Abstract
INTRODUCTION:
Immune dysfunction is important in aging and neurodegeneration; lacking clinically available tools limits research translation. We tested associations of cerebral spinal fluid (CSF) monocyte-to-lymphocyte ratio (MLR)— innate immune activation surrogate —with cognition in an aging and dementia cohort, hypothesizing that elevated MLR is associated with poorer executive functioning.
METHODS:
CSF MLR was calculated in well-characterized, genotyped participants enrolled in studies of aging and dementia at UCSF Memory and Aging Center (n=199, mean age 57.5 years, SD 11.9). Linear models tested associations with episodic memory and executive function (verbal fluency, speeded set-shifting).
RESULTS:
Aging was associated with higher CSF monocyte, lower lymphocyte counts, and higher MLRs (p<0.001). MLR was associated with verbal fluency (p<0.05) only.
DISCUSSION:
Using clinical labs, we show an inverse association between CSF MLR and executive function in aging and dementia, supporting the utility of clinical labs in capturing associations between innate immune dysfunction and neurodegeneration.
Keywords: Inflammation, Cognition, Executive Function, Cerebrospinal Fluid, Aging, Neurodegeneration
1. Background
An estimated 88 million Americans will be over age 65 by the year 2050 and one in ten older Americans have dementia1. As we still lack understanding about basic pathomechanisms, predictive and prognostic biomarkers, and effective treatments for dementia, these figures highlight an impending public health crisis. One growing area of research is in the role of the immune system in brain aging and disease. Often, important discoveries made in research take too long to enter into the sphere of clinical care. For this study, we formulated questions based on recent research and sought to answer the questions using generalized, clinic-based laboratory tests, routinely obtained in clinical settings.
Emerging studies show that changes in the immune system are inherent to the pathophysiology of aging2–4. Increasingly, the immune system is implicated in the pathogenesis of neurodegenerative diseases2–7, and this involvement may be one of the ways aging predisposes the brain to neurodegeneration. In the blood, declines in serum lymphocyte numbers and changes in cytokine levels are seen with age8. Within the central nervous system, aging is associated with the development of a detrimental pro-inflammatory milieu3 and changes in entry and exit of immune cell subtypes into the CNS9. Elevated serum inflammatory markers are used as indirect measures of immune activation or, depending on context, dysfunction10–13. However, the lack of cellular specificity, chemical lability, and physiological variability in levels, discourages use of these secreted factors as clinical biomarkers of disease states. Immune cells, on the other hand, have the great advantage of specificity. High serum neutrophil to lymphocyte ratios have been reported in Alzheimer’s disease (AD) and are associated with poor prognosis in Amyotrophic Lateral Sclerosis (ALS)11,14,15. Also, T cells isolated from CSF of individuals with AD may be implicated in AD pathologic processes16. CSF cells and inflammatory markers have also been associated with structural brain changes and cognitive decline in presymptomatic and early AD cases17. In Parkinson disease, elevated CSF lymphocyte count is associated with shorter survival18. Moreover, immune activation is linked to executive dysfunction19. Together, these support the role of immune dysregulation or activation in age-associated brain degenerative disease and cognitive dysfunction, however, most research has relied on expensive research assays not readily accessible in the clinic. The serum monocyte to lymphocyte ratio is commonly used as a general measure of innate immune activation20. Therefore, in this study, we wanted to use the clinically available CSF MLR measure to test the hypothesis that innate immune activation, captured by this ratio, is associated with executive dysfunction in a well-characterized neurodegenerative cohort. We tested the relationship between CSF cell counts and cognition, as well as the moderating effect of disease-causing genetic mutations on this relationship.
By analyzing standard cerebrospinal fluid cell counts as markers of immune system dysfunction, we take a first step toward translating findings from high accuracy, low availability research methods, to low sensitivity, generalizable clinical labs that all patients with CSF studies obtain in clinic. We hypothesize that higher MLR, representing innate immune activation, will be associated with worse cognition, with specificity for executive function.
2. Methods:
2.1. Participants:
The sample for this study consisted of all research participants enrolled in longitudinal natural history research studies at the University of California, San Francisco, Memory and Aging Center, enrolled in studies of aging, Alzheimer’s disease (AD), and frontotemporal dementia (FTD) with UCSF IRB approval and available clinical measures of interest included in analyses. Data included in this analysis were collected from January 2016 to December 2018. All research participants who completed a clinical assessment, nursing assessment, and lumbar puncture during the study period were included in the analyses. Within the framework of parent studies, after written informed consent was obtained, participants underwent neurological, neuropsychological, and functional assessments including informant interviews as described elsewhere21. Participants underwent structural imaging with brain MRI, peripheral blood, and cerebrospinal fluid collection. Clinical diagnosis was determined by multidisciplinary consensus conference using published diagnostic criteria for behavioral variant frontotemporal dementia (bvFTD)22, non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA)23, semantic variant primary progressive aphasia (svPPA)23, logopenic variant primary progressive aphasia (lvPPA)23, corticobasal syndrome (CBS)24, progressive supranuclear palsy (PSP)25,26, mild cognitive impairment (MCI)27, Alzheimer’s disease (AD)28, or were deemed clinically normal. All samples with complete clinical data and cerebrospinal fluid specimens were included in this study. Available clinical data used in models include demographic information, detailed history, physical and neurological examination, comprehensive neuropsychological assessments, and functional rating scales described previously21. Additional data includes genetic mutation status (carrier vs. non-carrier) for common disease-causing genes including MAPT, GRN, C9orf72, and APOE genotyping. All genetic testing was performed in the same laboratory at the University of California Los Angeles using standardized methods previously described21. Individual genetic mutations are not included to protect patient privacy. When clinical data were conflicting or absent, chart review was performed by a board-certified neurologist (AS, FE) to determine best clinical diagnosis. Participants were excluded if no CSF data was available or if clinical data was incomplete for variables needed for the statistical models testing primary hypotheses. If data from multiple time points existed, data from the closest time point to the CSF collection was used. For participants with multiple associated entries, any entries that were flagged by clinicians during data quality review were removed. Subsequently a single entry per participant for analysis using a selection criterion for the entry with (1) the highest CDR box sum score and (2) highest MLR value were selected for use in statistical models.
2.2. Cerebrospinal fluid samples:
Research participants underwent lumbar puncture as part of routine research protocols. Lumbar punctures were performed in the morning after an overnight fast with a 24-gauge Sprotte needle in the L3/4 or L4/5 space. Spinal fluid was collected in a polypropylene tube and analyzed at the UCSF Clinical Laboratories, Mission Bay Hematology. Analyses included appearance, glucose, protein, white and red blood cell counts, and percentage lymphocytes, monocytes, and neutrophils. Analyses were performed using a hemocytometer for cell count and wright stained cytocentrifuge preparation for differential at the UCSF Clinical Laboratory. Cell differentials were performed on concentrated samples and expressed as 0×106/L. Unusual results were flagged and reviewed by a pathologist. CSF variables in this analysis included CSF monocyte and lymphocyte counts as well as the MLR, which was calculated by dividing absolute monocyte count by absolute lymphocyte count.
