Abstract
This research aim was to assess the impact of the seed extracts of the date cultivars (Qatara, Barhi, and Ruthana) on rat’s liver steatosis, oxidative stress, and inflammation triggered by feeding a high-fat diet (HFD). The experimental design was based on random partitioning into two groups; one that received the standard diet and another that received the HFD diet. The HFD rats were orally administered Lipitor or date seed extracts at 300 or 600 mg/kg/day for 4 weeks. Accordingly, feeding rats HFD significantly increased body and liver weights, hepatic and serum lipid levels, glucose, insulin, HOMA-IR, liver function enzymes, and inflammation markers, and decreased oxidative stress enzymes. Oral administration of Barhi and Ruthana date seed extracts significantly decreased body and liver weights. Serum and liver total cholesterol TC, Triglycerides TGs, and free fatty acids FFAs were also decreased as were AST, ALT, MAD, leptin, and CRP, with a concomitant increase in SOD, GSH, and CAT. Furthermore, similar to Lipitor, oral administration of the extracts reduced inflammation markers such as TNF-α, serum CRP, IL-6, IL-1β, and leptin while increasing IL-10 and adiponectin levels. Histological observation revealed that extract administration improved hepatocyte and parenchymal structures and decreased lipid deposition. In conclusion, both Barhi and Ruthana seed extracts showed strong hepatoprotective, anti-inflammatory, and antioxidant effects against HFD-induced liver steatosis. And date seeds have other beneficial potential for prevention and treatment of various diseases, which can be studied in the future.
Keywords: Saudi Date Seeds, HFD, Hepatic Steatosis, Oxidative stress, Rats
1. Introduction
The most common form of dyslipidemia, or hyperlipidemia, is characterized by an altered circulatory lipid profile levels (Hill & Bordoni, 2021). It is a significant risk cause for cardiovascular disease (CVD) and is accountable for approximately 30% of all coronary heart diseases and 50% of cerebrovascular diseases worldwide, resulting in nearly 2.6 million deaths each year (World Health Organization WHO, 2021). The prevalence of hyperlipidemia in Middle Eastern and African countries was found to reach 70% (Raal et al., 2018), and in Saudi Arabia, it varies from 8.5 to 54.9%, based on location (Medani et al., 2018). High-fat diet (HFD) and obesity are major causes of hyperlipidemia, which negatively alter lipoprotein metabolism (Misar, 2020). It causes liver disorders by promoting Reactive oxygen species (ROS) production and oxidative stress increasing (Saryono et al., 2017). The increase in free radical activity is known to cause lipid peroxidation of cellular structures, resulting in serious molecular damage such as DNA breakdown, protein damage, and lipid peroxidation and triggering chronic inflammation, which has been linked to the initiation and/or the aggravation of many diseases (Al-Otaibi et al., 2020, López-Contreras et al., 2020), such as liver disorders. Oxidized LDL-C was also found to contribute to inflammatory processes in hepatocytes by increasing ROS synthesis, activating macrophages and endothelial cells, increasing apoptosis, and causing hepatic injury. It is well-recognized that the liver is involved in metabolism, detoxification, storage, and immunity. The liver is particularly susceptible to damage by HFD and hyperlipidemia, which disrupt liver functions by changing lipid and lipoprotein metabolism and causing fatty liver disease (Malhotra et al., 2020, Friedman et al., 2018). Hyperlipidemia and insulin resistance also contribute to the buildup of cholesterol, TGs, and free fatty acids (FFAs) in hepatocytes, leading to hepatic inflammation and fatty liver diseases (Malhotra et al., 2020).
The date palm (Phoenix dactylifera Linn), primarily farmed in the Middle East and North Africa regions since ancient times, is one of the major fruit trees worldwide (Al-Alawi et al., 2017). Saudi Arabia is currently one of the top world’s producers and exporters of date fruit. There are around 400 date varieties grown in Saudi Arabia (Abdul-Hamid et al., 2020). Date fruits have health-promoting effects due to their higher biologically active compound content (Alqarni et al., 2019, Khan et al., 2017). Depending on quality and variety, the seed constitutes 10–15% of the ripe date fruit weight (Ghnimi and Almansoori, 2015). It primarily comprises dietary fiber, protein, fat, carbohydrates, phenols, and minerals. The chemical makeup of date seeds varies with the cultivar, harvest period, post-harvest processes, and fertilizers employed. Date seeds have high levels of bioactive substances like phytosterols, phenolic acids, flavonoids, carotenoids, and tocopherols (Maqsood et al., 2020). Numerous research has demonstrated the antioxidant, hypolipidemic, anti-inflammatory, and antidiabetic properties of the bioactive compounds (Ayatollahi et al., 2020, Melek et al., 2019, Saryono et al., 2020). In the assessment of their hypolipidemic and antidiabetic impacts, Marghoob & Abdelmarouf (2016) discovered that Ajwa and Sukkari date seed extracts significantly enhanced lipid profiles and reduced oxidative indices while lowering glucose levels. Similar results were obtained by Saryono et al. (2017) in hypercholesterolemic rats following 21 days of administering date seed methanol extracts at various concentrations (0.25, 0.5, and 1.0 g/kg/day). Furthermore, Abiola et al. (2018) revealed that giving diabetic-induced rats 400 mg/kg of ethanolic date seed extract orally for 14 days reduced their TC, LDL-C, and TG levels. Data on the hepatoprotective effect of the Saudi date seeds are scarce and need extensive investigation. Accordingly, this research aimed to monitor the therapeutic influences of phenolic extracts from various date seed cultivars, primarily Qatath, Barhi, and Ruthana, on liver function as well as biomarkers of inflammation and oxidative stress in rats fed high-fat diets.
2. Materials and methods
2.1. The extraction process
The date palm cultivar fruits, Qatarah (QT), Barhi (BR), and Ruthana (RT) were purchased from the local markets from a certified supplier during the Rutab stage. The seeds were separated from the flesh manually. The seeds were cleaned in water to remove leftover flesh, dried in the sun, and then ground into powder. The bioactive compounds were extracted from the seed powder using a mixture of methanol:ethanol:water (40:40:20) with a 1:5 ratio of powder: solvent, following the method reported previously (Takaeidi et al., 2014). The extracts were concentrated in a rotatory evaporator and then lyophilized. The lyophilized samples were reconstituted in normal saline to the desired concentrations for use in animal experiments.
The standard hypolipidemic drug, Lipitor (Atorvastatin), was purchased from local markets and diluted to the required concentration in normal saline.
2.2. Animals
For this experiment, 72 healthy, adult male Wistar albino rats (weighing 150 ± 20 g) were obtained from the Experimental Animal Care Center at King Saud University, Riyadh, Saudi Arabia. Housing temperatures and relative humidity were respectively fixed at 22 ± 2 °C and 55 ± 5% relative humidity, with a light and dark duration, each 12 h long. The animals' control or HFD diets were accessible to rats freely during the whole experimental period. The Research Ethics Committee at King Saud University authorized the protocols employed in animal trials (Ethics Reference No: KSU-SE-22–07).
2.3. Diets
The diet standard and high-fat diets were prepared according to (AIN)-93 M method of the American Institute for Nutrition, as shown in Table 1 (Reeves, 1997, Woods et al., 2003).
Table 1.
Formulation of the control diet and high-fat diet (HFD) of rats.
| Ingredients | Standard diet (g/kg of diet) | High-fat diet (g/kg of diet) |
|---|---|---|
| Casein | 140.00 | 174.00 |
| L-Cystine | 1.8 | 1.8 |
| Corn Starch | 465.7 | 303.1 |
| Dextrinized corn starch | 155.00 | 115 |
| Sucrose | 100 | 89.9 |
| Fiber | 50.00 | 50.00 |
| Soybean Oil | 40.00 | 25.00 |
| Fat (Butter oil) | 20.00 | 216 |
| Mineral mix (AIN93-G) | 35.00 | 50.00 |
| Vitamin mix (AIN93-G) | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.01 | 0.01 |
2.4. Experimental design
After a one-week adaptation period on the control standard diet STD, rats were randomly allocated and then treated either with the control or HFD (8 rats for each), and the HFD included further treatments. The STD group received normal saline and the HFD groups were sorted into an additional 8 groups: a control HFD-fed group received normal saline; a second HFD-fed group received a safe dose of Lipitor (10 mg/kg) (Dhingra et al., 2014); and the remaining 6 HFD groups administrated QT, BR, or RT extracts orally in two doses of 300 mg or 600 mg/kg. The determined extract doses were drawn from previous reports (Chis et al., 2009; Adigun et al., 2016; Ahmed et al., 2016; Abiola et al., 2018). The treatment was conducted for 8 weeks, daily food consumption was monitored, and weekly final body weight was taken.
