Abstract
Vaccinia virus encodes two protein kinases (B1 and F10) and a dual-specificity phosphatase (VH1), suggesting that phosphorylation and dephosphorylation of substrates on serine/threonine and tyrosine residues are important in regulating diverse aspects of the viral life cycle. Using a recombinant in which expression of the H1 phosphatase can be regulated experimentally (vindH1), we have previously demonstrated that repression of H1 leads to the maturation of noninfectious virions that contain several hyperphosphorylated substrates (K. Liu et al., J. Virol. 69:7823–7834). In this report, we demonstrate that among these is a 25-kDa protein that is phosphorylated on tyrosine residues in H1-deficient virions and can be dephosphorylated by recombinant H1. We demonstrate that the 25-kDa phosphoprotein represents the product of the A17 gene and that A17 is phosphorylated on serine, threonine, and tyrosine residues during infection. Detection of phosphotyrosine within A17 is abrogated when Tyr203 (but not Tyr3, Tyr6, or Tyr7) is mutated to phenylalanine, suggesting strongly that this amino acid is the site of tyrosine phosphorylation. Phosphorylation of A17 fails to occur during nonpermissive infections performed with temperature-sensitive mutants defective in the F10 kinase. Our data suggest that this enzyme, which was initially characterized as a serine/threonine kinase, might in fact have dual specificity. This hypothesis is strengthened by the observation that Escherichia coli induced to express F10 contain multiple proteins which are recognized by antiphosphotyrosine antiserum. This study presents the first evidence for phosphotyrosine signaling during vaccinia virus infection and implicates the F10 kinase and the H1 phosphatase as the dual-specificity enzymes that direct this cycle of reversible phosphorylation.
Protein phosphorylation has emerged as a major regulator of numerous intracellular processes. Networks of kinases and phosphatases that add and remove phosphate to Ser, Thr, and Tyr residues regulate the orderly transition of eukaryotic cells through the cell cycle and activate checkpoints that halt this transition in response to intra- and extracellular stresses. Similarly, when extracellular signals contact membrane-bound receptors, the signal is often transduced to the nucleus by means of a cascade of phosphorylation events which converge on the mitogen-activated protein kinases. These reversible phosphorylation events can modulate such properties as protein stability, translocation to intracellular compartments, catalytic activity, and the ability to interact with other proteins, membranes, or nucleic acids. The number of kinases and phosphatases within mammalian cells is daunting, with estimates for each exceeding 1,000. Simpler eukaryotes such as fission and budding yeasts, which encode fewer kinases and phosphatases and are exceptionally amenable to genetic analysis, have proven somewhat simpler to decipher.
In recent years, it has become clear that bacteria and viruses also utilize reversible protein phosphorylation as a means of biological regulation. Our laboratory has been involved in an analysis of the two protein kinases (B1 and F10) and one protein phosphatase encoded by vaccinia virus. The B1 kinase (1, 15, 36) is expressed at early times after infection and appears to be essential for viral DNA replication (23, 24). B1 is known to phosphorylate several ribosomal proteins (2) and may also play a role in regulating subsequent phases of protein synthesis (12a). The F10 kinase, which is expressed at late times of infection and is the major kinase encapsidated within virions (14), is essential for the initiation of virion morphogenesis (37, 39). When infections with temperature-sensitive (ts) mutants containing lesions in the F10 gene are maintained at high temperature, no visible signs of virion morphogenesis are seen despite the unperturbed synthesis of late viral proteins.
The H1 phosphatase, which is also expressed at late times and encapsidated (9, 17), was the first phosphatase shown to be able to dephosphorylate Ser, Thr, and Tyr residues. A significant number of dual-specificity phosphatases have now been discovered, and they have been grouped into four classes (18). The H1 enzyme encoded by vaccinia virus is the prototype of class I, which contains enzymes encoded by poxviruses, baculoviruses, yeast, and human cells. Although the dual-specificity enzymes retain the active-site sequence motifs and the catalytic mechanism of Tyr-specific phosphatases, they resemble each other more closely than they do the traditional Tyr-specific phosphatases. We have previously described the construction of an inducible vaccinia virus recombinant in which expression of the H1 phosphatase is dependent on the inclusion of isopropyl-β-d-thiogalactopyranoside (IPTG) in the culture medium (17). Using this recombinant, we have shown that H1 is essential for ensuring the infectivity and transcriptional competence of nascent virions. As one approach to clarifying the role of H1 in ensuring virion infectivity, we have attempted to identify viral phosphoproteins that are H1 substrates. As we have previously reported, the preparation of 32P-labeled wild-type (wt) and H1-deficient virions facilitated the identification of 25-, 16-, and 11-kDa proteins that were hyperphosphorylated in H1-deficient virions. The 11-kDa species represents the DNA-binding protein encoded by the F18 gene (17) (designated F17 in the Copenhagen strain [11]); we have recently determined that the 16-kDa species represents the membrane protein encoded by the A14 gene (38). By demonstrating that recombinant H1 could reverse the hyperphosphorylation of these two proteins in vitro, we confirmed the enzyme-substrate relationship that was suggested by our genetic data. Both F18 and A14 are hyperphosphorylated on Ser residues in the absence of H1 expression. Because H1 has been shown to have dual specificity in vitro (9), and because Tyr phosphorylation is such an important feature of diverse regulatory networks, we were interested in determining whether any viral proteins would exhibit hyperphosphorylation on Tyr residues under conditions of H1 repression. In this report, we describe the identification and characterization of a virion component that is indeed phosphorylated on Tyr residues as well as on serine/threonine residues. Moreover, we provide evidence that the reversible phosphorylation of this protein, which is the product of the A17 gene, is regulated by the F10 kinase as well as the H1 phosphatase.
(Much of this work was presented at the American Society of Virology [19 to 23 July 1997, Bozeman, Mont.] and International Poxvirus [6 to 10 June 1998, St. Thomas, U.S. Virgin Islands] symposia; at the latter meeting, we learned that the laboratory of Bernard Moss [National Institutes of Health] has obtained similar results regarding the phosphorylation of A17 [3].)
MATERIALS AND METHODS
Cells and viruses.
BSC40 cells were maintained in Dulbecco modified Eagle medium (DMEM; GIBCO BRL) containing 5% fetal bovine serum (GIBCO BRL). wt vaccinia virus (WR strain), vindH1 (17), vindA17 (41) (vA17LΔ5; kindly provided by B. Moss), and ts15 and ts28 (gift from R. Condit) (5, 37) were amplified in monolayer cultures of BSC40 cells or suspension cultures of L cells. Viral stocks were prepared from cytoplasmic lysates of infected cells by ultracentrifugation through 36% sucrose; titrations were performed on BSC40 cells. In some cases, virions were further purified by banding on 25 to 40% sucrose gradients (17). For ts mutants, 31.5 and 39.5°C were used as the permissive and nonpermissive temperatures, respectively. For vindA17, permissive infections were performed in the presence of IPTG (5 μM). Where indicated, rifampin was added to a final concentration of 100 μg/ml.
Metabolic labeling of proteins. (i) Labeling with 32PPi.
Confluent BSC40 cell monolayers were rinsed with phosphate-buffered saline and infected with wt, vindH1, or ts28 (multiplicity of infection [MOI] of 2). Cells were refed with complete medium after a 30- to 60-min adsorption period. At 3 h postinfection (hpi), cells were rinsed with phosphate-free DMEM (ICN Biomedicals, Inc., Costa Mesa, Calif.) and fed with phosphate-free DMEM supplemented with 50 μCi of 32PPi (Dupont NEN, Boston, Mass.) per ml and 5% fetal calf serum that had been rendered phosphate free by dialysis against Tris-buffered saline (25 mM Tris-HCl [pH 7.4], 136 mM NaCl, 2.7 mM KCl). Cells were harvested at 17 hpi.
(ii) Labeling with [35S]methionine.
At 6 h after infection with vindH1, BSC40 cells were rinsed with methionine-free DMEM (ICN Biomedicals) and incubated for 60 min with methionine-free DMEM supplemented with 100 μCi of [35S]methionine (Dupont NEN) per ml.
Immunodetection analyses.
