Abstract
Intra-tumor heterogeneity is now arguably one of the most-studied topics in tumor biology, as it represents a major obstacle to effective cancer treatment. Since tumor cells are highly diverse at genetic, epigenetic, and phenotypic levels, intra-tumor heterogeneity can be assumed as an important contributing factor to the nullification of chemotherapeutic effects, and recurrence of the tumor. Based on the role of heterogeneous subpopulations of cancer cells with varying cell-cycle dynamics and behavior during cancer progression and treatment; herein, we aim to establish a comprehensive definition for adaptation of neoplastic cells against therapy. We discuss two parallel and yet distinct subpopulations of tumor cells that play pivotal roles in reducing the effects of chemotherapy: “resistant” and “tolerant” populations. Furthermore, this review also highlights the impact of the quiescent phase of the cell cycle as a survival mechanism for cancer cells. Beyond understanding the mechanisms underlying the quiescence, it provides an insightful perspective on cancer stem cells (CSCs) and their dual and intertwined functions based on their cell cycle state in response to treatment. Moreover, CSCs, epithelial–mesenchymal transformed cells, circulating tumor cells (CTCs), and disseminated tumor cells (DTCs), which are mostly in a quiescent state of the cell cycle are proved to have multiple biological links and can be implicated in our viewpoint of cell cycle heterogeneity in tumors. Overall, increasing our knowledge of cell cycle heterogeneity is a key to identifying new therapeutic solutions, and this emerging concept may provide us with new opportunities to prevent the dreadful cancer recurrence.
Keywords: Cancer stem cells (CSCs), Carcinoma, Cell cycle heterogeneity, Quiescence, Therapy resistance, Therapy tolerance, Tumor dormancy
Introduction
Tumor heterogeneity with particular emphasis on the cell cycle role
Cancer recurrence is defined as the return of cancer cells after a period of time, post-therapy.1 The remaining cancer cells after treatment cause cancer recurrence with a high risk of mortality. For instance, ovarian carcinoma is among the most common causes of death related to gynecologic tumors in female-specific cancers in the western world.2 Despite a huge progress in treatment strategies, including chemotherapy, radiotherapy and surgery, approximately 70% of ovarian carcinoma patients experience cancer recurrence.3, 4, 5 Similarly, 30%–55% of patients with non-small cell lung cancer (NSCLC) would suffer from a relapse6; and about 20% of pediatric acute lymphoblastic leukemia patients develop recurrence.7 Notably, cancer recurrence happens due to the proliferation of some sub-populations within a heterogeneous cancer cell population.8
Intra-tumor heterogeneity is a contributing factor to the nullification of chemotherapeutic effects, and recurrence of cancer; which represents a major obstacle to effective cancer treatment and personalized medicine.9,10
In both theoretical and experimental studies, it has been well-documented that approximately all cellular systems are heterogeneous.11,12 At many diverse levels and for several different reasons, heterogeneity can occur to improve functionality and survival. Based on the clonal evolution model and the action of Darwinian natural selection, carcinogenesis can be defined as an evolutionary process whereby somatic cells alter their phenotype by acquiring random (epi) mutations and clonally expanding the fittest new neoplastic population. This phenomenon, by repeated rounds of mutation and natural selection, can lead to the emergence of a malignant population, which is capable of migration and growth in remote sites.13 Heterogeneity is also generated through cellular differentiation. In the cancer stem cell (CSC) model, tumors are organized hierarchically, in a manner equivalent to the normal tissue hierarchy, which is supported by healthy stem cells. Therefore, cellular heterogeneity can be generated by CSCs through establishing a differentiation hierarchy and producing a range of distinct cell types present within the tumor.14 Moreover, by exerting various selective pressures, the tumor microenvironment (TME) can generate cellular diversity and heterogeneity in entirely different areas of the tumor.15,16 An intricate system with multiple layers of heterogeneity established by distinguished (epi) genome, transcriptome, proteome and functional properties of different cells can be generated by combining these three models, which can act together. Briefly, intra-tumor heterogeneity (also known as intra-lesion heterogeneity) reflects the variety of tumor cell populations within the same tumor specimen and can be defined in terms of different molecular and phenotypical profiles.17, 18, 19
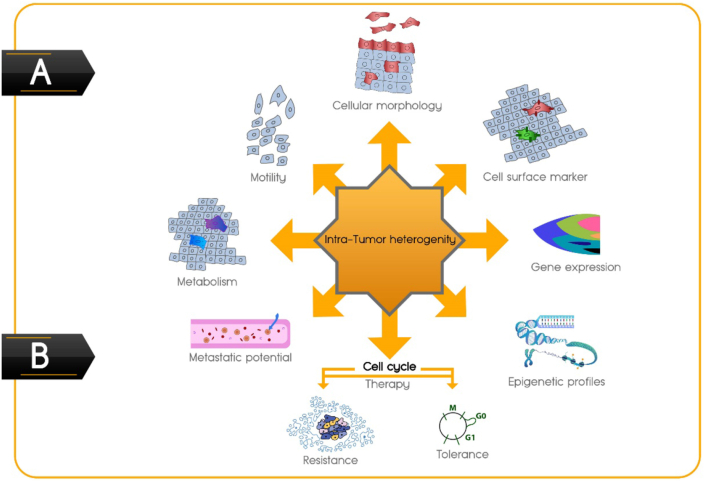
Despite our improved understanding of the biological complexity of intra-tumor heterogeneity in terms of cellular morphology, cell surface markers, gene expression, epigenetic variation, motility, metabolism, and metastatic potential,18,20 another aspect that should be taken into consideration is tumor cell cycle heterogeneity, which has lagged behind (Fig. 1A). By cell-cycle phase analysis at the single-cell level in solid tumors, different studies have demonstrated considerable evidence that distribution of cell cycle phases among cancer cells within a tumor is different and heterogeneous,21, 22, 23 which can be considered as one of the important facets of tumor heterogeneity. As indicated in a review by Douglas and Haddad, the cell-cycle duration of each cancer cell varies within the tumor mass, from 10 min to 50 h per cycle.24 Moreover, it was shown that even cancerous sister cells, which are more similar to unrelated cells, reach half the maximum variation of cell-cycle duration between 3 and 4 generations, producing more clonal heterogeneity regarding the cell cycle.25 Using a fluorescent ubiquitination-based cell cycle indicator (FUCCI) for monitoring tumor cells, Chittajallu et al could visualize G1, late G1/early S, S/G2, and mitosis in vivo.26 Yano et al studied tumor cells in a spatiotemporal manner through FUCCI imaging and established locational dependence of cell cycle dynamics and chemo-sensitivity. They found that 84% of cells were in the S/G2/M phases of the cell cycle in nascent tumors,27 while the quiescent cancer cells increased during the tumor growth.26,27 Moreover, in a tumor, access to nutrients, and oxygen as well as other factors such as cell density and proximity to particular stromal elements can influence the cycling behavior of cancerous cells.28, 29, 30
Figure 1.
