Abstract
Viruses can induce progressive neurologic disorders associated with diverse pathological manifestations, and therefore, viral infection of the brain can impair differentiated neural functions, depending on the initial viral tropism. We have previously reported that canine distemper virus (CDV) targets certain mouse brain structures, including the hypothalamus, early and selectively. Infected mice exhibit acute encephalitis, with late disease, characterized by motor impairment or obesity syndrome, appearing in some of the surviving mice several months after the initial viral replication. In the present study, we show viral persistence in the hypothalami of obese mice, as demonstrated by low, but still significant, levels of CDV nucleoprotein transcripts, associated with a dramatic decrease in F gene mRNAs. Given the pivotal role of the hypothalamus in obesity (eating behavior, energy consumption, and neuroendocrine function) and that of leptin, the adipose tissue-derived satiety factor acting through hypothalamic receptors, we analyzed the leptin networks in both obese and nonobese mice. The discrepancy found between the chronic and dramatic increase in blood leptin levels and the occurrence of obesity may be due to leptin resistance in the brain. In fact, expression of the long leptin receptor isoform, representing the functional leptin receptor, was specifically downregulated in the hypothalami of obese mice, explaining their inability to generate an adequate response to leptin in the brain. Intriguingly, during the acute phase of infection, its expression was increased in CDV-targeted structures in all infected mice and remained high in obese mice in all CDV-targeted structures, except for the hypothalamus. The biphasic change in hypothalamic leptin receptor expression seen during the progression of CDV-induced obesity provides a new paradigm for understanding mechanisms of neuroendocrinological, virus-induced abnormalities.
Neurotropic viruses can trigger transient or irreversible disorders in the central nervous system by altering the expression of neurotransmitters, neuropeptides, or receptors. Several lines of evidence demonstrate the links among viral replication and persistence in the brain, neuronal dysfunction (depending on the initial viral tropism), disordered homeostasis, and neurological diseases (16, 18, 22, 30, 43). We have previously described (7, 8) the development of late pathologies in Swiss mice inoculated with a highly neurovirulent strain of canine distemper virus (CDV), a lymphotropic and neurotropic negative-stranded RNA virus of the genus Morbillivirus, closely related to human measles virus (49, 65). Several months after inoculation, a substantial percentage of the infected mice that survive the effects of acute encephalitis develop motor impairments (paralysis or turning behavior) (5) or morbid obesity (7). The obesity syndrome, which includes pronounced hyperplasia of adipocytes in the liver and white adipose tissue, could be the consequence of viral replication in specific hypothalamic nuclei. In fact, by integrating central (neurotransmitters and neuropeptides) and peripheral (humoral, endocrine, and environmental) signals, the hypothalamus plays a key role in the control of eating behavior and energy consumption via its widely distributed targets, both inside and outside the brain. These bidirectional signals create a feedback loop for body weight regulation. Among the various factors involved, leptin, the protein product of the ob gene (66), produced almost exclusively in white adipose tissue, is thought to be crucial in this feedback loop by acting as a satiety factor on specific receptors mainly localized in the hypothalamus (13, 17, 20, 38, 51, 58). Thus, CDV infection of hypothalamic nuclei, acting by means of viral products or virus-induced molecules, could interfere with the hypothalamic cellular machinery and exert profound deleterious effects on the host’s physiology.
In the present study, to understand the pathogenesis of CDV-induced obesity, we analyzed viral transcription, viral persistence, and leptin receptor expression in the hypothalamus compared with other virus-targeted brain structures in both acutely infected and long-term-infected obese and nonobese mice. In addition, we measured levels of leptin and insulin in the blood as peripheral biological signals that are impaired in rodent obesity and the levels of which, by reflecting body mass, may indicate changes in the leptin network. Our main findings demonstrate that slow progressive CDV infection of several critical areas of the hypothalamus, which are involved in regulating food intake and body weight, triggers specific downregulation of the leptin receptor Ob-Rb in the hypothalami of obese mice despite a dramatic increase in blood leptin levels, thus leading to an obesity syndrome.
MATERIALS AND METHODS
Animals.
Four-week-old female outbred Swiss mice (Charles Rivers, Les Oncins, France) were housed according to European Economic Community (86/609/EEC) and French (Decree 87-848) animal care regulations in a temperature-controlled room with a fixed 12-h–12-h light-dark photoperiod. The animals were supplied ad libitum with standard laboratory chow and water throughout the experiment. The mice were weighed weekly once they appeared to start increasing in size. Weanling inbred C3H and BALB/c mice (H-2k and H-2d, respectively) were also used in some experiments and were treated as described above.
Experiment design.
A neurotropic variant of CDV was obtained from the Onderstepoort vaccinal strain (8) serially passaged in the suckling mouse brain. The infection titer, determined on Vero cells, was approximately 105 PFU/ml. Swiss mice were inoculated intracerebrally with neonatal mouse brain suspension (12th-passage homogenate used as viral stock) containing 200 to 1,000 PFU of the neuroadapted CDV strain. Brain homogenates from noninfected neonatal mice were inoculated into control (sham-inoculated) animals. Experiments were also performed with the inbred mouse strains C3H and BALB/c. During the early stage of acute meningoencephalitis (day 14 postinoculation [p.i.]), or during the late pathologies (5, 6, 8, 10, and 13 months p.i.), infected and sham-inoculated mice were perfused, under anesthesia, with cold phosphate-buffered saline (PBS), and then their brains were removed and used for in situ hybridization, immunohistochemistry, or RNA extraction. In addition, brain inoculations of 4-week-old Swiss mice with wild-type CDV (Onderstepoort strain) or a neuroadapted strain by several other routes (intraperitoneal, subcutaneous, footpad, or intranasal [either bilaterally or unilaterally]) were used for clinical evaluation and viral replication.
Infected mice were classified according to body weight, fat mass, and leptin and insulin levels. The lean mice had bodyweights similar to those of age-matched sham control mice (inoculated only with the vehicle). The preobese mice had plasma leptin levels of more than 20 μg/liter and showed a slight weight increase (nonsignificant). The obese mice gained weight (greater than the mean of the sham controls plus 1 standard deviation) and had increased insulin and leptin levels.
For RNA extraction, samples obtained from three sham-inoculated and three infected mice at 14 days p.i. (pooled microdissected brain structures) and from five mice (two sham-inoculated, one obese, and two lean infected mice without overt pathology) sacrificed at 5 months p.i. (individual samples from one obese mouse [70 g] and two lean mice [45 and 36 g] and pooled samples from two sham-inoculated mice [34 and 38 g]) were used. One sham-inoculated mouse (31 g) and two infected mice (one lean and one obese [39 and 73 g, respectively]) were sacrificed 13 months p.i. Two infected C3H mice, one showing turning movements (31 g) and one obese (49 g), were also kept for 6 months p.i. for RNA extraction. Finally, at 8 months p.i., six mice (two sham-inoculated and two nondiseased CDV-infected Swiss mice and one sham-inoculated and one nondiseased CDV-infected BALB/c mouse) were used as controls for leptin receptor expression.
For in situ hybridization and immunocytochemical (ICC) studies, brains from six mice (three sham inoculated and three infected) at 14 days p.i., three mice (one sham inoculated [43 g], one lean [38 g], and one obese [62 g]) at 5 months p.i., and another three mice (one sham inoculated [46 g], one lean [31 g], and one obese [64 g]) at 10 months p.i. were used.
At various times after inoculation, individual blood samples from several animals from each of the three mouse strains were taken for biochemical analysis.
For in situ hybridization or ICC studies, following decapitation, the brains were quickly removed, frozen rapidly on a plate chilled in liquid nitrogen, and stored at −80°C until use. Serial 14-μm-thick coronal sections, prepared with a cryostat microtome (−16°C), were collected on silane-coated glass slides (48) before being processed. For RNA extraction, microdissected brain structures were collected and processed as described below.
Blood insulin and leptin assays.
Serum or plasma samples were taken from noninfected or infected (obese, lean, or motor-diseased) mice from each of the three strains at various time intervals after inoculation (7 and 14 days p.i. and 1 to 13 months p.i.). Insulin levels were measured by radioimmunoassay (INSIK-5; Sorin Diagnostics France S.A.) as described previously (7). The mouse leptin radioimmunoassay (Wak ChemieMedical GmbH, Bad Homburg, Germany) was performed following the manufacturer’s recommendations, with a limit of detection of approximately 0.5 μg/liter. For both radioimmunoassays, the intra- and interassay coefficients of variation were, respectively, less than 8 and less than 10%. The data for leptin levels are presented as the mean and standard error and were analyzed by Student’s t test and analysis of variance, Fischer, and Scheffe tests.
Primer design.
The leptin receptor was originally described as an alternatively spliced single membrane-spanning domain showing homology with the class I cytokine receptor (58). The receptor, originally cloned from the choroid plexus, corresponds to the Ob-Ra splice variant, which has a short intracellular domain. In contrast, the splice variant Ob-Rb encodes a receptor with a long intracellular domain and is abnormally truncated in db/db mice. Reverse transcription (RT)-PCR was carried out with oligonucleotide primers that hybridize with either the sequence encoding the extracellular domain of the receptor (hybridizing with all splice variants of the leptin receptor gene mRNAs) or that encoding the long intracellular domain containing the putative intracellular signaling domain (Ob-Rb specific) (Table 1).
