Abstract
Gastroduodenal diseases have prevailed for a long time and more so due to dominance of gut bacteria Helicobacter pylori in most of the cases. But habitation by other gut microbiota in gastroduodenal diseases and the relationship between Helicobacter pylori and gastrointestinal microbiota in different gastroduodenal diseases is somewhat being unravelled in the current times. For this systematic review, we did a literature search of various gastroduodenal diseases and the effect on gut microbiota pertaining to it. A search of the online bibliographic databases PUBMED and PUBMED CENTRAL was carried out to identify articles published between 1977 and May 2022. The analysis of these selected studies highlighted the inhabitation of other gut microbiota such as Fusobacteria, Bacteroidetes, Streptococcaceae, Prevotellaceae, Fusobacteriaceae, and many others. Interplay between these microbiota and H. pylori have also been noted which suggested that gastroduodenal diseases and gut microbiota are intertwined by a symbiotic association regardless of the H. pylori status. The relationship between the gut microbiota and many gastroduodenal diseases, such as gastritis, gastric cancer, lymphomas, and ulcers, demonstrates the dysbiosis of the gut microbiota in both the presence and absence of H. pylori. The evolving ways for eliminating H. pylori are provided along with inhibiting qualities of other species on H. pylori. Most significant member of our gut system is Helicobacter pylori which has been associated with numerous diseases like gastric cancer, gastritis, duodenal ulcer.
Keywords: Gastroduodenal diseases, Helicobacter pylori, Gastric cancer, Duodenal ulcer, Gastritis, Prevotella, Gut microbiota
Introduction
Gastroduodenal diseases involving the stomach and duodenum have been responsible for a high disease burden in humans for thousands of years (Baron et al. 2002; Milivojevic et al. 2020). The most common diseases are gastritis (inflammation of the lining of the stomach), duodenal ulcer (stomach acid damages the lining of the digestive tract), duodenitis (inflammation of the duodenum), gastric cancer (malignant cells colonizing the lining of the stomach) etc. (Sipponen and Maaroos. 2015; Sitarz et al. 2018; Ahmed and Belayneh. 2019; Jenkins et al. 1985). Other common gastroduodenal disorders include functional dyspepsia, rumination syndrome, and belching disorders (Stanghellini et al. 2016). The most common symptoms are nausea, vomiting, chest discomfort, reflux symptoms, heartburn etc. (Tack et al. 2006). The pathogenesis of these gastroduodenal disorders is largely based on Helicobacter pylori (H. pylori), the most common disease-causing bacteria, as well as other factors such as the use of non-steroidal anti-inflammatory medicines (NSAIDs) (Murakami et al. 2009).
Numerous microbiomes form a symbiotic association with the host organism by residing in the gastrointestinal tract of humans and play an important role in metabolic, neuroendocrine, and immune systems (Chen et al. 2021). The intestinal microbiota is composed mainly of Gram-negative anaerobic bacilli that live in the gut microenvironment with the predominant genus(s) like Fusobacterium and Bacteroides (Benavides‑Ward et al. 2018) as well as Firmicutes, Actinobacteria, Proteobacteria and TM7 (Pei et al. 2004). Streptococcus, Haemophilus, Neisseria, Prevotella, and Veillonella are the fundamental microbes (Park et al. 2020). A study reported that Enterococcus, Streptococcus, Staphylococcus, Pseudomonas, and Stomatococcus are the most abundant genera (Monstein et al. 2000). There is an influence of external factors like geographical location on gut microbiota since it has been observed that inhabiting location has positive as well as negative association between Firmicutes and Bacteroidetes respectively (Suzuki and Worobey. 2014). H. pylori is a micro-aerophilic, spiral, gram-negative bacteria and is prevalent in peptic ulcer disease, gastric cancer (GC), and chronic atrophic gastritis (Prasad et al. 1994). It is one of the most studied and observed bacteria due to the fact that it manifests in a number of important diseases (Chen et al. 2021). Although most infections are asymptomatic, infected individuals (approximately 10–15%) develop inflammation, which leads to diseases such as gastric mucosa-associated lymphoid tissue lymphoma (MALT), gastritis, gastric carcinoma and it may increase the severity of infection that is caused by other gastroduodenal pathogens such as Vibrio cholera. 65–70% of the population is infected with H. pylori in India itself as a commensal and non-pathogenic mode (Ganguly et al. 2016). A study concluded that the proportion of H. pylori in the mucosa of patients is lower in duodenal ulcers and the diversity of the bacterial community is higher in the same disease (Chen et al. 2018a, b). So, it’s clear that mere colonization of gastric mucosa by H. pylori may not be the sole reason for causing gastroduodenal diseases and other factors may be involved. There have been many studies that indicate that the presence, as well as the absence of H. pylori, has an effect on the gut microbiota (Yang et al. 2019). H. pylori infection causes gastric microbial dysbiosis that may play a crucial role in carcinogenesis. Successful eradication of H. pylori restores gastric microbiota to a similar status as found in uninfected individuals and shows beneficial effects on gut microbiota (Guo et al. 2020). H. pylori and other non-H. pylori microbial species may coexist in the stomach resulting in potentially harmful pathological conditions. Therefore, there may be associations between these bacteria and gastroduodenal diseases, including peptic ulcer and stomach cancer (Abenavoli et al. 2021). This review paper demonstrates the association between H. pylori and gut microbiome in different gastroduodenal diseases and how gut microbiota is possibly affected either by the presence or absence of H. pylori in various gastroduodenal diseases.
Methodology
Search method and platforms
A search of the online bibliographic databases PUBMED and PUBMED CENTRAL was carried out to identify articles published between 1977 and May 2021. The terms/keywords used were gastric, stomach, gastrointestinal tract, gastric microbiota, microbiota, gastric cancer, atrophic gastritis, H. pylori, PPI, functional dyspepsia, non-ulcer dyspepsia, gastroesophageal reflux diseases, duodenitis, duodenal ulcer, nausea, vomiting. Synonyms and word variations were combined using the and/or function. In addition, we performed a full manual search of all review articles and published editorials and retrieved original studies. Manual searches of the reference list from the potentially relevant studies were performed to identify additional studies that may have been missed using the computer-assisted search strategy. Consensus statements were reached through discussion.
Inclusion criteria
Studies were included if they reported microbiota analysis on gastric fluid/aspirate using high-throughput sequencing such as 16S rRNA, etc. and provided information on the presence or abundance of microbial taxa (Fig. 1).
Fig. 1.
Flowchart depicting selection of studies. The studies were included from PUBMED and PubMed Central database using appropriate inclusion and exclusion criterias. 496 papers/articles were retrieved from which 138 studies were included
Exclusion criteria
Studies were excluded if they did not discuss patterns of individual bacterial taxa differences. Repetitive data were also excluded.
Results
Interaction between gut microbiota and gastroduodenal diseases
Gut microbiota refers to a group of mutualistic and pathogenic microorganisms that live in the digestive tract and play an important role in immunomodulation and defence against pathogens (Ubhayawardana et al. 2021). As a result, gastric microbes play a vital role to maintain gut equilibrium and functions (Belkaid and Hand. 2014). Dietary status, antibiotics use, infection in gastric mucosa, H. pylori colonization, immune system, and excess of hygienic lifestyle, all influence the composition of the gut microbiota and can cause dysbiosis (Ley et al. 2006; Kau et al. 2011). More than 80% of microorganisms are unculturable and therefore culture-based approaches provide a deficient and biased view of the richness of the gut microbiota (Wroblewski et al. 2016). Modern techniques particularly those based on high-throughput or next-generation sequencing technology, has transformed our knowledge of the gut microbiota. Bik et al. used modern molecular techniques to analyse microbiota from gut samples (genome sequencing using 16S rDNA clone library methodology) and found five major phyla: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Fusobacteria which confirmed the culture-based findings (Bik et al. 2006). The interaction between gut microbiota and various gastroduodenal diseases like ulcers, gastritis, gastric malignancy and lymphomas are described below.
Peptic ulcer
The most prevalent side effect of chronic H. pylori infection is peptic ulcer disease, accounting for 95% of duodenal ulcers (DU) and 70% of stomach ulcers (Ford et al. 2006). Infection with H. pylori is a major cause of peptic ulcers, contributing to more than 80% of cases (Fagoonee et al. 2019; Alam et al. 2014). According to prior research, ulcers cause changes in the gastrointestinal microbiota, including changes in aerobic and anaerobic bacterial species, fungal isolation, and culture (Li et al. 1995; Zhang et al. 1996). Several research has also looked into the involvement of non-H. pylori species in peptic ulcer disease (Da Cunha and Dharan. 2021; Devi et al. 2021). In a Malaysian investigation, patients with peptic ulcers and Streptococcus isolates were shown to have a strong connection (Khosravi et al. 2014).
Gastritis
Gastritis is described as an inflammation of the gastric mucosal lining (Rugge et al. 2011). One of the main types of this disease is chronic gastritis which is basically the chronic inflammation of the gastric mucosa and further has a subtype called atrophic chronic gastritis (Cheli et al. 1995; Sipponen and Maaroos. 2015). Chronic atrophic gastritis is caused by the invasion of H. pylori (Sipponen and Maaroos. 2015), which triggers a provocative reaction that affects gastric epithelial cells causing the breakdown of the gastrointestinal permeability (Gajewski et al. 2016), and cell death (Wan et al. 2016). Streptococcaceae, Helicobacteraceae, Prevotellaceae, and Fusobacteraceae were to be the main taxa used to alter the stomach microbial community in patients with chronic atrophic gastritis (Parsons et al. 2017). Additionally, Treponema, Tannerella, and Prevotella spp. were shown with a lower abundance rate in atrophic gastritis patients compared to healthy controls (Zhang et al. 2019).
