Abstract
Objective
Variations in prevalence and incidence of systemic lupus erythematosus (SLE) within a geographically defined area of central Sweden over a time period of 14 years were examined. Longitudinal differences in disease activity, laboratory test results, and damage accrual were investigated.
Methods
Adults (aged ≥18 years) residing in Östergötland County between 2008 and 2021 (mean adult population: 357,000 citizens) with confirmed SLE were identified and followed prospectively until death, December 31, 2021, or emigration. We estimated annual incidence per 100,000 inhabitants stratified by sex and age. Linear regression with year of diagnosis as the outcome assessed whether each clinical measurement at diagnosis varied over time.
Results
Prevalence on December 31, 2021, was 71.5 of 100,000 (87% female). One hundred twenty‐six new cases were identified during the study period, yielding a mean annual incidence of 3.0 of 100,000 inhabitants; this was higher in females (4.8/100,000) than in males (1.2/100,000). Mean age at diagnosis was 43.7 years (SD 17.3). Age at diagnosis and disease activity measures increased over the calendar year of diagnosis (P < 0.05) whereas disease manifestations, including lupus nephritis, did not vary significantly. Accrual of organ damage was demonstrated over time since diagnosis and stratified by sex, lupus nephritis, and corticosteroid‐related damage. Approximately 40% developed damage within 5 years.
Conclusion
SLE prevalence and incidence estimates remained constant over 14 years, and disease phenotypes at SLE onset were similar. SLE was diagnosed also among older individuals with a smaller female‐to‐male ratio. Estimates of prevalence and incidence were comparable to previous Scandinavian reports but lower than observed in registry data from the US and the UK.
SIGNIFICANCE & INNOVATIONS.
We examined variations in incidence and prevalence of adults with systemic lupus erythematosus (SLE), limited to cases meeting the 1982 American College of Rheumatology classification criteria and/or the Fries’ diagnostic principle, in a geographically defined area of central Sweden over a time period of 14 years.
The overall mean annual SLE incidence was 3.0 per 100,000 inhabitants (females 4.8 and males 1.2), and the prevalence on December 31, 2021, was 71.5 per 100,000 inhabitants (females 121.9 and males 17.4).
The female‐to‐male ratio of incident patients with SLE aged 55 years or older was different from those diagnosed at an earlier age.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic, multi‐system autoimmune disorder with highly heterogeneous clinical presentation, increased morbidity and mortality, and decreased health‐related quality of life (1). Although occurrence of SLE has been a subject of a series of epidemiological studies, incidence and prevalence figures vary considerably worldwide. Reasons for these discrepancies likely depend on several factors, including variations in race and ethnicity, access to and coverage of public health care, reliability of data, and the use of different SLE definitions (2).
Results of epidemiological studies performed in the Scandinavian countries, the UK, and the US have long been available, but data have also recently emerged from other parts of the world (3, 4, 5, 6, 7, 8, 9, 10). Worldwide, prevalence figures range from 12 to 200 cases per 100,000 inhabitants, both sexes combined, and annual incidence varies from 2 to 15 cases per 100,000 (11).
In Sweden, investigations of SLE epidemiology based on disease‐specific registries, hospital records, and laboratory databases have so far exclusively been published from the Lund–Orup health care district (6, 12). Nationwide data from 2010 exist, but those were based on the National Patient Register (administrative health data) showing a prevalence ranging from 46 per 100,000 for the strictest definition to 85 per 100,000 for the least strict definition (13). In addition, considerable variations between different counties were observed.
Herein, we investigated the distribution of SLE in Östergötland County and took advantage of the public health care organization and the Clinical Lupus Register in North‐Eastern Gothia (Swedish acronym “KLURING”—a regional quality and research registry that has enrolled practically all prevalent and incident SLE cases since 2008). The KLURING registry includes longitudinal follow‐up of all patients until emigration or death and has an estimated coverage of 97% or more in the area of Östergötland County (14).
