Abstract
Elevated inflammation is a risk factor for many psychiatric (e.g., depression) and somatic conditions (e.g., rheumatoid arthritis). Inflammation is influenced by psychosocial processes such as emotion regulation. Characterization of which emotion regulation characteristics impact inflammation could help refine psychosocial interventions aimed at normalizing health-harming inflammatory activity for individuals with psychiatric and somatic illnesses. To investigate this issue, we systematically reviewed the literature on associations between a variety of emotion regulation traits and inflammation. Out of 2816 articles identified, 38 were included in the final review. 28 (74%) found that (a) poor emotion regulation is associated with higher inflammation and/or (b) strong emotion regulation skills are associated with lower inflammation. Consistency of results differed as a function of the emotion regulation construct investigated and methodological characteristics. Results were most consistent for studies testing positive coping/social support seeking or broadly defined emotion regulation/dysregulation. Methodologically, studies testing reactivity to a stressor, adopting a vulnerability-stress framework, or using longitudinal data were most consistent. Implications for integrated, transdiagnostic psychoimmunological theories are discussed, as well as recommendations for clinical research.
Keywords: Emotion regulation, Inflammation, Stress, Immunology, Health
1. Introduction
Inflammation is a transdiagnostic correlate of many medical and psychiatric conditions (Dantzer et al., 2008; Michopoulos et al., 2016; Pearson et al., 2003; Saccaro et al., 2021; Sattar et al., 2003). Further, evidence suggests that inflammation has a causal effect on some of these health outcomes including depression (Capuron and Miller, 2004; Knight et al., 2022; Kuhlman et al., 2018; Moriarity, Kautz et al., 2020), ulcerative colitis (Ek et al., 2021), and osteoarthritis (Ek et al., 2021), positioning it to be a potentially important treatment target for a variety of disorders. Although inflammation-modulating biological treatments such as non-steroidal anti-inflammatory drugs, interferon-α therapy, and minocycline generally are considered primary interventions for inflammation-mediated conditions, psychosocial interventions such as cognitive-behavior therapy (CBT) and mindfulness meditation also have been shown to influence inflammatory biology (Black and Slavich, 2016; Shields et al., 2020). Inflammatory malleability to these biological and psychological interventions affords patients who suffer from inflammation-mediated disorders flexibility in treatment options. For example, biological interventions might be a useful adjunctive when individuals with both depression and elevated inflammation are struggling with the cognitive demands required to engage in evidence-based psychotherapy. Conversely, individuals for whom anti-inflammatory medications are contraindicated—or who refuse medication for other reasons—may benefit from psychosocial interventions (Shields et al., 2020).
Yet, the mere understanding that psychosocial interventions (e.g., cognitive-behavioral therapies) influence inflammation is insufficient to maximize therapeutic impact. It is necessary to explore which treatment targets of extant psychosocial interventions actually affect inflammatory biology. A nuanced understanding of which specific characteristics of psychosocial treatments reduce inflammation would have direct implications for treating inflammation-mediated mental and physical health problems and could help advance precision medicine approaches aimed at reducing risk for these conditions. Further, this work would integrate inflammatory mechanisms into existing, psychosocially oriented theories of psychiatric risk and resilience, which would guide theory development (Moriarity, 2021) and advance understanding of many complex, multifactorial health conditions.
Increasing the quantity and quality of emotion regulation skills, and decreasing emotional reactivity, is a shared goal of many psychotherapies (e.g., CBT, dialectical behavioral therapy, acceptance and commitment therapy), as strengthening emotion regulation aptitude can reduce distress in various areas of psychosocial functioning (Beatty et al., 2016; Ma and Fang, 2019). Indeed, skillful emotion regulation is associated with improved communication and social relationship functioning overall (Vater and Schröder, Abé, 2015). Additionally, individuals with advanced emotion regulation also are better able to select strategies that best align with their goals within the situational context (English et al., 2017). Given that inflammatory biology is reactive to increases in negative affect such as anger or anxiety (Carroll et al., 2011), it is plausible that improved emotion regulation also could influence inflammatory biology.
In fact, there are several extant theories/models implicating emotion regulation as a modulator of inflammation. The perseverative cognitions hypothesis is not specific to inflammation, but describes how perseverative (e.g., rumination, worry) reactions to unpleasant situations or emotions can simultaneously amplify the magnitude and duration of the physiological stress response-exacerbating downstream consequences for basal stress biology (Brosschot et al., 2006). Our team has extended this work to include cognitive vulnerabilities more generally in an immunocognitive model of psychopathology (Moriarity et al., 2018)-attempting to clarify discrepant results in stress/arousal→inflammation research by including emotion-modulating cognitive vulnerabilities as a moderator of this association. Others have described emotion regulation traits/abilities as a mediator of the negative emotionality→inflammation pathway (Renna, 2021) and suggested the possibility of bidirectional feedback loops between negative emotions, inflammation, and health outcomes. Although comprehensive tests of bidirectional relationships are lacking in this area, there is evidence from studies involving experimentally-administered endotoxin that inflammatory activity might increase negative reactivity (Dooley et al., 2018) and that certain health sequelae of inflammation (e.g., depression) are also predictive of future increases in inflammation (Moriarity, Kautz et al., 2020).
A few reviews have explored the association between emotion regulation and inflammation for specific emotion regulation constructs (e.g., see Szabo et al., 2022 for a scoping review on rumination and inflammation), but there have been no attempts to systematically review how a wide variety of emotion regulation characteristics are related to inflammatory biology. Given the range of both emotion regulation characteristics and inflammatory proteins, a systematic review of the associations between these two constructs is an important contribution to the field insofar as it would point to the therapeutic processes that are most relevant for reducing inflammation, a key health-damaging process. This is especially important given that the identification of cognitive targets that impact inflammatory biology could lead to the development of more precise psychological interventions for a variety of inflammation-mediated health outcomes (Moriarity, 2021).
We addressed this need by systematically reviewing for the first time all of the available evidence for associations between various emotion regulation characteristics and circulating inflammatory proteins in clinical (i.e., medical and psychiatric) and nonclinical samples. In addition to identifying which emotion regulation characteristics are associated with inflammatory biology, we assessed contextual factors that might influence the presence or absence of theoretically consistent associations to the extent possible (e.g., emotion regulation tested as a moderator of arousal-related characteristics and inflammation, emotion regulation as a trait vs. in the context of acute stress). Then, we used the reviewed evidence to formulate recommendations for clinical practice, and the integration of inflammation into theories of emotion regulation and psychopathology.
2. Method
2.1. Transparency and openness
This study was pre-registered (PROSPERO study protocol: CRD42021253574; link: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=253574) and conducted in accordance with PRISMA 2020 guidelines for systematic reviews (Page et al., 2021).
2.2. Search strategy and selection criteria
Several steps were used to identify and assess relevant articles for inclusion in this systematic review. First, PubMed and PsycInfo were searched for articles written in English and published until June 16th, 2022. Specific search terms are reported in Supplemental Table 1 and were filtered for human samples and articles available in English. One notable term that we did not include was “mindfulness”, given that the goal of mindfulness is to notice emotional states (among other things) rather than directly regulate them. Relatedly, broad-based immune terminology (e.g., “immune”, “immune response”, “immune activation”) were not included to ensure a focused review on inflammatory biology–streamlining attempts to connect the results of this review to specific clinical processes. Duplicate articles were removed. Then, abstracts and titles were screened for exclusion criteria. Studies passing this step had their full texts reviewed for inclusion criteria. To be included, studies had to be (1) empirical (i.e., no reviews), (2) based on human samples, (3) specifically test the association between a facet of emotion regulation and levels of inflammatory proteins (e.g., no gene expression or LPS-stimulated proteins), (4) not retracted, (5) devoid of critical analytic flaws (in the case that only some tests in a study were critically flawed, the appropriately conducted analyses are reported), and (6) published in a peer-reviewed academic journal (e.g., no pre-prints or unpublished theses/dissertations). If additional relevant articles were identified during the full-text review process, these steps were repeated to determine if the article should be included in the review.
Given the small number of studies testing the associations between identical emotion regulation measures + biomarkers, as well as a variety of other methodological disparities between studies (e.g., duration of assessment lags in longitudinal or experimental research, sample health characteristics, sample developmental stage, whether inflammatory proteins were measured in blood or saliva), it was determined that a quantitative meta-analysis would be inappropriate and risk contributing to growing concerns about lack of meta-analytic replicability (Sotola, 2022). To illustrate, the most popular protein in this review is CRP assayed in blood (23 studies) and the most common temporal design with CRP was cross-sectional (13 studies). Of these 13, the most popular emotion regulation construct was cognitive reappraisal (4 studies). Of these 4 studies only 3 used the same measure and all three featured fundamentally different samples (community adult, community adolescent, trauma-exposed veterans). Meta-analysis is an important tool for scientific advancement and we would rather stick to a narrative review that provides space for a detailed look into potential drivers of differing results (e.g., emotion regulation construct, longitudinal vs. cross-sectional designs) instead of potentially collapsing studies where different effect sizes are plausible. Hopefully this systematic review inspires more research on this topic so that the literature grows to a point that a meta-analysis would be more methodologically sound.
2.3. Data extraction
Study characteristics were independently extracted from the reviewed articles by three authors, and discrepancies were resolved by consensus discussion including all authors. The information extracted was sample size, sample characteristics (e.g., community, clinical), study design (e.g., cross-sectional, longitudinal, acute laboratory stressor), emotion regulation constructs, inflammatory proteins, inflammatory protein measurement method (i.e., blood, saliva, or sweat), results, test statistics and standardized effect sizes (when available), and covariates.
3. Results
The literature search identified 2816 articles. After exclusion of duplicates and irrelevant articles based on screening titles and abstracts, 67 full-text articles were screened, resulting in 38 studies included in the systematic review (see Fig. 1 for PRISMA flowchart outlining the study selection process). All included studies are systematically summarized in Table 1. Results are organized in the following subsections: emotion regulation (broadly defined), negative affectivity/emotional reactivity, emotional expression/suppression, perseverative cognitions and distraction/disengagement, cognitive reappraisal, and positive coping and support-seeking. Studies that tested several relevant emotion regulation constructs are described in multiple sections. For ease-of-reading, only significant associations (other than studies that only had null results) are highlighted below, but all relevant significant and null results are reported (with standardized effect sizes and metrics of significance, to the extent available) in Table 1.
Fig. 1.
