INTRODUCTION
Diabetes-related foot ulcers (DFUs) have a global prevalence of 6.3%, reaching 13% in North America [1]. DFUs have a dismal prognosis, with 5- and 10-year survival rates of approximately 50% and 25%, respectively [2, 3]. Infection, complicating >40% of DFUs, is often the coup-de-grace; nearly half of patients hospitalized with a diabetes-related foot infection (DFI) undergo amputation within 1 year [4, 5]. These risks disproportionately affect Black, Hispanic, Native, rural, and low-income communities [6, 7]. Lower extremity amputations due to DFUs are the third most costly diabetes complication and are feared by many patients more than death [8].
In 2012, the authors of the Infectious Diseases Society of America (IDSA) DFI guidelines declared that “The main problem currently is less our lack of full understanding of the problem as our failure to apply what we know works.” [9] This statement remains accurate a decade later. Multidisciplinary DFI teams can reduce major amputations, and similar teams have dramatically reduced attributable mortality for infective endocarditis [10, 11]. Despite this, inconsistent and unstructured collaboration between specialists that lead to breakdowns in shared decision-making remains common.
In this narrative review, we bring together experts in infectious diseases, endocrinology, podiatry, and vascular surgery to discuss shared decision-making in DFI care. We provide a focused overview of the comprehensive management of these patients, highlighting modern research spearheaded by our surgical colleagues and contemporary guidance from the International Working Group for the Diabetic Foot (IWGDF), arguably the lingua franca of DFI specialists [12]. Finally, we offer best practices for clinician–clinician and patient–clinician shared decision-making.
Notably, while this review reflects our expert opinion based on data largely published after the now-archived IDSA DFI guidelines, it is not intended to supplant those guidelines. This review is also written from the perspective of US-based clinicians and intended primarily for infectious diseases (ID) specialists. International colleagues may find some of our considerations regarding barriers to care less relevant, and a comprehensive review of surgical decision-making in DFI is outside this article's scope.
HOW ARE DIABETES-RELATED FOOT INFECTIONS AND THEIR OUTCOMES CLASSIFIED?
Lower limb amputations can be categorized as minor (at or below the ankle, sometimes also called foot amputation) or major (above the ankle). In retrospective DFI cohorts, minor amputation rates of 15%–30% and major amputation rates of 5%–20% are typical [7, 13, 14]. Concomitant peripheral artery disease (PAD) is the key, most consistently identified risk factor for major amputation and death [13, 15].
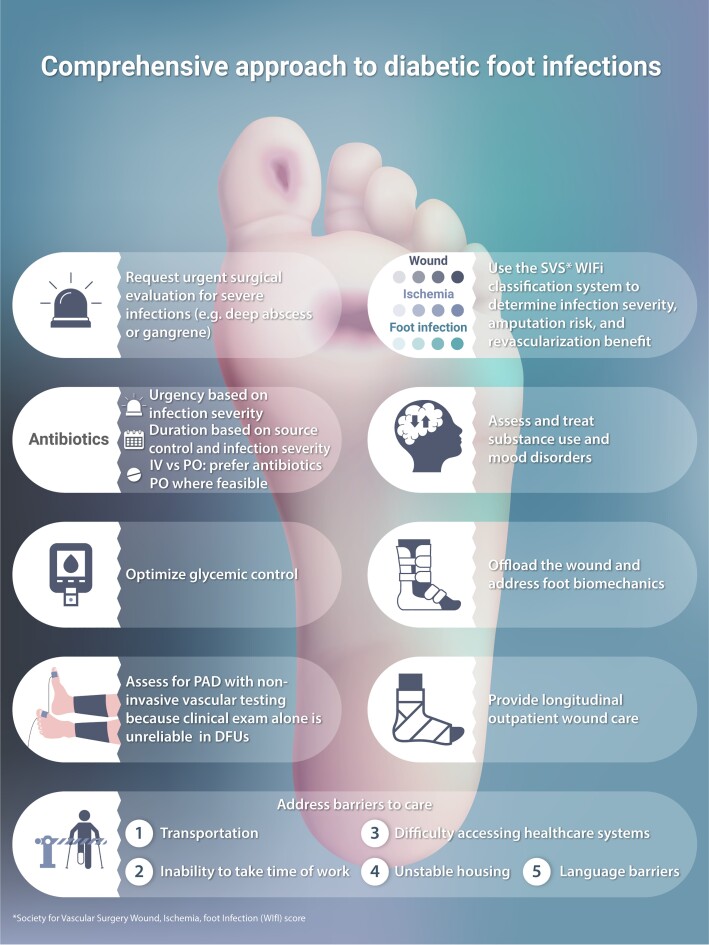
A number of DFU classification systems have been proposed, including the perfusion, extent/size, depth/tissue loss, infection and sensation (PEDIS), University of Texas, Wagner, sepsis, arteriopathy, denervation (SAD), Saint Elain, and IDSA criteria [16–19]; however, all have limitations in their differentiation of wounds, ischemia, and/or infection severity. The most modern and comprehensive criterion is the Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot infection) classification system, which iterates on the 2012 IDSA criterion with staging of the foot ulceration, the degree of accompanying gangrene, and the degree of accompanying limb ischemia [12, 20]. A straightforward approach to scoring and interpreting WIfI classifications is given in Tables 1 and 2.
Table 1.
Summary of the Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) Classification System for Diabetes-related Foot Infection
| W ound | Wound Appearance | Score |
| No ulcer | 0 | |
| Small, shallow ulcer without exposed bone unless distal toe; no gangrene | 1 | |
| Deeper ulcer with exposed bone/joint/tendon or shallow heel ulcer; gangrene limited to digits if present | 2 | |
| Extensive deep ulcer involving forefoot or midfoot or a deep, full-thickness heel ulcer; extensive gangrene | 3 |
| I schemia | Toe Pressure, Transcutaneous Oximetry, mmHg | Ankle Systolic Pressure, mmHg | Ankle-Brachial Indexa | Score |
| ≥60 | >100 | ≥0.8 | 0 | |
| 40–50 | 70–100 | 0.6–0.79 | 1 | |
| 30–39 | 50–70 | 0.4–0.59 | 2 | |
| <30 | <50 | <0.39 | 3 | |
| f oot Infection | Clinical Manifestations of Infection | … | ||
| No symptoms or signs of infection | 0 | |||
| Local infection; defined by >2 of local swelling/induration, erythema extending 0.5–2 cm from the ulcer, pain or tenderness, warmth, or purulent discharge, not due to some other inflammatory response, and without involvement of deeper tissues or systemic infection | 1 | |||
| Local infection as defined above, accompanied by erythema extending >2 cm from the ulcer or involving deeper tissues (eg, abscess, osteomyelitis, septic arthritis), without systemic signs of infection | 2 | |||
Total Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) class should be reported with the components of each score. For example, a patient with an ankle-brachial index (ABI) of 0.3 but no wound or signs of infection would be classified as WIfI 030.
ABI may be falsely elevated. The International Working Group for the Diabetic Foot guidance prefers use of toe pressure (TP) or transcutaneous oximetry, which have been shown to predict ulcer healing.
Table adapted from Mills et al [21].
Table 2.
Simplified Approach to Interpreting the Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) Classification for Diabetes-related Foot Infection Prognostication
| Low | Moderate | High | |
|---|---|---|---|
| What is the risk of major lower extremity amputation? | Nearly all classifications with a sum of scores <3a | Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) classifications that do not fit the low- or high-risk criteria shown | Nearly all classifications with a foot infection score of 3 (extensive local and systemic infection) OR a sum of scores >5b |
| What is the likelihood that revascularization would be beneficial if the infection can be controlled first? | All classifications with: • Ischemia scores of 0 • Ischemia scores of 1 with no wound and no more than local infection • Ischemia scores of 2 with no wound and no more than limited local infection |
Most other classifications with an ischemia score of 1 | Most other classifications with an ischemia score >2 |
Exceptions are classifications 003 and 021 (both moderate rather than low risk).