TABLE 1.
Sequences and locations of oligonucleotide primers used for RT-PCR, expected size of amplified products, and sequences of probes used in the hybridization of ampliconsa
| Target | 3′ Primer (5′-3′) | 5′ Primer (5′-3′) | Size (bp) | Probe (5′-3′) | Source or reference |
|---|---|---|---|---|---|
| GAPDH | 1030 - CATGTAGGCCATGAGGTCCACCAC - 1010 | 51 - TGAAGGTCGGTGTGAACGGATTTGGC - 76 | 979 | 497 - GGCTAAGCAGTTGGTGGT - 514 | 50 |
| Cyclophilin | 647 - CTTCAGTGAGAGCAGAGATTACAGG - 623 | 250 - ATAATGGCACTGGCGGCAGG - 269 | 395 | 392 - CAAGACTGAATGGCTGGATGGC - 409 | 25 |
| Ob-R | 1888 - GAACCTGGACCACATAGACTGC - 1866 | 1481 - GTCCATCTATTCATCCTACG - 1501 | 407 | 1633 - TTGGTGGCGAGTCAAGTGAACC - 1611 | 17 |
| Ob-Rb | 3330 - CACACCACTCTCTCTTCTGTTGACG - 3306 | 2843 - ATAAAGATGAGATGGTCCCAGCAGC - 2867 | 487 | 3068 - ACCCCTTGCTCTTCATCAGTTTCC - 3045 | 17 |
| CDV-F | 1990 - GCTGACATTCATTGCCTCCGATAC - 1967 | 1642 - CTGCTTGAGTGTCTGTTGGTAGCA - 1665 | 348 | 1929 - AATCAGCAACAACAAAGCCAGG - 1950 | 64 |
| CDV-NP | 564 - AATCCACCTTCTCATCTCCGAGTCG - 540 | 404 - AGGCTGGTTAGAGAATAAGG - 424 | 159 | 534 - AGTATCAGGAGCAGTCACCG - 515 | 49 |
| GFAP | 911 - AGTGCCTCCTGGTAACTGG - 893 | 616 - AGAGAGATTCGCACTCAATACGAGG - 640 | 277 | 688 - AAGTTTGCAGACCTCACAGACG - 709 | Mus GFAP acces-sion no. K01347 |
Sequences were selected (GenBank and DNA Data Bank), their specificity was verified by comparison with other known rodent mRNA sequences (EROD databank), and the corresponding oligonucleotides were synthesized and purified by Eurogentec (Seraing, Belgium).
RNA preparation.
At various time points after inoculation (see above), infected or uninfected mice were deeply anesthetized with pentobarbital (1 μl of a 6% solution per g) and then rapidly perfused with ice-cold 0.1 M PBS, pH 7.4. The brains were quickly removed, dissected at 4°C, and divided sagitally into two symmetric parts. Several precise anatomical landmarks allowed us to carefully dissect the structures (hypothalamus, hippocampus, cortex, mesencephalon, and cerebellum). Dissections were always performed in the same order to optimize reproducibility, and former histological and biochemical controls of the brain tissue pieces confirmed the presence of the corresponding structures. Total RNAs were extracted with RNAzol (Bioprobe, Montreuil sous Bois, France) according to the manufacturer’s recommendations, and RNA integrity was verified by denaturing 1% agarose gel electrophoresis and ethidium bromide staining (1). The RNAs were quantified by spectrophotometry at 260 nm, and their purities were estimated from the 260-280-nm absorbance ratio (1.8 to 2.0).
Semiquantification of viral transcripts and leptin receptor (Ob-R) gene mRNAs by RT-PCR.
RT was performed with either 500 ng or 1 μg of total RNAs from microdissected brain structures as the starting material. The RNAs were denatured (10 min at 70°C), and then first-strand cDNAs were synthesized at 42°C for 90 min in a final volume of 20 μl, containing 22 U of RNasin (Promega, Madison, Wis.); 10 mM dithiothreitol; 0.5 mM (each) dATP, dTTP, dGTP, and dCTP; 5 ng of oligo(dT)12–18 primer (Pharmacia Biotech); 1× RT buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL-Life Technologies).
PCR was performed on a BioMed thermal cycler, using 2 to 5 μl of a 1:5 or 1:10 dilution of the cDNA samples obtained by oligo(dT) priming. Coamplification was carried out with the ubiquitously and stably expressed housekeeping genes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (50) or cyclophilin (25). Parallel tests on separate amplifications were carried out in pilot experiments to check the efficacy of each amplification stage and to determine the exponential phase for each variable, i.e., the optimum number of cycles, the MgCl2 concentration, and the annealing temperature. The PCR mixture (final reaction volume, 50 μl) consisted of 1× PCR buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl) (Gibco BRL-Life Technologies), 1.5 to 3.0 mM MgCl2, 0.2 mM (each) deoxyribonucleoside triphosphate, 0.4 μM (each) specific 3′ and 5′ primers (Table 1), and 2 U of Taq DNA polymerase (Gibco BRL-Life Technologies). Samples were subjected to PCR (with a prior hot-start procedure) consisting of 18 to 32 cycles of 95°C for 45 s, 55 to 60°C for 45 s, and 72°C for 1 min for Ob-R, cyclophilin, and GAPDH gene and CDV mRNAs. Lack of contamination in PCR experiments was verified by omission of RT. To avoid pipetting inaccuracies and to maximize the reliability of the quantification of the amplified products, all samples to be compared were processed simultaneously for RT-PCR, using reagents from the same master mix. The leptin receptor gene amplification reaction was linear from cycle 22 to cycle 30 for both samples and, in one experiment, the Ob-Rb gene amplicon (487 bp) was sequenced to verify the accuracy of the process.
Ten microliters of coamplified products was then analyzed on a 2% Seakem-NuSieve agarose gel, and the amplicons were covalently bound to a 0.2-μm-pore-size Nytran nylon membrane (Schleicher and Schuell) by electrotransfer (15 V; 45 min) and subjected to DNA (Southern) blot hybridization analysis as previously described (4). Hybridization with specific internal probes, 5′ labeled with [γ-32P]ATP (Table 1), identified the Ob-R (either the extracellular domain or the long spliced intracellular domain), GAPDH, cyclophilin, or glial fibrillary acidic protein (GFAP) gene or CDV amplicons (nucleoprotein [NP] or F gene transcripts) in each cDNA sample.
Labeling was quantified by image analysis, phosphorimaging, and counting excised bands and was expressed as relative units, calculated as the ratio of (pixels [counts per minute] for the Ob-R gene amplicons)/(pixels [counts per minute] for the GAPDH, cyclophilin, or GFAP gene amplicons), corresponding to normalized values. The comparison between levels in infected brain structures and those in matched structures from sham-inoculated mice was then expressed as a percentage. CDV expression was evaluated as counts per minute of F gene transcripts versus counts per minute of NP gene transcripts, according to the gene order reflecting the transcription mode of morbilliviruses. This ratio gives an indication of the transcription rate in different brain structures in acutely diseased or late-diseased mice.
In situ hybridization: CDV detection and Ob-R gene mRNA expression.
Following in situ hybridization, CDV replication and Ob-R gene expression were detected with either an NP oligonucleotide probe, as previously described (6), or a 23-mer oligonucleotide (Eurogentec, Seraing, Belgium), complementary to bases 3068 to 3045 (17) of the mouse Ob-Rb gene mRNA sequence, as the RT-PCR internal probe (Table 1). Ten picomoles of oligonucleotides was 3′ end labeled (22 U of terminal deoxynucleotidyl transferase; Boehringer Mannheim, Meylan, France) with 17 pmol of [35S]dATP (1 h at 37°C) in 10 μl of 200 mM potassium cacodylate, 25 mM Tris-HCl [pH 6.6], 0.25 mg of bovine serum albumin/ml, and 2.5 mM cobalt chloride. The labeled probe (specific activity, 1,200 to 1,400 Ci/mmol) was used (2.5 nM/slide) in hybridization buffer (50% formamide, 3× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1× Denhardt solution, 0.02% bovine serum albumin, 100 μg of sonicated salmon sperm DNA/ml, 125 μg of yeast tRNA/ml, 10 mM dithiothreitol, 10% dextran sulfate, and 0.8% sarcosyl).
Brain slices were fixed in freshly prepared 4% paraformaldehyde (PFA)–0.1 M phosphate buffer, pH 7.4, and then dehydrated in graded ethanol solutions and hybridized (38°C) overnight. After several washes (2- to 0.5× SSC; 30 min each), the tissue sections, dehydrated in ethanol and air dried, were exposed for macroautoradiography (βmax; Amersham, les Ulis, France) for 1 week at 4°C. Analysis at the cellular level was performed by coating representative slices with K-5 emulsion (Ilford); after 3 weeks of exposure at 4°C, the slices were developed with D19 (Kodak-Pathé, Paris, France) and counterstained with cresyl violet.
