Abstract
Purpose:
Minimally invasive biomarkers have been used as important indicators of treatment response and progression in cancers such as prostate and ovarian. Unfortunately, all biomarkers are not prognostic in all cancer types and are often not routinely collected. Patient-reported outcomes (PRO) provide a non-obtrusive, personalized measure of a patient's quality of life and symptomatology, reported directly from the patient, and are increasingly collected as part of routine care. Previous literature has shown correlations between specific PROs (i.e., insomnia, fatigue) and overall survival. Although promising, these studies often only consider single time points and ignore patient-specific dynamic changes in individual PROs, which might be early predictors of treatment response or progression.
Experimental Design:
In this study, PRO dynamics were analyzed to determine if they could be used as interradiographic predictors of tumor volume changes among 85 patients with non–small cell lung cancer undergoing immunotherapy. PRO questionnaires and tumor volume scans were completed biweekly and monthly, respectively. Correlation and predictive analysis were conducted to identify specific PROs that could accurately predict patient response.
Results:
Changes in tumor volume over time were significantly correlated with dizziness (P < 0.005), insomnia (P < 0.05), and fatigue (P < 0.05). In addition, cumulative changes in insomnia could predict progressive disease with a 77% accuracy, on average 45 days prior to the next imaging scan.
Conclusions:
This study presents the first time that patient-specific PRO dynamics have been considered to predict how individual patients will respond to treatment. This is an important first step in adapting treatment to improve response rates.
Translational Relevance.
Current practice for most cancer types relies on invasive methods such as CT and MRI scans to follow a patient's disease trajectory. Patient-reported outcomes (PRO) provide a non-obtrusive, personalized measure of a patient's symptomatology and quality of life, reported directly from the patient. Prior studies have indicated associations of PROs with progression-free and overall survival. The goal of this was to evaluate whether changes in PROs could be used dynamically as interradiographic predictors of treatment response in non–small cell lung cancer (NSCLC). PRO and volume data from 85 patients with NSCLC undergoing immunotherapy were analyzed. Analysis found that changes in insomnia are correlated with volume dynamics and can predict progressive disease with a 77% accuracy, on average 45 days before the next volume scan. This study marks the first of our knowledge to dynamically utilize individual PROs as an early predictor of volume changes.
Introduction
Minimally invasive biomarkers such as PSA or CA-125 are easily accessible markers of tumor burden in prostate and ovarian cancers, respectively (1–3). Similar biomarkers are used in other cancer types, both for diagnostic and treatment response assessment. Tumor burden metrics are especially important for patients with lung cancer, who have some of the lowest survival rates among people with cancer. Of these patients, non–small cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer diagnoses. As most NSCLC develops without symptoms, 47% of patients present with advanced stage disease at diagnosis (4). Immunotherapy has been shown to exhibit durable and long-term survival benefit in 20% to 50% of patients with advanced stage NSCLC (5–7). Although blood protein markers such as tumor-associated antigens and autoantibodies have been identified as useful biomarkers in the diagnosis of lung cancer (8), few have been shown to allow for longitudinal assessment of treatment response in advanced lung cancer. Such biomarkers are crucially needed to predict, as early as possible, when patients are experiencing disease progression so treatment can be adapted accordingly.
Patient-reported outcomes (PRO) are quantitative measures of a patient's quality of life or symptomatology that are self-reported by the patient without interpretation from third parties (9). PROs are routinely collected using standardized questionnaires at various time points throughout a patient's care and are distinct from typical toxicity measures reported by the clinician. In addition to enabling patients to have more active roles in their treatment, the comprehensive evaluation of symptoms (e.g., fatigue, nausea, shortness of breath, and headache) provides a more holistic view of the patient experience that is often ignored when only considering traditional toxicity measures (9). The inclusion of PROs in routine clinical care continues to emerge as an important metric in precision medicine care among patients with cancer, which can be used in combination with standard biomarker and imaging data.
Recent studies have shown that temporal changes in PROs can be early indicators of clinically important events such as cancer development (10, 11), treatment progression (12, 13), and survival (14–16). A phase III randomized trial completed in 2017 compared a web-mediated follow-up system based on weekly self-scored patient symptoms versus routine surveillance among patients with advanced lung cancer. An alert email was automatically sent to the oncologist when self-scored symptoms matched predefined criteria for possible progression. Cancer progression was detected 5 weeks earlier and overall survival (OS) was extended by 9 months in the PRO group compared with the routine surveillance group (17).
The investigators presented a clinical example of 1 patient who benefited from the web-mediated follow-up in a subsequent 2018 paper (18). They demonstrated how the web-mediated follow-up helped to detect pulmonary embolism occurrence, relapse, and pseudo-progression to immunotherapy via PRO monitoring. Interestingly, imaging showed progressive disease despite an improvement in the patient's anorexia, dyspnea, and fatigue—all suggesting a good response to treatment. Treatment was continued and follow-up imaging showed tumor regression, confirming pseudoprogression. The web-mediated follow-up allowed the patient to remain on treatment for an additional year when imaging alone could have led physicians to stop an effective therapy (18).
