Abstract
Brain metabolism is a fundamental process involved in the proper development of the central nervous system and in the maintenance of the main higher functions in humans. As consequence, energy metabolism imbalance has been commonly associated to several mental disorders, including depression. Here, by employing a metabolomic approach, we aimed to establish if differences in energy metabolite concentration may underlie the vulnerability and resilience in an animal model of mood disorder named chronic mild stress (CMS) paradigm. In addition, we have investigated the possibility that modulation of metabolite concentration may represent a pharmacological target for depression by testing whether repeated treatment with the antidepressant venlafaxine may normalize the pathological phenotype by acting at metabolic level. The analyses were conducted in the ventral hippocampus (vHip) for its key role in the modulation of anhedonia, a core symptom of patients affected by depression. Interestingly, we showed that a shift from glycolysis to beta oxidation seems to be responsible for the vulnerability to chronic stress and that vHip metabolism contributes to the ability of the antidepressant venlafaxine to normalize the pathological phenotype, as shown by the reversal of the changes observed in specific metabolites. These findings may provide novel perspectives on metabolic changes that could serve as diagnostic markers and preventive strategies for the early detection and treatment of depression as well as for the identification of potential drug targets.
Subject terms: Stress and resilience, Emotion
Introduction
Brain metabolism is a fundamental process involved in the proper development of the central nervous system (CNS) and in the maintenance of the main higher functions in humans. Accordingly, energy metabolism imbalance has been commonly observed in several diseases of the CNS, such as mood disorders [1, 2]. In particular, major depressive disorder (MDD) in humans has been associated with alterations in mitochondrial activity and energy imbalance in the brain [3–7]. However, considering the inherent limitations of studying depression in humans several issues remain to be addressed and the preclinical approach can lay the foundation for further in-depth and targeted studies in patients.
Since stress events are key players both in the etiology and progression of MDD and other psychiatric disorders [8], several studies have investigated metabolite alterations in animal models of depression based on stress exposure and two recent comprehensive analyses of the available literature reveal the inadequate reproducibility of metabolomic studies [9, 10]. Actually, this conclusion is not surprising considering that the type of stressor, the time and the length of exposure, as well as the stress prediction, can recreate in rodents only an aspect of stress exposure (ie: social isolation, restraint stress) and consequently each model can reproduce specific symptoms that are paralleled by characteristic and diverse pattern of changes making it difficult to draw a clear conclusion.
Importantly, this view fully parallels the clinical nature of mood disorders that are characterized by high heterogeneity, as confirmed by the evidence that, often, subjects with the same diagnosis are profoundly different in the manifestation of symptoms and in the response to treatment.
Consequently, since years, our group have decided to employ the chronic mild stress (CMS) model of depression because it has an high transdiagnostic value, it meets all the three validity criteria (face-construct and predictive validity) [11] and it affects both emotional state and cognitive functions [12–16].
Accordingly, it is also useful to associate specific symptoms (behavioral phenotype) with specific molecular alterations, thus representing a powerful tool to identify diagnostic and predictive biomarkers and resulting into a better patient stratification and a more effective pharmacological approach.
On these bases, we and other groups have thoroughly characterized this model, demonstrating its validity in exploring either the mechanisms (neuroplasticity, HPA axis, inflammation, circadian rhythms), the development of several depressive-like behaviour (from the anhedonic phenotype to cognitive dysfunctions) or to test pharmacological strategies [12–20]. In addition, we have recently shown that mitochondrial dynamic in resilient versus vulnerable animals is a potential mechanism underlying the different response to stress exposure in the CMS animal model [21].
Here, we aimed to make a further step by employing a metabolomic approach to investigate potential differences in energy metabolite concentration that may help explain vulnerability and resilience to CMS exposure. Moreover, we explored the role of brain bioenergetic in the development of the pathological phenotype by testing whether repeated treatment with venlafaxine, a drug that is widely used in MDD [22] and that also has been demonstrated to be very effective on the anhedonic phenotype at preclinical levels [23, 24], would be able to normalize the depressive-like behaviour by modulating brain metabolism.
Based on our previous results showing that metabolic mechanisms involved in the stress response are differentially modulated in the ventral hippocampus (vHip) of vulnerable compared with resilient rats, we focused on this brain area that plays a key role in the modulation of anhedonia [16, 18, 21].
Our results suggest that vHip energy metabolism is implicated in the response to CMS exposure in terms of anhedonic phenotype and it may represent both an underestimated trait of depression and a novel target of chronic antidepressant action.
Material and methods
Animals
Adult male Wistar rats (5 weeks old) (Charles River, Germany) were brought into the laboratory one month before the start of the experiment. Animals were housed in standard laboratory conditions: food and water were freely available on a 12-h light/dark, constant temperature (22 ± 2 °C) and humidity (50 ± 5%). All procedures used in this study have conformed to the rules and principles of the 2010/63 European Communities Council Directive and have been approved by the Local Bioethical Committee (approval code: 4/2019; approval date 03/01/2019) at the Maj Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland. All efforts were made to minimize animal suffering, to reduce the number of animals used and the animal studies comply with the ARRIVE 2.0 guidelines.
Chronic mild stress protocol and pharmacological treatment
Before the starting of the experiment, the animals were trained to consume 1% sucrose solution; training consisted of nine 1 h baseline tests, conducted once weekly, in which sucrose was presented, in the home cage, following 14 h food and water deprivation. Subsequently, sucrose consumption was monitored, under similar conditions, at weekly intervals throughout the whole experiment by weighing pre-weighed bottles containing the sucrose solution, at the end of the test.
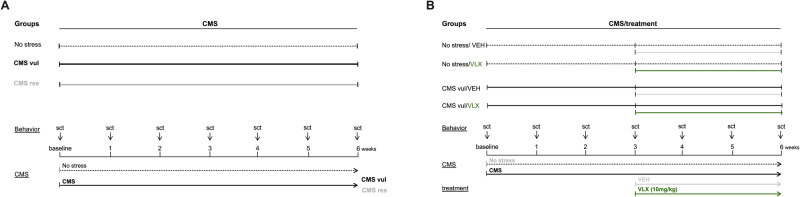
After the sucrose training, rats were randomly divided into two groups. One group of animals was single housed and subjected to a random combination of periods of stress of the duration of 10–14 h (food or water deprivation, 45-degree cage tilt, intermittent illumination every 2 h, soiled cage with 250 ml water in sawdust bedding, paired housing, low intensity stroboscopic illumination-150 flashes/min, and periods of no stress), the chronic mild stress procedure, for a period of 6 consecutive weeks. Animals of the No stress group were housed in separate rooms and had no contact with the stressed animals (Fig. 1A).