Atrophic gastritis
Atrophic gastritis is one of the most common life-long, serious and menacing illnesses in human beings and is a well-established precursor of intestinal-type Gastric cancer (Wang and Chen. 2020). A study reported that atrophic gastritis was prevalent in 33.4% of the general population and 31.6% in selected clinical situations (Marques-Silva et al. 2014). A study found that atrophic gastritis samples were dominated by Proteobacteria (62%) (Helicobacter being a member of this phylum), followed by Streptococcaceae (5%), Fusobacteriaceae (2%) and Prevotellaceae (2%) (Parsons et al. 2017). At the genus level, several differences were observed between normal and patients with H. pylori induced atrophic gastritis causing a decrease in the proportions of Tannerella (E. coli/Shigella/Salmonella), Treponema, and Prevotella. They also found that co-occurrence networks were more complicated in H. pylori-induced atrophic gastritis patients compared to those subjects who had H. pylori-induced gastritis. Clear negative relationships were observed between Helicobacter and genera such as Streptococcus, whilst Campylobacter, Prevotella, Haemophilus and Veillonella were amongst the most well-connected and influential bacteria observed in the stomachs of H. pylori atrophic gastritis patients (Parsons et al. 2017).
Gastric cancer
Gastric cancer is a foremost health problem across the world and has a high mortality rate after lung cancer (Ferlay et al 2010). There are multiple reasons behind the prevalence of GC such as infection by H. pylori as well as environmental factors like tobacco and smoking (Correa. 2013). Dysbiosis is characterized by changes in the microbiome compositions and functions (Levy et al. 2017). According to several research, microbial diversity is significantly decreased in inflammatory disorders and cancer (Ahn et al. 2013; Aviles-Jimenez et al. 2014; Gong et al. 2016). In many investigations, however, the abundance and variety of the stomach microbiome in GC tissues were found to be higher than in control tissues (Castaño-Rodríguez et al. 2017; Chen et al. 2019). Changes in the gastric microbiome in the early stage of gastric cancer featured adaptive functional and compositional changes and a simplified network. Host genetic backgrounds, H. pylori virulence and environmental changes may cause alterations of the gastric microbiome during the development and progression of gastric cancer. The microbial signature identified could serve as biomarkers for clinical assessment of gastric cancer risk in high-risk patients (Wang et al. 2020). The early stages of GC were enriched by Pseudoxanthomonas, Ochrobactrum, Novosphingobium, Anoxybacillus, and Ralstonia while Uruburuella, Burkholderia, Salinivibrio and Tsukamurella were present in high abundance in advance stages of GC (Wang et al. 2020). A new report gave knowledge about the microbial composition in various subtypes of GC which shows that Signet-ring cell carcinoma was abundant with Patescibacteria, Fusobacteria, Bacteroidetes, and adenocarcinoma was found to be enriched in Proteobacteria and Acidobacteria (Ravegnini et al. 2020). The frequency of pathogenic bacteria groups like Streptococcus, Peptostreptococcus, and Prevotella increased in patients having GC, but the number of Bifidobacterium decreased (Barra et al. 2021). Lactic acid-producing bacteria are also involved in the development of stomach cancer (Vinasco et al. 2019). A study from Taiwan found that Clostridium, Fusobacterium, and Lactobacillus species were frequently abundant in patients with gastric cancer, demonstrating a gastric cancer-specific bacterial signature. Their findings further showed that Clostridium colicanis and Fusobacterium nucleatum exhibited a diagnostic ability for gastric cancer (Hsieh et al. 2018). The interaction between microbial community regulates the equilibrium of the gut microbiome which also affect the illness related to the microenvironment. The lower abundance rate of H. pylori in tumoral sites resulted in a simplified network of bacterial interactions in the gastric microbiota. H. pylori, Prevotella copri and Bacteroides uniformis were significantly decreased, whereas Prevotella melaninogenica, Streptococcus anginosus and Propionibacterium acnes were increased in tumoral microhabitat (Liu et al. 2019).
MALT lymphoma
MALT lymphoma is a reduced malignant lymphoma which develops in the mucosa-associated lymphoid tissue (MALT) of regional lymph nodes organs like the gut, lungs, thyroid, salivary glands, skin, and liver (Tanaka et al. 2021). H. pylori is a known causative organism of this condition, found in around 90% of affected patients (Zullo et al. 2010). In a cross-sectional study, Burkholderia and Sphingomonas were found to be more frequent in MALT lymphoma patients compared to controls. While Prevotella and Veillonella were observed to be less common in H. pylori-negative MALT lymphoma individuals (Tanaka et al. 2021). Burkholderia can be considered as an opportunistic pathogen (Burns et al. 1996). H. pylori-negative MALT lymphoma patients exhibited altered gastric mucosal microbial compositions, suggesting that altered microbiota might be involved in the pathogenesis of H. pylori-negative MALT lymphoma.
Alterations in gut microbiota due to H. pylori infection
Normal microbiota
The fact that the human gut is colonized by numerous bacteria is known since time immemorial (Kusters et al. 2006). Initially, these bacteria were assumed to be contaminants that arose due to digested food rather than gastric colonizers until Barry Marshall and Robert Warren successfully isolated Helicobacter pylori (Marshall and Warren 1984). In the past, culturing and isolation were used to identify gut microbiota like Veillonella, Lactobacillus, and Clostridium spp., but the drawback was that only a small number of microbes can be cultured, thereby indicating that a lot were undiscoverable (Zilberstein et al. 2007). At present, with new-age technologies like random shotgun sequencing, microarrays, whole genome sequencing, 16S mRNA based techniques and others, researchers have discovered multiple taxa that may be present in the gut (Yang et al. 2021). At the phylum level, Firmicutes (42%), Bacteroidetes (24%), Actinobacteria (7%), Proteobacteria (17%), Fusobacteria (6%), and Verrucomicrobia are present in our stomach out of which Firmicutes and Bacteroidetes denote approximately 90% of the microbiome (Arumugam et al. 2011; Ndegwa et al. 2020). Lactobacillus, Bacillus, Clostridium, Enterococcus, Ruminicoccus are some that are present which represent Firmicutes genera while Bacteroides and Prevotella also prevail in the gut, representing Bacteroidetes genera (Arumugam et al. 2011). Along with these, oral and duodenum bacteria like Veillonella, Lactobacillus and Clostridium are also known to colonize the gut (Nardone et al. 2017). Based on OTUs (Operation Taxonomic Unit) analysis the most abundant families are Streptococcaceae (23%), Prevotellaceae (23%), Veillonellaceae (7%), Bacillales incertae sedis XI (4%), and Lachnospiraceae (3%) and Streptococcus (23%), Prevotella (22%), Veillonella (6%), Fusobacterium (5%), Gemella (4%), Neisseria (4%), and Haemophilus (4%) which represent top 7 genera (Ndegwa et al 2020). The gut microbiome may differ with age, gender, ethnicity, geographical factors, etc. (Haro et al. 2016; Lloyd et al. 2016; Yang et al. 2016). Fortunately, the risk of inflammation and over-proliferation by these bacteria is prevented by the acidic surroundings of the stomach (Howden and Hunt. 1987). But sometimes, these dynamics of the microbiome can be altered due to environmental factors and dietary factors, or aggravations of bacteria that are present (Nitert et al. 2020; Hasan et al. 2019).
Gut microbiota with H. pylori infection
A metagenomic shotgun sequencing study revealed alterations in gastric microbial taxa and function associated with H. pylori infection in the Chinese population which provided an insight into gastric microbial interactions and their potential role in the pathological process of gastric diseases (Wang et al. 2022). Another analysis of the mucosal lining of healthy individuals confirmed that Proteobacteria and Firmicutes were predominantly present followed by Actinobacteria, while Gemmatimonadetes, Bacteroidetes, and Deinococcus-Thermus were present in minute numbers (approximately < 3%) (Delgado et al. 2013). A 16S rRNA cloning and sequencing suggested that amongst Firmicutes there was a higher abundance of Streptococcus, Lactobacillus, Veillonella, Prevotella and amongst Proteobacteria- Epsilonproteobacteria, Neisseria, Haemophilus were present in higher amounts (Dicksved et al. 2009). H. pylori is found in the gastric surroundings of the stomach in various human populations across the world (Alzahrani et al. 2014). It has been suggested that factors like ethnicity and conditions of living may influence the abundance of H. pylori (Alberts et al. 2020; Laszewicz et al. 2014). There is a high prevalence of H. pylori infection in the Chinese population where the rate is as high as 40–60% which is why early diagnosis, as well as treatment, is important (Yang et al. 2019; Malfertheiner et al. 2017). A similar infection was also seen in adults and children (at a rate of 76.1% and 66.3% respectively) in Elazig which is situated in Turkey (Ozbey, et al. 2015). Even though more than 50% of the world’s population is affected by this infection, only a small portion of patients develop severe diseases like ulcers and stomach cancers, in a range of 11% -15% and less than 1% respectively (Martel et al. 2012; Mladenova et al. 2021). Furthermore, there are geographical factors which also influence H. pylori prevalence as there is a west- east divide: USA/Europe having up to 5–10% while Asian regions like India/China have 50–70% (Hunt et al. 2011).
In humans, the gut microbiota forms an intricate and active ecosystem and it is suggested that H. pylori infection may affect the same microbiome (Jo et al. 2016; Schulz et al. 2018). Usually, when H. pylori is present, it is the commonest organism in the gut in approximately 45–90% (Bik et al. 2006). It was further studied that if H. pylori is found in gut microbiota, it gains dominance which can alter the composition of microbiota in patients (Vasapolli et al. 2019; Llorca et al. 2017). At the genera level, Firmicutes, Actinobacteria, Proteobacteria, Bacteroides, TM7, Fusobacteria, Tenericutes, Spirochetes, and in the same study, Prevotella, Hemophilus, Veillonella, Fusobacterium, Streptococcus, and Neisseria were isolated via the culture-dependent method (Stojanovic et al. 2020). After extensive study, it was noticed that the same phyla as mentioned above were present in individuals who were H. pylori negative as well as positive with the only difference being in their abundance rate and it was reported that there was a higher abundance of Spirochetes and Acidobacteria (Maldonado-Contreras et al. 2011). H. pylori ( +) patients had a higher richness of Proteobacteria while a lower prevalence of Firmicutes, Actinobacteria, and Bacteroidetes (Bik et al. 2006). One of the general characteristics of the gut microbiome is an Enterotype (Arumugam et al. 2011) and studies found that H. pylori ( +) patients had a higher abundance of Prevotella which was categorized under enterotype 1 and H. pylori (-) patients had a higher prevalence of Bacteroides which was regarded as enterotype 2 (Wang et al. 2019a, b).