Rabbit polyclonal antiphosphotyrosine (anti-pTyr) serum was obtained from Transduction Laboratories (Lexington, Ky.); monoclonal anti-pTyr antibody was a generous gift from N. Tonks (Cold Spring Harbor Laboratories, Cold Spring Harbor, N.Y.). D. Hruby (Oregon Health Sciences Center, Corvallis) kindly provided rabbit anti-A17L and anti-L1 antisera. Rabbit anti-I3, anti-L4, and anti-A14 sera were developed in our laboratory (17, 25, 38). For immunoblot analysis, cell and virion extracts were prepared in the presence of 1 mM sodium orthovanadate (Sigma, St. Louis, Mo.) prior to fractionation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer to nitrocellulose (Schleicher & Schuell, Keene, N.H.) or Immobilon P (Millipore Corp., Bedford, Mass.) membranes. Transfer was performed in 3-[(cyclohexylamino)-1-propanesulfonic acid (CAPS) transfer buffer (10 mM CAPS in 10% methanol, pH 11.3). Primary sera are described above. Secondary antibodies (horseradish peroxidase [HRP]- or alkaline phosphatase-conjugated goat anti-rabbit or goat anti-mouse) were obtained from Bio-Rad (Richmond, Calif.) and used according the manufacturer’s instructions. Blots were developed colorimetrically or by enhanced chemiluminescence (ECL) (DuPont NEN or Pierce, Rockford, Ill.). For immunoprecipitation analysis, cells were rinsed with phosphate-buffered saline and lysed in 1× PLB (0.1 M NaPO4 [pH 7.4], 0.1 M NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate) containing 1 mM sodium orthovanadate (1.2 ml per 3 × 106 cells). Clarified lysates were incubated with primary serum for >4 h and then with protein A-Sepharose (Sigma) for 1.5 h; immunoprecipitates were then retrieved, washed extensively, and analyzed by SDS-PAGE and autoradiography or fluorography.
Virion fractionation.
wt and H1-deficient virions were purified from cytoplasmic lysates of infected cells by ultracentrifugation through 36% sucrose and subsequent banding on 25 to 40% sucrose gradients. Banded virions were concentrated by an additional ultracentrifugation; 109 particles were incubated at 37°C for 30 min in a 100-μl reaction mix containing 100 mM dithiothreitol (DTT), 100 mM Tris (pH 8.0), 0.1% Nonidet P-40 (NP-40), and 1 mM sodium orthovanadate. The permeabilized virions were partitioned into membrane and core fractions by centrifugation (14,000 × g, 1 h, 4°C).
Phosphatase treatment of H1-deficient virions.
H1-deficient virions (9 μg) were incubated with 1.5 μg of recombinant H1 phosphatase or the catalytically inert H1C110S mutant, which were prepared as previously described (17). Treatment was for 1 h at 37°C in 40 μl of 50 mM Tris (pH 8)–50 mM DTT–0.05% NP-40.
Construction of A17 alleles containing Tyr→Phe mutations: identification of Tyr-phosphorylated residue(s).
The wt A17 open reading frame (ORF) and five mutant alleles that would direct Tyr→Phe substitutions at codons 3, 6, 7, 3+6+7, or 203 were amplified by PCR. The 5′ primer introduced a ClaI site upstream of the ORF, and the 3′ primer introduced a BamHI site downstream of the ORF. These sites were used to insert the A17 sequences into a modified pUC under the transcriptional regulation of a strong, late poxvirus promoter, the cowpox ATI promoter (20, 38). The primers used for wt A17 were U (5′ CCATCG ATG AGT TAT TTA AGA 3′) and D (5′ GCGGATCC TTA ATA ATC GTC AGT ATT 3′). The primers used for Y3F A17 were 3 (5′ CCATCG ATG AGT TTC TTA AGA TAT TAC) and D. The primers used for Y6F A17 were 6 (5′ CCATCG ATG AGT TAT TTA AGA TTC TAC AAT 3′) and D. The primers used for Y7F A17 were 7 (5′ CCATCG ATG AGT TAT TTA AGA TAT TTC AAT ATG 3′) and D. The primers used for Y3,6,7F A17 were 367 (5′ CCATCG ATG AGT TTC TTA AGA TTC TTC AAT ATG CTT 3′) and D. The primers used for Y203F A17 were U and 203 (5′ GCGGATCC TTA AAA ATC GTC AGT ATT TAA AC 5′). The sequences of all of the constructs were determined and shown to be accurate, and the constructs were then used in transient transfection assays. By using Lipofectamine Plus (GIBCO BRL), 5 μg of each construct was applied to parallel cultures of BSC40 cells (1.2 × 106 cells) at 3 h after infection with vindA17 (MOI of 10) in the absence of IPTG. Cells were harvested at 30 hpi. As controls, cells were transfected with empty vector or left untransfected (with and without IPTG). Expression of the wt and mutant alleles of A17 within transfected cells was evaluated by immunoblot analysis using anti-A17 antiserum; the levels of tyrosine phosphorylation of the A17 proteins was assessed by immunoblot analysis using anti-pTyr antiserum.
Induction and purification of recombinant F10 kinase.
The F10 ORF was prepared by PCR using the HindIII F fragment of the vaccinia virus (WR strain) genome as a template and primers 1 (5′ CCGGATCCATATGTTAGTTGCCAATGAT 3′) and 2 (5′ TCGGATCCCTATTAGTTTCCGCCATTTAT 3′). The product generated with these primers contained the full-length F10 open reading frame, extending from the initiating ATG codon through the TAA termination codon (underlined). The upstream primer introduced an NdeI site that overlapped the initiating ATG codon, and the downstream primer introduced a BamHI site (boldface in each primer) downstream of the termination codon. The F10 ORF contains an internal NdeI site. Therefore, the ORF was cloned into pET16b (35) (Novagen, Madison, Wis.) in two steps: a C-terminal NdeI/BamHI fragment was cloned first, and then the N terminus was repaired by insertion of an NdeI/NdeI fragment in the proper orientation. The resulting clone encoded an F10 ORF predicted to contain a 27-amino-acid N-terminal extension that included a decahistidine tag. In this plasmid, the F10 ORF is under the regulation of the bacteriophage T7 promoter and the lac operator/repressor. pET16b-F10 was maintained in Escherichia coli BL21(DE3), where F10 expression was induced by the addition of IPTG. Mid-log-phase cultures were induced by the addition of IPTG (0.2 mM); ethanol (EtOH) was added (2%) to increase the fraction of F10 that would remain soluble. Induced cultures were placed on ice for an initial 30 min and then grown at 18°C with vigorous agitation for 48 h. Cultures were then harvested, and soluble lysates were prepared and subjected to batch chromatography on Ni2+-agarose (Qiagen, Valencia, Calif.). The resin was developed with increasing concentrations of imidazole; F10 was eluted from the column with 100 mM imidazole. The eluant was concentrated by sedimentation in a Centricon concentrator and stored at −80°C in 25% glycerol.
Kinase assays.
F10 kinase activity was verified by using myelin basic protein (MBP) as a substrate. Reaction mixes (25 μl) containing 2.5 μg of MBP, 5 μM [γ-32P]ATP (2.5 μCi/125 pmol), 50 mM Tris (pH 7.4), 10 mM MgCl2, 1 mM DTT, and 75 to 400 ng of pure F10 were incubated for 30 min at room temperature. Phosphorylated MBP was visualized by SDS-PAGE and autoradiography. To test whether F10 could phosphorylate sequences corresponding to the extreme C terminus of the A17 protein, the peptide N′ RRRTFNSLNTDDY C′ was synthesized (Protein Nucleic Acid Shared Facility, Medical College of Wisconsin). The purity and accuracy of the peptide were verified by high-pressure liquid chromatography and amino acid analysis. Kinase reaction mixes (25 μl) contained 75 ng of F10 kinase, peptide (5 to 500 μM), 5 μM [γ-32P]ATP (2.5 to 10 μCi/125 pmol), 50 mM Tris (pH 7.4), 10 mM MgCl2, and 1 mM DTT. Control reactions lacked the F10 kinase or the peptide substrate. After reactions were performed for the time indicated (10 or 30 min), they were spotted onto P81 phosphocellulose paper (Whatman, Ltd.) and washed in 75 mM orthophosphoric acid for 5 min with agitation (4, 21). Filters were then rinsed in acetone and left to air dry; the levels of radiolabeled peptide bound to the filter were quantitated by Cerenkov counting in a Beckman scintillation counter. The values obtained for control reactions lacking kinase or substrate were averaged and subtracted from the remaining experimental values. The data were plotted by using Sigma Plot 4.0 (SPSS Inc., Chicago, Ill.). Filters were also exposed overnight to Kodak MR film for autoradiographic visualization.