Alternatively, the following protocol of in situ tyramide signal amplification (TSA) (DuPont, NEN Life Science Products), originally described by Bobrow et al. (11), was used following the manufacturer’s recommendations to amplify a weak in situ signal. In this method, a biotinylated oligonucleotide (50 pmol); 3′ end labeled with 16-dUTP-biotin [Boehringer Mannheim]) is used, as described above, followed by incubation with streptavidin-horseradish peroxidase (SA-HRP). HRP catalyzes the deposition of biotinyl tyramide onto tissue sections that have been previously blocked and permits amplification of the signal by a second incubation with an SA-HRP complex, which binds to the deposited biotin. HRP was finally visualized by a diaminobenzidine procedure. Briefly, PFA-fixed brain tissue was subjected to in situ hybridization as described above and then washed twice with TNT (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.05% Tween 20), blocked for 30 min at room temperature in TNB buffer (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.5% DuPont blocking reagent), and incubated for 30 min at room temperature in SA-HRP (1:500 in TNB). After three washes in TNT, the slides were incubated at room temperature with 1:50 biotinyl-tyramide in amplification diluent and a second time (30 min at room temperature) with SA-HRP (1:100 in TNB), and then finally washed with TNT and subjected to routine diaminobenzidine staining (5 mg in 10 ml of 50 mM Tris [pH 7.6], 0.02% H2O2). Mouse (53) and rat (45) brain atlases were used to determine the anatomical locations of signals.
ICC.
Polyclonal rabbit anti-CDV antibodies and sera from patients with subacute sclerosing panencephalitis (SSPE) reacting with CDV antigens were used in indirect-immunofluorescence studies to detect CDV, while polyclonal goat antibodies against the Ob-R peptides, K20 or M18 (corresponding to residues 32 to 51 [N terminus] or 877 to 894 [C terminus], of the common form of mouse Ob-R [Santa Cruz Biotechnology, Santa Cruz, Calif.]) were used to identify structures and cells expressing Ob-R. Coronal brain sections, postfixed in acetone (10 min at −20°C) or 4% PFA, were incubated for 1 h at 37°C with 2 μg of anti-Ob-R antibodies/ml or with a 1:500 dilution of anti-CDV antibodies. After three PBS rinses, the sections were incubated in the dark for 45 min at room temperature with fluorescein isothiocyanate-conjugated secondary antibodies against rabbit, human, or goat immunoglobulin G (Biosys S.A., Compiegne, France) at a dilution of 1:200. When the primary antibodies were omitted, no immunoreactive signal was seen.
Histopathology.
Classical hematoxylin-eosin and cresyl violet staining was used to visualize the cell soma, processes, and anatomic nuclei and to show the absence of hypothalamic inflammatory lesions or white matter injury in the brains of any of the mice analyzed during the late stage of infection, except for mild edema in the periventricular area. Brain tissue was obtained from infected and noninfected mice at 14 days p.i. and from infected obese and lean littermates at 5 to 11 months p.i., by which time the obese animals had reached their maximal weights (60 to 75 g).
RESULTS
Clinical observations.
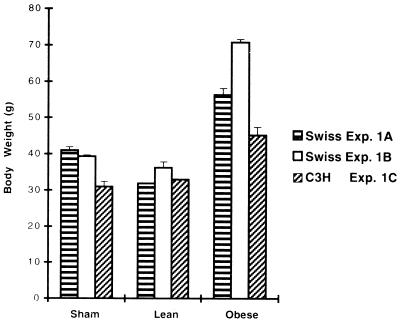
The postinfection obesity presented the features of a slow infection, as the significant increase in weight appeared only after an incubation period, with about 25% of the Swiss mice that survived acute meningoencephalitis becoming obese 4 to 5 months after intracerebral inoculation of the neuroadapted CDV strain. The mean body weights (presented in Fig. 1) of the mice used in three different experiments involving Swiss (two separate experiments, A and B) and C3H (experiment C) mice indicated a significant increase of 41 to 87% in obese mice (mean weights, 56.3, 70.8, and 45.2 g) compared to those of sham-inoculated mice (mean weights, 41, 39.3, and 31 g) and those of lean infected mice (mean weights, 31.8, 36.2, and 32.9 g) respectively, for each experiment. Thus, the obesity may be classically described as a fast increase in body weight (dynamic phase) followed by a plateau (static phase). The occurrence of obesity syndrome was correlated with the degree of neurovirulence of the viral strain, since a higher rate of mortality in the acute stage of infection resulted in a higher percentage of late obesity in the surviving mice (a mortality rate of 50 to 80% resulted in a 15 to 25% incidence of obese mice). Obesity seemed to be independent of the histocompatibility system, since both noninbred Swiss mice and inbred C3H or BALB/c (H-2k or H-2d, respectively) mice became obese after intracerebral inoculation with CDV. Finally, obesity was correlated with central viral infection, as mice inoculated intracerebrally with an inactivated virus did not exhibit any late pathology (unpublished results). In addition, prior vaccination with a vaccinia recombinant containing genes coding for CDV surface antigens was able to protect, partially or totally, against both acute encephalitis and the subsequent late obesity, indicating that the occurrence of obesity depends on the initial viral replication (54, 64). Moreover, inoculations with a CDV-neuroadapted strain by other routes (intraperitoneal, intranasal, footpad, and subcutaneous) or intracerebral inoculation of mice with wild-type CDV (Onderstepoort strain), never led to viral replication in the brain (absence of viral antigens and RNAs) and subsequent obesity (gain in bodyweight). Taking together, these observations are a strong indication that viral replication in the brain is a prerequisite for the development of obesity.
FIG. 1.
Body weight changes during the late stage of CDV infection. The effect of CDV infection on body weight in three different experiments with Swiss mice (experiments [Exp.] (A and B) and C3H mice (experiment C) are shown. The mice were weighed weekly over the period from 5 to 8 months after viral inoculation in experiment A (3 months of surveillance). In experiments B and C, the mice were examined over a period from 8 to 13 and 5 to 6 months after inoculation, respectively (corresponding to 5 and 2 months of surveillance, respectively, in B and C). The data are the mean (cumulative data) + standard error of the mean for each experiment. The number of mice in each experiment was as follows: (A) sham inoculated, n = 6; lean, n = 4; obese, n = 1; (B) sham inoculated, n = 5; lean, n = 2; obese, n = 1; (C) sham inoculated, n = 5; lean, n = 5; obese, n = 2. A significant increase in body weight was seen for obese mice versus either lean mice (P < 0.0002 [A], 0.0001 [B], and 0.005 [C]) or sham-inoculated mice (P < 0.001 [A], 0.0001 [B], and 0.005 [C]) (Student’s t test).
Viral expression in the hypothalamus.
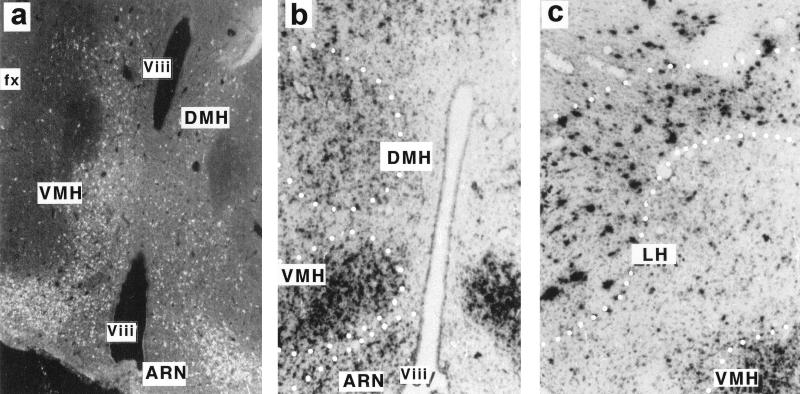
In the animals acutely infected 14 days p.i., hypothalamic nuclei, such as the ventromedian (VMH), dorsomedian (DMH), paraventricular nucleus (PVN), arcuate nucleus (ARN), and lateral (LH) hypothalamus, showed high expression of CDV proteins and RNAs (visualized by ICC and in situ hybridization, respectively [Fig. 2]), indicating that hypothalamic nuclei are permissive for viral tropism and viral replication. Viral transcripts or proteins could not be detected by in situ hybridization or ICC after 6 weeks p.i., but the use of the sensitive RT-PCR method permitted us to detect viral mRNAs in the hypothalamus during the late stage of infection (5 months p.i.). Expression of the NP gene (located at the 3′ end of the genome) and the F gene (located upstream in the first third of the genome) (52) was analyzed in more detail. The F/NP ratio reflects the characteristic transcriptional polarity of the abundance of morbillivirus mRNAs, determined by gene order, as a function of the distance of each gene from the 3′ end of the genome (15). The F/NP gene mRNA ratio in the hypothalamus rose to 34% during the acute stage of infection (14 days p.i.), similar to that seen in the other main viral target brain structures (hippocampus and mesencephalon) and in a neuroblastoma cell line acutely infected with CDV (Fig. 3 and Table 2), but fell to less than 0.6% in the hypothalami of obese mice (Table 2). As shown in Table 3, NP gene mRNAs could be detected in the hypothalami of obese (70 g) and preobese (45 g [but with high plasma leptin levels of 63 μg/liter]) Swiss mice (5 months p.i.) but not in infected nondiseased mice, and its transcription rate was up to one-half that in the acute stage of infection (56 and 49%, respectively), confirming a continuous transcriptional activity in the persistent state, as described by Zurbriggen et al. (68). At 13 months p.i., although weak expression of the NP gene was seen in the hypothalamus of one obese mouse (weight, 73 g; leptinemia, 75 μg/liter), we have never been able to detect F gene transcripts, despite increasing the amount of starting material or the number of PCR cycles (data not shown). Taken together, these results demonstrate that there was an attenuation of the transcription rate combined with drastic downregulation of gene expression for surface antigens, evidenced by restriction of the F viral gene by more than 10-fold compared to its expression in acute viral replication, and they substantiate the idea that late obesity might be linked to viral persistence in the hypothalamus.
