% | .028 |
| Enterococci | 27 | 9% | 21 | 9% | 1.000 |
| Other gram-positive | 10 | 3% | 3 | 1% | .166 |
| HACEK | 3 | 1% | 5 | 2% | .300 |
| Other gram-negative | 21 | 7% | 6 | 3% | .028 |
| Fungi | 10 | 3% | 3 | 1% | .166 |
| Polymicrobial bacteremia | 16 | 5% | 3 | 1% | .017 |
| Manifestations | |||||
| Systemic symptoms | 284 | 89% | 203 | 80% | .003 |
| Fever | 259 | 81% | 176 | 69% | .001 |
| Heart murmur | 135 | 42% | 124 | 49% | .129 |
| New heart murmur | 88 | 28% | 96 | 38% | .012 |
| Immunologic phenomena | 14 | 4% | 27 | 11% | .005 |
| Sepsis | 125 | 39% | 111 | 44% | .305 |
| Septic shock | 44 | 14% | 45 | 18% | .204 |
| Cerebral imaging performed due to neurologic symptoms | 147 | 46% | 187 | 74% | <.001 |
| Deficit | 28 | 9% | 115 | 45% | <.001 |
| Confusion/coma | 94 | 29% | 84 | 33% | .365 |
| Headache | 22 | 7% | 24 | 9% | .282 |
| Seizures | 2 | 1% | 6 | 2% | .147 |
| Days to cerebral imaging | 3 | 1–7 | 1 | 0–4 | <.001 |
| Embolic events prior to cerebral imaging | 79 | 25% | 74 | 29% | .255 |
| Cutaneous | 13 | 4% | 17 | 7% | .188 |
| Ocular | 9 | 3% | 15 | 6% | .092 |
| Thoracoabdominal | 63 | 20% | 61 | 24% | .222 |
| Infective endocarditis (final diagnosis) | 143 | 45% | 187 | 74% | <.001 |
| Positive Duke imaging criterion | 121 | 38% | 167 | 66% | <.001 |
Data are depicted as number/percentage or median/Q1–Q3.
Abbreviations: CEE, cerebral embolic events; HACEK, Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, Kingella kingae.
Cerebral Imaging in Patients With Infective Endocarditis
Among the 330 episodes with possible or definite IE, the median time from antibiotic treatment initiation to Cer-Im was 2 days (Q1–Q3: 0–5). In 175 (53%) the Cer-Im was performed in the absence of any neurologic symptom. At least 1 CEE was found on Cer-Im in 187 (57%) episodes (Table 3). Ischemic lesions (184; 98%) were the most commonly identified, followed by hemorrhagic lesions (35; 19%). For 67 (20%) episodes, the Duke's vascular criterion was fulfilled prior to the realization of the Cer-Im. Thus, 120 (36%) episodes met the vascular criterion due to the Cer-Im; 3 (1%) episodes were reclassified from rejected to possible IE and 25 (8%) episodes from possible to definite IE according to modified Duke criteria (Figure 2). Among episodes without neurologic symptoms, the vascular criterion was fulfilled by the Cer-Im in 34 (22%) episodes, leading to the reclassification of the episode from possible to definite IE in 4 (3%) episodes. A new surgical indication (in association with left-side vegetation >10 mm) for embolism prevention was established by the Cer-Im findings in 74 (22%) episodes (55 had valve surgery), 30 of which were asymptomatic (27 had valve surgery) (Table 3). The median time from Cer-Im to surgical intervention was 4 days (Q1–3: 1–14). Among the 19 episodes with a surgical indication, which did not receive valvular operation, surgery was contraindicated in 3 of them due to coma and in 1 due to hemorrhagic lesion.
Table 3.