Exceptions are classifications 003, 032, 103, 131, and 212 (all moderate rather than high risk).
Table adapted from Mills et al [21].
The WIfI classification offers prognostication on both the risk of amputation and potential benefit of revascularization among patients with DFUs [21, 22]. Although not DFI-specific, WIfI encompasses the major determinants of DFU prognosis and predicts DFU healing and minor amputations [21, 23, 24]; the infection scoring component of WIfI also predicts hospitalization and amputation [25]. The WIfI classification system is well validated in DFI and an ideal basis for evidence-based decision-making among ID specialists, surgeons, and patients. At a minimum, the competent ID specialist should be familiar with WIfI classification and its interpretation, as this common tool incorporates several of the key criteria beyond infection severity that inform our surgical colleagues’ decision-making.
WHAT ARE THE KEY COMPONENTS OF A COMPREHENSIVE APPROACH TO DIABETES-RELATED FOOT INFECTIONS?
Antimicrobial Therapy
Most diabetes-related foot infections are polymicrobial, with Staphylococcus aureus near universally the most prevalent organism [26, 27]. Other common pathogens include Enterobacterales, Enterococci, and Streptococci. Pseudomonas aeruginosa has a highly regional prevalence and is probably more common in tropical/subtropical regions and after antibiotic exposure. This variance has resulted in highly regional preferences for empiric antibiotic therapies [28]. Acknowledging this diversity and the limited data for specific regimens, we focus here on DFI treatment controversies that often frame decision-making discussions and for which new data are rapidly emerging.
Published data demonstrate that successful treatment of diabetes-related foot osteomyelitis (DFO) can routinely be accomplished without bony debridement. Truong et al identified 18 studies that evaluated nonoperative outcomes of DFO with a mean treatment success rate of 68.2% over a median of 15 months of follow-up. In contrast, the same systematic review identified 13 studies of surgical DFO treatment that reported a mean success rate of 85.7% over 19.5 months [29]. Important limitations of this meta-analysis include its use of primarily retrospective studies, introducing confounding by indication in patients selected for surgical vs nonoperative medical management, and substantial heterogeneity between studies in antibiotic management as well as definitions of disease and cure. While higher-quality evidence that demonstrates whether medical management is noninferior to surgical treatment at a clinically acceptable effect size would be welcome, these data indicate that nonoperative management can frequently be successful. Amputations often threaten a patient's independent mobility, particularly when the patient's preoperative functional status is already mediocre, and perversely may predispose to foot ulceration and infection elsewhere by shifting weight-bearing mechanics, putting new areas under threat. These patients are most likely to benefit from limb preservation efforts. In such cases, the modest improvement in infectious cure with surgical management may not be justified, given the risk of decline in functional status with amputation [30]. On the other hand, in patients with DFO that involves the distal phalanx or proximal interphlangeal (PIP) of a hammer toe or claw deformity, early surgery can both eradicate the infection and treat the underlying biomechanical issue with little threat to mobility and should be preferred.
Preserving independent mobility is key to patients’ prognoses, often central to their values and preferences, and must be weighed against antibiotic-associated adverse events. Antibiotics cannot restore devitalized tissue. Consequently, antibiotic therapy alone is not a reasonable strategy for infections that involve extensive tissue necrosis or copious undrained purulence, particularly when these have become sources of systemic infection. However, beyond these cases, a “therapeutic trial” of antibiotics is usually reasonable.
The noninferiority of oral to intravenous antibiotics for chronic osteomyelitis has been shown in 8 randomized, controlled trials (RCTs) and their meta-analyses [31]. The most robust was the Oral vs Intravenous Antibiotics for Bone and Joint Infection (OVIVA) noninferiority RCT, which found no difference in long-term cure with all-intravenous (IV) antibiotics vs a rapid transition to oral therapy [32]. While OVIVA did not report DFI-specific outcomes, modern RCTs of DFIs have used an IV-to-oral antibiotic switch strategy with IV durations as short as a median of 2 days, achieving high rates of cure [33–37].
Our general recommendation for oral antibiotics in DFO comes with a few caveats. First, the OVIVA's subgroup analyses identified a trend toward worse outcomes with oral vs IV among patients with no defined pathogen. Therefore, we suggest against empiric oral therapy in areas where there are no reliably susceptible oral options for Enterobacterales and S. aureus [32]. Second, the same analyses showed a trend toward poorer outcomes with oral penicillin vs IV therapy, possibly due to poorer adherence to oral penicillin’s frequent dosing and/or suboptimal bug–drug matches (eg, amoxicillin for osteomyelitis due to Enterococcus faecalis, whose penicillin minimum inhibitory concentrations are typically 10- to 100-fold higher vs Streptococci). While direct data to support this hypothesis are lacking, a recent large cohort found that maximizing oral beta-lactam dosing was a critical component of their noninferiority to other highly bioavailable antibiotics in gram-negative bacteremia [38]. We suggest selecting oral beta-lactams for DFO only with aggressive dosing and not in patients with obesity, augmented renal clearance, or other factors that limit drug exposures. Third, data for oral antibiotics in osteoarticular infections are not equal for each drug class. Combinations of rifampin with fluoroquinolones and trimethoprim-sulfamethoxazole are well studied in orthopedic infections [39]; data for other regimens are less robust, and their use requires expert judgment.
Finally, some experts advocate for routine addition of rifampin in DFO. This practice is supported by a large observational study that associated rifampin use with lower risks of amputation and death [40]. The forthcoming Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID) RCT seeks to compare rates of further amputation or death with 6 weeks of adjunctive rifampin vs placebo in patients with DFO that has been managed nonoperatively or features residual osteomyelitis and will provide better evidence to guide this practice [41].
Antimicrobial durations for DFI and DFO are not well defined by evidence and traditionally stratified by infection severity (mild vs moderate–severe), depth (soft tissue vs osteomyelitis), and extent of surgical debridement (none, partial, complete) [9]. When surgery has been performed to remove all infected bone and there is no residual soft tissue infection, antibiotics may be stopped within 48 hours [42]. Among patients with DFO managed nonoperatively, a large RCT demonstrated that 6 weeks of antibiotic therapy was noninferior to 12 weeks [36]. Two subsequent pilot RCTs with large noninferiority margins found similar rates of cure with 3 weeks vs 6 weeks of antibiotic therapy for DFO with residual osteomyelitis after debridement and 10 days vs 20 days of antibiotic therapy for moderate to severe DFI after debridement [37, 43]. Larger, confirmatory RCTs are ongoing [44]. A summary of reasonable durations of therapy for DFI based on these data is given in Table 3.
Table 3.
Typical Durations of Antimicrobial Therapy for DFI
| Degree and Management of Foot Infection | Duration |
|---|---|
| Osteomyelitis, managed nonoperatively | 6 weeks |
| Osteomyelitis, following debridement (but not curative amputation) | 3–6 weeksa |
| Soft tissue infection without osteomyelitis | 10–21 daysb |
| Soft tissue infection and/or osteomyelitis, following curative amputation | 0–48 hours |
Based on similar clinical outcomes with 3 vs 6 weeks of antimicrobials from a single pilot randomized, controlled trial (RCT).
Based on similar clinical outcomes with 10 vs 21 days of antimicrobials from a single pilot RCT. Given the limitations of this study, shorter durations may not be appropriate for patients with peripheral artery disease or who have not had highly sensitive imaging (eg, magnetic resonance imaging) to rule out osteomyelitis.