The findings from Denis and colleagues demonstrate the value of PROs in predicting patient responses to treatment. Here, we expand upon this work to investigate whether changes in PROs could be used dynamically as interradiographic predictors of treatment response in NSCLC. We hypothesize that patient-specific changes in insomnia can be used as an early predictor of progressive disease. The predictive power is assessed in 85 patients with NSCLC undergoing immunotherapy.
Materials and Methods
Clinical data and study design
Patients with NSCLC starting treatment with an immune checkpoint inhibitor at Moffitt Cancer Center were recruited between July 2017 and December 2019. Eligibility criteria included: (i) diagnosed with NSCLC, (ii) presence of a radiographically assessable disease at baseline, (iii) scheduled treatment with an immune checkpoint inhibitor, (iv) ability to read and speak English, (v) access to the Internet, and (vi) ability to provide informed consent. Written informed consent was obtained online or in person.
This study was conducted in accordance with the U.S. Common Rule and the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board (Advarra, Inc).
Patients were administered an item bank assessing 27 symptoms including symptomatic toxicities of immunotherapy (e.g., fatigue, muscle pain, rash) and symptoms associated with cancer progression (e.g., hemoptysis, pain, hoarseness, swelling of the face, fever, itchiness; ref. 19). Patients completed PRO surveys every 14 days using a 5-point interference response Likert scale (0 = not at all and 4 = very much).
Tumor volumes were measured from standard-of-care computerized tomography (CT) scans approximately every 30 days. Pseudo-progression seen during immunotherapy can be difficult to distinguish from actual disease progression. As such, treatment evaluation was completed using iRECIST criteria in line with the mode of treatment received (20). Under iRECIST criteria, progressive disease (PD) is defined as a 20% increase above the nadir of the sum of the maximum diameters of target lesions. This increase must be confirmed by a further increase in size at the next imaging assessment for patients treated with immunotherapy, resulting in the classification of confirmed progressive disease (iCPD) from unconfirmed progressive disease (iUPD; ref. 21). A 20% increase in the longest diameter translates to about a 73% increase in the tumor volume (22, 23).
Data analysis
The association between changes in tumor volume and individual symptoms was analyzed by pairing all survey responses (collected biweekly) taken within 1 week of volume assessment times (assessed monthly). To account for symptom severity differences between patients, each PRO was normalized to an individual patient's maximum severity experienced throughout the study. That is, if a patient's maximum severity for a particular symptom was four, each value was divided by four for this symptom such that his/her responses were within {0, 0.25, 0.5, 0.75, 1}. In line with prior PRO studies, the Pearson correlation coefficient was used to measure correlation between tumor volume responses and patient-reported symptoms throughout a patient's treatment. Changes between scans for PROs and volumes were then assessed in comparison to the previous paired data points. The Fisher exact test was used to determine the significance of associations between increases/decreases in volume and patient-reported symptoms over time.
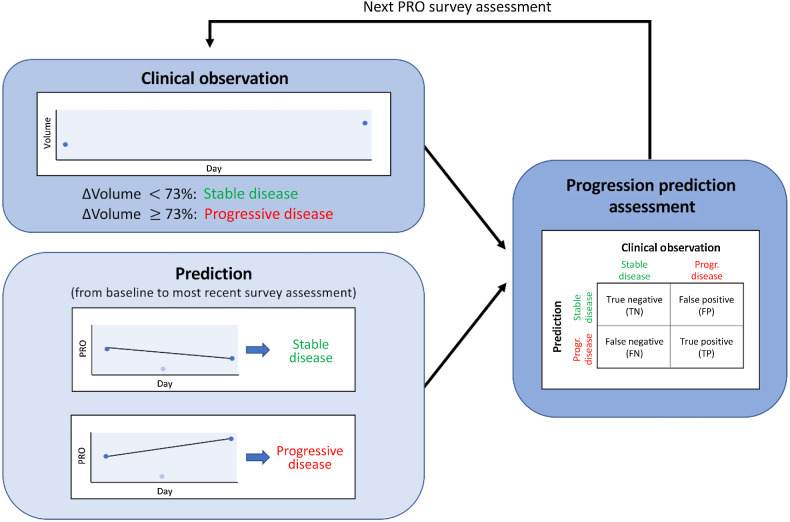
To evaluate the predictive potential of each PRO, we determined the change in each symptom from baseline to the most recent PRO assessment. Baseline is defined as the survey assessment obtained directly before the first volume within a prediction window. If the change did not indicate an increase in symptom severity (change less than or equal to zero), we predicted that the patient would continue to respond. Otherwise, iUPD was predicted. These predictions were compared with the actual tumor response at the next volume measurement. Predictions were made for each individual survey assessment between volumetric scans. The prediction flowchart is shown in Fig. 1.
Figure 1.
