Abstract
Purpose:
Patients with unresectable/metastatic chondrosarcoma have poor prognoses; conventional chondrosarcoma is associated with a median progression-free survival (PFS) of <4 months after first-line chemotherapy. No standard targeted therapies are available. We present the preclinical characterization of INBRX-109, a third-generation death receptor 5 (DR5) agonist, and clinical findings from a phase I trial of INBRX-109 in unresectable/metastatic chondrosarcoma (NCT03715933).
Patients and Methods:
INBRX-109 was first characterized preclinically as a DR5 agonist, with binding specificity and hepatotoxicity evaluated in vitro and antitumor activity evaluated both in vitro and in vivo. INBRX-109 (3 mg/kg every 3 weeks) was then evaluated in a phase I study of solid tumors, which included a cohort with any subtype of chondrosarcoma and a cohort with IDH1/IDH2-mutant conventional chondrosarcoma. The primary endpoint was safety. Efficacy was an exploratory endpoint, with measures including objective response, disease control rate, and PFS.
Results:
In preclinical studies, INBRX-109 led to antitumor activity in vitro and in patient-derived xenograft models, with minimal hepatotoxicity. In the phase I study, INBRX-109 was well tolerated and demonstrated antitumor activity in unresectable/metastatic chondrosarcoma. INBRX-109 led to a disease control rate of 87.1% [27/31; durable clinical benefit, 40.7% (11/27)], including two partial responses, and median PFS of 7.6 months. Most treatment-related adverse events, including liver-related events, were low grade (grade ≥3 events in chondrosarcoma cohorts, 5.7%).
Conclusions:
INBRX-109 demonstrated encouraging antitumor activity with a favorable safety profile in patients with unresectable/metastatic chondrosarcoma. A randomized, placebo-controlled, phase II trial (ChonDRAgon, NCT04950075) will further evaluate INBRX-109 in conventional chondrosarcoma.
Translational Relevance.
Chondrosarcoma is a rare, indolent, heterogeneous, malignant bone tumor. Conventional chondrosarcoma, which accounts for approximately 80% of chondrosarcomas, is largely resistant to chemotherapy and radiation; nonconventional chondrosarcoma (e.g., dedifferentiated chondrosarcoma) is generally associated with more aggressive growth. Surgical resection remains the only curative treatment, and options for patients with unresectable or metastatic chondrosarcoma are limited, with no improvement in outcomes observed in 30 years. In our study, INBRX-109, a novel, tetravalent DR5 agonist, demonstrated encouraging antitumor activity, including objective response and durable clinical benefit, and a favorable safety profile in patients with unresectable or metastatic chondrosarcoma. Our data suggest that DR5 is a valid target and that INBRX-109, with its favorable safety and efficacy profile, warrants further evaluation as a novel targeted therapy for chondrosarcoma. Additional ongoing studies, including a randomized phase II study, will confirm the activity of INBRX-109 in unresectable or metastatic chondrosarcoma.
Introduction
Chondrosarcoma, one of the over 100 different types of sarcoma (1), is a heterogeneous malignant tumor that forms cartilaginous matrix and accounts for approximately 20% of primary bone tumors (2). On the basis of histology, chondrosarcoma is classified as conventional or nonconventional, with clinical characteristics varying by subtype (2, 3). Primary conventional chondrosarcoma represents 85% to 95% of all chondrosarcoma cases and typically develops in the pelvis, femur, humerus, or ribs; less common sites include the mobile spine and craniofacial bones (2–4). Nonconventional chondrosarcoma is rare and includes clear cell, mesenchymal, and dedifferentiated chondrosarcoma (1–3). Mesenchymal and dedifferentiated chondrosarcoma are considered high grade and have a poor prognosis due to more aggressive growth and a higher risk of metastases than conventional chondrosarcoma; in contrast, clear cell chondrosarcoma is considered low grade (1–3).
In patients with conventional chondrosarcoma, complete surgical resection is the recommended management approach, although radiation therapy is used for select situations. However, treatment options for patients with unresectable or metastatic disease are greatly limited given that these tumors tend to be resistant to chemotherapy and radiation and that no standard targeted systemic therapies are available (5, 6). Prognosis in these patients is poor, with a median progression-free survival (PFS) of approximately 2.0 months with no systemic treatment (7). Chemotherapy has not greatly improved PFS rates, with studies reporting a median PFS of 2.5 months with doxorubicin and 3.6 months with doxorubicin in combination with cisplatin or ifosfamide (5). Although several agents have been evaluated for the treatment of unresectable or metastatic conventional chondrosarcoma, outcomes have not changed substantially in 30 years, highlighting the need for novel and effective therapies.
Death receptor 5 (DR5), a proapoptotic receptor that forms multimers on the cell surface and is activated after binding TRAIL, has long been a target of interest in oncology due to the differential sensitivity of cancerous over healthy cells to TRAIL-mediated killing (8, 9). The binding of trimeric TRAIL to three death receptors leads to clustering of DR5 and recruitment of the death-inducing signaling complex (DISC; refs. 10, 11). Within the DISC, procaspases-8 and procaspases-10 are autocatalytically cleaved, leading to activation of downstream effector caspase-3 and caspase-7, ultimately resulting in apoptosis (10–12). Although the trimer is the minimal functional unit for TRAIL activity, clustering of multiple receptor trimers at the cell–cell interface results in maximal apoptotic activity (13–15).
INBRX-109 is a third-generation, recombinant, humanized, agonistic antibody against DR5 that has been precisely engineered for optimal DR5 agonism and safety. INBRX-109 is based on a single-domain antibody (sdAb) platform and was designed to simultaneously engage four DR5 molecules and induce robust apoptotic signaling. Importantly, the tetravalent format of INBRX-109 was selected as an optimal balance of DR5 agonism on tumor versus normal cells, enabling cancer-biased cell death while avoiding hyperclustering and concomitant hepatotoxicity observed with previous multivalent DR5 candidates (16; Genmab; cited October 14, 2022). Available from: https://ir.genmab.com/static-files/be692ea1-1ee7-44d3-ab20-f0dda61ea843.
Early studies reported encouraging responses to DR5 agonists in patients with chondrosarcoma. In one case study, a patient achieved a sustained partial response and, eventually, a complete response (17); in a phase I study, a patient achieved a 20% reduction in measurable disease (18). On the basis of these early reports, we evaluated INBRX-109 in patients with chondrosarcoma as part of a phase I, first-in-human study in advanced solid tumors (NCT03715933; Supplementary Fig. S1). Here, we present the preclinical characterization of INBRX-109 as a DR5 agonist, followed by safety and efficacy findings in patients with unresectable or metastatic chondrosarcoma in the phase I study.
Patients and Methods
Preclinical
Cell lines
Multiple cell lines were used in the study: NoSpin HepaRG (Lonza; Catalog No. NSHPRG; RRID:CVCL_9720); H-EMC-SS (Millipore Sigma; Catalog No. 94042258; RRID:CVCL_1238); ExpiCHO-S (Thermo Fisher Scientific; Catalog No. A29127; RRID:CVCL_5J31); and Jurkat CD16a–V158 ADCC Bioassay Effector Cells (Promega; Catalog No. G701A). All cells were grown according to the manufacturers’ instructions.
Cytotoxicity assay
H-EMC-SS cells were plated at 10,000 cells/well in complete media [Ham's F12 (Sigma; Catalog No. N6658)/DMEM (Gibco; Catalog No. 11965–092) 1:1 + 10% FBS (Avantor, Catalog No. 97068–085) + 1× antibiotic-antimycotic (Gibco, Catalog No. 15240–062) and incubated overnight (37°C; 5% CO2). INBRX-109 (Inhibrx), bivalent anti-DR5 (Inhibrx), trivalent recombinant TRAIL (R&D Systems, Catalog No. 375-TL/CF), and hexavalent anti-DR5 (Inhibrx) were prepared in assay media and added at the indicated concentrations for 16 hours. Cells were incubated with CellTiter-Glo 2.0 (Promega; Catalog No. G924C) for 10 minutes at room temperature and luminescence (RLU) read on the Spectra Max M5e plate reader (Molecular Devices) and SoftMaxPro Data Acquisition and Analysis software v5.4 (Molecular Devices, RRID: SCR_014240). Percent survival was calculated as (experimental RLU/vehicle control RLU) × 100; percent cell death was calculated as (100 − percent survival).
Caspase-3/7 induction assay
H-EMC-SS cells were plated at 10,000 cells/well in complete media and incubated overnight (37°C; 5% CO2) with working dilutions of INBRX-109 ± Z-VAD-FMK (final concentration, 50 μmol/L; Promega, Catalog No. G7223B), bivalent anti-DR5, trivalent recombinant TRAIL, or hexavalent anti-DR5. Caspase-3/7 green (Essen BioScience, Catalog No. 4440) was then added at a final concentration of 2.5 μmol/L. The plate was incubated at room temperature for 5 minutes, and live-cell imaging was performed every 30 minutes for 16 hours using an Incucyte S3 Live-Cell Analysis System (10× objective; Sartorius, Catalog No. 4647; RRID: SCR_023147). Images were analyzed using Incucyte ZOOM 2018A software version 6.2.9200.0 (Sartorius). All data were background corrected using the no-treatment condition.
Generation of DR5-ExpiCHO-S cells
Full-length DR5 was cloned in frame into pRP (Inhibrx), with expression linked to citrine [pRP-DR5-FL-Cit (Inhibrx)]. ExpiCHO-S cells were transfected with a 3:1 ratio of PEI MAX high potency linear polyethylenimine hydrochloride (Polysciences, Inc., Catalog No. 24765–2) and pRP-DR5-FL-Cit; each was diluted separately in OptiMEM 1 (1x) + Glutamax Reduced Serum Medium (Gibco, Catalog No. 51985–034) before combining for 30 minutes at room temperature. The PEI-DNA solution was added to the cells, which were then incubated for 14 hours (37°C; 8% CO2). DR5-expressing cells were identified based on citrine expression by flow cytometry using an iQue Screener Plus (Intellicyt).
Binding competition assay
DR5-ExpiCHO-S cells were incubated with INBRX-109 ± 3.5 nmol/L His-tagged TRAIL (BioLegend, Catalog No. 752906) for 1 hour at 4°C in FACS buffer. Cells were washed and then resuspended in FACS buffer at 5 × 104 cells/well. Cells were transferred to a round-bottom 96-well plate, and supernatant was removed; then, 100 μL of serially diluted INBRX-109 or INBRX-109 plus TRAIL in FACS buffer was added to the cells. Cells were incubated for 1 hour at 4°C and protected from light; they were then washed two times with 150 μL FACS buffer. Cells were resuspended in 100 μL FACS buffer containing either Protein A-Alexa Fluor 647 (AF647) conjugate (1:1,000; Invitrogen, Catalog No. P21462) or anti-His tag APC-conjugated antibody (1:100; R&D Systems, Catalog No. IC050A; RRID:AB_10718109) to detect INBRX-109 or TRAIL, respectively. AF647 or APC on citrine-positive cells (median fluorescence intensity >105) was assessed by flow cytometry using an iQue Screener Plus.