The aims of this study were to estimate prevalence and incidence of adult SLE in Östergötland between the years 2008 and 2021, and to describe variations in clinical manifestations and laboratory features at diagnosis as well as accrual of damage over time since diagnosis.
PATIENTS AND METHODS
Study area and population
Swedish health care is public, tax‐funded, and offers universal access. This study was carried out in a geographical area located in the middle of Sweden (Figure 1A). Between 2008 and 2021, the mean adult population in the County of Östergötland was 357,000 inhabitants. The Rheumatology Clinic at the University Hospital of Linköping serves the entire adult population of Östergötland with rheumatological care (no private rheumatology service exists in the county). Importantly, SLE cases presenting with “pure renal lupus” (ie, lupus nephritis [LN] in the absence of other clinical SLE manifestations) are also seen by clinicians at our unit.
Figure 1.
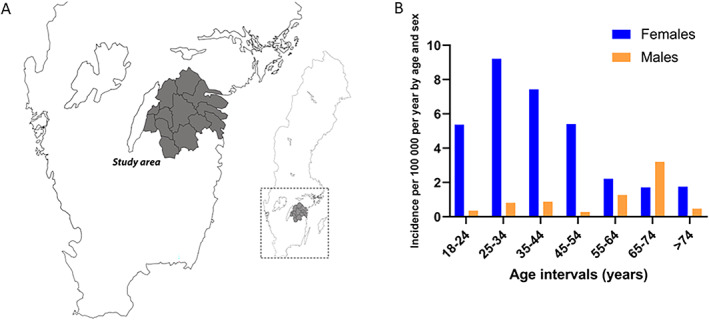
The right panel shows the map of Sweden, and the study area (Östergötland County) is depicted to the left (A). The left panel shows incidence estimates stratified by age intervals at SLE onset during the study period (B). SLE, systemic lupus erythematosus.
Case identification and definition, ascertainment of diagnosis and classification
From January 1, 2008, to December 31, 2021, individuals with an SLE diagnosis were identified from two main sources: a) the public health care organization's digital medical record (available since the beginning of 2008) with access to ICD codes (diagnoses) from all clinics in the county, as well as access to b) laboratory data from the central Clinical Immunology Unit at the University Hospital in Linköping, serving the entire county. Medical records of patients with a potential diagnosis of SLE were reviewed in detail by an experienced rheumatologist (Dr. Sjöwall) to ascertain correctness of diagnosis and fulfillment of classification criteria. True cases were defined as those with a clinical diagnosis of SLE combined with fulfillment of the 1982 American College of Rheumatology (ACR‐82) classification criteria and/or the Fries’ diagnostic principle (presence of antinuclear antibodies by immunofluorescence microscopy at least once plus involvement of at least two defined organ systems) (14).
All cases meeting ACR‐82 and/or Fries’ diagnostic principle were included in the KLURING research registry regardless of whether they had prevalent or incident SLE, and they were monitored prospectively at the Rheumatology Clinic, University Hospital of Linköping. Presence of concomitant antiphospholipid syndrome (APS) defined according to Miyakis et al was registered (15). Follow‐up included continuous assessment of disease manifestations, disease activity, annual estimation of accrual of organ damage, and registration of ongoing anti‐rheumatic drugs (ie, immunosuppressive agents, antimalarials, biologics, and corticosteroids).
Clinical disease activity was assessed using the physician global assessment and clinical SLE disease activity index‐2000 (cSLEDAI‐2K), excluding items for anti‐double‐stranded DNA binding and complement consumption (16). Laboratory monitoring included assessment of erythrocyte sedimentation rate (ESR) and plasma levels of C‐reactive protein (CRP), creatine kinase (CK) and creatinine for estimation of glomerular filtration rate (eGFR), as well as complement (C) proteins 3 and 4. Organ damage was assessed annually using the Systemic Lupus International Collaborating Clinics/ACR damage index (SDI) (16). Furthermore, in line with Gladman et al, damage was divided into separate organ domains according to its potential attribution to corticosteroid therapy (17).