Intra-tumor heterogeneity. (A) Different facets of intra-tumor heterogeneity with emphasis on cell cycle heterogeneity in cancer. (B) Two parallel and yet distinct adaption mechanisms of neoplastic cells to chemotherapeutic drugs.
Beaumont et al found that limited access to nutrients and oxygen (hypoxia) in certain tumor areas can induce the quiescent phase (G1 arrest) and affect the tumor cells sensitivity to chemotherapy.31 However, Granada et al showed that the cell cycle phase at the time of cisplatin addition was not predictive of outcome, and response to therapy was dependent on the dose; interestingly, they also suggested that slowly proliferating cells within tumors may be acutely vulnerable to chemotherapy.32 Yano et al also reported that the location within the tumor mass influences the cycling behavior of cancer cells. They showed that cancer cells far from vessels or in the center of the tumors were quiescent. In contrast, cancer cells near the surface of the tumor or tumor vessels were mostly cycling and growing.27,33
According to the cell cycle heterogeneity in cancer, the adaptation of neoplastic cells to chemotherapeutic drugs can be defined by two parallel and yet distinct mechanisms; “resistance and tolerance” (Fig. 1B).34
On the one hand, the “resistant population” of neoplastic cells continue to proliferate despite the presence of chemotherapeutic agents.8,35 For instance, it is demonstrated that some sub-populations of tumor cells may adopt a diversity of DNA repair mechanisms or rely on faulty checkpoints to reverse or escape chemical damages to DNA that occur by some drugs.36 Some other sub-populations have deficiencies in sensitivity to chemotherapeutic drugs depending on their relative cell cycle position, specifically when chemotherapeutic agents are used in combination.37 This phenomenon is called cell-cycle-mediated chemotherapeutic drug resistance. Besides, a multiscale computational study by Powathil et al indicated that cell-cycle-length-mediated chemotherapeutic resistance is mainly because of the currently available drugs, which target cancer cells in a specific phase (phases) of the cell cycle, thus missing some cells that are not in the targeted phase (phases).38
On the other hand, most chemotherapeutic drugs act on cells that are in rapidly proliferating phases (in the G1 or S-G2-M phase of the cell cycle); but similar to bacterial tolerance induced by the application of antibiotics, some sub-populations of cancer cells withdraw from the cell cycle and simply enter a dormant or quiescent state; and thereby survive the cytotoxic agents.28,39 Recently Larsen et al discovered that tumor cells could activate a nuclease that causes limited induction of DNA breaks at specific sites, which is coordinated with the process of DNA break repair. These self-inflicted DNA breaks trigger the G2 cell cycle checkpoint, preventing tumor cells from cycling and protecting them from death due to treatment-induced DNA damage.40 In light of the fact that quiescent cancer cells are often tolerant to conventional chemotherapy, these cells can be considered as “tolerant population”, and this phenomenon results in cancer progression and recurrence, which are considered as therapeutic challenges.41,42 Epithelial–mesenchymal transformed cells, CSCs, and circulating tumor cells (CTCs) are proved to share some common characteristics and show a cell cycle arrest phenotype,43,44 which can be implicated in our viewpoint of cell cycle heterogeneity in cancer cells and known as “tolerant populations”.
Since the phase of the cell cycle can determine whether a cancer cell can respond to a given drug, monitoring cell-cycle dynamics during tumor growth plays a pivotal role in improving our understanding of cancer and thereby developing a better treatment. Furthermore, development of new and effective therapeutic strategies that target both proliferating (resistant population) and quiescent (tolerant population) tumor cells remains an urgent medical requirement. Therefore, the main aim of this article is to establish a better understanding of the role of heterogeneous subpopulations of cancer cells with varying cell-cycle dynamics and behavior during cancer progression and treatment in order to facilitate discovering novel approaches to improve tumor therapy and prevent its recurrence.
Definition of “resistant populations” based on cell cycle heterogeneity in cancer therapy
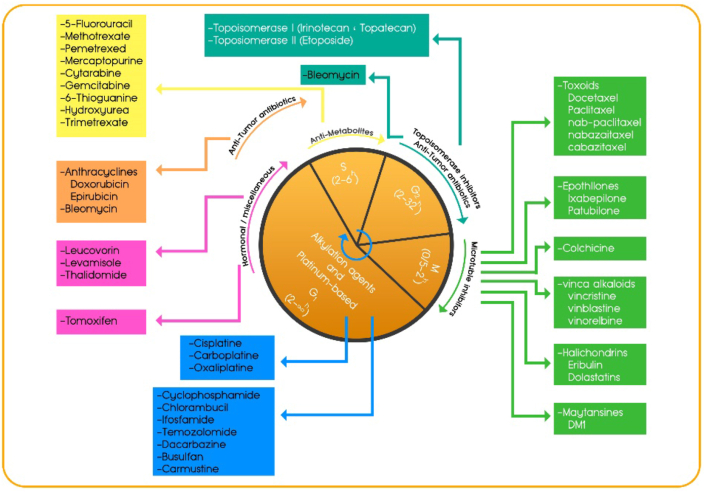
Chemotherapy continues to play a key role in the treatment plan of most patients with cancer. DNA-damaging chemotherapy has been the standard of care for over 40 years since the regulatory approval of cisplatin for testicular/bladder cancer treatment in 1978.45,46 DNA-damaging chemotherapy drugs aim to target rapidly proliferating cells through their cytotoxic effects, which alter the growth kinetics of malignant tissues, prompting downstream apoptotic events, either by directly interfering with DNA or by targeting the key proteins required for cell division. They are categorized in one of two ways by their biochemical properties or their effects on the cell cycle to induce DNA damage, and activate the intra-S-phase checkpoint to inhibit DNA synthesis leading to apoptosis or cellular senescence.47 The activity of chemotherapeutics which have diverse actions and cause different forms of DNA damage, varies in different cell cycle phases. Cycle-nonspecific drugs, such as alkylating agents, platinum-based agents like cisplatin and carboplatin exert their effects in all phases of the cell cycle and are effective for both high and low growth fraction malignancies. Cell cycle–specific drugs exert their effects within a specific phase of the cell cycle, which can cause cell injury only if present during a specific phase of the cell cycle48 (Fig. 2). Although chemotherapeutic treatments are highly toxic to the cell cycle and many tumors show an initial positive response to them, in some sub-populations of neoplastic cells, the response will not continue and often leaves a resistant residual tumor-cell population leading to recurrence with modest effects on overall survival.49 This poor drug performance and the emergence of drug resistance have been attributed to the extensive heterogeneity observed among cells of a single tumor (intra-tumoral heterogeneity) and between different patients (inter-tumoral heterogeneity). Both intrinsic (i.e., genetic) and extrinsic (i.e., microenvironmental) factors may contribute to the observed heterogeneity, which significantly affects tumor progression and therapeutic effectiveness.48
Figure 2.