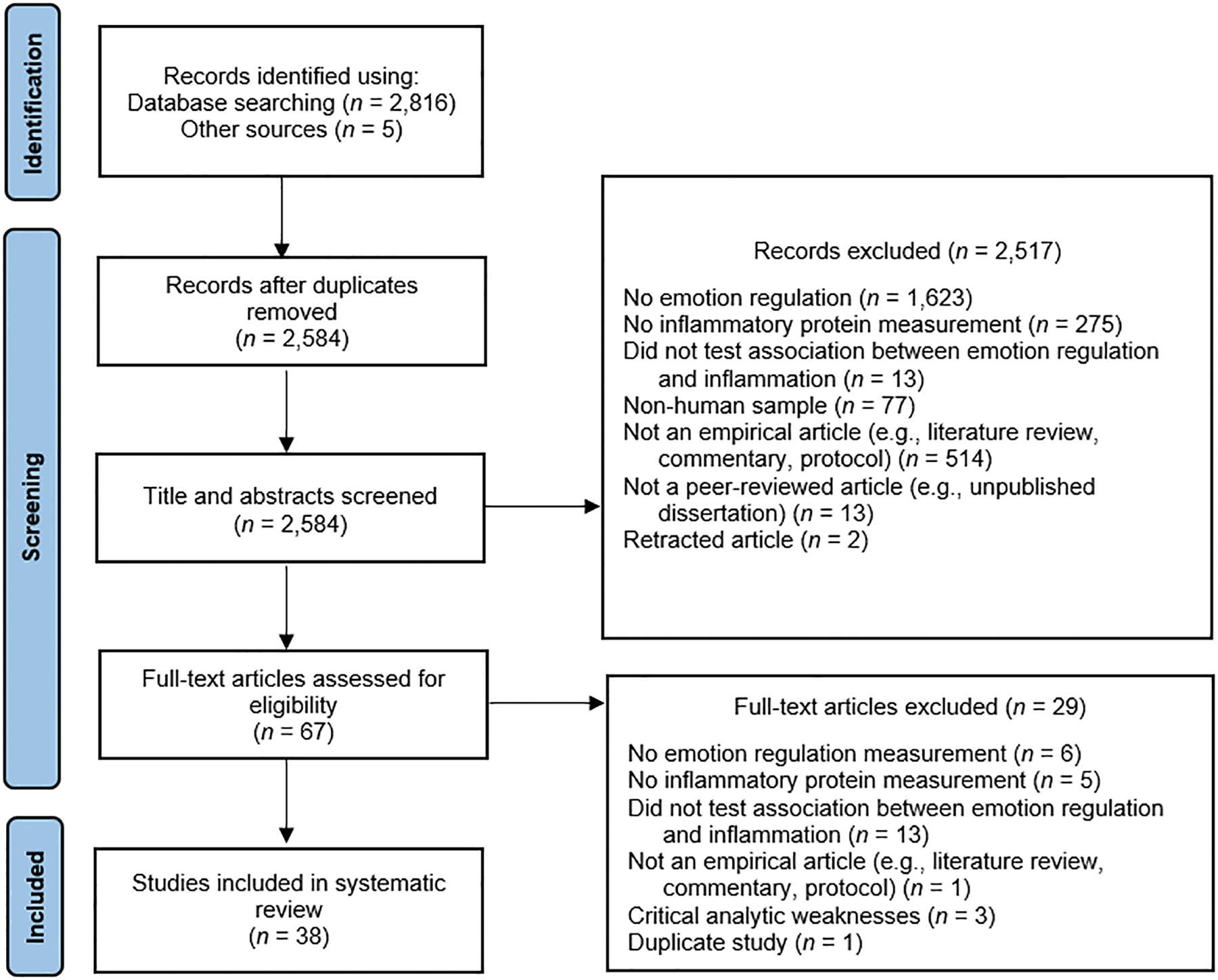
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram.
Table 1.
Summary of Studies Reviewed
| Authors (Year) | Sample Size | Sample Type & Age | Study Design | Inflammatory Proteins | Inflammatory Measurement Method | Emotion Regulation Variables | Emotion Regulation Category for Narrative Summary | Covariates | Results |
|---|---|---|---|---|---|---|---|---|---|
| Appleton et al. (2011) a | 379–400 (depending on model) | Community Mage 42.2 years, SD 1.8 years |
Longitudinal | CRP | Blood | Inappropriate self-regulation, distress proneness | Emotion Regulation/Dysregulation Negative Affectivity/Emotional Reactivity |
Age, sex, race, study site, smoking status, depression, education, BMI, gestational age, WISC score, childhood SES, childhood health. | ↑ Childhood inappropriate self-regulation → ↑ Adult CRP (p = .002) ↑ Childhood distress proneness → ↑ Adult CRP (p = .03) |
| Appleton et al. (2013) a | 379 | Community Mage 42.2 years, SD 1.7 years |
Cross-sectional | CRP | Blood | Emotional suppression, Cognitive reappraisal | Emotional Suppression Cognitive Reappraisal |
Age, race, gender, study site | ↑ Cognitive reappraisal →↓ CRP (p < .01) ↑ Suppression → ↑ CRP (p < .001). |
| Appleton et al. (2012) a | 388 | Community Mage 42.1 years, SD 1.7 years |
Longitudinal | CRP | Blood | Childhood distress proneness, inappropriate self-regulation | Emotion Regulation/Dysregulation Negative Affectivity/Emotional Reactivity |
Age, race, gender, study site, physical health, WISC score, BMI, depression, education, smoking status, gestational age | ↑ Childhood inappropriate self-regulation * ↓ income→ ↑ adult CRP (p < .05) ↑ Childhood distress proneness * ↓ income→ ↑ adult CRP (p < .05) ↑ Childhood distress proneness * middle income→ ↑ adult CRP (p < .05) |
| Castonguay et al. (2014) | 121 women | Clinical (breast cancer survivors) Mage 55.5 years, SD 11.0 years |
Cross-sectional | CRP | Blood | Health-related self-protection positive reappraisal | Cognitive Reappraisal | Age, education, smoking status, BMI, cancer stage, time since cancer diagnosis | ↓ Positive reappraisal * ↓ Capacity to disengage from unattainable goals→ ↑ CRP (β = .22, p = .01) Null: Positive reappraisal * Capacity to re-engagement with goals→ CRP (β = -.06, p=.55) |
| Chen et al. (2015) | 122 adolescents, 122 parents (tested separately) | Community Adolescent Mage 16.0 years, SD 1.2 years Parents Mage 46.7 years, SD 6.9 years |
Cross-sectional | Composite of CRP, IL-6 | Blood | Shift (positive reappraisal/reframing and cognitive restructuring), persist (developing purpose in life, holding onto hope future might be better) | Cognitive Reappraisal | Age, sex, ethnicity, waist circumference | ↑ Shift + persist * ↓ SES→ ↓ Composite (β = .18, p = .044) in adolescents but not parents (p = .72) |
| Collier et al. (2016) | 47 textile handcrafters | Community Mage 53.5 years, SD 14.0 years |
Experimental acute stressor- After recalling an upsetting situation randomized to a) writing exercise (rumination), b) quiet ego contemplation (neutral), c) textile art making | IL-1β | Saliva | Rumination | Perseverance vs. Distraction/Disengagement | None | ↑ IL-1B after negative mood induction only in rumination condition (p = .039) |
| Crosswell et al. (2022) | 182 | High risk community (92 high-stress caregivers of a child with autism, 91 controls without high stress who are caregivers of a neurotypical child) Mage 44.0 years |
Cross-sectional | CRP, IL-6 | Blood | Acceptance | Psychological Flexibility | Age, BMI | Null: Acceptance → CRP (β = .012, p = .806) Null: Acceptance → IL-6 (β = -.015, p = .552) Null: Acceptance * Parental stress → CRP (p > .05) Null: Acceptance * Parental stress → IL-6 (p > .05) |
| Dargél et al. (2017) b | 613 | Clinical- Bipolar disorder (type I, II, or Not Otherwise Specified) not in acute mood episode not in acute mood episode Mage 41.2 years, SD 12.4 years |
Cross-sectional | CRP | Blood | Emotional reactivity | Negative Affectivity/Emotional Reactivity | None | ↑ Emotion reactivity→ ↑ CRP (p < .001) |
| Dargél, et al. (2020) b | 1072 | Clinical- Bipolar disorder (type I, II, or Not Otherwise Specified) not in acute mood episode not in acute mood episode Mage 41.2 years, SD 12.4 years |
Cross-sectional | CRP | Blood | Emotional reactivity | Negative Affectivity/Emotional Reactivity | None | CRP higher in those with (M = 3.87, SD = 1.98) vs. without (M = 2.17, SD = 2.63) emotion hyper-reactivity (p < .001) |
| Denollet et al. (2003) | 42 males | Clinical-congestive heart failure Mage 57.9 years, SD 10.5 years |
Cross-sectional | TNF-α | Blood | "Type D personality" (negative affectivity and tendency to inhibit emotional expression in social situations) | Negative Affectivity/Emotional Reactivity Emotional Suppression |
None | ↑ Type D personality→ ↑TNF-α (d = .90, p = .003) |
| Gierlotka et al. (2015) | 65 | Clinical (33 PTSD, 32 control, all with mechanical injuries to extremities) PTSD Mage 37.7 years Control Mage 34.9 years |
Cross-sectional | IFN-γ, IL-6, IL-10, sIL-2, TNF-α | Blood | Perseverance, emotion reactivity | Negative Affectivity/Emotional Reactivity Perseverance vs. Distraction/Disengagement |
None | Null: All |
| Graham et al. (2009) | 84 | Community (married men and women) Mage 37.0 years, SD 13.1 years |
Longitudinal + experimental acute stressor—randomized to conflict or no-conflict | IL-6, TNF-α | Blood | Cognitive processing word use (underlying mechanism of emotion expression and disclosure) | Emotional Suppression | Gender, age, BMI, education, baseline inflammatory level, hostile interactions, positive interactions, marital quality, depression, hostility. | ↑ Cognitive word use during conflict → ↓ IL-6 24 hours later (β = -.26, p < .05) Null: Cognitive word use during conflict → TNF-a 24 hours later (β = -.18, p = .09) |
| Hladek et al. (2020) | 49 | Community Mage 50.9 years, SD 25.9 years |
Cross-sectional | IL-6, IL-10, TNF-α | Sweat patch | Coping self-efficacy (problem-solving, emotion regulation, social support) | Positive Coping/Social Support-Seeking | Age, sex, race, BMI, chronic diseases | ↓ Coping self-efficacy→ ↑ TNF-α (β = −.03, p = .028) ↓ Coping self-efficacy→ ↑ IL-10 (β = −.017, p = .007) Null: Coping self-efficacy→ ↑ IL-6 (β = −.22, p = .054) |
| Hoyt et al. (2013) | 41 men | Clinical- Prostate cancer Mage 66.6 years, SD 9.6 years |
Longitudinal | CRP, IL-6 | Blood | Emotion processing, emotion expression | Emotional Suppression | Age, ethnicity, BMI, # of months since completing primary cancer treatment | ↑ Emotional processing → ↓ IL-6 (β = -.66, .05 < p < .01) Null: Emotional processing → CRP (β = -.43, .05 < p < .10) Null: Emotional expression → IL-6 (β = .38, .05 < p < .10) Null: Emotional expression → CRP (β = .44, .05 < p < .10) |
| Järvelä-Reijonen et al. (2020) | 169 | Clinical (84 face-to-face treatment, 85 mobile treatment) Face-to-face Mage 51.0 years, SD 6.5 years Mobile Mage 48.8 years, SD 7.7 years |
Longitudinal | CRP, IL-1Ra, adiponectin | Blood | Psychological Flexibility (both general and weight-related) | Psychological Flexibility | Age, sex | Null: General acceptance → CRP (β = -.03, p = .772) Null: Weight-related acceptance → CRP (β = .16, p = .099) Null: General acceptance → IL-1Ra (β = .01, p = .888) Null: Weight-related acceptance → IL-1Ra (β = .00, p = .807) Null: General acceptance → adiponectin (β = .01, p = .946) Null: Weight-related acceptance → adiponectin (β = .02, p = .812) |
| Jones et al. (2022) | 279 | Community Mage 40.2 years, SD 6.2 years |
Longitudinal | CRP, IL-6 | Blood | Cognitive reappraisal, Expressive suppression | Emotional Suppression Cognitive Reappraisal |
Age, sex assigned at birth, racial/ethnic identity, time between assessments, inflammatory protein at baseline | ↑ Cognitive reappraisal→ ↓ IL-6 (β = -.155, p = .006) ↓ Cognitive reappraisal * ↑ Childhood trauma→ ↑ IL-6 (β = -.001, p = .012) ↓ Cognitive reappraisal * ↑ Childhood abuse→ ↑ IL-6 (β = -.001, p = .043) ↑ Expressive suppression * ↑ Childhood abuse→ ↑ IL-6 (β = .002, p = .011) ↑ Expressive suppression * ↑ Childhood abuse→ ↑ CRP (β = .001, p = .033) ↓ Cognitive reappraisal * ↑ Childhood neglect→ ↑ IL-6 (β = -.001, p = .009) ↓ Cognitive reappraisal * ↑ Childhood neglect→ ↑ CRP (β = -.002, p = .036) Null: Expressive suppression→ IL-6 (β = -.062, p = .294) Null: Expressive suppression * Childhood trauma → IL-6 (β = .001, p = .074) Null: Cognitive reappraisal→ CRP (β = -.056, p = .253) Null: Expressive suppression→ CRP (β = -.046, p = .362) Null: Cognitive reappraisal * Childhood trauma → CRP (β = -.005, p = .362) Null: Expressive suppression * Childhood trauma → CRP (β = .001, p = .117) Null: Cognitive reappraisal * Childhood abuse→ CRP (β = .001, p = .518) Null: Expressive suppression * Childhood neglect → CRP (β = .0003, p = .636) Null: Cognitive reappraisal * Childhood neglect→ IL-6 (β = -.0002, p = .472) |
| Jones et al. (2018) | 261 adolescents | Community Mage 14.6 years, SD 1.1 years |
Cross-sectional | CRP, IL-6 | Blood | Cognitive reappraisal, expressive suppression | Emotional Suppression Cognitive Reappraisal |