Type of Cerebral Embolic Events and Impact on Diagnosis and Management in Patients With Infective Endocarditis
| Total (n = 330) | Asymptomatic (n = 155) | Symptomatic (n = 175) | P* | ||||
|---|---|---|---|---|---|---|---|
| MRI | 194 | 59% | 78 | 50% | 116 | 66% | .004 |
| No cerebral embolic event | 143 | 43% | 95 | 61% | 48 | 27% | |
| Cerebral embolic event | 187 | 57% | 60 | 39% | 127 | 73% | <.001 |
| Ischemic lesions | 184 | 56% | 59 | 38% | 125 | 71% | <.001 |
| Intravenous thrombolysisa | 5 | 2% | 0 | 0% | 5 | 3% | .063 |
| Endovascular thrombectomy | 11 | 3% | 0 | 0% | 11 | 6% | .001 |
| Hemorrhagic lesions | 35 | 11% | 7 | 5% | 28 | 16% | .001 |
| Treatment of hemorrhagic lesions | 1 | <0.5% | 0 | 0% | 1 | 1% | 1.000 |
| Mycotic aneurysm | 18 | 6% | 7 | 5% | 11 | 6% | .629 |
| Treatment of mycotic aneurysm | 2 | 1% | 0 | 0% | 2 | 1% | .500 |
| Cerebral abscess | 6 | 2% | 2 | 1% | 4 | 2% | .688 |
| Drainage of abscess | 0 | 0% | 0 | 0% | 0 | 0% | |
| Treatment of any cerebral embolic event | 15 | 5% | 0 | 0% | 15 | 9% | <.001 |
| New cerebral embolic eventb | 120 | 36% | 34 | 22% | 86 | 49% | <.001 |
| Reclassification from rejected to possible endocarditis | 3 | 1% | 0 | 0% | 3 | 2% | .250 |
| Reclassification from possible to definite endocarditis | 25 | 8% | 4 | 3% | 21 | 12% | .001 |
| Operative indicationc | 74 | 22% | 30 | 19% | 44 | 25% | .235 |
| Valvular surgery performed | 55 | 17% | 27 | 17% | 28 | 16% | .768 |
Data are depicted as number/percentage.
Abbreviation: MRI, magnetic resonance imaging.
*Comparison between symptomatic and asymptomatic patients.
Thrombolysis was performed in patients for which the infective endocarditis (IE) suspicion was not substantiated the moment of the decision.
Not prior cutaneous, ocular, or thoracoabdominal embolic event.
In association with left-side valvular vegetation >10 mm.
Additional analyses were performed among 194 and 185 IE episodes, which had cerebral MRI and CT, respectively (Supplementary Tables 3 and 4). Among patients having MRI, 3 (2%) episodes were reclassified from rejected to possible IE and 17 (9%) from possible to definite IE. A new surgical indication (in association with left-side vegetation >10 mm) was established by the MRI findings in 53 (27%) episodes (40 had valve surgery), 18 of which were asymptomatic (14 had valve surgery).
The comparison of episodes with and without CEE are shown in Table 4. In the multivariable analysis (Supplementary Table 5), the factors associated with CEEs were mitral valve IE (P .002; aOR 2.27, 95% CI: 1.38–3.74), intracardiac abscess (P .003; aOR 1.89, 95% CI: 1.05–3.39), presence of neurologic symptoms (P < .001; aOR 4.36, 95% CI: 2.67–7.14), and presence of ocular EE prior to Cer-Im (P .036; aOR 5.60, 95% CI: 1.12–28.17).
Table 4.