Prediction flowchart. Each clinical observation is classified as stable disease if the volume increases by less than 73% or progressive disease if volume increases by more than 73%. The change in the PRO is computed from the baseline survey assessment (first survey prior to baseline volume) to the most current survey assessment. If the PRO decreases or remains the same, stable disease is predicted. Otherwise, progressive disease is predicted. The prediction is compared to the clinical volume observation.
Data availability
The clinical data used to conduct this study are available upon reasonable request to the corresponding author (RBN).
Results
Patient demographics
Of the 108 patients who provided informed consent, 85 patients completed surveys and had corresponding volumetric measurements and thus were included in the current analyses. Demographic and clinical treatment information is provided in Supplementary Table S1. The mean patient age was 66.0 years (SD = 8.1 years). Most patients were female (53%), White (94%), and diagnosed with stage 4 NSCLC (84%). The treatment most frequently given was pembrolizumab (47% of patients); however, other treatments were utilized for specific patients, such as atezolizumab, which has been approved for patients with stage II NSCLC as adjuvant therapy for PD-L1+ tumors (24). Supplementary Table S2 shows the representativeness of the study participants compared with the general population of patients with NSCLC.
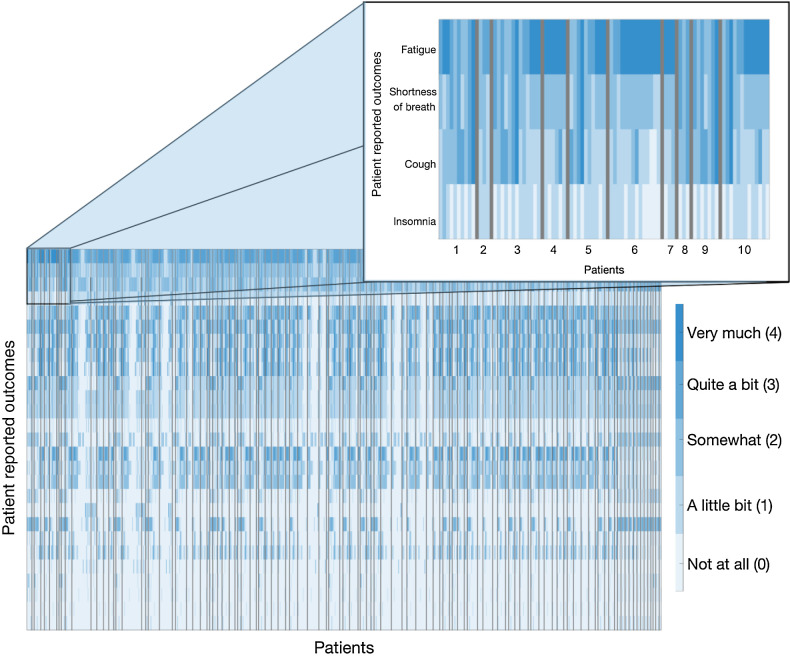
The average duration of patient participation was 170 days (SD = 115 days), with an average of 3 volume assessments (min = 2, max = 8) and 12 surveys completed (min = 3, max = 42) for each patient. Figure 2 presents the patient-specific responses for each symptom assessed throughout the duration of the study, ordered from most to least severe.
Figure 2.
Study patient-reported outcomes. Individual symptoms are separated by rows and are ranked here in descending order of average severity experienced. Patient responses are based on Likert scale ranging from “not at all” (light blue) to “very much” (dark blue). Responses for individual patients are separated by black lines. Longitudinal responses are shown for each patient (left to right).
Dizziness, insomnia, and fatigue are correlated with tumor volume
As previously noted, correlations were assessed using a Pearson correlation coefficient of PROs taken within 1 week of a tumor volume measurement, after being individually normalized to each patient's maximum on a 0 to 1 scale to account for variation in symptom tolerance. The strength and statistical significance of each correlation was assessed using the Pearson correlation coefficient. Our analysis found that volume was significantly correlated with dizziness (r = 0.27, P < 0.005), insomnia (r = 0.22, P = 0.01), and fatigue (r = 0.19, P = 0.03), indicating associations between volume and individual symptom severity. Statistically significant correlations were not found for the remaining symptoms. In line with previous studies, we also assessed the significance of the aggregate of dizziness, insomnia, and fatigue and found it not to be significantly correlated with volume. The correlation analysis results for the most severely experienced symptoms are presented in Table 1.
Table 1.