Fig. 1. Schematic representation of the experimental paradigms.
Experimental design of rats exposed to 6 weeks of chronic mild stress (CMS) (A) and treated with venlafaxine (VLX) for 3 weeks (B).
A subgroup of rats subjected to the CMS procedure demonstrated a gradual decrease in sucrose solution consumption to approximately 40% of pre-stress values, indicator of the development of the anhedonic phenotype, that was then named CMS vulnerable (CMS vul) group, whereas animals showing a similar hedonic phenotype to non-stressed animals (named CMS resilient) were defined CMS res group.
Rats of both the CMS vul and CMS res groups were subjected to the CMS protocol for 3 subsequent weeks (Fig. 1 A- experimental groups: No stress n = 16, CMS vul n = 16, CMS res n = 8).
Moreover, both non-stressed and CMS vul groups were further divided into two subgroups, and for the following 3 weeks they received, once a day, the administration of vehicle (VEH) (0,9% sterile saline, IP) or venlafaxine HCl (VLX) (10 mg/kg, per IP dissolved in 0,9% sterile saline) (Carbosynth Ltd., Compton, UK), while continuing the CMS procedure (Fig. 1B experimental groups: No stress/VEH n = 8, No stress/VLX n = 8, CMS vul/VEH n = 8, CMS vul/VLX n = 8). The daily injections were of 1 ml/kg of body weight.
The dose of VLX was chosen on the bases of our previous findings demonstrating its ability, with this regimen, to normalized the reduction of sucrose intake due to CMS exposure [23, 24]. VEH or VLX were administered at 10.00 am and the weekly sucrose tests were carried out 24 h following the last drug injections. 24 hours after the last injection, the animals were decapitated and ventral hippocampus (plates 34-43 according to the atlas of Paxinos and Watson [25]) was collected and stored at −80 °C.
Metabolomic analysis
We employed a liquid chromatography coupled to tandem mass spectrometry to obtain metabolomic data by using an API-3500 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA) coupled with an ExionLC™ AC System (AB Sciex, Framingham, MA, USA). 10 mg of ventral hippocampus were used for the analysis. Half tissue was smashed in 250 µl of ice-cold methanol/acetonitrile 50:50, while the second half was lysed in 250 µl of ice-cold water/methanol 20:80, respectively. Both solutions contained [U-13C6]-glucose (Merck Life Science S.r.l, Milano, Italy) 1 ng/µl and [U-13C5]-glutamine (Merck Life Science S.r.l, Milano, Italy) 1 ng/µl as internal standards. Lysates were spun at 2000g for 5 min at 4 °C and supernatants were then passed through a regenerated cellulose filter (4 mm Ø). Samples were then dried under N2 flow at 40 °C and resuspended in 100 µl of methanol for subsequent analysis.
A cyano-phase LUNA column (50 mm × 4.6 mm, 5 µm; Phenomenex, Torrance, CA, USA) by a 5 min run in negative ion mode with two separated runs was used to quantify energy metabolites. Protocol A: Samples lysed in acetonitrile/methanol were used to analyze lactate, malate, αKetoglutarate, phosphoenolpyruvate (PEP), dihydroxyacetone-P/glyceraldehyde-3P (DHAP/GAP), erytrose-4P (E4P), dTMP, dAMP, dIMP, dCTP, ITP and GTP. The mobile phase A was: water and phase B was: 5 mM ammonium acetate in MeOH and the gradient was 10% A and 90% B for all the analysis with a flow rate of 500 µl/min. Protocol B: samples lysed in water/methanol solution were used to analyse 3’, 5’-Cyclic GMP, Acetyl-CoA, ADP, AMP, ATP, cAMP, Citrate, CMP, CoA, CTP, dADP, dATP, dCDP, dCMP, dGDP, dGMP, dGTP, dITP, dTTP, dUMP, dUTP, FAD, Fructose 1,6-bisphosphate (Fructose bis-P), Fumarate, GDP, Glucose, Glucose-6P/Fructose-6P, GMP, IMP, Iso-citrate, malonyl-CoA, NAD+, NADH, NADP+, NADPH, oxaloacetate, pyruvate, ribose-xylulose-ribulose-5P (R-X-Ru-5P), succinate, succinyl-CoA, UDP, UMP and UTP. The mobile phase A was water and phase B was 5 mM ammonium acetate in MeOH and the gradient was 50% A and 50% B for all the analysis with a flow rate of 500 µl/min.
Carnitine quantification was performed on acetonitrile/methanol extracts by using a Varian Pursuit XRs Ultra 2.8 Diphenyl column. Samples were analysed by a 3 min run in positive ion mode and the mobile phase was 0.1% formic acid in MeOH.
The quantification of amino acids (AA) and biogenic amines (BA) was performed through previous derivatization. Briefly, 20 µl out of 100 µl of acetonitrile/methanol samples were collected and dried under N2 flow at 40 °C. Dried samples were resuspended in 50 µl of phenyl-isothiocyanate (PITC), EtOH, pyridine and water 5%:31.5%:31.5%:31.5% and then incubated for 20 min at RT, dried under N2 flow at 40 °C for 90 min and finally resuspended in 100 µl of 5 mM ammonium acetate in MeOH/H2O 50:50. Quantification of different amino acids was performed by using a C18 column (Biocrates, Innsbruck, Austria) maintained at 50 °C. The mobile phases for positive ion mode analysis were phase A: 0.2% formic acid in water and phase B: 0.2% formic acid in acetonitrile. The gradient was T0: 100%A, T5.5: 5%A, T7: 100%A with a flow rate of 500 µl/min. All metabolites analysed in the described protocols were previously validated by pure standards and internal standards were used to check instrument sensitivity.