Predictors of Embolic Events Detected by Cerebral Imaging Among Patients With Infective Endocarditis
| Without CEE (n = 143) | With CEE (n = 187) | P | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex | 109 | 76% | 137 | 73% | .610 |
| Age (y) | 65 | 48–74 | 67 | 55–75 | .358 |
| Comorbidities | |||||
| Atrial fibrillation | 32 | 22% | 39 | 21% | .787 |
| Congestive heart failure | 18 | 13% | 11 | 6% | .048 |
| Chronic obstructive pulmonary disease | 19 | 13% | 22 | 12% | .747 |
| Cirrhosis | 13 | 9% | 11 | 6% | .290 |
| Diabetes mellitus | 43 | 30% | 43 | 23% | .165 |
| Chronic kidney disease (moderate or severe) | 18 | 13% | 28 | 15% | .631 |
| Malignancy (solid organ or hematologic) | 14 | 10% | 14 | 7% | .551 |
| Obesity | 39 | 27% | 37 | 20% | .115 |
| Immunosuppression | 12 | 8% | 14 | 7% | .838 |
| Antithrombotic or anticoagulation treatment | 72 | 50% | 91 | 49% | .824 |
| Antithrombotic treatment | 44 | 31% | 51 | 27% | .540 |
| Anticoagulation treatment | 43 | 30% | 50 | 27% | .538 |
| Setting of infection onset | |||||
| Community or non-nosocomial healthcare-associated | 130 | 91% | 173 | 93% | |
| Nosocomial | 13 | 9% | 14 | 8% | .686 |
| Cardiac predisposing factors | 74 | 52% | 94 | 50% | .825 |
| Prosthetic valve | 39 | 27% | 57 | 30% | .543 |
| Timing of IE | |||||
| 2015–2017 (retrospective cohort) | 50 | 35% | 67 | 36 | |
| 2018–2022 (prospective cohort) | 93 | 65 | 120 | 42 | .908 |
| Microbiological identification | |||||
| Staphylococcus aureus | 64 | 45% | 77 | 41% | .575 |
| Coagulase negative staphylococci | 10 | 7% | 6 | 3% | .127 |
| Streptococci | 35 | 24% | 60 | 32% | .142 |
| Enterococci | 17 | 12% | 21 | 11% | .863 |
| Other gram-positive | 5 | 3% | 3 | 2% | .300 |
| HACEK | 6 | 4% | 5 | 3% | .541 |
| Other gram-negative | 5 | 3% | 3 | 2% | .300 |
| Intracellular pathogens | 0 | 0% | 3 | 2% | .261 |
| Fungi | 2 | 1% | 3 | 2% | 1.000 |
| Polymicrobial infection | 4 | 3% | 2 | 1% | .409 |
| No identification | 4 | 3% | 8 | 4% | .563 |
| Manifestations | |||||
| Systemic symptoms | 134 | 94% | 175 | 94% | 1.000 |
| Fever | 120 | 84% | 153 | 82% | .661 |
| Heart murmur | 90 | 63% | 116 | 62% | .909 |
| New heart murmur | 63 | 44% | 91 | 49% | .437 |
| Immunologic phenomena | 10 | 7% | 25 | 13% | .072 |
| Sepsis | 62 | 43% | 92 | 49% | .317 |
| Septic shock | 29 | 20% | 37 | 20% | 1.000 |
| Cerebral imaging performed due to neurologic symptoms | 48 | 34% | 127 | 68% | <.001 |
| Deficit | 11 | 8% | 71 | 38% | <.001 |
| Confusion/coma | 31 | 22% | 72 | 39% | .001 |
| Headache | 7 | 5% | 14 | 7% | .373 |
| Seizures | 0 | 0% | 4 | 2% | .136 |
| Days to cerebral imaging | 4 | 1–8 | 2 | 0–5 | <.001 |
| Embolic events prior to cerebral imaging | 53 | 37% | 67 | 36% | .819 |
| Cutaneous | 13 | 9% | 17 | 9% | 1.000 |
| Ocular | 2 | 1% | 14 | 7% | .010 |
| Thoracoabdominal | 43 | 30% | 55 | 29% | .904 |
| Site of infection | |||||
| Aortic valve | 74 | 52% | 94 | 50% | .825 |
| Mitral valve | 52 | 36% | 105 | 56% | <.001 |
| Other left-side site of infection | 1 | 1% | 1 | 1% | 1.000 |
| Tricuspid valve | 18 | 13% | 9 | 5% | .014 |
| Pulmonary valve | 7 | 5% | 1 | 1% | .023 |
| Multivalvular | 17 | 12% | 23 | 12% | 1.000 |
| CIED-IE | 14 | 10% | 7 | 4% | .039 |
| Type of left-side valve | |||||
| Native | 100 | 70% | 138 | 74% | .459 |
| Prosthetic | 34 | 24% | 52 | 28% | .449 |
| Positive imaging Duke criterion | 115 | 80% | 156 | 83% | .562 |
| Vegetation | 102 | 71% | 141 | 75% | .450 |
| Vegetation ≥10 mm | 61 | 43% | 92 | 49% | .002 |
| Abscess | 27 | 19% | 56 | 30% | .029 |
| Other lesionsa | 24 | 17% | 42 | 22% | .214 |
Data are depicted as number/percentage or median/Q1–Q3.
Abbreviations: CEE, cerebral embolic events; CIED-IE, cardiac implantable electronic device-related infective endocarditis; EE, embolic events; HACEK, Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, infective endocarditis.