Binding assays with H-EMC-SS cells were performed similarly using serial dilutions of INBRX-109, bivalent anti-DR5, His-tagged trivalent recombinant TRAIL, and hexavalent anti-DR5. Plates were incubated for 30 minutes at 4°C. Cells were washed twice with FACS buffer and resuspended in 100 μL FACS buffer containing a 1:2,000 dilution of propidium iodide (BioLegend, Catalog No. 421301) and respective antibodies. AF647-conjugated antihuman crystallizable fragment (Fc) antibody (1:1,000; Jackson ImmunoResearch, Catalog No. 709–605–098; RRID:AB_2340577) was used to detect anti-DR5 antibodies; AF647-conjugated mouse anti-His tag antibody (1:100; BioLegend, Catalog No. 362611; RRID:AB_2721401) was used to detect TRAIL.
Where indicated, raw mean fluorescence intensity values were exported to Excel and then graphed and analyzed using GraphPad Prism 9 (RRID:SCR_002798). All data were background corrected using secondary antibody–only wells. Apparent affinity (Kd in nmol/L) was determined using a One Site total analysis, using the following model: Y = Bmax × X/(Kd + X) + NS × X + Background.
Antibody-dependent cytotoxicity
Jurkat CD16a-V158 ADCC cells Bioassay Effector Cells were cocultured with DR5-ExpiCHO-S or ExpiCHO-S cells and then incubated with INBRX-109 or daratumumab (MedChemExpress, Catalog No. HY-P9915) along with Z-VAD-FMK (50 μmol/L). Assay plates were incubated at 37°C for 5.5 hours and then equilibrated at room temperature for 10 minutes. Luciferase activity of the effector cells was assayed using Bio-Glo reagent (Promega, Catalog No. G7940). Luminescence was measured using a SpectraMax L microplate reader (Molecular Devices). Curves were generated using GraphPad Prism 9 with a four-parameter fit [(agonist) vs. response-variable slope] using the following model equation: Y = Bottom + (X^HillSlope) × (Top–Bottom) / (X^HillSlope + EC50^HillSlope).
C1q complement binding ELISA
INBRX-109 or daratumumab was immobilized on 96-well MediSorp plates (Thermo Fisher Scientific, Catalog No. 467320) at 10 μg/mL in PBS and incubated for approximately 16 hours at 4°C. Assay plates were blocked for 2 hours at room temperature and incubated for 1 hour at room temperature with dilutions of pooled human complement serum (Innovative Research, Catalog No. ICSER10ML) followed by horseradish peroxidase–conjugated anti-C1q antibody (1:100; 1 hour at room temperature; Abcam, Catalog No. ab46191; RRID:AB_726908). Absorbance at 650 nm was measured approximately 5 minutes after adding peroxidase substrate (Seracare, 5210–0077) using an Emax microplate reader (Molecular Devices).
HepaRG toxicity assay
Cultured HepaRG cells were seeded at 6.3 × 104 cells/well and incubated for 24 hours (37°C; 5% CO2) before culturing for 3 more days in HepaRG Pre-Induction and Tox Medium (Lonza, Catalog No. MHPIT). Cells were then incubated for 20 minutes (37°C; 5% CO2) with INBRX-109, hexavalent compound, TAS266 analogue (Inhibrx), or TAS266 mut (Inhibrx) followed by media or intravenous immunoglobulin (IVIG; Grifols, NDC code 13533–800–72; final concentration, 10 mg/mL). Green caspase-3/7 reagent (final concentration, 1.25 μmol/L) was then added, and live-cell imaging was performed every 1 to 2 hours for 42 hours using an Incucyte S3 Live-Cell Analysis System (37°C, 5% CO2) and Incucyte ZOOM 2018A software. Endpoint cell viability was also measured at 42 hours with CellTiter-Glo 2.0. After equilibration to room temperature and addition of CellTiter-Glo reagent, assay plates were incubated for 10 minutes at room temperature. Luminescence was measured using the SpectraMax L microplate reader with a target wavelength of 470 nm and percent viability calculated as (experimental RLU/average untreated RLU) × 100.
InSphero InSight hepatotoxicity assay
Microtissues were treated with dilutions of INBRX-109 or a hexavalent agent for 7 days. Endpoint intracellular ATP content was measured with the CellTiter-Glo 2.0 Cell Viability Assay (Promega), and extracellular lactate dehydrogenase release was measured with the Bioluminescent LDH Release Toxicity Assay Kit (Promega, Catalog no. J2380), both according to InSphero AG internal technical operation procedures. The IC50, 95% CI, and Hill slope values were calculated using a nonlinear regression sigmoidal dose–response (variable slope) curve fitting with the bottom constrained to a constant value of 0.
Screen for and confirmation of preexisting ADAs
Screening for and confirmation of preexisting antidrug antibodies (ADA) was performed by BioAgilytix San Diego. Serum samples from healthy individuals (BioChemed, Inc.) and patients with cancer (Bioreclamation) were screened for the presence of preexisting ADAs against INBRX-109 or TAS266. 96-well microtiter plates (Meso Scale Diagnostics, Catalog no. L55XA) coated with INBRX-109 or TAS266 analogue were incubated with human serum samples followed by SULFO-TAG Labeled Anti-Hu Ig-LC (Thermo Fisher Scientific, Catalog no. MA1–34883; RRID:AB_10980148). Electrochemiluminescence was then measured. In the confirmatory assay, serum samples that were at or above the cut-point for each test article were diluted with either Assay Diluent or Assay Diluent spiked with INBRX-109 or TAS266 analogue and incubated for 1 hour at room temperature prior to addition to the plates. Percent inhibition (%INH) was calculated using the formula %INH = 100 × [1 − (sample ECLUspiked / sample ECLUunspiked)].
Binding to tumor necrosis factor receptor superfamily members
Recombinant proteins composed of the extracellular domain of human DR5, DR4, Decoy R1, or Decoy R2 fused to a mouse Fc were generated and diluted to 2 μg/mL using PBS for ELISA plate coating. Coating agents were added to 96-well MediSorp plates, and plates were incubated for 14 hours at 4°C. After washing and blocking, titrations of INBRX-109 or recombinant, human His-tagged TRAIL were added, and plates were incubated for 1 hour at room temperature. Peroxidase-conjugated antihuman IgG antibody (Jackson ImmunoResearch, Catalog no. 709–035–098; RRID:AB_2340494) and anti-His tag antibody (R&D Systems, Catalog no. MAB050H; RRID:AB_357354) were used to detect INBRX-109 and human His-tagged TRAIL, respectively. Peroxidase substrate was added, plates were incubated for 1 minute at room temperature, and sample A650 was measured using an Emax microplate reader.
DR5-ExpiCHO-S and Jurkat CD16a reporter cell binding
DR5-ExpiCHO-S cells or Jurkat CD16a reporter cells were incubated with a titration of INBRX-109 or daratumumab for 30 minutes at 4°C. Cells were washed and incubated with a 1:1,000 dilution of AF647-conjugated anti-human Fc antibody and a 1:2,000 dilution of propidium iodide for 20 minutes at 4°C. Cells were washed and resuspended in FACS buffer for analysis using an iQue Screener Plus flow cytometer. As described in the “Binding competition assay” section, detection of AF647 on citrine-positive cells was used to measure binding to DR5+ cells in the pool of transfected ExpiCHO-S cells.
Pharmacokinetics and immunogenicity
Blood samples were collected from all study patients to characterize the pharmacokinetic behavior of and the potential for immunogenicity against INBRX-109. Pharmacokinetic parameters were estimated via noncompartmental analysis using Phoenix WinNonlin version 8.3 (Certara, RRID:SCR_021370). Nonlinear mixed-effects modeling software (NONMEM, Version 7.3; ICON, RRID:SCR_016986), a software package for nonlinear mixed-effects analysis, was used for population pharmacokinetic modeling.
The method to quantify INBRX-109 in human serum was validated for clinical sample analysis and used the format described here. Wells were first coated with recombinant DR5 as the antigen capture (Inhibrx). INBRX-109 in standards, quality controls, and samples are bound to the capture antigen and subsequently detected with a ruthenium-labeled, drug-specific anti-idiotype antibody (Inhibrx). The method used the Meso Scale Discovery Electrochemiluminescence platform (Meso Scale Diagnostics); assay signal from calibrators were plotted, and a curve fit was generated using a 4PL fit model with 1/Y2 weighting for quantification of INBRX-109 in samples.
ADAs were detected and characterized using validated methods that follow the traditional three-tiered testing scheme. The first method used a direct bind format in which INBRX-109 coated to wells of a 96-well microtiter assay plate was used to capture ADAs in serum samples; ADAs were subsequently revealed with a ruthenium-labeled anti-human immunoglobulin light chain antibody (Thermo Fisher Scientific, MA1–34883; RRID:AB_10980148). Later in the study, a second method was validated. A bead-based sample pretreatment was used to purify ADAs from serum, which were then coated to wells of a multiarray high-bind plate (Meso Scale Diagnostics, Catalog no. L15XB) and directly detected using ruthenium-labeled INBRX-109. The sensitivity of anti-INBRX-109 antibody detection was increased approximately two-fold over the original method and was less than 10 ng/mL of ADA in serum.
In vivo patient-derived xenograft (PDX) models
INBRX-109 activity was evaluated at Champions Oncology, Inc., in low-passage chondrosarcoma PDX models CTG-1255 and CTG-2383. Histology of these models was consistent with conventional chondrosarcoma. Study mice were treated weekly for 3 weeks, with intravenous administration of vehicle control or 1 mg/kg INBRX-109. Effects on tumor growth were evaluated by twice-weekly tumor volume measurements. Tolerability was assessed by body weight loss (measured twice weekly) or animal morbidity/mortality. The study endpoint was reached when the mean tumor volume of the vehicle control group (uncensored) reached 1,500 mm³. All animal studies were approved by local regulatory authorities for animal welfare. All mice were kept in accordance with federal and state policies on animal research.
Data visualization and analysis
Data were graphed and analyzed using GraphPad Prism 9.
Clinical
Study design
The INBRX-109 phase I trial is an ongoing, open-label, three-part study evaluating INBRX-109 in patients with advanced/metastatic solid tumors that was initiated in November 2018. Part 1 (3+3 single-agent dose escalation) was completed in 2019. Patients were treated with dose levels of 0.3, 1, 3, 10, and 30 mg/kg. Intrapatient dose escalation was not permitted. INBRX-109 was administered as an intravenous infusion over 60 minutes every 21 days. INBRX-109 was well tolerated, with no significant toxicities observed; an MTD was not reached, and a dose of 3 mg/kg was chosen for further investigation.
In part 2 (single-agent dose expansion), INBRX-109 3 mg/kg i.v. every 3 weeks is being evaluated in several cancer types, including chondrosarcoma (cohort B4) and IDH1/IDH2-mutant conventional chondrosarcoma (cohort B6). Patients will be treated until disease progression or significant toxicity. Part 3 (combination dose expansion) is currently enrolling patients with various tumor types (Supplementary Fig. S1).
This study is being conducted in compliance with the Declaration of Helsinki, the International Council for Harmonisation Guidelines for Good Clinical Practice, and applicable national and local laws and regulatory requirements. The protocol was approved by the independent ethics committee or institutional review board at each site. All patients provided written informed consent before participation.