MultiQuant™ software (version 3.0.3, AB Sciex, Framingham, MA, USA) was used for data analysis and peak review of chromatograms. Raw areas were normalized by the median of all metabolite areas in the same sample. The data were then transformed by generalized log-transformation and Pareto scaled to correct for heteroscedasticity, reduce the skewness of the data, and reduce mask effects [26]. In detail, obtained values were transformed by generalized log (glog) as follows:
where a is a constant with a default value of 1 and x is the sample area for a given metabolites [27]. Then, obtained values underwent Pareto scaling as follows:
where xij is the transformed value in the data matrix (i (metabolites), j (samples)) and si is the standard deviation of transformed metabolite values [28]. Obtained values were considered as relative metabolite levels. Data processing and analysis were performed by MetaboAnalyst 5.0 web tool (https://www.metaboanalyst.ca [29]).
Metabolic pathway analysis
Metabolites were further analyzed by MetaboAnalyst 5.0 using the metabolic pathway analysis tool. The pathways provided had an impact factor >0, which was calculated by the relative importance of the metabolites. The p value was calculated from the enrichment analysis and the pathway was considered significantly related if it had a p value < 0.05.
Statistical analysis
The analyses of the sucrose consumption were performed with the two/three-way ANOVA with repeated measures, followed by Fisher’s Protected Least Significant Difference (PLSD). The results of the metabolomic analysis were analyzed with the one-way /two-way ANOVA and when appropriate, further differences were analyzed by the Fisher’s PLSD. A Priori sample size estimation was done considering the between-subjects factor of stress or stress and treatment, and each experimental group consists of at least 8 rats.
Partial least squares discriminant analysis (PLS-DA) identified differential metabolites between experimental groups. At the same time, the ranking of metabolites was performed by variable importance in projection (VIP), according to metabolite importance in discriminating groups. The permutation test was used with a maximum of 1000 permutations, with a separation distance test to assess the significance of class discrimination determined by PLS-DA. Classification and cross-validation were performed with a maximum of 5 components using the leave-one-out method.
Significance for all tests was assumed for p < 0.05. Data are presented as means ± standard error (SEM).
Results
6 weeks of CMS induced the development of vulnerable and resilient phenotype
As shown in Fig. 2, two-way ANOVA with repeated measures indicated a significant effect of stress on sucrose intake (F2-38 = 56.7).
Fig. 2. Behavioral characterization of rats exposed to 6 weeks of chronic mild stress (CMS).

Sucrose consumption test was performed at weekly intervals during the whole experiment. The data are the mean ± SEM of 8 independent determinations. ***p < 0.001 vs No stress. (Two-way ANOVA with repeated measures, Fisher’s PLSD).
Indeed, in line with our previous evidence [13, 21], already after one week of CMS, we observed a clear separation between resilient animals (grey line) showing an hedonic phenotype similar to the No stress group (black dashed line), and vulnerable rats (black line) showing a significant reduction of sucrose intake, proxy of the development of the anhedonic-like behavior. This difference was stable for the duration of the whole CMS procedure.
Metabolomic profile in the vHip of animals vulnerable and resilient to six weeks of CMS
We next studied the metabolomic profile of non-stressed, vulnerable and resilient rats following six weeks of CMS.
As shown in Fig. 3, we observed a different metabolomic profile that depicts vulnerability and resilience to CMS. The PLS-DA plot showed that No stress, CMS vul and CMS res were grouped into separated clusters (Fig. 3A). Results were statistically significant (p = 0.016) (Fig. S1A), with the R2 and Q2 (Q2 represents an estimation of the predictive ability of the model) of all tested combinations of components closest to 1 (Fig. S1B). PLS-DA and ANOVA (FDR < 0.05) identified the most important features which are the 25 metabolites represented in Fig. S1C.
Fig. 3. Metabolite levels in the vHip of rats vulnerable and resilient to 6 weeks of chronic mild stress.
A Partial least squares discriminant analysis. B Venn diagram illustrates the number of metabolomic features common and unique in CMS vulnerable and resilient rats. C Venn diagram illustrates the number of metabolic pathways common and unique in CMS vulnerable and resilient rats. D Metabolomic pathways analysis (MetaboAnalyst). The p value was calculated from the enrichment analysis. E Relative levels of nucleotides (NTPs) and deoxy nucleotides (dNTPs). F Relative levels of glycolysis metabolites. G Relative levels of acylcarnitines and ratio of C2-Carnitine, C2-Carnitine + C3-Carnitine, C16-Carnitine + C18-Carnitine and C0-Carnitine fold change levels. H Relative levels of amino acids. I Fold change of ATP energy change and of NAD+/NADH. Data in E–I are represented as violin plot with horizontal lines representing the mean and the quartiles. *p < 0.05, **p < 0.01, ***p < 0.001 vs No stress; #p < 0.05, ##p < 0.01 vs CMS vul (One-way ANOVA, Fisher’s PLSD).
The heat map represented in Fig. S2 showed the changes of several metabolites in No stress, CMS vul and CMS res groups, identified by the one-way ANOVA with Fisher’s PLSD with an FDR < 0.1.
Accordingly, as shown in Fig. 3B, stress exposure has significantly altered the concentration of 42 metabolites (Table S1). In particular, 22 were similarly changed in CMS vul and CMS res rats (black and white line-pattern), while 15 were selectively altered in CMS res (grey) and 6 in CMS vul (black).
The further metabolomic pathway analyses (Fig. 3C) revealed that CMS influences the function of 11 pathways (orange and blue line-pattern) independently from the hedonic phenotype, while 9 more pathways were specifically affected in resilient animals (orange). This means that 20 different intracellular mechanisms are needed to sustain resilience while only 12 underlie the vulnerability (Fig. 3D).
Going even deeper, as shown in Fig. 3E–H, we observed that the vulnerability and resilience to CMS were differently associated with alterations of metabolites of the classes of nucleotides-deoxynucleotides (NTPs-dNTPs) (UTP, ITP, dAMP, dIMP), glycolysis (fructose Bis-P, DHAP/GAP, PEP), acylcarnitines (C2-Carnitine, C14-Carnitine, C16-Carnitine, C16:1-Carnitine, C18:0-Carnitine, C18:1-Carnitine) and amino acids (Thr, Phe), as well as with the levels of succinate and sedoheptulose (Table S1).