Chemotherapy drugs that target the cell cycle. The cell cycle is divided into a number of phases G1, S, G2, and M, each of which can vary in length according to the type and growth rate of the cell. The activity of various classes of certain chemotherapy agents are optimal in different phases of the cell cycle as indicated in the picture.
Different heterogeneous cancer cell sub-populations can show various drug resistance, be categorized based on the time when it is developed, and be classified as intrinsic or acquired resistance.50 Intrinsic resistance is usually defined as the innate resistance, which exists prior to the treatment and usually results in reduced efficacy of the chemotherapy.51 Intrinsic resistance can happen via various mechanisms including (1) inherent genetic mutations in the tumor cells that result in decreased responsiveness of cancerous cells, such as triple negative breast cancer cells, to both chemotherapy and targeted therapy; (2) development of a resistant population such as cancer stem cells in heterogeneous tumors which will be selected upon drug treatment thus leading to relapse in later stages of therapy; (3) commencement of intrinsic pathways that are responsible for the detoxification under normal physiological conditions and used as defense against anticancer drugs.52 Acquired resistance can be identified by the gradual reduction of the anticancer efficacy of a drug following the treatment.51 This phenomenon can be the outcome of various cellular and molecular responses, including (1) activation of a second proto-oncogene that becomes the newly emerged driver gene; cancer cells can acquire resistance against targeted drugs by the generation of new mutations or alteration in the expression; (2) alterations in expression levels of the drug targets; (3) change in drug metabolism in the tumor cells; (4) upregulation of transmembrane transporters which efflux the drugs; (5) epigenomic changes and altered levels of microRNAs,53 which lead to variations in the downstream or upstream receptors; and (6) changes in tumor microenvironment after treatment. Therefore, it can be concluded that the “resistant populations” of neoplastic cells by using various underlying mechanisms, which are well described in a review by Haider et al, lead to chemotherapy failure in cancer.54 Moreover, a selected cell's spatial location within the tumor and intracellular interactions, including the evolution of the cell cycle within each cell, impact their decision to grow and divide.55 Further, the heterogeneity created by the perturbations in cell cycle dynamics can induce cell-cycle mediated drug resistance, dramatically reducing the efficacy of chemotherapeutic drugs. This intracellular heterogeneity, along with the heterogeneity created by external factors such as tumor microenvironment and drug transport limitations, significantly impair the cytotoxicity of combined chemotherapy.55 The sensibility of individual cancer cells to chemotherapy may differ due to haphazard differences. In vitro studies using genetically identical clonal cell lines, exposed to similar drug levels and corrected for cell cycle, found significant differences in chemotherapy sensitivity between individual cells;56, 57, 58 this highlights the fact that even genetically identical tumor cells show heterogeneous therapy responses due to poorly understood phenotypic heterogeneity, even when all external factors are controlled. Remarkably, it has been proposed that slowly dividing subpopulations may evade conventional cytotoxic therapies aimed at rapidly proliferating cells. Indeed, quiescent cancer stem cells could exist in some entities, arising from mutated tissue stem cells.59 However, aggressive cancers typically harbor strongly pro-proliferative genetic lesions incompatible with a quiescent phenotype.60 Thus, therapy resistance to rapidly growing tumors remains enigmatic. Nevertheless, Ryl et al showed that fast-proliferating tumor cells might resist cytotoxic treatment non-genetically by arresting within a favorable cell cycle window at the beginning of chemotherapy.61
Furthermore, the dosage and timing of drug delivery can be modified to affect chemotherapy response rates. Delivery of cell cycle-specific chemotherapeutic agents in intermittent doses to cells that have a shorter duration of cell cycle may yield a lower cytotoxicity. However, if a cell cycle-specific agent is given via continuous infusion, it may result in a more significant number of cells being killed. A transparent connection exists between the number of chemotherapeutic agents and the higher cell death rate.62 With our increased understanding of the fundamental mechanisms of tumorigenesis, cell cycle physiology, and apoptosis, we have come to a better insight into the impact of chemotherapy on cancerous and normal cells. Thus, individual chemotherapeutic agents with limited efficacy for most tumors and various side effects have not increased cure rates in cancer treatment.63 With this knowledge, by combination of single agents that have cytotoxic effects and induce cell cycle arrest, greater antitumor efficacies have been observed in patients.64 However, the rational choice of single agents for combination treatments and appropriate sequence and plan out for the administration of the drugs are critical to overcome cell-cycle-mediated drug resistance. This resistance phenomenon is common in various combination chemotherapeutic approaches. For instance, it was found that G1-arrested melanoma cells had disrupted apoptotic function induced by bortezomib (a protease inhibitor) and/or temozolomide (an alkylating agent). In contrast, apoptosis was induced in the same cells through G2/M arrest, because of their sensitivity to mitogen-activated protein kinase inhibitor (MAPKi).31,65 Pre-treatment of melanoma cells with MAPKi resulted in resistance to bortezomib and/or temozolomide (induced G1 arrest). Conversely, pre-treatment with temozolomide resulted in resistance to MAPKi (induced G2 arrest). Besides, even though bortezomib and temozolomide effectively induced apoptosis in proliferating melanoma cells, they are ineffective against drug-induced or