Abstract
Disclosure of HIV status is an important part of pediatric care. We studied disclosure and clinical outcomes in a multi-country Asian cohort of children and adolescents with HIV. Those 6–19 years of age who initiated combination antiretroviral therapy (cART) between 2008 and 2018, and who had at least one follow-up clinic visit were included. Data up to December 2019 were analyzed. Cox and competing risk regression analyses were used to assess the effect of disclosure on disease progression (WHO clinical stage 3 or 4), loss to follow‐up (LTFU; >12 months), and death. Of 1913 children and adolescents (48% female; median [IQR] age 11.5 [9.2–14.7] years at last clinic visit), 795 (42%) were disclosed to about their HIV status at a median age of 12.9 years (IQR: 11.8–14.1). During follow-up, 207 (11%) experienced disease progression, 75 (3.9%) were LTFU, and 59 (3.1%) died. There were lower hazards of disease progression (adjusted hazard ratio [aHR] 0.43 [0.28–0.66]) and death (aHR 0.36 [0.17–0.79]) for those disclosed to compared with those who were not. Disclosure and its appropriate implementation should be promoted in pediatric HIV clinics in resource-limited settings.
Keywords: HIV, disclosure, children, adolescents, Asia
INTRODUCTION
UNAIDS reported 1.7 million children aged 0–14 years were living with HIV in 2021 (UNAIDS, 2022). Increased access to antiretroviral therapy (ART) have resulted in substantial reductions in AIDS-related morbidity and mortality among children and adolescents (Hayfron-Benjamin et al., 2018). For children with perinatally acquired HIV, disclosure to them of their HIV diagnosis is a major challenge for families and health care practitioners. Previous studies have shown that children and youth living with HIV can benefit both physically and psychologically from being told about their HIV status, and that delayed disclosure or non-disclosure can result in sub-optimal adherence and consequently risks treatment failure (Vreeman et al., 2010; Montalto et al., 2017; Bulali et al., 2018; Ramos et al., 2018). Some studies have reported negative health outcomes of disclosure, such as emotional difficulties and internalized stigma, while others found no physical and mental health differences between those who were aware of their diagnosis and those who were not (Odiachi, 2017; Wiener et al., 2007).
In 2011, the World Health Organization (WHO) released guidelines on HIV disclosure in children, recommending disclosure when a child is 6–12 years (Krauss et al., 2011). The American Academy of Pediatrics (AAP) have also recommended that disclosure takes place prior to adolescence (Committee on Pediatric AIDS, 1999). However, despite these recommendations and the evidence on the importance of disclosure, many children living with HIV are unaware of their HIV status. In a systematic review on disclosure in resource-limited settings, the proportion of disclosed children in studies that reported prevalence data ranged widely from 0 to 69% (Vreeman et al., 2013). Younger age, perceived inability to understand the meaning of HIV infection, concerns over psychological harm, lack of social support, and lack of skills around how to disclose were the most common reasons mentioned by parents or caregivers for not disclosing to children in different studies (Arrivé et al., 2012; Vreeman et al., 2013; Odiachi, 2017; Shallo and Tassew, 2020).
Previously reported prevalence of HIV disclosure to children was 70% in Thailand (Sirikum et al., 2014) and 41% in India (Bhattacharya et al., 2011). In Thailand, caregivers were fearful of disclosure because of the perceived risks of stigma, discrimination and bullying (Siripong, A. et al., 2007) and possible emotional and behavioral consequences (Boon-yasidhi et al., 2016). Efforts to preserve family harmony and avoid stigma may have increased resistance to HIV disclosure (Jantarapakde et al., 2019).
The prevalence of disclosure and its impact on clinical response to ART are not well studied in Asia. In this study, we aimed to (1) describe the frequency of disclosure in children and adolescents living with HIV in an Asian regional cohort, and (2) assess the effect of disclosed HIV status on ART clinical outcomes, including disease progression, loss to follow-up (LTFU), and death.
METHODS
The TREAT Asia Pediatric HIV Observational Database (TApHOD) of the International epidemiology Databases to Evaluate AIDS (IeDEA) Asia-Pacific is a prospective cohort study conducted in 17 pediatric clinics across six countries: Cambodia (n=1), India (n=2), Indonesia (n=2), Malaysia (n=4), Thailand (n=5), and Vietnam (n=3). These sites are predominantly public or university-based pediatric HIV referral clinics, located in urban or semi-rural areas. For this study, we included patients 6–19 years of age who initiated combination ART (cART; defined as ≥3 antiretrovirals) between January 2008 through December 2018. Children were required to have initiated cART at least 12 months before the site-specific database closure date and have at least one clinic visit during follow-up between ages 6 to 19. We used all available follow-up data up to December 2019.
Ethics review
Ethics approval was obtained through the human research ethics committees at all participating sites, the data management and analysis center at the Kirby Institute (UNSW Sydney), and the coordinating center at TREAT Asia/amfAR (The Foundation for AIDS Research). Consent by parents or legal guardians and assent of the children and adolescents under care were not routinely obtained unless required by the local ethics committee (i.e., in some sites in India, Malaysia, and Thailand).
Definitions
Disclosure was defined as making the child or adolescent aware that they are living with HIV. Disease progression was defined as the first occurrence of a WHO clinical stage 3 or 4 during follow-up. Second-line ART was defined as the second triple-drug regimen with a change in drug class [e.g., nonnucleoside reverse transcriptase inhibitor (NNRTI) to protease inhibitor (PI)], excluding those exposed to mono/dual nucleoside reverse transcriptase inhibitor therapy and known to have been switched without failure of first-line therapy. Patients were considered LTFU when there was no contact with the clinic for >12 months before the site database closure date, with their follow-up period ending one year after their last clinic contact before their 20th birthday.
For laboratory and clinical measurements at cART initiation, we used the single closest value reported during a window period of six months prior to and one week after start. We extended the window period up to three months after cART initiation for weight and height measurements. Weight and height measurements were converted to age- and sex-adjusted z-scores. For characteristics at disclosure and at last clinic visit, the closest value during a window period of six months prior to each time point was used. Weight-for-age z-scores (WAZ) and height-for-age z-scores (HAZ) were calculated using WHO 1977 Standards and WHO 2007 Child Growth Standards, respectively (World Health Organization, 2007, 2019).