Furthermore, we observed increased energy charge and a trend of reduction of NAD+/NADH ratio in all stressed animals independently from the behavioral phenotype (Fig. 3I), whereas fatty acid β-oxidation was differently activated in vulnerable or resilient rats. Indeed, while in resilient rats we observed a decrease of C2-Carnitine, C14-Carnitine, C16-Carnitine, C16:1-Carnitine, C18:1-Carnitine that suggests a reduction in fatty acid degradation, vulnerable vHip showed increased C2-Carnitine/C0-Carnitine and (C2-Carnitine+C3-Carnitine)/C0-Carnitine ratio that are indirect hallmarks of boosted fatty acid β-oxidation (Fig. 3G). These data are further sustained by the activity of Carnitine palmitoyltransferase I (CptI), the key enzyme committing fatty acids towards mitochondrial catabolism, as evidenced by a trend to increase in the (C16:0-Carnitine+C18:0-Carnitine)/C0-Carnitine ratio (Fig. 3G).
Chronic treatment with venlafaxine normalized the anhedonic-like behavior observed in rats exposed to 6 weeks of chronic mild stress
Next, after 3 weeks of CMS, half of the CMS vul rats were treated with the antidepressant VLX for 3 subsequent weeks while continuing the CMS procedure (Fig. 1B).
As we previously observed [23, 24], CMS exposure and VLX treatment modulated the hedonic phenotype, as highlighted by the significant effect observed in the three-way ANOVA with repeated measures (F1-31 = 7.70) (Fig. 4).
Fig. 4. Behavioral characterization of rats exposed to 6 weeks of chronic mild stress (CMS) and treated with venlafaxine (VLX) for 3 weeks.

Sucrose consumption test was performed at weekly intervals in animals exposed to CMS and treated with VLX. The data are the mean ± SEM of 8 independent determinations. ***p < 0.001 vs No stress/VEH; ##p < 0.01, ###p < 0.001 vs CMS vul/VEH (three-way ANOVA with repeated measures, Fisher’s PLSD).
In detail, CMS induced a significant reduction of sucrose intake starting from the first week of stress vs No stress/VEH, effect that was maintained for the subsequent 5 weeks of CMS (black line). Chronic VLX treatment, starting from the first week of treatment (corresponding to the fourth week of CMS), significantly increased the levels of sucrose intake in CMS animals (green line), leading to a complete normalization of the anhedonic-like behavior induced by the CMS paradigm.
Chronic venlafaxine treatment reverted the alterations of several metabolites induced by 6 weeks of CMS
We next characterized the metabolomic profile of non-stressed and vulnerable rats treated for three weeks with vehicle or venlafaxine.
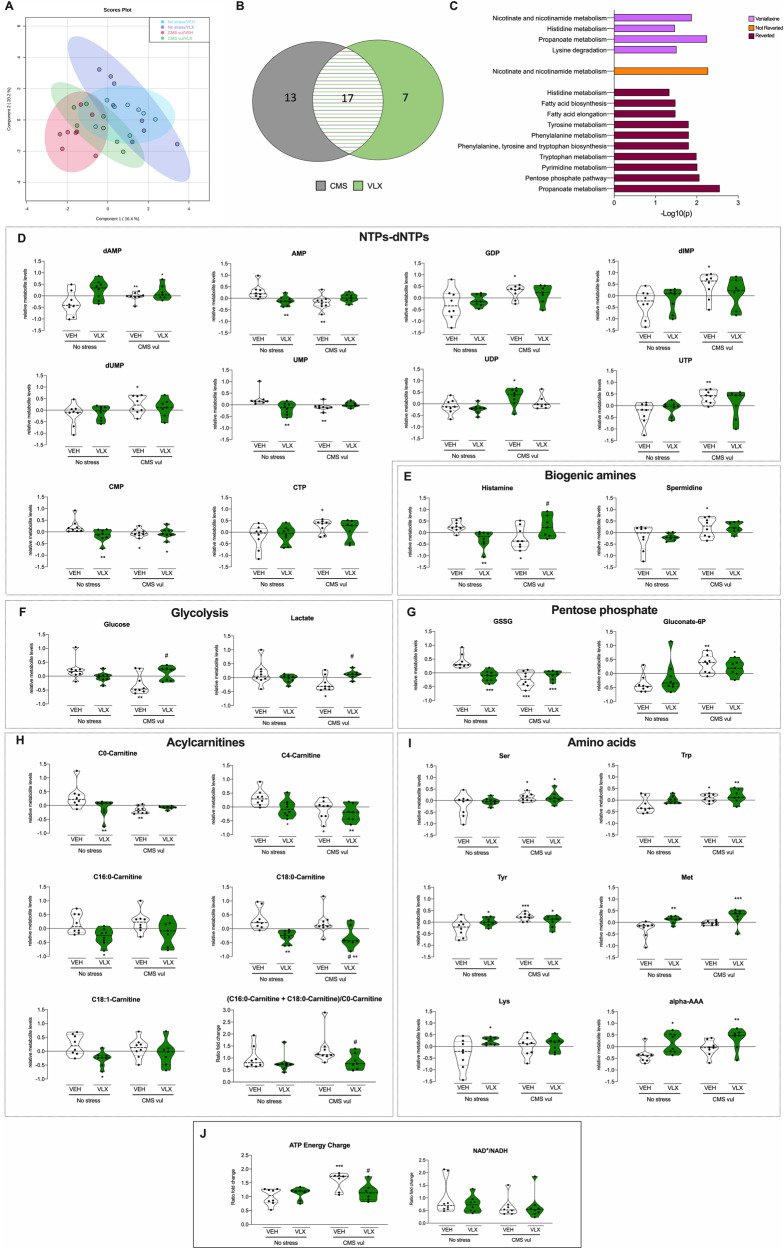
Figure 5 shows the metabolomic differences between stressed and non-stressed animals treated with vehicle or venlafaxine. The PLS-DA plot displayed that No stress/VEH and CMS vul/VEH were grouped into separated clusters, whereas CMS animals treated with VLX (CMS vul/VLX) showed an intermediate metabolic asset among No stress/VEH and CMS vul/VEH. Moreover, as expected, the No stress/VLX animals were similar to non-stressed untreated counterpart (Fig. 5A). As shown in the Fig. S1D, E, results were statistically significant, with the R2 and Q2 of all tested combinations of components closest to 1. PLS-DA and ANOVA (FDR < 0.1) identified the highly discriminant metabolites and the 25 metabolites represented in Fig. S1F are the most important features identified by PLS-DA.
Fig. 5. Metabolite levels in vHip modulated by chronic mild stress and normalized by venlafaxine (VLX) administration.