Preparation of figures.
For Fig. 1 to 4 and 6, scans of original autoradiographs and immunoblots were obtained with a Linotype-Hell Saphir Scanner. Images were adjusted to best resemble the original data with Photoshop 4.0 (Adobe Systems Inc., San Jose, Calif.) and then labeled by using Canvas 5.0 (Deneba Software, Miami, Fla.).
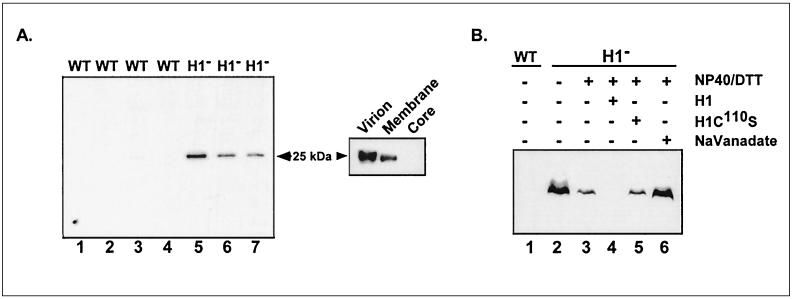
FIG. 1.
Identification of an encapsidated H1 substrate that is phosphorylated on Tyr residues. (A) A 25-kDa pTyrosine-containing protein is found within the membranes of H1-deficient virions. (Left) Seven independently prepared stocks of wt virions (lanes 1 to 4) and H1-deficient virions (lanes 5 to 7) (approximately 3 μg of each) were subjected to SDS-PAGE and immunoblot analysis. After incubation of the nitrocellulose filters with a polyclonal anti-pTyr serum and an HRP-conjugated secondary antiserum, ECL development allowed the immunoreactive proteins to be visualized on Kodak MR film. (Right) H1-deficient virions (4 μg) were partitioned into membrane and core fractions as described in Materials and Methods. pTyr-containing proteins were detected by immunoblot analysis as described above; only the relevant portion of the filter is shown. (B) The H1 phosphatase can dephosphorylate the 25-kDa protein in vitro. wt or H1-deficient virions (9 μg) were analyzed directly (lanes 1 and 2) or permeabilized with NP-40 plus DTT and then incubated for 1 h at 37°C in the absence (lane 3) or presence (lane 6) of 10 mM sodium orthovanadate or after the addition of 1.5 μg of active or catalytically inert H1 phosphatase (H1 [lane 4] and H1C100S [lane 5], respectively). The samples were then subjected to SDS-PAGE and immunoblot analysis with anti-pTyr serum as described above.
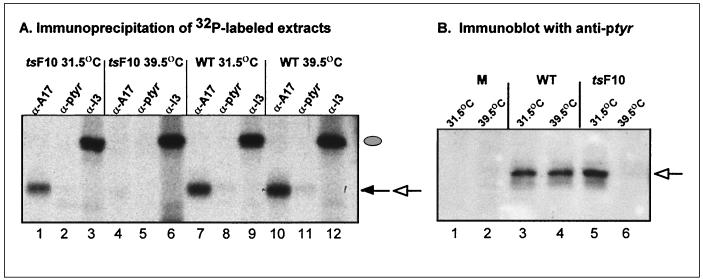
FIG. 4.
Phosphorylation of A17 is abrogated during nonpermissive infections with ts mutants defective in the F10 kinase. (A) Incorporation of 32PPi into A17 is not observed during nonpermissive infections with ts28 (tsF10). Confluent BSC40 cells were infected (MOI of 2) with wt virus or ts28 (tsF10) and maintained at the permissive (31.5°C) or nonpermissive (39.5°C) temperature. Cultures were metabolically labeled with 32PPi from 3 to 17 hpi, harvested, and subjected to immunoprecipitation with anti-A17 (lanes 1, 4, 7, and 10), anti-pTyr (lanes 2, 5, 8, and 11), or anti-I3 (lanes 3, 6, 9, and 12) serum. Incorporation of 32PPi into I3 (filled oval) was equivalent under all conditions, whereas incorporation of 32PPi into A17 (filled arrow) was lost in cells infected with ts28 at 39.5°C (compare lane 4 with lanes 1, 7, and 10). Since the H1 phosphatase was active during all of these infections, recovery of 32P-labeled A17 with anti-pTyr serum (open arrow) was minimal. (B) A17 does not contain immunoreactive pTyr in cells infected with ts28 under nonpermissive conditions. Cells were left uninfected or infected with wt virus or ts28 (tsF10) under permissive and nonpermissive conditions (MOI of 10). At 12 hpi, cells were harvested and subjected to immunoblot analysis with anti-pTyr serum. All of the immunoreactive pTyr in the A17 protein (open arrow) was lost during nonpermissive infections with ts28 (compare lane 6 with lanes 3 to 5).
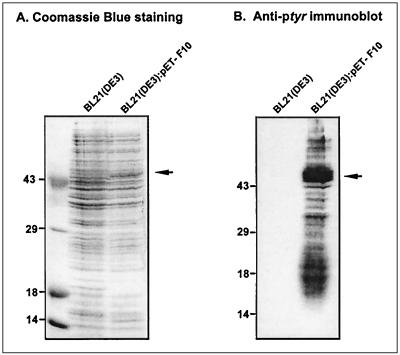
FIG. 6.
E. coli cultures induced to express active F10 kinase contain multiple proteins which display immunoreactive pTyr. Mid-log-phase cultures of E. coli BL21(DE3) and BL21(DE3):pET16b-F10 transformants were induced with IPTG as described in Materials and Methods. Cultures were harvested after 48 h of induction at 18°C, and total cell extracts were subjected to SDS-PAGE in duplicate. (A) One portion of the gel was stained with Coomassie blue to visualize total protein content; the two cultures displayed equivalent protein profiles. (B) The proteins in the other portion of the gel were transferred electrophoretically to nitrocellulose and subjected to immunoblot analysis with anti-pTyr serum. No immunoreactive pTyr was detected in the BL21(DE3) culture, whereas the culture expressing enzymatically active F10 kinase contained immunoreactive pTyr on numerous protein species. The arrow in panel A indicates a protein likely to represent the induced F10 species; in panel B, the arrow indicates a protein of similar electrophoretic mobility that is highly reactive with the anti-pTyr serum. In both panels, the electrophoretic profile of protein standards is shown at the left, with molecular masses indicated in kilodaltons.
RESULTS
The enzymatic properties of the vaccinia virus H1 phosphatase were originally characterized in vitro (9). The ability of the enzyme to remove the phosphate moiety from phosphoserine (pSer), phosphothreonine (pThr), and pTyr residues led to its classification as a dual-specificity phosphatase. These studies were complemented by our in vivo analyses of infections performed in the presence or absence of H1 expression. In the absence of H1 expression, nascent virions were greatly diminished in their infectivity and were found to contain at least three hyperphosphorylated proteins. Eleven- and 16-kDa proteins representing the products of the F18 and A14 genes (17, 38), respectively, were shown to be hyperphosphorylated on serine residues when H1 expression was repressed. The dual specificity of H1 in vitro suggested that Tyr phosphorylation might also play a role in the vaccinia virus life cycle. As a first step toward addressing this question, we investigated whether any virion proteins were phosphorylated on Tyr residues and, if so, whether this modification was regulated by the H1 phosphatase.
H1-deficient virions contain a 25-kDa protein that is phosphorylated on Tyr residues.