Perforation, dehiscence of prosthetic valve, fistula, pseudoaneurysm, aneurysm.
DISCUSSION
In the present study, performing Cer-Im in the diagnostic work-up of patients with suspected IE resulted in the discovery of CEEs in 44% of cases, but their identification had limited impact on the final diagnosis of IE. However, CEE detection frequently influenced subsequent surgical management, with 22% of IE patients meeting a new surgical indication following Cer-Im findings. To the best of our knowledge, this study is the largest to date trying to elucidate the role of Cer-Im in the diagnosis and subsequent management of patients with suspected IE.
As previously shown, the central nervous system was the most common site of embolization among patients with definite or possible IE. In fact, 57% of patients had a CEE, which was more common among symptomatic (73%) as compared to asymptomatic patients (39%). Previous studies reported wide variations in the prevalence of CEEs (22%–83%) [2, 4, 5, 7–14]. This wide range of incidence among previous studies was due the difference of inclusion criteria. Studies with higher incidence of reported CEEs included patients with neurologic symptoms [7], definite IE [4, 7, 9–11], with MRI [2, 7, 9–14], or Cer-Im performed as pre-operative screening [7–9].
The prevalence of CEEs (39%) among asymptomatic IE patients was significantly higher than previously reported (4%–30%) [4, 5, 8], whereas some studies presented higher rates (60%–80%) [2, 10–14]. These wide differences in prevalence may be explained by the various definitions of symptomatic lesions. Most studies defined lesions as symptomatic only in presence of focal neurologic deficit [4, 5, 11] but not in the presence of confusion, seizures, or headache as was the case for the present study. Therefore, our wider definition of neurologic symptoms may explain a higher prevalence of symptomatic CEE and a lower prevalence of asymptomatic CEE compared to previous studies.
The majority of CEEs were ischemic lesions (98%), which is in accordance to most previous studies [4, 6–9]. The few previous studies, which reported hemorrhagic lesions as the most common ones, also included microbleeds in the definition of hemorrhagic CEE, which was not the case in the present study [2, 10–13]. In a previous study, microbleeds presence was not associated with typical risk factors of embolization, such as vegetation size or type of pathogen, and showed a weak concordance with the presence of ischemic lesions, suggesting a difference process [13]. The results of our study were in line with a metanalysis including IE patients with cerebral MRI, which also identified ischemic lesions as the most common type of CEE. In all studies, mycotic aneurysms and abscesses were present on a minority of patients [6, 7, 10–13, 18].
Imaging studies for embolic events (cerebral MRI, whole body CT and/or 18F-FDG PET-CT) were part of the diagnostic algorithm in patients with a high clinical suspicion but still unproven IE, according to the ESC guidelines [15]. The role of cerebral MRI in diagnostic reclassification was previously studied among IE patients, and the results of cerebral MRI was found to lead to a change in classification in 5%–32% of patients [2, 3, 13]. In the present study, which is the only 1 having included patients with suspected IE and not only patients with an established diagnosis of IE, Cer-Im findings allowed diagnostic reclassification in 5% of patients only (1% among asymptomatic patients). Among 343 patients who benefited from MRI, diagnostic reclassification was also low. According to our results, there is little reason to recommend systematic Cer-Im for diagnostic purposes in asymptomatic patients with suspected IE.
Another potential impact of Cer-Im on patients with suspected IE, is the possibility to offer a specific treatment of CEEs’ complications, such as endovascular thrombectomy for ischemic lesions, drainage of abscess, or specific treatment of hemorrhagic lesions or mycotic aneurysms. Such interventions were indicated in 5% of our patients’ cohort, which was comparable to previous studies [16, 19]. None of these treatments was found to be indicated among asymptomatic patients with suspected IE, underlining the fact that a policy of systematic Cer-Im in asymptomatic patients with suspected IE is not warranted.