2.3. Cognitive Measures:
For the purposes of this analysis, we focused on aspects of cognition that have demonstrated sensitivity to typical brain aging and AD and FTLD-related neurodegenerative disease, including processing speed, executive functioning, and episodic memory28–31. Primary outcomes of interest included measures of executive functioning via verbal fluency (number of D words, L words and F words per 60 seconds) and speeded set-shifting performance (number of correct lines/second on the Modified Trail Making Test), as described in detail elsewhere31–33. The Short Form of the California Verbal Learning Test, Second Edition (CVLT-II), was used to assess episodic memory performance (number of words freely recalled after a 10-minute delay)34.
2.4. Statistical Analyses:
To investigate the relationship between MLR and cognitive outcome measures, we fit linear models using the open-source programming language R135,36. The models adjusted for genetic factors of disease including carrier status of disease-causing genetic mutations and APOE risk genotype, age, gender, education, and CDR box sum outcome as covariates. Specifically, we first fit models with the following formula to investigate possible interaction between MLR and each genetic factor of disease (Equation 1):
| (Eq 1) |
Where the asterisk “*” in the equation represents modeling the main effects as well as the interaction between MLR and the genetic factor of disease. Data was prepared for modeling using tidyverse37 for data frame manipulation. Linear models were fit using the “lm” function in the statspackage1. Type III analysis of variance (ANOVA) was performed on the fit linear models by the “Anova” function from the cars36 package to observe the significance of interaction (i.e. between MLR and the genetic factor of disease) and main effect terms (i.e. MLR and genetic factor of disease). After determining that the interaction terms were insignificant across all models, we removed the interaction terms and treated the genetic factors of disease as a covariate to improve the power of our analysis (Equation 2):
| (Eq 2) |
Type III ANOVA was performed on the new set of fit linear models for the significance of the relationship between MLR on the outcome measures. Data visualization was performed utilizing the ggplot4R package38. Plots of the relationship between MLR and outcome measures show the line generated by the fitted linear models as generated with Equation 2.
Statistical significance was defined as p<0.05 and statistical trends were defined as 0.05<p<0.10.
3. Results:
3.1. Demographics:
The final analyses included a total of 199 participants. Participant characteristics are shown in Table 1. Mean age was 58 (SD 12, 27–83 years). Mean CDR sum of boxes was 3 (SD 3, 0–16) (Supp. Fig.1). The majority of the sample was composed of white older adults with high level of education (Table 1). Clinical diagnoses, described in table 1, include: clinically normal; frontotemporal dementia (FTD), including behavioral variant FTD (bvFTD), corticobasal syndrome (CBS), progressive supranuclear palsy (PSP), nonfluent variant primary progressive aphasia (nfvPPA), semantic variant primary progressive aphasia (svPPA); Alzheimer’s disease, including logopenic variant primary progressive aphasia (lvPPA), and posterior cortical atrophy (PCA); mild cognitive impairment (MCI); and other, including Parkinson disease (PD), tuberous sclerosis complex associated neurocognitive disorder (TAND), amyotrophic lateral scelerosis (ALS), and chronic traumatic encephalopathy (CTE).
Table 1:
Demographics
| Participants | N=199 | |
|---|---|---|
| Age, mean (SD) years | 57.5 (11.9) | |
| Sex, n | Female | 103 |
| Male | 96 | |
| Education, mean (SD) years | 16.2 (2.5) | |
| Race/Ethnicity | 89.4% White | |
| Gene mutation carrier status, n | APO E4 | 51 |
| Genetic Carrier | 58 | |
| Unknown | 44 | |
| Clinical Diagnosis, n | Clinically Normal | 60 |
| MCI | 31 | |
| Alzheimer’s Disease* | 46 | |
| FTD† | 51 | |
| Other‡ | 11 | |
| CDR Sum of Boxes, mean (SD) | 2.8 (3.2) | |
AD includes lvPPA (8) and PCA (7)
FTD includes bvFTD (29), nfvPPA (9), svPPA (3), PSP (6) and CBS (4)
Other includes TAND (6), ALS (3), PD (1) and CTE (1)
Linear regression models including all participants found that CSF monocyte count was positively correlated with age (p=0.0005; standardized βage = 0.26; adjusted R2 = 0.064) (Fig 1A). Conversely, CSF lymphocyte count was negatively correlated with age (p=0.0001; standardized βage = −0.30; adjusted R2 = 0.084) (Fig 1B). CSF MLR was positively correlated with age (p=0.004; standardized βage = 0.22; adjusted R2 = 0.043) (Fig 1D). There was no significant correlation between CSF neutrophil count and age (Fig 1C) nor was there a relationship between CSF monocyte, lymphocyte, or neutrophil counts and sex (Fig 2). There was no effect of genetic status on CSF cell populations (Fig 3).
Figure 1: Correlation between Age and CSF Cell Counts.