FIG. 2.
Localization of CDV products in the hypothalami of infected mice during the acute stage of infection. Viral mRNAs and proteins are expressed in a unique pattern in the hypothalamus of an infected Swiss mouse (14 days p.i.). (a) Immunohistochemical localization of cells containing viral proteins with serum from an SSPE patient. Strong labeling is mainly seen in the peri-third ventral hypothalamic areas, especially in hypothalamic nuclei, such as the VMH, DMH, and ARN, while outside the hypothalamus, structures such as the fornix (fx) are completely unlabeled. (b) Viral mRNAs, demonstrated by in situ hybridization with a dATP 35S-3′-end-labeled NP oligonucleotide, are also found in hypothalamic nuclei, such as the VMH, DMH, and ARN. The distribution of labeling matched that for proteins, indicating active viral replication in the hypothalamus. (c) Viral NP gene mRNAs are also detected in neurons of the LH areas. The dotted lines represent schematic limits of the hypothalamic nuclei. Viii, third ventricle.
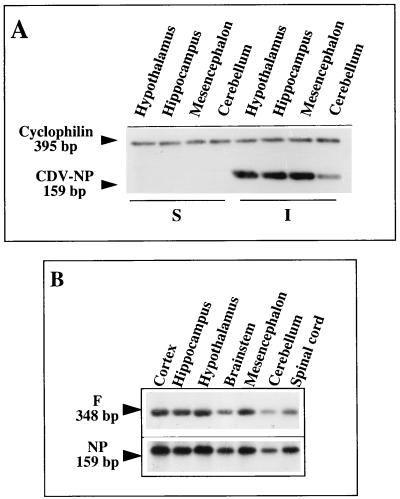
FIG. 3.
Analysis of CDV transcription in the main viral targets in the brain. RT-PCR coamplification of the NP gene and the housekeeping cyclophilin gene (A) and NP and F gene transcripts (B) in various brain structures of infected and noninfected (sham-inoculated) Swiss mice during the acute stage of infection (14 days p.i.) are shown. Visualization of NP (159-bp) and cyclophilin (395-bp) gene amplicons by ethidium bromide staining (A) shows that the same amount of amplified DNA was loaded in each lane and strongly suggests active viral replication in various brain structures, e.g., the hypothalamus. Southern blots (B), using internal probes specific for each viral amplicon (NP and F gene transcripts), allow semiquantification of the PCR products (image analysis, counting excised amplicons in counts per minute) (Tables 2 and 3). The autoradiographs were exposed for 10 min (NP gene amplicon) or 2 h (F gene amplicon), indicating the presence of different amounts of these two viral mRNAs, as expected from their positions on the viral genome. The expected sizes were 159 and 348 bp for the NP and F genes, respectively (Table 1).
TABLE 2.
Attenuation of viral transcriptional activity during the late stage of infectiona
| Time (p.i.) | Stage | F/NP ratio (%) in:
|
|||
|---|---|---|---|---|---|
| Hypothalamus | Hippocampus | Mesencephalon | NIE115c | ||
| 14 days | Acute | 34 | 24 | 25 | 34 |
| 5 months | Preobeseb | 7.3 | 5.2 | 5.9 | |
| Obese | 0.6 | 5.4 | 3.6 | ||
The F/NP ratio reflects the transcriptional mode of members of the genus Morbillivirus. This ratio was therefore analyzed in various brain structures of infected mice during the acute (14 days p.i.) or late (5 months p.i.) stage of infection. RT-PCR was carried out with coamplification of F and NP gene transcripts. The data are expressed as counts per minute of F gene mRNAs/counts per minute of NP mRNAs (relative units). PCR conditions: 3 mM MgCl2 and 30 cycles (95°C for 45 s, 60°C for 45 s, 72°C for 1 min). The transcription of F gene mRNAs in brain structures during acute infection corresponds to about one-third of that for NP gene mRNAs, as was also seen in a neuroblastoma cell line acutely infected with CDV. The data in the late stage of infection indicate a dramatic downregulation of the F gene, although NP gene transcription remains elevated (Table 3).
According to leptinemia of 63 μg/liter (weight, 45 g).
Neuroblastoma cell line.
TABLE 3.
Attenuation of NP gene transcription in obese mice during the late (5 months p.i.) versus the acute (14 days p.i.) stage of infectiona
| Structure | NP (late/acute) (%) in:
|
|
|---|---|---|
| Preobeseb | Obese | |
| Hypothalamus | 56 | 49 |
| Hippocampus | 39 | 23 |
| Mesencephalon | 44 | 22 |
RT-PCR was carried out on various microdissected structures from sham-inoculated and infected obese or nonobese mice. The RT-PCR schedule was 3 mM MgCl2 and 30 cycles (95°C for 45 s, 60°C for 45 s, 72°C for 1 min). The results are expressed as counts per minute for late NP gene mRNAs/counts per minute for acute NP gene mRNAs (normalized to the housekeeping gene) × 100. The data indicate that the NP gene transcription level in brain structures of obese mice remained elevated (up to 50%) compared to that during active viral replication (14 days p.i.).
According to leptinemia of 63 μg/liter (weight, 45 g).
Ob-R gene expression in the brain.
It was of interest to determine whether the development of virus-induced obesity was associated with hypothalamic dysfunction. Because of the hypothalamic pattern of expression of viral transcripts in the early stage of CDV infection (Fig. 2), we investigated leptin receptor (Ob-R) expression in the hypothalamus during both acute viral replication (14 days p.i.) and the late stages of infection (obese versus nonobese mice), using in situ hybridization, in situ TSA hybridization, ICC, and RT-PCR.