Correlation analysis between tumor volume and PROs for the most severely experienced symptoms.
| PRO | Correlation coefficient | P value | PRO | Correlation coefficient | P value |
|---|---|---|---|---|---|
| Dizziness | 0.27 | ** | Cough | −0.08 | 0.36 |
| Insomnia | 0.22 | * | Memory problems | 0.08 | 0.39 |
| Fatigue | 0.19 | * | Aching joints | −0.08 | 0.40 |
| Hand or feet numbness | −0.15 | 0.10 | Wheezing | −0.07 | 0.42 |
| Frequent urination | 0.15 | 0.10 | Concentration problems | 0.07 | 0.47 |
| Shortness of breath | 0.12 | 0.20 | Aching muscles | −0.05 | 0.61 |
| Nausea | −0.10 | 0.28 | Changes in the taste of food | −0.03 | 0.71 |
| Skin dryness | −0.09 | 0.32 | Itching | −0.03 | 0.75 |
| Sum of all PROs | −0.03 | 0.70 | Sum of dizziness, insomnia, and fatigue | 0.13 | 0.15 |
Changes in volume are associated with changes in insomnia
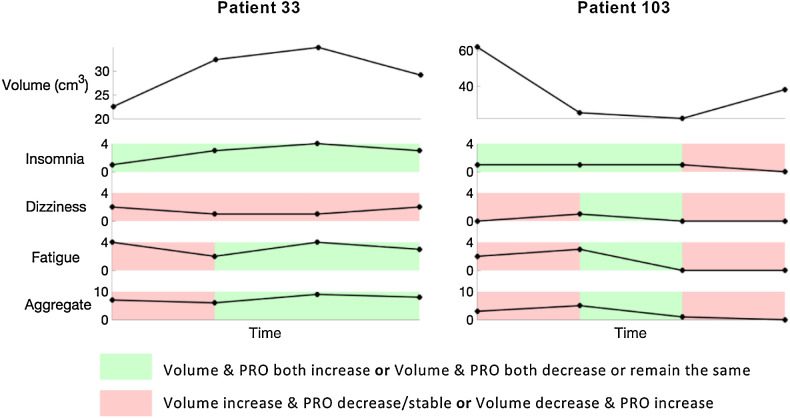
We next investigated whether individual changes in tumor volume across time were associated with changes in patients’ symptoms. The longitudinal changes of all paired tumor-volume measurements and PROs over time were assessed via a Fisher's exact test. Of all 27 symptoms, insomnia was the only symptom shown to be significantly associated with changes in volume (P = 0.019). Of interest, neither dizziness, fatigue, nor the symptom combination of the three resulted in a significant association (P = 0.41, 0.46, and 0.81, respectively). For illustrative purposes, results for Patients 33 and 103 are presented in Fig. 3. Patient 33 experienced two increases in volume, followed by a decrease. Changes in insomnia followed these tumor changes, whereas changes in dizziness, fatigue, and the combined symptoms did not. Tumor volume decreased at two assessments for Patient 103 and these dynamics matched well with changes in insomnia, but not in the other symptoms. Despite volume increasing at the last assessment, the patient reported a decrease in insomnia and no changes in dizziness, fatigue, or the combination of the three.
Figure 3.
Evaluation of association between volume and PRO changes. Response associations for Patients 33 and 103 for insomnia, dizziness, fatigue, and the combination of the three were analyzed using assessments taken within 1 week of each volume measurement. For Patient 33, changes in insomnia matched well with changes in volume. Changes in dizziness did not align with changes in volume, whereas fatigue and the aggregate had partial alignment. For Patient 103, initial changes in insomnia matched well with volume changes. The patient experienced a decline in all four PROs at the last assessment, despite an increase in volume.
Insomnia is an interradiographic predictor
To assess the predictive ability of PROs, we investigated if changes in PROs could predict when a patient would develop progressive disease. According to iRECIST, unconfirmed progressive disease (iUPD) first occurs when the sum of the longest diameters increases by more than 20% above the nadir, which translates to about a 73% increase in tumor volume. Upon confirmation via the subsequent increase in volume at the next scan, the disease is then classified as confirmed progressive disease (iCPD; see Materials and Methods for further details). Given a volume measurement, we determined the change from the first survey assessment following a tumor volume scan to each assessment after for all surveys taken between volume measurement i to measurement i + 1. If insomnia increased, iUPD was predicted at assessment i + 1. If insomnia decreased or remained the same, we predicted that the patient would have a continued response at i + 1. This process was repeated for all survey assessments recorded until the next volume measurement.