Inclusion and exclusion criteria
In part 1, patients were ages ≥18 years with advanced/metastatic or nonresectable solid tumors. In part 2, patients in cohort B4 were eligible if they had been diagnosed with chondrosarcoma, regardless of histology or grade. Patients in cohort B6 had grade 2 or grade 3 IDH1(R132)- or IDH2(R172)-mutant conventional chondrosarcoma. Patients were required to have archival tissue samples or fresh biopsies. No prior treatment with DR5 agonists was permitted. Patients with acute viral or toxic liver disease or chronic liver disease could not participate, with some exceptions.
Outcomes
The primary endpoint was safety and tolerability of INBRX-109. Treatment-emergent adverse events (AE) were recorded using Medical Dictionary for Regulatory Activities preferred terms and graded using Common Terminology Criteria for Adverse Events v5.0. Secondary endpoints were pharmacokinetics estimated using standard noncompartmental methods and incidence of ADAs. Exploratory endpoints are efficacy and potential predictive biomarkers. Efficacy measures included objective response by RECIST v1.1 criteria, disease control rate (DCR), duration of response, PFS, and calculation of the growth modulation index (GMI; ratio of time to progression with current therapy to time to progression with the last prior line of therapy). In this analysis, a modified GMI was determined using the ratio of PFS with INBRX-109 to prior treatment duration. Prior treatment duration was used since time to progression with previous therapy was not known for all patients.
Statistical analysis
The safety set comprised all patients who had received ≥1 dose of INBRX-109. Efficacy was determined in evaluable patients. The data cutoff for the analyses reported here was May 26, 2022. Time-to-event endpoints were estimated using the Kaplan–Meier method. Descriptive statistical analysis was used for other clinical and pharmacokinetic parameters.
Data availability
The data generated and/or analyzed in this study are not publicly available but are available upon reasonable request. Sharing of data is subject to protection of patient privacy and respect for the patient's informed consent. For more information on the process or to submit a request, contact clinicaltrials@inhibrx.com.
Results
Preclinical
Characterization of INBRX-109
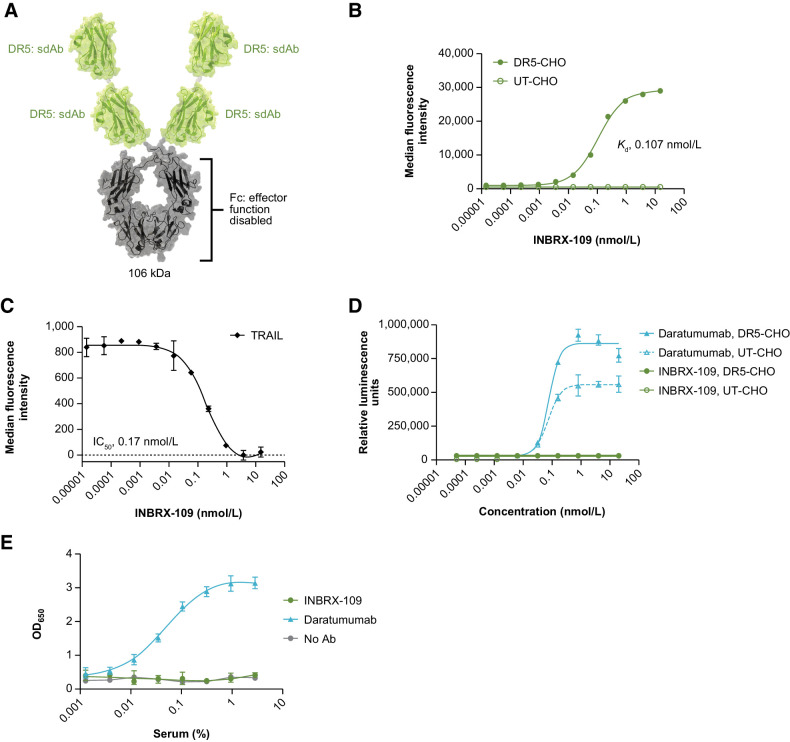
INBRX-109 is a 105.7-kDa recombinant, humanized, third-generation DR5 agonist that is built on a camelid-derived sdAb platform (Fig. 1A). INBRX-109 is composed of two identical sdAbs (heavy chain–only binding domains) joined end to end with an Fc domain based on human immunoglobulin G1 (IgG1) to create a molecule that homodimerizes into a tetravalent antibody targeting four DR5 receptors. To prevent the safety liability of excessive secondary clustering, the binding domains of INBRX-109 were humanized and specific modifications incorporated to eliminate recognition by preexisting ADAs. The Fc domain was engineered with a proprietary three amino acid deletion (Glu233, Leu234, and Leu235) near the hinge to prevent cross-linking and effector function through interaction with Fcγ receptors and complement, thereby constraining the activity of this molecule to that of a tetravalent agonist.
Figure 1.
INBRX-109 characterization. A, Schematic representation of INBRX-109 structure. B, Representative binding curve of INBRX-109 on ExpiCHO-S transfected with full-length human DR5; untransfected ExpiCHO-S cells served as a negative control. Apparent affinity of the observed binding interaction was determined using a One Site total analysis. C, INBRX-109 competition with TRAIL for binding to DR5 expressed by ExpiCHO-S cells. Detection of a constant concentration of TRAIL (3.5 nmol/L) in the presence of increasing concentrations of INBRX-109 is shown. D, ADCC capability of INBRX-109 was evaluated in the Promega Jurkat CD16a (V158) ADCC reporter assay using DR5-transfected ExpiCHO-S cells as targets and untransfected cells as negative controls. Daratumumab, the target of which is expressed on Jurkat cells, serves as a positive control. E, The ability of INBRX-109 to bind the complement component C1q contained within normal human serum was measured by ELISA. Daratumumab is used as a positive control. Abbreviations: Ab, antibody; ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; CHO, Chinese hamster ovary cells; DR5, death receptor 5; Fc, crystallizable fragment; Kd, equilibrium dissociation constant; sdAb, single-domain antibody; UT, untransfected.
INBRX-109 bound its target, DR5, in a dose-dependent manner with an apparent affinity of approximately 0.11 nmol/L (Fig. 1B) and blocked binding of TRAIL to DR5 with an IC50 of 0.17 nmol/L (Fig. 1C); no signal—and no cross-reactivity with hamster DR5—was detected in untransfected ExpiCHO-S cells (negative control). Binding of INBRX-109 was specific to DR5, with no binding to DR4 or either of the decoy receptors observed (Supplementary Fig. S2). No significant difference in INBRX-109 binding to DR5 was observed when TRAIL was present in solution (Supplementary Fig. S3). The potential for Fc receptor–mediated antibody-dependent cellular cytotoxicity (ADCC) with INBRX-109 was assessed using the ADCC Reporter Bioassay (Promega), a surrogate for primary cell ADCC activity, where a Jurkat reporter cell line was cocultured with target cells expressing high levels of DR5. Reporter activation was not observed with INBRX-109, consistent with a disabled effector function and the inability to mediate ADCC (Fig. 1D; Supplementary Fig. S4). In contrast, and as expected, reporter activation was observed with daratumumab, an antibody targeting CD38, a marker expressed on the Jurkat reporter cells (Fig. 1D; Supplementary Fig. S4). Binding of the complement C1q protein, a surrogate for complement-dependent cytotoxicity activity, was also absent for immobilized INBRX-109 (Fig. 1E), confirming effector silencing.
Antitumor activity in vitro and in vivo
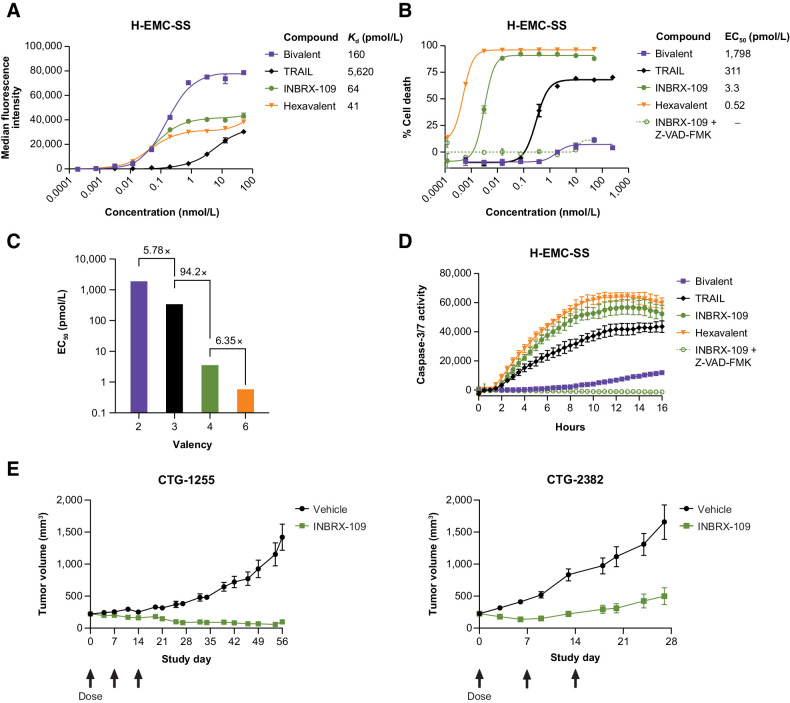
Given its high DR5 expression, the human extraskeletal myxoid chondrosarcoma cell line H-EMC-SS was used to characterize the antitumor activity of INBRX-109 in vitro (Fig. 2). The cytotoxicity of tetravalent INBRX-109 was compared with hexavalent and bivalent antibodies using the same sdAb within INBRX-109 as well as with recombinant, soluble TRAIL, a naturally occurring trimer. Although molecules with valencies ≥3 showed similar (within four-fold) high binding affinity to H-EMC-SS cells (Fig. 2A), their cytotoxic activities diverged considerably, demonstrating the impact of valency (Fig. 2B). Substantial increases in the potency of INBRX-109 and the hexavalent agent over trimeric TRAIL and the bivalent DR5 agonist were observed, indicating that increasing valency increases potency of signaling (Figs. 2B and C). Of note, the hexavalent comparator resulted in only a modest increase in potency over INBRX-109 (Fig. 2C).
Figure 2.
In vitro and in vivo antitumor activity. A, Representative dose-dependent binding of bivalent, trivalent (TRAIL), tetravalent (INBRX-109), and hexavalent anti-DR5 molecules on H-EMC-SS chondrosarcoma cells. B, Impact of valency on H-EMC-SS cell death. Death of H-EMC-SS cells 16 hours after treatment with the indicated concentrations of molecules of increasing valency was measured by CellTiter-Glo. Data were fit with a nonlinear four-parameter agonist concentration versus response curve, and calculated EC50 values are reported. Activity of INBRX-109 was assessed in the presence of the pan-caspase inhibitor Z-VAD-FMK. C, Impact of valency on potency. The fold improvement in potency from bivalent to trivalent, trivalent to tetravalent, and tetravalent to hexavalent is shown. D, Activation of caspase-3 and -7 in H-EMC-SS cells treated with bivalent, trivalent (TRAIL), tetravalent (INBRX-109), and hexavalent anti-DR5 molecules for the indicated durations of time was measured by real-time imaging on an Incucyte live cell imaging system. Activity of INBRX-109 was assessed in the presence or absence of the pan-caspase inhibitor Z-VAD-FMK. E, Tumor volume over time in animals harboring patient-derived chondrosarcoma tumors and treated with vehicle or INBRX-109 (1 mg/kg, i.v. once every week × 3 weeks starting on study day 0, as indicated by arrows). Each symbol represents the mean tumor volume of 8 animals, with error bars to denote SEM. Abbreviations: DR5, death receptor 5; Kd, equilibrium dissociation constant.
