Abstract
Introduction
Vascular complications like superior mesenteric artery (SMA) thrombosis and pancreaticoduodenal artery (PDA) pseudoaneurysm carry high morbidity and mortality. SMA provides the primary arterial supply to the small intestine and ascending colon. PDA aneurysms are extremely rare, accounting for only 2 % of all visceral artery aneurysms. We present a rare case of SMA thrombosis with concomitant PDA pseudoaneurysm.
Case presentation
Herein is the case of a 60-year-old male who presented with rectorrhagia, persistent generalized abdominal pain. After being diagnosed with colitis and mesenteric artery thrombosis based on a computed tomography (CT) scan, he was discharged from the hospital with rivaroxaban and mesalazin. However, he had to return to the hospital due to worsening of the symptoms. After a proper workout, SMA artery thrombosis with a concomitant PDA pseudoaneurysm was diagnosed for him. Therefore, he underwent surgery to stent the thrombosis and coil the pseudoaneurysm. His symptoms dramatically improved after the treatment.
Discussion
Angiography is the diagnostic and, with embolization, therapeutic procedure of choice, with surgery as a backup if embolization fails. However, even with these procedures, the mortality rate is high if the pseudoaneurysm ruptures.
Conclusion
In order to carry out the proper choice of surgical treatment before further complications occur, SMA thrombosis and PDA pseudoaneurysms must be investigated in each patient presenting with nonspecific abdominal pain, regardless of the risk factors.
Keywords: Superior mesenteric artery thrombosis, Pancreaticoduodenal artery pseudoaneurysm, Mortality, Thrombosis, Pseudoaneurysm
Highlights
-
•
Vascular complications carry a high morbidity and mortality rate.
-
•
Non-specific abdominal pain can be considered a warning sign for vascular complications.
-
•
SMA thrombosis and PDA pseudoaneurysms must be investigated in each patient presenting with nonspecific abdominal pain.
-
•
SMA thrombosis can occur with concomitant PDA pseudoaneurysms.
1. Introduction
Mesenteric artery thrombosis involves the gastrointestinal system's arterial blood flow. It is a serious condition with a high mortality risk that typically affects the SMA, which provides the small intestine and ascending colon with their primary arterial supply [1]. A reported 12.9 per 100,000 person-year incidence of mesenteric artery thrombosis has been noted [2]. The necessity of early diagnosis and treatment of SMA thrombosis is emphasized by the high mortality rate of this illness [3].
PDA pseudoaneurysms are uncommon vascular diseases with significant death rates after rupture. All pseudoaneurysms must be treated, regardless of size or symptoms, due to the significant potential for rupture and hemorrhage [4]. The presentation may be variable and include unspecific epigastric pain, nausea, vomiting, or hemorrhagic shock due to its rupture. While real aneurysm rupture typically results in retroperitoneal bleeding, pseudoaneurysm rupture is more likely to induce gastrointestinal (GI) bleeding [5]. In the following case report, we present a unique SMA thrombosis with a concomitant PDA pseudoaneurysm. The work was reported in accordance with the SCARE guidelines [6].
2. Case presentation
A 60-year-old man was admitted to our hospital with rectorrhagia and persistent generalized abdominal pain. The pain first appeared six weeks ago, was periodic, radiated slightly to the lower back, and was unrelated to eating. The rectorrhagia was one time with a small amount of blood. Anorexia, nausea, and periodic vomiting that contained bile were also reported. There were no obvious urinary symptoms. Gas passage was typical, and the latest bowel habit occurred within the day before. Additionally, the patient complained of a weight loss of 10 kg since the onset of his symptoms. He reported no previous medical or drug history except for using chlordiazepoxide for his sleeping disorder. He also had a history of left knee meniscus repair surgery 18 years ago.