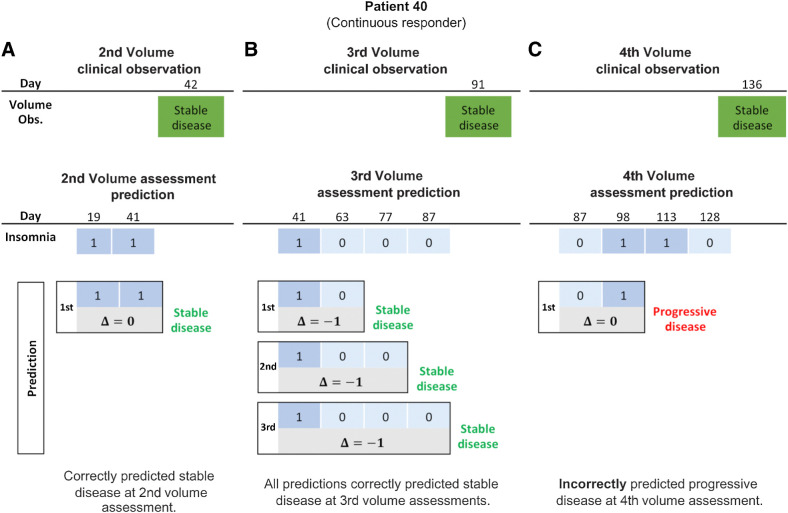
For illustrative purposes, the results for Patient 40 using insomnia as a predictor are shown in Fig. 4. Two surveys were completed between the first and second tumor assessments, maintaining a stable insomnia level of one (“a little bit”) for both. Thus, Δ = 1 − 1 = 0. Because Δ ≤ 0, we predicted that the patient would continue to respond. This was confirmed at the second volume assessment where the volume change indicated stable disease (Fig. 4A). Patient 40 completed three additional surveys between the second and third tumor measurements. From this, we see that
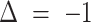 , so we predict that the patient will continue to respond. Comparing to the actual volume assessment at day 91, we see that the patient had stable disease. Thus, our prediction was correct (Fig. 4B). The third and fourth PRO assessments also show that
, so we predict that the patient will continue to respond. Comparing to the actual volume assessment at day 91, we see that the patient had stable disease. Thus, our prediction was correct (Fig. 4B). The third and fourth PRO assessments also show that
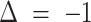 , further predicting stable disease which is confirmed by the volume assessement. The patient completed three surveys between the third and fourth tumor measurements. The change from the baseline insomnia at day 87 to the insomnia at day 98 showed an increase (
, further predicting stable disease which is confirmed by the volume assessement. The patient completed three surveys between the third and fourth tumor measurements. The change from the baseline insomnia at day 87 to the insomnia at day 98 showed an increase (
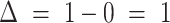 ), indicating progressive disease. However, this was incorrect as the volumetric response showed stable disease at day 136 (Fig. 4C).
), indicating progressive disease. However, this was incorrect as the volumetric response showed stable disease at day 136 (Fig. 4C).
Figure 4.
Response prediction for a continuous responder. A, Second assessment response prediction. The clinical volume observation showed stable disease at day 42. Stable insomnia on days 19 and 14 resulted in
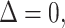 correctly predicting stable disease. B, Third assessment response prediction. The clinical volume observation showed stable disease at day 91. Predictions 1, 2, and 3 each correctly predicted stable disease. C, Fourth assessment response prediction. The clinical volume observation showed stable disease at day 136. Insomnia increased from 0 to 1 between days 87 and 98. Thus,
correctly predicting stable disease. B, Third assessment response prediction. The clinical volume observation showed stable disease at day 91. Predictions 1, 2, and 3 each correctly predicted stable disease. C, Fourth assessment response prediction. The clinical volume observation showed stable disease at day 136. Insomnia increased from 0 to 1 between days 87 and 98. Thus,
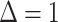 , predicting progressive disease. This was an incorrect prediction as the patient's volume showed stable disease.
, predicting progressive disease. This was an incorrect prediction as the patient's volume showed stable disease.
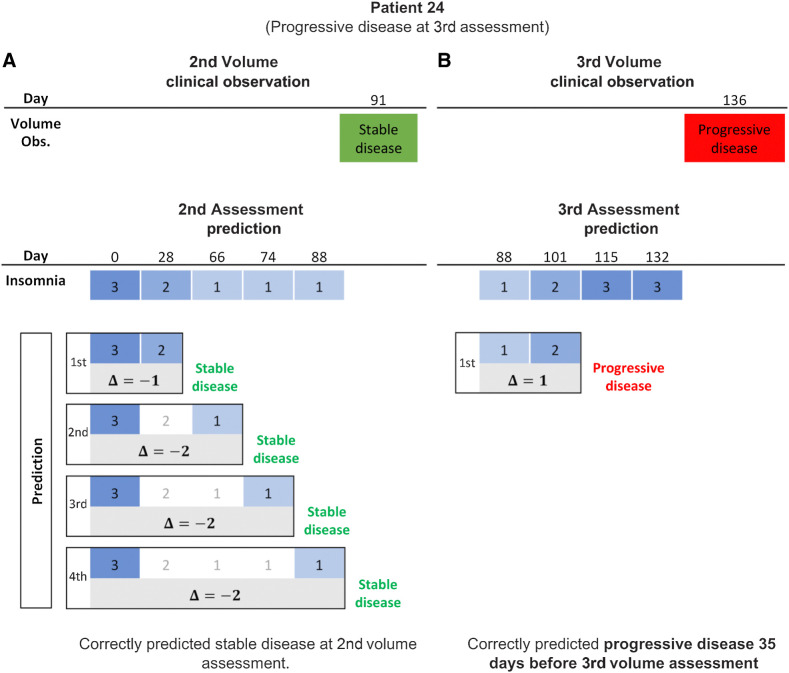
Prediction results for Patient 24, who developed progressive disease at the third volume assessment, are shown in Fig. 5. Insomnia decreased and then remained stable between tumor measurements one and two. These changes correctly predicted that the patient would have stable disease at day 91 (Fig. 5A). Insomnia increased from 1 to 2 between the second and third volume measurements and this change correctly predicted that Patient 24 would develop progressive disease (Fig. 5B). Overall, changes in insomnia between volume measurements predicted patient-specific response with a 77% accuracy. It should be noted that for Patient 24, the increase in insomnia correctly predicted progressive disease would occur 35 days before the next scan (Fig. 5B). Of the 85 patients included in the study, 19 patients developed iUPD. For 11 of these patients, changes in insomnia correctly predicted that they would develop iUPD on average 45 days before their next scan. Of the remaining 8 patients, 5 experienced zero or stable levels of insomnia throughout the duration of the study.