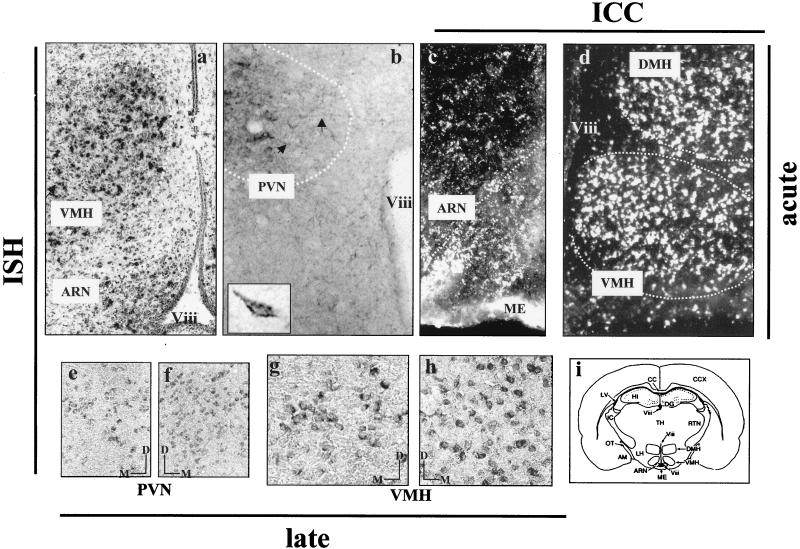
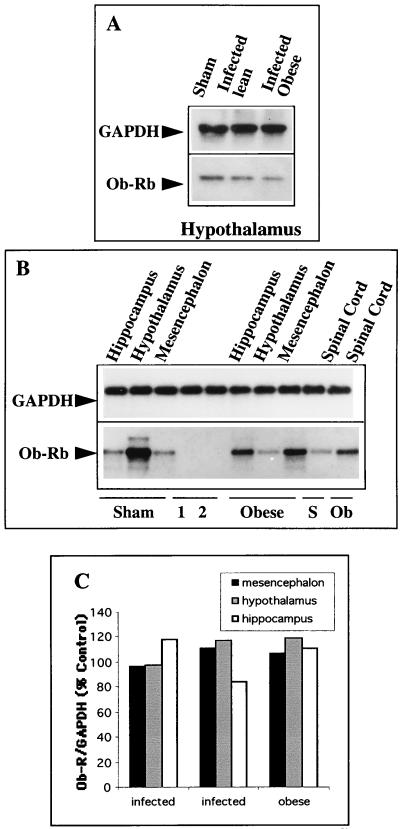
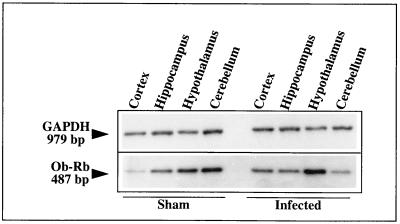
As described by Hakansson et al. (24), expression of proteins representing the common forms of the leptin receptors was seen in hypothalamic nuclei, especially in the DMH, VMH, and ARN (Fig. 4c and d). Using in situ procedures, we were also able to demonstrate expression of the long spliced variant, Ob-Rb. Ob-Rb gene mRNAs were localized to several hypothalamic nuclei (PVN, VMH, and ARN) (Fig. 4a and b) known to be implicated in the regulation of food intake and energy balance (23, 29, 38). This pattern of hypothalamic localization correlated with the distribution of CDV transcripts shown in Fig. 2. As shown by in situ TSA hybridization, leptin receptor expression was mainly seen in the bodies of cells, which, on the basis of their shapes, probably corresponded to neurons (Fig. 4b and e to h), and it seemed to be lower, both in terms of the number of Ob-Rb-expressing neurons and in terms of cellular concentration, in the hypothalamic areas of obese mice at 5 (not shown) and 10 months p.i. (Fig. 4e versus f and g versus h). This decrease in Ob-Rb expression in the hypothalami of obese mice, relative to that in other CDV-targeted structures, was confirmed by semiquantitative RT-PCR with coamplification of the leptin receptor gene and the housekeeping GAPDH gene, constitutively expressed, and was not significantly affected by viral infection (Fig. 5 and Table 4). This approach showed that, compared with the sham-inoculated mice, Ob-Rb expression in the hypothalami of obese mice (5 months p.i.) decreased by up to one-third (33%) while Ob-Rb gene mRNAs increased by up to twofold in the hippocampus and cortex. Furthermore, when Ob-Rb expression in the same samples was quantified by using the ratio of Ob-Rb counts per minute to GAPDH counts per minute (Fig. 5A), the decrease in its expression in the hypothalamus relative to that in the control was remarkably similar (31%). As shown in Fig. 5B, Ob-Rb gene mRNAs were also dramatically downregulated (up to 70%) in the hypothalami of obese Swiss mice at 13 months p.i., while the Ob-Rb levels in other brain structures, such as the hippocampus, mesencephalon, or spinal cord, increased compared to those in matched structures of sham-inoculated mice, in agreement with data obtained at 5 months p.i. The decrease of Ob-Rb in the hypothalami of obese mice versus that in sham-inoculated mice (13 months p.i.; quantified by phosphorimaging) was also seen when coamplifications were performed with either cyclophilin or GFAP (data not shown). No differences were seen in the sizes and sequences of Ob-Rb PCR products obtained from uninfected, lean, or obese mice, indicating an absence of mRNA sequence modification during persistent infection. It should be noted that the amounts of Ob-Rb gene expression, compared to that of the coamplified housekeeping gene (ratio determined by image analysis), were similar in two sham-inoculated (pooled) and two nonobese infected mice, with values of 76.6 ± 5.6, 47.0 ± 2.0, and 36.3 ± 4.6, respectively, for the hypothalamus, hippocampus, and cortex (P < 0.05). Expression of the long leptin receptor isoform (Ob-Rb) in the hypothalami of C3H mice at 6 months p.i. (obese mice compared to those with a turning behavior) was decreased by up to one-half (47%; data not shown), confirming the downregulation in the hypothalami of obese mice, which was independent of the mouse strain. Furthermore, analysis of leptin receptor expression in hypothalami from infected Swiss and BALB/c mice without any clinical signs at 8 months p.i. showed similar levels of Ob-Rb (expressed as a percentage of the normalized values, 93.7 and 94.1, respectively). In addition, when primers were designed to amplify extracellular sequences corresponding to the common leptin receptor, no significant modulation of its expression was seen, irrespective of the brain structures and mice (obese versus lean at 5 months p.i.) (Fig. 5C). We also analyzed Ob-Rb gene expression during the early stage of infection (14 days p.i.) (Table 5 and Fig. 6). Leptin receptor gene mRNAs were upregulated by up to twofold in the hypothalamus, as well as in almost all infected viral brain target structures except the cerebellum, which is a poorly permissive brain structure for CDV replication.
FIG. 4.
Localization of leptin receptors by ICC, in situ hybridization (ISH), and in situ TSA hybridization. ICC with antibodies reactive with all leptin receptor isoforms shows the presence of Ob-R proteins in the hypothalamic nuclei (VMH, DMH, and ARN) (c and d). In situ hybridization, using a dATP 35S (a) or 11-dUTP digoxigenin (b and e to h) 3′-end-labeled oligonucleotide designed to hybridize to mRNA coding Ob-Rb (Table 1), demonstrates the presence of these transcripts in hypothalamic areas of the Swiss mouse. Leptin receptor (Ob-Rb) expression is especially evident in the VMH and ARN (a) and in the PVN (b), as indicated by the schematic drawing of a coronal section (i) of the mouse brain at the level shown in panels a, c, and d, where most leptin receptor-positive cells are located. The cells were labeled at the level of the cell bodies (b and e to h); some neuronal processes were also decorated (b; indicated by arrows). The pictures are representative of the features seen in infected and uninfected mice during the acute stage of infection (a to d). The localization of Ob-R proteins matches that of Ob-Rb gene mRNAs. Panels a and d are in opposite orientations (left versus right hemisphere). The dotted lines represent schematic limits of the hypothalamic nuclei. Ob-Rb gene mRNAs were mainly found in neurons (with labeling at the cellular level [insert in panel b]) defined according to morphological criteria, namely, the sizes (larger than 20 μm) and the anatomical locations of labeled cells in precise hypothalamic nuclei (b and e to h), as seen with in situ TSA hybridization (see Materials and Methods). At 10 months p.i., the number of Ob-Rb-expressing cells seems to be lower in the hypothalamus of an obese mouse (e and g) than in that of a nonobese infected mouse (f and h). The bars in panels e to h indicate the medial (M) and dorsal (D) positions of the photographs. Magnification = ×28 (a and d), ×70 (b and c), ×112 (e to h), and ×280 (insert). ME, median eminence; TH, thalamus; HI, hippocampus; DG, dentate gyrus; AM, amygdala; IC, interne capsula; LV, lateral ventricle; Viii, third ventricle.
FIG. 5.
RT-PCR coamplification of leptin receptor and GAPDH genes during the late stage of infection. Total mRNAs (0.5 μg), extracted from infected (lean or obese) and sham-inoculated mice, were subjected to RT-PCR (26 to 30 cycles; 2 mM MgCl2) in a coamplification schedule (see the text), and the amplicons were loaded onto an agarose gel for electrophoresis, followed by electrotransfer and Southern blotting. The expected sizes were 487, 979, and 395 bp for Ob-Rb, GAPDH, and cyclophilin genes (Table 1). (A) RT-PCR of GAPDH and OB-Rb genes in the hypothalami of infected (lean and obese) and noninfected (sham-inoculated) Swiss mice (5 months p.i.). Quantification of the relative amount of Ob-Rb gene amplicon compared to the housekeeping gene amplicon (counts per minute of excised amplicons) indicates a decrease in Ob-Rb expression in the hypothalamus of an obese mouse similar (up to one-third) to that described in Table 4 for another experiment with the same hypothalamic RNAs as starting material, in which the values are expressed as pixels. (B) Coamplification of Ob-Rb and GAPDH in the hippocampus, mesencephalon, spinal cord, and hypothalamus of an infected obese Swiss mouse versus those of a sham-inoculated control (13 months p.i.) shows dramatic downregulation of the leptin receptor in the hypothalamus of the obese mouse concomitant with upregulation in other brain viral target structures. The data for the downregulation in the hypothalamus of the obese mouse (concomitant with upregulation in other brain structures) agree with those shown for obese Swiss mouse brains at 5 months p.i. (Table 4). 1 and 2, mRNA expression in an uninfected and an infected neuroblastoma cell line, respectively. (C) Expression of the leptin receptor Ob-R (common isoform) in the mesencephala, hypothalami, and hippocampi of infected obese and infected nonobese mice expressed relative to sham-inoculated Swiss mice (corresponding to 100%) at 5 months p.i. Similar levels are expressed irrespective of the mouse strain and brain structure, e.g., (averages as percentages of the controls), 104.8 ± 7.4, 111.5 ± 11.5, and 104.6 ± 17.9 for the mesencephalon, hypothalamus, and hippocampus, respectively.
TABLE 4.