2.5. Blood and tissue collection
After 8 weeks, a mixture of xylazine hydrochloride (10 mg/kg) and ketamine hydrochloride (100 mg/kg) was used to anesthetize all trial rats. To gather serum and plasma, blood samples were taken directly by puncturing the heart and placed into tubes without and with EDTA, respectively, before being centrifuged (1200 xg) for 10 min. For biochemical tests, all serum and plasma samples were kept at −80 °C and used afterward. The livers were then removed from each rat's abdomen, placed on ice, and sliced into small pieces before being cleaned in an ice-cold phosphate-buffered saline (PBS) solution (pH = 7.4). A liver portion was conserved in 10% buffered formalin before being sent to the pathology laboratory for analysis. Every remaining liver portion was subjected to swift freezing in liquid nitrogen and kept at −80 °C for future usage.
2.6. The extraction process of liver lipids
Lipids from frozen livers were extracted following the methanol/chloroform protocol established earlier (Folch et al., 1957). For the assay, 20 ml of a solvent mixture (methanol: chloroform; 1:2 v/v) was used to homogenize 0.5 g of each frozen liver part for 4–5 h in a tube. After adding 4 ml of normal saline and vortexing (2500 xg; 15 min), two phases appeared in the tube. The upper phase was separated from the bottom phase containing lipids, which was then dried and redissolved in 1 ml of isopropanol for further analysis.
2.7. Hepatic and serum lipid analyses
An assay kit (Cat. No. 10009582; Cayman Chemical, MI, USA) was employed to examine plasma glucose levels. Rat’s ELISA kit (Cat. No. 589501, Ann Arbor, MI, USA) was utilized to estimate plasma insulin levels. The following equation (Furukawa et al., 2019) was adopted to serve as the basis for calculating the HOMA-IR index: “HOMA-IR= (fasting plasma insulin levels (ng/ml) X fasting plasma glucose (mg/dl))/405”. A spectrophotometric assay kit (Cat. No. 10010303, Cayman Chemical, MI, USA, and ECCH-100, BioAssay Systems, CA, USA, respectively) was used to monitor TGs and CHOL levels in serum and liver, while assay kits (Cat. No. K4436, BioVision, CA, and Cat. No. USA79960, Crystal Chemicals, USA) were used to measure HDL-c and LDL-c levels. ELISA-specific kits were used to assess the serum concentrations of adiponectin, CRP, leptin, ALT, and AST (Cat. No. MBS068220; Cat No. MBS453159; Cat. No. MBS012834; Cat. No. MBS269614, and MBS264975; MyBiousorce, CA, USA). All measurements were carried out following the instructions provided by the kit manufacturers, for a total of 8 samples per group.
2.8. Preparation of liver tissue homogenates
To prepare a homogenate, 0.5 ml of ice-cold PBS (pH = 7.4) was added to a frozen liver sample (100 mg), homogenized, and then centrifuged (12,000 × g; 15 min; 4 °C). The obtained supernatant was stored at −20 ℃ until used for biochemical tests.
2.9. Biochemical measurements in the liver homogenates
The malondialdehyde (MDA) concentration in the liver homogenate was assessed using the assay kit with the Cat. No. 10,009,055 (Cayman, MI, USA). Total glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), tumor necrosis factor-alpha (TNF-α), and interleukin-10 and 6 (IL-6) levels in liver homogenate were determined using rats’ specific ELISA kits (Cat. NO. MBS265966; Cat. No. MBS036924, Cat. No MBS726781; Cat. No. BMS2507393, Cat. Mo. MBS034393; and Cat. No. MBS269892), which were procured from MyBioSource, CA, USA. All experiments were implemented following the manufacturer's guidance provided with each kit.
2.10. Histological analysis
Liver segments were kept safe for 24 h in a 10% buffered formalin solution. Following xylene deparaffinization, the liver tissue was rehydrated in ethanol solutions of varied percentages (100%, 95%, and 70%) sequentially. The segments were covered with paraffin wax and then split into slices (3–5 µM thickness) using a microtome. Hematoxylin and eosin staining was performed after mounting the slices on glass slides. Cover clips were used to secure the slides, which were then allowed 24 h to dry. A blind pathologist then photographed each image using a light microscope.
2.11. Statistical analysis
The data were shown as mean ± SD (standard deviation). The statistical software application GraphPad Prism was used to analyze the data (version 8, Australia). The variation between rat groups was analyzed by one-way ANOVA. Tukey's test was used as the post hoc test to determine differences between means at the significance level of P < 0.05.
3. Results
3.1. Food intake, body weight, fasting glucose, and insulin levels
Rats that received HFD revealed significantly higher food intake than rats that received the STD diet. Except for those given 300 mg and 600 mg/kg of RT extract, there was no significant difference among HFD samples. Similarly, HFD rats consumed higher amounts of food than STD rats. Feeding rats HFD increased liver weight compared to rats fed STD, except for rats administered Lipitor, which showed a significant reduction in their liver weights at p < 0.05 (Table 2). Rats fed HFD gained higher body and liver weights,as well as, elevated levels of fasting glucose, insulin, and HOMA-IR than control rats fed the STD (Table 2). There was no significant change in the levels of all these parameters between rats fed HFD and administered the Qatarah seed extracts (QT) (300 or 600 mg/kg dose) at p < 0.05. In contrast, HFD + Lipitor, HFD + Barhi (BR) (300 and 600 mg/kg), and HFD + Ruthana (RT) (300 mg/kg) seed extract-treated rats displayed lower body and liver weight gain, fasting glucose, insulin, and HOMA-IR as compared with HFD-fed rats (Table 2). In comparison to HFD + BR (300 or 600 mg/kg) and HFD + RT (300 mg/kg), HFD + Lipitor-treated rats showed significantly lower parameters at p < 0.05. Moreover, the results of the body and liver weights, fasting glucose, insulin, and HOMA-IR were not varied in rats treated with STD, HFD + Lipitor, and HFD + RT (600 mg/kg) (Table 2).
Table 2.
Changes in food intake, body weights, and fasting glucose and insulin in all groups of rats.
| STD | HFD | HFD + Lipitor | HFD + QT (300 mg/kg) | HFD + QT (600 mg/kg) | HFD + BR + (300 mg/kg) | HFD + BR (600 mg/kg) | HFD + RT (300 mg/kg) | HFD + RT (600 mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| Final body weight (g) | 324 ± 18 | 457 ± 28a | 358 ± 25ab | 433 ± 27ac | 463 ± 33ac | 398 ± 22abcde | 367 ± 18abdef | 364 ± 22abdef | 364 ± 22bdefgh |
| Food intake (g/rat/4wk) | 219 ± 16 | 322 ± 28a | 335 ± 22a | 334 ± 31a | 319 ± 24a | 344 ± 36a | 338 ± 32a | 327 ± 36a | 321 ± 31a |
| Liver weight (g) | 13.7 ± 1.1 | 19.5 ± 1.6a | 14.2 ± 1.4b | 20.1 ± 2.1ac | 19.6 ± 1.3ac | 17.5 ± 1.4abcde | 15.6 ± 1.2abcdef | 15 ± 1.1abcdef | 13 ± 1.1bdefgh |
| Glucose (mg/dl) | 97 ± 11.1 | 207 ± 17a | 110 ± 13b | 213 ± 7ac | 210 ± 15ac | 192 ± 8ac | 141 ± 12abcdef | 143 ± 16abcdef | 103 ± 8bdefgh |
| Insulin (µIU/ml) | 4.6 ± 0.8 | 7.3 ± 1.3a | 4.1 ± 1.1b | 7.5 ± 1.1ac | 7.1 ± 1.7ac | 7.2 ± 1.5ac | 5.9 ± 0.9abcdef | 5.5 ± 0.7abcdef | 4.4 ± 0.6bdefgh |
| HOMA-IR | 1.1 ± 0.2 | 3.7 ± 0.5a | 1.2 ± 0.2a | 3.8 ± 0.4ac | 3.7 ± 0.2ac | 3.4 ± 0.7ac | 2.2 ± 0.3abcdef | 1.9 ± 0.2abcdef | 1.2 ± 0.2bdefgh |
Data were analysed by one-way ANOVA followed by tukeys’ test. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), f: vs. HFD + Barhi (BR) seed extract (300 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (300 mg/kg).