Figure 5.
Response prediction for a progressive patient. A, Second assessment response prediction. The clinical volume observation showed stable disease at day 91. Prediction 1 shows that insomnia decreased from 3 to 2 at day 28, such that
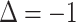 , correctly predicting stable disease. Insomnia continued to decrease, such that
, correctly predicting stable disease. Insomnia continued to decrease, such that
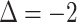 , correctly predicting stable disease for predictions 2 through 4. B, Third assessment response prediction. The clinical volume observation showed progressive disease at day 136. Insomnia increased from 1 to 2 at day 101, correctly predicting progressive disease 35 days before it was assessed via volumetric scan on day 136.
, correctly predicting stable disease for predictions 2 through 4. B, Third assessment response prediction. The clinical volume observation showed progressive disease at day 136. Insomnia increased from 1 to 2 at day 101, correctly predicting progressive disease 35 days before it was assessed via volumetric scan on day 136.
Discussion
The primary goal of this study was to determine whether PROs, which allow for frequent and noninvasive assessment of a patient's condition, could be used as a biomarker to dynamically predict early response to therapy and cancer progression. That is, could changes in PROs be indicative of a significant change in treatment response that might allow oncologists to intervene prior to progression? Analysis of all patient symptoms together showed high levels of several symptoms known to be common among patients with NSCLC (e.g., fatigue, shortness of breath, cough; refs. 25–28). Other symptoms such as blood in stool, hives, and vomiting were shown to not be frequently reported among patients. Subsequent analysis found that insomnia, dizziness, and fatigue were significantly correlated with tumor volume. Further analysis of associations between volume and symptom dynamics found that changes in insomnia were significantly associated with changes in tumor volume. Of all 27 PROs, insomnia was the only symptom found to be associated with volume changes. These findings align with previous studies that have shown insomnia to be associated with overall survival and treatment response (25, 29). To determine the predictive ability of insomnia, we investigated whether the changes between volume scans could be used to predict progressive disease. Results showed that changes in insomnia could predict patient response with a 77% accuracy, on average 45 days before the next volume scan.
The effects of sleep on cancerous growth have been previously reported with more than 78% of all circulating tumor cells being found in the resting/sleep phase as opposed to awake states (30). Furthermore, disruptions to the circadian rhythm, perpetuated by conditions such as insomnia and sleep apnea, have been shown to contribute to abnormal cell proliferation, increased gene mutations, and resistance to apoptosis. As such, circadian rhythm disruptions, specifically night shift work, are considered a potential carcinogen (Group 2A) by the WHO (30–33). Previous studies have shown that patients undergoing immunotherapy express high levels of insomnia (27, 34). Decreased insomnia has been found to be strongly associated with OS (25) and increased functional status, after controlling for pain, depression, fatigue, and dyspnea (26). Interestingly, sleep apnea simulated by intermittent hypoxia exposure has been shown to increase the expression of PD-1/PDL1 in immune cells, contributing to cancerous growth (35–37). Sleep apnea and other contributing factors in difficulty staying asleep (38, 39) provide reasoning behind the 77% predictive accuracy found for progressive tumor volume.
More objective measures of insomnia, such as actigraphy and wearable data for insomnia (40) and polysomnography for sleep apnea (41), might prove to be more useful measures of insomnia. Wearable devices can also provide more frequent data collection than traditional survey data (42, 43). Our study is an important first step in understanding how such data might potentially be used to tailor treatment and interventions in individual patients.
Limitations
Despite a predictive accuracy of 77%, the conclusions from these analyses have a few limitations. The study sample size is small (n = 85), a limitation especially apparent during the stratification of the sample by treatment response. This study should be repeated with more frequent tumor scans and PRO measurements to allow for an increased number of assessments per patient, as each patient only had an average of 2.5 assessments able to be paired. More frequent data collection may potentially highlight additional PROs or combinations of PROs that are predictive of treatment response. In addition, the size of the PRO assessment scale (5-point) limits prediction detail beyond iUPD. Because of the homogeneity of the sample (primarily White, non-Hispanic), care should be taken when applying these results to the heterogeneous population of all patients with NSCLC. Additional studies are needed to replicate these findings in independent samples.