hypoxia/serum-starved induced G1 arrested cells.66 Cell-cycle-mediated chemotherapeutic drug resistance has also been displayed for taxanes, which stabilize microtubules and induce G2/M cell cycle arrest before apoptosis.31,37 The cytotoxicity of paclitaxel is maximal at the G2/M phases of the cell cycle. Motwani et al found that pre-treatment of cancer cells with flavopiridol resulted in resistance to paclitaxel, because of an induced G1 arrest.66 Moreover, different factors such as the spatial distribution of cancer cells in the tumor mass, the timing of the drug delivery, the time between the doses of cytotoxic drugs, and the cell cycle and oxygen heterogeneity play important roles in determining the precise cytotoxic effectiveness of cell-cycle phase-specific chemotherapeutic drugs.55 For instance, Yano et al demonstrated that at the surface of a tumor, approximately 80% of the cells are in proliferating phase, but in deeper positions of the tumor, approximately 90% of the cells are in a dormant or quiescent state. Chemotherapeutic drugs act only on the surface cells of the tumor but quiescent cells remain, and thereby lead to resistance and tolerance to chemotherapy. Thus, after chemotherapy, a new set of proliferating cells would appear on the surface of the tumor.27
Definition of “tolerant population” based on cell cycle heterogeneity in cancer therapy
Differences between quiescent phase and other dividing/non-dividing states of the cell cycle
Although proliferation is one of the most conserved and fundamental traits of the cells, all cells, from prokaryotes to advanced eukaryotes, cease proliferation at some points during their life span in a controlled process termed cellular dormancy, which is often defined as a reversible non-dividing state of a cell.67, 68, 69
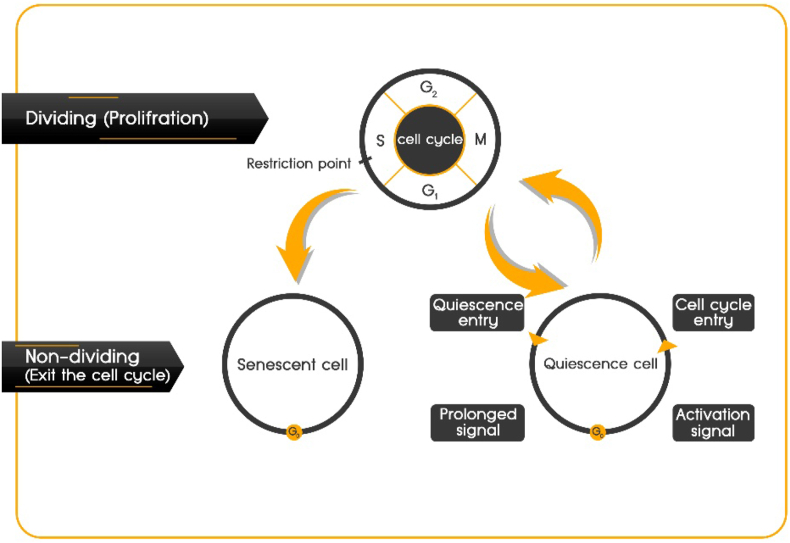
One of the most fundamental factors that contribute to this phenomenon during the life of unicellular organisms is environmental limitations. As cells divide only under appropriate conditions, some limitations, such as scarcity of nutrients, act as a selective pressure. Those unicellular organisms that could quit proliferation successfully will re-activate later on, in the more suitable conditions. However, in more advanced living organisms, such as mammals, many cells are found in the dormant phase and environmental limitations do not seem to contribute to this phenomenon, as some cells transfer to the dormant phase even in the presence of abundant resources.70, 71, 72, 73 Although dormant cells can enter the proliferative cell cycle in response to physiological growth signals, there are some non-dividing cells (e.g., senescent, or terminally differentiated cells) that are irreversibly arrested and they can no longer re-enter the cell cycle.74 Thus, three different non-dividing cell states can be described: quiescence, dormancy, and senescence (Fig. 3).
Figure 3.
Cellular quiescence. Although a continuous cell cycle resulting in proliferation might be considered the “default program” of cells, non-dividing cells can enter the G0 phase and can reversibly re-enter the cell cycle or become quiescent, losing the ability to cycle, and sometimes become senescent. Quiescence differs from other non-dividing states in that it can be reverted into proliferation in response to environmental signals.
Quiescence and dormancy are similar cell states and the distinction can be found in the way of re-entering the cell cycle. Quiescence, also referred to as G0, is a temporary pause of proliferation that will be resumed when conditions are favorable75 Dormancy is more persistent compared with quiescence, suggesting that dormancy refers to a deeper and longer state of quiescence.69,76 So, in this review, we will term both quiescent and dormant cells as quiescent cells when referring to non-dividing cells that have the ability to re-enter the cell cycle.
Senescent cells are metabolically active; however, their capacity to proliferate is lost, even under favorable conditions. So distinguishing quiescence from non-replicative senescence is accompanied by some confusion. Senescent cells are observed in an essentially permanent growth arrest induced by extrinsic and genotoxic stresses.77,78 Besides, these cells express some markers such as senescence-associated heterochromatin foci (SAHF), senescence-associated β-galactosidase and nuclear foci containing DNA damage proteins (DNA-SCARS), which are not found in quiescent cells.78 Furthermore, some reports that characterize senescence involve prolonged arrests in the G1 or G2 phases, differently from quiescent cells that are in G0 or G0/G1 transition.79,80
The importance of quiescent cells in healthy and cancerous tissues
In many different cell types and conditions, from distinct tissues in normal and aberrant situations (e.g., undifferentiated, differentiated, non-malignant and malignant cells), the ability to become quiescent is viable.81 For instance, tissue-specific stem cells (also known as adult stem cells) in different tissues use various strategies to ensure their maintenance over the organism's lifespan to contribute to tissue homeostasis and repair.82, 83, 84 One such strategy is to remain in a quiescent state, which is thought to protect the DNA from mutations acquired during successive rounds of cell division.85 Moreover, cellular quiescence provides protection against stress and toxicity, which is especially vital for these long-lived cells.86 As formation of tissues with renewal and regeneration capacity is critical for the body's homeostasis, adult stem cells that exist in a quiescent state can be found in many tissues. It can be concluded that reversible quiescent cells are fundamental to the tissue homeostasis process. Although proliferation might be considered as a default setting in tissues of higher organisms, cells retain a built-in program of quiescence, which is set off extrinsically.87 Thus, careful regulation of the balance between cellular quiescence and proliferation is necessary. Mis-regulation of this balance can lead to a wide range of hypo and hyper-proliferative diseases, such as fibrosis, autoimmune diseases, aging and cancer.