Age, sex, ethnicity, income, and BMI | Null: Chronic family stress * cognitive reappraisal→ CRP (β = -.079, p = .194) Null: Chronic family stress * suppression→ CRP (β = -.044, p = .472) Null: Chronic family stress * cognitive reappraisal→ IL-6 (β = .068, p = .256) Null: Chronic family stress * suppression→ IL-6 (β = -.005, p = .993) |
| Khan et al. (2020) | 606 | Clinical (trauma exposed veterans) Mage 58.0 years, SD 11.2 years |
Cross-sectional | Fibrinogen, CRP | Blood | Cognitive reappraisal, Expressive suppression | Emotional Suppression Cognitive Reappraisal |
Sex, age, race, education, income, creatinine, PTSD | ↑ Emotional suppression→ ↑ CRP (β = .11, p = .01) ↑ Emotional suppression→ ↑ Fibrinogen (β = 0.10, p = 0.02) Null: Cognitive reappraisal→ CRP (β = .03, p >.05) Null: Cognitive reappraisal → Fibrinogen (β = 0.01, p >.05) |
| Knight et al. (2022) | 162 | Community Mage 44.4 years, SD 11.2 years |
Cross-sectional, longitudinal | CRP + Cytokine composite (IL-1β, IL-6, IL-8, IL-10, TNF-α) | Blood | Rumination | Perseverance vs. Distraction/Disengagement | Age, BMI | Null-No gender-stratified relationships between rumination and CRP, the inflammatory composite, or individual cytokines |
| Lopez, et al. (2020) | 99 recently bereaved spouses | Community Mage 68.6 years, SD 10.7 years |
Cross-sectional | IL-2, IL-6, IL-17A, IFN-γ, TNF-α | Blood | Expressive suppression, cognitive reappraisal | Emotional Suppression Cognitive Reappraisal |
Age, sex, BMI, education, income, sleep, depression, medication, smoking status, Clinical conditions, physical activity, time since spouse passed away | ↑ Expressive suppression → ↑ IFN-γ (corrected p = .015) ↑ Expressive suppression → ↑ TNF-α (corrected p = .015) Null: Expressive suppression → IL-2, IL6, IL-17A Null: Cognitive reappraisal with all proteins. |
| Low et al. (2013) | 245 adolescents | Community Mage 15.7 years, SD 1.3 years |
Longitudinal-Daily diary | CRP | Blood | Positive engagement coping, disengagement coping | Perseverance vs. Distraction/Disengagement Positive Coping/Social Support-Seeking |
Age, race, sex. BMI, smoking status, income | ↑ Positive engagement coping * ↑ negative life events→ ↓ CRP (β = -.14, p = .013) ↑ Positive engagement coping * ↑ conflict life events→ ↓ CRP (β = -.12, p = .039) ↑ Positive engagement coping * ↑ daily interpersonal conflict/tension→ ↓ CRP (β = -.13, p = .039) Null: Disengagement coping * negative life events→ CRP Null: Disengagement coping * conflict life events→ CRP Null: Disengagement coping * daily interpersonal conflict/tension→ CRP |
| Master et al. (2009) | 22 | Community Mage 20.1 years, SD 1.5 years |
Acute stressor | IL-6 | Saliva | Emotion approach coping | Emotional Suppression | Baseline IL-6 | Null: Emotion approach coping → baseline IL-6 Null: Emotion approach coping → 25-minutes post stressor IL-6 Null: Emotion approach coping → 55-minutes post stressor IL-6 |
| Miller et al. (2013) | 34 preschoolers | Community Mage 4.1 years, SD 0.6 years |
Cross-sectional | IL-6, TNF-α | Blood | Emotion regulation, negative liability | Emotion Regulation/Dysregulation Negative Affectivity/Emotional Reactivity |
Age | ↑ Emotion regulation → ↑ TNF-α (p < .05) ↑ Negative lability → ↓ TNF-α (p < .05) Null: Emotion regulation → IL-6 Null: Negative lability → IL-6 |
| Mitchell & Christian (2019) | 67 | Clinical (pregnant) Mage 29.8 years, SD 5.3 years |
Longitudinal | IL-4, IL-6 | Blood | Perseverative thinking | Perseverance vs. Distraction/Disengagement | Race, BMI, gestational age, Clinical conditions | ↓ Repetitive thinking * ↑ SES→ ↓ IL-6 (∆R2 = .07, p = .03) Null: Repetitive thinking * SES→ IL-4 (p = .35) |
| Moriarity et al. (2018) c | 140 adolescents | Community Mage 16.5 years, SD 1.2 years |
Longitudinal | CRP, IL-6 | Blood | Rumination | Perseverance vs. Distraction/Disengagement | Age, BMI, timing of blood draw, SES, sex, race, time in study, baseline IL-6/CRP, baseline depression symptoms (when predicting depression) | ↑ Anxiety * ↑ Rumination→ ↑ IL-6 (p = .042) ↑ Anxiety * ↑ Rumination→ ↑ IL-6→ ↑ Depression (95% CI: .001-.004) Null: Anxiety * Rumination→ CRP (p = .745) Null: Anxiety * Rumination→ CRP→ Depression (95% CI: -.003-.002) |
| Moriarity et al. (2020a) | 109 | Community Mage 21.5 years, SD 2.1 years |
Longitudinal | Composite + individual proteins- CRP, IL-6, IL-8, TNF-α | Blood | Rumination on positive and negative affect | Perseverance vs. Distraction/Disengagement | Gender, race, age, BMI, birth control use, medication, time of day of blood draw | *only individual biomarkers (not the tested composite) are reported in this systematic review because no information was provided regarding the factor reliability/model fit of the inflammatory composite. Each of the interactions below was a follow-up of a significant interaction predicting the inflammatory composite ↑ Dampening of positive affect * ↓ reward responsiveness→ ↑ CRP (p = .022) ↑ Self-focused rumination on positive affect * ↑ reward responsiveness→ ↑ IL-8 (p = .013) Null: Dampening of positive affect * reward responsiveness→ IL-6 Null: Dampening of positive affect * reward responsiveness→ IL-8 Null: Dampening of positive affect * reward responsiveness→ TNF-α Null: Self-focused rumination on positive affect * reward responsiveness→ CRP Null: Self-focused rumination on positive affect * reward responsiveness→ IL-6 Null: Self-focused rumination on positive affect * reward responsiveness→ TNF-α |
| Moriarity et al. (2020b) c | 89 adolescents | Community Mage 18.3 years, SD 1.4 years |
Acute stressor | IL-6, IL-8 | Blood | Rumination, problem solving, distraction | Perseverance vs. Distraction/Disengagement Positive Coping/Social Support-Seeking |
Baseline protein levels, time, gender, income, race, age | ↑ Cognitive response ratio * ↑ Reward drive→ ↑ IL-6 (p = .002) Followed up with individual cognitive response styles ↑ Rumination * ↑ Reward drive→ ↑ IL-6 (p = .044) ↓ Problem solving * ↑ Reward drive→ ↑ IL-6 (p = .036) ↓ Distraction * ↑ Reward drive→ ↑ IL-6 (p = .008) Null: Cognitive response ratio * Reward drive→ IL-8 |
| Newton et al. (2017) | Study 1: 68 (45 rest group, 23 distraction group) Study 2: 68 (46 rest group, 22 distraction group) |
Community Study 1 Mage 20.8 years, SD 3.6 years Study 2 Mage 21.3 years, SD 3.8 years |
Experimental acute stressor- Study 1: Social stressor—randomized to rest or distraction Study 2: Angry autobiographical memory recall—randomized to rest or distraction Rest group: sitting quietly for 40 minutes to create opportunity for rumination Distraction group: 29 emotionally neutral puzzles Note: Amount of participants who actively ruminated during the rest condition was low in both studies (15 and 8, respectively) |
IL-1β, IL-6, TNF-α | Saliva | Rumination, negative emotion reactivity | Negative Affectivity/Emotional Reactivity Perseverance vs. Distraction/Disengagement |
None |
Study 1: ↑ Rumination→ ↑ IL-6 (β = .22, p ≤ .035) ↑ Negative emotion post-stressor→ ↑ IL-6 (β = .309, p = .012) ↑ Negative emotion post-stressor→ ↑ IL-1β (β = .288, p = .026) ↑ Negative emotion post-stressor → ↑ TNF-α (β = .255, p = .044) Null: Rumination → IL-1B (β = .011, p > .05) Null: Rumination → TNF-α (β = .064, p > .05). Study 2: Rest condition * ↑ Time→ ↑ IL-1B (p = .027) relative to distraction condition Null: Group * Time→ IL-6 (p = .434) Null: Group * Time→ TNF-α (p = .074) Null: Rumination → IL-6 (β = -.079, p > .05). Null: Rumination → IL-1B (β =-.014, p > .05) Null: Rumination → TNF-α (β =-.03, p > .05). Null: Negative emotion post-stressor→ IL-6 (p > .18) Null: Negative emotion post-stressor→ IL-1β (p > .18) Null: Negative emotion post-stressor → TNF-α (p > .18) |
| Powers et al. (2016) | 40 African American women | Clinical- Diabetes Mage 52.0 years, SD 7.6 years |
Cross-sectional | CRP | Blood | Emotion dysregulation | Emotion Regulation/Dysregulation | PTSD, depression, trauma, BMI | ↑ Emotion dysregulation→ ↑ CRP (β = .58, p < .001) |
| Prossin et al. (2011) | 28 females | Clinical (14 MDD, 14 controls) No age reported |
Acute stressor | IL-18 | Blood | Negative affective reactivity to sadness induction | Negative Affectivity/Emotional Reactivity | None | ↑ Negative affect post sadness induction → ↑ IL-18 (rs = .60, p=.03) for controls but not MDD |
| Segerstrom et al. (2017) | 120 | Community Mage 74.2 years, SD 5.6 years |
Longitudinal | IL-6 | Blood | Repetitive thought (worry, rumination, self-reproach, reflection, emotion processing) | Cognitive Reappraisal Perseverance vs. Distraction/Disengagement |
BMI, age, medication | ↑ Repetitive thought → ↓ IL-6 (p = .001) ↑ Repetitive thought * ↓ verbal IQ → ↓ IL-6 (p = .009) Quadratic effect of repetitive thought → IL-6 (p = .002), negative relationship between repetitive thought and IL-6 steepest at low levels of repetitive thought Null: Quadratic effect of repetitive thought * verbal IQ → IL-6 |
| Shimanoe et al. (2014) d | 7,873 | Community Men Mage 55.6 years, SD 8.3 years Women Mage 54.3 years, SD 8.2 years |
Cross-sectional | CRP | Blood | Emotion expression, emotion support seeking, positive reappraisal, problem solving, disengagement | Positive Coping/Social Support-Seeking Emotional Suppression Cognitive Reappraisal Perseverance vs. Distraction/Disengagement |