Even though the diagnostic impact of systematic Cer-Im is limited, its impact on patient's management is probably much more important. Indeed, Cer-Im together with the presence of a left-sided valvular vegetation >10 mm, established a new surgical indication for embolism prevention in 22% of IE patients (19% among asymptomatic patients). In the first randomized clinical trial of patients with large vegetations without heart failure, but at high-risk for EEs, early surgery resulted in a significantly lower rate of EEs, as compared to conventional treatment (0% vs 21%; P .005) [20]. Previous studies also reported the influence of Cer-Im on the surgical treatment strategy. In a metanalysis including IE patients, cerebral MRI findings led to changes in the surgical plan in 14% of patients (defined as establishing a new indication, or changes in surgical = planification, including advancement, cancelation or postponement). In the present study, only the establishment of a new surgical indication has been considered as the surgical endpoint.
As previously shown [21], the sensitivity of CT scan and MRI for the detection of hemorrhagic lesions, mycotic aneurysms, or cerebral abscess was comparable, but MRI was far more sensitive than CT scan for the detection of ischemic lesions. This underlines the importance of performing early MRI to accurately detect the whole spectrum of brain lesions potentially associated with IE.
The study has several limitations. First, because the study is a non-interventional one, the influence of Cer-Im on subsequent management was on discretion of the treating physician and infectious diseases specialist. Furthermore, in our center, valve surgery was offered on patients with vegetation >10 mm and EEs even if the EEs occurred before 5 days of appropriate antimicrobial treatment, based on the fact that although the risk of EEs decreases steadily during treatment, it remains high during the first 2 weeks of treatment. Thus, patients could benefit from the prevention of subsequent risk of EEs by an early cardiac surgery [20, 22, 23]; this difference in approach could limit generatability of our results in centers with different surgical practices. Second, cerebral angiography was not systematically performed and some mycotic aneurysms could have remained undiagnosed. Additionally, not all patients had a cerebral MRI, which is more sensitive than CT scan. Third, although we only evaluated the impact of Cer-Im on the establishment of new surgical indications, other important changes in management, such as advancement, cancelation, and postponement were not assessed. Furthermore, the impact of diagnostic or management modification on patient outcomes was not evaluated. Finally, even though Duke criteria were commonly used for the definition of IE, their diagnostic performance is moderate (sensitivity ∼80%) and should not replace clinical judgment [24]; thus, a final IE diagnosis was made after 2 months, incorporating endocarditis team appreciation.
In conclusion, our results do not support a policy of systematic Cer-Im in asymptomatic patients with suspected IE for diagnostic purposes, since it failed to substantially reclassify patients according to Duke criteria. On the other hand, together with the presence of a valvular vegetation >10 mm, Cer-Im led to the establishment of a new surgical indication for EEs’ prevention in one fifth of patients. Thus, systematic Cer-Im in asymptomatic IE patients may improve decision making, but more studies are needed to elucidate whether such changes in treatment strategy will eventually improve patient's prognosis in terms of a better survival.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Matthaios Papadimitriou-Olivgeris, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Benoit Guery, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Nicoleta Ianculescu, Department of Cardiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Vincent Dunet, Department of Medical Radiology, Service of Diagnostic and Interventional Radiology, Neuroradiology Unit, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Yosra Messaoudi, Department of Cardiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Silvia Pistocchi, Department of Medical Radiology, Service of Diagnostic and Interventional Radiology, Neuroradiology Unit, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Piergiorgio Tozzi, Department of Cardiac Surgery, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Matthias Kirsch, Department of Cardiac Surgery, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Pierre Monney, Department of Cardiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Notes
Author Contributions. P. M. and B. G. conceived the idea. M. P. O., B. G., N. I., V. D., Y. M., S. P., P. T., and M. K. collected the patients” data. P. M. supervised the project. M. P. O. performed the analysis and interpreted the results. M. P. O. wrote the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