Multimerization of DR5 leads to the formation of the DISC, in which caspase-8 is activated, leading to the subsequent activation of the downstream executioner caspase-3 and caspase-7 (10, 12, 19) and the coordinated destruction of the cell. Treatment of H-EMC-SS cells with INBRX-109 resulted in rapid activation of caspase-3 and caspase-7 (Fig. 2D). INBRX-109 induced substantially higher caspase activity than agents with lower valencies (i.e., TRAIL and a bivalent agonist), highlighting the impact of valency on executioner caspase activation. Consistent with our previous observations (Fig. 2B and C), the hexavalent comparator resulted in only a modest increase in caspase-3 and caspase-7 activation relative to that induced by INBRX-109 (Fig. 2D). The induction of caspase-3 and -7 and subsequent loss of cell viability could be inhibited by addition of a pan-caspase inhibitor, further indicating that the cytotoxicity induced by INBRX-109 was dependent on the activation of these executioner caspases (Fig. 2B and D). Antitumor activity was also demonstrated in vivo in chondrosarcoma PDX models (Fig. 2E). In evaluating a wide range of doses (0.1–10 mg/kg weekly) in mouse tumor models, INBRX-109 at a dose of 1 mg/kg weekly was generally the most efficacious and led to sustained tumor inhibition in two chondrosarcoma PDX models, with near-complete tumor regressions observed in one model.
Hepatotoxicity analyses
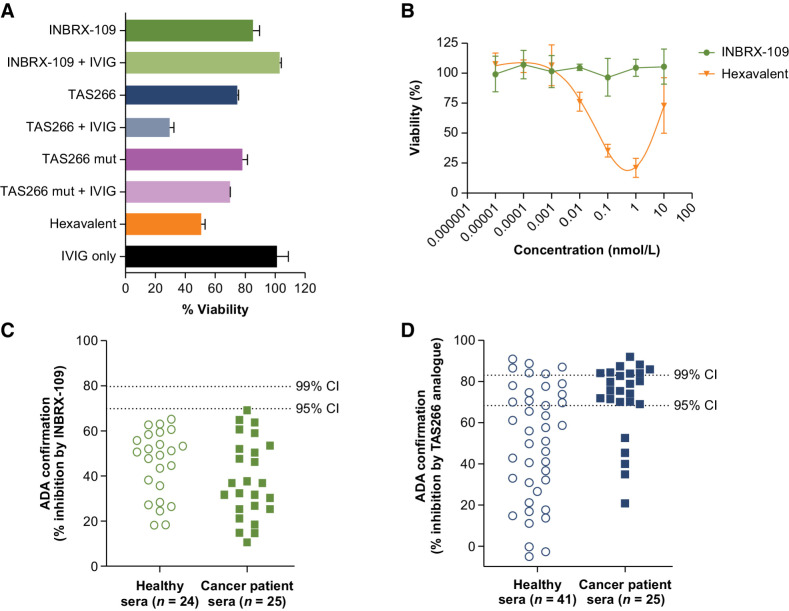
In Good Laboratory Practice toxicology studies in cynomolgus monkeys, INBRX-109 was well tolerated and the no-observed-adverse-effect levels were the highest doses tested (100 mg/kg weekly and 50 mg/kg every 2 weeks). Previous generations of DR5-targeted therapeutic antibodies, such as TAS266, have exhibited dose-limiting hepatotoxicity (16). In the case of TAS266, an sdAb-based tetravalent DR5 agonist, retrospective experiments revealed that hepatotoxicity correlated with the presence of preexisting ADAs to TAS266. A bivalent IgG ADA capable of recognizing TAS266 could bind two molecules, which would increase the functional valency of TAS266 from 4 to 8, leading to hyperclustering of DR5 and a greater potential for hepatotoxicity (16). INBRX-109 was engineered with proprietary modifications to remove preexisting ADA recognition sites and incorporates an Fc domain so that the C-terminal sdAb is not exposed. To demonstrate the effectiveness of these modifications, we evaluated the impact of clinical grade immunoglobulin (IVIG)—which is known to contain anti-sdAb ADAs—on the hepatotoxicity profile of INBRX-109 in HepaRG cells, an immortalized hepatic progenitor cell line that recapitulates hepatic cell biology and toxicity in vitro. By both CellTiter-Glo endpoint analysis of cell viability (Fig. 3A) and Incucyte real-time imaging of caspase-3 and caspase-7 activation (Supplementary Fig. S5A), the TAS266 analogue and INBRX-109 induced similarly low rates of HepaRG cell apoptosis in the absence of IVIG. The activity of INBRX-109 remained unchanged with the addition of IVIG. In contrast, the TAS266 analogue cross-linked by ADAs reduced the viability of HepaRG cells to approximately 30% (Fig. 3A). The ADA-mediated hepatotoxicity of the TAS266 analogue could be attenuated by engineering the molecule to include modifications from INBRX-109 that eliminate those ADA recognition sites (TAS266 mut), formally demonstrating that higher-order clustering, and not tetravalent valency, is responsible for TAS266 hepatotoxicity.
Figure 3.
Hepatotoxicity analyses. A, The impact of valency and ADA on the risk of hepatoxicity was evaluated in HepaRG cells. Percent viability, as measured by CellTiter-Glo and using average relative light unit value for untreated cell samples for normalization, 41 hours after treatment with INBRX-109, hexavalent sdAb, TAS266 analogue, and TAS266 mut (a re-engineered version of the TAS266 analogue with no ADA recognition sites) in the presence or absence of IVIG as a source of preexisting ADA. B, The impact of valency on cell viability of treated 3D human liver microtissues as determined using intracellular ATP content after 7 days of treatment measured by CellTiter-Glo. C and D, The existence of preexisting anti-sdAb antibodies to INBRX-109 (C) or an analogue of TAS266 (D) was assessed in an ELISA-based competitive inhibition assay in sera from healthy individuals and patients with cancer. C and D show preexisting anti-sdAb antibodies that were at or above the cutpoint in an initial screen. Serum samples from healthy individuals and patients with cancer that had low screen signal to INBRX-109 (C) or TAS266 (D) were not analyzed in the confirmatory assay and assumed negative for ADA. TAS266 is an sdAb-based therapeutic with known preexisting ADA reactivity. Abbreviations: ADA, antidrug antibody; IVIG, intravenous immunoglobulin; mut, mutant.
INBRX-109 was also evaluated in a more physiologically relevant 3D liver spheroid model (3D InSight Human Liver Microtissues, InSphero) composed of primary human hepatocytes pooled from 10 donors cocultured with nonparenchymal liver cells (i.e., mostly Kupffer cells and liver endothelial cells). A hexavalent variant of INBRX-109 was included to determine the impact of higher-order valency on hepatotoxicity. Treatment with up to 10 nmol/L of INBRX-109 resulted in no cell death as determined by total cellular ATP (7-day treatment; Fig. 3B). In contrast, treatment with the hexavalent molecule [up to 1 nmol/L (IC50, 41.3 pmol/L)] led to dose-dependent hepatotoxicity. At 10 nmol/L, the highest concentration tested, the hexavalent molecule had substantial but reduced hepatotoxicity, suggesting that at high concentrations, not all DR5 binding domains in the hexavalent molecule engage receptors, which results in a lower functional valency. Similar findings were observed when cell death was determined by lactate dehydrogenase release (Supplementary Fig. S5B).
The immunogenicity of INBRX-109 was also assessed in an ELISA-based ADA assay (Fig. 3C). The prevalence of preexisting anti-sdAb antibodies was assessed in sera from healthy individuals and patients with cancer. INBRX-109 had an ADA rate of 0%, whereas a TAS266 analogue had a combined ADA rate of 36% across healthy individuals and patients with cancer (Fig. 3D; Supplementary Table S1), indicating successful elimination of recognition sites for preexisting ADAs in INBRX-109.
The pharmacodynamic characteristics, in vitro and in vivo antitumor activity, and safety profile observed in the preclinical setting demonstrated the therapeutic potential of INBRX-109 in chondrosarcoma. A first-in-human phase I study in patients with advanced solid tumors was initiated (NCT03715933); safety and efficacy findings for INBRX-109 in chondrosarcoma are presented.
Clinical
Patients
A total of 149 patients with advanced solid tumors were included. In part 1 (Supplementary Fig. S1), patients received INBRX-109 at five escalating dose levels ranging from 0.3 to 30 mg/kg. Common tumor types were mesothelioma, chondrosarcoma, synovial sarcoma, and adenocarcinomas. Patients had received a median of 5.5 lines (range, 1–15 lines) of previous systemic therapies. INBRX-109 was well tolerated, and a maximum tolerated dose was not reached; 3 mg/kg was selected as the recommended phase II dose. Based on preclinical activity and clinical efficacy observed in part 1, eight cohorts were opened in part 2 of the study (single-agent expansion), including two cohorts of patients with chondrosarcoma; cohort B4 consisted of patients with any subtype of chondrosarcoma (n = 23), and cohort B6 included patients with IDH1/IDH2-mutant conventional chondrosarcoma (n = 12). Part 3 (combination therapy expansion) is ongoing.
Overall, 33 patients with chondrosarcoma (84.8% conventional) were included in this analysis (Table 1); patients were largely representative of the overall chondrosarcoma patient population (Supplementary Table S2). Most patients were male (78.8%). Among the 11 patients with a known IDH1/IDH2 mutation status, 10 (90.9%) carried IDH1 mutations. Median age was 59 years (range, 21–86 years); patients with IDH1/IDH2-mutant chondrosarcoma were younger (median age, 54 years) than those in the general chondrosarcoma cohort (median age, 63 years). Most patients had grade 2 chondrosarcoma (64.3%) and metastatic disease (87.9%). Almost all patients (97.0%) had undergone surgical treatment; 36.4% had received radiotherapy. Patients had received a median of one prior line of systemic therapy (range, 0–12 lines), with the most common prior systemic therapies being chemotherapy, immunotherapy, and molecularly targeted therapies. As expected, patients with IDH1/IDH2-mutant chondrosarcoma were more likely to have received immunotherapy or molecularly targeted therapy. Approximately one-quarter of patients (24.2%) had received no prior systemic therapy. As of the data cutoff (May 26, 2022), 9 of 33 patients with chondrosarcoma continued to receive study treatment.
Table 1.