Age, BMI, body fat, alcohol consumption, smoking, physical activity level, sleeping, occupation, working hours, years of schooling, perceived stress, social support | ↑ Disengagement → ↓ CRP in men but not women (partial η2 = 0.002, p = .027 and partial η2 < 0.001, p = .82, respectfully) ↑ Emotional support seeking→ ↓ CRP at high levels of stress in men only (pinteraction = .021; ptrend = .028). Null: Other emotional regulation strategies → CRP (analyses ran separately for men and women) Null: Other emotional regulation strategies * perceived stress → CRP (analyses ran separately for men and women) |
| Shimanoe et al. (2018) d | 7,256 | Community Women Mage 55.0 years, SD 8.1 years Men Mage 56.0 years, SD 8.0 years |
Longitudinal | CRP | Blood | Emotion expression, emotion support seeking, positive reappraisal, problem solving, disengagement | Emotional Suppression | Age, BMI, employment, alcohol consumption, smoking status, physical activity, sleep, medication, menopause status, HRT, social support, perceived stress | ↑ Positive reappraisal * ↑ perceived stress →↓ CRP in men (p = .007) ↑ Emotional expression → ↓ CRP in women (p = .024) Null: Other emotional regulation strategies → CRP (analyses ran separately for men and women) Null: Other emotional regulation strategies * perceived stress → CRP (analyses ran separately for men and women) |
| van Middendorp et al. (2005) | 60 | Clinical- Rheumatoid Arthritis Mage 59.0 years, SD 11.2 years |
Cross-sectional | IL-6 | Blood | Ambiguity, control, orientation, and expression | Negative Affectivity/Emotional Reactivity Emotional Suppression |
None | Null: Emotional regulation subscales →IL-6 were null after Bonferroni corrections (uncorrected results not described) |
| Woody et al. (2016) e | 34 women | Community Mage 20.7 years, SD 2.3 years |
Experimental acute stressor- randomized to 5 minutes of rumination or distraction | CRP, IL-6, TNF-α | Blood | Reflection | Perseverance vs. Distraction/Disengagement | Trait rumination, openness to experience, neuroticism, SES, BMI, condition | ↑ Reflection→ ↓ IL-6 (β = -.70, p = .007) Null: Reflection→CRP (β = .22, p = .407) Null: Reflection→ TNF-α (β = -.05, p = .869) |
| Yang et al. (2020) | 162 | Clinical (105 ADHD, 57 control) ADHD Median Age = 36.0 years (25th-75th% = 29.0–43.0 years) Control Median Age = 38 years 25th-75th% = 34–43 years |
Cross-sectional | CRP, SAA, sICAM-1, sVCAM-1 | Blood | Emotion regulation | Emotion Regulation/Dysregulation | Age, sex, BMI, ADHD medication, anti-inflammatory drugs, melatonin | ↑ Total emotion regulation difficulty → ↑ CRP (adjustedR2 = 0.25, p = 0.025) ↓ Effective emotion regulation strategies difficulty → ↑ CRP (adjustedR2 = 0.27, p = 0.006) Null: Total emotion regulation difficulty→ SAA Null: Total emotion regulation difficulty→ sICAM-1 Null: Total emotion regulation difficulty→ sVCAM-1 |
| Ysseldyk et al. (2018) | 54 women | Community Mage 21.2 years, SD 6.0 years |
Cross-sectional | IL-10, TNF-α | Blood | Rumination | Perseverance vs. Distraction/Disengagement | None | Null: Depressive rumination→ TNF-α (r = .24, p < .10) Null: Brooding rumination→ TNF-α (r = -.13, p > .05) Null: Reflective rumination→ TNF-α (r = .01, p > .05) Null: Depressive rumination→IL-10 (r = -.08, p > .05) Null: Brooding rumination→IL-10 (r = -.07, p > .05) Null: Reflective rumination→IL-10 (r = .10, p > .05) |
| Zoccola et al. (2014) e | 34 females | Community No age reported |
Experimental acute stressor—randomized to rumination or distraction after stressor | CRP, IL-6, TNF-α | Blood | Distraction, rumination | Perseverance vs. Distraction/Disengagement | BMI and baseline inflammatory protein values | Rumination group had linear increases in CRP (f2 = .22, p < .001). Linear trajectory of CRP differed between rumination and distraction groups (f2 = .18, p = .01). Distraction group had linear increases in CRP up to 43 minutes post stressor, after which they returned to baseline levels (f2 = .14, p = .03). Null: Group → IL-6 Null: Group → TNF-a |
Notes: Mage mean age, SD = standard deviation, BMI = body mass index, CRP = C-reactive protein, HRT = hormone replacement therapy, CRP = high-sensitivity C-reactive protein, IL = interleukin, IFN-γ = Interferon-gamma, IL-1Ra = interleukin-1 receptor antagonist, MDD = Major depressive disorder, PTSD = Post-traumatic stress disorder, SAA = Serum amyloid A, SES = socioeconomic status, sIL = soluble interleukin, sICAM-1 = soluble intercellular adhesion molecule 1, sVCAM-1 = soluble vascular cell adhesion molecule-1, TNF-α = Tumor necrosis factor alpha, WISC = Wechsler Intelligence Scale for Children
3.1. Study characteristics
Of the studies included in this review, 19 included cross-sectional data, 13 included longitudinal data, 8 included acute stressors, and 4 qualified as experimental designs. Twenty-six featured community samples, whereas the remaining 12 featured clinical samples (6 medical, 5 psychiatric, 1 combined medical and psychiatric). Regarding the assessment of inflammatory proteins, 34 studies used blood, 3 used saliva, and 1 used sweat. Only 6 studies featured nonadult samples (5 adolescent, 1 preschool-aged).
To provide structure for this review, seven emotion regulation categories were created. First, “Emotion Regulation/Dysregulation” describes results using measures that claimed to broadly capture the ability, or inability, to adaptively regulate emotions. The rest of the categories pertain to more specific emotion regulation skills/traits/strategies. Second, “Negative Affectivity/Emotional Reactivity”, includes studies evaluating (often through the use of mood-induction tasks) individual differences in the magnitude of emotional responses. Although these characteristics are theoretically different from regulation, we believe they are important to include in this review both because (a) they illustrate the inflammatory correlates of the emotional reactions that regulation traits modulate and (b) they are a marker of how successful an individual is at regulating their emotions. Third, “Expressive Suppression vs. Emotional Expression” describes studies investigating the tendency to keep emotions bottled up and away from others vs. openly communicating them. Fourth, “Cognitive Reappraisal” includes studies evaluating individuals’ ability or tendency to attempt to reframe stressful situations and/or negative emotions in a more neutral or positive light. Fifth, “Perseverance vs. Distraction/Disengagement” describes studies testing tendencies to ruminate or worry about stressful situations/emotions or, the inverse, to seek opportunities for disengagement from negative situations and emotions. Sixth, “Psychological Flexibility” covers research on either “psychological flexibility”—the ability to live in the present and change or persist behaviors aligned with values instead of emotions)—or its subcomponents such as acceptance of positive and negative aspects of life. Finally, “Positive Coping/Social Support Seeking” includes several studies of emotion regulation strategies commonly seen as adaptive (although this is influenced by the context of the stressor in question, discussed in further detail in the Discussion section).
3.2. Emotion regulation/dysregulation
Five studies investigated emotion regulation broadly. Difficulties with global emotion regulation were consistently associated with higher CRP in a mixed sample of adults with and without ADHD (Yang et al., 2020). When emotion regulation subscales were probed in this study, CRP was related specifically to limited access to effective emotion regulation strategies. A similar positive association between emotional dysregulation and CRP was observed in a small sample of Black American women with diabetes (Powers et al., 2016). Two studies from the New England Family Study corroborate this finding with a related construct called “inappropriate self-regulation” (reflecting emotional functioning in children whose behavior was unrestrained and impulsive) in childhood predicting adult inflammatory outcomes. Children with higher inappropriate self-regulation had higher CRP as adults (Appleton et al., 2011) and this association was stronger for participants who grew up in families with lower income (Appleton et al., 2012). Counter to hypotheses, a study of low-income preschoolers found that stronger parent-reported emotion regulation skills were associated with higher TNF-α cross-sectionally (Miller et al., 2013). It is worth noting that this study was the smallest (n = 34) and it is possible that such young participants may not have experienced enough cumulative emotionally-triggered inflammatory responses to shift their inflammatory baseline. Further, this also was the only study on this topic to not include covariates; therefore, it is possible that untested confounds drove this unexpected result. Finally, if socioeconomic status is an important moderator of the relation between emotion regulation and inflammatory proteins in children, restricting this sample to low-income children might have reduced the variability in the variables analyzed, influencing results via Berkson’s bias (i.e., conditioning sample recruitment based on levels of an analyzed variable).