A Partial least squares discriminant analysis. B Venn diagram illustrates the number of metabolomic features common and unique in stressed animals treated with VLX. C Metabolomic pathways analysis (MetaboAnalyst). The p value was calculated from the enrichment analysis. D Relative levels of mono-, di, and triphosphate nucleotides (NTPs) and of deoxy monophosphate nucleotides (dNTPs). E Relative levels of biogenic amines. F Relative levels of glycolysis metabolites. G Relative levels of metabolites of the pentose phosphate pathway. H Relative levels of acylcarnitines. I Relative levels of amino acids. J Fold change of ATP energy change and of NAD+/NADH. Data in D–J are represented as violin plot with horizontal lines representing the mean and the quartiles. *p < 0.05, **p < 0.01, ***p < 0.001 vs No stress/VEH; #p < 0.05 vs CMS vul/VEH (Two-way ANOVA, Fisher’s PLSD).
As a further step of the analysis, we investigated the metabolites affected by CMS exposure and chronic VLX treatment. Two-way ANOVA with Fisher’s PLSD with an FDR < 0.1 determined variation of several metabolite levels (Fig. 5, Fig. S3 and Table S2).
As shown in the Venn diagram (Fig. 5B) 17 (green and grey line-pattern) out of the 30 metabolites changed in CMS-vul rats were also modified by venlafaxine treatment, while 13 (grey) were still altered after 3 weeks of treatment and 7 were influenced by venlafaxine per se (green). Analyzing the pathways normalized by VLX and then potentially involved in the efficacy of this drug on the anhedonic phenotype we identified the pyrimidine metabolism, tyrosine metabolism, phenylalanine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, tricarboxylic acid cycle, tryptophan metabolism, pyruvate metabolism, fatty acid biosynthesis and elongation, propanoate metabolism and pentose phosphatase pathway (Fig. 5C).
In particular several metabolites of the classes of NTPs-dNTPs (AMP, GDP, UDP, UTP, CTP, dUMP, dIMP) (Fig. 5D), biogenic amines (histamine, spermidine) (Fig. 5E), (C16:0-Carnitine+C18:0-Carnitine)/C0-Carnitine ratio (Fig. 5H) and intermediates of the glycolysis (glucose, lactate) (Fig. 5F) were modulated in rats vulnerable to the CMS and these levels were reverted by the chronic treatment with venlafaxine. In line, also cAMP, histidine, fumarate, S-adenosylmethionine (SAME) were affected by stress and normalized by the pharmacological treatment (Table S2).
Similarly, the increased energy boost induced by CMS exposure, revealed by the increase of ATP energy charge, was normalized by the VLX administration (Fig. 5J).
On the contrary, other metabolites of the classes of NTPs-dNTPs (CMP, UMP, dAMP) (Fig. 4D), acylcarnitines (C0-Carnitine and C4-Carnitine) (Fig. 5H), intermediates of pentose phosphate pathway (GSSG, gluconate 6 P) (Fig. 5G), amino acids (ser, trp, tyr) (Fig. 5I), as well as succinate, dopa, erythrose-4P (E4P) and NAD+ (Table S2) were modulated by chronic stress and were not reverted by VLX treatment.
Finally, when considering the 7 metabolites affected by VLX treatment, but not by CMS exposure, we found that only long-chain acylcarnitines (C16:0-Carnitine, C18:0-Carnitine, C18:1-Carnitine) (Fig. 5H), amino acids (Met, Lys, alpha-AAA) (Fig. 5I) and putrescine (Table S2) were significantly modulated by the drug administration.
Discussion
In this study, we showed that vHip metabolism alterations are involved in the behavioral response to chronic stress exposure and contribute to the ability of the antidepressant venlafaxine to revert the pathological phenotype, as shown by the normalization of the changes observed in specific metabolites.
Specifically, our data suggest a different utilization of energy metabolites in vHip by resilient and vulnerable rats, with the latter preferring fatty acid utilization over glycolysis to produce ketone bodies and ultimately Acetyl-CoA as main energy source [30].
According to our previous studies [12–16, 18, 21], by exposing the animals to the sucrose consumption test, we were able to divide the population of stressed animals in two distinct subgroups, with vulnerable animals developing the anhedonic-like behavior while the resilient subgroup enduring resistance to the negative effects of stress that persisted throughout the entire 6 weeks procedure. Moreover, and in line with the literature [23, 31], we observed that venlafaxine treatment leads to a complete normalization of anhedonic-like behaviour.
By investigating the metabolic profile after 6 weeks of CMS in vulnerable versus resilient rats and based on the PLS-DA data, we demonstrated that the two groups are clearly different from each other and from non-stressed rats. Notably, resilient rats differ even more from non-stressed rats than vulnerable ones. This trend is different from that we observed previously in animals exposed to 2 weeks of CMS [21], suggesting that, although the behavioral phenotype is already established after 1 week of stress and remains quite stable, the underlying molecular processes, instead, change over time both in rats vulnerable and resilient, thus determining a scenario different from a shorter period of stress [21]. Interestingly, it appears that the modulation of 20 intracellular biochemical mechanisms and 37 metabolites is required to sustain resilience while a lower number of intracellular metabolic pathways and metabolites (12 and 28 respectively) underlie vulnerability. These outcomes confirm that resilience is an active coping response to stress and suggest that it is a more challenging condition to be achieved and be maintained than vulnerability. In fact, resilience is a positive adaptation after stressful situation that allows to maintain or restore normal functioning over time by successfully adapting to change, resisting the negative impact of stressors and avoiding occurrence of significant dysfunctions over time [32]. On the contrary, vulnerable subjects seem to reach a plateau, losing the ability to implement changes in response to unfavorable stimuli, which is reflected in the anhedonic phenotype. In agreement with this notion, several alterations detected in vulnerable rats after 2 weeks of CMS [21] returned to baseline at 6 weeks, while they either stayed the same or showed an opposite profile in the resilient rats, such as in the NAD+/NADH ratio and in the acylcarnitine family.
When investigating the energetic status, both vulnerable and resilient rats show an increased ATP energy charge and a trend of reduction of NAD+/NADH ratio, revealing the energetic susceptibility of vHip to external stressors [33]. However, differences that might support the diverse response to stress emerge when we break down the energetic status into the major classes of metabolites, such as glycolysis. In fact, fructose Bis-P, DHAP/GAP and PEP are all increased in the resilient but not in the vulnerable animals, indicating that a different metabolism of glucose between the two distinct phenotypes may represent a strategy to deal with stress. Indeed, these changes result in a significant activation of glycolysis pathway specifically in resilient animals. Moreover, the modulations of DHAP/GAP and sedoheptulose in resilient animals may suggest a possible increase of the pentose phosphate pathway to generate more NADPH to protect against oxidative stress [34].