Data management and statistical analysis
Detailed methods for cohort data management have previously been reported (Kariminia et al., 2011). Briefly, routinely collected clinical and program data were anonymized and then transferred to the Kirby Institute (UNSW, Sydney, Australia) for management and analysis on a biannual basis. Data were harmonized according to a common data exchange standard. Descriptive analyses were used to report demographic, immunological, and clinical characteristics of the study population at cART initiation by disclosure status. Incidence rates of disease progression, LTFU, and mortality were determined by dividing the number of events by person-years of observation. The beginning of follow-up (baseline) was from the date of cART initiation, or on the date of their 6th birthday for those who initiated cART before age 6. The end point was defined as the date of last contact before the site-specific database closure, first occurrence of WHO clinical stage 3 or 4, turning 19.9 years of age, LTFU, or death.
We used Cox regression analysis to assess predictors of first occurrence of WHO clinical stage 3 or 4 and mortality and competing risk regression based on Fine and Gray’s proportional sub-hazards model to identify predictors of LTFU (death as competing event) (Fine & Gray, 1999). Sex and calendar year at cART initiation were included as fixed variables. Age, CD4 cell count, WAZ, AIDS diagnosis, second-line cART, and disclosure status were included as time-updated variables. AIDS was only considered as a covariate for analysis when the outcome was LTFU or mortality. HAZ was not included as a co-variate because the proportion of missing data made it an unreliable measurement. For CD4 and WAZ, values were carried forward for one year if no subsequent measurements were recorded. We did not use multiple imputation to replace other missing data due to the relatively small numbers of covariates available. In the regression analyses, missing data were included as a separate category. We considered clinic as a random effect in the model. Covariates with a p-value of <0.20 in univariate analysis were included in a multivariate model. We selected the final model using a stepwise method and retained covariates with p-values of <0.05. The adjusted hazard ratios (aHR) and adjusted subdistribution hazard ratios (asHR) were reported with their 95% confidence intervals (95% CI). We tested the proportional hazard assumption using the estat phtest in Stata and no violation was observed. Sensitivity analyses were conducted by excluding patients without data on CD4 or WAZ. Data management and statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, TX, US).
RESULTS
As of December 2018, there were 7213 children and adolescents of all ages who had ever received care within the cohort. Of these, 1913 from 15 clinics met the inclusion criteria and were included in the analysis (48% female; median [interquartile range {IQR}] age, 11.5 [9.2–14.7] years at last clinic visit). These clinics were located in five countries: Vietnam (n=1156, 60%; 3 sites), Thailand (n=400, 21%; 5 sites), Indonesia (n=180, 9.4%; 2 sites), Malaysia (n=126, 6.6%; 4 sites), and India (n=51, 2.7%; 1 site). Seventy-nine percent of these sites were located in an urban setting. The median age at cART initiation was 5.8 years (IQR: 3.0–9.2); 52% initiated treatment before age 6 (Table 1). Of children and adolescents with known status, 74% were under the care of one or both parents. At cART initiation, for those with available data, the median CD4 count was 252 cells/mm3 (IQR: 50–621); 45% had CD4 <200 cells/mm3. HIV viral load was available in 411 (21%) children, who had a median log10 viral load of 5.2 copies/mL (IQR: 4.7–5.8). Thirty-three percent were severely underweight (WAZ <−3) and 26% were severely stunted (HAZ <−3) at cART initiation. Thirty-nine percent had experienced AIDS.
Table 1.
Characteristics of children and adolescents at cART initiation by subsequent HIV disclosure status in the IeDEA Asia-Pacific cohort (n=1913)
| All (N=1913) |
HIV disclosure between age 6–12 y (n=406) |
HIV disclosure between age 13–19 y (n=389) |
Not disclosed to (n=1118) |
|
|---|---|---|---|---|
| Female sex | 913 (48) | 224 (55) | 180 (46) | 509 (46) |
| Age (years) | ||||
| ≤5 | 986 (52) | 144 (35) | 55 (14) | 787 (70) |
| 5–9 | 541 (28) | 169 (42) | 114 (29) | 258 (23) |
| 10–14 | 322 (17) | 90 (22) | 159 (41) | 73 (6.5) |
| ≥15 | 64 (3.4) | 3 (0.7) | 61 (16) | 0 (0.0) |
| Median (IQR) | 5.8 (3.0–9.2) | 7.6 (4.7–9.7) | 10.9 (7.5–13.9) | 3.9 (1.7–6.4) |
| Facility setting | ||||
| Urban | 1512 (79) | 338 (83) | 281 (72) | 893 (80) |
| Mostly urban | 219 (12) | 49 (12) | 77 (20) | 93 (8.3) |
| Mostly rural | 182 (9.5) | 19 (4.7) | 31 (8.0) | 132 (12) |
| Facility level | ||||
| Healthcare center | 523 (27) | 90 (22) | 114 (29) | 319 (29) |
| University | 1390 (73) | 316 (78) | 275 (71) | 799 (71) |
| Primary care giver | ||||
| Available data, n (%) | 1497 (78) | 286 (70) | 288 (74) | 923 (83) |
| One/both parents | 1101 (74) | 194 (68) | 186 (65) | 721 (78) |
| Family/non-family members Grand parents | 334 (22) | 85 (30) | 84 (29) | 165 (18) |
| Foster care | 62 (4.1) | 7 (2.4) | 18 (6.3) | 37 (4.0) |