Four different preparations of purified wt virions and three different preparations of purified H1-deficient virions (1 to 3 μg of each) were fractionated by SDS-PAGE and transferred electrophoretically to nitrocellulose. After incubation with anti-pTyr antibodies, filters were developed with an HRP-conjugated secondary antibody and ECL. Figure 1A (left) shows that wt virions contained no proteins with detectable levels of pTyr; in contrast, each preparation of H1-deficient virions contained a tyr-phosphorylated protein with an apparent molecular weight (MW) of 25,000. Partitioning of H1-deficient particles into core and membrane fractions revealed that the 25-kDa protein was a component of the virion membrane (Fig. 1A, right). These data demonstrate a genetic relationship between repression of the H1 phosphatase and hyperphosphorylation of the 25-kDa protein on Tyr residues. To demonstrate a direct enzyme-substrate relationship, the ability of the residual encapsidated phosphatase and/or exogenously supplied H1 to dephosphorylate the 25-kDa protein was investigated. These data are presented in Fig. 1B. Lanes 1 and 2 demonstrate again the specific detection of a pTyr containing 25-kDa protein within H1-deficient virions. These virions contain approximately 2.4% of the levels of H1 protein found within wt particles (17). When these virions were incubated in the presence of NP-40 plus DTT, the level of pTyr within the 25-kDa protein decreased, presumably due to the activation of the residual phosphatase. Consistent with this interpretation, no diminution in the pTyr signal was observed (lane 6) when the incubation with NP-40 plus DTT was conducted in the presence of sodium orthovanadate, an inhibitor of the phosphatase (9). When treatment with NP-40 plus DTT was accompanied by the inclusion of recombinant H1 protein, the pTyr signal was lost completely (lane 4). This loss of phosphorylation was not seen when a catalytically inert form of the H1 protein (9, 17) was used instead (lane 5). (Appropriate controls confirmed that these treatments affected only the phosphorylation state of the virion proteins and not their integrity [not shown].) These data demonstrate that the 25-kDa protein can be dephosphorylated directly by the H1 phosphatase and is therefore a bona fide substrate. In concert with our previous analyses of H1’s role in regulating Ser phosphorylation of the F18 and A14 proteins, the data shown in Fig. 1 provide strong evidence that H1 is a dual-specificity enzyme in vivo and in vitro.
Our findings demonstrate that the 25-kDa protein is phosphorylated on Tyr residues in vivo and strongly imply that this modification is normally removed by the H1 phosphatase. To investigate this further, we harvested cells at various times after infection with wt virus or vindH1 and monitored the pTyr profile of the lysates by immunoblot analysis. No pTyr signal at 25 kDa was seen in uninfected cells; in infected cells, the signal was not present at early times postinfection but was easily detected at 8, 12, or 16 hpi (time course not shown; 12 hpi shown in Fig. 2B). As we expected, the signal seen in vindH1 infections performed in the absence of IPTG (i.e., no H1 expression) was significantly stronger than that seen in wt infections (compare lanes 2 and 4 in Fig. 2B). This pattern was not altered by the inclusion of rifampin, an inhibitor of virion morphogenesis, in the culture medium (not shown), implying that morphogenesis is not required for either the addition or removal of phosphate moieties onto Tyr residues of the 25-kDa protein (not shown).
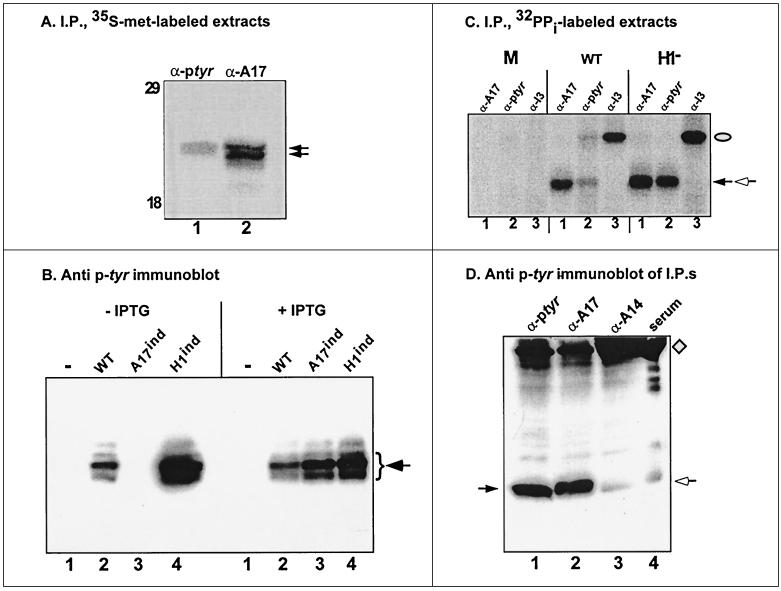
FIG. 2.
The 25-kDa pTyr-containing is the product by the A17 gene. (A) The proteins immunoprecipitated (I.P.) with anti-pTyr and anti-A17 sera comigrate. BSC40 cells were infected with vindH1 (MOI of 10) in the absence of IPTG and metabolically labeled with [35S]methionine. Lysates were subjected to immunoprecipitation with sera directed against pTyr or A17, as shown; after SDS-PAGE, radiolabeled proteins were visualized by fluorography. The electrophoretic profile of protein standards is shown at the left, with molecular masses indicated in kilodaltons; only the relevant portion of the autoradiograph is shown. (B) Detection of the pTyr-containing 25-kDa protein is dependent on expression of the A17 protein. BSC40 cells were left uninfected (lanes 1) or infected (MOI of 10) with wt virus (lanes 2) or recombinants in which expression of A17 (lanes 3) or H1 (lanes 4) is inducible. Duplicate infections were performed in the absence or presence of IPTG, the inducer. Cells were harvested at 12 hpi, and cell lysates were subjected to SDS-PAGE and immunoblot analysis with anti-pTyr serum. Only the relevant portion of the immunoblot is shown. (C) A17 is phosphorylated within infected cells. BSC40 cells were left uninfected or infected (MOI of 2) with wt virus or vindH1 in the absence of IPTG. Cells were radiolabeled with 32PPi from 3 to 17 hpi; cells were then harvested, and lysates were subjected to immunoprecipitation with anti-A17 (lanes 1), anti-pTyr (lanes 2), or anti-I3 (lanes 3). Phosphorylated I3 is indicated with a filled oval; the phosphoproteins immunoprecipitated with anti-A17 and anti-pTyr sera comigrate and are indicated with the filled and open arrows. (D) Immunoprecipitated A17 is recognized by anti-pTyr serum. BSC40 cells were infected with vindH1 in the absence of IPTG (MOI of 10); at 12 hpi, cells were harvested, and lysates were subjected to immunoprecipitation with rabbit polyclonal sera directed against pTyr, A17, or A14. Immunoprecipitates were then resolved on an SDS–15% polyacrylamide gel and transferred electrophoretically to nitrocellulose. The filter was incubated with a murine monoclonal antibody directed against pTyr, and immunoreactive proteins were visualized after ECL development. Lane 1 to 3, proteins precipitated with anti-pTyr, anti-A17, and anti-A14 sera, respectively; lane 4, normal rabbit serum. The arrow at the left indicates the strong immunoreactive signal in lanes 1 and 2. The diamond and open arrow at the right indicate the heavy and light chains of the rabbit immunoglobulin used in the immunoprecipitations; there is cross-species recognition of these proteins by the goat anti-mouse immunoglobulin G used in the immunoblot development.
The 25-kDa phosphoprotein is the product of the A17 gene.
Many structural components of vaccinia virions have already been identified. Among membrane proteins with MWs of approximately 25,000 the A17 and L1 proteins were possible candidates for the Tyr-phosphorylated species (22, 26, 41). We metabolically labeled vindH1-infected cells (without IPTG) with [35S]met and subjected cell lysates to immunoprecipitation with sera directed against pTyr, A17, L1, and L4 (a core component of 25 to 29 kDa). Comparison of the electrophoretic profile of the immunoprecipitates indicated that the protein doublet immunoprecipitated by the anti-pTyr serum comigrated with that immunoprecipitated by the anti-A17 serum (Fig. 2A). The migration of the other proteins was quite dissimilar (not shown). The comigration of the pTyr immunoprecipitate and A17 led us to investigate the pTyr profile of cells infected with an inducible recombinant in which A17 expression is dependent on the inclusion of IPTG in the culture medium (41). As shown in Fig. 2B, no 25-kDa protein was detected with the anti-pTyr serum in lysates harvested at 12 hpi from uninduced cultures (lane 3, −IPTG); inclusion of IPTG, however, restored expression of the pTyr-containing 25-kDa protein, as it restored expression of A17 (lane 3, +IPTG). This observation strongly suggested that the A17 protein was indeed the 25-kDa phosphorylated protein. The data shown in Fig. 2C and D extend this analysis. As shown in Fig. 2C, uninfected or infected cells were metabolically labeled with 32PPi and then subjected to immunoprecipitation analysis with sera directed against A17, pTyr, or I3. The latter, a virally encoded single-stranded-DNA-binding protein, serves as an internal control since it is phosphorylated on Ser residues in a manner which appears to be independent of the B1 and F10 kinases as well as the H1 phosphatase (25). The anti-A17 serum precipitated a phosphorylated species from both wt- and vindH1-infected cell extracts (lanes 1). The total levels of A17 phosphorylation appeared to be approximately twofold higher when H1 expression was repressed (compare lanes 1). The anti-pTyr serum immunoprecipitated a comigrating phosphoprotein from infected cell lysates; in this case, however, a dramatic increase in signal was seen when H1 expression was repressed (compare lanes 2). As expected, a 34-kDa phosphoprotein was seen when the I3 serum was used in immunoprecipitation analyses of wt- and vindH1-infected extracts (lanes 3); none of the sera immunoprecipitated any phosphorylated species from uninfected cells.