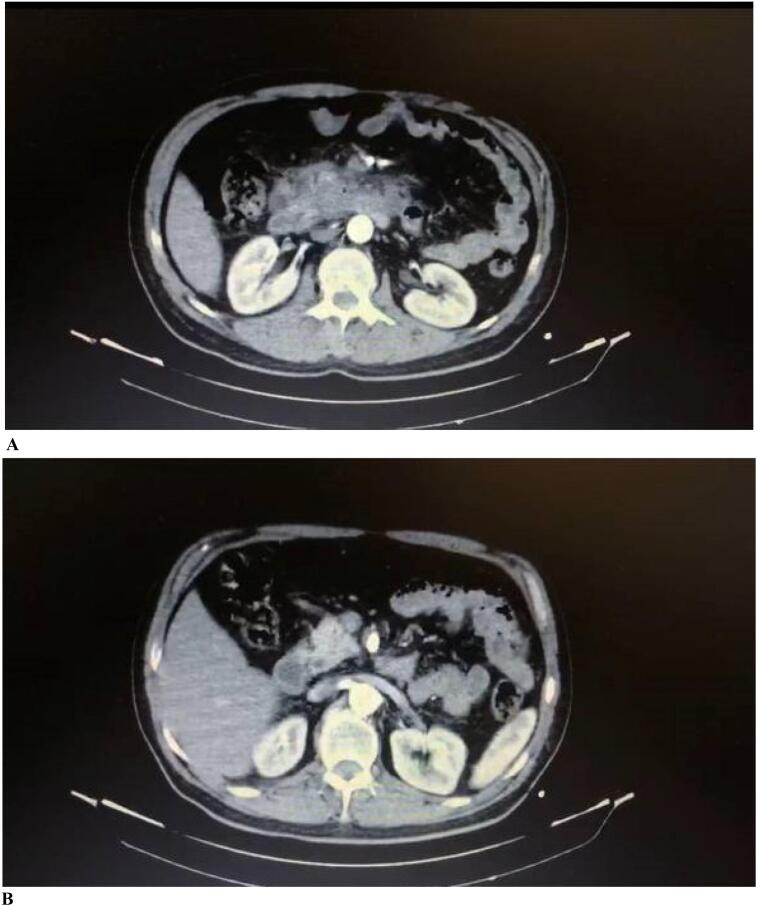
He had a prior hospital admission for this complaint during the early days of his symptoms. In the prior admission, a computed tomography (CT) scan of the abdomen with IV contrast was done for him, which revealed a collection with central necrosis and peripheral enhancement along with fat stranding measuring 120 ∗ 70 mm transaxially and 80 mm craniocaudally, with the origin of the duodenum at place D2 (the second part of the duodenum) in proximity to the pancreatic head and unicate process (Fig. 1A). This caused infiltration of the D2 and D3 walls as well as the stomach. The collection mentioned was suggested pseudoanurysm based on its vascular pattern. After the SMA artery's recognition, a thrombus of size 15 mm was also visible, which caused the artery to severely narrow (Fig. 1B). FNA (fine needle aspiration) from the collection at the pancreatic head and duodenum was carried out based on the findings of the CT scan. The fluid had a chocolate-colored appearance. No malignant cells were reported in the FNA result.
Fig. 1.
A. The collection with the origin of duodenum in proximity to the pancreatic head and unicate process.
Fig. 1B. Narrowing of the SMA artery.
He was discharged from that hospital on mesalazine and rivaroxaban after being diagnosed with colitis and mesenteric thrombosis. But as his pain got worse from 4 days ago, he had to rush to our emergency room.
In his initial physical examination during his admission to our hospital, He was found to be conscious, neither toxic nor ill. His mucosa was also not dry, and his sclera was not icteric. His lungs were clear during auscultation. His abdomen was soft and not distant during the examination. He had generalized tenderness upon superficial palpation of the abdomen. There was no guarding or rigidity present. No organomegaly was detected.