Bacterial species like Streptococcus spp., Staphylococcus spp., Neisseria spp., Bacillus spp., Veillonella spp., Fusobacterium, Lactobacillus spp. as well as Bacteroides fragilis and Peptostreptococcus anaerobius were seen in patients having H. pylori infection with respective to gastroduodenal ulcer and dyspepsia (Kato et al. 2006). A study concerned with gastritis showed that there was a relative increase in the prevalence of Bacteroidaceae and Enterobacteriaceae in H. pylori ( +) and (-) groups if considered at family and genus level, whereas the abundance of Lachnospiraceae, Bifidobacteriaceae, and Lactobacillaceae was observed in healthy individuals which led to a conclusion that gastritis can lead to a change in gut microbiota under H. pylori infection (Yang et al. 2019) leading to an observation that H. pylori ( +) patients may have less microbiome richness in comparison with H. pylori (-) (Gantuya et al. 2019). Notably, the interaction of Lactobacillus with Streptococcus along with H. pylori led to inflammation in the gastric region which could aggravate carcinogenesis (Aviles-Jimenez et al. 2014). H. pylori also plays an important role in carcinogenesis since its dominance lowers when the tumour transforms from benign to malignant indicating that H. pylori may hold importance only till pre-malignant state (Barra et al. 2021; Coker et al. 2018). It is very well known that H. pylori prevail as a dominant bacterium in non-atrophic gastritis as well as in normal gastric mucosa (Hooi et al. 2017). In GC, when H. pylori is dominant, the other microbiome significantly decreases and vice versa.
A study found a lower abundance of Bifidobacterium in the gastric microbiome concerning patients infected by H. pylori having gastric ulcers (Devi et al. 2021). Howardella, Holdemanella, Parasutterella, Pseudoflavonifractor, Haemophilus and Allisonella, were also found to be abundant in H. pylori-infected cases and these taxa represented 32.6% of the whole microbiome in a comprehensive cohort. Parasutterella was found to be decreased in H. pylori-infected patients (Frost et al. 2019). Remarkably, patients with atrophic gastritis showed an abundance of Gemella and Bosea and alterations in the amount of Streptococcus, Campylobacter, and Haemophilus, and in another observation, there was a decrease in Actinobacillus and Tannerella when the gut was treated with Proton Pump Inhibitors (PPI) (Parsons et al. 2017). When 16S rDNA profiling was analyzed in endoscopic biopsies of 39 patients, it was found that along with Helicobacter, bacterial groups like Lactobacillus, Halomonas, and Prevotella were prevalent and along with these, a negative correlation was drawn between Helicobacter and various bacterial genera like Bradyrhizobium, Methylobacterium, Halomonas, Meiothermus, Aeromonas, Cloacibacterium, Acidovorax, Bacillus, and Ralstonia (Das et al. 2017). A usual change/alteration that occurs amongst the gut microbiota due to H. pylori infection has been depicted in Fig. 2; Table 1. So, it’s clearly highly possible that H. pylori infection can alter the gastric microbiome and this alteration can be observed in various gastroduodenal diseases which would be described in the upcoming section.
Fig. 2.
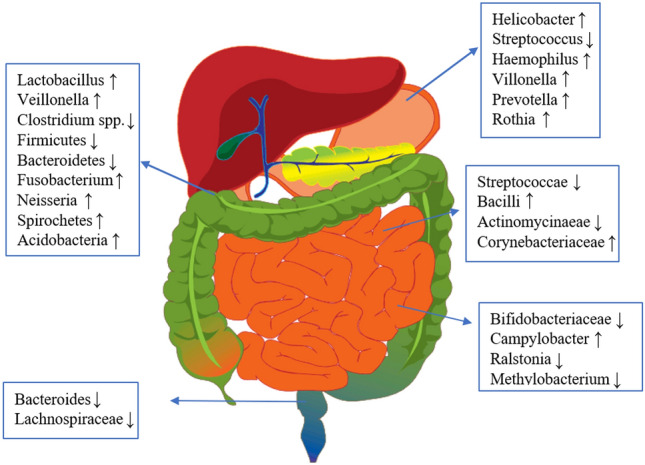
Various gut microbiota alterations due to infection by Helicobacter pylori. The “↑” arrow indicates the increase in abundance of bacterial species and the “↓” indicates the decrease in abundance of bacterial species
Table 1.
Summary of studies examining the relationships between gastric diseases and gut microbiota
| Disease conditions | Country | Sample size | Abundance of H. pylori in diseases | Microbial diversity | Taxon differences | References |
|---|---|---|---|---|---|---|
| NAG, IM, GC | Mexico | 5 NAG, 5 IM, 5 GC | ↓ | α-Diversity: NAG > IM > GC | ↑Lactobacillus, Lachnospiraceae from NAG, IM, to GC; ↓Saccaribacteria (TM7), Porphyromonas, Neisseria in GC | Aviles-Jimenez et al. 2014 |
| GC | Korea | 34 GC, 29 control | ↓ | No difference in α- and β-diversity | ↑Actinobacteria, Staphylococcus epidermidis in GC; ↑ nitrosating/nitrate‐reducing bacteria in GC (not statistically significant) | Jo et al. 2016 |
| Gastritis, IM, GC | Hong Kong | 8 Healthy control, 9 gastritis, 9 IM, 9 GC | ↑ |
↑ Shannon index in GC vs. gastritis ↓ phylogenetic diversity in GC vs. IM |
↑Flavobacterium, Klebsiella, Serratia marcescens, Stenotrophomonas, Achromobacter, Pseudomonas, Delftia, Ralstonia, Rhizobium, Elizabethkingia meningoseptica, Methyloversatiis, Gp4, Cytophagaceae in GC | Li et al. 2017 |
| GC, CG | China | 6 GC, 5 CG | ↓ | ↓Bacterial richness in GC, but not Shannon diversity index | ↑ Neisseria, Alloprevotella, Aggregatibacter, Streptococcus mitis and Porphyromonoas endodontalis in GC; ↓Sphingobium yanoikuyae in GC | Hu et al. 2012 |
| Gastritis, GC | Portugal | Discovery cohort: 81 gastritis, 54 GC | ↓ | ↓α-Diversity in GC | ↑Citrobacter, Clostridium, Lactobacillus, Achromobacter, and Rhodococcus in GC patients | Ferreira et al. 2018 |
| GC | China | 276 GC | ↓ | ↓α-Diversity in GC | ↓Prevotella copri and Bacteroides uniformis; ↑Prevotella melaninogenica, Streptococcus anginosus and Propionibacterium acnes | Liu et al. 2019 |
| GC | Korea | 288 GC, 288 control | ↑ | ↓α-Diversity in GC | ↑Prevotella copri and Propionibacterium acnes in GC; ↑ Lactococcus lactis in controls | Gunathilake et al. 2019 |
| GC, gastritis, atrophy, IM | Mongolia | 48 GC, 120 control (20 healthy, 20 gastritis, 40 atrophy, 40 IM) | ↓ | α-Diversity: normal > IM > GC > gastritis and atrophy | ↑Enterococcus, Lactobacillus, Carnobacterium, Glutamicibacter, Paeniglutamicibacter, Fusobacterium, and Parvimonas in GC | Gantuya et al. 2019 |
| Gastric ulcer, duodenal ulcer | China | Gastric ulcer, duodenal ulcer | ↑ | ↓α-Diversity in DU | ↑Helicobacter, Prevotella, Neisseria and Streptococcus | Chen et al. 2018a, b |
| Gastritis | Germany | Gastritis | ↑ | No significant difference | ↑Helicobacter, Streptococcus | Schulz et al. 2018 |
| Gastritis,IM, GC | China | Gastritis,IM, GC | ↓ | No significant difference | ↑Burkholderia, Enterobacter, and Leclercia, Clostridium, Fusobacterium in non-GC; ↑Lactobacillus in GC, C. colicanis and F. nucleatum represent diagnostic markers for GC | Hsieh et al. 2018 |
| Superficial gastritis, atrophic gastritis, IM, GC | China | 21 superficial gastritis, 23 atrophic gastritis, 17 IM, 20 GC | ↓ | ↓α-Diversity in GC and IM vs. SG | ↑oral flora, Peptostreptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua and Dialister pneumosintes in GC; ↓Vogesella, Comamonadaceae and Acinetobacter in GC | Coker et al. 2018 |
| Dyspepsia | Spain | Dyspepsia | ↓ | No significant difference | ↑Propionibacterium, Lactobacillus, Streptococcus and Staphylococcus | Delgado et al. 2013 |
| GC, dyspepsia | Sweden | 10 GC, 5 dyspepsia | ↓ | No significant difference | N/A | Dicksved et al. 2009 |
| GC, dyspepsia | Singapore and Malaysia | 12 GC, 20 dyspepsia | ↓ | ↑α-Diversity in GC | ↑Lactococcus, Veilonella, and Fusobacteriaceae (Fusobacterium and Leptotrichia) in GC | Castaño‐Rodríguez et al. 2017 |
| HP infection | India | HP infection suspected | ↑ | ↑α-Diversity | ↑Helicobacter,Lactobacillus, Halomonas and Prevotella | Das et al. 2017 |
| HP infection | China | HP infection suspected | ↑ | ↓α-Diversity | ↓Stenotrophomonas maltophilia, Stenotrophomonas unclassified, Variovorax unclassified, Chryseobacterium unclassified, Comamonas unclassified, and Pseudomonas stutzeri | Wang et al. 2022 |
GC gastric cancer, CG chronic gastritis, NAG non-atrophic gastritis, IM intestinal metaplasia, N/A not available. The “↑” arrow indicates the increase in H.pylori abundance/alpha diversity/ microbial diversity of the diseases and the “↓” indicates the decrease in H.pylori abundance/alpha diversity/ microbial diversity of the diseases
Gut microbiota without H. pylori infection