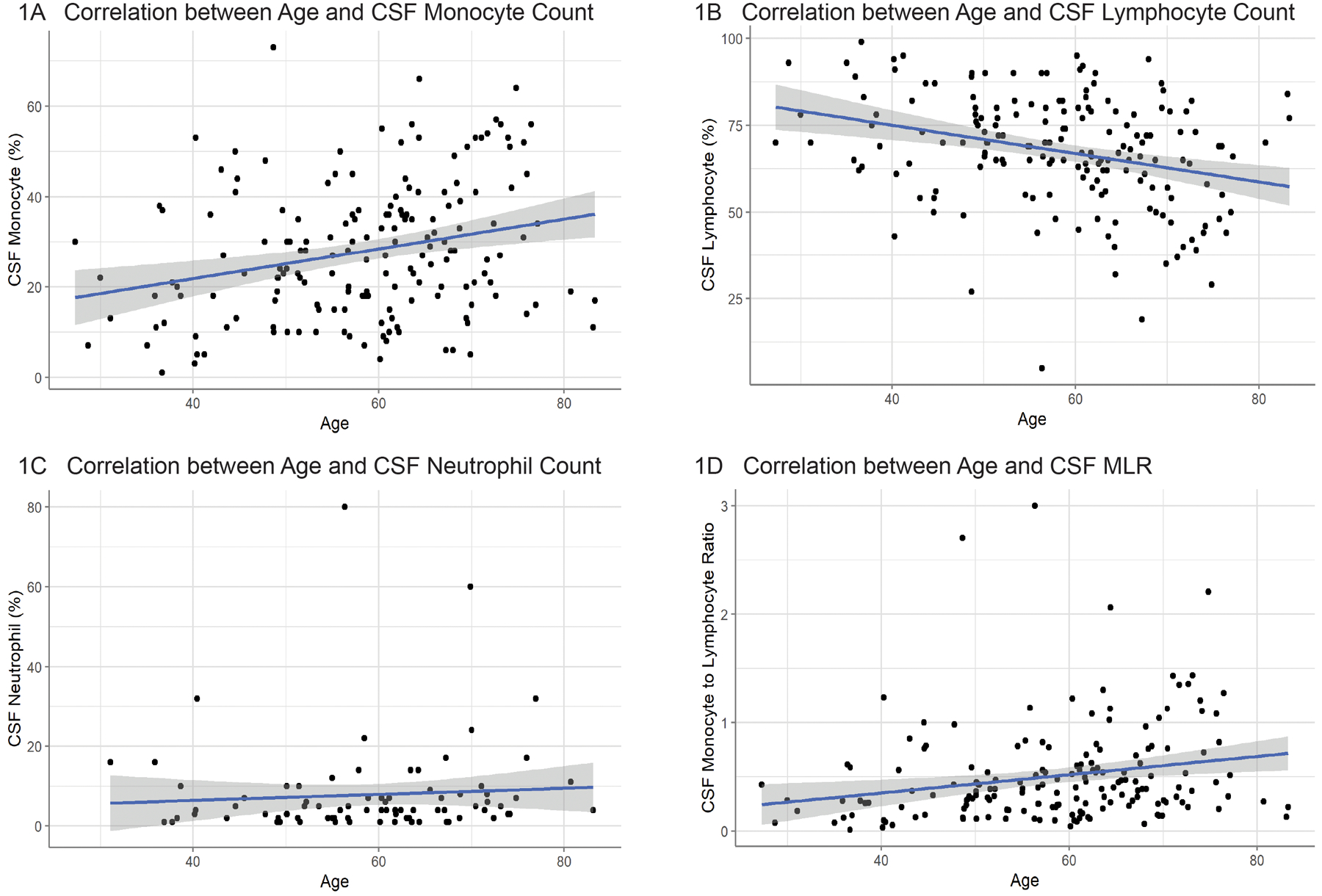
Correlation between subject age and CSF cell populations. Older subjects exhibited (A) a positive correlation with CSF monocyte population (n=173; p < 0.001) and (B) a negative correlation with CSF lymphocyte population (n=173; p < 0.0001). (C) There was no significant correlation between subject age and CSF neutrophil population (n=83). There was a positive correlation between age and the (D) CSF monocyte-to-lymphocyte ratio (n=173; p < 0.01).
Figure 2: Correlation between Sex and CSF Cell Counts.
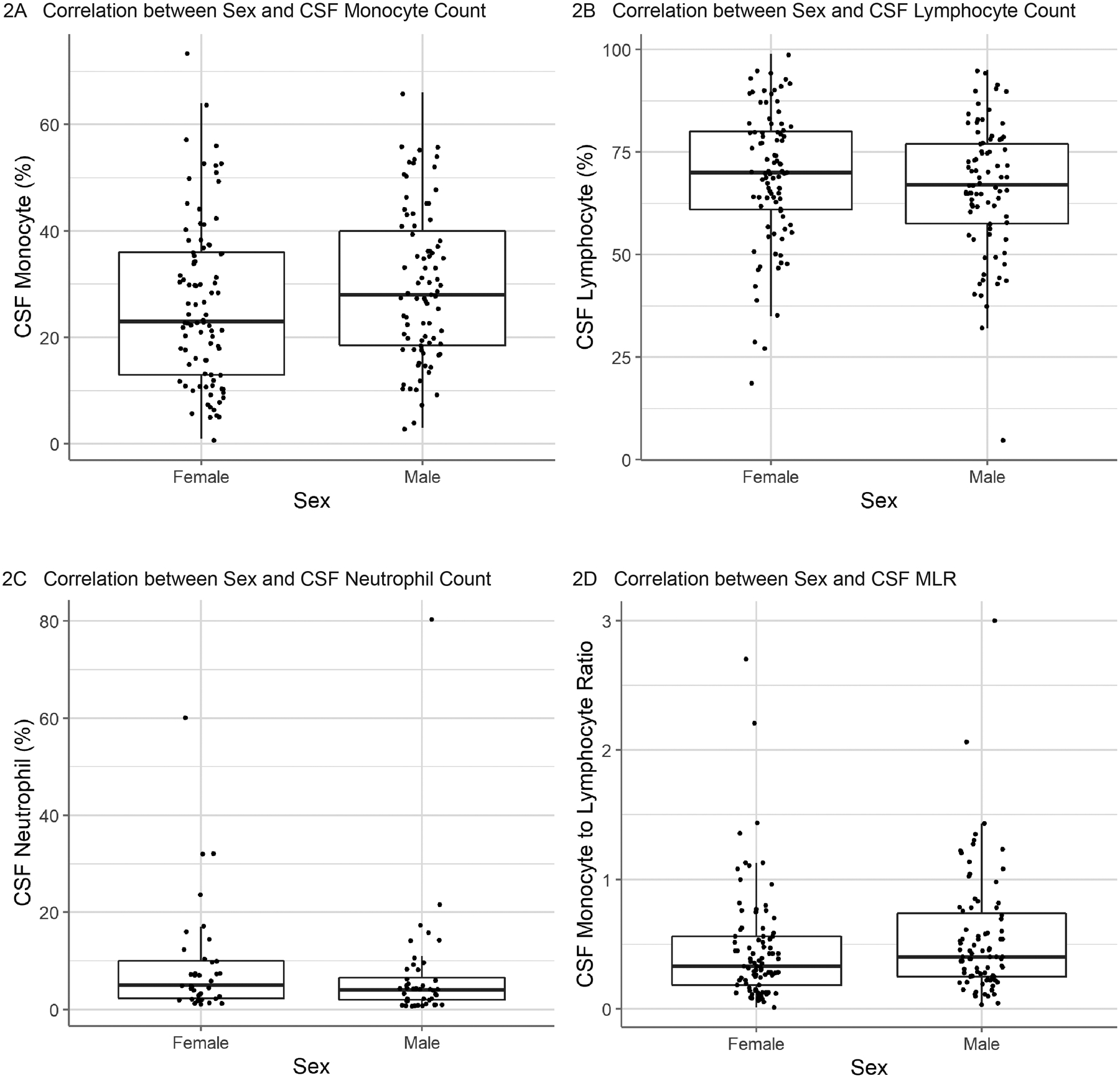
Correlation between sex and CSF cell populations. No significant correlation was observed between subject sex and (A) CSF monocyte population (n=173), (B) CSF lymphocyte population (n=173), or (C) CSF neutrophil population (n=82). There was not a significant correlation between subject sex and the (D) CSF monocyte-to-lymphocyte ratio (n=173).