Conclusions
Overall, this study demonstrates the potential value of PROs, specifically insomnia in NSCLC, as an inexpensive and easily accessible early marker of treatment response. Using this, oncologists may be able to intervene with alternative treatment options earlier to delay progression. Although the web-mediated follow-up conducted by Denis and colleagues aggregated all symptoms together (17), we demonstrated the predictive capability of insomnia alone and how it might be used to determine when a patient is progressing on treatment. We are confident that this is a promising first step in using PROs as a dynamic interradiographic predictor of patient-specific treatment response.
Future work will include investigating how PROs can be combined to make more accurate predictions for individual patients, as well as integration into a mathematical model to predict tumor volume change more definitively beyond disease progression.
Supplementary Material
Patient demographics table
Representativeness of study participants table
Acknowledgments
The research was supported in part by a Cancer Center Support Grant (CCSG) at the H. Lee Moffitt Cancer Center & Research Institute, awarded by the NCI (P30-CA76292), and by a Florida Biomedical Research Grant (21B12).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A. Bhatt reports a patent for the methods related to using patient-reported outcomes to predict patient-specific response to treatment in NSCLC that has been filed pending. M.B. Schabath reports other support from Bristol Myers Squibb outside the submitted work. H.S. Jim reports personal fees from SBR Bioscience and grants from Kite Pharma outside the submitted work. R. Brady-Nicholls reports a patent for using patient-reported outcome dynamics to predict response to immunotherapy in NSCLC pending. No disclosures were reported by the other authors.
Authors' Contributions
A.S. Bhatt: Formal analysis, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. M.B. Schabath: Conceptualization, resources, data curation, writing–original draft, writing–review and editing. A.I. Hoogland: Data curation, writing–review and editing. H.S.L. Jim: Conceptualization, resources, data curation, supervision, funding acquisition, writing–original draft, writing–review and editing. R. Brady-Nicholls: Conceptualization, resources, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Moradi A, Srinivasan S, Clements J, Batra J. Beyond the biomarker role: prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev 2019;38:333–46. [DOI] [PubMed] [Google Scholar]
- 2. Brady-Nicholls R, Nagy JD, Gerke TA, Zhang T, Wang AZ, Zhang J, et al. Prostate-specific antigen dynamics predict individual responses to intermittent androgen deprivation. Nat Commun 2020;11:1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charkhchi P, Cybulski C, Gronwald J, Wong FO, Narod SA, Akbari MR. CA125 and ovarian cancer: a comprehensive review. Cancers 2020;12:3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Kim D, Kim M, Lee Y, Ahn HK, Cho JH, et al. Long-term outcomes in patients with advanced and/or metastatic non–small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: multicenter, real-world data (KCSG LU20–11). Cancer 2022;128:778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rolfo C, Caglevic C, Santarpia M, Araujo A, Giovannetti E, Gallardo CD, et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. In Immunotherapy. Naing , A., Hajjar , J., Eds., Advances in experimental medicine and biology. Springer International Publishing: Cham, 2017. p. 97–125ISBN 978–3-31953156–4. [DOI] [PubMed] [Google Scholar]
- 7. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunotherapy Cancer 2018;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang H, Yang Y, Zhu Y, Chen H, Yang Y, Zhang L, et al. Blood protein biomarkers in lung cancer. Cancer Lett 2022;551:215886. [DOI] [PubMed] [Google Scholar]
- 9. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paparrizos J, White RW, Horvitz E. Screening for pancreatic adenocarcinoma using signals from web search logs: feasibility study and results. JOP 2016;12:737–44. [DOI] [PubMed] [Google Scholar]
- 11. White RW, Horvitz E. Evaluation of the feasibility of screening patients for early signs of lung carcinoma in web search logs. JAMA Oncol 2017;3:398–401. [DOI] [PubMed] [Google Scholar]
- 12. Kerrigan K, Patel SB, Haaland B, Ose D, Weinberg Chalmers A, Haydell T, et al. Prognostic significance of patient-reported outcomes in cancer. JCO Oncol Pract 2020;16:e313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barata A, Hoogland AI, Kommalapati A, Logue J, Welniak T, Hyland KA, et al. Change in patients’ perceived cognition following chimeric antigen receptor T-cell therapy for lymphoma. Transplant Cell Ther 2022;28:401.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Efficace F, Collins GS, Cottone F, Giesinger JM, Sommer K, Anota A, et al. Patient-reported outcomes as independent prognostic factors for survival in oncology: systematic review and meta-analysis. Value Health 2021;24:250–67. [DOI] [PubMed] [Google Scholar]
- 15. Mierzynska J, Piccinin C, Pe M, Martinelli F, Gotay C, Coens C, et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol 2019;20:e685–98. [DOI] [PubMed] [Google Scholar]