It is important to know whether there are microbiota apart from H. pylori in the upper gastro intestinal tract and if there are any studies that suggest the same microbiome may also lead to gastroduodenal diseases. At the phylum level, Firmicutes and Proteobacteria were found highly abundant whereas, Bacteroidetes, Actinobacteria and Fusobacteria were showing a low abundance rate. At the genus level Streptococcus, Prevotella, Neisseria, Haemophilus, and Porphyromonas, were commonly present in non-H. pylori sequences which were studied through 16S rRNA (Li et al. 2009). Consequently, it was found in a study that there was a noteworthy association between H. pylori ( +) status and increased abundance of non- H. pylori microbiota with Proteobacteria, Acidobacteria, and Spirochetes emerging as major microbiome among patients that were studied (Contreras et al. 2011). At the family level, comparative abundance was seen in Bradyhizobiaceae, Caulobacteraceae, Lactobacillaceae, and Burkholderiaceae in H.pylori (-) patients (Eun et al. 2014). Specifically in gastric cancer, there is a high prevalence of non- H. pylori bacteria like Klebsiella pneumoniae and Acinetobacter baumannii (Khosravi et al. 2014). Helicobacter species like H. cinaedi and H. mustelae were also observed in a 16S rRNA sequencing that was undertaken along with some other species like H. suis, H. heilmannii, H. felis, H. bizzozeronii, H. salomonis (Han et al. 2010; Margo Baele et al. 2009). Normally, in gastric cancer, there has been a gradual increase of Lactobacillus coleohominis, Klebsiella pneumoniae, or Acinetobacter baumannii and a decrease in Porphyromonas spp, Neisseria spp, Prevotella pallens, or Streptococcus sinensis (Jacome et al. 2016). In patients with hypochlorhydria, bacteria like Pseudomonas, Xanthomonas, Proteus, Klebsiella, Campylobacter jejuni were seen predominantly (Williams et al. 2006). The abundance of Achromobacter, Citrobacter, Phyllobacterium, and Rhodococcus was seen in patients with gastric cancer (Ferreira et al. 2018). In tumor tissues, at the species level, Propionibacterium acne, Streptococcus anginosus, and Prevotella melaninogenica were increased whereas H. pylori and Bacteroides uniformis were relatively decreased (Liu et al. 2019). Along with these, some bacteria are involved in the progression of gastric cancer like Peptostreptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua and Dialister pneumosintes (Ferreira et al. 2018). In some Iranian dyspeptic patients, H. salomonis had the highest prevalence (Shafaie et al. 2020). While other non- H. pylori bacteria that prevail in the gut are H. parahaemolyticus, H. parainfluenzae, and H. influenza, and V. rogasae, V. parvula, V. dispar, & V. atypia from Haemophilus and Veillonella spp., respectively (Miyata et al. 2019). We believe, that these bacteria are just the tip of the iceberg, and there is a whole lot that is left to unravel.
Role of Gut microbiota in the pathogenesis of Gastroduodenal diseases in the presence of H. pylori infection
Duodenal ulcer
Duodenal ulcer (DU) is one of the major gastroduodenal disease due to H. pylori infection. A study conducted by Wu et al. 2019, noticed that in samples with DU, the main phyla were Firmicutes, Bacteroidetes, and Proteobacteria at an abundance rate of 52.5%, 25.7%, and 15.7% respectively and all three together accounted for 93.9% of the total bacterial population. Further, they described that various phyla such as Actinobacteria, Gemmatimonadetes, Nitrospirae, Chlorobi, Thermi, WS3, and Caldithrix was found comparatively lower in patients with DU than in normal control groups. At genera level, Bacteroides (17.1%), Faecalibacterium (15.3%), Escherichia (7.8%), Ruminococcus (5.5%), Roseburia (4.7%), Prevotella (4.6%), Akkermansia (2.9%), Parabacteroides (2.4%), Bifidobacterium (2.0%), were observed being dominant and a unique increase was seen in Natronomonas genus which was present only in DU patients (Wu et al. 2019). Another study reported that genera like Lactobacillus, Bifidobacterium, Eubacterium, Bacteroides, and species such as Faecalibacterium prausnitzii, were found abundant in children suffering from DU (Kato et al. 2006). At the genus level, Neisseria, Streptococcus, Rothia, Staphylococcus were observed in higher abundance and Acinetobacter lwoffi, was an exclusive species that were detected among the samples (Hu et al. 2012). A study also found out that Bacteroidetes and Firmicutes covered the major proportion of DU patients with H. pylori ( +) status and remarkably, Prevotella (9.83%), Neisseria (5.03%), Streptococcus (3.96%), Veillonella (1.92%), Streptococcus (3.96%), and Porphyromonas (2.62%) were also present in the H. pylori ( +) samples. Additionally, at the genera level, Psychromonas, Frankia, Prevotella, and Porphyromonas were significantly increased in H. pylori ( +) patients (Chen et al. 2018a, b).
Gastritis
Gastritis is an inflammatory disorder of the stomach mucosa that manifests itself in a variety of ways in different people, depending on the host’s response. The colonisation of H. pylori in intestinal mucosa, causes changes in the gastric microbial community and disruptes the structure of the stomach microbiota (Maldonado-Contreras et al. 2011). H. pylori infection has also been linked to alterations in the large intestine flora (Lopetuso et al. 2014). A recent investigation on children found an interaction between modified intestinal microflora, H. pylori and gastritis. They showed that the changes in gut microbiota is due to changes in pH influenced by H. pylori presence and, causes compositional shift among native communities as a compensatory mechanism to translate unique functional genes that are involved in important metabolic pathways (Dash et al. 2021). The gut microbiota, which is altered by gastritis and H. pylori infection, was also found to alter the body’s baseline metabolic performance (Yang et al. 2019). The faecal microbiome was studied in H. pylori ( +) and (-) gastritis groups to assess the influence of H. pylori on the gut microbiome in children. Lactobacillales and Betaproteobacteria were observed with high abundance rate and Proteobacteria was found with less abundance among the H. pylori infected patients. At genus and family level, Streptococcus and Collinsella were showed high abundance rate in H. pylori positive relative to H. pylori negative group (Yang et al. 2019).
Gastric cancer
Gastric cancer (GC) is one of the major health problems across the world and numerous bacteria contribute to this disease. H. pylori can modify the gut environment, resulting in an alteration of the other residing microbiota such as in Bacteroidetes, Proteobacteria, and Firmicutes and the same alteration may further lead to GC (Mitchell et al 2017; Gao et al. 2018). At the phyla level, Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and Fusobacteria are the major bacteria in H. pylori ( +) patients and few Lactobacilli were found. The reason for this difference could be diversity in dietary behaviours or some methodological variations such as explicit experimental designs (Jo et al. 2016). It has been observed that Novosphingobium, Ralstonia, Ochrobactrum, Anoxybacillus, and Pseudoxanthomonas were seen thriving in the early stages of GC suggesting that they were active in bacteria communication and thus played central roles in the network (Wang et al. 2020). Contradicting this statement, another study found out that there were no noteworthy changes in the later as well early stages of GC in terms of gut microbiota, perhaps due to the differences in geographical areas as well as raw data that were taken (Liu et al. 2019). Patients with GC also showed an abundance in Achromobacter, Citrobacter, Clostridium, Rhodococcus, and Phyllobacterium than patients with gastritis and these bacteria exist in the intestinal mucosa as commensals but can be opportunistic pathogens (Ferreira et al. 2018). Gastric biopsies from a Colombian population revealed that major taxa abundant in gastric cancer cases were Leptotrichia wadei and Veillonella spp. from high risk region for development of gastric cancer, whereas Staphylococcus spp. were prevalent low risk regions (Hou et al. 2007). The bacterial taxa enriched in the cancer samples were predominantly represented by oral bacteria (such as Peptostreptococcus, Streptococcus, and Fusobacterium), while lactic acid-producing bacteria (such as Lactococcus lactis and Lactobacillus brevis) were more abundant in adjacent non-tumor tissues (Chen et al. 2019; Castano- Rodriguez et al. 2017). Interestingly, at the species level, it was detected that Prevotella melaninogenica, Streptococcus anginosus, and Propionibacterium acnes were increased that indicated their participation in GC tumorigenesis and P. acnes along with its products mainly short-chain fatty acids, activate a possible corpus-dominant lymphocytic gastritis and Bacteroides uniformis and Prevotella copri were reduced in GC when compared with normal (Liu et al. 2019). We know that Lactobacillus is an important bacterium that helps in dismissing different gastroduodenal disorders depending on the gender of the person as well as nutritional indicators (Salazar-Lindo et al. 2007). But along these lines, it has been mentioned that a major role in GC is played by Lactobacillus, Bifidobacterium, Streptococcus, and Lactococcus along with lactic-acid manufacturing bacteria-inducing mechanisms like the release of exogenous lactate which is a fuel source for cancer cells that promotes inflammation, angiogenesis, metastasis, immune evasion, epithelial-mesenchymal transition as well as production of reactive oxygen species (Vinasco et al. 2019). Species like Lactobacillus brevis and Lactococcus lactis were abundant in the neighbouring non-tumour area (Chen et al. 2019). A team of researchers studying gut microbiota profiles discovered that Propionibacterium acnes and Prevotella copri were the most abundant species which could aggravate GC (Gunathilake et al. 2019). However, in another study, P. acnes was abundant and P. copri was reduced in GC patients, suggesting that experimental analysis could differ on the basis of ethnicity and residential areas (Liu et al. 2019) which prompts us to think that additional studies are required to elucidate the role of P. copri in GC. As we know, symptoms of GC are not very well defined and this leads to patients being diagnosed with this disease when it has already reached an advanced stage (Luan et al. 2020). Ultimately, diagnosing and eradicating H. pylori is important to refrain from causing GC as well as other gastroduodenal diseases (Yang et al. 2021).