| cART before age 6 | 996 (52) | 144 (35) | 56 (14) | 796 (71) |
| cART initiation year | ||||
| 2008–2009 | 541 (28) | 139 (34) | 125 (32) | 277 (25) |
| 2010–2012 | 769 (40) | 163 (40) | 144 (37) | 462 (41) |
| 2013–2018 | 603 (32) | 104 (26) | 120 (31) | 379 (34) |
| Type of therapy | ||||
| cART-NNRTI | 1735 (91) | 381 (94) | 353 (91) | 1001 (90) |
| cART-PI | 129 (6.7) | 20 (4.9) | 24 (6.2) | 85 (7.6) |
| cART-II | 15 (0.8) | 2 (0.5) | 12 (3.1) | 1 (0.1) |
| cART-other | 34 (1.8) | 3 (0.7) | - | 31 (2.8) |
| Weight-for-age z-score | ||||
| Available data, n (%) | 1669 (87) | 339 (83) | 321 (83) | 1009 (90) |
| <−3 | 556 (33) | 97 (29) | 100 (31) | 359 (36) |
| −3 ≤ to <−2 | 317 (19) | 66 (19) | 60 (19) | 191 (19) |
| ≥−2 | 796 (48) | 176 (52) | 161 (50) | 459 (45) |
| Median (IQR) | −2.1 (−3.5, −1.0) | −1.9 (−3.2, −1.0) | −2.0 (−3.5, −1.0) | −2.2 (−3.7, −1.0) |
| Height-for-age z-score | ||||
| Available data, n (%) | 1563 (82) | 327 (81) | 318 (82) | 918 (82) |
| <−3 | 406 (26) | 73 (22) | 58 (18) | 275 (30) |
| −3 ≤ to <−2 | 419 (27) | 90 (28) | 96 (30) | 233 (25) |
| ≥−2 | 738 (47) | 164 (50) | 164 (52) | 410 (45) |
| Median (IQR) | −2.1 (−3.1, −1.1) | −2.0 (−2.8, −1.1) | −2.0 (−2.8, −1.0) | −2.2 (−3.2, −1.2) |
| WHO clinical stage | ||||
| Stages 1 and 2 | 1168 (61) | 263 (65) | 248 (64) | 657 (59) |
| Stages 3 and 4 | 745 (39) | 143 (35) | 141 (36) | 461 (41) |
| CD4 count (cells/mm3) | ||||
| Available data, n (%) | 1598 (84) | 334 (82) | 324 (83) | 940 (84) |
| <200 | 721 (45) | 165 (49) | 180 (56) | 376 (40) |
| 200–499 | 381 (24) | 103 (31) | 91 (28) | 187 (20) |
| ≥500 | 496 (31) | 66 (20) | 53 (16) | 377 (40) |
| Median (IQR) | 252 (50–621) | 204 (43–417) | 149 (23–365) | 336 (69–812) |
| CD4 percentage | ||||
| Available data, n (%) | 1489 (78) | 300 (74) | 310 (80) | 879 (79) |
| <10 | 697 (47) | 150 (50) | 174 (56) | 373 (42) |
| 10–24 | 603 (40) | 132 (44) | 113 (36) | 358 (41) |
| ≥25 | 189 (13) | 18 (6.0) | 23 (7.4) | 148 (17) |
| Median (IQR) | 11.0 (3.0–19.1) | 9.9 (2.6–17.1) | 8.4 (2.0–15.0) | 12.8 (3.7–21.4) |
| HIV viral load (copies/mL) | ||||
| Available data, n (%) | 411 (21) | 97 (24) | 112 (29) | 202 (18) |
| Median log10 (IQR) | 5.2 (4.7–5.8) | 5.1 (4.6–5.6) | 5.0 (4.5–5.4) | 5.5 (5.0–6.1) |
| WHO stages 3 and 4 | 207 (11) | 57 (14) | 55 (14%) | 95 (8%) |
IQR: interquartile range; cART: combination antiretroviral therapy; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; INSTI: integrase strand transfer inhibitor
Of 1913 children and adolescents in the analysis, 795 (42%) had been informed of their HIV status. The proportion of children and adolescents who had been disclosed to was 35% in lower middle-income countries (India, Indonesia, Vietnam) and 59% in upper middle-income countries (Malaysia, Thailand). The median age at disclosure was 12.9 years (IQR: 11.8–14.1). At cART start, compared with children who remained unaware of their HIV status, those who were disclosed to were older (median age: 8.8 vs. 3.9 years), had a higher proportion with CD4 <200 cells/mm3 (52% vs. 40%), and had a lower proportion who were under the care of biological parents (66% vs. 78%) (Table 1). At disclosure, 6.8% of children and adolescents had previously experienced WHO clinical stage 3 and 4 compared with 3.6% at last visit for those who remained unaware of their HIV status (Table 2). At last clinic visit, the median (IQR) age was 15.8 (IQR: 13.2–16.9) years for those who were disclosed to and 10.3 (IQR: 8.5–11.8) for those who were not. Of 815 adolescents aged >12 years at the last visit, 688 (84%) were reported to have been disclosed to that they were living with HIV (Figure 1).
Table 2.
Characteristics at disclosure or at last clinical contact among children and adolescents living with HIV in the IeDEA Asia-Pacific cohort
| Disclosed to (n=795) |
Not disclosed to (n=1118) |
P value | |
|---|---|---|---|
| Age years, Median (IQR) | 12.9 (11.8–14.1) | 10.3 (8.5–11.8) | <0.001 |
| 6–9 | 73 (9.2) | 512 (46) | |
| 10–14 | 606 (76) | 566 (51) | |
| 15–19 | 116 (15) | 40 (3.6) | |
| CD4 count (cells/mm3) | |||
| Overall, median | 579 (348–850) | 726 (424–1045) | <0.001 |
| Available data | 502 (63) | 393 (35) | |
| 6–9 years, median | 649 (287–965) | 749 (308–1102) | |
| Available data | 52 (71) | 197 (38) | |
| 10–14 years, median | 596 (363–868) | 725 (497–990) | |
| Available data | 382 (68) | 181 (32) | |
| 15–19 years, median | 491 (264 −651) | 576 (342–881) | |
| Available data | 68 (59) | 15 (38) | |
| HIV viral load (copies/mL) | |||
| Overall median | 1.6 (1.3–3.0) | 1.3 (1.2–1.6) | <0.001 |
| Available data | 360 (45) | 412 (37) | |
| 6–9 years, median | 1.7 (1.6–3.0) | 1.3 (1.3–1.6) | |
| Available data | 31 (42) | 179 (35) | |
| 10–14 years, median | 1.5 (1.3–2.6) | 1.3 (1.3–1.6) | |
| Available data | 281 (46) | 221 (39) | |
| 15–19 years, median | 3.4 (1.4–4.7) | 1.5 (1.3–1.6) | |
| Available data | 48 (41) | 12 (30) | |
| WHO clinical stages 3 and 4 | |||
| Overall | 54 (6.8) | 40 (3.6) | 0.001 |
| 6–9 years | 7 (9.6) | 27 (5.3) | |
| 10–14 years | 39 (6.4) | 12 (2.1) | |
| 15–19 years | 8 (6.9) | 1 (2.5) |
Note: Wilcoxon test was used to compare continuous data and Pearson chi-square or Fisher’s exact test, as appropriate, to compare categorical data. IQR: interquartile range
Figure 1.