Baseline demographics and patient characteristics of patients with chondrosarcoma.
| Category | Chondrosarcoma, anya (n = 23) | IDHmt conventional chondrosarcomab (n = 10) | Total (n = 33) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 57.6 (16.7) | 50.9 (14.8) | 55.6 (16.24) |
| Median (range) | 63.0 (25.0–86.0) | 54.0 (21.0–67.0) | 59.0 (21.0–86.0) |
| Sex, n (%) | |||
| Male | 18 (78.3) | 8 (80.0) | 26 (78.8) |
| Female | 5 (21.7) | 2 (20.0) | 7 (21.2) |
| ECOG performance status, n (%) | |||
| 0 | 2 (8.7) | 1 (10.0) | 3 (9.1) |
| 1 | 21 (91.3) | 9 (90.0) | 30 (90.9) |
| Race, n (%)c | |||
| White | 19 (82.6) | 10 (100.0) | 29 (87.9) |
| Black or African American | 1 (4.3) | 0 | 1 (3.0) |
| Asian | 3 (13.0) | 0 | 3 (9.1) |
| Cancer stage, n (%) | |||
| I | 0 | 0 | 0 |
| II | 1 (4.3) | 0 | 1 (3.0) |
| III | 0 | 0 | 0 |
| IV | 18 (78.3) | 8 (80.0) | 26 (78.8) |
| Unknown | 4 (17.4) | 2 (20.0) | 6 (18.2) |
| Histological subtype | |||
| Conventional | 18 (78.3) | 10 (100) | 28 (84.8) |
| Dedifferentiated | 3 (13.0) | 0 | 3 (9.1) |
| Mesenchymal | 1 (4.3) | 0 | 1 (3.0) |
| Extraskeletal myxoidd | 1 (4.3) | 0 | 1 (3.0) |
| Histological grade, n (%)e | n = 16 | n = 10 | n = 26 |
| Grade 2 | 9 (56.3) | 8 (80.0) | 17 (65.4) |
| Grade 2/3 | 2 (12.5) | 2 (20.0) | 4 (15.4) |
| Grade 3 | 5 (31.3) | 0 | 5 (19.2) |
| IDH1/IDH2 mutation status, n (%) | |||
| IDH1 | 1 (4.3) | 9 (90.0) | 10 (30.3) |
| IDH2 | 0 | 1 (10.0) | 1 (3.0) |
| No or unknown | 22 (95.7) | 0 | 22 (66.7) |
| Disease status at study entry, n (%) | |||
| Metastatic | 20 (87.0) | 9 (90.0) | 29 (87.9) |
| Locally advanced | 3 (13.0) | 1 (10.0) | 4 (12.1) |
| Prior anticancer surgery, n (%) | |||
| Yes | 22 (95.7) | 10 (100.0) | 32 (97.0) |
| No or missing | 1 (4.3) | 0 | 1 (3.0) |
| Prior anticancer radiation, n (%) | |||
| Yes | 8 (34.8) | 4 (40.0) | 12 (36.4) |
| No or missing | 15 (65.2) | 6 (60.0) | 21 (63.6) |
| No. of lines of prior systemic therapy, median (range) | 1.0 (0–12) | 1.5 (0–4) | 1.0 (0–12) |
| Prior systemic therapy, n (%)f | |||
| Chemotherapy | 10 (43.5) | 4 (40.0) | 14 (42.4) |
| Immunotherapy | 8 (34.8) | 5 (50.0) | 13 (39.4) |
| Molecularly targeted therapy | 4 (17.4) | 6 (60.0) | 10 (30.3) |
| Biologicsg | 2 (8.7) | 0 | 2 (6.1) |
| None | 7 (30.4) | 1 (10.0) | 8 (24.2) |
Note: Data cutoff: May 26, 2022.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IDHmt, isocitrate dehydrogenase 1/2 mutant.
aIncludes 1 patient from dose-escalation cohort A4 (INBRX-109 10 mg/kg) and 22 patients from dose-expansion cohort B4 (INBRX-109 3 mg/kg). Five patients did not have conventional chondrosarcoma but are included because this was not an exclusion criterion at the time of their enrollment.
bTwo patients were excluded from the analysis due to taking prohibited medication (n = 1) or having dedifferentiated chondrosarcoma (n = 1).
cPatients could select >1 category for race.
dPatient was diagnosed with chondrosarcoma and described as having chondrosarcoma-like features per investigator assessment. Patient was allowed to enroll since inclusion in this cohort was dependent on diagnosis and not tumor histology.
eHistological grade was determined using pathology reports reviewed on an individual patient basis. Only conventional chondrosarcoma and evaluable patients were included.
fPatients could have received >1 systemic therapy.
gBiologics included ozuriftamab vedotin (BA3021) and DeltaRex-G (n = 1 for each).
Pharmacokinetic and immunogenicity profile
Following intravenous infusion, peak INBRX-109 concentrations (Cmax), which increased in an approximately dose-proportional manner across the single-agent dose-escalation dose range of 0.3 to 30 mg/kg, were observed at or near the end of the infusion (Supplementary Fig. S6). The mean terminal elimination half-life after the first dose ranged from ≈2 to 8 days (Supplementary Table S3). Early unaudited pharmacokinetic data from single-agent dose-expansion and combination cohorts showed no significant difference in INBRX-109 pharmacokinetics in patients with different tumor types or in combination with various anticancer agents. The expansion dose of 3 mg/kg every 3 weeks was selected based on clinical efficacy and safety data as well as pharmacokinetic/pharmacodynamic modeling of nonclinical and clinical data.
Preliminary immunogenicity assessments indicated that treatment-emergent ADAs against INBRX-109 were present in at least half of study patients; however, most samples were of low titer, and no significant impact on INBRX-109 pharmacokinetics was observed in any ADA-positive patient.
Safety
Treatment-related AEs occurred in 46.3% of all enrolled patients and were predominantly grade 1 or 2; 12.1% of patients had an AE of grade ≥3 (Table 2). The most common (in >5% of patients) treatment-related AEs were fatigue (18.8%; grade ≥3, 1.3%), increased alanine aminotransferase (10.7%; 4.0%), increased aspartate aminotransferase (8.7%; 2.7%), nausea (8.7%; 0.7%), and diarrhea (6.0%; 2.0%).
Table 2.
Frequent (in ≥3 patients) treatment-emergent and treatment-related adverse events.
| Chondrosarcomaa (n = 35) | Other tumors (n = 114) | All patients (N = 149) | ||||
|---|---|---|---|---|---|---|
| Preferred term, n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| ≥1 TEAE | 7 (20.0) | 2 (5.7) | 62 (54.4) | 16 (14.0) | 69 (46.3) | 18 (12.1) |
| Fatigue | 2 (5.7) | 0 | 26 (22.8) | 2 (1.8) | 28 (18.8) | 2 (1.3) |
| Alanine aminotransferase increased | 4 (11.4) | 0 | 12 (10.5) | 6 (5.3) | 16 (10.7) | 6 (4.0) |
| Nausea | 1 (2.9) | 0 | 12 (10.5) | 1 (0.9) | 13 (8.7) | 1 (0.7) |
| Aspartate aminotransferase increased | 3 (8.6) | 0 | 10 (8.8) | 4 (3.5) | 13 (8.7) | 4 (2.7) |
| Diarrhea | 0 | 0 | 9 (7.9) | 3 (2.6) | 9 (6.0) | 3 (2.0) |
| Pyrexia | 0 | 0 | 6 (5.3) | 1 (0.9) | 6 (4.0) | 1 (0.7) |
| Blood alkaline phosphate increased | 1 (2.9) | 0 | 4 (3.5) | 1 (0.9) | 5 (3.4) | 1 (0.7) |
| Constipation | 0 | 0 | 4 (3.5) | 0 | 4 (2.7) | 0 |
| Vomiting | 0 | 0 | 4 (3.5) | 1 (0.9) | 4 (2.7) | 1 (0.7) |
| Blood bilirubin increased | 2 (5.7) | 1 (2.9) | 2 (1.8) | 0 | 4 (2.7) | 1 (0.7) |
| Thrombocytopenia | 0 | 0 | 3 (2.6) | 1 (0.9) | 3 (2.0) | 1 (0.7) |
| Headache | 0 | 0 | 3 (2.6) | 0 | 3 (2.0) | 0 |
| Decreased appetite | 0 | 0 | 3 (2.6) | 0 | 3 (2.0) | 0 |
| Alopecia | 0 | 0 | 3 (2.6) | 0 | 3 (2.0) | 0 |
| Infusion-related reaction | 0 | 0 | 3 (2.6) | 1 (0.9) | 3 (2.0) | 1 (0.7) |
Note: TEAEs were recorded using MedDRA preferred terms and graded according to CTCAE v5.0. Related AEs are those considered to be possibly, probably, or likely/certain to be related to INBRX-109 by the principal investigator. AEs are presented based on descending frequency in any-grade AEs seen in all patients. Data cutoff: May 26, 2022.
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent AE.
aIncludes 1 patient from dose-escalation cohort A4 (INBRX-109 10 mg/kg), 22 patients from dose-expansion cohort B4 (INBRX-109 3 mg/kg), and 12 patients from dose-expansion cohort B6 (IDH1/IDH2-mutant chondrosarcoma; INBRX-109 3 mg/kg).
Among patients with chondrosarcoma, 20.0% had ≥1 treatment-related AE; 5.7% of AEs were grade ≥3 (Table 2). The most common (in >5% of patients) treatment-related AEs in patients with chondrosarcoma were increased alanine aminotransferase (11.4%; grade ≥3, 0%), increased aspartate aminotransferase (8.6%; 0%), increased blood bilirubin (5.7%; 2.9%), and fatigue (5.7%; 0%). Among patients with frequent treatment-related AEs, no grade 4 or 5 events were reported.
Given the potential for hepatotoxicity, liver-related AEs associated with INBRX-109 treatment were also assessed. Most patients (93.3%) had no or low-grade liver-related AEs associated with INBRX-109 treatment; grade ≥3 events were reported in 10 patients (6.7%). Similarly, liver-related AEs in patients with chondrosarcoma were mostly low grade; grade ≥3 events were reported in 2 patients. Two deaths occurred due to hepatic failure and were temporally associated with INBRX-109 treatment; 1 patient had mesothelioma, and 1 had IDH2-mutant chondrosarcoma. At the time of INBRX-109 initiation, the patient with mesothelioma was taking high doses of acetaminophen and antibiotics for the treatment of urinary tract infections, urosepsis, and pneumonia. The patient died 2 weeks after study start. The patient with chondrosarcoma experienced a significant increase in liver function tests 7 days after the first dose of INBRX-109. The patient was treated with steroids and plasmapheresis but experienced liver failure and died. It was later discovered that this patient had been self-medicating with high doses of fenbendazole, a veterinary antihelminthic that has been linked to hepatic dysfunction (20); the patient had received no prior systemic therapies.