The vital signs were as follows: Blood pressure was 110/70 mmHg, Pulse rate was 86 per minute, Respiratory rate was 16 per minute, and body temperature was 37 °C. Afterwards, an NG tube was inserted, 1.5 l of GI contents came back to the NG bag, and the abdominal pain was alleviated shortly after the procedure.
He was subsequently brought to the surgical ward. The results of the laboratory tests after serum therapy were as follows: CBC and platelet count were in the normal range: PH = 7.359, Pco2 = 47.4, HCO3 = 25.1, and po2 = 99.8. Additionally, an INR of 1.7 was found. Following that, two units of FFP were ordered. When the patient was questioned regarding any coagulopathy disorders once more, he gave a negative response. The mentioned INR was thought to be an adverse effect of Rivaroxaban use. The Partial prothrombin time (PTT) was reported 33 s (normal range: 25–35 s). The Amylase and lipase levels were within the normal range.
Abdominal ultrasound demonstrated that there was fat stranding with hazy boundaries in the pancreatic head. The ultrasound also revealed a hypo-hetero echo area with a 128 mm diameter and a 300 ml volume around the pancreas neck, which is indicative of a hematoma. Additionally, an echogenic region within the SMA artery lumen prior to branching was observed, which may be causing partial occlusion and represents partial artery thrombosis. Endoscopy revealed one large ulcer, severe mucosal erythema, and stenosis in the distal region of the duodenum (D2) that appeared to be brought on by external pressure.
Cholangiopancreatography (MRCP) showed that the pancreatic and bile ducts appeared to be normal. Additionally, there was evidence of a pseudoaneurysm of the celiac artery branches surrounding the head of the pancreas and a hematoma around the duodenum. Embolization was advised based on the location of the hematoma and the existence of the pseudoaneurysm. The decision was made to operate on the patient in order to coil the pseudoaneurysm and stent the SMA artery at the location of its stenosis based on the imaging results and the diagnosis of SMA thrombosis and PDA pseudoaneurysm.
The patient underwent an angiography of the organs in order to stent the SMA artery and coil the pseudoaneurysm; under general anesthesia, the femoral area of the right leg and brachial area of the left arm were cannulated, and a 7F sheet was then placed. A pigtail catheter was inserted using a hydrophilic guidewire. Aortogram was done from two views. The SMA artery measured roughly 8 cm in length. Guide wires were used to determine the SMA artery's origin, and then a cover stent 38 for the origin and a Bare stent 38 were used to stent the remaining portion (Video 1.A). The SMA artery and all of its branches were enhanced during control angiography. In the next step, the celiac artery and, following that, the hepatic artery was cannulated. The gastroduodenal artery was identified and cannulated during a selective angiography that was carried out with the aid of a guidewire. The pseudoaneurysm was coiled using four nester coils after a microcatheter was introduced (Video 1.B). The pseudoaneurysm in the control angiogram was sealed. All of the catheters and guidewires were eventually removed, along with the sheet. The patient was stable following surgery. He resumed oral food three days later, and all of his gastrointestinal complaints resolved dramatically. The patient was discharged from the hospital in good condition. During the follow-up visit one month after, he regained his original weight and had no other complaints.
3. Discussion
According to reports, the prevalence of SMA thrombosis is 12.9 per 100,000 person-years [7]. The coexistence of SMA artery thrombosis and PDA pseudoaneurysms is extremely rare. To our knowledge, this is the first case reported with the mentioned complications. The occlusion of the SMA artery may be caused by in-situ thrombosis of the vessel, which most frequently results from underlying atherosclerotic disease, or embolic occlusion from a remote source, which may happen in people with atrial fibrillation [8]. SMA thrombosis cases are frequently older than 60 years and present with symptoms like nausea, vomiting, abdominal pain, and weight loss [3]. A further misleading factor in this case was the occurrence of SMA artery occlusion despite the absence of established risk factors such as atrial fibrillation, heart illness, coagulopathy disorders, inflammatory bowel disease (IBD), and others. Concern was solely raised regarding age.