Figure 3: Correlation between Genetic Status and CSF Cell Counts.
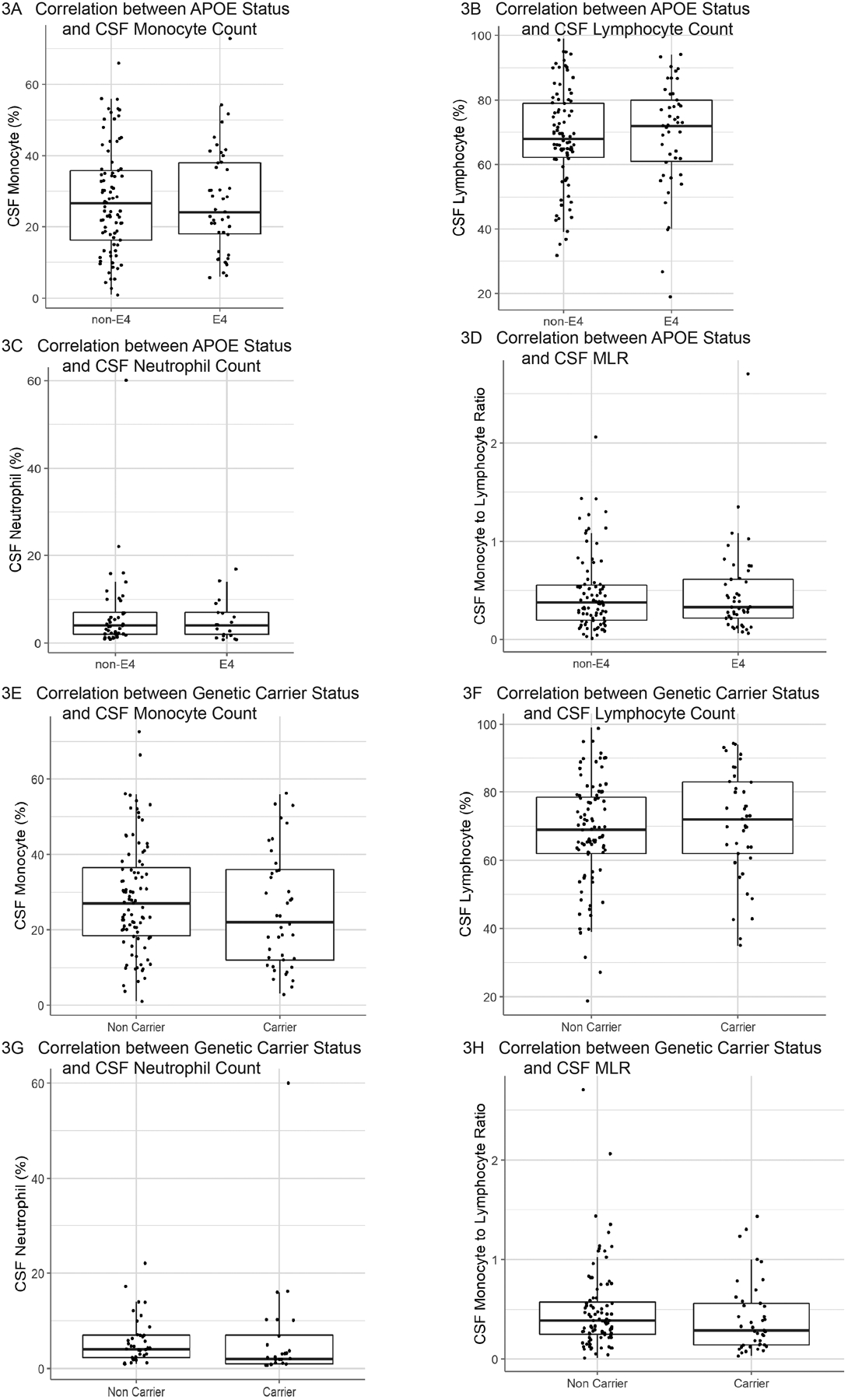
Correlation between ApoE4 genetic status and CSF cell populations (A-D). No significant correlation was observed between the ApoE4 genetic status and (A) CSF monocyte population (n=131), (B) CSF lymphocyte population (n=131), or (C) CSF neutrophil population (n=62). There was not a significant correlation between the ApoE4 genetic status and the (D) CSF monocyte-to-lymphocyte ratio (n=131).
Correlation between genetic status for monogenic disease genes (carrier vs non-carrier) and CSF cell populations (E-H). No significant correlation was observed between the presence of monogenic disease mutations and (E) CSF monocyte population (n=132), (F) CSF lymphocyte population (n=132), or (G) CSF neutrophil population (n=63). There was not a significant correlation between the presence of monogenic disease mutations and the (H) CSF monocyte-to-lymphocyte ratio (n=132).
3.2. Immune Activation and Cognition:
To investigate the association of MLR to cognition, linear regression was performed while controlling for age, sex, education, clinical impairment (CDR Box Sum score), and APOE risk genotype or carrier status as covariates. The analysis revealed that MLR was significantly associated with verbal fluency, for all letters tested (D, L and F words), when controlling for either APOE risk genotype (D words p=0.04; L words p=0.02; F words p=0.05) or carrier status (D words p=0.03; L words p=0.01; F words p=0.05) (Fig 4). Mean verbal fluency scores were 11.25 (SD=6.28), 11.69 (SD=5.76), and 11.54 (SD=5.47) for D words, F words and L words respectively (Supp. Fig. 2). In contrast, similar linear models did not reveal any significant relationship between MLR and Modified Trail Making Test or episodic memory.
Figure 4: Correlation between CSF MLR and Measures of Executive Function.