Surgery
Surgical treatment of DFI is performed in a stepwise approach, starting as distally as possible, with the goal of achieving functional limb preservation [45, 46]. Most mild and superficial DFI, meaning DFI that does not extend below the subcutaneous fascia and does not include osteomyelitis or abscess, can be managed conservatively. Urgent or emergency surgery is needed in severe infection and involves incision and drainage of deep plantar spaces, debridement of devitalized tissue, and sometimes toe or partial foot amputation. Although every effort should be made to avoid major amputation, emergency open below-knee amputation may be necessary for severe life-threatening infections, particularly necrotizing fasciitis. Temporization of infection should take precedence over PAD evaluation in patients who require emergent or urgent source control. In non–life-threatening DFIs, surgery is typically deferred to allow for PAD evaluation and treatment. Depending on local expertise and available resources, definitive toe/foot surgery may be performed during or after revascularization.
The level of amputation is determined based on the extent of infection, tissue viability, tissue perfusion, and the functionality of the amputation. Amputation of a single digit or 2 consecutive digits, with or without removal of the metatarsal head (ray amputation), is generally well tolerated. In patients who require amputation of 3 or more digits, a transmetatarsal amputation (TMA) is a better option. The main challenge with a TMA and other more proximal mid-foot amputations (such as Lisfranc and Chopart amputations) is the high biomechanical failure rate (30%–60%) [47]. Although the reoperation risk is greater than for a below-knee amputation, TMA yields a superior functional outcome and is preferred in ambulatory patients. Major amputation is required in patients with TMA failure [48, 49]. In selected patients with open TMA without a viable posterior flap, which is necessary for closure, negative pressure wound therapy (“wound vac”) can be used until the application of a partial thickness skin graft or healing by secondary intention [50].
PAD affects 30%–50% of patients with DFUs and is associated with significantly worse ulcer healing, amputation, and mortality [15]. In the EURODIALE prospective DFU cohort (n = 1088), concurrent infection was a key predictor of limb loss in patients with PAD [15]. Timely PAD assessment and treatment are paramount. Revascularization, whether open or endovascular, increases limb perfusion and limb salvage rates in patients with ischemic ulcers [51], while delayed revascularization is associated with an increased risk of limb loss and mortality [52].
Clinical examination (pulse palpation, capillary refill time) is insufficient to rule out PAD; therefore, all patients with DFUs (especially those without clearly palpable pulses) should undergo noninvasive vascular testing. The ankle-brachial index (ABI) can be inaccurate due to falsely elevated ankle pressure. IWGDF guidelines recommend tests that predict healing, such as toe pressure measurement, transcutaneous oximetry (TcPO2), or skin perfusion pressure. In a systemic review, an ankle pressure of 70 mmHg or a combination of ABI <0.5 and ankle pressure <50 mmHg was associated with risk of major amputation, whereas toe pressures of ≥45 mmHg, TcPO2 >25 mmHg, and skin perfusion pressures >40 mmHg were associated with healing [53]. The ischemic grading of the WIfI classification can identify patients who could benefit from revascularization [54, 55].
Other Care Components
Many evidence-based interventions for DFI are nonpharmacologic, particularly regarding long-term care for accompanying ulcers. These provide ID clinicians with the opportunity to embrace our roles as masters of internal medicine and complex problem solvers rather than single-faceted antibiotic sommeliers. To that end, we offer a checklist for comprehensive DFI care (Table 4). At a minimum, all such patients need wound care, offloading, PAD assessment/treatment, and glycemic control [12, 56]. Multidisciplinary DFU care improves outcomes; delayed referral to DFU services is associated with poorer healing and more amputations [10]. Although there are no standards regarding the components of a DFU multidisciplinary team, most experts suggest that these teams, at a minimum, include podiatrists and vascular surgeons to deliver wound care, offloading, and PAD care, as well as physical therapists to optimize functional outcomes (the “toe flow and go” model). We further suggest incorporating social workers and/or case managers into the team, with the specific goals of identifying and addressing barriers to care.
Table 4.
To-Do List for a Comprehensive Approach to Diabetes-related Foot Infection Care
| Surgical debridement: If present, drain deep purulence and excise necrotic tissue. Assess risk of amputation using clinically validated criteria (WIfI). If elevated, request surgical evaluation and risk-benefit/shared decision-making |
| Peripheral artery disease: Obtain relevant vascular studies (eg, toe pressure measurements); request vascular surgery evaluation if patient is likely to benefit from revascularization (ie, by Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) classification system WIfI classification) |
|
Antibiotic therapy: Once patient is stabilized and has responded to initial antimicrobial therapy, select an appropriate oral (or IV) definitive antimicrobial regimen, with considerations including: • Results of deep tissue cultures or the local epidemiology and antibiogram if cultures are not available • Duration appropriate to the degree of infection and surgical management provided • Social factors, including the ability to adhere to the regimen (eg, affordability, pill burden, ability to store and administer IV antibiotics, and travel to infusion centers) and whether giving IV antibiotic therapy inpatient or via skilled nursing facility facilitates access to other needed care (eg, wound care) |
| Offloading: Provide the patient with either a non-removable device (eg, total contact cast) or removable device (ie, surgical boot) to provide mechanical offloading of the diabetes-related foot wound; consider surgical offloading referral for select patients who do not heal with mechanical devices |
| Wound care: Secure longitudinal outpatient follow-up with a wound care specialist who can provide serial assessment, debridement, and appropriate dressings or negative pressure wound therapy. Ensure the patient has access to adequate wound care supplies upon discharge and at each follow-up visit |
|
Glycemic control: Initiate or intensify diabetes treatment to achieve the HbA1c goal to optimize wound healing: • The HbA1c goal for most adults is <7% (comparable continuous glucose monitoring targets are time in range >70% with time below range <4%). Higher or lower glycemic targets may be appropriate based on the individual's comorbidities and risk of hypoglycemia |
|
Concurrent foot pathology: Identify and address other conditions that provide a bacterial portal of entry into the foot or otherwise predispose to infection: • Treat onychomycosis and tinea pedis if present • Offer compression garments and recommend leg elevation if venous stasis is present and degree of peripheral arterial disease allows • Recommend daily moisturizer to areas of dry, cracked skin • Arrange longitudinal follow-up every 3 months, preferably by a podiatrist, for secondary prevention and early detection of ulcers/infection |
|
Other key comorbidities:
• Offer pharmacotherapy and referral to local evidence-based programs for tobacco cessation if patient has any active tobacco use • Initiate pharmacotherapy (eg, buprenorphine-naltrexone for opiate use disorder, selective serotonin reuptake inhibitor for major depression) or request psychiatry consultation for untreated mood or substance use disorders |
| Barriers to care: Mitigate social factors likely to impede the patient's adherence to treatment (see Table 5) |
Abbreviation: IV, intravenous; WIfI, Society for Vascular Surgery's WIfI (Wound, Ischemia, Foot Infection) classification system.
DFU wound care focuses on removing devitalized tissue by physical (eg, sharp debridement with a scalpel), biochemical (eg, hydrogels such as INTRASITE or Purilon gels), or biological (eg, larvae) methods. Although head-to-head comparisons between methods are lacking, IWGDF guidelines recommend sharp debridement absent contraindications such as pain or ischemia, partially due to lower cost [57]. Secondary analyses of clinical trials and large observational studies suggest that frequent debridement (≤7 days compared with longer intervals) may produce better healing [58–60].
Following debridement, wound dressings are applied to keep the wound clean and absorb exudate without drying the wound. Dressing choice should be individualized and may need to be modified as the wound evolves. Although wet-to-dry saline dressings are common, they may increase viable tissue loss vs alternatives. A plethora of “advanced” dressings have entered the market, but few are supported by RCTs [61]. Moreover, patients with DFIs are generally excluded from these RCTs, making it unclear whether and when to deploy these products for DFIs.