Brain leptin receptor (Ob-Rb) expression in brain structures of Swiss mice during the persistent stage of infection (5 months p.i.)a
| Mouse type | Expression (%) in:
|
||
|---|---|---|---|
| Cortex | Hippocampus | Hypothalamus | |
| Sham | 31 | 45 | 84 |
| Infected lean | 39 | 47 | 75 |
| Infected lean | 39 | 49 | 71 |
| Infected obese | 54 | 71 | 56 |
| Obese/sham (%) | 174 | 157 | 67 |
RT-PCR was carried out by coamplification of Ob-Rb and GAPDH. The products were loaded and then transferred (Southern blotting) and hybridized with a specific radiolabeled internal probe. The labeling of amplicons was quantified by image analysis (NIH Image software), and Ob-Rb was expressed as relative units of Ob-Rb–GAPDH (normalized values). Modulation of Ob-Rb corresponds to the expression in brain structures of infected mice compared to that in the matched structures of sham-inoculated mice (as a percentage) and indicates a downregulation of Ob-Rb in the hypothalamus of the obese mouse, whereas its expression increases in the other brain structures. The data are representative of two sham-inoculated mice (pooled structures), two separate infected lean mice, and one obese mouse.
TABLE 5.
Leptin receptor expression (Ob-Rb) in various brain structures during the acute stage of infection (14 days p.i.)a
| Mouse type | Expression (%) in:
|
||
|---|---|---|---|
| Cortex | Hippocampus | Hypothalamus | |
| Sham | 39 | 71 | 85 |
| Infected | 82 | 105 | 168 |
| Infected/sham (%) | 210 | 147 | 284 |
Total RNAs were extracted from each pooled structure from three sham-inoculated and three infected mice. DNA products were loaded, transferred (Southern blotting), and hybridized with a specific radiolabeled internal probe. Densitometric analysis was performed after autoradiography and quantified by image analysis (NIH Image software). Ob-Rb expression was calculated as the Ob-Rb/GAPDH ratio in structures from infected mice relative to that in the same structures from noninfected mice (normalized values). Note the upregulation of Ob-Rb expression in the brain structures of infected versus sham-inoculated mice (almost twofold in the cortex and hypothalamus). The data for the sham-inoculated mice showed similar leptin receptor expression in the acute and late stages of infection in the cortex (39/36.3 ± 4.6) and hypothalamus (85/76.6 ± 5.6).
FIG. 6.
RT-PCR coamplification of leptin receptor Ob-Rb and GAPDH genes during the acute stage of infection. Total RNAs were extracted from the hippocampi, cortexes, cerebella, and hypothalami of three sham-inoculated and three infected mice. RT was performed on 0.5 μg of total pooled RNA, which was then diluted 1/10 and subjected to 28 cycles of PCR (95°C for 45 s, 60°C for 45 s, and 72°C for 1 min), using 3 mM MgCl2. Note the upregulation of Ob-Rb expression in the hypothalami of infected mice compared to that in sham-inoculated mice. The results for each brain structure (ratio of Ob-Rb counts per minute to GAPDH counts per minute in infected structures relative to those in sham-inoculated mouse structures) are given in Table 5.
In conclusion, the decrease in expression of the long spliced variant, Ob-Rb, could be demonstrated only in the hypothalami of obese mice. This downregulation of Ob-Rb, which is considered to be the functional receptor responsible for transducing the signal in the hypothalamus, suggests that either selective dysfunction or loss of a subset of hypothalamic neurons, or both, might be induced by CDV infection.
Blood leptin and insulin levels.
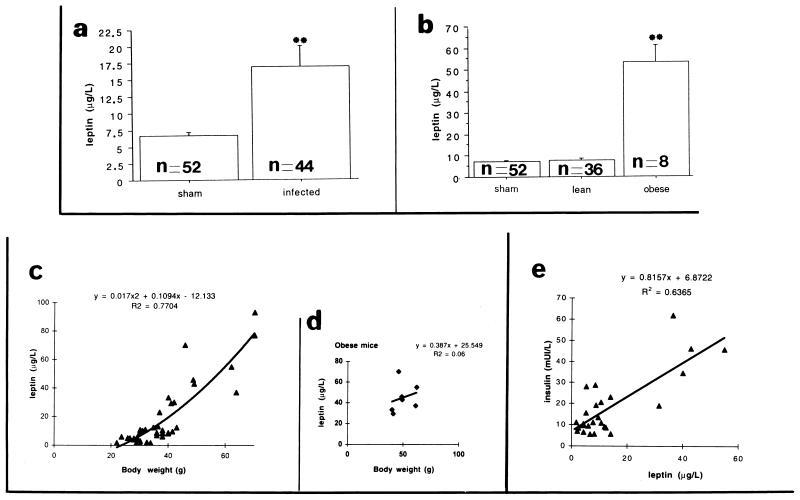
It was possible that leptin might be implicated in the appearance and maintenance of obesity, either by changes in its concentration or following alteration or mutation of the functional hypothalamic receptor (60, 63). To further clarify the effect of CDV infection and any changes in the hypothalamic areas involved in leptin negative feedback on body weight, serum leptin levels were measured in infected obese (n = 8) or lean (n = 36) mice and sham-inoculated (n = 52) mice, with a wide range of bodyweights and at different postinoculation times (up to 13 months p.i.).
As shown in Fig. 7, the average basal leptin levels in sham-inoculated or infected mice (taking all infected mice together) were 6.7 (n = 52) and 16.9 (n = 44) μg/liter, respectively (Fig. 7a). When the values for lean and obese infected mice were considered separately, a significant difference was seen between sham-inoculated and lean infected mice (7.1 [n = 52] and 7.8 [n = 36] μg/liter, respectively) and obese mice (53.4 μg/liter [n = 8]) (Fig. 7b). The figure also shows the weak correlations between leptin and insulin levels (R2 = 0.6365 [Fig. 7e]) and leptin level and body weight (R2 = 0.7704 [Fig. 7c]) when all samples are considered. Furthermore, in obese mice, blood leptin level seemed to be independent of body weight (R2 = 0.06 [Fig. 7d]). In agreement with other rodent models of obesity (either genetic deficits or hypothalamic injury) (35, 36), leptin levels were significantly increased in mice rendered obese by CDV infection. Certain features, such as hyperleptinemia and hyperinsulinemia, seem to be consistently present in all studies of obese mice.
FIG. 7.
Effect of CDV infection on blood leptin levels over the following 13 months. The data are the means ± standard error for uninfected (n = 52) and infected Swiss mice, either taken as a whole (n = 44) (a) or separated into lean (n = 36) and obese (n = 8) groups (b). The average basal leptin levels in sham-inoculated and infected mice (taking all infected mice together) were 6.7 and 16.9 μg/liter, respectively (a). When the values for lean and obese infected mice were considered separately, a marked difference was seen between sham-inoculated and infected lean mice (7.1 and 7.8 μg/liter, respectively) and obese mice (53.4 μg/liter) (b). A significant difference (P < 0.0001) was found between obese and sham-inoculated or obese and lean mice by analysis of variance, Fisher, and Scheffe tests. The data show the relationship between blood insulin (results for 26 samples) and leptin levels (e) and between blood leptin level and body weight (c and d), respectively; no strict correlation is seen between insulin and leptin levels (R2 = 0.6365) or leptin levels and body weight (R2 = 0.7704). Moreover, there is no strict relationship between blood leptin levels and body weight (R2 = 0.06) for the seven samples obtained from the obese mice analyzed (d), indicating an independent change in these two parameters.
DISCUSSION
Several months after intracerebral inoculation of mice with a highly neurovirulent CDV strain, an obesity syndrome was seen in a substantial portion of the surviving mice. The obesity syndrome was unambiguously related to viral replication, since vaccination against CDV protected the mice from challenge-mediated acute and late disease (54, 64). Moreover, obesity was never observed when CDV could not replicate in the brain (a nonneuroadapted strain or absence of viral transport from peripheral sites of injection to the brain). Anatomical and physiological evidence support the involvement of the CDV-infected hypothalamus in the occurrence of this late and progressive neuroendocrinological disease. In other models, it has been suggested that viral infection and subsequent viral persistence may alter differentiated functions of neural cells even in the absence of inflammation, cell lysis, or infiltrating immune cells (5, 16, 18, 30, 34, 41, 42). In the dog (its natural host), CDV causes acute noninflammatory demyelination and late paralysis due to a persistent virus infection, giving rise to motor and behavioral disturbances several weeks, months, or even years after the initial systemic disease (10, 39, 47).
We have previously shown that, at an early stage, CDV targets selected brain structures, such as the hippocampus, catecholaminergic nuclei, and hypothalamic nuclei, regardless of the site of injection in the brain (6). CDV infection of the hypothalamus may be a key event in the pathogenesis of the slow and progressive obesity syndrome. Thus, high levels of viral material were expressed in the hypothalami of all CDV-infected mice and low levels of viral transcripts were detected in the hypothalami of obese mice up to 1 year after inoculation, during the late stage of the disease. In addition, the progressive decrease in the CDV transcriptional rate in the hypothalamus mainly affects the expression of mRNAs coding for F protein rather than the nucleoprotein. In fact, the F surface protein of morbilliviruses plays an essential role in infectivity, enabling the virus to fuse with the host cell membrane and to spread rapidly from cell to cell. Recent molecular genetic studies have unraveled a range of mechanisms by which defective expression of M, HA, or F proteins may lead to viral persistence in brain cells under conditions not allowing its identification by immune surveillance mechanisms. Thus, the decrease in the level of F gene mRNAs could be a prerequisite for development of CDV persistence, as demonstrated in SSPE, a human infection caused by the measles virus, which is closely related to CDV (33). Using this strategy, CDV could escape immunologic surveillance, which would explain why we have been unable to see any hallmarks of inflammation, i.e. glial activation or infiltrating cells, in the hypothalami of acutely diseased or late-diseased mice. The hypothalamus, by segregating viral material, may therefore act as a reservoir of “hidden” virus in the brain. This noncytolytic CDV infection of the hypothalamus could, in turn, impede specific hypothalamic functions, leading to obesity. A similar scenario has been proposed for the effect of lymphocytic choriomeningitis virus infection and persistence on pituitary function, leading to disordered growth hormone synthesis, growth retardation, and hypoglycemia (18, 42).