3.2. Changes in serum and liver lipids
Table 3 shows the results of the contents of CHO, TGs, and FFAs in lipids obtained from rat serum and liver. In comparison to rats fed the STD, the blood serum and liver lipids of rats fed the HFD revealed significantly greater content of CHOL, TG, and FFA, but their HDL-c content was lower at p < 0.05. All hepatic and serum lipid parameters were significantly non-different at p < 0.05 between rats fed HFD and rats fed HFD + QT seed extracts (300 and 600 mg/kg). In comparison to control rats, HFD + Lipitor and HFD + RT (600 mg/kg)-treated rats had significantly greater TGs, CHOL, and FFA levels in the serum, together with greater amounts of serum LDL-c and hepatic TGs, CHOL, and FFAs. Moreover, the STD, HFD + Lipitor, and HFD + RT (600 mg/kg)-treated rats revealed non-significant variations concerning all these studied lipid parameters at p < 0.05. When compared with control, HFD + Lipitor, and HFD + RT (600 mg/kg)-treated rats, the HFD + BR (300 and 600 mg/kg) and HFD + RT (300 mg/kg)-treated rats contained significantly higher quantities of serum and liver TGs, CHOL, and FFAs, but they contained lower quantities of the serum HDL-c at p < 0.05.
Table 3.
Changes in serum and hepatic lipids in all groups of rats.
| STD | HFD | HFD + Lipitor | HFD + QT (300 mg/kg) | HFD + QT (600 mg/kg) | HFD + BR + (300 mg/kg) | HFD + BR (600 mg/kg) | HFD + RT (300 mg/kg) | HFD + RT (600 mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| Serum | |||||||||
| TGs (mg/dl) | 47 ± 5.1 | 145 ± 14.2a | 53 ± 7.4b | 153 ± 13.1ac | 149 ± 15.6ac | 121 ± 11.2abcde | 91 ± 8.6abcdef | 96 ± 7.8abcdef | 51 ± 6.1bdefgh |
| CHOL (mg/dl) | 72 ± 6.4 | 188 ± 13.1a | 75 ± 6.7b | 174 ± 14.3ac | 193 ± 13.5a | 121 ± 9.2abcde | 98 ± 6.5 abcdef | 101 ± 11 abcdef | 67 ± 5.4bdefgh |
| LDL-c (mg/dl) | 37.4 ± 4.2 | 95.2 ± 7.5a | 39.9 ± 5.1b | 101 ± 9.4ac | 97.2 ± 6.8ac | 81.2 ± 7.4abcde | 65.1 ± 5.8abcdef | 72 ± 7.3abcdef | 35.6 ± 4.4bdefgh |
| HDL-c (mg/kg) | 23.5 ± 3.3 | 11.4 ± 2.1a | 26.5 ± 4.1b | 13.2 ± 3.7ac | 11.8 ± 2.4ac | 15.6 ± 1.3abcde | 18.7 ± 3.1abcdef | 16.8 ± 2.8abcde | 24.3 ± 3.1bdefgh |
| FFAs (µmol/l) | 345 ± 31 | 783 ± 49a | 382 ± 39b | 798 ± 59ac | 804 ± 73ac | 615 ± 43abcde | 493 ± 35abcdef | 512 ± 53abcdef | 362 ± 29bdefgh |
| Liver | |||||||||
| TGs (mg/g) | 3.2 ± 0.4 | 7.7 ± 1.3a | 2.9 ± 0.5b | 8.1 ± 1.1ac | 7.8 ± 1.6ac | 6.1 ± 1.3 abcde | 5.1 ± 0.8abcdef | 4.9 ± 0.7abcdef | 3.1 ± 0.4bdefgh |
| CHOL (mg/g) | 0.5 ± 0.07 | 2.2 ± 0.4a | 0.6 ± 0.1b | 2.4 ± 0.5ac | 2.1 ± 0.4ac | 1.6 ± 0.4 abcde | 1.1 ± 0.2abcdef | 0.93 ± 0.2abcdef | 0.6 ± 0.2bdefgh |
| FFAs (µmol/g) | 38 ± 4.1 | 103 ± 9.1a | 41 ± 6.4b | 95 ± 8.3ac | 99 ± 6.3ac | 84 ± 6.7abcde | 60 ± 6.1abcdef | 56 ± 5.9abcdef | 44 ± 5.6bdefgh |
Data were analysed by one-way ANOVA followed by tukeys’ test. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), f: vs. HFD + Barhi (BR) seed extract (600 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (600 mg/kg).
3.3. Liver functions
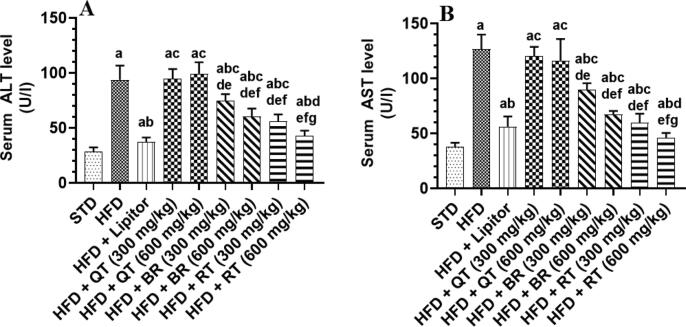
Fig. 1A and B depicts the results of the liver function indicators. Rats fed the HFD had significantly greater concentrations of serum ALT and AST than rats fed the STD. The ALT and STD levels were not significantly different (at p < 0.05) in rats given HFD or HFD + QT seed extract (300 or 600 mg/kg). Compared to the model HFD-fed rats, all other treated HFD rats had significantly lower serum levels of ALT and AST. However, they nevertheless remained noticeably higher than those explored in the rats that intake STD. As compared to rats treated with HFD + Lipitor, HFD + BR (300 and 600 mg/kg), and HF + RT (300 mg/kg), the treatment of rats with HFD + RT (600 mg/kg) led to the greatest, most significant decline in the contents of serum ALT and AST at p < 0.05.
Fig. 1.
Levels of alanine aminotransferase and aspartate aminotransferase in serum of untreated and treated rats. One-way ANOVA and Tukey's test were employed to statistically analyze the results. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), f: vs. HFD + Barhi (BR) seed extract (600 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (600 mg/kg).
3.4. Hepatic markers of oxidative stress
The liver MDA content was greatly higher in rats fed the HFD than in STD, while GSH, SOD, and CAT levels were lower (p < 0.05). In the HFD rat group and the HFD + QT (300 mg/kg) and HFD + QT (600 mg/kg)-treated groups, similar non-significant hepatic levels of all these biochemical parameters were found (Fig. 2A-D). When HFD-fed rats were compared with HFD + QT (300 mg/kg) and HFD + QT (600 mg/kg)-treated rats, similar non-significant hepatic levels of all these biochemical parameters were found (Fig. 2A-D). In HFD + Lipitor, HFD + BR (300 and 600 mg/kg), and HFD + RT (300 and 600 mg/kg)-treated rats, a significant decrease in the MDA content with a simultaneous increase in the hepatic contents of SOD, GSH, and CAT was discovered, considering HFD-fed rats serving as the reference group. Compared with the other groups, the livers of HFD + RT (600 mg/kg) were found to have lower MDA levels versus higher simultaneous levels of SOD, GSH, and CAT, with the most noticeable improvements yet to be witnessed (Fig. 2A-D).