Efficacy
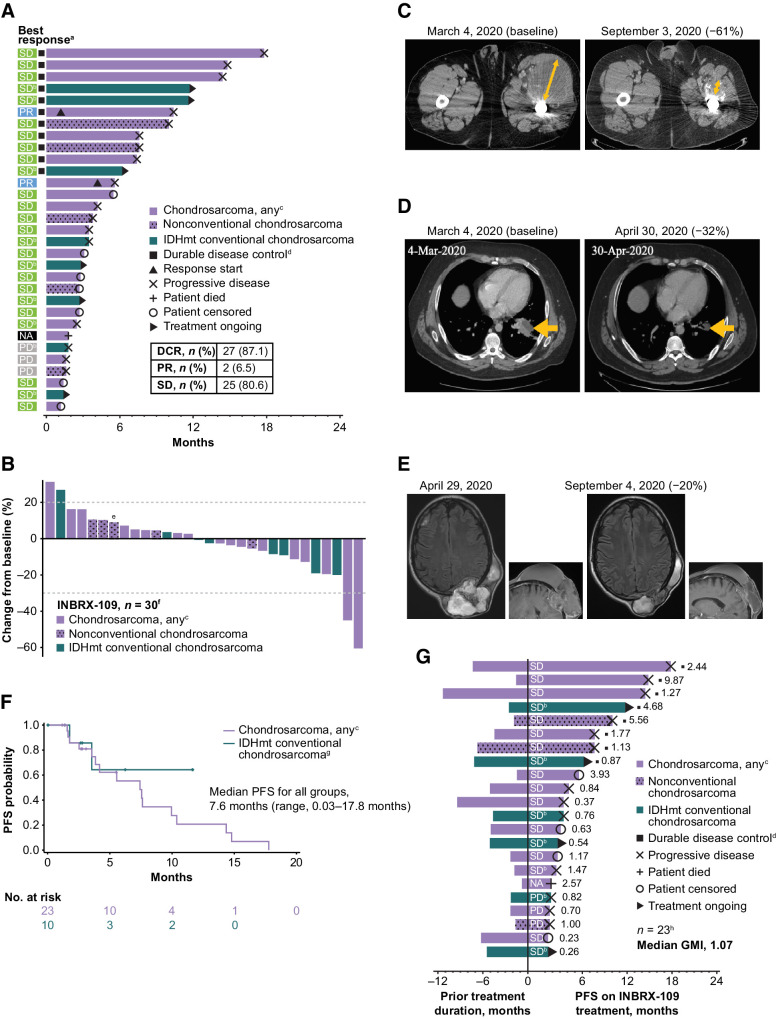
Among evaluable patients with chondrosarcoma (n = 31), the DCR—defined as best overall response of complete response, partial response (PR), or stable disease (SD) up to the first event of progression or death—was 87.1% (27/31; Fig. 4A). The DCR was 88.5% (23/26) in evaluable patients with conventional chondrosarcoma and 80.0% (4/5) in those with nonconventional subtypes. Although it is rare for chondrosarcoma tumors to shrink with currently available therapy, 2 patients (both with conventional chondrosarcoma) achieved PR (objective response rate, 6.5%). The observed clinical benefit was durable: 11 of 27 patients who achieved disease control had a clinical benefit lasting ≥180 days; the longest duration of SD was 17.8 months. Of the 25 patients who experienced SD, 14 (56.0%; 13/14 with conventional chondrosarcoma) had decreases from baseline in tumor size (Fig. 4B). Disease control was observed in patients with and without IDH1/IDH2 mutations. Five patients were treated for >12 months, including 2 with an IDH1/IDH2 mutation.
Figure 4.
Clinical efficacy of INBRX-109 in chondrosarcoma. A, Best tumor response and time receiving treatment up to the first event of progression or death. B, Best response up to data cutoff date. C and D, Representative baseline and posttreatment contrast-enhanced CT scans of patients who experienced a PR. Orange arrows show the diameter of target lesions. E, Representative baseline and posttreatment contrast-enhanced CT scans of a patient who experienced SD. All patients in C–E had grade 3, metastatic, conventional chondrosarcoma. F, PFS by Kaplan–Meier analysis. Median follow-up was 11.6 months. Crosses indicate censored data. G, Mean modified growth modulation index (modified GMI), as determined by the ratio of PFS with INBRX-109 to prior treatment duration. Best response to treatment with INBRX-109 is indicated within each bar, and the ratio of PFS on INBRX-109 to prior treatment duration is indicated at the right of each bar. Data cutoff: May 26, 2022. Abbreviations: GMI, growth modulation index; IDHmt, isocitrate dehydrogenase 1/2 mutant; NA, no postbaseline scan available; PD, progressive disease. aA total of 31 patients were included in the analysis. Four patients from cohort B6 were excluded for taking prohibited medications (n = 1), not having conventional chondrosarcoma (n = 1), or not having first scan data (n = 2). bPatient has a mutation in IDH1 (R132) or IDH2 (R172). cOne patient is from dose-escalation cohort A4 and received INBRX-109 at a dose of 10 mg/kg; all other patients are from dose-expansion cohort B4 (INBRX-109 3 mg/kg). Patients with nonconventional chondrosarcoma are indicated with a stippled pattern. dDurable disease control is SD, PR, or CR for >6 months. ePatient's first tumor assessment during treatment showed PD. A second scan post PD showed tumor shrinkage, and the best change from baseline up to data cutoff is shown; patient was not receiving a subsequent therapy at the time of the second scan. fA total of 30 patients were evaluable. Five patients were excluded for taking prohibited medications (n = 1; cohort B6), not having conventional chondrosarcoma (n = 1; cohort B6), not having first scan data (n = 2; cohort B6), or due to death (n = 1; cohort B4). gTwo patients were excluded due to taking prohibited medication (n = 1) or having dedifferentiated chondrosarcoma (n = 1). hOverall, 23 of 35 patients were included in this analysis. One patient had a long prior treatment duration of 84 months (cohort B6) and, although included in the analysis, is not included in the figure (GMI, 0.03). Patients were excluded for taking prohibited medications (n = 1; cohort B6), not having conventional chondrosarcoma (n = 1; cohort B6), or having a treatment duration that could not be estimated. (n = 10). Partial start and stop dates were ignored.
Representative CT scans of the 2 patients who achieved a PR are presented in Figs. 4C and D. Both patients had grade 3, metastatic, IDH1/IDH2 wild-type, conventional chondrosarcoma and had received no prior therapies before starting INBRX-109. The first patient, a 29-year-old man, experienced a 61% decrease in target lesions after starting treatment with INBRX-109; the response was durable, and he continued to receive study treatment for 45 weeks. The second patient, a 57-year-old woman, experienced a 32% decrease in target lesions after seven treatment cycles.
Representative CT scans of a patient who achieved SD are presented in Fig. 4E. This patient was a 55-year-old man with grade 3, metastatic, conventional chondrosarcoma who had received four prior lines of therapy, including pazopanib, nivolumab, and pazopanib plus nivolumab. The patient had a 20% decrease in target lesions after initiating INBRX-109; disease control was maintained for 17.8 months (Fig. 4A).
Median PFS among patients with chondrosarcoma (n = 33) was 7.6 months (range, 0.03–17.8 months; median follow-up, 11.6 months; Fig. 4F). The 3- and 6-month PFS rates were 82% and 58%, respectively. Median PFS was not reached in the cohort of patients with IDH1/IDH2-mutant conventional chondrosarcoma.
To further examine its antitumor activity, we determined the growth-modulating effect of INBRX-109 in chondrosarcoma. Growth modulation can be measured by calculating the GMI, or von Hoff ratio (21), which is defined as the ratio of the time to progression (TTP) with a new treatment versus TTP with the prior line; the patient serves as their own control (21). In our study, TTP with the last therapy was not available. To circumvent this, we used a modified analysis that determined the ratio of TTP with INBRX-109 versus prior treatment duration (modified GMI). Among 23 evaluable patients, the median modified GMI was 1.07 (range, 0.03–9.87; Fig. 4G). Overall, 54.5% of patients had a modified GMI of >1, 36.3% had a modified GMI of >1.33 (i.e., 33% improvement), and 31.8% had a modified GMI of >1.5 (i.e., 50% improvement), further indicating that INBRX-109 has antitumor activity in chondrosarcoma.
Discussion
Drug development for rare diseases is challenging. INBRX-109, a precisely engineered, tetravalent, DR5 agonist, has been designed to overcome the limitations of earlier-generation agonists that lacked efficacy or ceased development due to hepatoxicity. This study demonstrated that INBRX-109 had encouraging antitumor activity in chondrosarcoma and a manageable safety profile, suggesting that DR5 is a valid target in the treatment of this tumor.
DR5 has long been of interest in oncology due to its ability to induce programmed cell death through the extrinsic apoptosis pathway in a cancer-biased manner (8, 9). The first DR5 agonist to enter the clinic, dulanermin (recombinant TRAIL), showed some promising clinical activity in chondrosarcoma; however, rapid clearance and decoy receptor binding limited its activity (9, 17, 22–24). Several bivalent antibodies targeting DR5 were also developed on the basis of promising in vitro and in vivo preclinical data (25–28). Unfortunately, the clinical activity for bivalent antibodies was underwhelming, likely due to requiring Fc receptor engagement for DR5 clustering and activity (29, 30). To circumvent this requirement, a second-generation DR5 agonist, TAS266, was developed using camelid-derived sdAbs to create a tetravalent molecule designed to more efficiently cluster DR5 in the absence of Fc receptor engagement (31). However, preexisting ADAs toward this molecule led to additional higher-order DR5 clustering that was presumably responsible for the observed hepatotoxicity that led to the cessation of a phase I trial (16).
INBRX-109 also has a tetravalent configuration and induces DR5-mediated apoptosis without the need for additional cross-linking. In preclinical studies, DR5 receptor clustering by INBRX-109 induced death in H-EMC-SS cells (Fig. 2). This chondrosarcoma-like line has high DR5 expression, making it well suited for characterizing the activity of INBRX-109, and was previously used to evaluate an anti-DR5 pentameric IgM antibody that is currently in clinical development (32).
With the activity of INBRX-109 as a DR5 agonist established in vitro, we examined its activity in PDX mouse models of various cancers, including chondrosarcoma. The encouraging results that we observed (Fig. 2E), along with prior studies that suggested that targeting DR5 in this tumor type could be beneficial (17, 18), motivated our clinical evaluation of INBRX-109. In patients with metastatic or locally advanced chondrosarcoma, INBRX-109 demonstrated antitumor activity (Fig. 4) and, unlike TAS266, minimal hepatotoxicity both in vitro (Fig. 3) and in the clinical setting (Table 2).
Although both tetravalent and hexavalent anti-DR5 antibodies show superior cytotoxic activity to bivalent antibodies and trimeric TRAIL in the absence of Fc receptor binding, increasing valency carries with it an increased risk of activity on normal healthy tissue, as was seen with HexaBody-DR5/DR5 (GEN1029), a mixture of two DR5-specific antibodies with a hexamerization-enhancing Fc mutation [ref. 33; Genmab. (cited August 8, 2022). Available from: https://ir.genmab.com/static-files/60467b61-f6ed-4196-ae15–455d57d9f919.]. In August 2019, the FDA issued a partial clinical hold on a phase I/II study of HexaBody DR5/DR5 (NCT03576131) due to liver toxicity. The clinical hold was lifted in October 2019; however, development of the drug was later stopped due to what the sponsor considered to be a narrow therapeutic index [Genmab. (cited August 8, 2022). Available from: https://ir.genmab.com/static-files/be692ea1–1ee7–44d3-ab20-f0dda61ea843]. In our study, the hexavalent comparator showed robust cross-linking–independent hepatocyte cytotoxicity in vitro (Fig. 3). In contrast, INBRX-109 demonstrated little induction of the DR5-mediated apoptotic pathway in hepatocytes, suggesting that a tetravalent molecule may have achieved an optimal combination of activity and safety.