These results indicated that A17 was indeed phosphorylated in vivo, presumably on both serine/threonine and tyrosine residues, with the levels of the latter modification being exquisitely sensitive to the action of the H1 phosphatase. We have performed phosphoamino acid analysis on comparable immunoprecipitates (not shown). The phosphoproteins retrieved with anti-A17 serum contained pSer, pThr, and pTyr, with the latter reaching significant levels only in samples prepared from vindH1-infected cultures. Moreover, the protein immunoprecipitated with the anti-pTyr serum also contained pSer, pThr, and pTyr. These data show that individual molecules of the 25-kDa phosphoprotein, which we believe to be A17, are multiply phosphorylated on serine/threonine and tyrosine residues.
As a final proof that the 25-kDa Tyr-phosphorylated protein was indeed A17, we performed the experiment illustrated in Fig. 2D. Extracts prepared from vind-H1-infected cells were subjected to immunoprecipitation with anti-pTyr, anti-A17, and anti-A14 (as a negative control; another negative control, anti-L1, is not shown) sera. The immune complexes were then fractionated by SDS-PAGE, transferred to nitrocellulose, and probed with a murine monoclonal antibody directed against pTyr. As shown in Fig. 2D, the proteins retrieved by immunoprecipitation with either anti-pTyr or anti-A17 were recognized strongly by the anti-pTyr monoclonal antibody, providing a final confirmation that the A17 protein is indeed phosphorylated on tyrosine residues in vivo.
On which tyrosine is A17 phosphorylated?
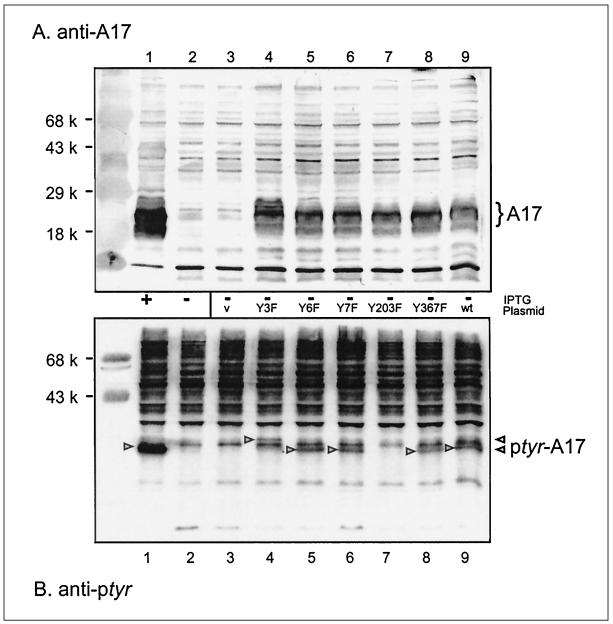
To aid us in identifying which kinase was responsible for phosphorylating A17, and to set the stage for understanding how A17 phosphorylation might regulate its function, we were interested in determining which Tyr residue was the site of phosphorylation. Our analysis took advantage of information regarding A17’s topology as well as its evolutionary conservation. First, the A17 protein is highly hydrophobic; it is thought to be inserted into the endoplasmic reticulum membrane cotranslationally and is predicted to span the bilayer two to four times (13). The N and C termini are the least hydrophobic in nature and are predicted to be exposed and project into the cytosol and/or the interior of the virion. Second, if phosphorylation of A17 on tyrosine residues is functionally significant, we predicted that the Tyr residue(s) involved would be conserved throughout poxviruses. We therefore aligned the deduced amino acid sequence of the vaccinia virus A17 protein with that from molluscum contagiosum virus, a highly divergent poxvirus (32, 33). We identified the Tyr residues at positions 3, 6, 7, and 203 as being evolutionarily conserved and located outside the membrane-spanning domains and therefore reasonable candidates for the site(s) of phosphorylation. We used overlap PCR mutagenesis to construct A17 alleles predicted to contain Tyr→Phe substitutions at these positions. Plasmids containing the wt allele and those directing Y3F, Y6F, Y7F, Y3+6+7F, and Y203F substitutions under the regulation of a strong cowpox promoter were constructed (20). Cells were then infected with vindA17 in the absence of IPTG so that endogenous A17 synthesis was repressed, and the plasmids encoding the various A17 alleles were introduced by lipofection. Parallel plates received empty plasmid or no DNA as controls, and a final plate was infected in the presence of IPTG to induce endogenous A17 synthesis. At 30 hpi, cells were harvested and lysates were fractionated and transferred to membranes. Duplicate filters were developed with anti-A17 and anti-pTyr sera.
As shown in Fig. 3A, a strong A17-specific signal was seen in untransfected cultures infected in the presence (lane 1) but not in the absence (lane 2) of IPTG. The A17 signal was restored in cultures carrying plasmids encoding the wt, Y3F, Y6F, Y7F, Y367F, and Y203F alleles (lanes 9, 4, 5, 6, 7, and 8, respectively). The protein encoded by the Y3F allele appeared to migrate more slowly than the other forms of A17 (lane 4). Introduction of empty vector did not restore an anti-A17 reactive signal (lane 3). Figure 3B addresses the pTyr content of the plasmid-encoded proteins. The endogenous protein expressed in the culture infected in the presence of IPTG yielded the strongest signal (lane 1). A clear signal was also seen in uninduced cultures expressing the wt, Y3F, Y6F, Y7F, and Y367F proteins (lanes 9, 4, 5, 6, and 8, respectively). Again, the protein encoded by the Y3F allele had an altered mobility (lane 4). Most importantly, however, introduction of the plasmid encoding the Y203F allele (lane 7) failed to stimulate any increase in the pTyr signal above the basal level present in uninduced and untransfected cultures (lanes 2 and 3). We have obtained this result in multiple independent experiments. Replacement of Tyr203 with Phe, therefore, does not compromise A17 synthesis or accumulation but abrogates the phosphorylation of A17 on Tyr residues. The simplest interpretation of these data is that Tyr203, the C-terminal amino acid, is the only Tyr residue on which A17 is phosphorylated.
FIG. 3.
Mutation of Tyr203 to Phe abrogates the phosphorylation of A17 on Tyr residues. BSC40 cells were infected with an inducible A17 recombinant (MOI of 10) in the presence (lanes 1) or absence (lanes 2 to 9) of IPTG. Infected cells were left untransfected (lanes 1 and 2) or transfected with empty plasmid (lane 3) or a plasmid encoding wt A17 (lane 9) or A17 containing the following amino acid substitution: Y3F (lane 4), Y6F (lane 5), Y7F (lane 6), Y203F (lane 7), or Y367F (lane 8). Samples were harvested at 30 hpi, and cell lysates were subjected to SDS-PAGE and immunoblot analysis. (A) Analysis with anti-A17 serum. The A17 species expressed from the genomic (lane 1) or transfected DNA (lanes 4 to 9) are indicated by the brace at the right of panel A. Note that the Y3F protein migrates somewhat more slowly than the other A17 proteins. Prestained protein standards are shown in the leftmost lane, with their MWs indicated. (B) Analysis with anti-pTyr serum. The A17 proteins containing immunoreactive pTyr are indicated by arrowheads; note that the Y3F protein migrates somewhat more slowly than the other pTyr-containing A17 proteins. No immunoreactive pTyr was detected in the A17 protein containing the Y203F substitution.
Phosphorylation of A17 on Ser/Thr and Tyr residues is dependent on the vaccinia virus F10 kinase in vivo.