Although obesity is a complex disease triggered by intrinsic (mutations of single or multiple genes) or extrinsic (hormones or feeding) factors (32, 58, 62), the role of the hypothalamus in the occurrence of the obese phenotype has been clearly demonstrated by its chemical or surgical destruction (12, 26, 44, 61). CDV-induced obesity seems to be independent of the genetic characteristics of the mouse strain, since both outbred (Swiss) and inbred (BALB/c and C3H) mice exhibited similar CDV-induced clinical features. Intriguingly, obese mice had elevated levels of leptin, an adipose tissue-derived secreted hormone, in the blood (66). Leptin, structurally related to cytokines (67), is a satiety factor encoded by the ob gene and secreted almost exclusively by adipocytes. Acting by inhibiting eating behavior, it exerts pleiotropic effects in the periphery (mainly in the regulation of adipose tissue mass) and in the brain, where it acts through several hypothalamic effectors (58). Increased serum leptin levels have also been described in genetic or chemical-induced rodent or human obesity, and they correlate with the body mass index (36). The appearance of obesity, despite high levels of circulating leptin, in CDV-infected obese mice suggests an ineffective leptin response in the brain. The inefficiency of leptin in counteracting the occurrence of obesity may be due to a deficiency of transport into the brain (2) or to defective brain receptors (14). In fact, leptin regulates body weight via brain targets, and five isoforms of the leptin receptor (Ob-R), encoded by a single gene, are predicted to exist. Mutation, abnormal dimerization, affinity changes, postreceptor signal transduction, or effector response changes of leptin receptors may compromise the effectiveness of leptin (9, 17, 32, 59). Interestingly, expression of Ob-Rb, the long isoform implicated in the functional effect of leptin, was mostly found in those hypothalamic nuclei (VMH, PVN, and ARN) which contained CDV products during the early stage of infection.
During the acute stage of CDV infection, Ob-Rb gene mRNAs were upregulated by viral replication in all CDV-targeted structures. This upregulation might be related to the presence of proinflammatory cytokines induced by CDV replication during the acute stage of infection (4). These cytokines might regulate the metabolism of the whole body and food intake by acting on both ob gene transcription (27, 28, 46) and the electrical activity of hypothalamic glucose-sensitive neurons (19). In addition, Ob-Rb shows substantial homology with the signaling domain (gp130) of the type I cytokine receptor family, which transduces the signal via the Jak-STAT pathway (3, 32, 58, 60). Thus, leptin and cytokines, which have similar receptors, might contribute to leptin receptor upregulation in neural cells. During the late stage of infection, Ob-Rb expression was dramatically decreased in the hypothalami of obese mice while its expression was unchanged in the hypothalami of infected nonobese mice compared with that in sham-inoculated mice. The specific decrease in Ob-Rb gene mRNA levels in the hypothalami of obese mice is probably crucial in triggering the obesity syndrome, since a similar decrease has been seen in the hypothalami of mice rendered obese by hypothalamic lesions with gold thioglucose (23). CDV per se might downregulate leptin receptor expression in the hypothalamus, since it is able to downregulate other membrane receptors, such as endothelin and adrenoreceptors (31, 37). Other mechanisms connected to hypothalamic infection with CDV, such as a decrease in the transcriptional rate of the Ob-Rb gene, degradation of Ob-Rb gene mRNAs, leading to affinity changes, neuronal death of a subset of cells expressing Ob-Rb, and high leptin levels that can hyperpolarize glucose-receptive hypothalamic neurons (55), could be also involved in hypothalamic leptin network impairment. On the other hand, the possibility that other CDV-impaired hypothalamic intrinsic factors, such as neuropeptides or monoamines, are indirectly involved in the Ob-Rb decrease and in the genesis of CDV-induced obesity in mice cannot be ruled out, since (i) pharmacological or lesional disturbances of hypothalamic catecholaminergic neurons contribute to the development of obesity (56, 57), (ii) Ob-Rb can be colocalized in hypothalamic neurons synthesizing catecholamines (24), and (iii) a decrease in catecholamine synthesis in the hypothalami of mice rendered obese by CDV infection has been described (reference 40 and unpublished results). Even if several mechanisms could be involved in CDV-induced obesity, the reason for the selective hypothalamic vulnerability of certain mice to CDV infection, with some infected mice escaping brain-mediated disease, remains to be fully investigated.
In conclusion, CDV infection might alter hypothalamic integrity and, subsequently, the leptin network. The marked and sustained obesity induced by CDV infection in mice is a unique paradigm for understanding how environmental factors, such as viruses, may alter hypothalamic homeostasis and trigger brain-mediated disorders. Currently, human obesity is among the most important health risk factors in developed countries; our data, obtained from animals, suggest that certain neuroendocrinological human diseases could be related by a history of viral infection (21).
ACKNOWLEDGMENTS
We thank Hubert Vidal for advice and stimulating discussion. We are grateful to Tom Barkas for critical evaluation of the English.
This work was supported by grants from INSERM-INRA and the Rhône-Alpes Region.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, et al. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. Analysis of RNA by Northern and slot blot hybridization; pp. 1–14. [Google Scholar]
- 2.Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 3.Baumann H, Morella K K, White D W, Dembski M, Baillon P S, Kim H, Lai C E, Tartaglia L A. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. doi: 10.1016/0165-5728(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 5.Bencsik A, Akaoka H, Giraudon P, Belin M F, Bernard A. Inhibition of tyrosine hydroxylase expression within the substantia nigra of mice infected with canine distemper virus. J Neuropathol Exp Neurol. 1997;56:673–685. [PubMed] [Google Scholar]
- 6.Bernard A, Fèvre-Montange M, Bencsik A, Giraudon P, Wild T, Confavreux C, Belin M F. Brain structures selectively targeted by canine distemper virus in a mouse model of infection. J Neuropathol Exp Neurol. 1993;52:471–480. doi: 10.1097/00005072-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bernard A, Zwingelstein G, Meister R, Wild T F. Hyperinsulinemia induced by canine distemper virus infection of mice and its correlation with the appearance of obesity. Comp Biochem Physiol. 1988;91B:691–696. doi: 10.1016/0305-0491(88)90193-9. [DOI] [PubMed] [Google Scholar]
- 8.Bernard A, Wild T F, Tripier M F. Canine distemper infection in mice: characterization of a neuro-adapted virus strain and its long-term evolution in the mouse. J Gen Virol. 1983;64:1571–1579. doi: 10.1099/0022-1317-64-7-1571. [DOI] [PubMed] [Google Scholar]
- 9.Bjorbaek C, Elmquist J K, Frantz J D, Shoelson S E, Flier J S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 10.Blakemore W F, Summers B A, Appel M J G. Evidence of oligodendrocyte infection and degeneration in canine distemper encephalomyelitis. Acta Neuropathol. 1989;77:550–553. doi: 10.1007/BF00687258. [DOI] [PubMed] [Google Scholar]
- 11.Bobrow M N, Harris T D, Shaughnessy K J, Litt G J. Catalyzed reporter deposition, a novel method for signal amplification. Application to immunoassays. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 12.Bray G A, York D A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59:719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- 13.Campfield L A, Smith F J, Gulsez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 14.Caro J F, Kolaczynski J W, Nyce M R, Ohannesian J P, Opentanova I, Goldman W H, Lynn R B, Zhang P L, Sinha M K, Considine R V. Decreased cerebrospinal fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo R, Redmann G, Baczko K, Ter Meulen V, Billiter M. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987;160:523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- 16.Ceccaldi P E, Fillion M P, Ermine A, Tsiang H, Fillion G. Rabies virus selectively alters 5-HT1 receptor subtypes in rat brain. Eur J Pharmacol. 1993;245:129–138. doi: 10.1016/0922-4106(93)90120-x. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, Duyk G M, Tepper R I, Morgenstern J P. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 18.de La Torre J C, Borrow P, Oldstone M B A. Viral persistence and disease: cytopathology in the absence of cytolysis. Br Med Bull. 1991;47:838–851. doi: 10.1093/oxfordjournals.bmb.a072515. [DOI] [PubMed] [Google Scholar]
- 19.De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995;27:15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- 20.Devos R, Richards J G, Campfield L A, Tartaglia L A, Guisez Y, van der Heyden J, Tavernier J, Plaetinck G, Burn P. OB protein binds specifically to the choroid plexus of mice and rats. Proc Natl Acad Sci USA. 1996;93:5668–5673. doi: 10.1073/pnas.93.11.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhurandhar N V, Kulkarni P R, Ajinkya S M, Sherikar A A, Atkinson R L. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 22.Dunn A J, Powell M L, Moreshead W V, Gaskin J M, Hall N R. Effects of Newcastle disease virus administration to mice on the metabolism of cerebral biogenic amines, plasma corticosterone and lymphocyte proliferation. Brain Behav Immun. 1987;1:216–230. doi: 10.1016/0889-1591(87)90024-9. [DOI] [PubMed] [Google Scholar]