3.3. Negative affectivity/emotional reactivity
Ten of the studies reviewed analyzed the associations between intensity of negative emotional responses and inflammatory proteins. The above-referenced investigations from the New England Family Study also evaluated emotional reactivity in the form of temperamental “distress proneness” (i.e., the tendency to be emotionally labile and easy to frustrate) in children significantly predicting adult CRP concentrations (Appleton et al., 2011), which was stronger for children in low- and middle-income families relative to their high-income peers (Appleton et al., 2012). Further, parallel to the results with perceived emotion regulation broadly, the above-referenced study of pre-school aged children found that higher negative lability predicted elevated TNF-α cross-sectionally (Miller et al., 2013). Negative affectivity also was associated with higher TNF-α in a study of the immunological correlates of “Type D personality” (defined as a combination of negative affectivity and tendency to inhibit emotional expression in social situations) in men with congestive heart failure (Denollet et al., 2003). Elevated emotional reactivity also was associated with higher levels of CRP in euthymic patients with bipolar disorder using both continuous methods (Dargél et al., 2017) and clinical cut-offs (Dargél et al., 2020) of emotional reactivity. However, two of the cross-sectional studies with clinical samples found null associations. One case-control study of posttraumatic stress disorder (PTSD)—in which all participants, regardless of whether they had PTSD, had mechanical injuries to extremities—found no association between emotional reactivity and five inflammatory proteins (Gierlotka et al., 2015). Similarly, a study of patients with rheumatoid arthritis found no association between emotional orientation (described as attending to, valuing, and experiencing emotions intensely) and IL-6 (van Middendorp et al., 2005).
Two of the studies reviewed tested associations between acute emotional reactivity and inflammatory proteins. The first, a case-control study of females with major depressive disorder (MDD) found that increased sadness reactivity during a sadness induction predicted greater IL-18 increases in controls but not participants with MDD (Prossin et al., 2011). The lack of association in participants with MDD might have been due to ceiling effects because individuals with MDD had higher IL-18 and negative affect compared to controls at baseline and the sample was small (n = 12 per group). Another study found negative affect post-Trier Social Stress Test was associated with greater IL-6, IL-1β, and TNF-α, but emotional reactivity to an angry autobiographical memory recall was not associated with any of these three proteins (Newton et al., 2017). Importantly, both studies randomized participants to a distraction group or a resting group to create the opportunity for rumination.
3.4. Expressive suppression vs. emotional expression
Twelve articles evaluated the association between tendencies to either express or suppress emotions and inflammatory biology. A cross-sectional study using data from the New England Family Study found that adults reporting higher levels of expressive suppression had higher levels of CRP (Appleton et al., 2013). Similarly, expressive suppression was positively associated with CRP and fibrinogen in trauma-exposed veterans (Khan et al., 2020), IFN-γ and TNF-α in recently bereaved spouses (Lopez et al., 2020), and TNF-α in men with congestive heart failure (contextualized as “Type D” personality, as described in the study above; Denollet et al., 2003). Two studies evaluated expressive suppression as a moderator of childhood adversity. The first observed null main effects of expressive suppression on changes in CRP and IL-6 but found that expressive suppression interacted with childhood abuse such that expressive suppression amplified the positive association between child abuse and increases in CRP and IL-6 (Jones et al., 2022). The second explored whether expressive suppression also interacted with chronic family stress, which was not supported in a separate cross-sectional dataset (Jones et al., 2018). The rheumatoid arthritis study described above also did not find associations between IL-6 and subcategories of emotion regulation related to ambivalence or resistance to expressing emotions (van Middendorp et al., 2005).
Consistent with evidence that suppressing emotions might elevate inflammatory profiles, several studies indicated that processing and expressing emotions is associated with lower levels of circulating inflammatory proteins. For example, a small longitudinal study of men who had undergone radical prostatectomy or radiation therapy for prostate cancer in the previous two years found that higher emotional processing predicted lower IL-6 four months later (Hoyt et al., 2013). One study randomized dyads of married men and women to “conflict” or “no conflict” discussion with their partner and assessed changes in IL-6 and TNF-α over 24 h. Higher cognitive processing word use, an indicator of emotion regulation, during conflictual conversation was associated with less steep IL-6 increases 24-hours post-discussion (Graham et al., 2009). However, another acute stressor study found no associations between a related construct, trait “emotion approach coping” (described as a combination of purposeful emotional processing and emotional expression), and changes in salivary IL-6 (Master et al., 2009). It is important to note that this study was small (n = 22) and that emotional approach coping was measured at a different study visit than the stressor and inflammation measurements (the study provided no information on the average length of time between these visits), which may play a role in the lack of association observed. Interestingly, two studies from the same sample suggested that the association between some emotional regulation characteristics and inflammatory biology might be sex-specific and might also differ between cross-sectional and longitudinal modeling approaches. A cross-sectional analysis for the INTERHEART study found null gender-stratified associations for a variety of emotion regulation-CRP associations (including emotional expression; Shimanoe et al., 2014); however, a later longitudinal analysis using the same data found that higher emotional expression was associated with less CRP over time in women (Shimanoe et al., 2018).
3.5. Cognitive reappraisal
Nine studies tested whether positive cognitive reappraisal, the technique of reinterpreting emotionally arousing situations to reduce negative emotions, is associated with inflammatory biology. The cross-sectional New England Family study that found that expressive suppression was associated with higher CRP also found that the tendency to positively reappraise negative scenarios was associated with lower CRP (Appleton et al., 2013). A longitudinal sample observed consistent findings, with higher cognitive reappraisal being associated with decreases in IL-6, but not CRP, over time (Jones et al., 2022). A related construct, “shift-and-persist” (a combination of reappraisal and hopefulness/purpose), was associated with lower CRP and IL-6 (modeled as a composite) in adolescents but not parents (Chen et al., 2015). Conversely, no evidence of a direct association between cognitive reappraisal and inflammatory proteins (including CRP) was found in a study of recently bereaved spouses (Lopez et al., 2020), a large community sample (Shimanoe et al., 2014), or a sample of trauma exposed veterans (Khan et al., 2020), all of which were cross-sectional.
Five studies evaluated cognitive reappraisal as a moderator to buffer stress or stress-generative characteristics. Similar to the pattern of results for emotional expression in the INKHEART study described above, cognitive reappraisal did not interact with stress to predict CRP in cross-sectional data (Shimanoe et al., 2014), but this interaction was significant when predicting change in CRP over time (Shimanoe et al., 2018). Specifically, cognitive reappraisal buffered the risk that elevated stress in the past year had on longitudinal changes in CRP for men. Cognitive reappraisal also buffered the association between childhood neglect on change in IL-6 and CRP as well as the relation between childhood abuse and trauma on IL-6 (Jones et al., 2022). Lower cognitive reappraisal (focused on health-related cognitions) also interacted with an inability to disengage from unattainable goals (but not the capacity to re-engage with goals) to be associated with elevated CRP in female breast cancer survivors (Castonguay et al., 2014). Finally, cognitive reappraisal was not a moderator of the cross-sectional relation between chronic family stress and CRP in a community sample (Jones et al., 2018).
3.6. Perseverance vs. distraction/disengagement
The most commonly assessed emotion regulation characteristics were related to perseverative cognitive styles. Interestingly, all four studies that tested main effects between perseverative cognitive styles and inflammatory biology in observational data (cross-sectional or gender-stratified analyses in longitudinal data) found null results (Gierlotka et al., 2015; Knight et al., 2022; Ysseldyk et al., 2018) and one study found associations in the opposite direction than hypothesized (Segerstrom et al., 2017). With respect to the latter, this study also found a significant interaction between repetitive thoughts and verbal IQ predicting IL-6 in an unexpected direction. Specifically, IL-6 was relatively stable for those with higher IQs, regardless of repetitive thought, but was negatively associated with repetitive thought at lower IQ levels. Notably, the average IQ of the sample was almost 1 standard deviation higher than would be expected (IQ tests are normed to have a mean of 100 and SD of 15). Additionally, as part of an exploratory analysis, a quadratic relation between rumination and IQ was found such that the inverse association between repetitive thought and IL-6 was strongest at lower levels of repetitive thought.
Three studies that measured acute inflammatory reactivity to acute laboratory stressors found results more consistent with theory that perseverative thoughts about negative situations and emotions would increase levels of inflammatory proteins. One study of female college undergraduates who completed a social stress task found that random assignment to an instructed rumination condition led to steeper increases in CRP compared to an instructed distraction condition (Zoccola et al., 2014). A different study using a social stressor that randomized some participants to a rest condition to make the opportunity for rumination or a distraction condition found that although no group difference was observed, higher trait rumination was associated with increases in salivary IL-6 after a social stressor (Newton et al., 2017). The total number of people in the rest condition who reported ruminating in post-stressor assessments was low (8 out of 45 people), which might have attenuated observable group-level effects relative to studies that instructed participants to ruminate. This publication also featured a second study with an angry autobiographical memory stressor and randomization to the same conditions and found that the rest/rumination group experienced steeper increases in IL-1β relative to the distraction condition. The final experiment randomly assigned textile handcrafters to: (a) a writing exercise designed to induce rumination, (b) a neutral ego contemplation, or (c) textile art making (hypothesized to be a positive emotional experience) after instructed recall of an upsetting memory (Collier et al., 2016). Significant increases in IL-1β only were observed in the rumination condition.
Several of the studies by the authors of this review investigated perseverative thinking styles as moderators of the relation between arousal-modulating characteristics and inflammatory biology in a vulnerability-stress conceptualization. For example, a more perseverative cognitive response style ratio (quantified as rumination/[distraction + problem-solving]) amplified the relation between reward drive (the domain of reward functioning related to intensity and arousal in the pursuit of rewards) and increases in IL-6 (but not IL-8) to a social stress task (Moriarity et al., 2020b). When this interaction was followed-up with an investigation of individual cognitive styles, all 3 subscales interacted with reward drive to predict change in IL-6. Specifically, high rumination amplified the association between reward drive and increases in IL-6 (the other subscale results are described in thematically appropriate sections, below). Another study in a community sample of adults selected for high or moderate reward sensitivity found that self-focused rumination aimed to promote positive affect interacted with high reward sensitivity to predict IL-8 (Moriarity et al., 2020a). Conversely, higher levels of perseverative thought aimed at dampening positive affect interacted with low reward sensitivity to predict higher CRP. Finally, in the same sample of adolescents as the first study of this paragraph, higher rumination amplified the association between baseline anxiety symptoms and increases in IL-6 (but not CRP) over time (Moriarity et al., 2018). Further, data supported a moderated mediation in which changes in IL-6 partially mediated the association between baseline anxiety and changes in depression and this indirect effect was amplified in adolescents who tend to ruminate on negative affect. Another study from a different research team found that low levels of repetitive negative thinking interacted with higher socioeconomic status (SES) to predict less IL-6 in a longitudinal sample of pregnant women (Mitchell & Christian, 2019).