Based on the cellular quiescence concept in cancer biology, there are some quiescent cancer cell populations, which are considered as a therapeutic challenge since they confer dormancy in the tumor, hence circumventing inherent anti-neoplastic surveillance system and standard-of-care cancer therapeutics including chemotherapy and radiotherapy. Since majority of the therapeutics target actively proliferating cancer cells, these populations eventually develop quiescent nature as a mechanism of survival leading to cancer progression under both niche and therapeutic pressures.49 The presence of these quiescent cancer cell populations, “tolerant populations”, is associated with recurrence, metastasis, and poor clinical outcome, suggesting that these quiescent populations may play a crucial role in the process of cancer progression and relapse. Therefore, it is specifically important to study cancerous cells not only with regard to uncontrolled growth and proliferation but also in the context of cellular quiescence.68,88,89
Quiescence is a molecularly varied, non-terminal and tissue-specific state that can be activated and maintained by the cell microenvironment.90 Therefore, the microenvironment regulates cellular behavior and fate, including the quiescence: proliferation balance.91 Changes in the microenvironment can re-activate cells in a controllable fashion or stimulate the cell to stop proliferation and enter the quiescence program. However, this reciprocal exchange between the cell and its microenvironment can be disrupted, which results in the appearance of cancer. Moreover, both clinical and experimental evidences show that cancerous cells are maintained in a non-proliferating quiescent state as single cells or as micro-metastases for long time intervals before re-activation and growing out into macro-metastatic states,28,39,92 and it is not far from believing that we are all cancer survivors rather than cancer-free individuals because of harboring quiescent malignant cells in our organs.93 This phenomenon may occur at early stages of the tumorigenesis or following the chemotherapeutic intervention. Re-activating these quiescent cells leads to tumor progression and relapse, which may occur after very long periods.94
Conventional therapies preferentially target fast-dividing cells, leaving quiescent cancer cells largely insensitive to these treatments. When chemotherapy drugs act on tumor cells, quiescent cell populations could flexibly have a modulation at genetic95, 96, 97 and epigenetic98, 99, 100 levels to keep a quiescent state. Thus, these quiescent cancer cells avoid apoptosis and also conserve strength for further relapse because they could shelter from the lesion and diminish energy expenditure and substrate consumption. Antitumor drug tolerance is a temporary condition, which can occur after drug stimulation. Although this condition can revert after the cessation of cytotoxic stimuli, in the presence of continuous drug stimulation or other cellular stresses such as hypoxia, it stabilizes into an enduring drug-tolerant state.101,102 Consequently, it can be argued that the quiescent state is an initiative response of tumor cells rather than a passive defense to chemotherapy.
As previously mentioned, quiescence is also crucial for maintaining adult stem cells. Quiescent adult stem cells resist environmental or radiation-induced stress and can regenerate their whole lineage after an insult.103,104 Similarly, tumors often efficiently regenerate their multiple levels of heterogeneity in response to radiotherapy or chemotherapy, which targets mostly proliferating cells. This observation prompted the hypothesis of the existence of CSCs as a key compartment of intra-tumoral heterogeneity and suggested that quiescence contributes to the ability of tumors to relapse after treatment.105, 106, 107 In both preclinical and clinical samples, various analyses have revealed that chemotherapeutic agents successfully eliminated the bulk population of non-CSCs, while in multiple cancer types, considerable numbers of CSCs remained intact.108,109
Cancer stem cells with dual function in response to chemotherapeutic drugs: resistance and tolerance
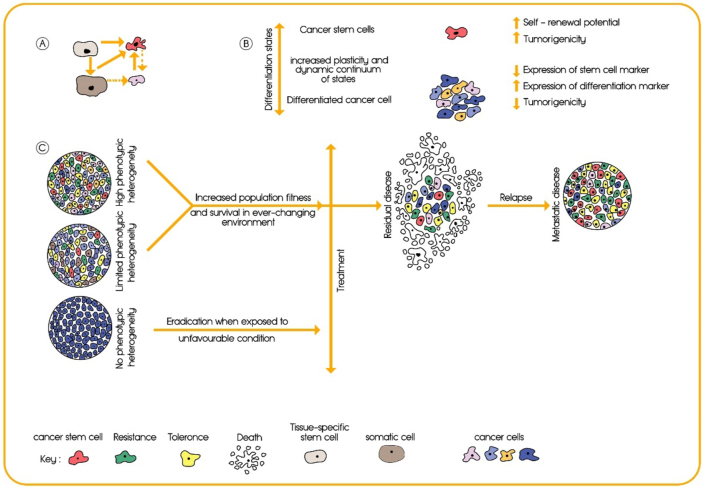
Contrary to what was thought in the past, tumors consist of heterogeneous populations, both at levels of mutational landscape and cellular phenotype; and just a distinct, and rare cell population within tumor has the capability to repopulate an entire tumor mass from a few hundred cells. These cells characteristically resemble normal stem cells (NSCs) by some common features, including self-renewal and multi-lineage differentiation.110, 111, 112 These cancer stem-like cells or CSCs have enhanced tumor-initiating capacity and metastatic potential (Fig. 4A, C).113 Although we reviewed cancer resistance to therapy in the context of the stem cell theory of cancer, it should be taken into consideration that the concept of cancer stem cells does not resolve the age-old controversy of two opposing hypotheses of the origin of cancer, namely the stem cell hypothesis versus the dedifferentiation or re-programming hypothesis. These concepts are well discussed in other scientific literature, such as in a review article written by Trosko.114 Moreover, Ryl et al discovered that arrest in an early cell-cycle phase supports later re-entry into unhindered proliferation providing an exciting twist to the cancer stem cell hypothesis. They suggested that the transient G1 arrest of cells born at the start of chemotherapeutic treatment ultimately supports therapy resistance.61
Figure 4.
Tumor heterogeneity and the function of CSCs in resistance to therapy. (A) Tumor initiation as a key cellular event in progression of cancer. (B) The difference between cancer stem cells and differentiated cancer cells in various aspects. (C) Schematic representation of tumor cell heterogeneity: A homogeneous tumor will be eradicated in response to a selective pressure such as chemotherapy, whereas a heterogeneous tumor is more likely to contain various subpopulations of cells including CSCs, resistant, and tolerant populations. Usually, a first-line therapy is chosen based on the histological subtype, stage of the tumor, presence of biomarkers, and other clinical data. If the first-line therapy does not target CSCs, resistant, and tolerant populations, the mass of the tumor will be reduced but these cells will remain (mostly) unaffected, leading to progression, relapse and metastasis of the cancer.
Since the initial identification of acute myeloid leukemia CSCs in 1997,115 CSCs have been isolated from many cancers, including breast, brain, colon, pancreas, liver, prostate, lung, head and neck, ovarian, and gastric cancers.113,116, 117, 118, 119, 120, 121, 122, 123, 124, 125 Considerable and strong pieces of evidence show that CSCs play a pivotal role in chemotherapeutic resistance as a “resistant population”. These cells have a higher intrinsic resistance to conventional chemotherapies than other cancer cells, and can be the source of post-therapy relapse, as they have the ability to self-renew and to differentiate into the heterogeneous lineages of tumor cells (Fig. 4B).60,113,126 Besides, upregulation of survival mechanisms in cancer cells and deregulation of apoptotic and cell death signaling pathways, especially in CSCs, result in resistance to diverse drugs in a wide variety of cancers.127, 128, 129, 130, 131
As we discussed before, there is an association between the quiescent phase of cancer cells and anti-tumor drug tolerance. Accumulating evidence indicates that some cancer cells with combined features of stemness, and dormancy have been identified in several tumors including pancreatic carcinoma,132,133 ovarian cancer,134 melanoma,135 lung cancer,136 and chronic myelogenous leukemia (CML).137 Moreover, quiescent CSCs have been identified in acute leukemia,138 glioblastoma,139, 140, 141 breast142 and colorectal143,144 cancers. Furthermore, Tu et al reviewed cancer dormancy and quiescence in the stem cell theory context, and they discuss that when we consider a unified theory of cancer, dormancy may be one of the more illuminating examples to illustrate the prevalence and pertinence of this theory.145
Based on the concept of cell cycle heterogeneity in cancer, CSCs can be considered as a neoplastic population that acquired adaption to chemotherapeutic drugs as two parallel and yet distinct populations: “resistant and tolerant”.