In this phase I study, INBRX-109 was well tolerated and demonstrated a manageable safety profile in patients with chondrosarcoma. Most treatment-related AEs in patients with chondrosarcoma or in those with other tumors were low grade. Among those with chondrosarcoma who experienced frequently reported AEs, only 1 patient had a grade 3 event (increased blood bilirubin). Similarly, liver-related AEs were mainly grade 1 or 2, indicating that the INBRX-109 design minimizes hepatotoxicity, unlike earlier DR5 agonists. Two deaths occurred, both due to hepatic failure. Both patients had several comorbidities and/or were taking concomitant medications, including fenbendazole, a prohibited treatment linked to hepatotoxicity (20). Therefore, the deaths were considered as possibly related to INBRX-109 treatment by the investigators. To better understand any treatment-related effects on liver function, hepatotoxicity will continue to be closely monitored in future studies of INBRX-109.
INBRX-109 demonstrated single-agent activity in chondrosarcoma—including in the traditionally chemotherapy-resistant conventional chondrosarcoma subtype—with a DCR of 87.1%; 2 patients achieved a PR (6.5%; Fig. 4). Although a direct comparison is not possible, this rate was much higher than the DCR of 31.3% observed in patients treated with placebo (n = 16) in a randomized phase II study in advanced chondrosarcoma (7). These suggest that the observed DCR in our study was due to the activity of INBRX-109 and not the indolent nature of the disease. Similar to our study, in the phase II study most patients had conventional chondrosarcoma; only 2 patients in the placebo arm had nonconventional subtypes (7).
As expected in sarcomas (34, 35), few objective responses by RECIST criteria were observed. However, 25 of 31 patients (80.6%) achieved SD, regardless of IDH1/IDH2 mutation status, with many of these patients having decreases in tumor size (Fig. 4). Disease control was durable for >6 months in 11 of 27 patients (40.7%). We acknowledge that, due to the indolent nature of chondrosarcoma, the use of RECIST criteria may lead to an overinterpretation of the DCR for this tumor indication. In the study by Duffaud and colleagues (7), 5 of 16 patients (31.3%) in the placebo arm had tumor shrinkage. However, in our study, tumor shrinkage was observed in 16 of 30 evaluable patients (53.3%; 56% of patients with SD; Fig. 4B). These data suggest activity of INBRX-109 in chondrosarcoma.
These findings with INBRX-109 are notable given the lack of effective therapies in this setting and the lower DCR seen with targeted single-agent therapies in recent studies. A phase I study of ivosidenib (IDH1 inhibitor) in patients with IDH1-mutant advanced solid tumors reported a DCR of 40% (no complete response or PR) in 21 patients with advanced chondrosarcoma (36). Ivosidenib is considered a systemic treatment option for patients with IDH1-mutant chondrosarcoma despite no objective response observed in the phase I study (36), highlighting the importance of SD in this setting and the need for more effective treatment options. Similarly, a phase II study of pazopanib in patients with unresectable or metastatic conventional chondrosarcoma (N = 47) reported a DCR of 42% at week 16, with 1 patient achieving a PR (37); olaparib, a PARP inhibitor, led to a DCR of 40% in 5 patients with chondrosarcoma in a phase II, open-label study in patients with metastatic solid tumors (38).
Given the limitations with RECIST criteria, other measures of activity, such as nonprogression and tumor growth delay, have been explored and are widely considered to be appropriate indicators of activity in sarcomas (39, 40). In our study, nonprogression was determined by PFS, which has been suggested as a measure of success for targeted therapies in sarcomas (21, 34, 35, 41). Patients treated with INBRX-109 had a median PFS of 7.6 months, which was substantially longer than what has been observed with chemotherapy (usually <4 months; ref. 5) and other recently evaluated targeted therapies, including ivosidenib (5.6 months) (36) and the tyrosine kinase inhibitor regorafenib (≈5 months; ref. 7). Median PFS in patients treated with pazopanib was similar (7.9 months; ref. 37).
The GMI, which measures tumor growth delay, has also been considered by some to be a way of determining success in studies of sarcomas (39). In a study evaluating concordance between the GMI and efficacy outcomes in advanced sarcomas, a higher GMI correlated with better outcomes, including survival (39). A GMI of >1 was considered a sign of activity, and a GMI of >1.33 (i.e., 33% improvement) was considered a more conservative threshold (21). In our study, 36.3% and 31.8% of patients had modified GMIs of >1.33 and >1.5, respectively, suggesting that INBRX-109 has an antitumor effect and can delay tumor growth in patients with chondrosarcoma. However, our modified analysis used the time receiving prior line of treatment rather than TTP, which could make interpretation of the results difficult given the potential confounders. For example, physicians may change a patient's treatment before progression if they believe a new treatment regimen will lead to greater clinical benefits or tolerability. Overall, INBRX-109 not only demonstrated clinical activity by RECIST criteria (objective response and tumor shrinkage) but also by nonprogression and tumor growth delay, which are two additional measures considered to be appropriate indicators of activity in sarcomas (39, 40).
Together with previous findings, our data suggest that DR5 is a valid target in the treatment of chondrosarcoma. Although the mechanisms driving tumor response to INBRX-109 are not known, enhanced sensitivity of chondrosarcoma to DR5 agonism could play a role. It is not uncommon for tumors to develop resistance to DR5 agonism due to prior exposure to TRAIL expressed on infiltrating immune cells (42). However, immune cell infiltration into conventional chondrosarcoma is poor (43–45), making chondrosarcoma less likely to develop resistance to DR5 agonism; this provides support for the use of DR5 agonists in this tumor type. Another possible mechanism involves the activity of DR5 agonism on tumor-associated, suppressive immune cell populations. Immune infiltrates in chondrosarcoma are largely composed of immunosuppressive macrophages (44, 45), which have been shown to express functional DR5 (46). In a study of patients with advanced cancers (NCT02076451), the DR5 agonist DS8273a reduced the tumor-associated, myeloid-derived suppressor cell population (47), suggesting that INBRX-109 could be acting on this suppressive immune cell population in chondrosarcoma. Given that tumor DR5 expression could not be assessed in patients at baseline, additional studies are needed to better understand the mechanism of action of INBRX-109 in chondrosarcoma and the link between DR5 levels on tumor and/or tumor-infiltrating immune cells and response to INBRX-109. To further evaluate INBRX-109 and address these open questions, a randomized, blinded, placebo-controlled, phase II trial of INBRX-109 in unresectable or metastatic conventional chondrosarcoma (ChonDRAgon; NCT04950075) is currently enrolling in the United States and Europe. In addition, the phase I study (NCT03715933) is now exploring clinical activity of INBRX-109 in patients with nonconventional chondrosarcoma. This cohort is still enrolling, and findings will be reported in a separate manuscript.
In conclusion, INBRX-109 demonstrated a manageable safety profile and led to a high DCR of 87.1% in patients with metastatic or advanced chondrosarcoma; 2 patients achieved PR, and approximately half of the patients achieved tumor shrinkage. Importantly, median PFS with INBRX-109 was 7.6 months, which is a substantial improvement over results with currently available therapies. However, given the indolent nature of the disease, randomized controlled studies, like the ongoing ChonDRAgon study, are needed to validate these early findings. Overall, our study suggests that DR5 is a valid target in chondrosarcoma and indicates that INBRX-109 has antitumor activity in unresectable or metastatic chondrosarcoma, regardless of IDH1/IDH2 mutation status. Importantly, findings from this study fill a highly unmet need in a rare disease and provide support for continued evaluation of INBRX-109 in unresectable or metastatic chondrosarcoma.
Supplementary Material
Supplementary Tables, Figures, and References
Acknowledgments
This study was funded by Inhibrx. We thank the patients and their families; Maria Diab, R. Donald Harvey, Robert G. Maki, Lee Hartner, Joseph Chao, Mark Agulnik, and all phase I INBRX-109 trial investigators; Miranda Fox, Kevin Bayer, Michelle Darling, Klaus Wagner, Quinn Deveraux, John Timmer, and the rest of the Inhibrx research team; Bruno Filippi and the rest of the InSphero team; and all other personnel involved in the study for their participation and contributions. We also thank Karen Chinchilla, PhD, of ArticulateScience, LLC, for providing medical writing assistance, which was funded by Inhibrx. V. Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center, acknowledges support of The Jacquelyn A. Brady Fund, and is supported by a US NIH grant (no. R01CA242845 and R01CA273168). The MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (no. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (no. P30 CA016672).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
V. Subbiah reports grants from Inhibrx during the conduct of the study; reports support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty Ltd.; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi Sankyo, R-Pharm US, and Jazz Pharmaceuticals; and other relationship with Peerview and Medscape. A.P. Conley reports grants and personal fees from Inhibrx during the conduct of the study; personal fees from Aadi Bioscience, Applied Clinical Intelligence, and Guidepoint Global; grants from Eli Lily, EpicentRx, NCI, Nant Pharma; and other support from OncLive and Medscape outside the submitted work. B.A. Wilky reports personal fees from Adaptimmune, Adcendo, Daiichi Sankyo, Epizyme, Polaris, Springworks, and Boehringer Ingelheim outside the submitted work. A. Tolcher reports personal fees and other support from AbbVie, Inc., Aclaris Therapeutics, Agenus, Inc., Asana Biosciences, Ascentage, Axlmmune, Bayer, BluPrint Oncology, Daiichi Sankyo, Inc., Gilde Healthcare Partners, HBM Partners, IDEA Pharma, Immuneering, Immunomet Therapeutics, Inc., Impact Therapeutics US, Inc., Karma Oncology B.V., Kirilys Therapeutics, Inc., Lengo Therapeutics, Inc., Link Immunotherapeutics, Mekanistic Therapeutics, Menarini Ricerche, Mersana, Nanobiotix, Novo Nordisk Inc., Nerviano Medical Sciences S.r.I. (NMS), Nurix Therapeutics, Ocellaris Pharma, Inc. & Eli Lilly, Partner Therapeutics, Pfizer Inc., Qualigen Therapeutics, Pierre Fabre, Roche, Ryvu Therapeutics, Seattle Genetics (Seagen), SK Life Science, SOTIO Biotechnology Co., Spirea Limited Inc., Sunshine Guojian Pharmaceutical (Shanghai) Co., Ltd., Transcenta Therapeutics Inc., Transgene, Trillium Therapeutics Inc., Verastem Oncology, VRise Therapeutics, Inc., and Zentalis Pharmaceuticals during the conduct of the study; personal fees and other support from Adagene, Inc., Aro Biotherapeutics, BioInvent, Boehringer Ingelheim International GmbH, Bright Peak Therapeutics, Deka Biosciences, Eleven Bio, Elucida, EMD Serono/Merck KGaA, HiberCell, Inc., Ikena Oncology, Immunome, Janssen Global Services, LLC, NBE Therapeutics, Pelican, Jazz Pharmaceuticals, Pieris Pharma, Pyxis Oncology, Senti Biosciences, Vincerx, ZielBio, Inc., Zymeworks Biopharmaceuticals Inc., Mirati, and Roche outside the submitted work; and also has a patent for Ascentage Pharma issued and a patent for Zentalis issued. N.J. Lakhani reports other support from Inhibrx, Alpine Biosciences, Janssen, GSK, Celgene, Constellation, CytomX, Gilead, Helsinn, Jounce, KSQ Therapeutics, Loxo/Lilly, Macrogenics, Alkermes, Regeneron, Repare Therapeutics, Servier, Shattuck Labs, Sapience Therapeutics, Symphogen, Odonate, Tizona, and Mersana and personal fees and other support from Ikena and SK Life Science during the conduct of the study. D. Berz reports personal fees from Jazz Pharma, Mirati, EMD, and Sun Pharma outside the submitted work. W. Crago reports nonfinancial support from ArticulateScience, LLC, during the conduct of the study; other support from Inhibrx, Inc. outside the submitted work; and also has a patent for Various Patents with Inhibrx, Inc. pending and issued. M. Holcomb reports other support from Inhibrx, Inc. outside the submitted work and also has a patent for DR5 agonist and IAP antagonist combination therapy pending. A. Hussain reports other support from ArticulateScience, LLC, during the conduct of the study; other support from Inhibrx, Inc. outside the submitted work; and also has a patent for Multivalent and Multispecific DR5-Binding Fusion Proteins issued. C. Veldstra reports personal fees from Inhibrx, Inc. outside the submitted work and ownership of Inhibrx, Inc. stock. L. Senne reports other support from Inhibrx, Inc. outside the submitted work. K.M. Willis reports other support from ArticulateScience, LLC, during the conduct of the study; other support from Inhibrx, Inc. outside the submitted work; and also has a patent for Multivalent and Multispecific DR5-Binding Fusion Proteins issued. B.P. Eckelman reports personal fees from Inhibrx Inc. outside the submitted work and also has a patent for US Patent 11,117,973 issued and a patent for US Patent 10,308,720 issued. No disclosures were reported by the other authors.