Finally, some studies evaluated tendencies to disengage from perseverative cognitions as a protective factor against elevated inflammation. For example, a small study of adult women featuring a social stress task followed by either 5 min of directed rumination or distraction found that trait reflection, a characteristic of “intellectual self-attentiveness” described as an emotionally-neutral cognition compared to the negative focus of rumination, was negatively associated with change in IL-6 post stressor (Woody et al., 2016). A study in the same sample (referenced above; Zoccola et al., 2014) found that participants in the distraction group had shorter inflammatory spikes that started to return to baseline by the end of the visit (roughly 50 min post-stressor), unlike the rumination group whose IL-6 did not decrease in the timeframe assessed. A separate study pairing an acute stressor (an angry autobiographical memory) with a resting/rumination and distraction condition found that participants in the distraction condition had less steep increases in IL-1β post-stressor (Newton et al., 2017). Further, the tendency to cognitively respond to negative emotion with distraction buffered the positive association between reward drive and increases in IL-6 post-social stressor in adolescents (Moriarity et al., 2020b). The above-referenced cross-sectional study in the INTERHEART cohort found that the tendency to disengage from stressful situations and negative emotions was associated with lower CRP concentrations in men, but not women (Shimanoe et al., 2014). Conversely, a daily diary study in adolescents did not support a hypothesized buffering interaction between various negative life events and disengagement predicting CRP (Low et al., 2013).
3.7. Psychological flexibility
Two studies tested either psychological flexibility or acceptance (a skill that helps foster psychological flexibility). One cross-sectional analysis examining acceptance in a case-control study of high-stress caregivers of children with Autism and non-high stress caregivers of neurotypical children did not find significant associations between acceptance and either CRP or IL-6 (Crosswell et al., 2022). Interactions between acceptance and parental stress predicting these proteins were also null. A separate study of overweight or obese, high-stress individuals tested post-Acceptance and Commitment Therapy levels of psychological flexibility (both general and specific to weight-related difficulties) predicting levels of CRP, IL-1 receptor agonist, and adiponectin 6 months later also found null results (Järvelä-Reijonen et al., 2020).
3.8. Positive coping/social support seeking
Several other studies investigated emotion regulation characteristics hypothesized to be negatively associated with inflammatory proteins. For example, the daily diary study described above found that, unlike disengagement, positive engagement coping (the tendency to change focus to positive qualities of life, keep a sense of humor, strategize how to handle the situation, focus on self-improvement) buffered the positive association between (a) conflictual life events, (b) daily interpersonal conflicts/tension, and (c) total negative life events and CRP (Low et al., 2013). Further, one study found that reward drive predicted greater increases in IL-6 post stressor for adolescents with low trait problem solving when feeling negative emotions relative to adolescents with greater proclivities to problem solve (Moriarity et al., 2020). Additionally, adults low in coping self-efficacy (confidence in one’s ability to navigate difficult situations through emotion regulation, problem-solving, and social support) had higher TNF-α and IL-10 (Hladek et al., 2020). Finally, high tendency to seek emotional support socially was associated with lower CRP in adults with high levels of perceived stress (Shimanoe et al., 2014).
4. Discussion
This systematic review summarizes the findings of 38 studies that investigated associations between emotion regulation and inflammatory proteins. Broadly, there was support for the hypothesis that emotion regulation abilities are related to differences in inflammatory activity. Specifically, 74% of studies found results consistent with the hypothesis that difficulty regulating emotion was associated with elevated inflammatory biology whereas skillful emotion regulation was associated with lower inflammation. However, the consistency of empirical support differed as a function of the specific aspects of emotion regulation examined and study methodology (Table 2), supporting the decision to not aggregate these studies in a quantitative, meta-analytic review.
Table 2.
Percentage of Studies with Theoretically Consistent Results.
| Category | % (Fraction) |
|---|---|
| Total Reviewed | 74% (28/38) |
| Emotion Regulation Constructs | |
| Positive Coping/Social Support-Seeking | 100% (4/4) |
| Emotion Regulation/Dysregulation | 100% (5/5) |
| Negative Affectivity/Emotional Reactivity | 80% (8/10) |
| Emotional Suppression | 71% (5/7) |
| Perseverance vs. Distraction/Disengagement | 71% (10/14) |
| Emotional Expression | 60% (3/5) |
| Cognitive Reappraisal | 56% (5/9) |
| Psychological Flexibility | 0% (0/2) |
| Methodological Characteristics | |
| Acute Stressor | 88% (7/8) |
| Vulnerability-Stress* | 81% (17/21) |
| Longitudinal | 77% (10/13) |
| Not Vulnerability-Stress | 69% (15/22) |
| Cross-sectional | 63% (12/19) |
Note: *Vulnerability-Stress is defined as either (a) testing emotion regulation as a predictor of inflammatory reactivity to an acute stressor or (b) testing whether emotion regulation moderates the association between reported stress or a stress-modulating variable (e.g., anxiety symptoms) and inflammatory biology. Also, note that some individual studies fit into multiple categories.
Relations between broadly defined emotion regulation/dysregulation and inflammatory proteins were observed in all five articles reviewed, although one of these studies found an association in the direction opposite to hypotheses and results from the other four studies (i.e., better emotion regulation was associated with higher concentrations of inflammatory proteins). Higher negative affectivity and emotional reactivity were associated with elevated levels of inflammatory proteins in eight of 10 studies reviewed. Of the articles testing associations between tendencies to suppress vs. express emotions and inflammatory proteins, five of seven supported the hypothesis that suppressing emotions would be related to elevated inflammatory biology; whereas a less convincing proportion (three of five) found that emotional expression was associated with lower concentrations of inflammatory proteins. It is possible that “expressing emotions” is not a specific enough construct to reliably associate with inflammatory outcomes. For example, “expressing emotions” could equally take the form of collaborative, healthy discussion in a productive manner with friends or expressing emotions in a hostile, confrontational manner. Slightly above half (five of nine) of the studies that examined cognitive reappraisal supported an association between reappraisal of negative situations and emotions as a protective factor against higher inflammation. The most common emotion regulation category reviewed was perseverative cognition vs. distraction/disengagement. Of the 14 studies reviewed, ten found evidence for a relation between these emotion regulation characteristics and inflammatory biology, three found null results, and one found results opposite of the hypothesized direction (e.g., more repetitive thought led to lower concentrations of inflammation in individuals with lower verbal IQ scores; Segerstrom et al., 2017). Neither of the two studies testing psychological flexibility (either generally or the subcomponent of acceptance) found significant associations with inflammatory biology. Plausibly, this might be due to psychological flexibility/acceptance being skills that open up the ability to engage with alternate responses to emotion; thus, they could facilitate inflammatory modulation emotion regulation but themselves are not sufficient. But, with only two published studies in this category, more work is needed. The remaining emotion characteristics were grouped into a “positive coping/social support-seeking” category. All four included studies supported that these traits predicted lower concentrations of inflammatory proteins. In sum, the evidence from this systematic review supports that emotion regulation is a modulator of inflammatory biology.
4.1. Integration of inflammation into emotion regulation models of risk
Given the breadth of physical and psychological health outcomes associated with emotion regulation and inflammation, it is critical for future work to consider integrated, multi-level frameworks of risk to develop theory. Establishment of etiological theories integrating malleable psychological (e.g., emotion regulation) and biological (e.g., inflammatory biology) constructs are critical for maximally comprehensive, and maximally flexible, healthcare (Moriarity, 2021). One such model tested by three studies in this review is an immunocognitive model of psychopathology (Moriarity et al., 2018, 2020b, 2020a), in which cognitive vulnerabilities (e.g., emotion regulation) amplify the impact of stress or stress-modulating characteristics (e.g., anxiety) on inflammation in ways that increase risk for psychopathology (e.g., depression). However, it is plausible to consider that this mechanistic pathway also might be relevant for many, if not all, inflammation-mediated disease processes (e.g., rheumatoid arthritis).
Clinically, identifying both psychosocial and biological treatment targets better facilitates comprehensive health care and coordination among medical and psychological members of a treatment team. Fully characterizing a risk pathway provides flexibility for idiosyncratic needs of patients. For example, if dysphoria or fatigue are obstacles to treatment adherence targeting emotion regulation (e.g., cognitive-behavioral therapies), understanding that reducing inflammation might improve these symptoms (Moriarity et al., 2022; Moriarity, Kautz et al., 2020) could warrant consideration of anti-inflammatory adjunctive medications. Conversely, some clients are unable, or unwilling, to take anti-inflammatory medications for an immune-mediated disease (e.g., arthritis, HIV/AIDS). Targeting inflammation using emotion regulation skills in a psychosocial intervention (e.g., cognitive behavioral therapies) might be an effective means of symptom reduction. Reduced inflammation might even be a biological mediator of some of the beneficial outcomes of psychosocial interventions (Shields et al., 2020).
In addition to treatment implications, it is critical to consider how these findings might facilitate fostering resiliency. This perspective helps health providers reduce illness recurrence and can inform organizational strategy (e.g., first year college orientation activities and resources) and policy change (e.g., educational materials provided to public schools). This review covers several emotion regulation skills (e. g., cognitive reappraisal, problem solving, distraction, social support-seeking) that might buffer the impacts of stress on inflammatory biology and inflammation-related outcomes. Although all of these skills plausibly could reduce the negative emotional impact of an event/stressor, social support-seeking (only evaluated in one reviewed study) might be a particularly promising emotion regulation skill for future research given empirical work suggesting that social stressors are particularly strongly associated with inflammatory stress responses (Dickerson et al., 2009; Slavich et al., 2020). Social Safety Theory argues that social safety schemas (socially-specific schemas about social safety or threat) are of particular importance for biological and psychological health (Slavich, 2020, 2022, Slavich et al., 2023).
4.2. Methodological implications
Across different emotion regulation traits, several methodological characteristics seemed to be associated with results in the hypothesized directions (i.e., difficulty with emotion regulation relating to higher concentrations of inflammatory proteins).
4.3. Longitudinal data
Longitudinal datasets investigating change in inflammatory proteins as a function of emotion regulation traits resulted in a greater proportion of results in the direction hypothesized relative to cross-sectional studies (77% vs. 63%), especially for studies testing perseverative thinking. For studies with repeated measures, this could be partially due to the benefit of being able to account for baseline levels of inflammatory proteins and focus on within-person change. Given the necessity of longitudinal data for analyses to have the potential causal relevance necessary to build and evaluate models of risk and resilience, this finding highlights the importance of collecting multiple timepoints of emotion regulation and inflammatory data in future research. Importantly, this distinction also suggests temporal specificity (Moriarity and Alloy, 2021)—, namely the possibility of the strength of the association between emotion regulation and inflammatory biology to change over time. This physiometric information is critical to design future research studies testing the extent to which emotion regulation characteristics influence trajectories of inflammation, in both observational and intervention studies.