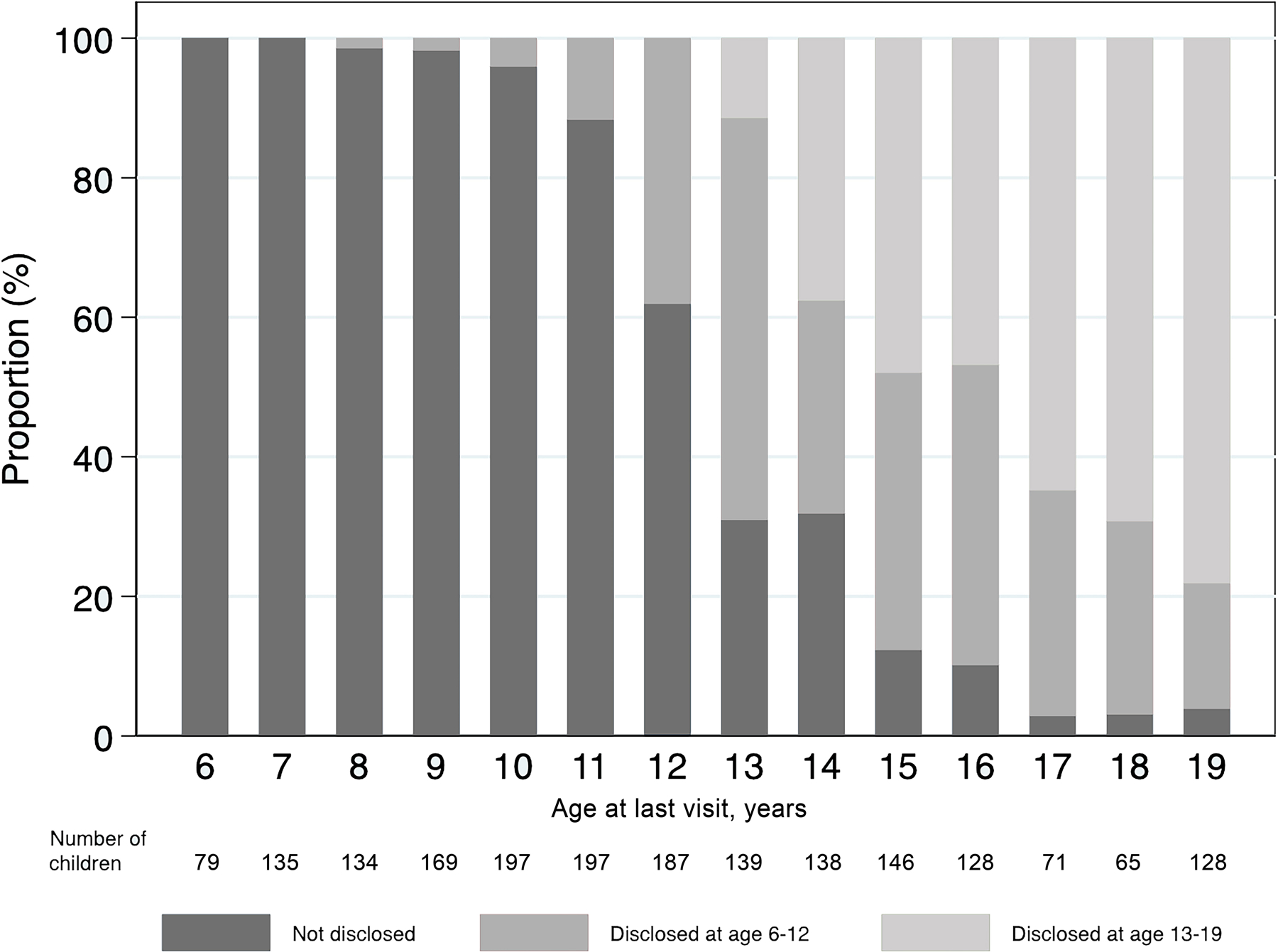
HIV disclosure status at the last clinic visit in children and adolescents 6–19 years of age after cART initiation in the IeDEA Asia-Pacific cohort (n = 1913).
There were 207 (11%) patients who experienced disease progression to WHO clinical stage 3 or 4 over a median follow-up period of 4.6 years (IQR: 2.4–6.7). Of these, 16% had pulmonary tuberculosis, 12% had severe recurrent bacterial pneumonia, and 6% had persistent oral candidiasis. Over a median follow-up period of 4.7 years (IQR: 2.3–4.6), 75 (3.9%) were LTFU and 59 (3.1%) died. The median age at death was 9.4 years (IQR: 7.3–12.7). Overall, compared to those who were not disclosed to, those who were disclosed to had a similar proportion of LTFU (3.9% vs. 3.9%), but lower proportion of death (0.9% vs. 4.7%). At five years of follow-up, the probability of being retained in care was higher for those who were disclosed to (98% vs. 89%, Log rank p <0.001) (Figure 2).
Figure 2.