The F10 gene is one of two within the viral genome that encodes a protein kinase. The F10 kinase (also known as VRK2) is expressed at late times of infection and is encapsidated within virions. Previous studies from our laboratory and others have demonstrated a crucial role for the kinase in regulating the earliest steps of viral morphogenesis (37, 39). When cells infected nonpermissively with ts mutants carrying defects in the F10 kinase (ts28 and ts15) were examined by electron microscopy, none of the hallmarks of viral morphogenesis were observed. Cleared areas of cytoplasm devoid of cellular organelles were seen, but no crescents, immature virions (IV), or mature virions (IMV) were found. This block to morphogenesis occurs despite the synthesis of the full complement of late viral proteins.
Since the F10 kinase appears to drive virion morphogenesis, and since A17 is a multiply phosphorylated protein which is also essential for virion morphogenesis (26, 41), we investigated whether disruption of F10 function had an impact on the phosphorylation of A17. We compared the phosphorylation profiles of A17 in cells infected with either wt virus or ts28 (tsF10) at both 31.5°C (permissive temperature) and 39.5°C (nonpermissive for ts28). Infected cells were metabolically labeled with 32PPi, and extracts were subjected to immunoprecipitation with sera directed against A17, pTyr, and 13 (Fig. 4A). Whereas 32P-labeled A17 was retrieved from cells infected with wt virus at both temperatures (lanes 7 and 10), this signal was lost in cells infected nonpermissively with ts28 (compare lane 4 with lane 1). Thus, the global phosphorylation of A17 appears to be genetically dependent on the F10 kinase. No phosphorylated signal was seen when lysates from cells infected nonpermissively with ts28 were subjected to immunoprecipitation with anti-pTyr (lane 5). However, the signal seen in permissively infected cells or cells infected with wt virus was also minimal (lanes 2, 8, and 11), since the H1 phosphatase is active during these infections. To better address the question of whether the phosphorylation of A17 on Tyr residues is also dependent on a wt allele of the F10 kinase, extracts of infected cells were subjected to immunoblot analysis with anti-pTyr antiserum (12, 19). When this more sensitive assay was used (Fig. 4B), a striking deficit in the Tyr phosphorylation of A17 was seen in cells infected nonpermissively with ts28. These highly reproducible data were also seen when other ts mutants carrying lesions in F10 (e.g., ts15) were tested. (Development of parallel blots with anti-A17 serum confirmed that the loss of phosphorylation was not due to a decrease in the levels of A17 protein [not shown].) Thus, although F10 has previously been characterized as a Ser/Thr kinase, its genetic inactivation abrogates the phosphorylation of A17 on Tyr as well as on Ser/Thr residues.
Recombinant F10 kinase phosphorylates a peptide derived from the C terminus of A17.
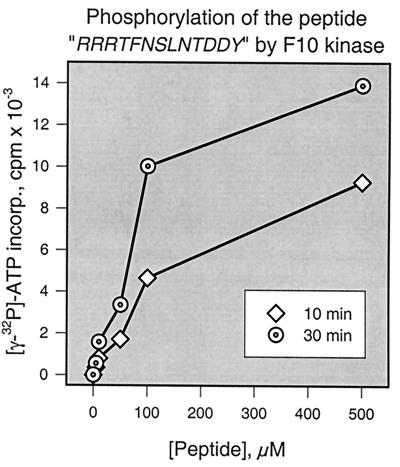
The data discussed above and shown in Fig. 4 demonstrate that the phosphorylation of A17 on Ser/Thr and Tyr residues is genetically dependent on the F10 kinase. Since F10 has not previously been shown to be a dual-specificity kinase, a determination of whether the enzyme plays a direct or indirect role in A17’s phosphorylation is of high priority. F10 might indeed phosphorylate A17 with dual specificity. Alternatively, F10 might phosphorylate A17 on Ser/Thr residues and, by so doing, convert it into a good substrate for a cellular tyrosine kinase. Finally, F10 might not phosphorylate A17 at all, but its role in initiating virion morphogenesis might be required to position A17 for phosphorylation by a cellular kinase. Direct tests of whether A17 is a substrate for F10 would help in discriminating between these possibilities; however, A17 is a transmembrane protein with only short exposed regions, and thus producing soluble, recombinant protein is difficult if not impossible. Since we defined Tyr203 as being essential for tyrosine phosphorylation of A17, we chose instead to synthesize a peptide corresponding to the C terminus of A17 and encompassing residues 194 to 203. An N-terminal basic extension was added to the peptide to confer an affinity for P81 phosphocellulose paper and so facilitate assays of the phosphorylation of this peptide by F10 (4, 21). The sequence of the peptide was, therefore, N′ RRRTFNSLNTDDY C′; the peptide contains four potential phosphorylation sites shown in boldface. Recombinant F10 was tested for its ability to phosphorylate this peptide in vitro; Fig. 5 provides a graphic representation of the kinase assay. F10 was indeed able to phosphorylate the peptide in a manner that was dependent on both the reaction duration and the substrate concentration. Although these results clearly suggested that F10 could phosphorylate the C terminus of A17 directly, their interpretation should be tempered by the fact that the in vitro phosphorylation reaction was extremely inefficient. Only a fraction of the substrate was shown to undergo modification, and the residue(s) phosphorylated by F10 has not yet been identified.
FIG. 5.
Recombinant F10 kinase phosphorylates a peptide representing the C terminus of the A17 protein. Recombinant F10 kinase (74 ng) was incubated with [γ-32P]ATP and various concentrations (0, 5, 10, 50, 100, or 500 μM) of a peptide substrate whose sequence comprised a basic N-terminal extension followed by 10 amino acids representing residues 194 to 203 of the A17 protein. Reactions were performed for 10 or 30 min, as indicated. To quantitate the incorporation of radiolabel, samples were spotted onto P81 paper, washed extensively, and analyzed by Cerenkov counting as described in Materials and Methods.
E. coli induced to express F10 contains multiple proteins which react with anti-pTyr antiserum.
The genetic data presented above suggested that F10 might indeed be a dual-specificity protein kinase. To test whether F10 has tyrosine kinase activity, we exploited the fact that bacteria do not contain tyrosine kinases and therefore have no immunoreactive pTyr. The appearance of immunoreactive pTyr upon expression of a kinase in E. coli has become a classic approach to discovering tyrosine kinase activity (16). The tyrosine kinase activity of dual specificity kinases is often highly substrate specific, and an unbiased scan of all bacterial proteins has frequently been more profitable than individual testing of peptides or proteins. We therefore prepared mid-log-phase cultures of BL21(DE3) and BL21(DE3):pET16b-F10 and subjected them to identical inductions: addition of IPTG (to 0.2 mM) and EtOH (to 2%), incubation on ice for 30 min, and vigorous agitation at 18°C for 48 h. Bacteria were harvested and disrupted, and crude lysates were fractionated by SDS-PAGE. The overall protein profiles were compared after staining with Coomassie blue, and the pTyr content was determined by immunoblot analysis using a polyclonal anti-pTyr serum. After ECL development, the immunoblots were stained with amido black to ensure that equal amounts of protein were present in the samples. As shown in Fig. 6, the results were dramatic. Whereas no immunoreactive pTyr was seen in the BL21(DE3) cultures, the F10-expressing cultures contained several strongly immunoreactive species. The major species had an apparent MW of ≈55,000 and may therefore represent autophosphorylated F10. We have obtained consistent results from several independent inductions and analyses and feel that these data demonstrate that the F10 kinase does indeed have the ability to phosphorylate Tyr residues in vivo.
DISCUSSION
The studies described in this report clearly demonstrate that the A17 protein is phosphorylated on Ser, Thr, and Tyr residues in vivo. This phosphorylation is genetically dependent on a wt allele of the F10 kinase and does not occur during nonpermissive infections performed with ts mutants which carry lesions in the F10 gene. The link between A17 and F10 is intuitively appealing, since both play a role in the earliest stages of virion morphogenesis (26, 27, 37, 39, 41). In vaccinia virus morphogenesis, membranes of the intermediate compartment are thought to be recruited to the cytoplasmic sites where viral proteins are concentrated. The first visual sign of morphogenesis is the appearance of membrane crescents, which then elongate and develop into spherical IV. Dense nucleoids then form within the IV, and finally the virion matures into a brick-shaped particle that encloses a biconcave core (IMV). In the absence of a wt allele of the F10 kinase, none of these intermediates or IMV are seen, and the most striking feature is the presence of large, cleared-out areas of the cytoplasm that are devoid of organelles. In the absence of A17 expression, dense aggregates appear within the cleared cytoplasm, but no crescents, IV, or IMV are seen.