Fig. 2.
Levels of hepatic oxidative stress markers in all groups of rats. One-way ANOVA and Tukey's test were employed to statistically analyze the results. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), f: vs. HFD + Barhi (BR) seed extract (600 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (600 mg/kg).
3.5. Leptin, adiponectin, and C-reactive protein (CRP)
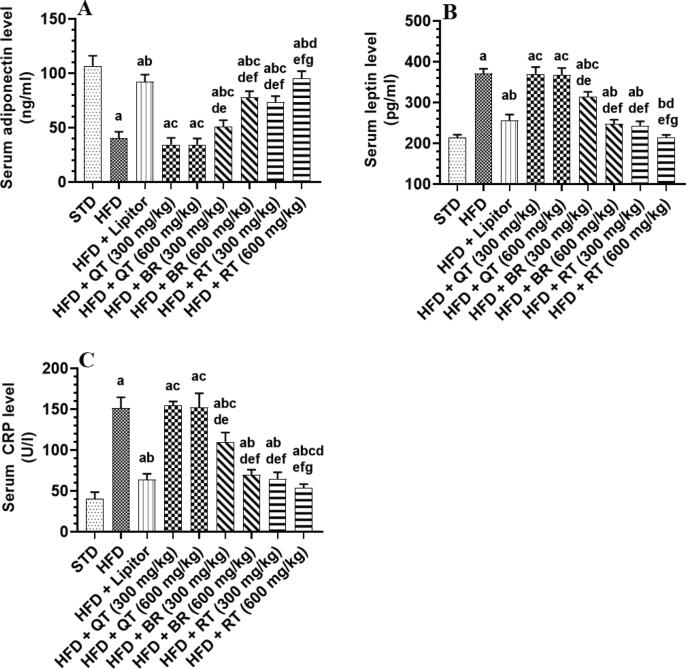
The analysis of blood serum from HFD-fed rats illustrated a significant decrease in adiponectin and an increase in CRP and leptin contents when compared to STD-fed rats, which were statistically similar when compared to rats received HFD + QT (300 and 600 mg/kg). As compared to the HFD group, the blood of rats treated with HFD + Lipitor, HFD + BR (300 and 600 mg/kg), and HFD + RT (300 mg/kg) contained decreased CRP and leptin amounts and increased adiponectin amounts (p < 0.05). HFD + RT (600 mg/Kg) treatment increased blood serum levels of adiponectin while decreasing levels of CRP and leptin, which was significantly different from the other treated groups (Fig. 4A-C).
Fig. 4.
Levels of leptin, adiponectin, and C-reactive protein (CRP) in the serum of all groups of rats. One-way ANOVA and Tukey's test were employed to statistically analyze the results. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), f: vs. HFD + Barhi (BR) seed extract (300 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (300 mg/kg).
3.6. Hepatic markers of inflammation
The contents of the hepatic markers, TNF-α, IL-6, and IL-1β, increased significantly in HFD rats compared to STD rats, while IL-10 levels decreased, as shown in Fig. 3A-D. These values were the same as those in HFD + QT (300 and 600 mg/kg). The contents of all of these parameters varied significantly from those evaluated in the STD-fed rats and were significantly higher in the other treated rat groups than in the HFD-fed rat group. Yet when compared to rats treated with HFD + Lipitor, HFD + BR (300 and 600 mg/kg), and HFD + RT (300 mg/kg), rats administered HFD + RT (600 mg/kg) showed the greatest significant reductions in the levels of all these inflammatory markers (Fig. 3A-D).
Fig. 3.
Levels of hepatic markers of inflammation in all groups of rats. One-way ANOVA and Tukey's test were employed to statistically analyze the results. All values were presented as mean ± SD (n = 8 rats/group) and were considered significantly different at p < 0.05. a: vs. STD, b: vs. HFD, c: vs. HFD + Lipitor, d: vs. HFD + Qatarah (QT) seed extract (600 mg/kg), e: vs. HFD + Qatarah (QT) seed extract (300 mg/kg), f: vs. HFD + Barhi (BR) seed extract (600 mg/kg), g: vs. HFD + Barhi (BR) seed extract (300 mg/kg). h: vs. HFD + Ruthana (RT) seed extract (600 mg/kg).
3.7. Histological findings
Fig. 5A-E displays the microscopic images of the control and treated rat Livers. The control and Lipitor-treated rat livers displayed typical histological traits with sound central vein hepatocytes and sinusoids. The livers of HFD, HFD, and HFD + Qatarah (QT) (300 or 600 mg/kg)-treated rats showed similar morphological changes, including loss of the parenchyma, increased fat granules filling the majority of the hepatocyte cytoplasm (large vacuoles) (long black arrow) with pyknotic nuclei (short arrow), and increased immune infiltrate (arrowhead). The structure of the hepatocytes and parenchyma in the livers of all other treated groups significantly improved, with a decrease in fat accumulation in immune cell infiltrates. The HFD-fed rats that received Ruthana seed extract showed the greatest improvement (see Fig. 5, Fig. 6).
Fig. 5.
Histological features taken from some groups of rats: A and B: were taken from control (STD) and Lipitor-treated rats showing normally sized central vein surrounded by normal hepatocytes (long black arrow) and sinusoids (short black arrow). C, D and E: were taken from HFD-fed, HFD + Qatarah (QT) (600 mg/kg), and HFD + Qatarah (QT) (300 mg/kg)-treated rats, respectively and showing almost similar morphological changes including loss of the parenchyma, increased fat granules filling the majority of the hepatocyte cytoplasm (large vacuoles) (long black arrow) with pyknotic nuclei (short arrow) and increased immune infiltrate (arrowhead).
Fig. 6.
Histological features are taken from some groups of rats: FandG: were taken from HFD + Barhi (BR) (300 mg and 600 mg/kg), respectively, and show a significant improvement in the structure of the livers. In both sections, there was a significant increase in the number of normal hepatocytes (long black arrow) and a decrease in the number of fat-vocalized cells. In HFD + Barhi (600 mg/kg)-treated rats, the number of cells with full-fat accumulation in the cytoplasm (arrowhead) and neutrophils infiltrate (red arrow) was significantly higher than those observed in HFD + Barhi (300/mg/kg)-treated rats (arrowhead). On the contrary, the number of hepatocytes with partial fat accumulation (short black arrow), as well as the number of infiltrating neutrophils (red arrow), were significantly higher in HFD + Barhi (300 mg/kg). H: was taken from HFD + Ruthana (RT) seed extract (600 mg/kg) and exhibited pathological findings that were nearly identical to those seen in HFD + Barhi (300 mg/kg). I: were taken from HFD + Ruthana seed extract (300 mg/kg) and demonstrated the greatest improvement in hepatocyte structure. The majority of the hepatocytes appeared normal (long black arrow) with the presence of some hepatocytes with partial fat accumulates (short black arrow). However, some inflammatory cells were seen around the central vein (red arrow).