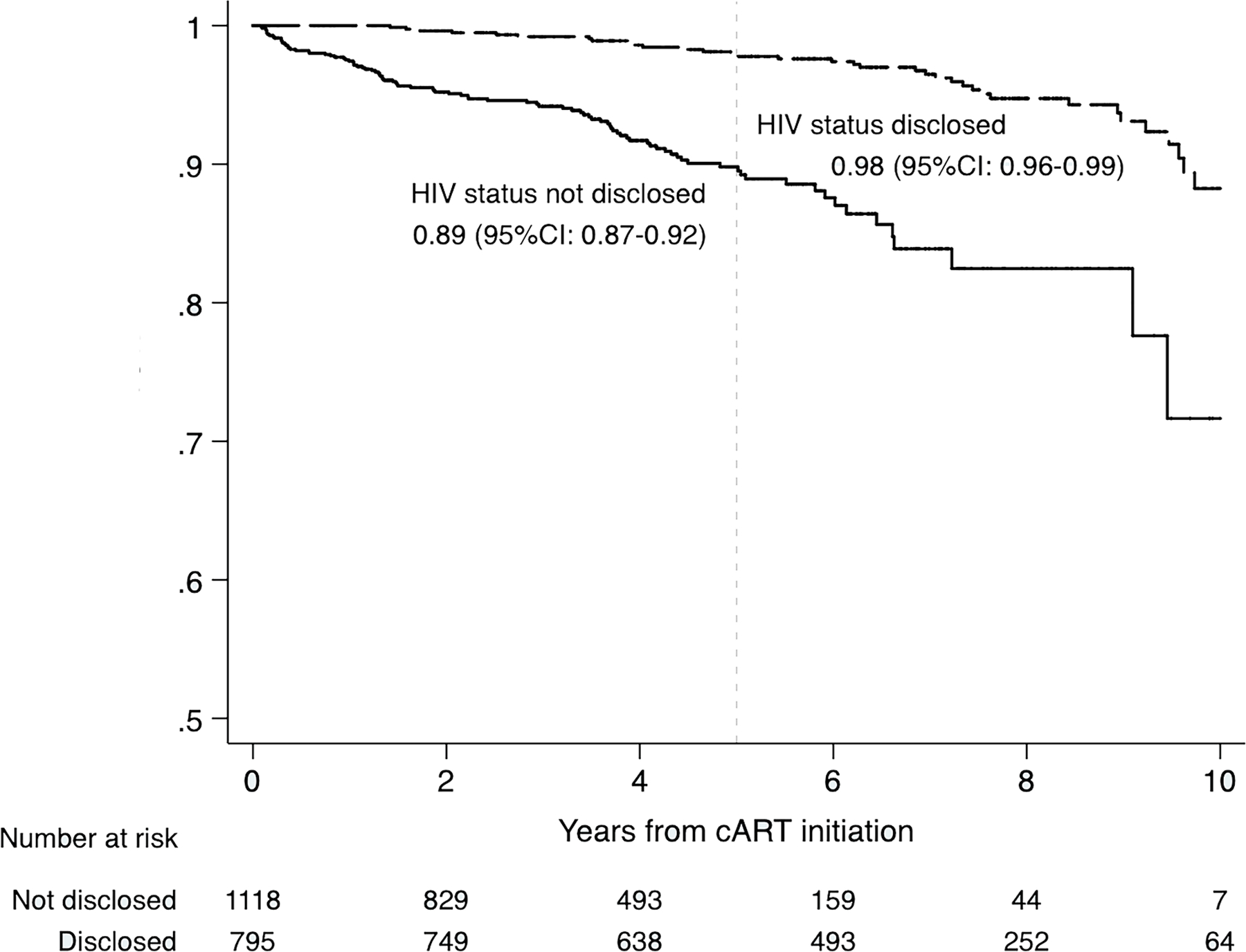
Probability of retention in HIV care by disclosure status in children and adolescents 6–19 years of age after cART initiation in the IeDEA Asia-Pacific cohort (n = 1913).
Predictors associated with disease progression, LTFU, and death
In the survival analysis, disclosure was protective against disease progression (aHR 0.43, 95% CI: 0.28–0.66) (Table 3). The hazard was lowest among children with CD4 ≥500 cells/mm3 (aHR 0.11, 95% CI: 0.06–0.18 vs. those with <200 cells/mm3) and among those with WAZ ≥−2 (aHR 0.27, 95% CI: 0.21–0.35 vs. WAZ <−3). The hazard of being LTFU was lower for those with higher CD4 during follow-up (lowest asHR for ≥500 cells/mm3 0.30, 95% CI: 0.18–0.49 vs. those with less than 200 cells/mm3). LTFU was not significantly associated with disclosure (asHR 0.61, 95% CI: 0.28–1.34). With death as a competing event, the hazard of LTFU was significantly increased among those aged 15 to 19 (asHR 2.48, 95% CI: 1.03–5.94 vs. those less than 10 years old) and later start of cART (2013 to 2018) (asHR 4.28, 95% CI: 2.66–6.90 vs. started earlier [2008 to 2009]) (Table 3). The hazard of death was lower for those who were disclosed to (aHR 0.36, 95% CI: 0.17–0.79) (Table 3). The hazard of death was also lowest among patients with CD4 count ≥500 cells/mm3 (lowest aHR 0.01, 95% CI: 0.01–0.04 vs. CD4 count <200 cells/mm3), and with WAZ ≥−3 to <−2 (aHR 0.20, 95% CI: 0.11–0.37 vs. WAZ <−3). The risk of death was highest for those who experienced an AIDS diagnosis during follow-up (aHR 2.26, 95% CI: 1.17–4.37).
Table 3.
Predictors associated with disease progression, LTFU, and mortality in children and adolescents 6–19 years of age after ART initiation in the IeDEA Asia-Pacific cohort (n=1913)
| Disease progression | LTFU | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | WHO clinical stage 3 and 4 (n=207) | aHR (95% CI) |
p-value | LTFU (n=75) | asHR (95% CI) |
p-value | Deaths (n=59) | aHR (95% CI) |
p-value |
| Sex | |||||||||
| Male | 108 | 1.00 | 41 | 1.00 | 33 | 1.00 | |||
| Female | 99 |
1.04
(0.75–1.44) |
0.820 | 34 |
0.87
(0.45–1.67) |
0.681 | 26 |
0.96
(0.59–1.55) |
0.870 |
| Current age, years a | 0.231 | 0.028 | 0.162 | ||||||
| 6–9 | 126 | 1.00 | 19 | 1.00 | 33 | 1.00 | |||
| 10–14 | 74 |
0.92
(0.62–1.36) |
0.679 | 26 | 0.87 (0.51–1.50) |
0.627 | 19 |
0.55
(0.23–1.27) |
0.162 |
| 15–19 | 7 |
0.43
(0.16–1.14) |
0.091 | 30 | 2.48 (1.03–5.94) |
0.042 | 7 |
0.79
(0.23–2.76) |
0.712 |
| HIV disclosed a | |||||||||
| No | 186 | 1.00 | 44 | 1.00 | 52 | 1.00 | |||
| Yes | 21 | 0.43 (0.28–0.66) |
<0.001 | 31 | 0.61 (0.28–1.34) |
0.219 | 7 | 0.36 (0.17–0.79) |
0.011 |
| Year of first cART | 0.420 | <0.001 | 0.100 | ||||||
| 2008–2009 | 73 | 1.00 | 22 | 1.00 | 27 | 1.00 | |||
| 2010–2012 | 80 |
0.85
(0.60–1.21) |
0.369 | 29 | 1.80 (0.87–3.71) |
0.113 | 20 |
0.69
(0.44–1.07) |
0.099 |
| 2013–2018 | 54 |
0.81
(0.57–1.16) |
0.247 | 24 | 4.28 (2.66–6.90) |
<0.001 | 12 |
0.54
(0.20–1.46) |
0.227 |
| Currently on second-line cART a | |||||||||
| No | 180 | 1.00 | 57 | 1.00 | 50 | 1.00 | |||
| Yes | 27 |
1.23
(0.73–2.0) |
0.440 | 18 |
1.29
(0.64–2.62) |
0.479 | 9 |
0.75
(0.36–1.57) |
0.442 |
| Current weight-for-age z-score a | <0.001 | 0.615 | <0.001 | ||||||
| <−3 | 90 | 1.00 | 16 | 1.00 | 38 | 1.00 | |||
| −3 ≤ to <−2 | 37 | 0.42 (0.31–0.55) |
<0.001 | 14 |
0.84
(0.43–1.62) |
0.597 | 6 | 0.20 (0.11–0.37) |
<0.001 |
| ≥−2 | 64 | 0.27 (0.21–0.35) |
<0.001 | 41 |
0.80
(0.54–1.20) |
0.289 | 15 | 0.28 (0.14–0.57) |
<0.001 |
| Missing | 16 | - | 4 | - | 0 | ||||
| Ever diagnosed with AIDS a , b | |||||||||
| No | - | - | 28 | 1.00 | 10 | 1.00 | |||
| Yes | - | - | 47 |
1.36
(0.68–2.70) |
0.384 | 49 | 2.26 (1.17–4.37) |
0.015 | |
| Current CD4 cell count (cells/mm3) a | <0.001 | <0.001 | <0.001 | ||||||
| <200 | 98 | 1.00 | 14 | 1.00 | 45 | 1.00 | |||
| 200–499 | 28 | 0.19 (0.13–0.26) |
<0.001 | 16 | 0.49 (0.26–0.92) |
0.027 | 9 | 0.12 (0.06–0.24) |
<0.001 |
| ≥500 | 60 | 0.11 (0.06–0.18) |
<0.001 | 40 | 0.30 (0.18–0.49) |
<0.001 | 4 | 0.01 (0.01–0.04) |
<0.001 |
| Missing | 21 | - | 5 | - | 1 | ||||
aHR: adjusted hazard ratio; asHR: adjusted subdistribution hazard ratio; 95% CI: 95% confidence interval; cART: combination antiretroviral therapy
Disclosure was our variable of interest and was retained in the final models regardless of its statistical significance. Covariates in italics were not included in the final model. Their aHR were presented individually in the multivariate model adjusted for significant predictors.
Age, HIV disclosure, on second line cART, weight-for-age z-score, AIDS diagnosis, CD4 count are considered time-dependent variables where each patient can contribute to more than one category.
AIDS was only considered as a covariate when the outcome was loss to follow-up or death
In the complete case analyses for death and lost to follow-up, we excluded 122 (6.4%) patients without data on CD4 or WAZ. These analyses changed the aHR for different covariates only digitally. In the complete case analysis of disease progression in which we excluded 151 (7.9%) patients without data on CD4 or WAZ, the aHR for the covariate disclosure increased from 0.43 (95% CI: 0.28–0.66) to 0.57 (95% CI: 0.41–1.07).
DISCUSSION