An alternative to wound dressings, wound vac promotes granulation tissue formation via growth factor upregulation [61, 62]. Two negative pressure wound therapy trials (total n = 277) included participants with DFI following amputations, both showing higher healing rates in the intervention arms [63, 64]. Another large RCT (n = 335) enrolled participants with long-standing, large uninfected ulcers (median ulcer duration of 198 days and area of 13.5 cm2 in the intervention arm) and found that 16-week healing rates increased (43% vs 29%) with wound vac therapy [64]. Although negative pressure wound therapy for DFUs is generally safe, we suggest carefully monitoring patients at risk for loss to follow-up. In our experience, delays in changing the device/wound interface (“sponge”), which is typically exchanged weekly, can lead to infections.
There has been long-standing interest in using oxygen (systemically or topically) for DFUs, given the high prevalence of micro- and macrovascular disease and consequent tissue hypoxemia [62]. Systemic hyperbaric oxygen therapy for DFUs remains highly controversial, given its high cost and mixed data from underpowered and poorly designed RCTs. Although the 2019 IWGDF guidelines recommend systemic hyperbaric oxygen therapy for patients with nonhealing ischemic ulcers, multiple studies (including 2 RCTs) suggest that this practice offers no benefit [65–67]. Topical oxygen therapy seems more promising, with a meta-analysis of RCTs published since 2010 including 4 studies (total n = 494) and finding topical oxygen associated with increased 12-week healing rates (relative risk, 1.59; 95% confidence interval, 1.07–2.37) [68]. Of note, these RCTs included the optimal standard of care (including debridement and offloading) across arms. Consequently, topical oxygen or other advanced modalities may not be value-added unless basic interventions are also performed.
Offloading (ie, reducing pressure at the ulcer site) is a cornerstone of neuropathic DFU treatment and can be accomplished using devices or surgical intervention [69]. Nonremovable knee-high offloading devices (fiberglass total contact cast or nonremovable knee-high walkers) increase healing rates for forefoot and midfoot plantar ulcers and appear superior to removable devices in a recent meta-analysis [70, 71]. However, nonremovable devices are generally avoided in cases of significant infection or ischemia and may be unsuitable for patients who have difficulty adhering to frequent clinic follow-ups due to transportation or other barriers [72]. Removable offloading devices can also improve healing if worn regularly, but nonadherence is common. Addressing concerns about balance and falling may increase adherence [73, 74]. There is growing interest in monitoring offloading adherence and providing real-time reinforcement via wearable devices [75–77]. Finally, several surgical offloading techniques (eg, Achilles tendon lengthening) exist, with variable levels of evidence supporting their use; these procedures are reserved for patients who do not heal with mechanical offloading [69, 70]. Whatever offloading modality is chosen, patients with DFIs generally should not leave the office/hospital with the same footwear they walked in with.
Tighter glycemic control has been associated with decreased DFU and amputation risk in clinical trials [78, 79]. However, there are surprisingly little data from clinical trials regarding the impact of glycemic control on DFU or DFI outcomes, and while tight control might improve wound healing, data are insufficient to recommend specific glycemic targets in this population [80–82]. HbA1c values ≥8.0% and correlating fasting blood glucose of ≥126 mg/dL are associated with increased lower extremity amputation risk among patients with DFUs, and HbA1c control between 7% and 8% was associated with optimal DFU healing at 1 year in observational studies [83]. Continuous glucose monitors (CGMs) offer an opportunity to assess more nuanced glycemic control metrics compared with monitoring with traditional glucometers and HbA1c measurements. One observational study that used CGMs after surgical debridement of DFO found that more time spent in the blood glucose range of 70–180 mg/dL and less time spent below that range were associated with lower major amputation rates [83]. More research is needed to determine if CGMs can improve DFI outcomes.
Care fragmentation and loss to follow-up after discharge are common for patients with DFIs, particularly in health systems with no “one-stop shop” clinic with all required providers. Only 53.8% of patients hospitalized with a DFU attended a DFU-related outpatient appointment within 30 days of hospital discharge in an Atlanta, Georgia–based urban hospital with no single clinic incorporating all DFU providers [84]. Guideline-concordant DFU care may be even harder to achieve in rural settings, where referrals to urban providers are often challenging and delayed [85]. Although evidence-based interventions proven to mitigate the sources of DFI health disparities are scarce, we propose solutions based on our collective experience (Table 5). Last, we suggest that providers in health systems with a large DFU burden, particularly where health disparities are prevalent, establish limb salvage programs. Although daunting, there is guidance on how to build a scalable program by starting with a “hot foot line.” This is a single point of contact to whom acute diabetes-related foot complications discovered in the emergency department or inpatient wards can be reported, leading to a rapid assessment by a member of the limb salvage team who then consults the most appropriate surgical specialist). These teams can make patient care less burdensome and more effective [87].
Table 5.
Common Barriers to Care and Potential Solutions
| Barrier to Care | Potential Solution |
|---|---|
| Inadequate access to transportation/limited time off work | • Verify whether insurance includes nonemergency medical transportation benefits • Refer for local taxi/bus voucher programs • Offer telehealth appointments • Consolidate clinic appointments with other specialists on campus (ie, arrange multiple same-day visits) to limit travel needs • Organize a multidisciplinary DFU/DFI clinic • Organize a mobile DFU clinic to perform home calls or rotate between high-burden communities [86] |
| Inadequate access to healthcare providers | • Ensure patient has registered for the care system's electronic patient portal (eg, to review laboratory results, track appointments, and exchange secure messages with the treatment team; ideally set up via mobile phone), if available • Define a single DFI/DFO point of contact (eg, ID nurse clinic coordinator) who can triage patient concerns and connect them to other members of the treatment team; provide their contact information at first encounter • Use nursing visits and other healthcare professionals (eg, pedorthists) to the capacity of their license • Reserve appointment slots in the ID clinic for early post-discharge follow-up of DFI/DFO patients or create a weekly nurse- or physician-led telehealth clinic for timely post-discharge evaluation |
| No stable housing | • Consult with social worker for patient's access to local publicly funded housing, respite care services, shelters, and similar services • Employ outpatient intravenous antimicrobial therapy to facilitate temporary housing at a nursing facility |
| Poverty | • Ask patients if they have adequate wound care supplies and dispense at the visit if not • Ask patients if they have offloading footwear; keep a supply of donated footwear in clinic to distribute if not • Screen for food insecurity and refer to food banks • Identify lower-cost therapeutic alternatives for DFI medications in your area (eg, GoodRx coupons for local pharmacies or mail-order options via CostPlusDrugs.com) • Preferentially use low-cost oral antibiotics if available to adequately cover the isolated pathogens |
| Insecure employment | • Offer doctor's notes for each visit • Inquire about nature of patient's employment; offer letters of support for duty modifications (eg, reduced walking/standing) to promote foot healing |
| Not fluent in English | • Ensure clinic has on-demand access to telephonic medical translation services with a loud, good audio quality speakerphone • Provide written patient instructions in the patient's preferred language at the point of care using medical translation services • Allow patients to record all or a portion of the visit (eg, the summary guidance and Q&A conversation at the end) for later reference |
Abbreviations: DFI, diabetes-related foot infection; DFO, diabetes-related foot osteomyelitis; DFU, diabetes-related foot ulcer; ID, infectious diseases.
Assessing Clinical Response
Along with resolution of the cardinal sign of inflammation, reduction in wound size is the key marker of response in DFIs; only 45% of DFIs heal within 12 months [88]. Since antibiotic therapy for DFIs, even with osteomyelitis, should seldom extend beyond 6 weeks, most patients will have unhealed ulcers at antibiotic cessation. All ulcers are colonized by bacteria, and antibiotic therapy for uninfected ulcers does not improve healing. In short, antibiotics are meant to treat the infection, not to heal the ulcer, and a persistent wound alone does not indicate further benefit from continued antibiotics [89].