Authors' Contributions
V. Subbiah: Resources, supervision, investigation, writing–original draft, writing–review and editing. S.P. Chawla: Resources, supervision, investigation, writing–original draft, writing–review and editing. A.P. Conley: Resources, supervision, investigation, writing–original draft, writing–review and editing. B.A. Wilky: Resources, supervision, investigation, writing–original draft, writing–review and editing. A. Tolcher: Resources, supervision, investigation, writing–original draft, writing–review and editing. N.J. Lakhani: Resources, supervision, investigation, writing–original draft, writing–review and editing. D. Berz: Resources, supervision, investigation, writing–original draft, writing–review and editing. V. Andrianov: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. W. Crago: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. M. Holcomb: Conceptualization, data curation, formal analysis, methodology, writing–original draft, project administration, writing–review and editing. A. Hussain: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. C. Veldstra: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. J. Kalabus: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. B. O'Neill: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. L. Senne: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. E. Rowell: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. A.B. Heidt: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing-original draft, writing–review and editing. K.M. Willis: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. B.P. Eckelman: Conceptualization, data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing.
References
- 1. WHO Classification of Tumours editorial board, editor. WHO classification of tumours. Soft tissue and bone tumours. 5 ed. Volume3. Lyon: IARC Press; 2020. [Google Scholar]
- 2. Unni KK, Inwards CY. Dahlin's bone tumors: general aspects and data on 10,165 cases. Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010, vii. p.402. [Google Scholar]
- 3. Picci P, Manfrini M, Donati DM, Gambarotti M, Righi A, Vanel D, et al. Diagnosis of musculoskeletal tumors and tumor-like conditions: clinical, radiological and histological correlations-the Rizzoli case archive. Springer; 2020. [Google Scholar]
- 4. Monga V, Mani H, Hirbe A, Milhem M. Non-conventional treatments for conventional chondrosarcoma. Cancers (Basel) 2020;12:1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Maldegem A, Conley AP, Rutkowski P, Patel SR, Lugowska I, Desar IME, et al. Outcome of first-line systemic treatment for unresectable conventional, dedifferentiated, mesenchymal, and clear cell chondrosarcoma. Oncologist 2019;24:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Tsuchiya H. Therapeutic targets and emerging treatments in advanced chondrosarcoma. Int J Mol Sci 2022;23:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duffaud F, Italiano A, Bompas E, Rios M, Penel N, Mir O, et al. Efficacy and safety of regorafenib in patients with metastatic or locally advanced chondrosarcoma: results of a non-comparative, randomised, double-blind, placebo controlled, multicentre phase II study. Eur J Cancer 2021;150:108–18. [DOI] [PubMed] [Google Scholar]
- 8. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999;5:157–63. [DOI] [PubMed] [Google Scholar]
- 9. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999;104:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther 2005;4:139–63. [DOI] [PubMed] [Google Scholar]
- 11. Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci 2005;118:265–7. [DOI] [PubMed] [Google Scholar]
- 12. Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2000;2:241–3. [DOI] [PubMed] [Google Scholar]
- 13. Valley CC, Lewis AK, Mudaliar DJ, Perlmutter JD, Braun AR, Karim CB, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces death receptor 5 networks that are highly organized. J Biol Chem 2012;287:21265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan L, Fu TM, Zhao W, Zhao L, Chen W, Qiu C, et al. Higher-order clustering of the transmembrane anchor of DR5 drives signaling. Cell 2019;176:1477–89.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF, et al. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci U S A 2015;112:5679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papadopoulos KP, Isaacs R, Bilic S, Kentsch K, Huet HA, Hofmann M, et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother Pharmacol 2015;75:887–95. [DOI] [PubMed] [Google Scholar]
- 17. Subbiah V, Brown RE, Buryanek J, Trent J, Ashkenazi A, Herbst R, et al. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther 2012;11:2541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 2010;16:1256–63. [DOI] [PubMed] [Google Scholar]
- 19. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997;326:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamaguchi T, Shimizu J, Oya Y, Horio Y, Hida T. Drug-induced liver injury in a patient with nonsmall cell lung cancer after the self-administration of fenbendazole based on social media information. Case Rep Oncol 2021;14:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs—twenty-first Richard and Hinda Rosenthal Foundation award lecture. Clin Cancer Res 1998;4:1079–86. [PubMed] [Google Scholar]
- 22. Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol 2010;28:1527–33. [DOI] [PubMed] [Google Scholar]
- 23. Soria JC, Márk Z, Zatloukal P, Szima B, Albert I, Juhász E, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4442–51. [DOI] [PubMed] [Google Scholar]
- 24. Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 2001;299:31–8. [PubMed] [Google Scholar]
- 25. Zeng Y, Wu XX, Fiscella M, Shimada O, Humphreys R, Albert V, et al. Monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells in vitro and inhibits tumor growth in vivo. Int J Oncol 2006;28:421–30. [DOI] [PubMed] [Google Scholar]
- 26. Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol 2008;19:1060–7. [DOI] [PubMed] [Google Scholar]
- 27. Sharma S, de Vries EG, Infante JR, Oldenhuis CN, Gietema JA, Yang L, et al. Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Invest New Drugs 2014;32:135–44. [DOI] [PubMed] [Google Scholar]
- 28. Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther 2010;9:618–31. [DOI] [PubMed] [Google Scholar]
- 29. Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 2011;19:101–13. [DOI] [PubMed] [Google Scholar]
- 30. Li F, Ravetch JV. Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement. Proc Natl Acad Sci U S A 2012;109:10966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huet HA, Growney JD, Johnson JA, Li J, Bilic S, Ostrom L, et al. Multivalent nanobodies targeting death receptor 5 elicit superior tumor cell killing through efficient caspase induction. MAbs 2014;6:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang BT, Kothambawala T, Wang L, Matthew TJ, Calhoun SE, Saini AK, et al. Multimeric anti-DR5 IgM agonist antibody IGM-8444 is a potent inducer of cancer cell apoptosis and synergizes with chemotherapy and BCL-2 inhibitor ABT-199. Mol Cancer Ther 2021;20:2483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Overdijk MB, Strumane K, Beurskens FJ, Ortiz Buijsse A, Vermot-Desroches C, Vuillermoz BS, et al. Dual epitope targeting and enhanced hexamerization by DR5 antibodies as a novel approach to induce potent antitumor activity through DR5 agonism. Mol Cancer Ther 2020;19:2126–38. [DOI] [PubMed] [Google Scholar]
- 34. Livingston JA, Hess KR, Naing A, Hong DS, Patel S, Benjamin RS, et al. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase I clinical trials. Oncotarget 2016;7:64421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760–4. [DOI] [PubMed] [Google Scholar]
- 36. Tap WD, Villalobos VM, Cote GM, Burris H, Janku F, Mir O, et al. Phase I study of the mutant IDH1 inhibitor ivosidenib: safety and clinical activity in patients with advanced chondrosarcoma. J Clin Oncol 2020;38:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow W, Frankel P, Ruel C, Araujo DM, Milhem M, Okuno S, et al. Results of a prospective phase 2 study of pazopanib in patients with surgically unresectable or metastatic chondrosarcoma. Cancer 2020;126:105–11. [DOI] [PubMed] [Google Scholar]
- 38. Eder JP, Doroshow DB, Do KT, Keedy VL, Sklar JS, Glazer P, et al. Clinical efficacy of olaparib in IDH1/IDH2-mutant mesenchymal sarcomas. JCO Precis Oncol 2021;5:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penel N, Demetri GD, Blay JY, Cousin S, Maki RG, Chawla SP, et al. Growth modulation index as metric of clinical benefit assessment among advanced soft tissue sarcoma patients receiving trabectedin as a salvage therapy. Ann Oncol 2013;24:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol 2010;11:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mick R, Crowley JJ, Carroll RJ. Phase II clinical trial design for noncytotoxic anticancer agents for which time to disease progression is the primary endpoint. Control Clin Trials 2000;21:343–59. [DOI] [PubMed] [Google Scholar]
- 42. Sag D, Ayyildiz ZO, Gunalp S, Wingender G. The role of TRAIL/DRs in the modulation of immune cells and responses. Cancers (Basel) 2019;11:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kostine M, Cleven AH, de Miranda NF, Italiano A, Cleton-Jansen AM, Bovee JV. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod Pathol 2016;29:1028–37. [DOI] [PubMed] [Google Scholar]
- 44. Iseulys R, Anne GB, Corinne B, Gonzague DBP, Marie K, Jean-Yves B, et al. The immune landscape of chondrosarcoma reveals an immunosuppressive environment in the dedifferentiated subtypes and exposes CSFR1+ macrophages as a promising therapeutic target. J Bone Oncol 2020;20:100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simard FA, Richert I, Vandermoeten A, Decouvelaere AV, Michot JP, Caux C, et al. Description of the immune microenvironment of chondrosarcoma and contribution to progression. Oncoimmunology 2017;6:e1265716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liguori M, Buracchi C, Pasqualini F, Bergomas F, Pesce S, Sironi M, et al. Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget 2016;7:41662–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res 2017;23:2942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables, Figures, and References
Data Availability Statement
The data generated and/or analyzed in this study are not publicly available but are available upon reasonable request. Sharing of data is subject to protection of patient privacy and respect for the patient's informed consent. For more information on the process or to submit a request, contact clinicaltrials@inhibrx.com.