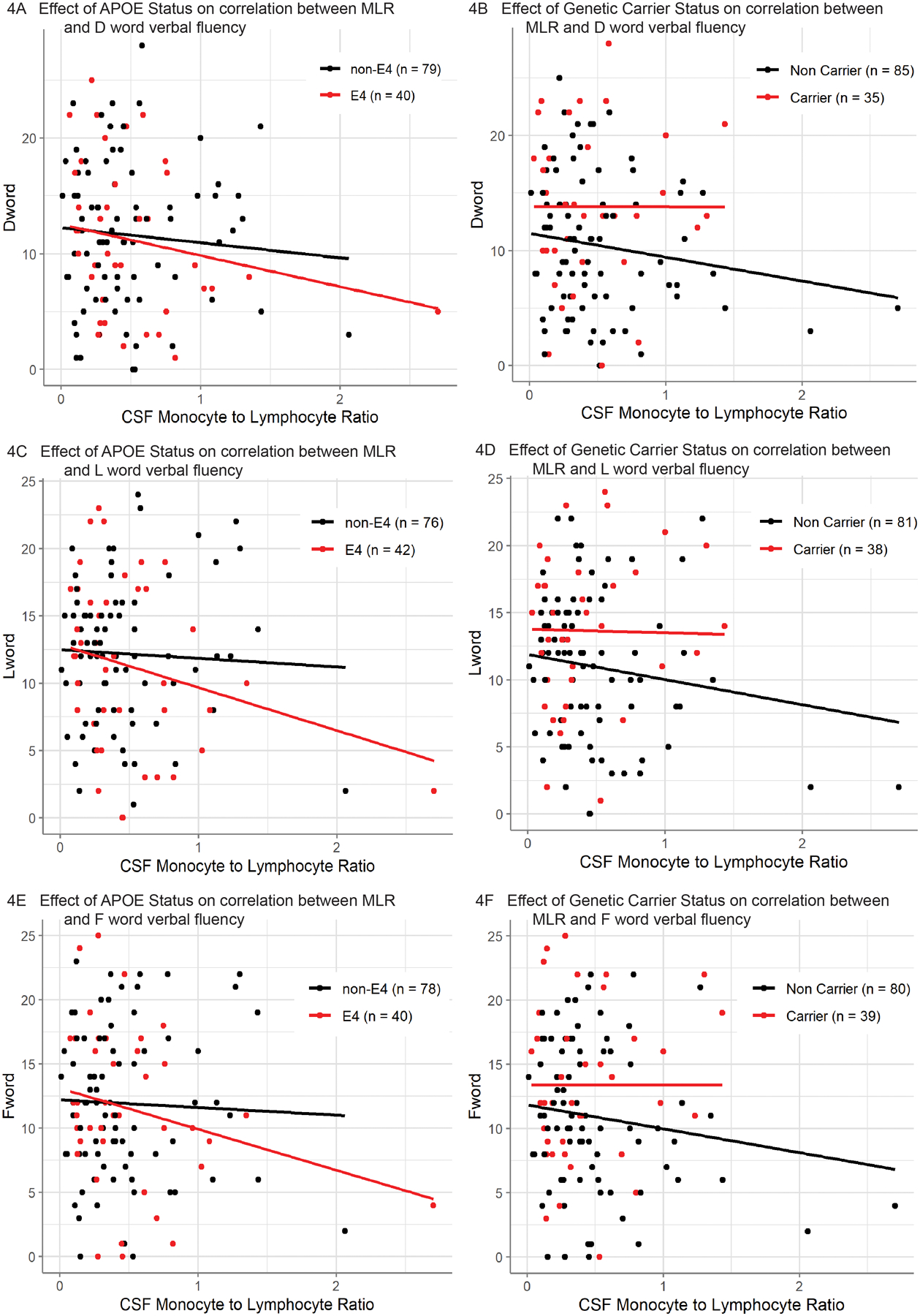
Fig [4A-B]. Correlation between CSF monocyte-to-lymphocyte ratio and performance on [D Word] cognitive test after controlling for age, sex, education, and CDR Box Sum score as covariates. Additionally, the effect of (A) ApoE4 (n=119) or (B) monogenic disease carrier (n=120) statuses were also included (red: genetic mutation, black: no genetic mutation). Importantly, neither ApoE4 nor monogenic disease carrier status had a significant interaction with CSF monocyte-to-lymphocyte ratio on [D Word] performance. The plotted lines show the best fit for the corresponding model’s prediction of each subject. There was a significant negative correlation between CSF monocyte-to-lymphocyte ratio and [D Word] performance when ApoE4 status is included as a covariate (p < 0.05) and when monogenic disease carrier status was included as a covariate (p < 0.05).
Fig [4C-D]. Correlation between CSF monocyte-to-lymphocyte ratio and performance on [L Word] cognitive test after controlling for age, sex, education, and CDR Box Sum score as covariates. Additionally, the effect of (C) ApoE4 (n=118) or (D) monogenic disease carrier (n=119) statuses were also included (red: genetic mutation, black: no genetic mutation). Importantly, neither ApoE4 nor monogenic disease carrier status had a significant interaction with CSF monocyte-to-lymphocyte ratio on [L Word] performance. The plotted lines show the best fit for the corresponding model’s prediction of each subject. There was a significant negative correlation between CSF monocyte-to-lymphocyte ratio and [L Word] performance when ApoE4 status is included as a covariate (p < 0.05) and when monogenic disease carrier status was included as a covariate (p < 0.05).
Fig [4E-F]. Correlation between CSF monocyte-to-lymphocyte ratio and performance on [F Word] cognitive test after controlling for age, sex, education, and CDR Box Sum score as covariates. Additionally, the effect of (E) ApoE4 (n=118) or (F) monogenic disease carrier (n=119) statuses were also included (red: genetic mutation, black: no genetic mutation). Importantly, neither ApoE4 nor monogenic disease carrier status had a significant interaction with CSF monocyte-to-lymphocyte ratio on [F Word] performance. The plotted lines show the best fit for the corresponding model’s prediction of each subject. There was a significant negative correlation between CSF monocyte-to-lymphocyte ratio and [F Word] performance when ApoE4 status is included as a covariate (p < 0.05) and when monogenic disease carrier status was included as a covariate (p < 0.05).
4. Discussion:
Despite advances in understanding of the pathogenesis in neurodegenerative disorders, the field still lacks easily accessible diagnostic and predictive biomarkers. Inflammation and immune activation are implicated in brain aging across neurodegenerative diseases4,5,13,32,39–45. In this study, we leverage readily available, standard clinical laboratory data and use them as surrogate measures of immune activation to explore the association between immune activation and clinical impairment in aging, autosomal dominant genetic forms of FTLD and sporadic Alzheimer’s disease, with and without APOE4 genotype. We found that executive function—specifically verbal generativity performance—but not episodic memory, was negatively associated with the CSF MLR (Fig 5).
Figure 5: Graphical Summary.