Inflammatory marker trends (eg, C-reactive protein and sedimentation rate) do not correlate with DFI or DFO outcomes, and we do not routinely use them to inform treatment decisions [90, 91]. Similarly, there are no studies that demonstrate a predictive value of serial imaging, and data from other forms of osteomyelitis suggest routine follow-up imaging may not add value beyond clinical judgment alone [31]. We reserve serial imaging for patients with concerns about progressing infection at follow-up rather than reimaging routinely. When patients with known or suspected DFIs do not respond to therapy, clinicians should consider alternative diagnoses (eg, Charcot), review indications for surgery (eg, undrained abscess), consider repeating cultures with optimal specimens (eg, bone biopsy off antibiotics), and, most importantly, ensure that the cornerstones of DFU therapy, that is, wound offloading and care, PAD assessment, and treatment, are being implemented. The success of further management will hinge on identifying and addressing the reason for an initial, suboptimal response.
Finally, there is little evidence to inform the optimal follow-up interval, modality, or duration for DFIs. However, because frequent debridement (usually defined as at least weekly) is associated with increased DFU healing, weekly follow-up for outpatients with DFIs until at least some improvement occurs seems ideal [58–60]. Early and more frequent ID follow-up (in-person or remotely) is indicated for patients on antibiotics who require closer monitoring (eg, linezolid and outpatient parenteral antibiotic therapy) [92]. Ulcer recurrence is common: approximately 40% of healed DFUs recur within 12 months, and osteomyelitis carries a 5-fold increase in the odds of ulcer recurrence [2]. Thus, patients with DFIs need life-long foot care, with the 2019 IWGDF guidelines recommending foot evaluations at least every 3 months after treatment of acute infection [12].
HOW DO WE ENGAGE IN SHARED DECISION-MAKING CONVERSATIONS ABOUT DFI MANAGEMENT
With Surgeons
Most nonproductive conversations about DFI management between ID and surgical colleagues fall into 1 of 3 categories. The first is inadequate or absent communication between specialists (eg, consults placed and received electronically or via ancillary staff, with no direct conversation between specialists, or conversations that occur solely between house staff who lack the necessary experience to communicate concerns accurately across teams and who, in any case, lack the authority to negotiate on their team's behalf). The second is conflicting appraisals of the medical and/or surgical prognoses (eg, the likelihood of cure with vs without immediate surgical intervention or the likelihood of response with conservative debridement vs a minor or major amputation). The third is conflicting valuation of antibiotic vs surgical stewardship principles.
We frequently encounter scenarios where house staff or patients attempt to relay information about another team's care plan but misconstrue or omit key details that render our colleagues’ assessment puzzling or scenarios in which medical team hierarchy becomes a roadblock to compromise. A phone call between senior team members (ideally, attending to attending) in which the specialist shares their own assessment and asks open-ended questions is the best first step to address perceived conflicts and is often sufficient.
When ID and surgical specialists genuinely do have conflicting care plans for patients with DFIs, we find these most often derive from different assessments of the patient's prognosis and specifically the perceived need for surgery and/or the degree of intervention needed. Framing such conversations around objective data and validated measures of prognosis (eg, the presence of gangrene or undrained abscess; WIfI classification), as well as the degree to which the proposed surgery would impact the patient's current mobility and potential for wound healing, can help the team reach consensus. Often, the surgical specialist can offer additional insight into the patient's revascularization potential, biomechanics, and other factors that inform their judgment.
Occasionally, patients have a marginal likelihood of cure with nonoperative or conservative surgical management, and the ID and surgical specialists may have disagreements based on genuine conflict over the relative importance of antibiotic stewardship vs the potential to avoid surgery (and often, preserve the patient's functional status). The presence of extensive wet gangrene/necrosis, undrained abscess, or progressive infection during antimicrobial therapy is a relatively strict indication for surgical debridement. In the case of wet gangrene, which describes infection with a component of progressive necrotizing soft tissue infection, surgery should be performed urgently, whereas dry gangrene, in which necrosis is driven primarily by ischemia, is usually not a surgical emergency. However, neither form of gangrene is likely to resolve with antimicrobial therapy alone. Indeed, delays in source control have been associated with more proximal amputation and prolonged hospitalization [93, 94], and deferring indicated major amputation has been associated with substantially increased risks of mortality and poor wound healing [95]. Beyond such cases, we encourage humility among our ID colleagues. The literature demonstrates that many patients with DFIs, even when complicated by osteomyelitis, can achieve clinical cure with antibiotic therapy alone. It may be helpful to frame antibiotic therapy in such cases as a “therapeutic trial,” with early outpatient follow-up and rediscussion among the care team if no clinical improvement has occurred.
Alternatively, in patients with DFIs who have marginal prospects for limb salvage, the most fruitful approach is often not to attempt to withhold antibiotics but rather to ensure they are being given in the context of a comprehensive strategy for DFI care, optimizing the likelihood of clinical success, and ensuring that medical management is exhausted before functionally limiting amputations are pursued. For example, revascularization can play a critical role in limiting the extent of amputation. Patients who undergo revascularization before, compared with after, a second minor amputation have a markedly lower risk of major amputation [96]. Despite this, less than 70% of patients undergo vascular studies prior to amputation, and only approximately 60% undergo attempted revascularization prior to amputation, with marked racial and rural disparities in this metric [97].
Finally, multidisciplinary teams that facilitate provider–provider shared decision-making (and regular multidisciplinary meetings to discuss complex cases) improve care and have been associated with reduction in major amputation [96]. These teams seldom rely on, or attribute their results to, cutting-edge technology. Instead, they share a strong common goal (limb salvage), have a clear understanding of the roles and responsibilities of each member (often via a care algorithm), and communicate effectively, generating mutual trust [98]. These relationships are built starting with face-to-face introductions, cultivated with frequent communication outside of the medical record and documentation, and develop best with stable team membership over time [96].
With Patients
Effective, shared decision-making with patients starts with a clear understanding of their individual values and priorities. Along with infection, amputation is commonly the most feared complication of diabetes-related foot disease [99]. Therefore, just as ID clinicians may view relapsed infection after attempted medical management as a failure state, patients may view amputation as the primary failure state to be avoided. Unsurprisingly, it is often difficult to convince patients that early aggressive surgical management for DFI is indicated, particularly when patients have not been adequately counseled about the reasoning behind that recommendation, have low trust in the healthcare system, or have clinicians who have not adequately built therapeutic rapport. Some may refuse surgery until they observe conservative therapy fail firsthand.
We recommend engaging in shared decision-making with patients using the same clear, systematic approach to the risks and benefits that we use with colleagues. When considering prolonged antibiotic therapy for patients with DFI, we suggest explicitly describing the balance of limb and function at risk with the likelihood and potential consequences of antibiotic-related adverse events. For example, a nonambulatory patient with heel DFO that does not threaten their current functional status may not benefit from antibiotic therapy, as untreated osteomyelitis seldom leads to sepsis, whereas an ambulatory patient with heel DFO may warrant aggressive antibiotic therapy (in addition to all other elements of foot care) because heel DFO presents high risk for a major amputation. Similarly, a patient who has no suitable oral antimicrobial options and advanced diabetes-related nephropathy (ie, someone for whom preservation of long-term vascular access is paramount) would be hard-pressed to opt for prolonged antimicrobials than a patient with a safe, low-risk oral option. Clinicians should also consider the foot territory at risk when discussing further antimicrobial therapy. For example, a patient with possible residual DFO after a TMA may warrant more aggressive antibiotic therapy compared with a patient with possible residual DFO after a partial toe amputation because TMA failure is likely to lead to a major amputation, whereas a post-partial toe amputation can likely be addressed with another minor amputation. Patients require counseling that delayed surgical management is either clearly futile and/or likely harmful in 3 situations: acute life-threatening infection (eg, necrotizing fasciitis), clearly failing antimicrobial therapy (eg, worsening soft tissue and/or systemic infection after several days of antimicrobials) and when extensive gangrene, tissue necrosis, or undrained purulence are present.