Cell cycle-based resistance of CSCs to conventional chemotherapies
Generally, most chemotherapies induce an apoptotic response in the bulk of rapidly proliferating cell populations, typically by damaging the DNA and/or inhibiting mitotic division. The non-responsiveness or insensitivity of slowly or non-dividing cancer cells or CSCs to the treatments is termed as chemo-resistance, which can either be an intrinsic or an acquired resistance.49 Different mechanisms contributing to resistance to conventional chemotherapies and target-specific anticancer agents have been reported in CSCs, which are well discussed in various articles (Table 1).146, 147, 148
Table 1.
Various mechanisms related to CSC-mediated therapy resistance.
| Different mechanisms contributing to chemo-resistance of CSCs |
|---|
| High expression of ABC transporters (P-gp, BCRP, and MRP5) |
| Increased survival signals |
| Interaction of CSCs and their niche |
| Elevated cytoprotective pathways |
| Elevated DNA repair |
| Enhanced DNA damage response and ROS scavenging |
| Dysregulated anti-apoptotic proteins (upregulation of c-FLIP, IAPs, and BCL-2 family) |
| Aberrant stemness signaling pathways |
| Increased autophagy |
| Increased quiescence |
| ALDH activity |
| Apoptosis evasion mechanisms |
Moreover, accumulating pieces of evidence suggest that CSCs are resistant to DNA damaging therapies through various strategies such as regulation of the cell cycle and increasing DNA repair capacity.149, 150, 151 As mentioned earlier, DNA damage response (DDR) refers to a complex network of molecular circuitries that detect and control specific forms of DNA damage. Both normal and malignant stem cells rely on a very strong DDR, which while it is beneficial in healthy tissues, it can be detrimental, as it favors CSCs survival and their resistance to therapy.152 Thus, such robust DDRs can clarify elevated cell cycle-based resistance of CSCs to DNA-damaging agents. Furthermore, CSCs preferentially activate cytoprotective pathways of DDRs, favoring the survival of CSCs even when experiencing sizable genetic lesions.153 In particular, there is evidence that CSCs efficiently sense and signal endogenous/exogenous DNA lesions, which results in operating robust DNA repair and tolerance systems. The DDR mechanisms cause cell cycle arrest at specific checkpoints and recruit and activate the DNA repair proteins to mend the DNA breaks induced by chemotherapy. Bao et al found that CD133+ glioblastoma CSCs preferentially activate DNA damage checkpoint response through the ATM kinase and checkpoint kinases 1 and 2 (CHK1 and CHK2).154 Preferential activation of the repair mechanism and anti-apoptotic pathways were observed in cancerous stem cells, where the group protein BMI1 and ATM kinase preferentially activated the DNA double-strand break (DSB) repair machinery.155 Similar DNA repair associated genes like MGMT and BRCA-1 have been expressed in CSCs derived from glioblastoma and pancreatic tumors.156 CSCs efficiently resolve DSBs since they have developed superior HR (Homologous recombination) or NHEJ (Non-homologous end joining) repair systems.157, 158, 159 In vitro investigations suggest that in lung, ovarian and breast cancers this is accompanied by constitutive up-regulation of some HR and/or players, such as RAD51.141,160,161 Some types of CSCs also have elevated DNA single-strand break (SSB) repair properties, possibly linked to mechanisms such as up-regulation of APEX1 in breast CSCs or increased PARP1 activity in glioma stem cells.162,163 Consequently, lung and ovarian CSCs efficiently resolve DNA cross-links induced by cisplatin.164,165
Quiescent state in CSC population as a mean of survival mechanism
CSCs can survive in unfavorable stressful tumor microenvironments by harnessing quiescent state (Fig. 5). When the environment becomes conducive for tumor progression, these CSCs may be re-activated to proliferate and differentiate into mature cancer cells. For regulation of stem cell quiescence, several signaling molecules are responsible including tumor suppressors P53, RB, cyclin-dependent protein kinase inhibitors such as P21, P27, P57, NOTCH-related pathways and some micro-RNAs.166 These factors develop several adaptive metabolic responses that allow SC quiescent cells to survive in stressful microenvironments, maintain their genomic integrity and attribute to tumor recurrence or relapse. For instance, hitherto, evidences from scientific reports show that P53 plays a dual role after exposure to cytotoxic treatments, by either activating apoptosis or launching processes directing to DNA repair and survival of the cells.167 In the alternative pathway, P53 would provide both time and tools to reverse drug-induced DNA damage, namely, by mediating G1 arrest and activates a series of DNA repair related molecules and signal pathways.167 After completion of DNA repair, residual tumor cells could exit from quiescent state and re-enter into cell cycle, eventually contributing to therapy failure and tumor relapse.
Figure 5.
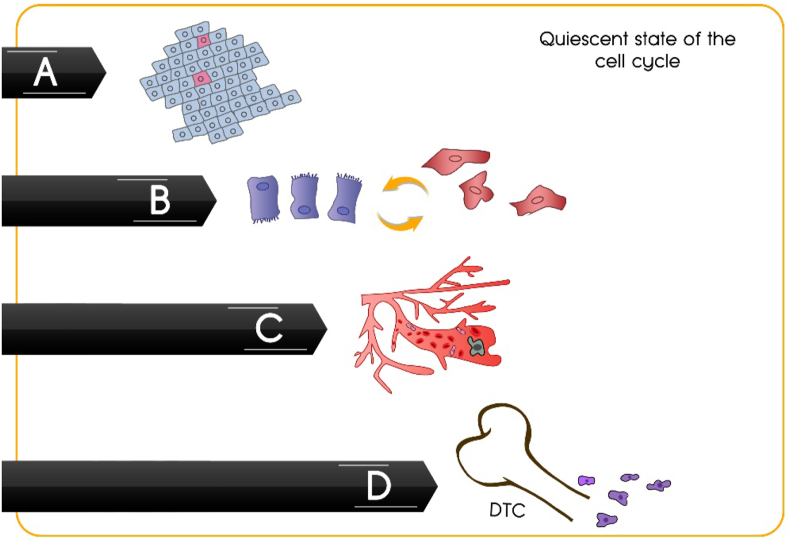
A pivotal biological link between important cancer cells. Quiescence is a common feature among: (A) cancer stem cells, (B) epithelial–mesenchymal transformed cells, (C) circulating tumor cells and (D) disseminated tumor cells.