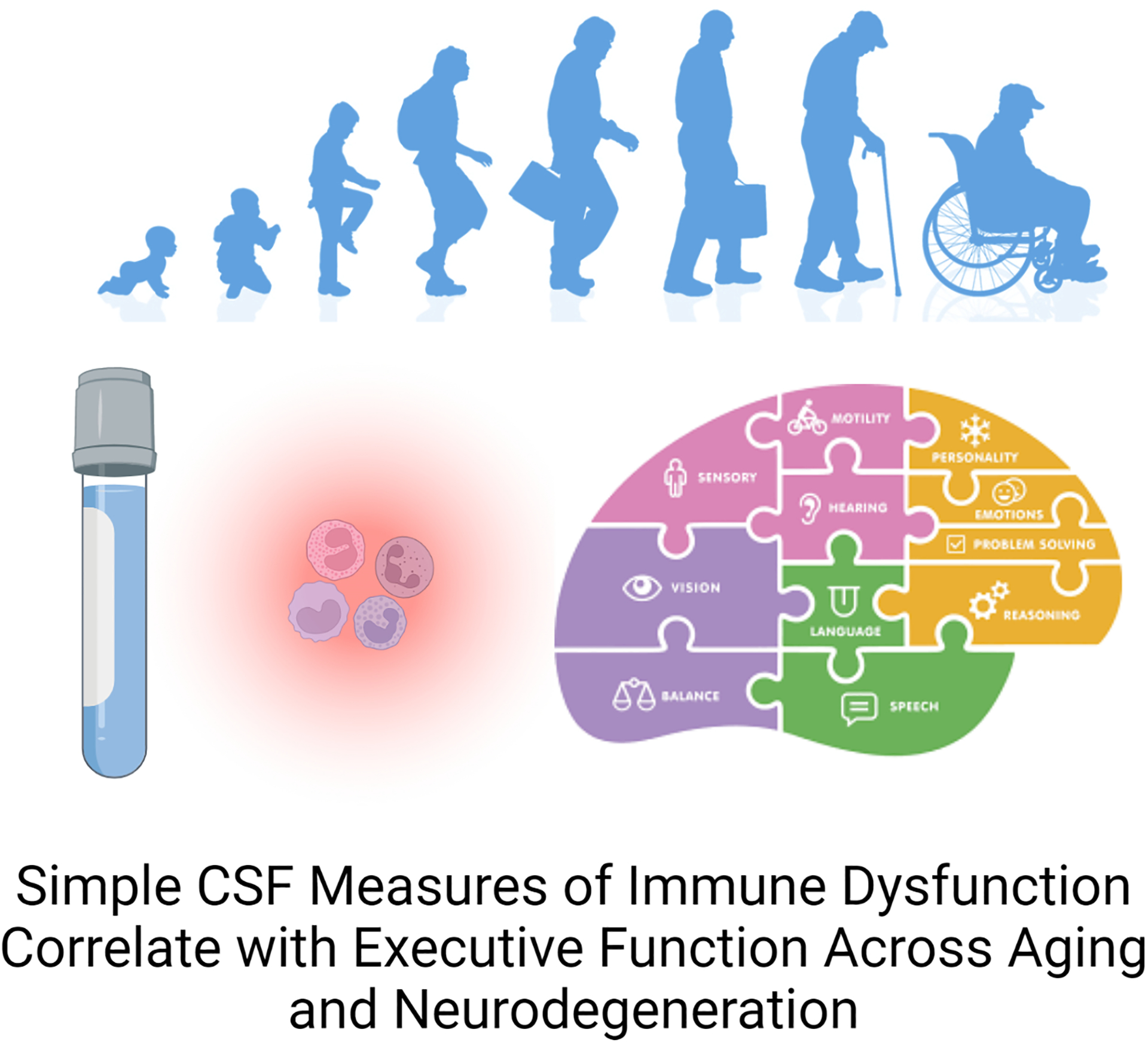
Immune cell counts in CSF correlate with measures of executive function in aging and neurodegeneration.
Cerebrospinal fluid pleocytosis has been observed in many different diseases including human immunodeficiency virus, multiple sclerosis, and Parkinson disease12,14,18,46. These diseases may share certain cellular pathogenesis involving the immune system and may portend disease outcome12,18. Several studies have investigated serum monocyte or neutrophil to lymphocyte ratios as clues to the role of the immune system in cerebrovascular disease, Alzheimer’s disease and ALS14,15,46. Direct assays of the CSF in neurodegenerative diseases provides an indirect, close look at intracerebral disease processes. While it is not possible to comment on causative, versus reactive, based on correlated models, CSF cell counts readily available in clinical labs, suggest that there is involvement of immune system across a diversity of neurodegenerative disease categories. More cells in the brain compartment, and higher counts in CSF could reflect higher entry, lower exit, or both.
Interestingly, drops in circulating lymphocytes in the blood and changes in the immune system composition is one of the hallmarks of aging8. Immunosenecsence is an emerging mechanism invoked in the neurodegenerative disease process7. While this phenomenon has been well studied in the periphery, despite mounting evidence of the role of immune dysregulation in neurodegenerative diseases, this has not been well studied in humans, using cerebrospinal fluid cells. We use CSF from a well-characterized sample of participants across the aging and neurodegenerative disease spectrum to demonstrate that even simple clinical labs, with limited sensitivity and coarse specificity, provide support for innate immune involvement in neurodegenerative disease. In the first study of its kind, our findings indicate that, similar to peripheral blood, CSF lymphocyte levels may decline with age.
Across two main cognitive domains investigated in this study—executive function and episodic memory—executive function, specifically verbal generativity, seems most sensitive to changes in the immune state within our mixed cohort. Consistent with this, mouse models show that inflammation has a selective impact on executive functions while sparing memory47. Additionally, this finding is supported by observations from other neurological diseases where executive dysfunction is prominent, including both primary autoimmune conditions such as multiple sclerosis and autoimmune encephalitis, as well as other neurodegenerative disorders such as vascular-related cognitive impairment6,10,12,48,49. Many studies have similarly found an association with peripheral markers of inflammation and executive function broadly as well as verbal fluency specifically, though none have reported this finding within the CSF50–54. Moreover, it has also been demonstrated that immune activation alters brain connectivity specifically in the executive network while sparing the default mode network associated with memory as well as the salience network associated with emotional regulation55,56. This finding may support network vulnerability and neuroanatomical correlations between areas of the executive network vasculature most exposed to peripheral influences. However, a retrospective study in a sample of convenience has limitations and potential sample biases that are not known and accounted for in analyses. Therefore, future prospective studies would be better suited to confirm and further explore test specificity of various cognitive domains with CNS immune dysfunction. Inclusion of imaging and more specific disease state biomarkers, such as A/T/N for AD57 may help further clarify causal relationships between immune activation and neurodegeneration, at least in AD.
The most important limitation of this study is that we had to rely on a sample of convenience and cross-sectional data, limiting the interpretation of our findings in relation to disease course and causal inferences. Use of a sample of convenience also meant that our sample lacked diversity, with mainly whites from high socioeconomic environments. We hope to extend this study in the future, by including diverse populations, larger sample sizes and incorporating measures of vascular and brain barrier function, A/T/N biomarkers, and longitudinal assessments to begin to differentiate causal from reactive immune responses in neurodegenerative diseases and the effect of genetic mutations on this relationship.