Another significant alteration that emerged from our study concerns the class of acylcarnitines, known to be indirect indicators of fatty acid catabolism occurring in mitochondria and known to be altered in depression [35]. Recent studies have shown that lipid metabolism, and more specifically fatty acids β-oxidation, plays a significant role in several adult brain functions [36–39]. Here, we observed that many carnitines, both short- and long chain-acylcarnitines, are downregulated in the vHip of resilient animals, while the ratio C2-Carnitine/C0-Carnitine and (C2-Carnitine + C3-Carnitine)/ C0-Carnitine are increased in vulnerable animals. These data indicate that the vulnerability to 6 weeks of CMS seems to be associated with a boosted fatty acid β-oxidation, an effect that contributes to the increase in the ATP energy charge in this specific subgroup of stressed animals. On the opposite, the augmentation of the ATP energy that was observed also in resilient rats seems to originate from an increase of glycolysis.
Interestingly, the shift from using glucose to fatty acid as energy source has been already observed under several brain pathological conditions [40–44]. Indeed, production of acyl-CoA through upregulated β-oxidation results in negative feedback to pyruvate, which results in decreased glucose metabolism, leading to a vicious cycle disrupting the homeostasis [45].
In support to our hypothesis, the modulation of CptI activity is involved in the progression of ALS [46] and in the development of the anhedonic phenotype in the CMS model [47].
Among the amino acids analyzed, Phe and Thr were significantly increased in resilient animals, indicating a possible need of these amino acids to produce more neurotransmitters and neuromodulators [48], whereas with respect to nucleotides-deoxynucleotides, the changes we observed may be related to alteration in neurogenesis mechanisms, as observed in the ventral hippocampus of stressed rats [49–51]. Further, we found that succinate was decreased specifically in vulnerable rats indicating a reduced production of the intermediates of the Krebs cycle and in line it has been found that antidepressants were able to modulate succinate dehydrogenase activity thus increasing energetic metabolism in different rat brain regions [52].
Considering the pharmacological treatment, non-stressed animals and CMS vulnerable animals treated with vehicle show a different metabolic pattern, while CMS vulnerable animals treated with VLX show an intermediate metabolic pattern compared with the two previous groups, as showed by the PLS-DA plot and confirmed by heatmap analysis. Moreover, as expected, the No stress/VLX animals were similar to the non-stressed untreated counterpart, highlighting that venlafaxine has a selective effect on the metabolic profile under pathological, but not physiological, conditions.
Interestingly, 17 out of the 30 metabolites changed in CMS vulnerable animals were also modified by venlafaxine treatment. In particular several metabolites of the classes of NTPs-dNTPs, biogenic amines and intermediates of the glycolysis were reverted by the chronic treatment with the venlafaxine. Interestingly, the antidepressant drug has normalized (C16:0-Carnitine+C18:0-Carnitine)/C0-Carnitine ratio, index of CptI activity, and glucose and lactate levels. Glucose is the primary source of energy for the mammalian brain [53] and it is tightly coupled with the regulation of cell death [54–56]. Moreover, even if lactate has long been associated with ischemia [57], more recent evidence demonstrates its importance for maintaining brain health [58]. These findings suggest that the efficacy of venlafaxine as antidepressant may in part depend on restoring normal levels of these metabolites crucial for healthy brain function.
Our study holds some potential limitations. First, although it is well-established that stress-related disorders occur with different frequency depending on sex, our studies were conducted only in male rats; thus, we do not know if our findings can be extended to female rats. However, given the present findings showing that energy metabolism does play a role in stress response, we will plan our future studies including females.
We are aware that we have used only one drug and further studies investigating the effect of other antidepressants may provide useful information and increase the impact of the potential role of brain metabolism in the treatment of psychiatric disorders. However, as mentioned in the introduction, VLX was chosen because of its ability to normalize the anhedonic phenotype [23, 24, 31] and also as one of the most effective approaches for the treatment of major depression [22].
Third, we have conducted the metabolomic approach only in one brain region. However, the rationale for this choice derives from our previous studies that indicated the ventral hippocampus as the main area to discriminate between resilient and vulnerable animals in molecular terms. Moreover, in attempt to find biomarkers of vulnerability or resilience we will next focus on the analysis of blood in stressed animals. Another limitation from a metabolic point of view is to unequivocally prove that vulnerability imposes the switch from glucose to fatty acids as main fuel source in the ventral hippocampus while the resilient condition continues to support the glucose utilization. To achieve this goal, in vivo, metabolic tracing experiments represent the gold standard. These are technically difficult experiments to achieve in vivo and nowadays they can only be performed in isolated brain-derived cells by losing the contribution of key cellular interaction occurring in the hippocampus (i.e. neurons with astrocytes).
Fourth, we focused on only one specific behavioral phenotype. Indeed, despite the heterogeneity of symptom manifestation in psychiatric disorders, we believe that the best approach is to focus on one deficit at a time. Therefore, the identification of anomalies associated, in this case, with anhedonia could be indicative for patients presenting such alterations and could be used to better stratify the population.
To summarize, we found that both resilient and vulnerable rats are characterized by higher ATP energy charge and Acetyl-CoA compared to No stressed rats. However, while in the vulnerable animals the energy support is obtained from the utilization of fatty acids, highlighting a possible deregulation of the mechanisms that regulate the utilization of glucose as primary source to produce energy within the brain, resilience, instead, seems to be achieved by an increase of glycolysis.
Finally, the normalization of ATP load following venlafaxine administration seems to be associated with normalization of CptI activity and of both glucose and lactate levels. Accordingly, the concomitant treatment with a specific inhibitor of CptI during stress exposure is efficient in ameliorating the development of the anhedonic phenotype [47].
These findings may provide novel perspectives on metabolic changes that could serve as diagnostic markers and preventive strategies for the early detection and treatment of MDD as well as the identification of potential drug targets.
Moreover, we also provide new insights on the metabolic mechanism of action of venlafaxine in the context of stress-related disorders, suggesting that it may promote adaptive mechanisms that are essential for the ability to modulate different pathological domains linked with psychiatric disorders.