References
- 1. Mohananey D, Mohadjer A, Pettersson G, et al. . Association of vegetation size with embolic risk in patients with infective endocarditis: a systematic review and meta-analysis. JAMA Intern Med 2018; 178:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duval X, Iung B, Klein I, et al. . Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152:497–504, W175. [DOI] [PubMed] [Google Scholar]
- 3. Champey J, Pavese P, Bouvaist H, et al. . Is brain angio-MRI useful in infective endocarditis management? Eur J Clin Microbiol Infect Dis 2016; 35:2053–8. [DOI] [PubMed] [Google Scholar]
- 4. Thuny F, Avierinos JF, Tribouilloy C, et al. . Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J 2007; 28:1155–61. [DOI] [PubMed] [Google Scholar]
- 5. Snygg-Martin U, Gustafsson L, Rosengren L, et al. . Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis 2008; 47:23–30. [DOI] [PubMed] [Google Scholar]
- 6. Monteiro TS, Correia MG, Golebiovski WF, Barbosa GIF, Weksler C, Lamas CC. Asymptomatic and symptomatic embolic events in infective endocarditis: associated factors and clinical impact. Braz J Infect Dis 2017; 21:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okazaki S, Yoshioka D, Sakaguchi M, Sawa Y, Mochizuki H, Kitagawa K. Acute ischemic brain lesions in infective endocarditis: incidence, related factors, and postoperative outcome. Cerebrovasc Dis 2013; 35:155–62. [DOI] [PubMed] [Google Scholar]
- 8. Champey J, Pavese P, Bouvaist H, et al. . Cerebral imaging in infectious endocarditis: a clinical study. Infect Dis 2016; 48:235–40. [DOI] [PubMed] [Google Scholar]
- 9. Chakraborty T, Scharf E, Rabinstein AA, et al. . Utility of brain magnetic resonance imaging in the surgical management of infective endocarditis. J Stroke Cerebrovasc Dis 2017; 26:2527–35. [DOI] [PubMed] [Google Scholar]
- 10. Hess A, Klein I, Iung B, et al. . Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol 2013; 34:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper HA, Thompson EC, Laureno R, et al. . Subclinical brain embolization in left-sided infective endocarditis: results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study. Circulation 2009; 120:585–91. [DOI] [PubMed] [Google Scholar]
- 12. Iung B, Klein I, Mourvillier B, et al. . Respective effects of early cerebral and abdominal magnetic resonance imaging on clinical decisions in infective endocarditis. Eur Heart J Cardiovasc Imaging 2012; 13:703–10. [DOI] [PubMed] [Google Scholar]
- 13. Iung B, Tubiana S, Klein I, et al. . Determinants of cerebral lesions in endocarditis on systematic cerebral magnetic resonance imaging: a prospective study. Stroke 2013; 44:3056–62. [DOI] [PubMed] [Google Scholar]
- 14. Chakraborty T, Scharf E, DeSimone D, et al. . Variable significance of brain MRI findings in infective endocarditis and its effect on surgical decisions. Mayo Clin Proc 2019; 94:1024–32. [DOI] [PubMed] [Google Scholar]
- 15. Habib G, Lancellotti P, Antunes MJ, et al. . 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 16. Ahn Y, Joo L, Suh CH, et al. . Impact of brain MRI on the diagnosis of infective endocarditis and treatment decisions: systematic review and meta-analysis. AJR Am J Roentgenol 2022; 218:958–68. [DOI] [PubMed] [Google Scholar]
- 17. Friedman ND, Kaye KS, Stout JE, et al. . Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 18. Yang A, Tan C, Daneman N, et al. . Clinical and echocardiographic predictors of embolism in infective endocarditis: systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:178–87. [DOI] [PubMed] [Google Scholar]
- 19. Meshaal MS, Kassem HH, Samir A, Zakaria A, Baghdady Y, Rizk HH. Impact of routine cerebral CT angiography on treatment decisions in infective endocarditis. PLoS One 2015; 10:e0118616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang DH, Kim YJ, Kim SH, et al. . Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366:2466–73. [DOI] [PubMed] [Google Scholar]
- 21. Vitali P, Savoldi F, Segati F, et al. . MRI Versus CT in the detection of brain lesions in patients with infective endocarditis before or after cardiac surgery. Neuroradiology 2022; 64:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheggi V, Alterini B, Olivotto I, et al. . Embolic risk stratification and prognostic impact of early surgery in left-sided infective endocarditis. Eur J Intern Med 2020; 78:82–7. [DOI] [PubMed] [Google Scholar]
- 23. Kim DH, Kang DH, Lee MZ, et al. . Impact of early surgery on embolic events in patients with infective endocarditis. Circulation 2010; 122:S17–22. [DOI] [PubMed] [Google Scholar]
- 24. Habib G, Derumeaux G, Avierinos JF, et al. . Value and limitations of the duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol 1999; 33:2023–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.