The field lacks clinically reliable biomarkers for immune activation in neurodegeneration. Here we use clinical labs to test the association of innate immune dysfunction with cognitive impairment in aging, neurodegenerative disease, FTLD, and AD. We describe age-related differences in cell counts, which fit with previously described peripheral blood changes8. We also provide data showing a unique cognitive profile of executive dysfunction—specifically impaired phonemic fluency—associated with immune system activation in both genetic and sporadic forms of neurodegenerative diseases. This suggests the possibility of common cellular immune pathways in brain degeneration. Although future investigations need higher depth in characterization of cells, in light of the phenotypic diversity of immune cells across coarse categorical buckets, such as mononuclear cells, lymphocytes, and neutrophils. This study provide support for the value of investigating CNS immune cells across neurodegenerative disease categories and linking back findings to labs that can be implemented in clinic and translated to improving clinical classification, prognostications, and ultimately therapeutics.
Supplementary Material
Research in Context.
Systematic Review: The authors conducted a review of the literature using PubMed and available posters, presentations, and abstracts. While there is a growing interest in the role of inflammation in the initiation and propagation of the neurodegeneration, clinical assays are limited. Moreover, knowledge about changes in cellular profiles in CSF with age, disease, and disease outcomes is lacking. The relevant articles have been appropriately cited.
Interpretation: We describe a large sample of CSF cell profiles in a diverse aging and neurodegenerative cohort, establish the utility of clinically available tests as proxies of inflammation, and we correlate CSF cell profiles with cognitive measures to begin to bridge the gap between research and clinic.
Future Directions: Future directions include correlating CSF profiles directly with research assays of inflammation to further validate this approach. Additionally, we hope to correlate CSF profiles with patient outcomes in neurodegenerative disorders and relate this to underlying disease mechanisms.
Acknowledgements/Conflicts/Funding Sources
The authors would like to express their gratitude to the patients who participated in the ongoing research that made this study possible. We also thank the clinicians and research coordinators who collected the data that was used in this study. This work was funded by the National Institute on Aging and Department of Veterans Affairs IK2CX002180, Larry L. Hillblom Foundation 2019A012SUP and New Vision Research (to F.M.E.). Participant recruitment and data collection at UCSF was funded by the NIH-NIA ADRC (P50AG023501) and PPG (P01AG019724) (to B.L.M.), and Hillblom Aging Network for the Prevention of Age-Associated Cognitive Decline study grants (2140-A-004-NET) (to J.H.K.).
This article was partially prepared while Dr. Snyder was employed at UCSF. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. Drs. Snyder, Chou, and Lindbergh and Ms. Grant have no relevant disclosures. Dr. Elahi serves as associate editor for the Alzheimer’s & Dementia Journal. Dr. Kramer has received royalties from Pearson, Inc. in addition to the grants to the institution listed above. Dr. Miller is the recipient of grants/contracts P30AG062422, P01AG019724, R01AG057234 from the National Institutes of Health/NIA. As an additional disclosure, he serves as Medical Director for the John Douglas French Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia (UK) and Board Member for the American Brain Foundation (ABF).
Contributor Information
Allison Snyder, National Institute of Neurological Disorders and Stroke, 35 Convent Drive, Building 35, 2A-1010, Bethesda, MD 20892, USA..
Harli Grant, Memory and Aging Center, Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Austin Chou, Brain and Spinal Injury Center, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Cutter A. Lindbergh, Department of Psychiatry, University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Joel H. Kramer, Memory and Aging Center, Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Bruce L. Miller, Memory and Aging Center, Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Fanny M. Elahi, Memory and Aging Center, Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA; Departments of Neurology, Neuroscience, and Pathology, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Icahn School of Medicine at Mount Sinai, Annenberg 20-58, Box 1137, One Gustave L. Levy Place, New York, NY 10029.
References
- 1.On the Front Lines: Primary Care Physicians and Alzheimer’s Care in America.
- 2.Hammond TR, Marsh SE, Stevens B. Immune Signaling in Neurodegeneration. Immunity. 2019;50(4):955–974. doi: 10.1016/j.immuni.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson MA, Banks WA. Age-associated changes in the immune system and blood–brain barrier functions. Int J Mol Sci. 2019;20(7). doi: 10.3390/ijms20071632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noori A, Mezlini AM, Hyman BT, Serrano-Pozo A, Das S. Systematic review and meta-analysis of human Transcriptomics reveals Neuroinflammation, deficient energy metabolism, and Proteostasis failure across Neurodegeneration. Neurobiol Dis. Published online December 19, 2020:105225. doi: 10.1016/j.nbd.2020.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCauley ME, Baloh RH. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019;137(5):715–730. doi: 10.1007/s00401-018-1933-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elahi FM, Harvey D, Altendahl M, Casaletto KB, Fernandes N, Staffaroni AM, Maillard P, Hinman JD, Miller BL, DeCarli C, Kramer JH EJG. Endothelial-derived exosomes demonstrate a link between endothelial innate inflammation and brain dysfunction and injury in aging. biorxiv. Published online June 21, 2019:670083. doi: 10.1101/670083 [DOI] [Google Scholar]
- 7.Costantini E, Angelo CD’, Reale M. The Role of Immunosenescence in Neurodegenerative Diseases. Published online 2018. doi: 10.1155/2018/6039171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Kim J, Metter EJ, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immunity and Ageing. 2016;13(1):24. doi: 10.1186/s12979-016-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener. 2013;8(1). doi: 10.1186/1750-1326-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright CB, Moon Y, Paik MC, et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40(11):3466–3471. doi: 10.1161/STROKEAHA.109.559567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Gutierrez J, Meier IB, et al. Circulating inflammatory biomarkers are related to cerebrovascular disease in older adults. Neurol Neuroimmunol Neuroinflamm. 2019;6(1):521. doi: 10.1212/NXI.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeman D, Adam P, Kalistová H, Sobek O, Anděl J, Anděl M. Cerebrospinal fluid cytologic findings in multiple sclerosis: A comparison between patient subgroups. Acta Cytol. 2001;45(1):51–59. doi: 10.1159/000327187 [DOI] [PubMed] [Google Scholar]
- 13.Bettcher BM, Johnson SC, Fitch R, et al. Cerebrospinal Fluid and Plasma Levels of Inflammation Differentially Relate to CNS Markers of Alzheimer’s Disease Pathology and Neuronal Damage. Journal of Alzheimer’s Disease. 2018;62(1):385–397. doi: 10.3233/JAD-170602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SJJ, Hong YHH, Kim SMM, Shin JYY, Suh YJ, Sung JJJ. High neutrophil-to-lymphocyte ratio predicts short survival duration in amyotrophic lateral sclerosis. Sci Rep. 2020;10(1). doi: 10.1038/s41598-019-57366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuyumcu ME, Yesil Y, Oztürk ZA, et al. The Evaluation of Neutrophil-Lymphocyte Ratio in Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2012;34(2):69–74. doi: 10.1159/000341583 [DOI] [PubMed] [Google Scholar]