- 23.Fei H, Okano H J, Li C, Lee G H, Zhao C, Darnell R, Friedman J M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakansson M L, Brown H, Ghilardi N, Skoda R C, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasel K W, Sutcliffe J G. Nucleotide sequence of a cDNA coding for mouse cyclophilin. Nucleic Acids Res. 1990;18:4019. doi: 10.1093/nar/18.13.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hervey G R. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959;145:336–352. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holden R J, Pakula I S. The role of tumor necrosis factor α in the pathogenesis of anorexia and bulimia nervosa, cancer cachexia and obesity. Med Hypotheses. 1996;47:423–438. doi: 10.1016/s0306-9877(96)90153-x. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil G S, Sargill N S, Spiegelman B M. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 29.Huang X F, Koutcherov I, Lin S, Wang H Q, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- 30.Klavinskis L, Oldstone M B A. Lymphocytic choriomeningitis virus selectively alters differentiated but not housekeeping functions: block in expression of growth hormone gene is at the level of transcriptional initiation. Virology. 1989;168:232–235. doi: 10.1016/0042-6822(89)90262-6. [DOI] [PubMed] [Google Scholar]
- 31.Koschel K, Muenzel P. Persistent paramyxovirus infections and behaviour of beta-adrenergic receptors in C6 rat glioma cells. J Gen Virol. 1980;47:513–517. doi: 10.1099/0022-1317-47-2-513. [DOI] [PubMed] [Google Scholar]
- 32.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 33.Liebert U G, Baczko K, Budka H, ter Meulen V. Restricted expression of measles virus protein in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986;67:2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- 34.Lipkin W, Battenberg E L F, Bloom F E, Oldstone M B A. Viral infection of neurons can depress neurotransmitter mRNA levels without histologic injury. Brain Res. 1988;451:333–339. doi: 10.1016/0006-8993(88)90779-2. [DOI] [PubMed] [Google Scholar]
- 35.Maffei M, Fei H, Lee G H, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman J M. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maffei M, Halaas J, Ravusin E, Pratley R E, Lee G H, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern P A, Friedman J M. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 37.Meissner N N, Koschel K. Down regulation of endothelin receptor mRNA synthesis in C6 rat astrocytoma cells by persistent measles virus and canine distemper virus infections. J Virol. 1995;69:5191–5194. doi: 10.1128/jvi.69.8.5191-5194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercer J G, Hoggard N, Williams L M, Lawrence C B, Hannah L T, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 39.Müller C E, Fatzer R S, Beck K, Vandevelve M, Zurbriggen A. Studies on canine distemper virus persistence in the central nervous system. Acta Neuropathol. 1995;89:438–445. doi: 10.1007/BF00307649. [DOI] [PubMed] [Google Scholar]
- 40.Nagashima K, Zabriskie J, Lyons M. Virus-induced obesity in mice: association with a hypothalamic lesion. J Neuropathol Exp Neurol. 1992;51:101–109. doi: 10.1097/00005072-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Oldstone M B A, Sinha Y N, Blount P, Tishon A, Rodriguez M, von Wedel M, Lampert P W. Virus induced alterations in homeostasis: alterations in differentiated functions of infected cells in vivo. Science. 1982;218:1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- 42.Oldstone M B A, Rodriguez M, Daughaday W, Lampert P. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature. 1984;307:278–281. doi: 10.1038/307278a0. [DOI] [PubMed] [Google Scholar]
- 43.Oliver K R, Brennan P, Fazakerley J K. Specific infection and destruction of dopaminergic neurons in the substantia nigra by the Theiler’s virus. J Virol. 1997;71:6179–6182. doi: 10.1128/jvi.71.8.6179-6182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olney J W. Brain lesions, obesity and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London, England: Academic Press; 1986. [Google Scholar]
- 46.Plata-Salaman C R, Vasselli J R, Sonti G. Differential responsiveness of obese (fa/fa) and lean (Fa/Fa) Zucker rats to cytokine-induced anorexia. Obes Res. 1997;5:36–42. doi: 10.1002/j.1550-8528.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 47.Raine C S. On the development of CNS lesions in natural canine distemper virus encephalomyelitis. J Neurol. 1976;30:13–28. doi: 10.1016/0022-510x(76)90251-3. [DOI] [PubMed] [Google Scholar]
- 48.Rentrop M, Knapp B, Winter H, Schweizer J. Amino alkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986;18:271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- 49.Rozenblatt S, Eizenberg O, Ben-Levy R, Lavie V, Bellini W J. Sequence homology within morbilliviruses. J Virol. 1985;53:684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabath D E, Broome H E, Prystowsky M B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin-2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. Identification of targets of leptin action in rat hypothalamus. J Clin Investig. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidhu M S, Husar W, Cook S D, Dowling P C, Udem S A. Canine distemper terminal and intergenic non-protein coding nucleotide sequences: completion of the entire CDV genome sequence. Virology. 1993;193:66–72. doi: 10.1006/viro.1993.1103. [DOI] [PubMed] [Google Scholar]
- 53.Sidman R L, Angevine J B, Pierce E T. Atlas of the mouse brain and spinal cord. Cambridge, Mass: Harvard University Press; 1971. [Google Scholar]
- 54.Sixt N, Cardoso A, Vallier A, Fayolle J, Buckland R, Wild T F. Canine distemper virus DNA vaccination induces humoral and cellular immunity and protects against a lethal intracerebral challenge. J Virol. 1998;72:2516–2518. doi: 10.1128/jvi.72.11.8472-8476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spanswick D, Smith M A, Groppi V E, Logan S D, Ashford M L J. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1998;392:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi A, Ishimaru H, Ikarashi Y, Maruyama Y. Aspects of hypothalamic neuronal systems in VMH lesion-induced obese rats. J Autonom Nerv Syst. 1994;48:213–219. doi: 10.1016/0165-1838(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi A, Ishimaru H, Ikarashi Y, Maruyama Y. Decreased norepinephrine and preservation of acetylcholine in the hypothalamus of VMH obese rats. Brain Res Bull. 1995;36:97–99. doi: 10.1016/0361-9230(94)00173-x. [DOI] [PubMed] [Google Scholar]
- 58.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K J, Smutko J S, Mays G G, Woolf E A, Monroe C A, Tepper R I. Identification and expression cloning of a leptin receptor, Ob-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 59.Ur E, Grossman A, Després J P. Obesity results as a consequence of glucocorticoid induced leptin resistance. Horm Metab Res. 1996;28:744–747. doi: 10.1055/s-2007-979891. [DOI] [PubMed] [Google Scholar]
- 60.Vaisse C, Halaas J L, Horvath C M, Darnell J E, Stoffel M, Friedman J M. Leptin activation of STAT3 in hypothalamus of wild type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 61.Weingarten H P, Chang P K, McDonald T J. Comparison of the metabolic and behavioral disturbances following paraventricular and ventromedial-hypothalamic lesions. Brain Res Bull. 1985;14:551–559. doi: 10.1016/0361-9230(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 62.West D. Genetics of obesity in human and animal models. Obesity. 1996;25:801–813. doi: 10.1016/s0889-8529(05)70355-8. [DOI] [PubMed] [Google Scholar]
- 63.White D W, Kuropatwinski K K, Devos R, Baumann H, Tartaglia L A. Leptin receptor (Ob-R) signaling. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 64.Wild T F, Bernard A, Spehner D, Villeval D, Drillien R. Vaccination of mice against canine distemper virus induced encephalitis with vaccinia virus recombinants encoding measles or canine distemper virus antigens. Vaccine. 1993;11:438–444. doi: 10.1016/0264-410x(93)90285-6. [DOI] [PubMed] [Google Scholar]
- 65.Yamanouchi K. Comparative aspects of pathogenicity of measles, canine distemper, and rinderpest viruses. Jpn J Med Sci Biol. 1980;33:41–66. doi: 10.7883/yoken1952.33.41. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Proenca R, Maffie M, Barone M, Leopold L, Friedman J M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Basinski M B, Beals J M, Briggs S L, Churgay L M, Clawson D K, DiMarchi R D, Furman T C, Hale J E, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;372:425–432. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 68.Zurbriggen A, Graber H U, Wagner A, Vandevelde M. Canine distemper virus persistence in the nervous system is associated with noncytolytic selective virus spread. J Virol. 1995;69:1678–1686. doi: 10.1128/jvi.69.3.1678-1686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]