Finally, as in discussions with surgical colleagues, often the ID clinician's most useful input is not to gatekeep reasonably indicated antimicrobials but to ensure they are being given as part of a comprehensive approach to DFI so that the infection resolves and retreatment can be avoided. To that end, general exhortations about the importance of adhering to therapy are mostly useless. Nonadherence more often stems from material conditions such as poor insurance coverage, inability to stop working or caregiving to adhere to offloading, and inability to obtain timely post-discharge appointments or communicate with clinicians as issues arise [100]. Specifically elucidating and addressing these barriers will not only optimize the odds of cure but can help build rapport and set up the clinician and patient for a productive future conversation about amputation should conservative management fail.
CONCLUSIONS
DFI is common and can devastate patients’ mobility, independence, and quality of life. This disease disproportionately affects marginalized communities, with social determinants of health frequently complicating treatment. Antimicrobial therapy is just one component of comprehensive DFI care, which also involves assessing and treating peripheral vascular disease, caring for wounds, offloading, addressing biomechanics, controlling diabetes, treating contributing comorbidities, and exploring and mitigating the patient's barriers to care.
Of note, we have primarily addressed barriers to care in DFI from the perspective of individual patients and clinicians rather than discussing social determinants of health at the community or national level. This approach was taken to emphasize opportunities for ID clinicians to take direct action to address social factors for their individual patients. However, the sources of health inequity in DFI, differential access to medical care mediated by insurance status, English fluency, transportation, and insecurity of employment and housing, ultimately require effective interventions at the level of healthcare policy, systems, and communities [101]. Expanded paid medical leave, subsidized or free access to medical transportation and care, subsidized or free housing, and other policies successfully implemented in other nations would undoubtedly improve DFI outcomes in the United States.
Indeed, German microbiologist and epidemiologist Rudolf Virchow recognized the limits of individual practitioners in addressing health disparities with socioeconomic origins nearly 200 years ago. He called for “social medicine, that is, political prioritization of the health of the community, particularly the poor, and for physicians to be its advocates [102]. Modern data support Virchow's contention: the removal of access to podiatric care for Medicaid patients led to an astonishing 37% increase in hospitalization for DFIs in 1 state, while a decade later, the expansion of access to foot care through the Affordable Care Act resulted in a 33% reduction in major amputations among minoritized patients in early adopting states [103, 104]. Today, we can rise to this challenge individually by advocating for policies such as Medicaid expansion, increasing the effectiveness and capacity of existing resources by forming multidisciplinary teams, ensuring that triage pathways promote equitable access to our expertise, and helping individual patients tap into resources beyond our individual discipline.
Patients with DFIs that involve substantial undrained purulence, gangrene, necrotizing infection, or infection clearly worsening on antimicrobial therapy have relatively strict indications for surgery. Beyond these, many patients can achieve durable cure nonoperatively. Whether to pursue surgical debridement in such cases depends on the territory of the foot under threat and the accompanying threat to mobility surgery entails, whether vascularization of the foot is or can be made adequate for wound healing and the patient's values and preferences (ie, regarding avoiding amputation vs avoiding rehospitalization and prolonged care for infection). These decisions should be made in concert with both the patient and surgeon. We find that the most effective framing for these conversations is a systematic consideration of the patient's modifiable and nonmodifiable prognostic factors (informed by validated tools such as the WIfI classification) and the relative risks and benefits of antimicrobial and surgical options, as detailed above.
When conflict arises out of shared decision-making (either between colleagues or with patients), simple miscommunication, conflicting prognostications, and genuine conflicts in values and preferences (eg, a surgical colleague’s emphasis on preservation of mobility or a patient's emphasis on avoidance of amputation vs an ID clinician's emphasis on eradication of the infection with minimal necessary antimicrobial exposure) are common sources. Specifically identifying and addressing these sources often aids the group in reaching consensus. We emphasize that in some cases of conflicting values where surgery is not strictly indicated, the ID clinician may bring the most value to the patient's care by framing antimicrobials as a “therapeutic trial” to be reevaluated at early clinic follow-up and by ensuring that antimicrobials are being given as part of a comprehensive treatment strategy to preserve the patient's functional status via limb salvage. Finally, we recommend developing multidisciplinary DFI teams that can centralize patient care, foster longitudinal relationships and trust between clinicians, and optimize outcomes.
Contributor Information
Nicolas W Cortes-Penfield, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
David G Armstrong, Department of Surgery, University of Southern California, Los Angeles, California, USA.
Meghan B Brennan, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
Maya Fayfman, Division of Endocrinology and Metabolism, Emory University, Atlanta, Georgia, USA; Department of Medicine, Grady Memorial Hospital, Atlanta, Georgia, USA.
Jonathan H Ryder, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Tze-Woei Tan, Department of Surgery, University of Southern California, Los Angeles, California, USA.
Marcos C Schechter, Department of Medicine, Grady Memorial Hospital, Atlanta, Georgia, USA; Division of Infectious Diseases, Emory University, Atlanta, Georgia, USA.
Notes
Financial support. D. G. A. is supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and reports that this work is partially supported by the National Science Foundation’s Center to Stream Healthcare in Place. T. W. T. reports the following support for this work: 1K23DK122126 from National Institute of Diabetes and Digestive and Kidney Disease, Vascular Cures Collaborative Patient-Centered Research grant, and a Society of Vascular Surgery Foundation award (supplement to 1K23DK122126).