4. Discussion
HFD is a major cause of obesity and nonalcoholic steatohepatitis (NASH), among other disorders (He et al., 2020). The most frequent reason for liver cirrhosis is NASH. Serval studies have demonstrated the effectiveness and safety of different plants in preventing and treating diseases. This research looked into the potential impact of date seed extracts on liver function, inflammation, and oxidative stress in HFD-fed rats. Typically, HFD is often linked to higher rates of obesity, hyperlipidemia, insulin resistance, alterations in liver functions, steatosis, and fibrosis, which all lead to NASH (He et al., 2020). Our investigation findings, which are comparable to similar earlier findings (Antunes et al., 2016, Zabielski et al., 2018), demonstrate that rats given the HFD had significantly higher levels of blood and liver lipids, fasting glucose, and insulin, HOMA-IR, and body and liver weights. The weights of the rats' bodies and livers were reduced by Barhi and Ruthana seed extracts, as were their contents of fasting blood glucose, insulin, and HOMA-IR. They also led to a significant reduction in TC, TG, LDL-C, and VLDL-C levels and a significant rise in HDL-C levels. A 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitor, which is termed Lipitor, contributes to lowering lipid contents in both people and animals (Sehra et al., 2017). Furthermore, Ruthana seed extracts at a higher dose (600 mg/kg) significantly reduced the lipid profile markers to the normal range, which is comparable to the Lipitor drug. The current findings agree with previous studies, which revealed that the extract from date seed prevented obesity, lowered blood glucose and insulin levels, protected against insulin resistance, eased the hyperlipidemia associated with HFD in experimental animals, and had a comparable effect to statins in improving lipid profiles (Alahmadi and Banayah, 2021, Ayatollahi et al., 2020, Abiola et al., 2018).
Similarly, Jubayer et al. (2020) reported improvement in the lipid profile of hypercholesterolemic humans after the consumption of 200 mg of milled date seed for 90 days and accordingly advised using it as a supplement for managing hypercholesterolemia. The liver can essentially regulate glucose and lipid metabolism. HFD and long-term hyperlipidemia alter lipid metabolism in hepatocytes by elevating the expression and activity of transcription factors like SREBP-1c that regulate lipid biosynthesis, thereby affecting the synthesis of liver lipids and lipoproteins and causing hepatocellular injury and inflammation. So it suggested that the hypoglycemic and hypolipidemic effects of dates can be explained by decreasing the expression and activity of transcription factors like SREBP-1c.
Phytochemicals are secondary plant metabolites with bioactivities that are produced during plant growth. Some of them, like phenolics, phytosterols, and amino acids, play important roles in protecting cells from damage and modulating the genetic pathways (Bachar et al., 2021, Martinez et al., 2017). It has been noted that the date seed contains different bioactive compounds with therapeutic effects, such as phenolic acid, flavonoids, carotenoids, tocopherols, and carotenoids (Sultan, 2019). The efficiency of these components is demonstrated by their antioxidant, anti-hyperglycemic, anti-inflammatory, and anti-hyperlipidemic activities. They were reported to improve lipid profiles, stimulate insulin secretion, inhibit enzymes such as glucosidase, amylase, and pancreatic lipase, and enhance the overexpression of genes involved in lipid metabolism (Barquero et al., 2020, Trautwein and McKay, 2020, Sekhon-Loodu and Rupasinghe, 2019). HFD is associated with fat deposition in hepatocytes, elevated fatty acid oxidation in liver lipids, and mitochondrial overburden, resulting in the production of large amounts of ROS (He et al., 2020). Prolonged accumulation of these endogenous ROS in the liver can cause chronic oxidative stress (OS), which in turn promotes NASH. This condition is made worse by the production of more lipid peroxides in cells, the depletion of domestic antioxidants, and the dysfunction of antioxidant enzymes (Bikri et al., 2021, López-Contreras et al., 2020). Therefore, stopping the chain oxidation reaction that leads to liver damage is critical. In our research, liver enzymes, MDA, and free radical amounts were significantly elevated in rats after feeding HFD, corresponding with lower lipid peroxidation and reduced tissue damage biomarker levels like GSH, SOD, and CAT.
In contrast, animals treated with Barhi and Ruthana seed extracts revealed significantly lower liver enzyme and lipid peroxidation levels, which corresponded with enhanced hepatic antioxidant potential. These results agreed with previous research findings reporting that animals treated with date seed extract can restore the function of the liver by lowering oxidative damage and improving antioxidant enzymes without causing toxicity even after large doses of date seed extract (Bikri et al., 2021, Bouhlali et al., 2021, Saryono and Proverawati, 2019). Natural antioxidants from dietary sources are key determinants in the defense against OS. HFD was reported to negatively impact antioxidant enzyme contents and activities (Rivera-Mancía et al., 2018). Furthermore, in NASH, oxidized LDL-C induces acute inflammation and severe hepatic injury that leads to hepatic steatosis and fibrosis (Takahashi and Fukusato, 2017). Different varieties of date seeds showed higher antioxidant activity than date flesh and other fruits (Djaoudene et al., 2019). Polyphenols in seed extract have shown their ability to prevent lipid oxidation and endothelial dysfunction, boost antioxidant capacity, and regulate genes involved in lipogenesis and lipid oxidation through antioxidant activity by enhancing the activity of arylesterase and paraoxonase, which protect against lipid oxidation and preserve the HDL-C function (Bijami et al., 2020, Sultan, 2019). The occurrence of polyphenols and oleic acid, which repair and shield hepatocytes from ROS by acting as hydrogen donors, free radical scavengers, or reducing agents, is solely accountable for the seed extract’s antioxidative potential (Eze et al., 2019, Metoui et al., 2019, Bouhlali et al., 2020). The effects of date seed extracts are associated with their chemical and phytochemical composition.
Adiponectin and leptin are adipokines essential for body weight regulation. They serve as NASH diagnostic biomarkers. Elevating leptin with decreased adiponectin levels due to obesity leads to increased liver lipid storage, which boosts liver steatosis (Marques et al., 2021). In NASH, leptin was found to increase hepatic ROS generation and production of the pro-inflammatory cytokine that enhances liver cirrhosis (Polyzos et al., 2016). In contrast, adiponectin promotes FA oxidation in the liver and downregulates pro-inflammatory cytokines, thus preventing liver damage (Adolph et al., 2017). An elevation in inflammatory markers inhibits the production of adiponectin while increasing leptin levels; this is consistent with the current study's findings, which showed a rise in leptin levels and a drop in adiponectin levels in rats fed HFD induced by administrating Barhi and Ruthana seed extracts. Inflammation contributes to the development of NASH, and pro-inflammatory cytokines develop complications like fibrosis and cirrhosis (Mastroianni et al., 2014). An imbalance between inflammatory and anti-inflammatory cytokines is observed to be associated with HFD and obesity, leading to a state of chronic inflammation (Kondo et al., 2018). TNF-α, IL-6, IL-1β, and CRP levels were found to be elevated in the livers of rats fed HFD, in contrast to IL-10; however, administration of Barhi and Ruthana seed extracts reversed the effect of HFD markers. According to several studies, date seeds may have anti-inflammatory properties in both people and animals (Barakat et al., 2020, Maqsood et al., 2020, Saryono et al., 2020). This effect is the consequence of the phenolic compounds in seed extract, as they can stabilize lysosomal membranes, boost nitric oxide production, reduce the synthesis of CRP and fibrinogen, and inhibit the oxidative stress-related processes of free radicals and protein denaturation (Bouhlali et al., 2020).
Histopathological examinations highlighted the harmful effects of hyperlipidemia on the liver. The HFD-treated rats showed disturbances in normal liver architecture, including injured hepatocytes and macro-vesicular steatosis, while the administration of Barhi and Ruthana date seed was found to improve hepatic lobules and nearly restore the liver with the normal architecture of hepatocytes. In contrast, Qatara seed extract showed no significant effect in all markers or liver tissues, probably due to its low phytochemical content. Many factors influence the phytochemical composition of date seeds, including genetic diversity, ripening stage, geographic origin, fertilizers, soil type, water availability, harvesting timings, diseases, and storage conditions (Bouhlali et al., 2018).
5. Conclusions
The findings of this research verified the hepatoprotective impacts of date seed extracts, primarily through their antioxidant potential, which inhibits the hepatic OS and enhances the endogenous antioxidant system. Hyperlipidemia, inflammation, and OS are known to play key roles in NASH. Therefore, it is reasonable to conclude that date seed extracts may help to prevent this disease through their hypolipidemic, anti-inflammatory, and antioxidant effects. Lesser-known date seed varieties of Saudi dates need to be screened and evaluated for their potential health-promoting phytochemicals that can be utilized in treating and preventing the disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend thanks to the Researchers Supporting Project number (RSP2023R84), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Arwa Ali Zarie, Email: 438202908@student.ksu.edu.sa.