Effect of gut microbiota on H. pylori
While numerous articles have suggested that H. pylori affect the other gut microbiota, few studies suggest that the inhabiting gut microbiota also has an impact on H. pylori e.g. Bifidobacterium bifidum has been found active in in-vitro studies related to H. pylori and has been observed to inhibit H. pylori (Chenoll et al. 2011). As the diversity of intestinal microflora (other than H. pylori) increases, the level of H. pylori colonization decreases (Lertpiriyapong et al. 2014). Lactobacillus casei has been reported to decrease the growth and colonization of H. pylori in gastric cancer (Sgouras et al. 2004). On the other hand, other studies have proved contradictory results about Lactobacillus. Lactobacillus supplementation can effectively eradicate H. pylori (Yu et al. 2019) and reduce the chance of GC development (Yang et al. 2021). In another study, L. plantarum ZDY 2013 pre-treatment prevented an increase in inflammatory cytokines (e.g., IL-1β and IFN-γ) and inflammatory cell infiltration in gastric cancer induced by H. pylori infection (Pan et al. 2016). The study by Ferreira et al. approved a notable decline in the abundance of H. pylori and a significant increase in the genera Achromobacter, Clostridium, Citrobacter, Rhodococcus, and Lactobacillus in Portuguese patients with GC in comparison with chronic gastritis (Ferreira et al. 2018). Sheh and Fox in their review article stated that in a certain research study, there were few differences in the microbiota of 10 gastric cancer patients that were observed. While 8 out of 10 gastric cancer patients were H. pylori-positive, the abundances of H. pylori were very low, perhaps reflecting the changes in the gastric niche that occur with gastric cancer, and the altered stomach was colonized by multiple Streptococcus sp., including S. mitis, S. parasanguinis and S. bovis (Sheh and Fox 2013; Dicksved et al. 2009). In certain clinical trials, the incidence of gastrointestinal disorders declined after ingestion of Lactobacilli, and in some cases, H. pylori levels decreased, although H. pylori infection was hardly ever fully eradicated (Canducci et al. 2000). Furthermore, a study suggested that L. acidophilus CRL 639 might be involved in the inhibition of H. pylori in gastrointestinal diseases (Lorca et al. 2001). Another research stated that lactic acid produced by L. casei strain Shirota is involved in the inhibition of the bacterial urease system and consecutively leads to the reduced ability of H. pylori to survive at a low pH in the absence of urea in gastritis (Sgouras et al. 2004). But still, most studies suggest the effect of H. pylori on gut microbiota, but very few discussions about the impact of gut microbiota on H. pylori (Dash et al. 2021) amongst gastroduodenal diseases, which is a subject that needs to be dealt with in-depth. All the findings have been summarised in Table 2.
Table 2.
Summary of inhibitory effects of other bacterial species on H.pylori
| Bacteria | Impact on H.pylori | References |
|---|---|---|
| Bifidobacterium bifidum | Inhibits H. pylori growth in in-vitro studies | Chenoll et al. (2011) |
| Lactobacillus casei | Decreases growth and colonization of H. pylori in gastric cancer | Sgouras et al. (2004) |
| Lactobacillus spp. | Contradictory results; supplementation can effectively eradicate H. pylori and reduce the chance of gastric cancer development in some studies | Yu et al. (2019); Yang et al. (2021) |
| Lactobacillus plantarum | Pre-treatment prevents an increase in inflammatory cytokines and inflammatory cell infiltration in gastric cancer induced by H. pylori infection | Pan et al. (2016) |
| Achromobacter spp. | Notable decline in H. pylori abundance observed in Portuguese patients with gastric cancer compared to chronic gastritis | Ferreira et al. (2018) |
| Clostridium spp. | Notable decline in H. pylori abundance observed in Portuguese patients with gastric cancer compared to chronic gastritis | Ferreira et al. (2018) |
| Citrobacter spp. | Notable decline in H. pylori abundance observed in Portuguese patients with gastric cancer compared to chronic gastritis | Ferreira et al. (2018) |
| Rhodococcus spp. | Notable decline in H. pylori abundance observed in Portuguese patients with gastric cancer compared to chronic gastritis | Ferreira et al. (2018) |
| Lactobacillus spp. | Notable increase in Lactobacillus abundance observed in Portuguese patients with gastric cancer compared to chronic gastritis | Ferreira et al. (2018) |
| Streptococcus spp. | Altered stomach in gastric cancer patients colonized by multiple Streptococcus species, including S. mitis, S. parasanguinis, and S. bovis | Sheh and Fox (2013); Dicksved et al. (2009) |
| Lactobacillus spp. | Ingestion associated with a decline in gastrointestinal disorders and, in some cases, decreased H. pylori levels, although complete eradication is rare | Canducci et al. (2000) |
| Lactobacillus acidophilus | Involved in the inhibition of H. pylori in gastrointestinal diseases | Lorca et al. (2001) |
| Lactobacillus casei | Lactic acid produced by L. casei strain Shirota inhibits the bacterial urease system, leading to reduced survival ability of H. pylori in low pH conditions without urea | Sgouras et al. (2004) |
Advancing strategies for the eradication of Helicobacter pylori
Eradication of H. pylori in reducing the incidence of gastroduodenal diseases has been discussed in numerous studies (Chen et al. 2018a, b; Martin-Nunez et al. 2020). A metagenomic shotgun sequencing study showed that the alterations in the gut microbiota after the eradication therapy could become the basis for developing new prognostic markers (Khusnutdinova et al. 2016). Antibiotic resistance plays a main role in H. pylori eradication so much so that they alter the gut microbiota indicating long-lasting changes (Yap et al 2016). A meta-analysis conducted by a team of researchers noted that after the eradication, Proteobacteria population initially increased and then was brought back to the original level, whereas Actinobacteria decreased further when compared to the baseline (Ye et al. 2020). A recent study undertaken in China (Yan et al. 2020) suggested that out of 2610 patients that enrolled, eradication took place successfully in 1999 of them (76.6%), providing some room for positive results after eradication. After eradication, there have been instances where the body mass index increased significantly (Upala et al. 2017), functional dyspepsia was improved (Osawa et al. 2005), and the Bacteroidetes to Firmicutes ratio decreased significantly (Guo et al. 2020). A study showed that H. pylori exhibits antibiotic resistance with geographical differences with total resistance to metronidazole, levofloxacin, clarithromycin, amoxicillin, and tetracycline being high in one of the regions in China leading to the fact that it is probably one of the main reasons why it is difficult to eradicate (Wang et al. 2019a). There exists a study suggesting that microbiome restoration therapies by using faecal microbiota transplantation can be used possibly to cure gastroduodenal disease, but more insight is required into this (Malikowski et al. 2017). A study from India highlighted the use of concomitant therapy (pantoprazole 80 mg, amoxicillin 2000 mg, clarithromycin 1000 mg, and metronidazole 1000 mg daily in divided doses) for the eradication of H.pylori in place of standard triple therapy (Jha et al. 2019). Another recent study from India showed that LOAD regimen (levofloxacin 250 mg OD, omeprazole 40 mg BD, nitazoxanide 500 mg BD, and doxycycline 100 mg OD) can help in better eradication of H.pylori than standard triple therapy (Raina et al. 2021). In a randomized clinical trial based on population of Europe and The United States, vonoprazan triple and dual therapy were superior to lansoprazole triple therapy (Chey et al. 2022). Hence there are various studies suggesting perturbations in gut microbiota after H. pylori eradication. But studying the long-term effects of eradication is still awaited.
Significance
There are plentiful research papers and review articles describing the relationship between H. pylori and gastroduodenal diseases, but very few interlink H. pylori and gut microbiota in various gastroduodenal diseases, which is what we have tried to establish in this review article. H. pylori as a human pathogen is known, but its detailed interaction with other gut microbiota is still yet to be unravelled and for satisfying that purpose, we have classified its interaction under different sub-headings. We have also tried to describe the colonization of non-H. pylori microbiota in the gut system and possible outcomes if H. pylori were to be eradicated. Furthermore, if we were to talk about the biological plausibility of various gut microbiota for the treatment of H. pylori-related gastroduodenal diseases, there have been a few studies that suggest the same. For example, various B. bifidum strains have been labelled to produce beneficial effects, including antibacterial features against pathogens like H. pylori which causes gastritis and DU (Chenoll et al. 2011). Lactobacillus acidophilus (Bhatia et al. 1989), Lactobacillus casei strain Shirota (Sgouras et al. 2004), Bacillus subtilis (Pinchuk et al. 2001), and even Weissella confusa (Nam et al. 2002) have rendered an antagonistic effect against H. pylori which causes gastroduodenal diseases. Saccharomyces boulardii is another unique probiotic known to survive in gastric acidity and is not adversely affected or inhibited by antibiotics or does not alter or adversely affect the normal microbiota, has been employed to prevent gastroduodenal disorders (Tomičić et al. 2016). A research study also found out that Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus can possibly eradicate Helicobacter pylori thereby preventing severe stages of gastritis (Tongtawee et al. 2015). Another study showed that Lactobacillus plantarum APSulloc 331,261, can be used for alleviating gastric inflammation in gastric ulcer (Park and Lee 2020). A study proved the potential role of Lactobacillus reuteri in H. pylori eradication by accessing the urease activity before and after 4–6 weeks of therapy which reported a significant drop in H. pylori levels after probiotic administration (Yuan et al. 2021). Another study found that after probiotics supplemented eradication treatment, pathogenic bacteria like Fusobacterium and Campylobacter decreased. The microbial diversity was closer to H. pylori-negative subjects than in the other quadruple therapy group (Viazis et al. 2022). Thus, further research surrounding this topic may yield better results.