References
- 1. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017; 49:106–16. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017; 376:2367–75. [DOI] [PubMed] [Google Scholar]
- 3. Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes Obes Metab 2023; 25:36–45. [DOI] [PubMed] [Google Scholar]
- 4. Jia L, Parker CN, Parker TJ, et al. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PLoS One 2017; 12:e0177916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richard JL, Lavigne JP, Got I, et al. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab 2011; 37:208–15. [DOI] [PubMed] [Google Scholar]
- 6. Brennan MB, Powell WR, Kaiksow F, et al. Association of race, ethnicity, and rurality with major leg amputation or death among Medicare beneficiaries hospitalized with diabetic foot ulcers. JAMA Netw Open 2022; 5:e228399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan TW, Shih CD, Concha-Moore KC, et al. Disparities in outcomes of patients admitted with diabetic foot infections. PLoS One 2019; 14:e0211481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hicks CW, Canner JK, Karagozlu H, et al. Quantifying the costs and profitability of care for diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2019; 70:233–40. [DOI] [PubMed] [Google Scholar]
- 9. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54:e132–73. [DOI] [PubMed] [Google Scholar]
- 10. Musuuza J, Sutherland BL, Kurter S, Balasubramanian P, Bartels CM, Brennan MB. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J Vasc Surg 2020; 71:1433–46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009; 169:1290–8. [DOI] [PubMed] [Google Scholar]
- 12. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020; 36(Suppl 1):e3266. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhary N, Huda F, Roshan R, Basu S, Rajput D, Singh SK. Lower limb amputation rates in patients with diabetes and an infected foot ulcer: a prospective observational study. Wound Manag Prev 2021; 67:22–30. [PubMed] [Google Scholar]
- 14. Pena G, Kuang B, Edwards S, Cowled P, Dawson J, Fitridge R. Factors associated with key outcomes in diabetes related foot disease: a prospective observational study. Eur J Vasc Endovasc Surg 2021; 62:233–40. [DOI] [PubMed] [Google Scholar]
- 15. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia 2008; 51:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004; 20 (Suppl 1):S90–5. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998; 21:855–9. [DOI] [PubMed] [Google Scholar]
- 18. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981; 2:64–122. [DOI] [PubMed] [Google Scholar]
- 19. Macfarlane RMJ, William J. Classification of diabetic foot ulcers: the S(AD) SAD system. The Diabetic Foot 1999; 2:123–31. [Google Scholar]
- 20. Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020; 36 (Suppl 1):e3280. [DOI] [PubMed] [Google Scholar]
- 21. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014; 59:220–34.e2. [DOI] [PubMed] [Google Scholar]
- 22. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019; 58:S1–S109.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hicks CW, Canner JK, Mathioudakis N, et al. The Society for Vascular Surgery wound, ischemia, and foot infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg 2018; 68:1096–103. [DOI] [PubMed] [Google Scholar]
- 24. van Reijen NS, Ponchant K, Ubbink DT, Koelemay MJW. Editor's choice—the prognostic value of the WIfI classification in patients with chronic limb threatening ischaemia: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2019; 58:362–71. [DOI] [PubMed] [Google Scholar]
- 25. Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Diseases Society of America's diabetic foot infection classification system. Clin Infect Dis 2007; 44:562–5. [DOI] [PubMed] [Google Scholar]
- 26. Schechter MC, Ali MK, Risk BB, et al. Percutaneous bone biopsy for diabetic foot osteomyelitis: a systematic review and meta-analysis. Open Forum Infect Dis 2020; 7:ofaa393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis 2021; 21:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004; 39:885–910. [DOI] [PubMed] [Google Scholar]
- 29. Truong DH, Bedimo R, Malone M, et al. Meta-analysis: outcomes of surgical and medical management of diabetic foot osteomyelitis. Open Forum Infect Dis 2022; 9:ofac407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds 2014; 13:211–9. [DOI] [PubMed] [Google Scholar]
- 31. Spellberg B, Aggrey G, Brennan MB, et al. Use of novel strategies to develop guidelines for management of pyogenic osteomyelitis in adults: a WikiGuidelines group consensus statement. JAMA Netw Open 2022; 5:e2211321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipsky BA, Baker PD, Landon GC, Fernau R. Antibiotic therapy for diabetic foot infections: comparison of two parenteral-to-oral regimens. Clin Infect Dis 1997; 24:643–8. [DOI] [PubMed] [Google Scholar]
- 34. Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis 2004; 38:17–24. [DOI] [PubMed] [Google Scholar]
- 35. Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care 2014; 37:789–95. [DOI] [PubMed] [Google Scholar]
- 36. Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open-label controlled randomized study. Diabetes Care 2015; 38:302–7. [DOI] [PubMed] [Google Scholar]
- 37. Gariani K, Pham TT, Kressmann B, et al. Three weeks versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: a prospective, randomized, noninferiority pilot trial. Clin Infect Dis 2021; 73:e1539–e45. [DOI] [PubMed] [Google Scholar]
- 38. Mponponsuo K, Brown KA, Fridman DJ, et al. Highly versus less bioavailable oral antibiotics in the treatment of gram-negative bloodstream infections: a propensity-matched cohort analysis. Clin Microbiol Infect 2022;29:490–7. [DOI] [PubMed] [Google Scholar]
- 39. Schechter MC, Sax PE, Cortés-Penfield N. What is the best oral therapy for Staph aureus osteomyelitis? NEJM Evidence 2022; 1:EVIDtt2200119. [DOI] [PubMed] [Google Scholar]
- 40. Wilson BM, Bessesen MT, Doros G, et al. Adjunctive rifampin therapy for diabetic foot osteomyelitis in the Veterans Health Administration. JAMA Netw Open 2019; 2:e1916003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bessesen MT, Doros G, Henrie AM, et al. A multicenter randomized placebo controlled trial of rifampin to reduce pedal amputations for osteomyelitis in veterans with diabetes (VA INTREPID). BMC Infect Dis 2020; 20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossel A, Lebowitz D, Gariani K, et al. Stopping antibiotics after surgical amputation in diabetic foot and ankle infections—a daily practice cohort. Endocrinol Diabetes Metab 2019; 2:e00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Truong T, Gariani K, Richard JC, et al. Moderate to severe soft tissue diabetic foot infections: a randomized, controlled, pilot trial of post-debridement antibiotic treatment for 10 versus 20 days. Ann Surg 2022; 276:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waibel F, Berli M, Catanzaro S, et al. Optimization of the antibiotic management of diabetic foot infections: protocol for two randomized controlled trials. Trials 2020; 21:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armstrong DG, Lipsky BA. Diabetic foot infections: stepwise medical and surgical management. Int Wound J 2004; 1:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fisher TK, Scimeca CL, Bharara M, Mills JL, Sr., Armstrong DG. A step-wise approach for surgical management of diabetic foot infections. J Vasc Surg 2010; 52(3 Suppl):72s–5s. [DOI] [PubMed] [Google Scholar]
- 47. Ammendola M, Sacco R, Butrico L, Sammarco G, de Franciscis S, Serra R. The care of transmetatarsal amputation in diabetic foot gangrene. Int Wound J 2017; 14:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suckow BD, Goodney PP, Cambria RA, et al. Predicting functional status following amputation after lower extremity bypass. Ann Vasc Surg 2012; 26:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ordaz A, Trimm C, Pedowitz J, Foran IM. Transmetatarsal amputation results in higher frequency of revision surgery and higher ambulation rates than below-knee amputation. Foot Ankle Orthop 2022; 7:24730114221112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holloway JJ, Lauer K, Kansal N, Bongard F, Miller A. A novel approach to limb salvage: healing transmetatarsal amputations without a viable plantar flap. Ann Vasc Surg 2021; 70:51–5. [DOI] [PubMed] [Google Scholar]
- 51. Hinchliffe RJ, Brownrigg JRW, Andros G, et al. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev 2016; 32 (Suppl 1):136–44. [DOI] [PubMed] [Google Scholar]
- 52. Li Q, Birmpili P, Johal AS, et al. Delays to revascularization for patients with chronic limb-threatening ischaemia. Br J Surg 2022; 109:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brownrigg JR, Hinchliffe RJ, Apelqvist J, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev 2016; 32 (Suppl 1):128–35. [DOI] [PubMed] [Google Scholar]
- 54. Mayor J, Chung J, Zhang Q, et al. Using the society for vascular surgery wound, ischemia, and foot infection classification to identify patients most likely to benefit from revascularization. J Vasc Surg 2019; 70:776–85.e1. [DOI] [PubMed] [Google Scholar]
- 55. Hicks CW, Canner JK, Sherman RL, Black JH III, Lum YW, Abularrage CJ. Evaluation of revascularization benefit quartiles using the wound, ischemia, and foot infection classification system for diabetic patients with chronic limb-threatening ischemia. J Vasc Surg 2021; 74:1232–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020; 36 (Suppl 1):e3283. [DOI] [PubMed] [Google Scholar]