- 16. Turner K, Brownstein NC, Thompson Z, El Naqa I, Luo Y, Jim HSL, et al. Longitudinal patient-reported outcomes and survival among early-stage non-small cell lung cancer patients receiving stereotactic body radiotherapy. Radiother Oncol 2022;167:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denis F, Basch E, Septans A-L, Bennouna J, Urban T, Dueck AC, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA 2019;321:306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denis F, Koontz BF, Letellier C. Application and benefits of web-mediated symptom reporting for patients undergoing immunotherapy: a clinical example. Case Rep Oncol 2018;11:763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Webster KA, O'Connor ML, Hansen AR, Kircher S, Jim HSL, Dicker AP, et al. Development of a functional assessment of chronic illness therapy item library and primary symptom list for the assessment of patient-reported adverse events associated with immune checkpoint modulators. J Cancer Metastasis Treat 2020;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guibert N, Mazieres J, Delaunay M, Casanova A, Farella M, Keller L, et al. Monitoring of KRAS-mutated CtDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma. Oncotarget 2017;8:38056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. IRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldmacher GV, Conklin J. The use of tumour volumetrics to assess response to therapy in anticancer clinical trials. Br J Clin Pharmacol 2012;73:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 24. Research C. for D.E. and FDA approves atezolizumab as adjuvant treatment for nonsmall cell lung cancer. FDA 2023. [Google Scholar]
- 25. Badaoui S, Shahnam A, McKinnon RA, Abuhelwa AY, Sorich MJ, Hopkins AM. The predictive utility of patient-reported outcomes and performance status for survival in metastatic lung cancer patients treated with chemoimmunotherapy. Transl Lung Cancer Res 2022;11:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen M-L, Yu C-T, Yang C-H. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer 2008;62:391–400. [DOI] [PubMed] [Google Scholar]
- 27. McLouth LES, Lycan TW, Levine BJ, Gabbard J, Ruiz J, Farris M, et al. Patient-reported outcomes from patients receiving immunotherapy or chemo-immunotherapy for metastatic non-small cell lung cancer in clinical practice. Clin Lung Cancer 2020;21:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whisenant MS, Williams LA, Garcia Gonzalez A, Mendoza T, Shi Q, Cleeland C, et al. What do patients with non–small-cell lung cancer experience? Content domain for the MD Anderson Symptom Inventory for Lung Cancer. JCO Oncology Practice 2020;16:e1151–60. [DOI] [PubMed] [Google Scholar]
- 29. Ke W, Zhang L, Dai Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (IrAEs). Thoracic Cancer 2020;11:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ball H, Nagrath S. Cancer cells spread aggressively during sleep. Nature 2022;607:33–34. [DOI] [PubMed] [Google Scholar]
- 31. Basit H, Damhoff TC, Huecker MR. Sleeplessness and circadian disorder. In StatPearls. StatPearls Publishing: Treasure Island (FL), 2022. [PubMed] [Google Scholar]
- 32. Zhou L, Zhang Z, Nice E, Huang C, Zhang W, Tang Y. Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Hematol Oncol 2022;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shafi AA, Knudsen KE. Cancer and the circadian clock. Cancer Res 2019;79:3806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooley ME. Symptoms in adults with lung cancer: a systematic research review. J Pain Symptom Manage 2000;19:137–53. [DOI] [PubMed] [Google Scholar]
- 35. Cubillos-Zapata C, Almendros I, Díaz-García E, Toledano V, Casitas R, Galera R, et al. Differential effect of intermittent hypoxia and sleep fragmentation on PD-1/PD-L1 upregulation. Sleep 2020;43:zsz285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cubillos-Zapata C, Balbás-García C, Avendaño-Ortiz J, Toledano V, Torres M, Almendros I, et al. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology 2019;24:684–92. [DOI] [PubMed] [Google Scholar]
- 37. Polasky C, Steffen A, Loyal K, Lange C, Bruchhage K-L, Pries R. Redistribution of monocyte subsets in obstructive sleep apnea syndrome patients leads to an imbalanced PD-1/PD-L1 cross-talk with CD4/CD8 T cells. J Immunol 2021;206:51–58. [DOI] [PubMed] [Google Scholar]
- 38. Benetó A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev 2009;13:287–93. [DOI] [PubMed] [Google Scholar]
- 39. Luyster FS, Buysse DJ, Strollo PJ. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med 2010;06:196–204. [PMC free article] [PubMed] [Google Scholar]
- 40. Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev 2002;6:113–24. [DOI] [PubMed] [Google Scholar]
- 41. Rundo JV. Obstructive sleep apnea basics. CCJM 2019;86:2–9. [DOI] [PubMed] [Google Scholar]
- 42. Ong JC, Crawford MR, Wallace DM. Sleep apnea and insomnia: emerging evidence for effective clinical management. Chest 2021;159:2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang EM, Gillespie EF, Shaverdian N. Truthfulness in patient-reported outcomes: factors affecting patients’ responses and impact on data quality. Patient Relat Outcome Meas 2019;10:171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient demographics table
Representativeness of study participants table
Data Availability Statement
The clinical data used to conduct this study are available upon reasonable request to the corresponding author (RBN).