Magdi A. Osman, Email: magdios@ksu.edu.sa.
Ghedeir M. Alshammari, Email: aghedeir@ksu.edu.sa.
Amro B. Hassan, Email: ahassan2ks.c@ksu.edu.sa.
Abu ElGasim Ahmed Yagoub, Email: amohammed4@ksu.edu.sa.
Mohammed Abdo Yahya, Email: mabdo@ksu.edu.sa.
References
- Abdul-Hamid N.A., Mustaffer N.H., Maulidiani M., Mediani A., Ismail I.S., Tham C.L., Shadid K., Abas F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2020;19(2):151–160. doi: 10.1016/j.jssas.2018.08.004. [DOI] [Google Scholar]
- Abiola T., Dibie D., Akinwal O.J., Shomuyiwa O.A. Assessment of the Antidiabetic Potential of the Ethanolic Extract of Date Palm (Phoenix Dactylifera) Seed in Alloxan-Induced Diabetic Rats. J. Diabetes Metab. 2018;9:784. doi: 10.4172/2155-6156.1000784. [DOI] [Google Scholar]
- Adolph T., Grander C., Grabherr F., Tilg H. Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmadi A.A., Banayah H.M. Ajwa Seeds (Phoenix dactylifera L.) Suspension Exerted Antidiabetic and Antihyperlipidemic Effects Against Streptozotocin-Induced Diabetes in Rats by Downregulating Insulin Expression in the Pancreatic Beta Islets. Journal of Contemporary Medical Sciences. 2021;7(4) doi: 10.22317/jcms.v7i4.1058. [DOI] [Google Scholar]
- Al-Alawi R., Al-Mashiqri J.H., Al-Nadabi J.S.M., Al-Shihi B.I., Baqi Y. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Frontiers. Plant Sci. 2017;8(June) doi: 10.3389/fpls.2017.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Otaibi S.N., Alshammari G.M., AlMohanna F.H., Al-Khalifa A.S., Yahya M.A. Antihyperlipidemic and hepatic antioxidant effects of Leek leaf methanol extract in high fat diet-fed rats. All Life. 2020;13(1):373–385. doi: 10.1080/26895293.2020.1792355. [DOI] [Google Scholar]
- Alqarni M.M.M., Osman M.A., Al-Tamimi D.S., Gassem M.A., Al-Khalifa A.S., Al-Juhaimi F., Mohamed Ahmed I.A. Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J. Food Biochem. 2019;43(8):1–12. doi: 10.1111/jfbc.12933. [DOI] [PubMed] [Google Scholar]
- Antunes L., Elkfury J., Jornada M., Foletto K., Bertoluci M. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Archives of Endocrinology and Metabolism. 2016;60:138–142. doi: 10.1590/2359-3997000000169. [DOI] [PubMed] [Google Scholar]
- Ayatollahi S.A., Sharifi-Rad M., Roointan A., Baghalpour N., Salehi B., Shinwari Z.K., Khalil A.T., Sharifi-Rad J. Antidiabetic activity of date seed methanolic extracts in alloxan-induced diabetic rats. Pakistan Vet. J. 2020;39(4):583–587. doi: 10.29261/pakvetj/2019.099. [DOI] [Google Scholar]
- Bachar, S. C., Mazumder, K., Bachar, R., Aktar, A., and Al Mahtab, M. 2021. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV . In Frontiers in Pharmacology (Vol. 12). https://www.frontiersin.org/article/10.3389/fphar.2021.732891. [DOI] [PMC free article] [PubMed]
- Barakat A.Z., Hamed A.R., Bassuiny R.I., Abdel-Aty A.M., Mohamed S.A. Date palm and saw palmetto seeds functional properties: antioxidant, anti-inflammatory and antimicrobial activities. J. Food Meas. Charact. 2020;14(2):1064–1072. doi: 10.1007/s11694-019-00356-5. [DOI] [Google Scholar]
- Barquero S., Tresserra-Rimbau A., Vitelli Storelli F., Doménech M., Salas-Salvadó J., Martín V., Rubín García M., Buil-Cosiales P., Corella D., Fitó M., Romaguera D., Vioque J., Alonso-Gómez Á., Wärnberg J., Alfredo M., Serra-Majem L., Tinahones F., Lapetra J., Pinto X., Estruch R. Dietary Polyphenol Intake is Associated with HDL-Cholesterol and A Better Profile of other Components of the Metabolic Syndrome: A PREDIMED-Plus Sub-Study. Nutrients. 2020;12:689. doi: 10.3390/nu12030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijami, A., Rezanejad, F., Oloumi, H., and Mozafari, H. 2020. Minerals, antioxidant compounds and phenolic profile regarding date palm (Phoenix dactylifera L.) seed development. Scientia Horticulturae, 262(November 2019), 109017. https://doi.org/10.1016/j.scienta.2019.109017.
- Bikri S., Aboussaleh Y., Abuelezz N., Benmhammed H., Berrani A., Louragli I., Ahami A.O.T. Phenolic fraction concentrate of Phoenix dactylifera L. seeds: A promising antioxidant and glucose regulator. Journal of Pharmacy and Pharmacognosy Research. 2021;9(6):921–936. [Google Scholar]
- Bouhlali E. dine T., El Hilaly J., Ennassir J., Benlyas M., Alem C., Amarouch M.Y., Filali-Zegzouti Y. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. Journal of King Saud University - Science. 2018;30(4):519–526. doi: 10.1016/j.jksus.2017.08.011. [DOI] [Google Scholar]
- Bouhlali, E. D. T., Derouich, M., Hmidani, A., Bourkhis, B., Khouya, T., Filali-Zegzouti, Y., and Alem, C. 2021. Protective Effect of Phoenix dactylifera L. Seeds against Paracetamol-Induced Hepatotoxicity in Rats: A Comparison with Vitamin C. Scientific World Journal, 2021. https://doi.org/10.1155/2021/6618273. [DOI] [PMC free article] [PubMed]
- Bouhlali E. dine T., Hmidani A., Bourkhis B., Khouya T., Ramchoun M., Filali-Zegzouti Y., Alem C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon. 2020;6(2):e03436. doi: 10.1016/j.heliyon.2020.e03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaoudene O., López V., Cásedas G., Les F., Schisano C., Bachir Bey M., Tenore G.C. Phoenix dactylifera L. seeds: A by-product as a source of bioactive compounds with antioxidant and enzyme inhibitory properties. Food Funct. 2019;10(8):4953–4965. doi: 10.1039/c9fo01125k. [DOI] [PubMed] [Google Scholar]
- Eze C., Ezinwanne N., Ozioko A., Martina C., Chineye N., Evurani S., Uchechi L. Evaluation of Antimicrobial Activities of Crude Methanol Extract of Phoenix dactylifera Seeds on Clinical Isolates of Different Strains of E. coli. International Journal of Biochemistry Research and Review. 2019:1–7. doi: 10.9734/ijbcrr/2019/v25i130066. [DOI] [Google Scholar]
- Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghnimi S., Almansoori R. Quality Evaluation of Coffee-Like Beverage from Date Seeds (Phoenix dactylifera, L.) J. Food Process. Technol. 2015;6(12) doi: 10.4172/2157-7110.1000525. [DOI] [Google Scholar]
- He Y., Yang T., Du Y., Qin L., Ma F., Wu Z., Ling H., Yang L., Wang Z., Zhou Q., Ge G., Lu Y. High fat diet significantly changed the global gene expression profile involved in hepatic drug metabolism and pharmacokinetic system in mice. Nutr. Metab. 2020;17 doi: 10.1186/s12986-020-00456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.F., Bordoni B. Hyperlipidemia. In StatPearls Publishing. 2021 https://www.ncbi.nlm.nih.gov/books/NBK559182/ [PubMed] [Google Scholar]
- Jubayer F., Kayshar S., Rahaman M. Effects of Ajwa date seed powder on serum lipids in humans: A randomized, double-blind, placebo-controlled clinical trial. Journal of Herbal Medicine. 2020;24(November) doi: 10.1016/j.hermed.2020.100409. [DOI] [Google Scholar]