Because F10 and A17 function along the same morphogenetic pathway, an enzyme-substrate relationship seems quite reasonable. However, F10 has been characterized as a Ser/Thr kinase and has not previously been shown to phosphorylate substrates on Tyr residues as well. The dual-specificity class of protein kinases is poorly understood in terms of diagnostic sequence motifs and biochemical properties. The mammalian mitogen-activated protein kinase kinases that phosphorylate the Thr and Tyr residues within the TXY motif of their substrates (8, 16) are among the best-studied examples of such enzymes. Dual-specificity kinases resemble Ser/Thr kinases in their primary sequences (16). However, they possess the ability to undergo autophosphorylation, or to direct the phosphorylation of exogenous substrates, on Tyr residues. Tyr phosphorylation has typically been detected by monitoring immunoreactivity with anti-pTyr antisera and has often been identified upon expression of candidate kinases in bacteria. Although F10 does have some of the conserved motifs of a Ser/Thr protein kinase, it appears to lack several of the others and may therefore have unique properties (14). Recombinant F10 has previously been shown to phosphorylate exogenous substrates and to undergo autophosphorylation on Ser and Thr residues. In this report we provide new evidence that multiple proteins containing immunoreactive pTyr are found within bacterial strains expressing F10; the most abundant of these is likely to be F10 itself (Fig. 5B). Together, these data strongly suggest that F10 is a dual-specificity kinase.
Although we have not addressed the site(s) on which A17 undergoes Ser/Thr phosphorylation, our data argue that Tyr203 is the only site of Tyr phosphorylation. Mutation of this terminal residue to Phe leads to the synthesis and accumulation of A17 protein that lacks any detectable pTyr. (Although we favor the direct interpretation of these data, it remains formally possible that the Tyr203→Phe substitution causes a structural change that prevents phosphorylation of residues elsewhere.) The nine residues upstream of Tyr203 (underlined) contain three potential sites (boldfaced) for Ser/Thr phosphorylation (N′ TFNSLNTDDY 3′), making this region a reasonable target for a dual-specificity kinase. We have shown that F10 has the capacity to phosphorylate a peptide representing the 10 C-terminal amino acids of A17, albeit inefficiently. This lack of efficiency is often seen with peptide substrates, suggesting that a strong kinase-substrate interaction involves contacts with regions distal from the target residue. It will be of obvious interest to pursue a further biochemical analysis of F10’s apparent dual specificity and to analyze its interactions with A17 and other potential substrates.
Hyperphosphorylation of A17 is seen when expression of the H1 phosphatase is repressed. The total amount of 32P incorporated into intracellular A17 (as assessed by immunoprecipitation with anti-A17 serum) is increased only twofold when H1 expression is blocked. However, the levels of phosphorylated A17 that can be retrieved by precipitation with anti-pTyr sera increase dramatically upon repression of the phosphatase. Immunoblot analysis reveals that intracellular A17 is indeed phosphorylated on Tyr residues during wt infections, albeit at a level significantly lower than that seen in the absence of H1 expression. Since the A17 encapsidated within purified, wt virions does not contain any immunoreactive pTyr, dephosphorylation must normally occur during or after morphogenesis. H1-deficient virions, in contrast, contain A17 that retains tyrosine phosphorylation.
The demonstration that the H1 phosphatase is responsible for dephosphorylation of tyrosine residues on A17 as well as Ser/Thr residues on F18 and A14 (17) provides a clear demonstration that H1 is indeed a dual-specificity phosphatase. The levels of pTyr within A17 are exquisitely sensitive to the levels of H1, suggesting that A17 is an excellent substrate for H1 and a poor substrate for cellular phosphatases. Interestingly, the residues preceding Tyr203, 194TFNSLNTDDY203, contain several potential sites of Ser/Thr phosphorylation. It has previously been shown that dual-specificity phosphatases act preferentially on diphosphorylated substrates in which the pTyr residue is in proximity to pSer or pThr and that the dephosphorylation of the Tyr residue is the first and most rapid step in the reaction (7, 18). Moreover, the acidic character of the residues preceding the Tyr residue in the N′ TFNSLNTDDY C-terminal sequence are a common feature of preferred sites for Tyr dephosphorylation.
Although phosphorylation of A17 has not previously been reported, a significant amount is known about the topology of the protein. A17 is predicted to insert its central, hydrophobic region into the lipid bilayer during translation on membrane-bound polysomes (13). Residues 1 to 63 and 158 to 203 are the only residues predicted to extend beyond the membrane and are therefore the most likely to sustain phosphorylation and to participate in protein-protein interactions. The 15 N-terminal amino acids of A17 are proteolytically removed during virion morphogenesis, with cleavage occurring at the diagnostic Ala-Gly-Ala motif found at the processing site of several virion proteins (28, 40). This processing converts the 23-kDa precursor into the mature 21-kDa form of A17.
As mentioned above, A17 is essential for early stages of IMV morphogenesis; in the absence of A17 expression, morphogenesis arrests prior to the formation of crescents, IV, or IMV (26, 27, 41). Similar results have been reported for the A14 protein, another component of the virion membrane (31, 38). This functional overlap suggests that some interaction of the A17 and A14 proteins is likely; indeed, such an association has been reported (30). A17 is also thought to recruit the viral A27 protein (p14) to the surface of IMV, where the latter plays an essential role in enabling a subset of IMV to become wrapped in membranes of the trans-Golgi network (28, 29). These wrapped virions mature into the cell-associated and extracellular enveloped virions that are responsible for cell-to-cell and distal spread of the virus. p14 interacts only with the N-terminally processed, 21-kDa form of A17. Whether phosphorylation or dephosphorylation of A17 affects its interaction with p14 is not yet known.
The role of A17 in anchoring p14 relies on A17 being exposed on the external surface of the virion. The N and C termini of A17 have indeed been shown to be accessible to protease digestion within postnuclear supernatants of infected cells and in purified IMV (13). However, analysis by immunoelectron microscopy has indicated that the N and C termini of A17 are exposed on the inner surface of crescents and IV, with little or no A17 exposed on the outer surface (13, 41). Moreover, A17 is virtually undetectable on IMV when a C-terminus-specific antiserum is used, although the same antibody easily detects encapsidated A17 in immunoblot assays. Presumably, immunoreactive epitopes on the outer surface of crescents and IV are masked, perhaps because of steric interference due to protein-protein interactions, conformational changes, or posttranslational modification. Comparable mechanisms might contribute to the masking of C-terminal epitopes in IMV. The immunoelectron microscopic data do indicate, however, that some molecules of A17 are clearly exposed on the inner surface of the virion membrane.
A full understanding of the topology of the A17 molecule within the virion awaits a clarification of the biogenesis and structure of the membrane itself. Early studies suggested that a single bilayer enclosing the virion was formed de novo in the cytoplasm (6). Although the concept of de novo membrane formation remains controversial, a recent study provides careful measurements that provide support for the presence of a single 5-nm-thick membrane surrounding the virion (10). In contrast, other investigators have hypothesized that membranes from the intermediate compartment (between the endoplasmic reticulum and Golgi apparatus) become tightly apposed and curve to form the oval membrane that defines IV (34). In this scenario, the virion is delimited by two adjacent lipid bilayers, with the cytoplasmic face of one facing outward and the cytoplasm face of the other facing inward. The latter model could easily account for the cumulative findings that the termini of A17 are exposed on both the inner and outer faces of the virion membrane. In any case, it is quite possible that the reversible phosphorylation of the C terminus of A17 affects the internal as well as the external structure of the virion.
Further analysis of the phosphorylation state of A17 and its dephosphorylation by H1 is clearly of interest. Reversible phosphorylation of the C-terminal region might well regulate protein-protein interactions which are essential in driving virion morphogenesis; alternatively, such modifications might mediate A17’s recruitment of p14, the association of IMV with the Golgi apparatus, and the subsequent formation of enveloped virus. Dephosphorylation does not appear to be an essential step in morphogenesis, since H1-deficient virions mature normally. The dephosphorylation of A17, however, might contribute to virion stability or infectivity, since these parameters are affected by the repression of H1.