Outcome measures and statistical analysis
The population alive and living in Östergötland County, Sweden, on December 31, 2021, aged 18 or older was obtained from national census data (Statistics Sweden: www.scb.se) and used as the denominator for prevalence calculations (n = 372,174). The adult Östergötland population on December 31 for each year was used as the denominator to determine annual incidence. Incidence was estimated per 100,000 inhabitants stratified by sex and age. Prevalent cases were all individuals meeting inclusion criteria who had received SLE diagnosis in 2021, or earlier, living in Östergötland as of December 31, 2021. Incident cases were all cases who received their first ever SLE diagnosis in 2008 or later and who were followed prospectively until death by December 31, 2021, or emigration. We used linear regression with calendar year of diagnosis as the outcome to assess whether each clinical measurement at diagnosis varied over time. The SDI score was displayed over time graphically, stratified by subject characteristics (year of diagnosis, sex, LN) and source of damage (corticosteroid‐caused or not) using loess regression and smoothing in R (ggplot). Possible differences between two groups of patients were analyzed using Student's t‐test.
Ethical considerations
All included subjects provided oral and written informed consent. The study protocol was approved by the Regional Ethics Review Board in Linköping (M75–08/2008).
RESULTS
Prevalence estimates
There were 266 individuals with prevalent lupus included. The prevalence of SLE on December 31, 2021, was 71.5 per 100,000 inhabitants (86.1% females), with higher prevalence in females (121.9 per 100,000) compared with males (17.4 per 100,000). The mean age was 56.5 (standard deviation [SD] 16.7) years; the mean age for females was 55.9 (SD 16.6) and 59.9 for males (SD 17.7). LN was observed in 78 of 266 patients (29.3%) and APS was observed in 42 of 266 (15.8%) patients. During the study period, 13 individuals who fulfilled our stipulated inclusion criteria were lost or did not provide informed consent. Of the 13 lost patients, only four were defined as incident cases with onset of SLE during the study period.
Incidence estimates
During the study period 2008 to 2021, 126 new SLE cases were diagnosed, of whom 16 (12.7%) fulfilled the Fries’ diagnostic principle in the absence of meeting ACR‐82 criteria. However, all 16 turned out to satisfy the 2012 Systemic Lupus International Collaborating Clinics classification criteria at last follow‐up (18, 19). In total, 80.2% were females and 81.8% of Caucasian ethnicity. This yielded a mean annual incidence of 3.0 per 100,000 inhabitants. Incidence showed a decreasing but nonsignificant trend over the study period. As illustrated in Figure 1B, higher incidences were observed in females (4.8 per 100,000) than in males (1.2 per 100,000). The mean age at diagnosis was 43.7 (SD 17.3) years (Table 1). In subjects diagnosed at an age of 55 years or more, the female‐to‐male ratio was smaller than in those diagnosed at younger ages (Figure 1B).
Table 1.
Demographics of newly diagnosed adult patients with SLE from 2008 to 2021 and incidence of SLE per 100,000 per year by age and sex in Östergötland, Sweden
| Incident SLE, 2008‐2021 | |
|---|---|
| N | 126 |
| Age at diagnosis (y), mean (SD) | 43.7 (17.3) |
| Age (y), min, max | 18, 80 |
| Female sex, % | 80.2 |
| Caucasian ethnicity, % | 81.7 |
| Incidence per 100,000 per year | 3.0 |
| Incidence by sex | |
| Female | 4.8 |
| Male | 1.2 |
| Incidence by age | |
| 18‐24 years old | 2.9 |
| 25‐34 years old | 4.8 |
| 35‐44 years old | 4.1 |
| 45‐54 years old | 2.8 |
| 55‐64 years old | 1.7 |
| 65‐74 years old | 2.6 |
| >74 years old | 1.4 |
Abbreviations: SD, standard deviation; SLE, systemic lupus erythematosus.