- 16.Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020 577:7790. 2020;577(7790):399–404. doi: 10.1038/s41586-019-1895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janelidze S, Mattsson N, Stomrud E, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018;91(9):e867–e877. doi: 10.1212/WNL.0000000000006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bäckström D, Granåsen G, Domellöf ME, et al. No Title. Neurology. 2018;91(22). doi: 10.1212/WNL.0000000000006576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culley DJ, Snayd M, Baxter MG, et al. Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: Possible implications for delirium. Front Aging Neurosci. 2014;6(JUN):107. doi: 10.3389/FNAGI.2014.00107/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (United States). 2018;97(26). doi: 10.1097/MD.0000000000011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(11):2957. doi: 10.1093/BRAIN/AWN234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456. doi: 10.1093/BRAIN/AWR179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006. doi: 10.1212/WNL.0B013E31821103E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496. doi: 10.1212/WNL.0B013E31827F0FD1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.L I, A Y, C D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- 26.H GU, R G, S M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/MDS.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A MS, D ST, D D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/J.JALZ.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M GM, K DS, C H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/J.JALZ.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salthouse TA. The Processing-Speed Theory of Adult Age Differences in Cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- 30.Wecker NS, Kramer JH, Hallam BJ, Delis DC. Mental flexibility: Age effects on switching. Neuropsychology. 2005;19(3):345–352. doi: 10.1037/0894-4105.19.3.345 [DOI] [PubMed] [Google Scholar]
- 31.Kramer JH, Jurik J, Sha SJ, et al. Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, and Alzheimer Disease. Cognitive and Behavioral Neurology. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- 32.Lindbergh CA, Casaletto KB, Staffaroni AM, et al. Systemic Tumor Necrosis Factor-Alpha Trajectories Relate to Brain Health in Typically Aging Older Adults.; 2019. doi: 10.1093/gerona/glz209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staffaroni AM, Brown JA, Casaletto KB, et al. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. Journal of Neuroscience. 2018;38(11):2809–2817. doi: 10.1523/JNEUROSCI.3067-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test - second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology. 2006;21(5):413–420. doi: 10.1016/j.acn.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 35.R Foundation for Statistical Computing. R Core Team: A Language and Environment for Statistical Computing. Published online 2020.
- 36.Fox J, Weisberg S. An R Companion to Applied Regression. Third. Sage; 2019. [Google Scholar]
- 37.Wickham H, Averick M, Bryan J, et al. Welcome to the {tidyverse}. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 38.Wickham H Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York; 2016. https://ggplot2.tidyverse.org [Google Scholar]
- 39.Broce I, Karch CM, Wen N, et al. Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies. PLoS Med. 2018;15(1). doi: 10.1371/journal.pmed.1002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19(10):610–621. doi: 10.1038/s41583-018-0055-7 [DOI] [PubMed] [Google Scholar]
- 41.Heneka MT, Kummer MP, Latz E. Innate Immune Activation in Neurodegenerative Disease. Vol 14. Nature Publishing Group; 2014. doi: 10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- 42.Ljubenkov PA, Miller Z, Mumford P, et al. Peripheral Innate Immune Activation Correlates With Disease Severity in GRN Haploinsufficiency. Front Neurol. 2019;10:1004. doi: 10.3389/fneur.2019.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasqualetti G, Brooks DJ, Edison P. The Role of Neuroinflammation in Dementias. Curr Neurol Neurosci Rep. 2015;15(4):17. doi: 10.1007/s11910-015-0531-7 [DOI] [PubMed] [Google Scholar]
- 44.Bettcher BM, Neuhaus J, Wynn MJ, et al. Increases in a Pro-inflammatory Chemokine, MCP-1, Are Related to Decreases in Memory Over Time. Front Aging Neurosci. 2019;11(FEB):25. doi: 10.3389/fnagi.2019.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues MCO, Sanberg PR, Cruz LE, Garbuzova-Davis S. The innate and adaptive immunological aspects in neurodegenerative diseases. J Neuroimmunol. 2014;269(1–2):1–8. doi: 10.1016/j.jneuroim.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 46.Bogorodskaya M, Lyass A, Mahoney T, et al. Utilization of absolute monocyte counts to predict cardiovascular events in people living with HIV. HIV Med. 2021;22(4):314–320. doi: 10.1111/HIV.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Culley DJ, Snayd M, Baxter MG, et al. Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: Possible implications for delirium. Front Aging Neurosci. 2014;6(JUN):107. doi: 10.3389/FNAGI.2014.00107/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cepok S Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(11):2169–2176. doi: 10.1093/brain/124.11.2169 [DOI] [PubMed] [Google Scholar]
- 49.Casaletto KB, Elahi FM, Staffaroni AM, et al. Cognitive aging is not created equally: differentiating unique cognitive phenotypes in “normal” adults. Neurobiol Aging. 2019;77:13–19. doi: 10.1016/j.neurobiolaging.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin T, Liu GA, Perez E, et al. Systemic inflammation mediates age-related cognitive deficits. Front Aging Neurosci. 2018;10(AUG):236. doi: 10.3389/FNAGI.2018.00236/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsland AL, Petersen KL, Sathanoori R, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68(6):895–903. doi: 10.1097/01.PSY.0000238451.22174.92 [DOI] [PubMed] [Google Scholar]
- 52.Boots EA, Castellanos KJ, Zhan L, et al. Inflammation, Cognition, and White Matter in Older Adults: An Examination by Race. Front Aging Neurosci. 2020;12. doi: 10.3389/FNAGI.2020.553998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang Y, Lunetta KL, Mez J, et al. Association of blood cell parameters of peripheral inflammation with cognitive function. Alzheimer’s & Dementia. 2021;17(S5):e051838. doi: 10.1002/ALZ.051838 [DOI] [Google Scholar]
- 54.Tegeler C, O’Sullivan JL, Bucholtz N, et al. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol Aging. 2016;38:112–117. doi: 10.1016/J.NEUROBIOLAGING.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 55.Nusslock R, Brody GH, Armstrong CC, et al. Higher Peripheral Inflammatory Signaling Associated With Lower Resting-State Functional Brain Connectivity in Emotion Regulation and Central Executive Networks. Biol Psychiatry. 2019;86(2):153–162. doi: 10.1016/J.BIOPSYCH.2019.03.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y, Zhou M, Jia X, et al. Inflammation Disrupts the Brain Network of Executive Function After Cardiac Surgery. Ann Surg. Published online July 2021. doi: 10.1097/SLA.0000000000005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535. doi: 10.1016/J.JALZ.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