- Khan F., Khan T.J., Kalamegam G., Pushparaj P.N., Chaudhary A., Abuzenadah A., Kumosani T., Barbour E., Al-Qahtani M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017;17(1):1–10. doi: 10.1186/s12906-017-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Abe I., Gotoh K., Fukui A., Takanari H., Ishii Y., Ikebe Y., Kira S., Oniki T., Saito S., Aoki K., Tanino T., Mitarai K., Kawano K., Miyoshi M., Fujinami M., Yoshimura S., Ayabe R., Okada N., Takahashi N. Interleukin 10 Treatment Ameliorates High-Fat Diet-Induced Inflammatory Atrial Remodeling and Fibrillation. Circ. Arrhythm. Electrophysiol. 2018;11(5):e006040. doi: 10.1161/CIRCEP.117.006040. [DOI] [PubMed] [Google Scholar]
- López-Contreras, A. K., Martínez-Ruiz, M. G., Olvera-Montaño, C., Robles-Rivera, R. R., Arévalo-Simental, D. E., Castellanos-González, J. A., Hernández-Chávez, A., Huerta-Olvera, S. G., Cardona-Muñoz, E. G., and Rodríguez-Carrizalez, A. D. 2020. Importance of the Use of Oxidative Stress Biomarkers and Inflammatory Profile in Aqueous and Vitreous Humor in Diabetic Retinopathy. In Antioxidants (Vol. 9, Issue 9). 10.3390/antiox9090891. [DOI] [PMC free article] [PubMed]
- Malhotra, P., Gill, R. K., Saksena, S., and Alrefai, W. A. 2020. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases . In Frontiers in Medicine (Vol. 7). https://www.frontiersin.org/article/10.3389/fmed.2020.00467. [DOI] [PMC free article] [PubMed]
- Maqsood S., Adiamo O., Ahmad M., Mudgil P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020;308(September 2019) doi: 10.1016/j.foodchem.2019.125522. [DOI] [PubMed] [Google Scholar]
- Marques, V., Afonso, M. B., Bierig, N., Duarte-Ramos, F., Santos-Laso, Á., Jimenez-Agüero, R., Eizaguirre, E., Bujanda, L., Pareja, M. J., Luís, R., Costa, A., Machado, M. V, Alonso, C., Arretxe, E., Alustiza, J. M., Krawczyk, M., Lammert, F., Tiniakos, D. G., Flehmig, B., … Rodrigues, C. M. P. 2021. Adiponectin, Leptin, and IGF-1 Are Useful Diagnostic and Stratification Biomarkers of NAFLD . In Frontiers in Medicine (Vol. 8). https://www.frontiersin.org/article/10.3389/fmed.2021.683250. [DOI] [PMC free article] [PubMed]
- Martinez, K. B., Mackert, J. D., and McIntosh, M. K. 2017. Chapter 18 - Polyphenols and Intestinal Health (R. R. B. T.-N. and F. F. for H. A. Watson (ed.); pp. 191–210). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-805376-8.00018-6.
- Mastroianni C., Lichtner M., Mascia C., Zuccalà P., Vullo V. Molecular Mechanisms of Liver Fibrosis in HIV/HCV Coinfection. Int. J. Mol. Sci. 2014;15:9184–9208. doi: 10.3390/ijms15069184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medani K., Mansour M., Mohamed E., Alfhaid F., Alghamdi T., Sami W., Abdalla S., Zahrani M. Prevalence and Risk Factors of Hypercholesterolemia in Majmaah, Saudi Arabia. Majmaah Journal of Health Sciences. 2018;7(2):34. doi: 10.5455/mjhs.2018.01.006. [DOI] [Google Scholar]
- Melek F.R., Saleh D.O., Medhat A.M., Farrag A.R.H., Ghaly N.S., Baraka S.M. ANTIDIABETIC and ANTIOXIDANT ACTIVITIES of Phoenix dactylifera L. Seed extract in streptozotocin-induced diabetic rats. Malaysian. J. Biochem. Mol. Biol. 2019;22(1):53–59. [Google Scholar]
- Metoui M., Essid A., Bouzoumita A., Ferchichi A. Chemical Composition, Antioxidant and Antibacterial Activity of Tunisian Date Palm Seed. Pol. J. Environ. Stud. 2019;28(1):267–274. doi: 10.15244/pjoes/84918. [DOI] [Google Scholar]
- Misar Wajpeyi S. Analysis of Etiological Factors of Dyslipidemia -A Case Control Study. International Journal of Ayurvedic Medicine. 2020;11:92–97. doi: 10.47552/ijam.v11i1.1340. [DOI] [Google Scholar]
- Polyzos S., Aronis K., Kountouras J., Raptis D., Vasiloglou M., Mantzoros C. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59 doi: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- Raal F.J., Alsheikh-Ali A.A., Omar M.I., Rashed W., Hamoui O., Kane A., Alami M., Abreu P., Mashhoud W.M. Cardiovascular risk factor burden in Africa and the Middle East across country income categories: a post hoc analysis of the cross-sectional Africa Middle East Cardiovascular Epidemiological (ACE) study. Archives of Public Health = Archives Belges de. Sante Publique. 2018;76:15. doi: 10.1186/s13690-018-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- Rivera-Mancía S., Jiménez-Osorio A.S., Medina-Campos O.N., Colín-Ramírez E., Vallejo M., Alcántara-Gaspar A., Cartas-Rosado R., Vargas-Barrón J., Pedraza-Chaverri J. Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions. Int. J. Environ. Res. Public Health. 2018;15(12):2687. doi: 10.3390/ijerph15122687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saryono S., Eliyan J., Herdiati D., Khikmatullah A.A., Silvana C.P., Adi H.P. Anti-atherogenic properties of Deglet Noor Date seeds (Phoenix dactylifera) Methanol extract on Diet-Induced Hypercholesterolemic Rats. IOP Conference Series: Materials Science and Engineering. 2017;172:12046. doi: 10.1088/1757-899X/172/1/012046. [DOI] [Google Scholar]
- Saryono W., Isworo A., Sarmoko. Anti-inflammatory activity of date palm seed by downregulating interleukin-1β, TGF-β, cyclooxygenase-1 and -2: A study among middle age women. Saudi Pharmaceutical Journal. 2020;28(8):1014–1018. doi: 10.1016/j.jsps.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saryono S., Proverawati A. Hepatoprotective effect of date seeds works through the antioxidant mechanism: A systematic review. Annals of Tropical Medicine and Public Health. 2019;22(11) doi: 10.36295/ASRO.2019.221139. [DOI] [Google Scholar]
- Sehra D., Sehra S., Sehra S.T. Cardiovascular pleiotropic effects of statins and new onset diabetes: is there a common link: do we need to evaluate the role of KATP channels? Expert Opin. Drug Saf. 2017;16(7):823–831. doi: 10.1080/14740338.2017.1338269. [DOI] [PubMed] [Google Scholar]
- Sekhon-Loodu S., Rupasinghe H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front Nutr. 2019;25(6):53. doi: 10.3389/fnut.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan F. Anti-Obesity and Hypolipidemic Effects of Ajwa Date Seed Compared to Simvastatin in Butter Fed Dyslipidemic Rats. Proceedings of Shaikh Zayed Medical Complex Lahore. 2019;33(2):6–12. doi: 10.47489/p000s332z7051-7mc. [DOI] [Google Scholar]
- Takahashi, Y., and Fukusato, T. 2017. Chapter 13 - Animal Models of Liver Diseases (P. M. B. T.-A. M. for the S. of H. D. (Second E. Conn (ed.); pp. 313–339). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-809468-6.00013-9.