- 57. Elraiyah T, Domecq JP, Prutsky G, et al. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J Vasc Surg 2016; 63(2 Suppl):37S–45S.e2. [DOI] [PubMed] [Google Scholar]
- 58. Tettelbach WH, Cazzell SM, Hubbs B, Jong JL, Forsyth RA, Reyzelman AM. The influence of adequate debridement and placental-derived allografts on diabetic foot ulcers. J Wound Care 2022; 31(Sup9):S16–s26. [DOI] [PubMed] [Google Scholar]
- 59. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol 2013; 149:1050–8. [DOI] [PubMed] [Google Scholar]
- 60. Karavan M, Olerud J, Bouldin E, Taylor L, Reiber GE. Evidence-based chronic ulcer care and lower limb outcomes among Pacific Northwest veterans. Wound Repair Regen 2015; 23:745–52. [DOI] [PubMed] [Google Scholar]
- 61. Vas P, Rayman G, Dhatariya K, et al. Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev 2020; 36 (Suppl 1):e3284. [DOI] [PubMed] [Google Scholar]
- 62. Boulton AJM, Armstrong DG, Löndahl M, et al. New evidence-based therapies for complex diabetic foot wounds. Arlington, VA: American Diabetes Association, 2022. [PubMed] [Google Scholar]
- 63. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005; 366:1704–10. [DOI] [PubMed] [Google Scholar]
- 64. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008; 31:631–6. [DOI] [PubMed] [Google Scholar]
- 65. Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care 2013; 36:1961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fedorko L, Bowen JM, Jones W, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes Care 2016; 39:392–9. [DOI] [PubMed] [Google Scholar]
- 67. Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower-extremity ulcers in patients with diabetes: results of the DAMO(2)CLES multicenter randomized clinical trial. Diabetes Care 2018; 41:112–9. [DOI] [PubMed] [Google Scholar]
- 68. Carter MJ, Frykberg RG, Oropallo A, et al. Efficacy of topical wound oxygen therapy in healing chronic diabetic foot ulcers: systematic review and meta-analysis. Adv Wound Care (New Rochelle) 2022; 12:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bus SA, Armstrong DG, Gooday C, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020; 36 (Suppl 1):e3274. [DOI] [PubMed] [Google Scholar]
- 70. Lazzarini PA, Jarl G, Gooday C, et al. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diabetes Metab Res Rev 2020; 36 (Suppl 1): e3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Health Quality Ontario . Fibreglass total contact casting, removable cast walkers, and irremovable cast walkers to treat diabetic neuropathic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser 2017; 17: 1–124. [PMC free article] [PubMed] [Google Scholar]
- 72. Raspovic A, Landorf KB. A survey of offloading practices for diabetes-related plantar neuropathic foot ulcers. J Foot Ankle Res 2014; 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ababneh A, Finlayson K, Edwards H, Lazzarini PA. Factors associated with adherence to using removable cast walker treatment among patients with diabetes-related foot ulcers. BMJ Open Diabetes Res Care 2022; 10:e002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Crews RT, Shen BJ, Campbell L, et al. Role and determinants of adherence to off-loading in diabetic foot ulcer healing: a prospective investigation. Diabetes Care 2016; 39:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Souza J, Escadas S, Baxevani I, Rodrigues D, Freitas A. Smart wearable systems for the remote monitoring of selected vascular disorders of the lower extremity: a systematic review. Int J Environ Res Public Health 2022; 19:15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Park C, Mishra R, Vigano D, et al. Smart offloading boot system for remote patient monitoring: toward adherence reinforcement and proper physical activity prescription for diabetic foot ulcer patients. J Diabetes Sci Technol 2023; 17:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Armstrong D. Smart boot use to measure offloading adherence. Available at: https://clinicaltrials.gov/ct2/show/NCT04460573?term=david+armstrong&cond=diabetic+foot+ulcer&draw=2&rank=2. Accessed 23 December 2022.
- 78. Boyko EJ, Zelnick LR, Braffett BH, et al. Risk of foot ulcer and lower-extremity amputation among participants in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2022; 45:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg 2016; 63(2 Suppl):22S–8S.e2. [DOI] [PubMed] [Google Scholar]
- 80. Fernando ME, Seneviratne RM, Tan YM, et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev 2016; 2016: Cd010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol 2011; 131: 2121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang ZX, Zhang HH, Huang Y, et al. Association of time in range with postoperative wound healing in patients with diabetic foot ulcers. Int Wound J 2022; 19:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yin X, Zhu W, Liu C, et al. Association of continuous glucose monitoring-derived time in range with major amputation risk in diabetic foot osteomyelitis patients undergoing amputation. Ther Adv Endocrinol Metab 2022; 13:20420188221099337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mahgoub U, Magee MJ, Heydari M, et al. Outpatient clinic attendance and outcomes among patients hospitalized with diabetic foot ulcers. J Diabetes Complications 2022; 36:108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sutherland BL, Pecanac K, Bartels CM, Brennan MB. Expect delays: poor connections between rural and urban health systems challenge multidisciplinary care for rural Americans with diabetic foot ulcers. J Foot Ankle Res 2020; 13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. San Antonio Vascular and Endovascular Clinic . SAVE: the San Antonio Vascular and Endovascular Clinic. Available at: https://thesaveclinic.com/. Accessed 8 November 2022.
- 87. Khan T, Shin L, Woelfel S, Rowe V, Wilson BL, Armstrong DG. Building a scalable diabetic limb preservation program: four steps to success. Diabet Foot Ankle 2018; 9: 1452513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ndosi M, Wright-Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med 2018; 35:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother 2015; 16:821–32. [DOI] [PubMed] [Google Scholar]
- 90. van Asten SA, Jupiter DC, Mithani M, La Fontaine J, Davis KE, Lavery LA. Erythrocyte sedimentation rate and C-reactive protein to monitor treatment outcomes in diabetic foot osteomyelitis. Int Wound J 2017; 14:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pham TT, Wetzel O, Gariani K, et al. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes Obes Metab 2021; 23:637–41. [DOI] [PubMed] [Google Scholar]
- 92. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68: e1–e35. [DOI] [PubMed] [Google Scholar]
- 93. Faglia E, Clerici G, Caminiti M, Quarantiello A, Gino M, Morabito A. The role of early surgical debridement and revascularization in patients with diabetes and deep foot space abscess: retrospective review of 106 patients with diabetes. J Foot Ankle Surg 2006; 45:220–6. [DOI] [PubMed] [Google Scholar]
- 94. Tan JS, Friedman NM, Hazelton-Miller C, Flanagan JP, File TM Jr. Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation? Clin Infect Dis 1996; 23:286–91. [DOI] [PubMed] [Google Scholar]
- 95. Zhou S, Schmidt BM, Henig O, Kaye KS. Deferring amputation in diabetic foot osteomyelitis: doing more harm than good? Open Forum Infect Dis 2021; 8:ofab184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lin JH, Jeon SY, Romano PS, Humphries MD. Rates and timing of subsequent amputation after initial minor amputation. J Vasc Surg 2020; 72:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vemulapalli S, Greiner MA, Jones WS, Patel MR, Hernandez AF, Curtis LH. Peripheral arterial testing before lower extremity amputation among Medicare beneficiaries, 2000 to 2010. Circ Cardiovasc Qual Outcomes 2014; 7:142–50. [DOI] [PubMed] [Google Scholar]
- 98. Sutherland BL, Pecanac K, LaBorde TM, Bartels CM, Brennan MB. Good working relationships: how healthcare system proximity influences trust between healthcare workers. J Interprof Care 2022; 36:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wukich DK, Raspovic KM, Jupiter DC, et al. Amputation and infection are the greatest fears in patients with diabetes foot complications. J Diabetes Complications 2022; 36:108222. [DOI] [PubMed] [Google Scholar]
- 100. Fayfman M, Schechter MC, Amobi CN, et al. Barriers to diabetic foot care in a disadvantaged population: a qualitative assessment. J Diabetes Complications 2020; 34:107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 2023; 46:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brown TM, Fee E. Rudolf Carl Virchow: medical scientist, social reformer, role model. Am J Public Health 2006; 96:2104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Skrepnek GH, Mills JL, Armstrong DG. Foot-in-wallet disease: tripped up by “cost-saving” reductions? Diabetes Care 2014; 37:e196–7. [DOI] [PubMed] [Google Scholar]
- 104. Tan TW, Calhoun EA, Knapp SM, et al. Rates of diabetes-related major amputations among racial and ethnic minority adults following Medicaid expansion under the Patient Protection and Affordable Care Act. JAMA Netw Open 2022; 5:e223991. [DOI] [PMC free article] [PubMed] [Google Scholar]