Conclusions
H. pylori, gut microbiota and gastroduodenal diseases are interlinked by a symbiotic association. With the coming of culture independent techniques, specifically those dependent on next generation sequencing technology, high throughput technology has broadened our research aspect regarding gut microbiota. Regardless of any gastroduodenal disease, the major phyla existing in them are: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria and Fusobacteria. The most important member of our gut system is H. pylori which has been associated with various diseases like gastric cancer, gastritis, duodenal ulcer, to name a few. Dietary factors, antibiotic usage, H. pylori colonization, all influence the residing gut microbiota. As previously mentioned, inflammatory diseases and gastric cancer have lower levels of microbial diversity, while H. pylori (-) individuals have higher levels of microbial richness than H. pylori ( +) individuals. Certainly, there are aspects like population studies, etc. which need to be thoroughly carried out, although, there have been major improvements in research techniques, we still need to study gut microbiota composition which is unaffected by prevalence of H. pylori so as to contribute to personalized medicine approaches, as well as study positive and negative viewpoints after H. pylori eradication.
Acknowledgements
We thank Amity University for providing the infrastructure and support to carry out the work. We also thank Akshita Mathur for her valuable suggestions during the revision of the manuscripts.
Abbreviations
- DU
Duodenal ulcer
- GC
Gastric cancer
- H. pylori
Helicobacter pylori
Author contributions
RD and KD have given the concept of Cross talk between Helicobacter pylori and Gastrointestinal microbiota. PS and SP have written the manuscript. PW has done the statistical analysis. AM has critically revised the manuscript. SM and RD have finalized the manuscript. KD has conceptualized the idea clinically and critically corrected the manuscript.
Funding
The manpower (PS) for the project has been funded by Department of Science and Technology, Govt. of India (EMR/2016/003676).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Footnotes
Prateek Sharma is First Author.
Contributor Information
Prateek Sharma, Email: prateek.sharma18@student.amity.edu.
Prisha Warikoo, Email: prishaw22@gmail.com.
Akshita Mathur, Email: akshita.mathur1@s.amity.edu.
Shweta Mahant, Email: shwetabanerjeemahant@gmail.com.
Kunal Das, Email: drkunaldas@yahoo.com.
Rajashree Das, Email: rdas@amity.edu, Email: rajashreepatra79@yahoo.co.in.
References
- Abenavoli L, Scarpellini E, Luzza F (2021) The role of gut microbiota in gastrointestinal diseases: the heart of the matter. Minerva Gastroenterol (Torino) 67(4):312–313. 10.23736/S2724-5985.21.03022-9 [DOI] [PubMed]
- Ahmed S, Belayneh YM. Helicobacter pylori and Duodenal Ulcer: systematic review of controversies in causation. Clin Exp Gastroenterol. 2019;12:441. doi: 10.2147/CEG.S228203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–1911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MR, Ahmed EU, Rahman MZ, Islam ASMN, Khan MMR, Ahmed DS, et al. A study on healing of peptic ulcer disease after eradication of Helicobacter pylori infection. Banglad Med J. 2014;43:84–89. doi: 10.3329/bmj.v43i2.21388. [DOI] [Google Scholar]
- Alberts CJ, Jeske R, de Martel C, den Hollander WJ, Michel A, Prins M, Waterboer T. Helicobacter pylori seroprevalence in six different ethnic groups living in Amsterdam: The HELIUS study. Helicobacter. 2020;25(3):e12687. doi: 10.1111/hel.12687. [DOI] [PubMed] [Google Scholar]
- Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol WJG. 2014;20(36):12767. doi: 10.3748/wjg.v20.i36.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of Gastric cancer. Sci Rep. 2014;4:4202. doi: 10.1038/srep04202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baele M, Pasmans F, Flahou B, Chiers K, Ducatelle R, Haesebrouck F. Non-Helicobacter pylori helicobacters detected in the stomach of humans comprise several naturally occurring Helicobacter species in animals. FEMS Immunol Med Microbiol. 2009;55(3):306–313. doi: 10.1111/j.1574-695X.2009.00535.x. [DOI] [PubMed] [Google Scholar]
- Baron JH, Sonnenberg A. Hospital admissions for peptic ulcer and indigestion in London and New York in the 19th and early 20th centuries. Gut. 2002;50(4):568–570. doi: 10.1136/gut.50.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra WF, Sarquis DP, Khayat AS, Khayat BCM, Demachki S, Anaissi AKM, de Assumpção PP. Gastric cancer microbiome. Pathobiology. 2021;88(2):156–169. doi: 10.1159/000512833. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Ward A, Vasquez-Achaya F, Silva-Caso W, Aguilar-Luis MA, Mazulis F, Urteaga N, del Valle-Mendoza J. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res Notes. 2018;11(1):1–7. doi: 10.1186/s13104-018-3565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia SJ, Kochar NEENA, Abraham PHILIP, Nair NG, Mehta AP. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol. 1989;27(10):2328–2330. doi: 10.1128/jcm.27.10.2328-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci. 2006;103(3):732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JL, Jonas M, Chi EY, Clark DK, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun. 1996;64(10):4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625–1629. doi: 10.1046/j.1365-2036.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- Castaño-Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-16289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli R, Testino G, Giacosa A, Cornaggia M. Chronic gastritis: its clinical and physiopathological meaning. J Clin Gastroenterol. 1995;21(3):193–197. doi: 10.1097/00004836-199510000-00005. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu W, Lee A, He J, Huang B, Zheng W, Chen S. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xia C, Li Q, Jin L, Zheng L, Wu Z. Comparisons between bacterial communities in mucosa in patients with gastric antrum ulcer and a Duodenal Ulcer. Front Cell Infect Microbiol. 2018;8:126. doi: 10.3389/fcimb.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Wang A, Chu AN, Gong YH, Yuan Y. Mucosa-associated microbiota in Gastric Cancer tissues compared with non-cancer tissues. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021 doi: 10.1080/19490976.2021.1909459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoll E, Casinos B, Bataller E, Astals P, Echevarría J, Iglesias JR, Balbarie P, Ramón D, Genovés S. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl Environ Microbiol. 2011;77(4):1335–1343. doi: 10.1128/AEM.01820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022;163(3):608–619. doi: 10.1053/j.gastro.2022.05.055. [DOI] [PubMed] [Google Scholar]
- Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67(6):1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Gastric cancer: overview. Gastroenterol Clin. 2013;42(2):211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha T, Dharan M. Acute peptic ulcer disease caused by non-Helicobacter pylori-Helicobacter. Cureus. 2021 doi: 10.7759/cureus.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Pereira V, Saxena S, Ghosh TS, Anbumani D, Bag S, Mande SS. Gastric microbiome of Indian patients with Helicobacter pylori infection, and their interaction networks. Scient Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-15510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash NR, Khoder G, Nada AM, Al Bataineh MT. Correction: Exploring the impact of Helicobacter pylori on gut microbiome composition. Plos one. 2021;16(8):e0256274. doi: 10.1371/journal.pone.0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Dekker Nitert M, Mousa A, Barrett HL, Naderpoor N, De Courten B. Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado S, Cabrera-Rubio R, Mira A, Suárez A, Mayo B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol. 2013;65(3):763–772. doi: 10.1007/s00248-013-0192-5. [DOI] [PubMed] [Google Scholar]
- Devi TB, Devadas K, George M, Gandhimathi A, Chouhan D, Retnakumar RJ, Chattopadhyay S. Low Bifidobacterium abundance in the lower gut microbiota is associated with Helicobacter pylori-related gastric ulcer and Gastric Cancer. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.631140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Jácome E, Libânio D, Borges-Canha M, Galaghar A, Pimentel-Nunes P (2016) Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria: a systematic review. Revista Española de Enfermedades Digestivas 108(9):530–540. 10.17235/reed.2016.4261/2016 [DOI] [PubMed]
- Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol. 2009;58(4):509–516. doi: 10.1099/jmm.0.007302-0. [DOI] [PubMed] [Google Scholar]
- Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, Kim JF. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter. 2014;19(6):407–416. doi: 10.1111/hel.12145. [DOI] [PubMed] [Google Scholar]
- Fagoonee S, Pellicano R. Helicobacter pylori: molecular basis for colonization and survival in gastric environment and resistance to antibiotics. A short review. Infect Dis. 2019;51(6):399–408. doi: 10.1080/23744235.2019.1588472. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67(2):226–236. doi: 10.1136/gutjnl-2017-314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AC, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003840.pub5. [DOI] [PubMed] [Google Scholar]
- Frost F, Kacprowski T, Rühlemann M, Bang C, Franke A, Zimmermann K, Lerch MM. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-56631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski A, Mnich E, Szymañski K, Hinc K, Obuchowski M, Moran AP et al (2016) Helicobacter pylori antigens, acetylsalicylic acid, LDL and 7- ketocholesterol – their potential role in destabilizing the gastric epithelial cell barrier. An in vitro model of Kato III cells. Acta Biochim Pol 63, 145–152. 10.18388/abp.2015_1122 [DOI] [PubMed]