In sum, this report brings together the A17, F10, and H1 proteins, all of which are essential for the production of infectious viral progeny. F10 initiates virion morphogenesis and mediates the phosphorylation of A17 on Ser/Thr and Tyr residues. These events are localized on the membranes of the endoplasmic reticulum, intermediate compartment, and developing virions, and unraveling their intricacies will have relevance not only to vaccinia virus morphogenesis but also to questions of organelle biogenesis and vesicle trafficking. H1 reverses much of the action of F10, and its cumulative actions are key to ensuring that nascent virions are infectious. The amenability of vaccinia virus to genetic and biochemical dissection should facilitate a thorough analysis of this intriguing network of protein phosphorylation.
ACKNOWLEDGMENTS
This work was supported by a grant to P.T. from the NIH (5R01 GM 53601) and by a special group of donors from the Dorothy Rodbell Cohen Foundation. M.D. was a fellow of the Charles H. Revson Foundation.
We thank D. Hruby, E. Wolffe, B. Moss, M. Esteban, D. Pickup, and N. Tonks for graciously providing reagents.
REFERENCES
- 1.Banham A H, Smith G L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992;191:803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- 2.Beaud G, Sharif A, Topa-Masse A, Leader D P. Ribosomal protein S2/Sa kinase purified from HeLa cells infected with vaccinia virus corresponds to the B1R protein kinase and phosphorylates in vitro the viral ssDNA-binding protein. J Gen Virol. 1994;75:283–293. doi: 10.1099/0022-1317-75-2-283. [DOI] [PubMed] [Google Scholar]
- 3.Betakova T, Wolffe E J, Moss B. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L kinase. J Virol. 1999;73:3534–3543. doi: 10.1128/jvi.73.5.3534-3543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casnellie J E. Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Methods Enzymol. 1991;200:115–121. doi: 10.1016/0076-6879(91)00133-h. [DOI] [PubMed] [Google Scholar]
- 5.Condit R C, Motyczka A, Spizz G. Isolation, characterization and physical mapping of temperature sensitive mutants of vaccinia virus. Virology. 1983;128:429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- 6.Dales S, Mosbach E H. Vaccinia as a model for membrane biogenesis. Virology. 1968;35:564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- 7.Denu J M, Zhou G, Wu L, Zhao R, Yuvaniyama J, Saper M A, Dixon J E. The purification and characterization of a human dual-specific protein tyrosine phosphatase. J Biol Chem. 1995;270:3796–3803. doi: 10.1074/jbc.270.8.3796. . (Erratum, 270:10358.) [DOI] [PubMed] [Google Scholar]
- 8.Dhanasekaran N, Reddy E P. Signaling by dual specificity kinases. Oncogene. 1998;17:1447–1455. doi: 10.1038/sj.onc.1202251. [DOI] [PubMed] [Google Scholar]
- 9.Guan K, Broyles S S, Dixon J E. A tyr/ser phosphatase encoded by vaccinia virus. Nature. 1991;350:359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- 10.Hollinshead M, Vanderplasschen A, Smith G L, Vaux D J. Vaccinia virus intracellular mature virions contain only one lipid membrane. J Virol. 1999;73:1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson G P, Goebel S J, Paoletti E. An update on the vaccinia virus genome. Virology. 1993;196:381–401. doi: 10.1006/viro.1993.1494. [DOI] [PubMed] [Google Scholar]
- 12.Kamps M P, Sefton B M. Most of the substrates of oncogenic viral tyrosine protein kinases can be phosphorylated by cellular tyrosine protein kinases in normal cells. Oncogene Res. 1988;3:105–115. [PubMed] [Google Scholar]
- 12a.Kovacs, G. Personal communication.
- 13.Krijnse-Locker J, Schleich S, Rodriguez D, Goud B, Snijder E J, Griffiths G. The role of a 21-kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment. J Biol Chem. 1996;271:14950–14958. doi: 10.1074/jbc.271.25.14950. [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Broyles S S. Vaccinia protein kinase 2: a second essential serine/threonine kinase encoded by vaccinia virus. Proc Natl Acad Sci USA. 1994;91:7653–7657. doi: 10.1073/pnas.91.16.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S, Chen W, Broyles S S. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992;66:2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg R A, Quinn A M, Hunter T. Dual-specificity protein kinases: will any hydroxyl do? Trends Biochem Sci. 1992;17:114–119. doi: 10.1016/0968-0004(92)90248-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu K, Lemon B, Traktman P. The dual-specificity phosphatase encoded by vaccinia virus, VH1, is essential for viral transcription in vivo and in vitro. J Virol. 1995;69:7823–7834. doi: 10.1128/jvi.69.12.7823-7834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martell K J, Angelotti T, Ullrich A. The “VH1-like” dual-specificity protein tyrosine phosphatases. Mol Cell. 1998;8:2–11. [PubMed] [Google Scholar]
- 19.Morla A O, Wang J Y. Protein tyrosine phosphorylation in the cell cycle of BALB/c 3T3 fibroblasts. Proc Natl Acad Sci USA. 1986;83:8191–8195. doi: 10.1073/pnas.83.21.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel D D, Ray C A, Drucker R P, Pickup D J. A poxvirus-derived vector that directs high levels of expression of cloned genes in mammalian cells. Proc Natl Acad Sci USA. 1988;85:9431–9435. doi: 10.1073/pnas.85.24.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ploegh H L. Phosphorylation and phosphatases. In: Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 13.7.15–13.7.17. [Google Scholar]
- 22.Ravanello M P, Hruby D E. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J Virol. 1994;68:6401–6410. doi: 10.1128/jvi.68.10.6401-6410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rempel R E, Anderson M K, Evans E, Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rempel R E, Traktman P. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992;66:4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochester S C, Traktman P. Characterization of the single-stranded DNA binding protein encoded by the vaccinia virus I3 gene. J Virol. 1998;72:2917–2926. doi: 10.1128/jvi.72.4.2917-2926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez D, Esteban M, Rodriguez J R. Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis. J Virol. 1995;69:4640–4648. doi: 10.1128/jvi.69.8.4640-4648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez D, Risco C, Rodriguez J R, Carrascosa J L, Esteban M. Inducible expression of the vaccinia virus A17L gene provides a synchronized system to monitor sorting of viral proteins during morphogenesis. J Virol. 1996;70:7641–7653. doi: 10.1128/jvi.70.11.7641-7653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez D, Rodriguez J R, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez J F, Smith G L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990;18:5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez J R, Risco C, Carrascosa J L, Esteban M, Rodriguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez J R, Risco C, Carrascosa J L, Esteban M, Rodriguez D. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J Virol. 1998;72:1287–1296. doi: 10.1128/jvi.72.2.1287-1296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senkevich T G, Bugert J J, Sisler J R, Koonin E V, Darai G, Moss B. Genome sequence of a human tumorigenic poxvirus: prediction of specific host responses-evasion genes. Science. 1996;273:813–816. doi: 10.1126/science.273.5276.813. [DOI] [PubMed] [Google Scholar]
- 33.Senkevich T G, Koonin E V, Bugert J J, Darai G, Moss B. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology. 1997;233:19–42. doi: 10.1006/viro.1997.8607. [DOI] [PubMed] [Google Scholar]
- 34.Sodeik B, Doms R W, Ericsson M, Hiller G, Machamer C E, van’t Hof W, van Meeer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studier F W, Rosenberg A L, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 36.Traktman P, Anderson M K, Rempel R E. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J Biol Chem. 1989;264:21458–21461. [PubMed] [Google Scholar]
- 37.Traktman P, Caligiuri A, Jesty S A, Liu K, Sankar U. Temperature-sensitive mutants with lesions in the vaccinia virus F10 kinase undergo arrest at the earliest stage of virion morphogenesis. J Virol. 1995;69:6581–6587. doi: 10.1128/jvi.69.10.6581-6587.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traktman, P., K. Liu, B. Unger, R. Rollins, and S. A. Jesty. Elucidating the essential role of the A14 phosphoprotein in vaccinia morphogenesis: construction and characterization of a tetracycline-inducible recombinant. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 39.Wang S, Shuman S. Vaccinia virus morphogenesis is blocked by temperature-sensitive mutations in the F10 gene, which encodes protein kinase 2. J Virol. 1995;69:6376–6388. doi: 10.1128/jvi.69.10.6376-6388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead S S, Hruby D E. Differential utilization of a conserved motif for the proteolytic maturation of vaccinia virus proteins. Virology. 1994;200:154–161. doi: 10.1006/viro.1994.1174. [DOI] [PubMed] [Google Scholar]
- 41.Wolffe E J, Moore D M, Peters E J, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]