- Trautwein E.A., McKay S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients. 2020;12(9):2671. doi: 10.3390/nu12092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S.C., Seeley R.J., Rushing P.A., D'Alessio D., Tso P. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2021. Noncommunicable diseases. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- Zabielski P., Hady H., Chacinska M., Roszczyc K., Kowalski J., Blachnio-Zabielska Agnieszka B. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Abtahi-Evari S.H. Protective effect of Galegaofficinalis extract on streptozotocin-induced kidney damage and biochemical factor in diabetic rats. Crescent Journal of Medical and Biological Sciences. 2017;4(June):108–114. [Google Scholar]
- Abul-Soad, A. A., Jain, S. M., and Jatoi, M. A. 2017. Biodiversity and Conservation of Date Palm (Vol. 3, Issue December 2017). https://doi.org/10.1007/978-3-319-66426-2_12.
- Al-Farsi M., Alasalvar C., Al-Abid M., Al-Shoaily K., Al-Amry M., Al-Rawahy F. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007;104(3):943–947. doi: 10.1016/j.foodchem.2006.12.051. [DOI] [Google Scholar]
- Al-Farsi M.A., Lee C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108(3):977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Ali-Mohamed A.Y., Khamis A.S.H. Mineral ion content of the seeds of six cultivars of Bahraini date palm (Phoenix dactylifera) J. Agric. Food Chem. 2004;52(21):6522–6525. doi: 10.1021/jf030518x. [DOI] [PubMed] [Google Scholar]
- Al-Meqbaali F., Habib H., Othman A., Al-Marzooqi S., Al-Bawardi A., Pathan J.Y., Hilary S., Souka U., Al-Hammadi S., Ibrahim W., Platat C. The antioxidant activity of date seed: Preliminary results of a preclinical in vivo study. Emirates Journal of Food and Agriculture. 2017;29(11):822–832. doi: 10.9755/ejfa.2017.v29.i11.1477. [DOI] [Google Scholar]
- Ardekani M.R.S., Khanavi M., Hajimahmoodi M., Jahangiri M., Hadjiakhoondi A. Comparison of antioxidant activity and total phenol contents of some date seed varieties from Iran. Iranian Journal of Pharmaceutical Research. 2010;9(2):141–146. doi: 10.22037/ijpr.2010.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestri S., Nascimbeni F., Romagnoli D., Lonardo A. The independent predictors of NASH and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, ALT and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Dig. Liver Dis. 2015;47:e229. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- Basheikh K., Felemban A., Felemban M., AlRaddadi R., Alnuqali E., Abaalkhail B., Alshareef K. Prevalence of dyslipidemia and its associated factors among employees of primary health care centers, Jeddah, Saudi Arabia. International Journal of Medical Science and Public Health. 2016;5(5):946. doi: 10.5455/ijmsph.2016.22012016333. [DOI] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A., Idelchik M. del P.S., Melendez J.A. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal S., Jeandidier N., Seyfritz E., Bietiger W., Péronet C., Moreau F., Pinget M., Maillard E., Sigrist S. Featured Article: Oxidative stress status and liver tissue defenses in diabetic rats during intensive subcutaneous insulin therapy. Experimental Biology and Medicine (Maywood. N.J.) 2016;241(2):184–192. doi: 10.1177/1535370215603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaoudene O., Mansinhos I., Gonçalves S., Jara-Palacios M.J., Bachir bey M., Romano A. Phenolic profile, antioxidant activity and enzyme inhibitory capacities of fruit and seed extracts from different Algerian cultivars of date (Phoenix dactylifera L.) were affected by in vitro simulated gastrointestinal digestion. S. Afr. J. Bot. 2021;137:133–148. doi: 10.1016/j.sajb.2020.10.015. [DOI] [Google Scholar]
- Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elumalai A., Jain S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, T., Patel, A., and Purohit, M. 2022. Chapter 11 - Selection of experimental models mimicking human pathophysiology for diabetic microvascular complications (R. C. B. T.-A. in A. E. and M. Sobti (ed.); pp. 137–177). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-323-90583-1.00034-9.
- Gawrieh S., Chalasani N. Pharmacotherapy for Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2015;35:338–348. doi: 10.1055/s-0035-1562951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghnimi S., Almansoori R. Quality Evaluation of Coffee-Like Beverage from Date Seeds (Phoenix dactylifera. L.). Journal of Food Processing and Technology. 2015;6(12) doi: 10.4172/2157-7110.1000525. [DOI] [Google Scholar]
- González-Aguilar G., Robles-Sánchez R.M., Martínez-Téllez M.A., Olivas G.I., Alvarez-Parrilla E., De La Rosa L.A. Bioactive compounds in fruits: Health benefits and effect of storage conditions. Stewart Postharvest Review. 2008;4(3) doi: 10.2212/spr.2008.3.8. [DOI] [Google Scholar]
- Hussain M.I., Farooq M., Syed Q.A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.) – A review. Food. Bioscience. 2020;34(December 2019) doi: 10.1016/j.fbio.2019.100509. [DOI] [Google Scholar]
- Li J., Luo J., Xu H., Wang M., Zhu J., Shi H., Haile A.B., Wang H., Sun Y. Fatty acid synthase promoter: Characterization, and transcriptional regulation by sterol regulatory element binding protein-1 in goat mammary epithelial cells. Gene. 2015;561(1):157–164. doi: 10.1016/j.gene.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Patil I.Y., Jiang T., Sancheti H., Walsh J.P., Stiles B.L., Yin F., Cadenas E. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One. 2015;10(5):e0128274–e. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwafemi Omoniyi Oguntibeju Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. International Journal of Physiology, Pathophysiology and Pharmacology. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- Shokri F., Shokoohi M., Abadi A., Kalarestaghi H. The Ameliorative Effect of Galega officinalis Extract on Histological Damages, Oxidative Stress Induced by Torsion-Detorsion in Adult Rats’ Ovarian. International Journal of Women’s Health and Reproduction Sciences. 2019;7:119–123. doi: 10.15296/ijwhr.2019.19. [DOI] [Google Scholar]
- Souli I., Jemni M., Rodríguez-Verástegui L.L., Chaira N., Artés F., Ferchichi A. Phenolic composition profiling of Tunisian 10 varieties of common dates (Phoenix dactylifera L.) at tamar stage using LC-ESI-MS and antioxidant activity. J. Food Biochem. 2018;42(6) [Google Scholar]
- Temitope, A., DC, D., OJ, A., and OA, S. 2018. Assessment of the Antidiabetic Potential of the Ethanolic Extract of Date Palm (Phoenix Dactylifera) Seed in Alloxan-Induced Diabetic Rats. Journal of Diabetes and Metabolism, 09(01), 1–9. https://doi.org/10.4172/2155-6156.1000784.
- Walenbergh S.M.A., Koek G.H., Bieghs V., Shiri-Sverdlov R. Non-alcoholic steatohepatitis: The role of oxidized low-density lipoproteins. J. Hepatol. 2013;58(4):801–810. doi: 10.1016/j.jhep.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Wiseman, H. (2013). Phytochemicals: Health Effects (B. B. T.-E. of H. N. (Third E. Caballero (ed.); pp. 47–51). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-375083-9.00227-0.
- Wong V.W.S., Chan W.K., Chitturi S., Chawla Y., Dan Y.Y., Duseja A., Fan J., Goh K.L., Hamaguchi M., Hashimoto E., Kim S.U., Lesmana L.A., Lin Y.C., Liu C.J., Ni Y.H., Sollano J., Wong S.K.H., Wong G.L.H., Chan H.L.Y., Farrell G. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017—Part 1: Definition, risk factors and assessment. Journal of Gastroenterology and Hepatology (Australia) 2018;33(1):70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- Yaribeygi H., Atkin S.L., Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019;234(2):1300–1312. doi: 10.1002/jcp.27164. [DOI] [PubMed] [Google Scholar]
- Zhang C.R., Aldosari S.A., Vidyasagar P.S.P.V., Shukla P., Nair M.G. Health-benefits of date fruits produced in Saudi Arabia based on in vitro antioxidant, anti-inflammatory and human tumor cell proliferation inhibitory assays. J. Saudi Soc. Agric. Sci. 2017;16(3):287–293. doi: 10.1016/j.jssas.2015.09.004. [DOI] [Google Scholar]