In this study of children and adolescents receiving care at pediatric HIV clinics in Asia, 42% knew that they were living with HIV at a median age of disclosure of 12.9 years with the earliest age at 8.0 years. The risk of disease progression and death was observed to be lower in children and adolescents who had been disclosed to. The prevalence of disclosure in our cohort was consistent with those of studies in India (Bhattacharya et al., 2011), Ethiopia (Abegaz et al., 2019), and South Africa (Madiba, 2012), but it was low compared with the 70% reported in a study in Thailand, which included a larger number of older children (median age of 14.8 years) (Sirikum et al., 2014). The reported proportion of children who have been told about their HIV status was within the wide range reported in a systematic review participated by low- and middle-income countries in Africa and Asia (0% to 69% among children with mean age 8.1 to 13.5) (Vreeman et al., 2013). Similarly, a review of studies in North America and Europe (Pinzon-Iregui et al., 2013) indicated that the proportion of children and adolescents who had knowledge of their HIV status varied widely from 18% to 75%. HIV disclosure may be more challenging in some contexts due to the stigma against people living with HIV and local traditions and norms that discourage discussing sexual health issues. For example, in a global investigation of perceptions of HIV-related stigma among individuals living with HIV (Nachega et al., 2012), the proportion of respondents who expressed strong concerns about others knowing their HIV status was highest in the Asia-Pacific (43%) and lowest in Africa (16%).
In our study, 16% of adolescents older than 12 reported being unaware of their HIV-positive status. This may be an overestimate because as observed in a study conducted in the United Kingdom (Dorrell & Katz, 2013), adolescents living with HIV may remain silent about their status due to the stigma attached to the infection. In the majority of studies included in a review addressing disclosure among children and adolescents, parents and health workers advised that discussion regarding HIV should be started at age 10 and completed by the mid-teens (Sahay, 2013). Delayed or avoidance of HIV disclosure at a time of increased experimentation with sexual activity and substance use may restrict the use of appropriate measures to prevent onward HIV transmission.
Disclosed children and adolescents in our cohort were less likely to die during the follow-up period than non-disclosed children. A study in Romania (Ferris et al., 2007) among children and adolescents aged 5–17 years and in Kenya (Ngeno et al., 2019) among adolescents aged 10–14 years, also reported that those who were aware of their HIV status had less than half the risk of death compared with those who remained unaware. The authors attributed the survival benefit to improved treatment adherence, although previous studies assessing the association between disclosure and adherence reported mixed results (Arage et al., 2014; Cluver et al., 2015).
Children and adolescents in our study who were disclosed to also appeared less likely to experience subsequent clinical disease progression over the follow-up period. Similarly, the Romanian study (Ferris et al., 2007) described that HIV disclosure was associated with delayed disease progression, characterized by an endpoint of CD4 decline. However, another Kenyan study (Vreeman et al., 2014) did not find an association between disclosure status and clinical indicators like CD4 count and WHO disease stage. Although we did not find an association between HIV disclosure and LTFU, a multicenter study conducted in West Africa (Vreeman et al., 2013) showed that HIV disclosure was associated with better retention in care among younger adolescents (median age 10.4 years). However, as data relating to LTFU are complicated by the different definitions of LTFU used, direct comparisons may be less reliable.
There are a number of limitations to this study. The main challenge was the observational nature of the study and the risk of incomplete and inconsistent data reporting, including with regards to variability in diagnosing and reporting WHO clinical staging. As disclosure reporting was passive, it may have been under or overreported, depending on the interpretation of the definition of disclosure by local clinical staff. There also was potential misclassification of death as LTFU because of the risk of unascertained mortality. In addition, survival bias may have been present in the analysis because we excluded those who died before age six. Although we adjusted our model for a large number of potential confounding variables, our study lacks information on factors such as psychological functioning (e.g., childhood trauma, social support, stressful life events) and treatment adherence prior to or during follow-up. This may have affected our outcomes and our observed associations may be subject to residual confounding. Research gaps not addressed through our study include examining whether disease progression triggers or is a consequence of disclosure, and the extent to which delays in disclosure negatively impact treatment adherence and retention in care.