According to the cancer recurrence perspective, an important term that should be considered is tumor mass dormancy, which is defined as the period of time during which populations of tumor cells remain undetectable until the recurrence of a clinically evident disease.168 Although certain cancers demonstrate long periods of tumor mass dormancy (prostate cancer, breast cancer), other neoplasms are characterized by considerably shorter relapse times (pancreatic carcinoma, lung cancer).168,169 In breast cancer, about 25% of CSCs were detected to be quiescent and the remaining 75% cycled very slowly compared to more differentiated cancer cells.170 Conversely, after exposure to ionizing radiation, many non-stem cells died or entered a dormant state, while at the same time the quiescent CSC population was recruited into the cell cycle.170 Based on the fact that the emergence of quiescent drug-tolerant CSC population has been detected and quantified in multiple tumors upon treatment with chemotherapeutics or targeted agents including cisplatin, erlotinib, and gefitinib in NSCLC, lapatinib in breast cancer, the RAF kinase inhibitor AZ628 in melanoma and colorectal cancer, and the MET inhibitor PF-2341066 in MET-amplified gastric cancer,102 it can be concluded that the quiescent state is a fundamental feature and specialized cycling behavior of CSCs, which shields them from anti-proliferative agents and is a contributing factor towards their chemo tolerance manner.89 Therefore, this challenge needs to be taken into consideration and should be addressed by developing effective therapeutic approaches that also target these CSCs, without which a complete cure may well not be expected.
Autophagy is a manifestation of CSCs intertwined behavior as resistant and tolerant populations
Autophagy is another important feature of cancer cells which has a specific role in dictating chemo resistance171, 172, 173, 174 and also is required during quiescence.175,176 CSCs show a high level of autophagy, which contributes to their survival and therapy resistance.177, 178, 179 Apart from promoting resistance to chemotherapy, this critical catabolic pathway determines cell fate by targeting degradation of paramount transcription factors such as P53 and FOXO3A, or by enforcing quiescent growth arrest. Moreover, high level of autophagy in CSCs maintains their pluripotency, allows them to cope with low nutrients and hypoxia in the tumor microenvironment to maintain metabolic fitness.178 For instance, in the presence of nutritional stress, cancer cells secrete factors that inhibit the PI3K pathway, which results in dormancy and autophagy induction.180 Besides, autophagy regulates CSCs migration and invasion, and helps them escape immune surveillance.178,181
Distant metastasis and recurrence by cells that remain quiescent and resistant to chemotherapy
As intra-tumor heterogeneity is accumulated in the quiescent phase of the cell cycle, it leads to a more malignant phenotype of tolerant populations and filial generations. An interesting association between stemness and dormancy, together with enhanced migratory features, has been reported in early metastatic cells which are largely responsible for tumor dissemination.182, 183, 184, 185 Besides, drug resistant and tolerant cell populations are not only detected within the bulk tumor but also are found in distant organs as DTCs (disseminated tumor cells), which have been known as the factors required for metastasis.
Metastasis contributes to poor prognosis in many types of cancers and is the key cause of cancer-related death.186 This cascade follows a series of steps that tumor cells need to take to reach distant organs and eventually form metastasis.187 These steps include evasion from the primary tumor, invasion into the lymphatic or hematogenous vasculature, survival in the circulation, arrest and extravasation into the secondary organ site, initiation of metastatic growth at that site, and maintenance of growth into macrometastases.188,189 When tumor cells leave the primary site and enter the bloodstream, they become circulating tumor cells.190 CTCs must overcome a number of physiological barriers to disseminate. These barriers faced by CTCs can be solved by the potential of tumor cells (and future CTCs) to undergo epithelial-to-mesenchymal transition (EMT) which supports them in acquisition of stem cell-like traits.191
EMT and its reversed process, mesenchymal-to-epithelial transition (MET), are fundamental processes in embryonic development and tissue repair, but confer malignant properties to carcinoma cells including invasive behavior, cancer stem cell activity, and greater resistance to chemotherapy and immunotherapy.187,192 It has been suggested that cells transiently enter cell cycle arrest as a prerequisite to facilitate EMT, and EMT-transformed cells are linked with decreased proliferation and show a cell cycle arrest phenotype.193, 194, 195, 196 EMT induces the tumor cells to exhibit stem cell-like characteristics which promote cells to invade surrounding tissues and exhibit therapeutic resistance.197,198 Thus, epithelial–mesenchymal transformed cells exhibit an elevated capability in migration and invasion. Consequently, these cells tend to infiltrate into the circulation system and disseminate through the bloodstream which contributes to the inhibition of anoikis and the formation of CTCs.199, 200, 201, 202 For instance, a tumor cell undergoing EMT downregulates E-cadherin which enables separation from neighboring epithelial cells and upregulates matrix metalloproteinase (MMP) activity, which simplifies navigation via the local ECM and entry to the microvasculature.203,204 It has been shown that 62% of CTC-positive patients with metastatic breast cancer, had CTCs that were positive for at least one of three EMT markers (AKT2, PI3K, and TWIST1).205 Besides, in a mouse model of breast cancer metastasis, it was demonstrated that the EMT signature detected in CTCs is not limited to one or two transcripts or molecules,206 but instead involved considerable changes in expression levels of several genes, for instance in lung cancer (at least 76 genes).207,208
However, once CTCs reach and lodge in a distant organ, they are termed disseminated tumor cells. DTCs are in a state of dormancy and can be resistant to different treatment strategies including chemotherapy, targeted therapy, and hormonal therapy.29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212 These paramount features of DTCs are crucially dependent on the target organ niche, which plays an important role in sustaining their survival, regulating their growth, and conferring resistance to therapy.191,213 For instance, a recent study indicated that disseminated breast cancer cells are shielded from chemotherapeutic treatment by integrin-mediated interactions irrespective from their cell cycle status.209 Moreover, disseminated tumor cells can reside in a patient's organs for long periods as they enter a long-lasting quiescent state in responding to homeostatic signals from the host microenvironment.211 Indeed, in melanoma, breast, and prostate cancers, these dormant tumor cells may enter into a quiescent state, only to ‘awaken’ after years or even decades and form deadly metastases.188
In summary, there are multiple biological links between CSCs, epithelial–mesenchymal transformed cells, CTCs and DTCs including phenotypic overlaps. Moreover, these cells share one fundamental property i.e., they are mostly in a quiescent state of the cell cycle (Fig. 5).28,191,214 Thus, these emerging concepts may provide us with new opportunities to prevent lethal recurrences and deeper understanding of these intertwined processes may facilitate the development of novel therapies in the future.