The age at SLE diagnosis appeared to increase (P < 0.05) during the study period. Concomitant APS was confirmed in 15 of 126 (11.9%) patients. Age was not significantly different among patients with or without concomitant APS. Renal disorder (LN), defined according to the 7th ACR‐82 criterion, was observed in 36 of 126 (28.6%) at onset of SLE. The mean age at diagnosis was similar among those with and without LN at SLE onset. Of the 36 patients with LN at diagnosis, nine were males (25%). The average age of males with LN was 59.9 (SD 17.1) years compared with the age of females with LN which was 38.3 (SD 13.4) years. No obvious gender difference was observed regarding renal histopathology as 23 of 27 (85%) women had proliferative LN (in some cases in combination with a membranous component) and 4 of 27 displayed pure membranous LN, whereas 8 of 9 (89%) men showed proliferative LN and only 1 man pure membranous LN. However, with regard to complement consumption in patients with new‐onset biopsy‐proven LN, we observed significantly lower C3 (mean values 0.92 vs. 1.08 g/L; P = 0.0003) and C4 (mean values 0.15 vs. 0.24 g/L; P = 0.003) concentrations over time in females compared with males. Still, renal function assessed by absolute eGFR over time did not show any significant difference (mean values 83.2 vs. 79.6 ml/min; P = 0.45) between females and males.
Disease activity, organ involvement, and laboratory items at diagnosis
Among the incident cases, disease activity measures (physician global assessment and cSLEDAI‐2K) at diagnosis increased slightly during the study period (P < 0.05), but none of the laboratory items changed significantly. LN, as well as involvement of other organ systems (eg, fulfilled classification criteria), at disease onset did not vary significantly over calendar period.
Damage accrual from diagnosis and onward
Acquired damage for the first 5 years of SLE of patients diagnosed 2008 to 2017 is demonstrated with alluvial plots (individuals diagnosed 2018 or later had less than 5 years’ duration of SLE). In Figure 2A, organ damage for all incident patients is shown; 41.8% of patients had acquired any damage at the 5‐year evaluation. Supplementary Figure 1 illustrates damage accrual by the 2008 to 2012 and 2013 to 2017 intervals. Furthermore, we stratified damage by sex and LN at SLE onset in Figures 2B and 2C. Finally, damage in organ domains with probable (cardiovascular, peripheral vascular disease, neuropsychiatric, diabetes) and definite (ocular, musculoskeletal) attribution to corticosteroid therapy are shown in relation to damage in domains with independent association with corticosteroid use (renal, pulmonary, gastrointestinal, skin, premature gonadal failure, malignancy) (Figure 2D) (17).
Figure 2.
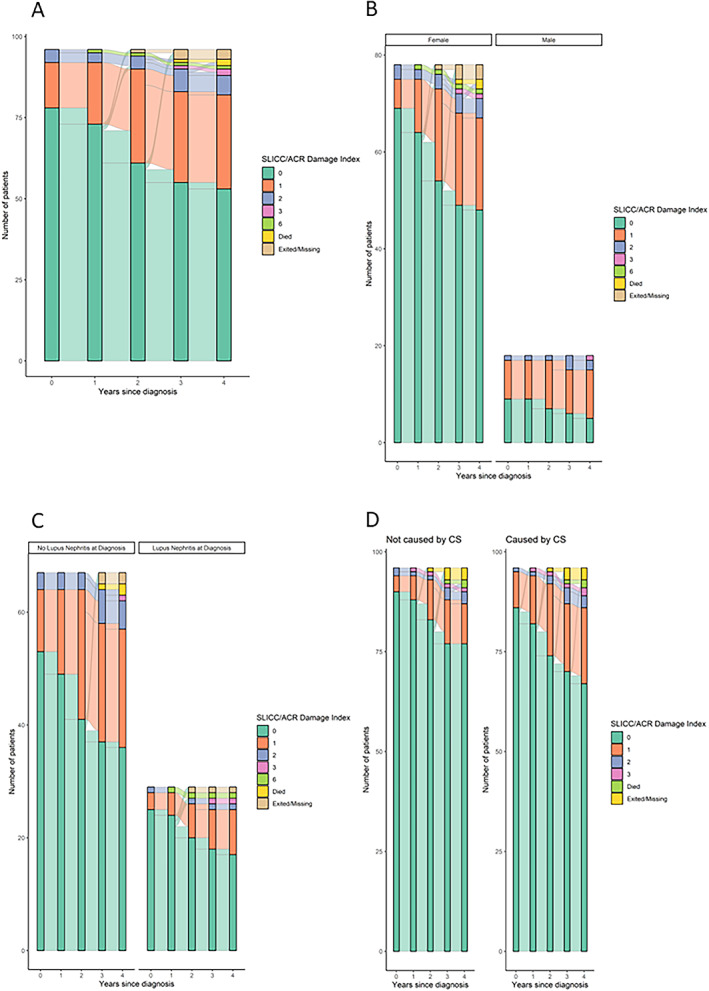
Damage accrual in patients with incident SLE diagnosed 2008‐2017 are demonstrated using alluvial plots in different ways: For all new patients diagnosed 2008 to 2017 (A); stratified by sex (B) and LN (C); and, in (D), damage in organ domains with probable and definite attribution to CS therapy is illustrated. CS, corticosteroid; LN, lupus nephritis; SLE, systemic lupus erythematosus.