CONCLUSIONS
In our Asia regional cohort, most of the children and adolescents with HIV were disclosed to about their HIV status as older adolescents. They experienced reduced mortality and decreased risk of experiencing WHO clinical stage 3 and 4 after disclosure compared to those who had not been disclosed to. The importance of disclosure and global recommendations for how and when this can be done need to be emphasized in local clinical practice guidelines in order to encourage pediatric HIV providers to work with caregivers to start this process at younger ages. Our findings support the need to review reasons for delayed disclosure, and to develop interventions to ensure that adolescents are informed about their HIV status as part of long-term HIV care management in Asia.
Funding acknowledgement
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Site investigators and cohorts
PS Ly, V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N Kumarasamy, E Chandrasekaran, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; A Kinikar, V Mave, S Nimkar, I Marbaniang, BJ Medical College and Sassoon General Hospitals, Maharashtra, India; DK Wati, D Vedaswari, IB Ramajaya, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati, D Muktiarti, Cipto Mangunkusumo – Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; SM Fong, M Lim, F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff, P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; TJ Mohamed, MR Drawis, Department of Pediatrics, Women and Children Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy, KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk, V Sirisanthana, L Aurpibul, Department of Pediatrics, Faculty of Medicine, and Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; P Ounchanum, R Hansudewechakul, S Denjanta, A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon, P Kosalaraksa, P Tharnprisan, T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Puthanakit, S Anugulruengkit, W Jantarabenjakul, R Nadsasarn, Department of Pediatrics and Center of Excellence for Pediatric Infectious Diseases and Vaccines, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; K Chokephaibulkit, K Lapphra, W Phongsamart, S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong, QT Du, CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do, TM Ha, VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen, DM Tran, HTT Tran, TTT Giang, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn, JL Ross, T Suwanlerk, TREAT Asia/amfAR - The Foundation for AIDS Research, Bangkok, Thailand; MG Law, A Kariminia, The Kirby Institute, UNSW Sydney, NSW, Australia
Footnotes
DISCLOSURE STATEMENT
AHS receives grants to her institution from ViiV Healthcare. No other conflicts of interest were reported by the author(s).
REFERENCES
- Abegaz BF, Walle TA, & Tilahun AD (2019). HIV positive status disclosure and associated factor among HIV infected children in pediatric ART clinics in Gondar town public health facilities, North West Ethiopia, 2018. Journal of Infection and Public Health, 12(6), 873–877. 10.1016/j.jiph.2019.05.018 [DOI] [PubMed] [Google Scholar]
- Arage G, Tessema GA, & Kassa H (2014). Adherence to antiretroviral therapy and its associated factors among children at South Wollo Zone Hospitals, Northeast Ethiopia: A cross-sectional study. BMC Public Health, 14(1), 365. 10.1186/1471-2458-14-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivé E, Dicko F, Amghar H, Aka AE, Dior H, Bouah B, Traoré M, Ogbo P, Dago-Akribi HA, Eboua TKF, Kouakou K, Sy HS, Alioum A, Dabis F, Ekouévi DK, Leroy V, & for the Pediatric IeDEA West Africa Working Group. (2012). HIV Status Disclosure and Retention in Care in HIV-Infected Adolescents on Antiretroviral Therapy (ART) in West Africa. PLoS ONE, 7(3), e33690. 10.1371/journal.pone.0033690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Dubey AP, & Sharma M (2011). Patterns of Diagnosis Disclosure and its Correlates in HIV-Infected North Indian Children. Journal of Tropical Pediatrics, 57(6), 405–411. 10.1093/tropej/fmq115 [DOI] [PubMed] [Google Scholar]
- Boon-yasidhi V, Naiwatanakul T, Chokephaibulkit K, Lolekha R, Leowsrisook P, Chotpitayasunond T, & Wolfe M (2016). Effect of HIV diagnosis disclosure on psychosocial outcomes in Thai children with perinatal HIV infection. International Journal of STD & AIDS, 27(4), 288–295. 10.1177/0956462415579590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulali RE, Kibusi SM, & Mpondo BCT (2018). Factors Associated with HIV Status Disclosure and Its Effect on Treatment Adherence and Quality of Life among Children 6–17 Years on Antiretroviral Therapy in Southern Highlands Zone, Tanzania: Unmatched Case Control Study. International Journal of Pediatrics, 2018, 1–10. 10.1155/2018/8058291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluver LD, Hodes RJ, Toska E, Kidia KK, Orkin FM, Sherr L, & Meinck F (2015). ‘HIV is like a tsotsi. ARVs are your guns’: Associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS, 29(Supplement 1), S57–S65. 10.1097/QAD.0000000000000695 [DOI] [PubMed] [Google Scholar]
- Committee on Pediatric AIDS. (1999). Disclosure of Illness Status to Children and Adolescents With HIV Infection. Pediatrics, 103(1), 164–166. 10.1542/peds.103.1.164 [DOI] [PubMed] [Google Scholar]
- Dorrell J, & Katz J (2013). “I knew I had something bad because no-one spoke about it” – disclosure discovery: Experiences of young people with perinatally acquired HIV in the UK. Vulnerable Children and Youth Studies, 8(4), 353–361. 10.1080/17450128.2013.774453 [DOI] [Google Scholar]
- Ferris M, Burau K, Schweitzer AM, Mihale S, Murray N, Preda A, Ross M, & Kline M (2007). The influence of disclosure of HIV diagnosis on time to disease progression in a cohort of Romanian children and teens. AIDS Care, 19(9), 1088–1094. 10.1080/09540120701367124 [DOI] [PubMed] [Google Scholar]