Supplementary information
Author contributions
PB: conceptualization, formal analysis, data curation, investigation, writing original draft. MA: methodology, formal analysis. MTG: formal analysis. EM: formal analysis. PG: methodology. ML: methodology. EL: methodology. FF: review and editing. MP: methodology, review and editing. NM: data curation, review and editing. FC: conceptualization, visualization, writing original draft.
Funding
The behavioral part of the study was supported by the statutory activity of the Maj Institute of Pharmacology Polish Academy of Sciences (Krakow, Poland). The molecular study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca to FC (PRIN2017, Project number 2017MLC3NF), by the Ministry of University and Research (MUR) Progetto Eccellenza (2023–2027) to the Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano and partially by the Italian Ministry of Health with Ricerca Corrente and 5×1000 funds to NM Maria Teresa Gallo was supported by cycle XXXVII of the doctorate in Pharmacological Biomolecular Sciences, Experimental and Clinical, Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01633-0.
References
- 1.Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–85. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- 2.Giménez-Palomo A, Dodd S, Anmella G, Carvalho AF, Scaini G, Quevedo J, et al. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front Psychiatry. 2021;12:546801. doi: 10.3389/fpsyt.2021.546801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu X, Ke S, Wang Q, Zhuang T, Xia C, Xu Y, et al. Energy metabolism in major depressive disorder: Recent advances from omics technologies and imaging. Biomed Pharmacother. 2021;141:111869. doi: 10.1016/j.biopha.2021.111869. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald K, Krishnan A, Cervenka E, Hu G, Guadagno E, Trakadis Y. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am J Med Genet Part B Neuropsychiatr Genet. 2019;180:122–37. doi: 10.1002/ajmg.b.32680. [DOI] [PubMed] [Google Scholar]
- 5.Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, et al. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281–95. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triebelhorn J, Cardon I, Kuffner K, Bader S, Jahner T, Meindl K, et al. Induced neural progenitor cells and iPS-neurons from major depressive disorder patients show altered bioenergetics and electrophysiological properties. Mol Psychiatry. 2022. 10.1038/s41380-022-01660-1. [DOI] [PMC free article] [PubMed]
- 7.Pu J, Liu Y, Zhang H, Tian L, Gui S, Yu Y, et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol Psychiatry. 2021;26:4265–76. doi: 10.1038/s41380-020-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci. 2012;109:17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pu J, Liu Y, Gui S, Tian L, Yu Y, Wang D, et al. Effects of pharmacological treatment on metabolomic alterations in animal models of depression. Transl Psychiatry. 2022;12:175. doi: 10.1038/s41398-022-01947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pu J, Liu Y, Gui S, Tian L, Yu Y, Song X, et al. Metabolomic changes in animal models of depression: a systematic analysis. Mol Psychiatry. 2021;26:7328–36. doi: 10.1038/s41380-021-01269-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese F, Brivio P, Gruca P, Lason-Tyburkiewicz M, Papp M, Riva MA. Chronic Mild Stress-Induced Alterations of Local Protein Synthesis: A Role for Cognitive Impairment. ACS Chem Neurosci. 2017;8:817–25. doi: 10.1021/acschemneuro.6b00392. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese F, Brivio P, Sbrini G, Gruca P, Lason M, Litwa E, et al. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: The potential role for glucocorticoid receptor signalling. J Psychopharmacol. 2020;34:420–8. doi: 10.1177/0269881119895547. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese F, Savino E, Papp M, Molteni R, Riva MA. Chronic mild stress-induced alterations of clock gene expression in rat prefrontal cortex: modulatory effects of prolonged lurasidone treatment. Pharm Res. 2016;104:140–50. doi: 10.1016/j.phrs.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Brivio P, Sbrini G, Tarantini L, Parravicini C, Gruca P, Lason M, et al. Stress Modifies the Expression of Glucocorticoid-Responsive Genes by Acting at Epigenetic Levels in the Rat Prefrontal Cortex: Modulatory Activity of Lurasidone. Int J Mol Sci. 2021;22:6197. doi: 10.3390/ijms22126197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brivio P, Buoso E, Masi M, Gallo MT, Gruca P, Lason M, et al. The coupling of RACK1 with the beta isoform of the glucocorticoid receptor promotes resilience to chronic stress exposure. Neurobiol Stress. 2021;15:100372. doi: 10.1016/j.ynstr.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brivio P, Gallo MT, Gruca P, Lason M, Litwa E, Fumagalli F, et al. Resilience to chronic mild stress-induced anhedonia preserves the ability of the ventral hippocampus to respond to an acute challenge. Eur Arch Psychiatry Clin Neurosci. 2022. 10.1007/s00406-022-01470-0. [DOI] [PMC free article] [PubMed]
- 19.Rossetti A, Paladini MS, Colombo M, Gruca P, Lason-Tyburkiewicz M, Tota-Glowczyk K, et al. Chronic Stress Exposure Reduces Parvalbumin Expression in the Rat Hippocampus through an Imbalance of Redox Mechanisms: Restorative Effect of the Antipsychotic Lurasidone. Int J Neuropsychopharmacol. 2018;21:883–93. doi: 10.1093/ijnp/pyy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossetti AC, Papp M, Gruca P, Paladini MS, Racagni G, Riva MA, et al. Stress-induced anhedonia is associated with the activation of the inflammatory system in the rat brain: Restorative effect of pharmacological intervention. Pharmacol Res. 2016. 10.1016/j.phrs.2015.10.022. [DOI] [PubMed]
- 21.Brivio P, Audano M, Gallo MT, Gruca P, Lason M, Litwa E, et al. Metabolomic signature and mitochondrial dynamics outline the difference between vulnerability and resilience to chronic stress. Transl Psychiatry. 2022;12:87. doi: 10.1038/s41398-022-01856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutens B, Yrondi A, Rampon C, Guiard BP. Psychopharmacological properties and therapeutic profile of the antidepressant venlafaxine. Psychopharmacology. 2022;239:2735–52. doi: 10.1007/s00213-022-06203-8. [DOI] [PubMed] [Google Scholar]
- 23.Bialek K, Czarny P, Wigner P, Synowiec E, Barszczewska G, Bijak M, et al. Chronic Mild Stress and Venlafaxine Treatment Were Associated with Altered Expression Level and Methylation Status of New Candidate Inflammatory Genes in PBMCs and Brain Structures of Wistar Rats. Genes. 2021;12:667. doi: 10.3390/genes12050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wigner P, Synowiec E, Jóźwiak P, Czarny P, Bijak M, Białek K, et al. The Effect of Chronic Mild Stress and Venlafaxine on the Expression and Methylation Levels of Genes Involved in the Tryptophan Catabolites Pathway in the Blood and Brain Structures of Rats. J Mol Neurosci. 2020;70:1425–36. doi: 10.1007/s12031-020-01563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sixth Edition. San Diego, California USA: Elsevier Acad Press; 2007.