DISCUSSION
The single‐center cohort KLURING is well suited for epidemiological studies of SLE. Having only one health care organization providing care to patients with rheumatological disease in the county facilitated the identification of patients and helped in avoiding selection bias. The coverage of patients with confirmed diagnosis of SLE in the catchment area of Östergötland is almost complete during the calendar years 2008 to 2021. This kind of population‐based study with very high coverage of patients, longitudinal follow‐up, and assessment of organ damage is indeed scarce but not entirely unique in Scandinavia (12, 20).
The overall SLE incidence in Östergötland County, Sweden, was estimated as 3.0 per 100,000 inhabitants during the study period. Based on registry data, Elfving et al reported slightly lower incidence in Finland (2000‐2007) whereas Danish (1995‐2011) and Norwegian data (1999‐2008) are very similar to our observations (7, 8, 9). In contrast to high‐quality epidemiological SLE studies from the UK and the US using registry data, showing an overall incidence of approximately 5 per 100,000 and rising incidence, the current study as well as other Scandinavian investigations, and a recent very large study from the UK using registry data, have reported stable or slightly decreased incidence over time (4, 5, 9, 21, 22, 23). In this context, it must be emphasized that the spectrum of ethnicity is different in Scandinavian countries compared with the UK and the US (89% Caucasian herein). Because ethnicity may be linked to genetic vulnerability to (and severity of) SLE as well as linked to health care access, it cannot be excluded that demographic differences may yield diverse observations in relation to epidemiology (1, 2, 3).
By applying a similar approach as the current study with confirmed cases meeting the ACR‐82 criteria and/or Fries’ diagnostic principle, Ingvarsson et al reported high incidence in the Lund–Orup health care district, in Southern Sweden, during the calendar years 1981 to 1993 (5.0 per 100,000), but the incidence decreased to 2.8 per 100,000 inhabitants during 1994 to 2006 (6). The latter incidence is comparable to our findings from Östergötland. The authors speculate that both improved retrieval sources and lifestyle changes might underlie the observed decreased SLE incidence during these decades (6).
We found that incidence estimates were more than 11 times higher in females compared with males in the age interval 25 to 34 years. Interestingly, among older patients, the female‐to‐male ratio was less pronounced, and in the age interval 65 to 74 years more males than females were diagnosed with SLE. In fact, similar trends have been seen in studies from Southern Sweden, Norway, Finland, and the UK, possibly indicating an important role for gonadal steroids in the debut of SLE (5, 7, 8, 12).
The observed presence of renal involvement (LN) in patients with recently diagnosed SLE were constant during the study period. Yet both the overall percentage of affected patients with SLE and the higher prevalence among male patients with SLE were in line with findings from Sweden and Denmark (9, 24). The disease activity measures cSLEDAI‐2K and physician global assessment, assessed by a limited number of physicians at our unit, showed significantly higher scores calendar year of diagnosis. Because the assessments were not accompanied by increasing levels of ESR, CRP, and CK or decreasing levels of C3 and C4, the results should be interpreted with caution.