- Ganguly M, Sarkar S, Ghosh P, Sarkar A, Alam J, Karmakar BC, Mukhopadhyay AK. Helicobacter pylori plasticity region genes are associated with the gastroduodenal diseases manifestation in India. Gut Pathog. 2016;8(1):1–10. doi: 10.1186/s13099-016-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantuya B, El-Serag HB, Matsumoto T, Ajami NJ, Oyuntsetseg K, Azzaya D, Yamaoka Y. Gastric microbiota in Helicobacter pylori-negative and-positive gastritis among high incidence of gastric cancer area. Cancers. 2019;11(4):504. doi: 10.3390/cancers11040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JJ, Zhang Y, Gerhard M, Mejias-Luque R, Zhang L, Vieth M, Pan KF. Association between gut microbiota and Helicobacter pylori--related gastric lesions in a high-risk population of gastric cancer. Front cell infect microbiol. 2018;8:202. doi: 10.3389/fcimb.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Gong X, Wang L, Yu X, Dong Q. Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol Res Pract. 2016;2016:6951091. doi: 10.1155/2016/6951091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunathilake MN, Lee J, Choi IJ, Kim YI, Ahn Y, Park C, Kim J. Association between the relative abundance of gastric microbiota and the risk of GC: a case-control study. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-50054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhang Y, Gerhard M, Gao JJ, Mejias-Luque R, Zhang L, Pan KF. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of GC. Gut. 2020;69(9):1598–1607. doi: 10.1136/gutjnl-2019-319696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HS, Lee KY, Lim SD, Kim WS, Hwang TS. Molecular identification of Helicobacter DNA in human gastric adenocarcinoma tissues using Helicobacter species-specific 16S rRNA PCR amplification and pyrosequencing analysis. Oncol Lett. 2010;1(3):555–558. doi: 10.3892/ol_00000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE. 2016;11(5):e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, Ng SC. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Hou L, El-Omar EM, Chen J, Grillo P, Rabkin CS, Baccarelli A, Chow WH. Polymorphisms in Th1-type cell-mediated response genes and risk of GC. Carcinogenesis. 2007;28(1):118–123. doi: 10.1093/carcin/bgl130. [DOI] [PubMed] [Google Scholar]
- Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28(1):96. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, Li C. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Scient Rep. 2018;8(1):1–11. doi: 10.1038/s41598-017-18596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, He LH, Di Xiao GDL, Gu YX, Tao XX, Zhang JZ. Bacterial flora concurrent with Helicobacter pylori in the stomach of patients with upper gastrointestinal diseases. World J Gastroenterol: WJG. 2012;18(11):1257. doi: 10.3748/wjg.v18.i11.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, Van der Merwe S, Organization WG (2011) Helicobacter pylori in developing countries. World gastroenterology organisation global guideline. J Gastrointest Liver Dis JGLD 20(3): 299–304 [PubMed]
- Jenkins D, Goodall A, Gillet FR, Scott BB. Defining duodenitis: quantitative histological study of mucosal responses and their correlations. J Clin Pathol. 1985;38(10):1119–1126. doi: 10.1136/jcp.38.10.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SK, Mishra MK, Saharawat K, Jha P, Purkayastha S, Ranjan R. Comparison of concomitant therapy versus standard triple-drug therapy for eradication of Helicobacter pylori infection: A prospective open-label randomized controlled trial. Indian J Gastroenterol. 2019;38:325–331. doi: 10.1007/s12664-019-00949-4. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Kim J, Kim N, Park JH, Nam RH, Seok YJ, Jung HC. Analysis of gastric microbiota by pyrosequencing: minor role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. Helicobacter. 2016;21(5):364–374. doi: 10.1111/hel.12293. [DOI] [PubMed] [Google Scholar]
- Kato S, Fujimura S, Kimura K, Nishio T, Hamada S, Minoura T, Oda M. Non-Helicobacter bacterial flora rarely develops in the gastric mucosal layer of children. Dig Dis Sci. 2006;51(4):641–646. doi: 10.1007/s10620-006-3185-0. [DOI] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi Y, Dieye Y, Poh BH, Ng CG, Loke MF, Goh KL, Vadivelu J. Culturable bacterial microbiota of the stomach of Helicobacter pylori positive and negative gastric disease patients. Scient World J. 2014 doi: 10.1155/2014/610421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusnutdinova D, Grigoryeva T, Abdulkhakov S, Safina D, Siniagina M, Markelova M, Ismagilova R. Gut microbiome shotgun sequencing in assessment of microbial community changes associated with H. pylori eradication therapy. BioNanoScience. 2016;6:585–7. doi: 10.1016/j.dib.2017.07.070. [DOI] [Google Scholar]
- Kusters JG, Van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łaszewicz W, Iwańczak F, Iwańczak B, Annabhani A, Bała G, Bąk-Romaniszyn L, Zimnicki P. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv Med Sci. 2014;59(1):147–150. doi: 10.1016/j.advms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Lertpiriyapong K, Whary MT, Muthupalani S, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54–63. doi: 10.1136/gutjnl-2013-305178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Li Z, Xiang Y, Yi C, Zhang J. Common stomach stomach qualitative and quantitative study. Chin Intern Med. 1995;34:408–410. [Google Scholar]
- Li XX, Wong GLH, To KF, Wong VWS, Lai LH, Chow DKL, Ding C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PloS One. 2009;4(11):e7985. doi: 10.1371/journal.pone.0007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shao L, Liu X, Ji F, Mei Y, Cheng Y, Ling Z. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with GC. EBioMedicine. 2019;40:336–348. doi: 10.1016/j.ebiom.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca L, Pérez-Pérez G, Urruzuno P, Martinez MJ, Iizumi T, Gao Z, Alarcón T. Characterization of the gastric microbiota in a pediatric population according to Helicobacter pylori status. Pediatr Infect Dis J. 2017;36(2):173–178. doi: 10.1097/INF.0000000000001383. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J, Abu-Ali G, Huttenhower C. The Healthy Human Microbiome. Genome Med. 2016;8(1):1–11. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Scaldaferri F, Franceschi F, Gasbarrini A. The gastrointestinal microbiome–functional interference between stomach and intestine. Best Pract Res Clin gastroenterol. 2014;28(6):995–1002. doi: 10.1016/j.bpg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Lorca GL, Wadström T, Font de Valdez G, Ljungh Å. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr Microbiol. 2001;42:39–44. doi: 10.1007/s002840010175. [DOI] [PubMed] [Google Scholar]
- Luan F, Li X, Cheng X, Huangfu L, Han J, Guo T, Ji J. TNFRSF11B activates Wnt/β-catenin signaling and promotes Gastric Cancer progression. Int J Biol Sci. 2020;16(11):1956. doi: 10.7150/ijbs.43630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, Karaoz U, Contreras M, Blaser MJ, Dominguez-Bello MG. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011;5(4):574–579. doi: 10.1038/ismej.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O’morain CA, Gisbert JP, Kuipers EJ, Axon AT, El-Omar EM. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- Malikowski T, Khanna S, Pardi DS. Fecal microbiota transplantation for gastrointestinal disorders. Curr Opin Gastroenterol. 2017;33(1):8–13. doi: 10.1097/MOG.0000000000000326. [DOI] [PubMed] [Google Scholar]
- Marques-Silva L, Areia M, Elvas L, Dinis-Ribeiro M. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26(4):378–387. doi: 10.1097/MEG.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Martín-Núñez GM, Cornejo-Pareja I, Roca-Rodríguez MDM, Clemente-Postigo M, Cardona F, Fernández-García JC, Tinahones FJ. Helicobacter pylori eradication treatment causes alterations in the gut microbiota and blood lipid levels. Front Med. 2020;7:417. doi: 10.3389/fmed.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, Milosavljevic T. Burden of gastroduodenal diseases from the global perspective. Curr Treat Opt Gastroenterol. 2020 doi: 10.1007/s11938-020-00277-z. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Derakhshan MH, Wirz AA, Orange C, Ballantyne SA, Going JJ, McColl KE. The gastric acid pocket is attenuated in Helicobacter pylori infected subjects. Gut. 2017;66(9):1555–1562. doi: 10.1136/gutjnl-2016-312638. [DOI] [PubMed] [Google Scholar]
- Miyata N, Hayashi Y, Hayashi S, Sato K, Hirai Y, Yamamoto H, Sugano K (2019) Lipopolysaccharides from non-Helicobacter pylori gastric bacteria potently stimulate interleukin-8 production in gastric epithelial cells. Clin Transl Gastroenterol 10.14309/ctg.0000000000000024 [DOI] [PMC free article] [PubMed]
- Mladenova I. Clinical relevance of Helicobacter pylori Infection. J Clin Med. 2021;10:3473. doi: 10.3390/jcm10163473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monstein HJ, Tiveljung A, Kraft CH, Borch K, Jonasson J. Profiling of bacterial flora in gastric biopsies from patients with Helicobacter pylori-associated gastritis and histologically normal control individuals by temperature gradient gel electrophoresis and 16S rDNA sequence analysis. J Med Microbiol. 2000;49(9):817–822. doi: 10.1099/0022-1317-49-9-817. [DOI] [PubMed] [Google Scholar]
- Murakami K, Okimoto T, Kodama M, Tanahashi J, Yasaka S, Inoue K, Fujioka T. Helicobacter pylori and NSAID-induced gastric ulcer in a Japanese population. J Gastroenterol. 2009;44(19):40–43. doi: 10.1007/s00535-008-2259-5. [DOI] [PubMed] [Google Scholar]
- Nam H, Ha M, Bae O, Lee Y. Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl Environ Microbiol. 2002;68(9):4642–4645. doi: 10.1128/AEM.68.9.4642-4645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone G, Compare D, Rocco A. A microbiota-centric view of diseases of the upper gastrointestinal tract. Lancet Gastroenterol Hepatol. 2017;2(4):298–312. doi: 10.1016/S2468-1253(16)30108-X. [DOI] [PubMed] [Google Scholar]
- Ndegwa N, Ploner A, Andersson AF, Zagai U, Andreasson A, Vieth M, Ye W (2020) Gastric microbiota in a low–Helicobacter pylori prevalence general population and their associations with gastric lesions. Clin Transl Gastroenterol 10.14309/ctg.0000000000000191. [DOI] [PMC free article] [PubMed]
- Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metabol. 2005;90(1):10–16. doi: 10.1210/jc.2004-1330. [DOI] [PubMed] [Google Scholar]
- Ozbey G, Dogan Y, Demiroren K, Ozercan IH. Prevalence of Helicobacter pylori in children in eastern Turkey and molecular typing of isolates. Braz J Microbiol. 2015 doi: 10.1590/S1517-838246220140234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M, Wan C, Xie Q, Huang R, Tao X, Shah NP, Wei H. Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection. J Dairy Sci. 2016;99(2):970–981. doi: 10.3168/jds.2015-10510. [DOI] [PubMed] [Google Scholar]