- 26.Ghaffari MH, Jahanbekam A, Sadri H, Schuh K, Dusel G, Prehn C, et al. Metabolomics meets machine learning: Longitudinal metabolite profiling in serum of normal versus overconditioned cows and pathway analysis. J Dairy Sci. 2019. 10.3168/jds.2019-17114. [DOI] [PubMed]
- 27.Durbin BP, Hardin JS, Hawkins DM, Rocke DM. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics. 2002;18:S105–10. doi: 10.1093/bioinformatics/18.suppl_1.S105. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics. 2006. 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed]
- 29.Pang Z, Zhou G, Ewald J, Chang L, Hacariz O, Basu N, et al. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc. 2022;17:1735–61. doi: 10.1038/s41596-022-00710-w. [DOI] [PubMed] [Google Scholar]
- 30.Guzmán M, Blázquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Ess Fat Acids. 2004;70:287–92. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Wigner P, Synowiec E, Czarny P, Bijak M, Jóźwiak P, Szemraj J, et al. Effects of venlafaxine on the expression level and methylation status of genes involved in oxidative stress in rats exposed to a chronic mild stress. J Cell Mol Med. 2020;24:5675–94. doi: 10.1111/jcmm.15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babić R, Babić M, Rastović P, Ćurlin M, Šimić J, Mandić K, et al. Resilience in Health and Illness. Psychiatr Danub. 2020;32:226–32. [PubMed] [Google Scholar]
- 33.Bigio B, Mathé AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci. 2016;113:7906–11. doi: 10.1073/pnas.1603111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuehne A, Emmert H, Soehle J, Winnefeld M, Fischer F, Wenck H, et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell. 2015;59:359–71. doi: 10.1016/j.molcel.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Deng K, Xue Y, Yang R, Yang R, Gong Z, et al. Carnitine and Depression. Front Nutr. 2022;9:853058. doi: 10.3389/fnut.2022.853058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce KD, Zsombok A, Eckel RH. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front Endocrinol. 2017;8:60. doi: 10.3389/fendo.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int. 2014;2014:472459. doi: 10.1155/2014/472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schönfeld P, Reiser G. Why does Brain Metabolism not Favor Burning of Fatty Acids to Provide Energy? - Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J Cereb Blood Flow Metab. 2013;33:1493–9. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taïb B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T. Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem. 2013. 10.1074/jbc.M113.506238. [DOI] [PMC free article] [PubMed]
- 40.Adibhatla RM, Hatcher JF. Altered Lipid Metabolism in Brain Injury and Disorders. Lipids Health Dis. Dordrecht: Springer Netherlands. p. 241–68. [DOI] [PMC free article] [PubMed]
- 41.Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S-J, Zhang J, Choi AMK, Kim HP. Mitochondrial Dysfunction Induces Formation of Lipid Droplets as a Generalized Response to Stress. Oxid Med Cell Longev. 2013;2013:1–10. doi: 10.1155/2013/925804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuang J-C, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, et al. Chronic social defeat stress disrupts regulation of lipid synthesis. J Lipid Res. 2010;51:1344–53. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cermenati G, Audano M, Giatti S, Carozzi V, Porretta-Serapiglia C, Pettinato E, et al. Lack of Sterol Regulatory Element Binding Factor-1c Imposes Glial Fatty Acid Utilization Leading to Peripheral Neuropathy. Cell Metab. 2015;21:571–83. doi: 10.1016/j.cmet.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front Mol Neurosci. 2018;11:10. doi: 10.3389/fnmol.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trabjerg MS, Andersen DC, Huntjens P, Oklinski KE, Bolther L, Hald JL, et al. Downregulating carnitine palmitoyl transferase 1 affects disease progression in the SOD1 G93A mouse model of ALS. Commun Biol. 2021;4:509. doi: 10.1038/s42003-021-02034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mørkholt AS, Wiborg O, Nieland JGK, Nielsen S, Nieland JD. Blocking of carnitine palmitoyl transferase 1 potently reduces stress-induced depression in rat highlighting a pivotal role of lipid metabolism. Sci Rep. 2017;7:2158. doi: 10.1038/s41598-017-02343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hüfner K, Galffy M, Egeter J, Giesinger JM, Arnhard K, Oberacher H, et al. Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines. Brain Sci. 2020;10:322. doi: 10.3390/brainsci10060322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–61. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levone BR, Cryan JF, O’Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress. 2015;1:147–55. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scaini G, Santos PM, Benedet J, Rochi N, Gomes LM, Borges LS, et al. Evaluation of Krebs cycle enzymes in the brain of rats after chronic administration of antidepressants. Brain Res Bull. 2010;82:224–7. doi: 10.1016/j.brainresbull.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–97. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danial NN, Gramm CF, Scorrano L, Zhang C-Y, Krauss S, Ranger AM, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–6. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 55.King A, Gottlieb E. Glucose metabolism and programmed cell death: an evolutionary and mechanistic perspective. Curr Opin Cell Biol. 2009;21:885–93. doi: 10.1016/j.ceb.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Mergenthaler P, Kahl A, Kamitz A, van Laak V, Stohlmann K, Thomsen S, et al. Mitochondrial hexokinase II (HKII) and phosphoprotein enriched in astrocytes (PEA15) form a molecular switch governing cellular fate depending on the metabolic state. Proc Natl Acad Sci. 2012;109:1518–23. doi: 10.1073/pnas.1108225109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taher M, Leen WG, Wevers RA, Willemsen MA. Lactate and its many faces. Eur J Paediatr Neurol. 2016;20:3–10. doi: 10.1016/j.ejpn.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Proia P, Di Liegro C, Schiera G, Fricano A, Di Liegro I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int J Mol Sci. 2016;17:1450. doi: 10.3390/ijms17091450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.