The prevalence of SLE in Östergötland was 71.5 per 100,000 inhabitants on December 31, 2021, which is very similar to what was reported from 2006 by Ingvarsson et al (65 per 100,000) and well in line with previous data based on the Swedish National Patient Register from 2010 (6, 13). A small proportion of patients initially meeting the Fries’ diagnostic principle only later fulfilled the more sensitive (but less specific) Systemic Lupus International Collaborating Clinics classification criteria (18, 19). Duarte‐García et al observed considerably higher prevalence (data from 2015, 97.4 per 100,000) from Olmsted County, Minnesota, and similar numbers were reported from the UK (data from 2012, 97.0 per 100,000) (5, 21).
In this study, accrual of organ damage was carefully assessed annually by one rheumatologist. The trajectories did not show a clear difference in acquired damage during the two time intervals (2008‐2012 vs. 2013‐2017), underlining the need for more effective therapies with fewer side‐effects. The findings that patients with LN at SLE onset appear to accrue more damage over time are well in line with previous observations. Although limited by the number of males, our data indicate that men develop more damage early compared with females and the gender difference seems to remain over time. It is an open question whether this is related to patients’ delay or to the avoidance of physicians to suspect and diagnose SLE in males with mild skin and joint involvement until overt manifestations—such as serositis or proteinuria—become apparent (24, 25). For the whole study population, the average SDI score in females was 0.18 and 0.48 in males during the first year. But when restricting to individuals aged 55 or older at diagnosis, the average SDI score in females was more similar to the average in men (0.41 vs. 0.44, respectively). To properly address this issue further, future studies with higher statistical power should examine damage accrual with regard to gender by adjusting for age.
In our study population, damage related to corticosteroid use increased similarly to damage in other organ domains during the first 4 to 5 years. However, after that, damage less associated with corticosteroids seemed to diminish, whereas damage in the ocular and musculoskeletal domains increased steadily. This finding also highlights the need for and implementation of efficacious drugs with steroid‐sparing properties.
Our study has limitations, particularly related to size of the study population of the included patients. However, whereas the study population was limited, the size is comparable to other studies from Scandinavia, and the 14 years of follow‐up is longer than in many comparable studies. Strengths of the study include that Swedish health care is public, tax‐funded, and offers universal access. In comparison to studies using ICD codes only, these diagnoses are made by clinicians and not based on administrative health data. Furthermore, it was a single‐center study, and patients were seen by a limited number of rheumatologists (n ≤10) likely yielding a high degree of agreement in both clinical diagnostics and evaluation of laboratory data, and assessment of organ damage was done by one rheumatologist only.
To conclude, we present high‐quality data on epidemiology of SLE based on confirmed cases from a tertiary referral center in a Swedish county over a period of 14 years. Prevalence was estimated to be 71.5 per 100,000 inhabitants (87% female), and mean annual incidence was 3.0 per 100,000 inhabitants. A smaller female‐to‐male ratio was observed in patients diagnosed later in life. Despite public and tax‐funded health care with universal access, accrual of damage appears to be a persisting problem because more than 40% had acquired damage within 5 years. Furthermore, we show that incident cases with male sex and LN accrued more damage over time, and a significant proportion of the acquired damage can be attributed to the use of corticosteroids.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Arkema and Sjöwall had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Arkema, Simard, Sjöwall.
Acquisition of data
Saleh, Sjöwall.
Analysis and interpretation of data
Arkema, Saleh, Simard, Sjöwall.
ROLE OF THE STUDY SPONSOR
The funding sources had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by the funding sources.
Supporting information
Disclosure Form
Figure S1: Supplementary Figure
ACKNOWLEDGMENTS
We are sincerely grateful to all the participating patients and we thank all the clinicians at the Rheumatology Clinic, University Hospital of Linköping, for their efforts.