- Park CH, Lee SK. Exploring esophageal microbiomes in esophageal diseases: a systematic review. J Neurogastroenterol Motility. 2020;26(2):171. doi: 10.5056/jnm19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Cho D, Huang E, Seo JY, Kim WG, Todorov SD, Ji Y, Holzapfel WH. Amelioration of alcohol induced gastric ulcers through the administration of Lactobacillus plantarum APSulloc 331261 isolated from green tea. Front Microbiol. 2020;11:420. doi: 10.3389/fmicb.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons BN, Ijaz UZ, D’Amore R, Burkitt MD, Eccles R, Lenzi L, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13:e1006653. doi: 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci. 2004;101(12):4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Mégraud F, Urdaci MC. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemotherapy. 2001;45(11):3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Mathan M, Chandy G, Rajan DP, Venkateswaran S, Ramakrishna BS, Mathan VI. Prevalence of Helicobacter pylori in southern Indian controls and patients with gastroduodenal disease. J Gastroenterol Hepatol. 1994;9(5):501–506. doi: 10.1111/j.1440-1746.1994.tb01281.x. [DOI] [PubMed] [Google Scholar]
- Raina H, Sainani R, Parray A, Asharaf U, Raina MA. Efficacy of levofloxacin, omeprazole, nitazoxanide, and doxycycline (LOAD) regimen compared with standard triple therapy to eradicate Helicobacter pylori infection: a prospective randomized study from a tertiary hospital in India. Gastroenterol Hepatol Bed to Bench. 2021;14(4):342. [PMC free article] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M, Figueiredo C, Smet A, Hansen R, Kupcinskas J, Rokkas T, Hold GL. Systematic review: gastric microbiota in health and disease. Aliment Pharmacol Ther. 2020;51(6):582–602. doi: 10.1111/apt.15650. [DOI] [PubMed] [Google Scholar]
- Ravegnini G, Fosso B, Saverio V, Di Sammarini G, Zanotti F, Rossi G, et al. Gastric adenocarcinomas and signet-ring cell carcinoma: unraveling Gastric Cancer complexity through microbiome analysis-deepening heterogeneity for a personalized therapy. Int J Mol Sci. 2020;21:9735. doi: 10.3390/ijms21249735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, Di Mario F. Gastritis: the histology report. Dig Liver Dis. 2011;43:S373–S384. doi: 10.1016/S1590-8658(11)60593-8. [DOI] [PubMed] [Google Scholar]
- Salazar-Lindo E, Figueroa-Quintanilla D, Caciano MI, Reto-Valiente V, Chauviere G, Colin P, Lacteol Study Group Effectiveness and safety of Lactobacillus LB in the treatment of mild acute diarrhea in children. J Pediatric Gastroenterol Nutrit. 2007;44(5):571–576. doi: 10.1097/MPG.0b013e3180375594. [DOI] [PubMed] [Google Scholar]
- Schulz C, Schütte K, Koch N, Vilchez-Vargas R, Wos-Oxley ML, Oxley AP, Pieper DH. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut. 2018;67(2):216–225. doi: 10.1136/gutjnl-2016-312904. [DOI] [PubMed] [Google Scholar]
- Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Mentis Α. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004;70(1):518–526. doi: 10.1128/AEM.70.1.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaie S, Kaboosi H, Ghadikolaii FP. Prevalence of non Helicobacter pylori gastric Helicobacters in Iranian dyspeptic patients. BMC Gastroenterol. 2020;20(1):1–7. doi: 10.1186/s12876-020-01331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4(6):505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50(6):657–667. doi: 10.3109/00365521.2015.1019918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. GC: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Suzuki TA, Worobey M. Geographical variation of human gut microbial composition. Biol Let. 2014;10(2):20131037. doi: 10.1098/rsbl.2013.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Takahide Tanaka, Yuich Matsuno, Torisu Takehiro, Hiroki Shibata, Atsushi Hirano, Junji Umeno, Keisuke Kawasaki, Shin Fujioka, Yuta Fuyuno, Tomohiko Moriyama, Motohiro Esaki, Takanari Kitazono. Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine. 2021;100(38):e27287. doi: 10.1097/MD.000000000002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomičić ZM, Čolović RR, Čabarkapa IS, Vukmirović ĐM, Đuragić OM, Tomičić RM. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed Res. 2016;43(2):103–110. doi: 10.5937/FFR1602103T. [DOI] [Google Scholar]
- Tongtawee T, Dechsukhum C, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, Loyd RA, Panpimanmas S. Improved Helicobacter pylori eradication rate of tailored triple therapy by adding Lactobacillus delbrueckii and Streptococcus thermophilus in Northeast region of Thailand: a prospective randomized controlled clinical trial. Gastroenterol Res Pract. 2015 doi: 10.1155/2015/518018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhayawardana DLN, Fernando SS, Gunasekara TDC, Weerasinghe GGY, Weerasekera MM, Weerasekara DD. Human Stomach Microbiota: Effects on Health and Disease. 2021 doi: 10.4038/sljid.v11i1.8331. [DOI] [Google Scholar]
- Upala S, Sanguankeo A, Saleem SA, Jaruvongvanich V. Effects of Helicobacter pylori eradication on insulin resistance and metabolic parameters: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(2):153–159. doi: 10.1097/MEG.0000000000000774. [DOI] [PubMed] [Google Scholar]
- Vasapolli R, Schütte K, Schulz C, Vital M, Schomburg D, Pieper DH, Malfertheiner P. Analysis of transcriptionally active bacteria throughout the gastrointestinal tract of healthy individuals. Gastroenterology. 2019;157(4):1081–1092. doi: 10.1053/j.gastro.2019.05.068. [DOI] [PubMed] [Google Scholar]
- Viazis N, Argyriou K, Kotzampassi K, Christodoulou DK, Apostolopoulos P, Georgopoulos SD, Stogiannou D. A four-probiotics regimen combined with a standard Helicobacter pylori-eradication treatment reduces side effects and increases eradication rates. Nutrients. 2022;14(3):632. doi: 10.3390/nu14030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinasco K, Mitchell HM, Kaakoush NO, Castano-Rodriguez N (2019) Microbial carcinogenesis: Lactic acid bacteria in GC. Biochimica et Biophysica Acta (BBA)-Rev Cancer 1872(2):188309. 10.1016/j.bbcan.2019.07.004 [DOI] [PubMed]
- Wan XK, Yuan SL, Wang YC, Tao HX, Jiang W, Guan ZY, Liu CJ. Helicobacter pylori inhibits the cleavage of TRAF1 via a CagA-dependent mechanism. World J of Gastroenterol. 2016;22(48):10566. doi: 10.3748/wjg.v22.i48.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chen XZ. Prevalence of atrophic gastritis in southwest China and predictive strength of serum gastrin-17: A cross-sectional study (SIGES) Sci Rep. 2020;10(1):4523. doi: 10.1038/s41598-020-61472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo Q, Yuan Y, Gong Y. The antibiotic resistance of Helicobacter pylori to five antibiotics and influencing factors in an area of China with a high risk of GC. BMC Microbiol. 2019;19(1):1–10. doi: 10.1186/s12866-019-1517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li Y, Zhong H, Ding Q, Lin Y, Tang S, Liu X. Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Open Bio. 2019;9(9):1552–1560. doi: 10.1002/2211-5463.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xin Y, Zhou J, Tian Z, Liu C, Yu X, Dong Q. Gastric mucosa-associated microbial signatures of early GC. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang T, Lu Y, Wang C, Wu Y, Li J, Ma J. Helicobacter pylori infection affects the human gastric microbiome, as revealed by metagenomic sequencing. FEBS Open Bio. 2022;12(6):1188–1196. doi: 10.1002/2211-5463.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, McColl KE. proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- Wroblewski LE, Peek RM. Helicobacter pylori, cancer, and the gastric microbiota. Stem Cells Pre-Neoplasia Early Cancer Upper Gastrointestinal Tract. 2016 doi: 10.1007/978-3-319-41388-4_19. [DOI] [PubMed] [Google Scholar]
- Wu L, Wang Z, Sun G, Peng L, Lu Z, Yan B, Yang Y. Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in Duodenal Ulcer. Scient Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-49415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TL, Gao JG, Wang JH, Chen D, Lu C, Xu CF. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J Gastroenterol. 2020;26(32):4846. doi: 10.3748/wjg.v26.i32.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, Suerbaum S. Different gastric microbiota compositions in two human populations with high and low GC risk in Colombia. Sci Rep. 2016;6(1):1–10. doi: 10.1038/srep18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang J, Xu J, Wei X, Yang J, Liu Y, Gai Z. Helicobacter pylori infection aggravates dysbiosis of gut microbiome in children with gastritis. Front Cell Infect Microbiol. 2019 doi: 10.3389/fcimb.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhou X, Liu X, Ling Z, Ji F. Role of the gastric microbiome in GC: from carcinogenesis to treatment. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.641322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TWC, Gan HM, Lee YP, Leow AHR, Azmi AN, Francois F, Vadivelu J. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PloS one. 2016;11(3):e0151893. doi: 10.1371/journal.pone.0151893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Shao X, Shen R, Chen D, Shen J. Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: a systematic review and meta-analysis. Helicobacter. 2020;25(4):e12713. doi: 10.1111/hel.12713. [DOI] [PubMed] [Google Scholar]
- Yu M, Zhang R, Ni P, Chen S, Duan G. (2019) Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: A meta-analysis of randomized controlled trials. PLoS One. 2:14 (10):e0223309. 10.1371/journal.pone.0223309 [DOI] [PMC free article] [PubMed]
- Yuan Z, Xiao S, Li S, Suo B, Wang Y, Meng L, Zhou L. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter. 2021;26(6):e12848. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yu C, Liu W, et al. Changes of gastric microenvironment in patients with gastrointestinal diseases. Chin J Microbiol. 1996;3:22–25. [Google Scholar]
- Zhang S, Shi D, Li M, Li Y, Wang X, Li W. The relationship between gastric microbiota and gastric disease. Scand J Gastroenterol. 2019;54:391–396. doi: 10.1080/00365521.2019.1591499. [DOI] [PubMed] [Google Scholar]
- Zilberstein B, Quintanilha AG, Santos MA, Pajecki D, Moura EG, Alves PRA, Gama-Rodrigues J. Digestive tract microbiota in healthy volunteers. Clinics. 2007;62(1):47–54. doi: 10.1590/s1807-59322007000100008. [DOI] [PubMed] [Google Scholar]
- Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa–associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8(2):105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.