4.4. Acute stressor designs
Testing emotion regulation as a predictor of inflammatory reactivity to an acute stressor also provided a higher proportion of theory-consistent results relative to naturalistic, cross-sectional studies (88% vs. 63%). Similar to longitudinal data, this may be due to the ability to account for baseline levels of inflammatory proteins and quantify within-person change. Acute stressors also provide the opportunity to evaluate specific contexts (e.g., social stressors) that might be most impactful to train emotion regulation skills and mitigate impact on inflammatory outcomes. Further, randomization to various conditions with instructed engagement in particular emotion regulation strategies (e.g., rumination vs. distraction; Woody et al., 2016; Zoccola et al., 2014)) can facilitate direct comparison of different emotion regulation options people use in response to a common stressor. Acute stressors also provide an important opportunity to test state emotion regulation/dysregulation as a predictor of inflammatory reactivity, whereas other designs typically rely on self-report of trait emotion regulation/reactivity. Given recent research on how individuals transition between using various emotion regulation strategies (Daniel et al., 2022), one important future direction that might be testable in an acute stressor design would be to evaluate how individual differences in emotion regulation transitions relate to inflammatory outcomes. Additionally, given evidence that different affective reactions have different inflammatory correlates (Carroll et al., 2011), future acute stressor studies should collect data on specific affective responses in addition to generalized distress.
4.5. Vulnerability-stress framework
Another factor that was associated with high theoretical consistency of results was the evaluation of emotion regulation characteristics (especially perseverative thinking) in the context of either stress (e.g., perceived stress, childhood trauma) or a variable that modulates stress responses or exposure (e.g., anxiety symptoms). Specifically, 81% of such studies found theoretically consistent results relative to 69% of the remaining studies. Given that the use of emotion regulation characteristics is contingent on emotional responses/exposure to emotionally-salient events, moderation studies might be particularly relevant for advancing theory on the association between emotion regulation, inflammation, and related health outcomes. It is important to note that by “moderation” studies we both refer to statistical moderation, as well as study designs that allow for tests of emotion regulation in the context of experienced emotion that can be regulated (e.g., acute stressor designs).
4.6. Context matters: actionable stressors
One critical detail that is not included in any of the reviewed articles, and thus could not be evaluated in this systematic review, is how specific contextual details of a stressor influence which emotion regulation skills/traits might be adaptive. Specifically, the ability of an individual to successfully intervene on a stressor by taking action might influence the long-term usefulness of a given emotion regulation strategy (Ford and Troy, 2019), a distinction taught as a foundational perspective in several psychotherapeutic frameworks (e.g., dialectical behavioral therapy). For example, problem-solving and/or emotional expression might be most adaptive in situations where an individual’s actions can change the situation (e.g., discussing boundaries and separating household responsibilities with a roommate). On the other hand, emotion-focused strategies like cognitive reappraisal, acceptance, and social support seeking might be best suited for stressors out of an individual’s realm of influence (e.g., the loss of a loved one). Future research and theory development should be careful to consider the limits of agency in emotionally-arousing situations to ensure maximum clinical-translatability. Two strategies for incorporating this nuance in future research are (a) comparing the interaction between different types of stressors and different emotion regulation traits and/or (b) using “common stressor” designs in which all participants experience the same, naturally occurring stressor.
4.7. Clinical research
Finally, much information could be gained from intervention research targeting emotion regulation characteristics. It is worth noting that several intervention studies that plausibly target emotion regulation were found during the initial literature search and excluded because they did not specifically test whether reductions in dysfunctional emotion regulation covaried with reductions in inflammatory biology. Given that the behavioral foci of many psychosocial interventions could have impacts on inflammatory biology (e.g., changes to appetite, substance use, or diet), there are many opportunities for confounds in psychosocial intervention studies unless specifically analyzed to test whether reductions in maladaptive emotion regulation are associated with inflammation. Therefore, we look forward to secondary data analysis on this topic in the future that, if results support this line of inquiry, can inform the design of intervention studies specifically created to test these research questions. To the extent that sample size might be a concern for readers with access to relevant data, we refer them to integrative data analysis (which facilitates the combination of different datasets) as a potential resource (Curran and Hussong, 2009) to aggregate multiple relevant datasets.
5. Conclusion
This systematic review of 2816 studies broadly found support for an association between a variety of emotion regulation constructs and inflammatory biology. We propose that integrated, multi-level theories of disease risk incorporating emotion regulation and inflammation as inter-related risk factors might result in more comprehensive treatment plans that provide flexibility for client needs and preferences. Across emotion regulation domains, theory-consistent results (i.e., difficulties with emotion regulation being associated with higher concentrations of inflammatory proteins) seemed to be more likely with longitudinal data, studies leveraging acute stressors, and/or studies testing vulnerability-stress models of risk for elevated inflammation.
Supplementary Material
Acknowledgments
Daniel P. Moriarity was supported by National Research Service Award F32 MH130149, an APF Visionary Grant, and grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. Lydia G. Roos and George M. Slavich were also supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. Rachel F. L. Walsh was supported by a National Science Foundation Graduate Research Fellowship. Lauren B. Alloy was supported by National Institute of Mental Health grants R01 MH101168 and R01 MH123473. We thank all researchers involved in the studies reviewed as well as Erin Dunning forher contributions to early stages of this project. This study was pre-registered (PROSPERO study protocol: CRD42021253574; link: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=253574) and conducted in accordance with PRISMA 2020 guidelines for systematic reviews (Page et al., 2021).
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neubiorev.2023.105162.
Data availability
No data were used for the research described in the article.
References
- Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD, 2013. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation (psyh). Health Psychol 32 (7), 748–756. 10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Kubzansky LD, 2012. The association between childhood emotional functioning and adulthood inflammation is modified by early-life socioeconomic status. Health Psychol 31 (4), 413–422. 10.1037/a0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Gilman SE, Kubzansky LD, 2011. Emotional functioning at age 7 years is associated with c-reactive protein in middle adulthood. Psychosom. Med 73 (4), 295–303. 10.1097/PSY.0b013e31821534f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty L, Koczwara B, Wade T, 2016. Evaluating the efficacy of a self-guided Web-based CBT intervention for reducing cancer-distress: a randomised controlled trial. Support. Care Cancer 24 (3), 1043–1051. 10.1007/s00520-015-2867-6. [DOI] [PubMed] [Google Scholar]
- Black DS, Slavich GM, 2016. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci 1373 (1), 13–24. 10.1111/nyas.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF, 2006. The perseverative cognition hypothesis: a review of worry prolonged stress-related physiological activation and health. J. Psychosom. Res 60 (2), 113–124. 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2004. Cytokines and psychopathology: lessons from interferon-α. Biol. Psychiatry 56 (11), 819–824. 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL, 2011. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav. Immun 25 (2), 232–238. 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay AL, Wrosch C, Sabiston CM, 2014. Systemic inflammation among breast cancer survivors: the roles of goal disengagement capacities and health-related self-protection. Psycho-Oncol 23 (8), 878–885. 10.1002/pon.3489. [DOI] [PubMed] [Google Scholar]
- Chen E, McLean KC, Miller GE, 2015. Shift-and-persist strategies: associations with socioeconomic status and the regulation of inflammation among adolescents and their parents. Psychosom. Med 77 (4), 371–382. 10.1097/PSY.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier ADF, Wayment HA, Birkett M, 2016. Impact of making textile handcrafts on mood enhancement and inflammatory immune changes. Art. Ther 33 (4), 178–185. 10.1080/07421656.2016.1226647. [DOI] [Google Scholar]
- Crosswell AD, Sagui-Henson S, Prather AA, Coccia M, Irwin MR, Epel ES, 2022. Psychological resources and biomarkers of health in the context of chronic parenting stress. International Journal of Behavioral Medicine 29 (2), 175–187. 10.1007/s12529-021-10007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Hussong AM, 2009. Integrative data analysis: the simultaneous analysis of multiple data sets. Psychol. Methods 14 (2), 81–100. 10.1037/a0015914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel KE, Moulder RG, Teachman BA, Boker SM, 2022. Stability and spread: a novel method for quantifying transitions within multivariate binary time series data. Behav. Res. Methods 10.3758/s13428-022-01942-0. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9 (1), 46–56. 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargél AA, Godin O, Etain B, Hirakata V, Azorin J-M, M’Bailara K, Bellivier F, Bougerol T, Kahn J-P, Passerieux C, Aubin V, Courtet P, Leboyer M, Henry C, 2017. Emotional reactivity functioning and C-reactive protein alterations in remitted bipolar patients: clinical relevance of a dimensional approach. Aust. N. Z. J. Psychiatry 51 (8), 788–798. 10.1177/0004867417691850. [DOI] [PubMed] [Google Scholar]
- Dargél AA, Volant S, Brietzke E, Etain B, Olié E, Azorin J, Gard S, Bellivier F, Bougerol T, Kahn J, Roux P, Aubin V, Courtet P, Leboyer M, Henry C, 2020. Allostatic load emotional hyper-reactivity and functioning in individuals with bipolar disorder. Bipolar Disord 10.1111/bdi.12927. [DOI] [PubMed] [Google Scholar]
- Denollet J, Conraads VM, Brutsaert DL, De Clerck LS, Stevens WJ, Vrints CJ, 2003. Cytokines and immune activation in systolic heart failure: the role of Type D personality. Brain Behav. Immun 17 (4), 304–309. 10.1016/S0889-1591(03)00060-6. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME, 2009. Psychobiological responses to social self threat: functional or detrimental? (psyh). Self Identit 8 (2–3), 270–285. 10.1080/15298860802505186. [DOI] [Google Scholar]
- Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE, 2018. The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neurosci. Biobehav. Rev 94 (March), 219–237. 10.1016/j.neubiorev.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek WE, Karlsson T, Höglund J, Rask-Andersen M, Johansson Å, 2021. Causal effects of inflammatory protein biomarkers on inflammatory diseases. eabl4359 Sci. Adv 7 (50). 10.1126/sciadv.abl4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English T, Lee IA, John OP, Gross JJ, 2017. Emotion regulation strategy selection in daily life: the role of social context and goals. Motiv. Emot 41 (2), 230–242. 10.1007/s11031-016-9597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford BQ, Troy AS, 2019. Reappraisal reconsidered: a closer look at the costs of an acclaimed emotion-regulation strategy. Curr. Dir. Psychol. Sci 28 (2), 195–203. 10.1177/0963721419827526. [DOI] [Google Scholar]