Supported by grants from the Swedish Rheumatism Association, the Region Östergötland (ALF Grants), the Gustafsson Foundation, and the King Gustaf V's 80‐year Anniversary foundation. Dr. Sjöwall was supported by King Gustaf V and Queen Victoria's Freemasons foundation.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11585.
REFERENCES
- 1. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 2. Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol 2018;30:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barber MR, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol 2021;17:515–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta‐analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med 2021;8:e000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rees F, Doherty M, Grainge M, et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999‐2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ingvarsson RF, Bengtsson AA, Jönsen A. Variations in the epidemiology of systemic lupus erythematosus in southern Sweden. Lupus 2016;25:772–80. [DOI] [PubMed] [Google Scholar]
- 7. Elfving P, Puolakka K, Kautiainen H, et al. Incidence of systemic lupus erythematosus in Finland, 2000‐2007, a nationwide study. Clin Exp Rheumatol 2014;32:953–5. [PubMed] [Google Scholar]
- 8. Lerang K, Gilboe I, Garen T, et al. High incidence and prevalence of systemic lupus erythematosus in Norway. Lupus 2012;21:1362–9. [DOI] [PubMed] [Google Scholar]
- 9. Hermansen ML, Lindhardsen J, Torp‐Pedersen C, et al. Incidence of systemic lupus erythematosus and lupus nephritis in Denmark: a nationwide cohort study. J Rheumatol 2016;43:1335–9. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka Y, O'Neill S, Li M, et al. Systemic lupus erythematosus: targeted literature review of the epidemiology, current treatment, and disease burden in the Asia Pacific region. Arthritis Care Res 2022;74:187–98. [DOI] [PubMed] [Google Scholar]
- 11. Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford). 2017;56:1945–61. [DOI] [PubMed] [Google Scholar]
- 12. Stahl‐Hallengren C, Jonsen A, Nived O, et al. Incidence studies of systemic lupus erythematosus in Southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J Rheumatol 2000;27:685–91. [PubMed] [Google Scholar]
- 13. Simard JF, Sjöwall C, Ronnblom L, et al. Systemic lupus erythematosus prevalence in Sweden in 2010: what do national registers say? Arthritis Care Res 2014;66:1710–7. [DOI] [PubMed] [Google Scholar]
- 14. Frodlund M, Dahlström O, Kastbom A, et al. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: analysis of a regional Swedish register. BMJ Open 2013;3:e003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 16. Arora S, Isenberg DA, Castrejon I. Measures of adult systemic lupus erythematosus: disease activity and damage. Arthritis Care Res 2020;72 Suppl 10:27–46. [DOI] [PubMed] [Google Scholar]
- 17. Gladman DD, Urowitz MB, Rahman P, et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 18. Ighe A, Dahlstrom O, Skogh T, et al. Application of the 2012 systemic lupus international collaborating clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res Ther 2015;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voss A, Green A, Junker P. Systemic lupus erythematosus in Denmark: clinical and epidemiological characterization of a county‐based cohort. Scand J Rheumatol 1998;27:98–105. [DOI] [PubMed] [Google Scholar]
- 21. Duarte‐García A, Hocaoglu M, Valenzuela‐Almada M, et al. Rising incidence and prevalence of systemic lupus erythematosus: a population‐based study over four decades. Ann Rheum Dis 2022;81:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haukeland H, Moe SR, Brunborg C, et al. S09.2 Gender differences in trends of incidence in systemic lupus erythematosus in Norway; estimates from a population‐based cohort. Lupus Sci Med 2022;9. [Google Scholar]
- 23. Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co‐occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population‐based cohort study of 22 million individuals in the UK. Lancet 2023;401:1878–90. [DOI] [PubMed] [Google Scholar]
- 24. Ramírez Sepúlveda JI, Bolin K, Mofors J, et al. Sex differences in clinical presentation of systemic lupus erythematosus. Biol Sex Differ 2019;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simard JF, Chaichian Y, Rizk N, et al. Are we missing lupus in males? Evidence of cognitive bias from a randomized experiment in the United States. Am J Epidemiol 2022;191:230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Figure S1: Supplementary Figure