CT angiography is the gold standard for diagnosis and has a sensitivity of 96 % and a specificity of 94 % in detecting acute and chronic forms of mesenteric ischemia [7]. When diagnosed, patients need immediate intervention with aggressive fluid resuscitation, anticoagulation medications, and laparotomy, if peritoneal irritation signs are present [8]. The cause of the PDA pseudoaneurysm remains unknown in this case; it could have resulted from pancreatitis or a trauma during the FNA sampling. In addition to removing the infarcted bowel, acute SMA occlusion is treated by embolectomy, thromboendarterectomy, or bypass grafting. Anticoagulation and vasodilators are frequently used as conservative treatments for multiple, small, distal SMA emboli [9]. Nevertheless, the thrombosis was located close to the proximal root of the SMA; therefore, discharging the patient on anticoagulation did not alleviate the symptoms.
4. Conclusion
In order to carry out the proper choice of surgical treatment before further complications occur, SMA thrombosis and PDA pseudoaneurysms must be investigated in each patient presenting with nonspecific abdominal pain, regardless of the risk factors.
The following are the supplementary data related to this article.
Stenting the artery
Coiling of the artery
Insufflation and deployment of the stent
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Our case report is exempt from ethical approval in our institution. The reason is, it is case report only.
Funding
No funding.
Guarantor
Corresponding author: Delaram Sakhaei
Islamic Azad University of Medical Sciences, Sari Branch, Sari, Iran. Email address: deli.sakhaei@gmail.com
Research registration number
Not applicable.
CRediT authorship contribution statement
MM contributed to the research concept and design, analysis and interpretation. HM contributed to the data collection and writing the manuscript. EP contributed to the data collection and treatment design of the study. DS contributed to writing the manuscript and reviewer of the manuscript. MZ contributed to the writing the manuscript and reviewer of the manuscript. SI contributed to reviewer of the manuscript. All authors reviewed the final draft of the manuscript.
Declaration of competing interest
No conflicts of interest.
Acknowledgment
Not applicable.
References
- 1.Liao G., Chen S., Cao H., Wang W., Gao Q. Review: acute superior mesenteric artery embolism: a vascular emergency cannot be ignored by physicians. Medicine (Baltimore) 2019;98(6) doi: 10.1097/MD.0000000000014446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin. Vasc. Surg. 2010;23(1):4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Cheung S., Quiwa J.C., Pillai A., Onwu C., Tharayil Z.J., Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao S.B., Johnson D.B., Bonatti H.J.R. Pseudoaneurysm of the pancreaticoduodenal artery associated with duodenal diverticulitis. Case Rep. Surg. 2019;2019:2831234. doi: 10.1155/2019/2831234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitrovic Jovanovic M., Tadic B., Jankovic A., Stosic K., Lukic B., Cvetic V., et al. Endovascular treatment of a pseudoaneurysm of the posterior inferior pancreaticoduodenal artery as a complication of chronic pancreatitis: a case report. J. Int. Med. Res. 2022;50(2) doi: 10.1177/03000605221083441. 3000605221083441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Olson M.C., Fletcher J.G., Nagpal P., Froemming A.T., Khandelwal A. Mesenteric ischemia: what the radiologist needs to know. Cardiovasc. Diagn. Ther. 2019;9(Suppl. 1):S74. doi: 10.21037/cdt.2018.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta P.K., Natarajan B., Gupta H., Fang X., Fitzgibbons R.J., Jr. Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery. 2011;150(4):779–787. doi: 10.1016/j.surg.2011.07.079. [DOI] [PubMed] [Google Scholar]
- 9.Kuhelj D., Kavcic P., Popovic P. Percutaneous mechanical thrombectomy of superior mesenteric artery embolism. Radiol. Oncol. 2013;47(3):239–243. doi: 10.2478/raon-2013-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stenting the artery
Coiling of the artery
Insufflation and deployment of the stent