- Gierlotka Z, Sobiś J, Misiołek H, Kunert Ł, Misiołek A, Gorczyca PW, 2015. Relationships between various temperament dimensions levels of selected cytokines and posttraumatic stress disorder (ptsd) in males incurred as a result of mechanical injuries of lower extremities. Psychiatr. Pol 49 (4), 697–708. 10.12740/PP/26614. [DOI] [PubMed] [Google Scholar]
- Graham JE, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK, 2009. Cognitive word use during marital conflict and increases in proinflammatory cytokines (psyh). Health Psychol 28 (5), 621–630. 10.1037/a0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladek M, Gill J, Lai C, Lorig K, Szanton S, 2020. High Coping self-efficacy associated with lower sweat inflammatory cytokines in adults: a pilot study. Biol. Res. Nurs 22 (1), 75–81. 10.1177/1099800419870607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Stanton AL, Bower JE, Thomas KS, Litwin MS, Breen EC, Irwin MR, 2013. Inflammatory biomarkers and emotional approach coping in men with prostate cancer. Brain Behav. Immun 32, 173–179. 10.1016/j.bbi.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvelä-Reijonen E, Puttonen S, Karhunen L, Sairanen E, Laitinen J, Kolehmainen M, Pihlajamäki J, Kujala UM, Korpela R, Ermes M, Lappalainen R, Kolehmainen M, 2020. The effects of Acceptance and Commitment Therapy (ACT) intervention on inflammation and stress biomarkers: A randomized controlled trial. International Journal of Behavioral Medicine 10.1007/s12529-020-09891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Marsland AL, Gianaros PJ, 2022. Do trait-level emotion regulation strategies moderate associations between retrospective reports of childhood trauma and prospective changes in systemic inflammation? smi.3205 Stress Health 10.1002/smi.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Lam PH, Hoffer LC, Chen E, Schreier HMC, 2018. Chronic family stress and adolescent health: the moderating role of emotion regulation. Psychosom. Med 80 (8), 764–773. 10.1097/PSY.0000000000000624. [DOI] [PubMed] [Google Scholar]
- Khan AJ, O’Donovan A, Neylan TC, Gross JJ, Cohen BE, 2020. Suppression but not reappraisal is associated with inflammation in trauma-exposed veterans. Psychoneuroendocrinology 122. 10.1016/j.psyneuen.2020.104871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EL, Majd M, Graham-Engeland JE, Smyth JM, Sliwinski MJ, Engeland CG, 2022. Depressive symptoms and other negative psychological states relate to ex vivo inflammatory responses differently for men and women: cross-sectional and longitudinal evidence. Physiol. Behav 244. 10.1016/j.physbeh.2021.113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dooley LN, Boyle CC, Haydon MD, Bower JE, 2018. Within-subject associations between inflammation and features of depression: using the flu vaccine as a mild inflammatory stimulus. Brain Behav. Immun 69, 540–547. 10.1016/j.bbi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Brown RL, Wu E-LL, Murdock KW, Denny BT, Heijnen C, Fagundes C, 2020. Emotion regulation and immune functioning during grief: testing the role of expressive suppression and cognitive reappraisal in inflammation among recently bereaved spouses. Psychosom. Med 82 (1), 2–9. 10.1097/PSY.0000000000000755. [DOI] [PubMed] [Google Scholar]
- Low CA, Matthews KA, Hall M, 2013. Elevated c-reactive protein in adolescents: roles of stress and coping. Psychosom. Med 75 (5), 449–452. 10.1097/PSY.0b013e31828d3f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Fang S, 2019. Adolescents’ mindfulness and psychological distress: the mediating role of emotion regulation. Front. Psychol 10, 1358. 10.3389/fpsyg.2019.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master SL, Amodio DM, Stanton AL, Yee CM, Hilmert CJ, Taylor SE, 2009. Neurobiological correlates of coping through emotional approach. Brain Behav. Immun 23 (1), 27–35. 10.1016/j.bbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T, 2016. Inflammation in fear- and anxiety-based disorders: ptsd gad and beyond. Neuropsychopharmacology 42 (April), 1–53. 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Lumeng CN, Delproposto J, Florek B, Wendorf K, Lumeng JC, 2013. Obesity-related hormones in low-income preschool-age children: implications for school readiness. Mind Brain Educ 7 (4), 246–255. 10.1111/mbe.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Christian LM, 2019. Repetitive negative thinking, meaning in life, and serum cytokine levels in pregnant women: Varying associations by socioeconomic status. J. Behav. Med 42 (5), 960–972. 10.1007/s10865-019-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, 2021. Building a replicable and clinically-impactful immunopsychiatry: methods phenotyping and theory integration. Brain Behav. Immun. Health 16. 10.1016/j.bbih.2021.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Alloy LB, 2021. Back to basics: the importance of measurement properties in biological psychiatry. Neurosci. Biobehav. Rev 123, 72–82. 10.1016/j.neubiorev.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Slavich GM, Alloy LB, Olino TM, 2022. Hierarchical inflammatory phenotypes of depression: a novel approach across five independent samples and 27,730 adults. S0006322322015268 Biol. Psychiatry 10.1016/j.biopsych.2022.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, McArthur BA, Ellman LM, Coe CL, Abramson LY, Alloy LB, 2018. Immunocognitive model of depression secondary to anxiety in adolescents. J. Youth Adolesc 47 (12), 2625–2636. 10.1007/s10964-018-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Ng T, Titone MK, Chat IK, Nusslock R, Miller GE, Alloy LB, 2020a. Reward responsiveness and ruminative styles interact to predict inflammation and mood symptomatology. Behav. Ther 51 (5), 829–842. 10.1016/j.beth.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Kautz MM, Mac Giollabhui N, Klugman J, Coe CL, Ellman LM, Abramson LY, Alloy LB, 2020. Bidirectional associations between inflammatory biomarkers and depressive symptoms in adolescents: potential causal relationships. Clin. Psychol. Sci 8 (4), 690–703. 10.1017/CBO9781107415324.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Ng T, Curley E, Mcarthur BA, Ellman LM, Coe CL, Abramson LY, Alloy LB, 2020b. Reward sensitivity cognitive response style and inflammatory response to an acute stressor in adolescents. J. Youth Adolesc 49 (10), 2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Lyle KB, Szabo YZ, Miller JJ, Warnecke AJ, 2017. Salivary cytokine response in the aftermath of stress: an emotion regulation perspective (psyh). Emotion 17 (6), 1007–1020. 10.1037/emo0000156. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D, 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed 372, n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F, 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation 107 (3), 499–511. 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Powers A, Michopoulos V, Conneely K, Gluck R, Dixon H, Wilson J, Jovanovic T, Pace TWW, Umpierrez GE, Ressler KJ, Bradley B, Gillespie CF, 2016. Emotion dysregulation and inflammation in african-american women with type 2 diabetes. Neural Plast 2016, 8926840 10.1155/2016/8926840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Koch AE, Campbell PL, McInnis MG, Zalcman SS, Zubieta J-K, 2011. Association of plasma interleukin-18 levels with emotion regulation and μ-opioid neurotransmitter function in major depression and healthy volunteers. Biol. Psychiatry 69 (8), 808–812. 10.1016/j.biopsych.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Renna ME, 2021. A review and novel theoretical model of how negative emotions influence inflammation: the critical role of emotion regulation. Brain Behav. Immun. Health 18, 100397. 10.1016/j.bbih.2021.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C, 2021. Inflammation anxiety and stress in bipolar disorder and borderline personality disorder: a narrative review. Neurosci. Biobehav. Rev 127, 184–192. 10.1016/j.neubiorev.2021.04.017. [DOI] [PubMed] [Google Scholar]
- Sattar N, McCarey DW, Capell H, McInnes IB, 2003. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108 (24), 2957–2963. 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Reed RG, Scott AB, 2017. Intelligence and interleukin-6 in older adults: the role of repetitive thought. Psychosom. Med 79 (7), 757–762. 10.1097/PSY.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, Slavich GM, 2020. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 77 (10), 1031–1043. 10.1001/jamapsychiatry.2020.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanoe C, Hara M, Nishida Y, Nanri H, Otsuka Y, Horita M, Yasukata J, Miyoshi N, Yamada Y, Higaki Y, Tanaka K, 2018. Coping strategy and social support modify the association between perceived stress and C-reactive protein: a longitudinal study of healthy men and women. Stress. Int. J. Biol. Stress 21 (3), 237–246. 10.1080/10253890.2018.1435638. [DOI] [PubMed] [Google Scholar]
- Shimanoe C, Otsuka Y, Hara M, Nanri H, Nishida Y, Nakamura K, Higaki Y, Imaizumi T, Taguchi N, Sakamoto T, Horita M, Shinchi K, Tanaka K, 2014. Gender-specific associations of perceived stress and coping strategies with C-reactive protein in middle-aged and older men and women. Int. J. Behav. Med 21 (5), 821–832. 10.1007/s12529-013-9341-y. [DOI] [PubMed] [Google Scholar]
- Slavich GM, 2020. Social safety theory: a biologically based evolutionary perspective on life stress health and behavior. Annu. Rev. Clin. Psychol 16, 265–295. 10.1146/annurev-clinpsy-032816-045159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, 2022. Social safety theory: understanding social stress disease risk resilience and behavior during the covid-19 pandemic and beyond. Curr. Opin. Psychol 45, 101299 10.1016/j.copsyc.2022.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Giletta M, Helms SW, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ, 2020. Interpersonal life stress inflammation and depression in adolescence: testing social signal transduction theory of depression. Depress. Anxiety 37 (2), 179–193. 10.1002/da.22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Roos LG, Mengelkoch S, Webb CA, Shattuck EC, Moriarity DP, & Alley JC (2023). Social Safety Theory: Conceptual foundation, underlying mechanisms, and future directions. Health psychology review, 17(1), 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotola LK, 2022. Garbage in, garbage out? evaluating the evidentiary value of published meta-analyses using z-curve analysis. Collabra Psychol 8 (1), 32571. 10.1525/collabra.32571. [DOI] [Google Scholar]
- Szabo YZ, Burns CM, Lantrip C, 2022. Understanding associations between rumination and inflammation: a scoping review. Neurosci. Biobehav. Rev 135, 104523 10.1016/j.neubiorev.2022.104523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Middendorp H, Geenen R, Sorbi MJ, van Doornen LJP, Bijlsma JWJ, 2005. Neuroendocrine-immune relationships between emotion regulation and health in patients with rheumatoid arthritis. Rheumatology 44 (7), 907–911. 10.1093/rheumatology/keh626. [DOI] [PubMed] [Google Scholar]
- Vater A, Schröder–Abé M, 2015. Explaining the Link between personality and relationship satisfaction: emotion regulation and interpersonal behaviour in conflict discussions. Eur. J. Personal 29 (2), 201–215. 10.1002/per.1993. [DOI] [Google Scholar]
- Woody A, Figueroa WS, Benencia F, Zoccola PM, 2016. Trait reflection predicts interleukin-6 response to a social-evaluative stressor. Brain Behav. Immun 52, 27–31. 10.1016/j.bbi.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Yang LL, Stiernborg M, Skott E, Söderström Å, Giacobini M, Lavebratt C, 2020. Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur. Neuropsychopharmacol 41, 118–131. 10.1016/j.euroneuro.2020.10.005. [DOI] [PubMed] [Google Scholar]
- Ysseldyk R, McQuaid RJ, McInnis OA, Anisman H, Matheson K, 2018. The ties that bind: ingroup ties are linked with diminished inflammatory immune responses and fewer mental health symptoms through less rumination. PloS One 13 (4), e0195237. 10.1371/journal.pone.0195237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola PM, Figueroa WS, Rabideau EM, Woody A, 2014. Differential effects of post-stressor rumination and distraction on C-reactive protein in healthy women. Health Psychol 33 (12), 1606–1609. 10.1037/hea0000019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were used for the research described in the article.