- Fine JP, & Gray RJ (1999). A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, 94(446), 496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- Hayfron-Benjamin A, Obiri-Yeboah D, Ayisi-Addo S, Siakwa PM, & Mupepi S (2018). HIV diagnosis disclosure to infected children and adolescents; challenges of family caregivers in the Central Region of Ghana. BMC Pediatrics, 18(1), 365. 10.1186/s12887-018-1330-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantarapakde J, Pancharoen C, Teeratakulpisarn S, Mathajittiphan P, Kriengsinyot R, Channgam T, Pengnonyang S, Plodgratok P, Lakhonphon S, Luesomboon W, Jadwattanakul T, Avihingsanon A, Ananworanich J, Ungaro P, & Phanuphak P (2019). An Integrated Approach to HIV Disclosure for HIV-Affected Families in Thailand. Journal of the International Association of Providers of AIDS Care (JIAPAC), 18, 232595821983102. 10.1177/2325958219831021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariminia A, Chokephaibulkit K, Pang J, Lumbiganon P, Hansudewechakul R, Amin J, Kumarasamy N, Puthanakit T, Kurniati N, Nik Yusoff NK, Saphonn V, Fong SM, Razali K, Nallusamy R, Sohn AH, & Sirisanthana V (2011). Cohort Profile: The TREAT Asia Pediatric HIV Observational Database. International Journal of Epidemiology, 40(1), 15–24. 10.1093/ije/dyp358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss B, Letteney S, Baets A. de, Murugi J, Okero FA, & World Health Organization. (2011). Guideline on HIV disclosure counselling for children up to 12 years of age http://whqlibdoc.who.int/publications/2011/9789241502863_eng.pdf [PubMed]
- Madiba S (2012). Patterns of HIV Diagnosis Disclosure to Infected Children and Family Members: Data from a Paediatric Antiretroviral Program in South Africa. World Journal of AIDS, 02(03), 212–221. 10.4236/wja.2012.23027 [DOI] [Google Scholar]
- Montalto GJ, Sawe FK, Miruka A, Maswai J, Kiptoo I, Aoko A, Oreyo C, Obiero E, Korir S, Bii SK, Song KX, & Kunz AN (2017). Diagnosis disclosure to adolescents living with HIV in rural Kenya improves antiretroviral therapy adherence and immunologic outcomes: A retrospective cohort study. PLOS ONE, 12(10), e0183180. 10.1371/journal.pone.0183180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Morroni C, Zuniga JM, Sherer R, Beyrer C, Solomon S, Schechter M, & Rockstroh J (2012). HIV-Related Stigma, Isolation, Discrimination, and Serostatus Disclosure: A Global Survey of 2035 HIV-Infected Adults. Journal of the International Association of Physicians in AIDS Care, 11(3), 172–178. 10.1177/1545109712436723 [DOI] [PubMed] [Google Scholar]
- Ngeno B, Waruru A, Inwani I, Nganga L, Wangari EN, Katana A, Gichangi A, Mwangi A, Mukui I, & Rutherford GW (2019). Disclosure and Clinical Outcomes Among Young Adolescents Living With HIV in Kenya. Journal of Adolescent Health, 64(2), 242–249. 10.1016/j.jadohealth.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odiachi A (2017). The Impact of Disclosure on Health and Related Outcomes in Human Immunodeficiency Virus-Infected Children: A Literature Review. Frontiers in Public Health, 5, 231. 10.3389/fpubh.2017.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Iregui MC, Beck-Sague CM, & Malow RM (2013). Disclosure of Their HIV Status to Infected Children: A Review of the Literature. Journal of Tropical Pediatrics, 59(2), 84–89. 10.1093/tropej/fms052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JV, Mmbaga BT, Turner EL, Rugalabamu LL, Luhanga S, Cunningham CK, & Dow DE (2018). Modality of Primary HIV Disclosure and Association with Mental Health, Stigma, and Antiretroviral Therapy Adherence in Tanzanian Youth Living with HIV. AIDS Patient Care and STDs, 32(1), 31–37. 10.1089/apc.2017.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay S (2013). Coming of Age with HIV: A Need for Disclosure of HIV Diagnosis among Children/Adolescents. Journal of HIV/AIDS and Infectious Diseases, 1–8. 10.17303/jaid.2013.103 [DOI]
- Shallo Seifadin Ahmed, & Tassew Mesfin. (2020). HIV positive status disclosure and its associated factors among children on antiretroviral therapy in West Shoa Zone, Western Ethiopia, 2019: A mixed method cross-sectional study. Journal of Multidisciplinary Healthcare, 13, 507–517. 10.2147/JMDH.S258851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikum C, Sophonphan J, Chuanjaroen T, Lakonphon S, Srimuan A, Chusut P, Do TC, Prasitsuebsai W, Puthanakit T, Ananworanich J, Bunupuradah T, & on behalf of HIV-NAT 015 study team. (2014). HIV disclosure and its effect on treatment outcomes in perinatal HIV-infected Thai children. AIDS Care, 26(9), 1144–1149. 10.1080/09540121.2014.894614 [DOI] [PubMed] [Google Scholar]
- Siripong A, Bunupuradah T, Apateerapong W, Boonrak P, Pancharoe C, & Ananworanich J (2007). Attitudes of Thai caregivers of children with HIV infection towards HIV disclosure. Vulnerable Children and Youth Studies, 2(3), 191–197. 10.1080/17450120701593696 [DOI] [Google Scholar]
- UNAIDS. (2022). Global HIV statistics—Fact sheet 2022 https://www.unaids.org/en/resources/fact-sheet
- Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, & Nyandiko WM (2013). Disclosure of HIV status to children in resource‐limited settings: A systematic review. Journal of the International AIDS Society, 16(1), 18466. 10.7448/IAS.16.1.18466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeman RC, Nyandiko WM, Ayaya SO, Walumbe EG, Marrero DG, & Inui TS (2010). The Perceived Impact of Disclosure of Pediatric HIV Status on Pediatric Antiretroviral Therapy Adherence, Child Well-Being, and Social Relationships in a Resource-Limited Setting. AIDS Patient Care and STDs, 24(10), 639–649. 10.1089/apc.2010.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeman RC, Scanlon ML, Mwangi A, Turissini M, Ayaya SO, Tenge C, & Nyandiko WM (2014). A Cross-Sectional Study of Disclosure of HIV Status to Children and Adolescents in Western Kenya. PLoS ONE, 9(1), e86616. 10.1371/journal.pone.0086616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener L, Mellins CA, Marhefka S, & Battles HB (2007). Disclosure of an HIV Diagnosis to Children: History, Current Research, and Future Directions. Journal of Developmental & Behavioral Pediatrics, 28(2), 155–166. 10.1097/01.DBP.0000267570.87564.cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2007). Growth reference data for 5–19 years
- World Health Organization. (2019). Child growth standards https://www.who.int/tools/child-growth-standards/software