Conclusion
Tumor heterogeneity with differential layers of complexity can be defined in terms of cellular morphology, cell surface markers, gene expression, epigenetic variation, motility, metabolism, metastatic potential, and cell cycle state. Given the role of cell cycle heterogeneity, it is conceivable that tumor cells exhibit differential drug sensitivity based on their residence in specific cell cycle phases. Cell cycle heterogeneity can give rise to chemo-resistant and chemo-tolerant subpopulations within a tumor whose interaction can result in unexpected responses.
Due to the cell cycle heterogeneity, resistant populations of tumor cells continue to proliferate despite the presence of classical chemotherapeutic agents and/or novel targeted drugs. It was demonstrated that this sub-population of tumor cells adopts a diversity of DNA repair mechanisms, reverses or escapes chemical damages to DNA by relying on faulty checkpoints and avoiding apoptosis, and shows cell-cycle-mediated drug resistance. The evidence provided suggests that therapeutic strategies using single chemotherapeutic agents are most likely to lead to eventual treatment failure due to the presence of chemo-resistant population as the drug kills sensitive cancer cells but resistant cells continue to proliferate. Thus, combinational therapies are strongly preferred as tumors are heterogeneous in different aspects. However, since cancer is a cell cycle heterogeneous dynamic target, cell populations should be thoroughly characterized at various times, in order to rationally choose single agents for combination treatments and appropriate sequence and plan out for the administration of the drugs to overcome cell-cycle-mediated drug resistance.
Furthermore, according to the evidence presented concerning cell cycle heterogeneity and the fact that some cancer populations eventually develop quiescent nature as a mechanism of survival and cancer progression under both niche and therapeutic pressures, it is especially important to study cancerous cells not only with regard to uncontrolled growth and proliferation but also in the context of cellular quiescence. The presence of quiescent cancer cell populations (tolerant population) is associated with recurrence, metastasis, and poor clinical outcome, suggesting that these quiescent populations play a crucial role in the process of cancer progression and relapse.
Therapeutic strategies directed against quiescent cancer cell populations are divided in three main work streams: the first type is “sleeping strategies” which are to maintain neoplastic cells in a state of quiescence by suppressing proliferative signals with different drugs.215,216 Moreover, induction of senescence in quiescent cells by repression of autophagy can be used as a safer approach,217,218 though it was shown that the transition from autophagy to senescence induces considerable heterogeneity at single cell level.219 The second type of strategies directed against quiescent cells is “awakening strategies” which are to stimulate neoplastic cells to re-enter the cell cycle in order to improve the efficacy of anti-proliferative drugs.220,221 The third strategy involves eliminating quiescent/tolerant cell population while dormant, which can be a difficult but not impossible challenge. For instance, elimination of quiescent cells has been achieved in brain tumors with mithramycin.222
In conclusion, based on the cell cycle heterogeneity concept, understanding the mechanisms of chemo-resistance and chemo-tolerance are mandatory in order to improve the effectiveness of cancer therapies. Because of the discoveries made in the last decade, drug resistant and tolerant populations of cancer cells in their different contexts are starting to appear as treatable targets. However, increasing efforts are required to explore the mechanisms that regulate chemo-resistance/tolerance of cancer cells in either primary tumors, pre-metastatic niches, or overt metastases in order to find new therapeutic approaches.
Conflicts of interests
There are no conflicts of interest to declare. All authors contributed to the discussion and writing of the manuscript.
Acknowledgements
We appreciate the contribution and support of Ferdowsi University of Mashhad (FUM) for providing the opportunity to carry out this study.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Santos-de-Frutos K., Djouder N. When dormancy fuels tumour relapse. Commun Biol. 2021;4(1):747. doi: 10.1038/s42003-021-02257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momenimovahed Z., Tiznobaik A., Taheri S., et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L., Xie H.J., Li Y.Y., et al. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review) Oncol Rep. 2022;47(4):82. doi: 10.3892/or.2022.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood R.D., Morgan R.D., Edmondson R.J., et al. First-line management of advanced high-grade serous ovarian cancer. Curr Oncol Rep. 2020;22(6):64. doi: 10.1007/s11912-020-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleki E.H., Bahrami A.R., Sadeghian H., et al. Discovering the structure–activity relationships of different O-prenylated coumarin derivatives as effective anticancer agents in human cervical cancer cells. Toxicol In Vitro. 2020;63 doi: 10.1016/j.tiv.2019.104745. [DOI] [PubMed] [Google Scholar]
- 6.Uramoto H., Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connor D., Sibson K., Caswell M., et al. Early UK experience in the use of clofarabine in the treatment of relapsed and refractory paediatric acute lymphoblastic leukaemia. Br J Haematol. 2011;154(4):482–485. doi: 10.1111/j.1365-2141.2011.08752.x. [DOI] [PubMed] [Google Scholar]
- 8.Housman G., Byler S., Heerboth S., et al. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lüönd F., Tiede S., Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. 2021;125(2):164–175. doi: 10.1038/s41416-021-01328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asli N. Molecular dissection of intra-tumoral heterogeneity in human epithelial carcinoma. J Stem Cell Res Med. 2016;1 [Google Scholar]
- 11.Altschuler S.J., Wu L.F. Cellular heterogeneity: do differences make a difference? Cell. 2010;141(4):559–563. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsasser W.M. Outline of a theory of cellular heterogeneity. Proc Natl Acad Sci U S A. 1984;81(16):5126–5129. doi: 10.1073/pnas.81.16.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowell P.C. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 14.Prasetyanti P.R., Medema J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sayes N., Vito A., Mossman K. Tumor Heterogeneity: a great barrier in the age of cancer Immunotherapy. Cancers (Basel) 2021;13(4):806. doi: 10.3390/cancers13040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson D.A., Kessenbrock K., Davis R.T., et al. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 2018;20(12):1349–1360. doi: 10.1038/s41556-018-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramón Y.C.S., Sesé M., Capdevila C., et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berl). 2020;98(2):161–177. doi: 10.1007/s00109-020-01874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnquist C., Watson R.A., Protheroe A., et al. Tumor heterogeneity: does it matter? Expert Rev Anticancer Ther. 2019;19(10):857–867. doi: 10.1080/14737140.2019.1667236. [DOI] [PubMed] [Google Scholar]
- 19.Lenz G., Onzi G.R., Lenz L.S., et al. The origins of phenotypic heterogeneity in cancer. Cancer Res. 2022;82(1):3–11. doi: 10.1158/0008-5472.CAN-21-1940. [DOI] [PubMed] [Google Scholar]
- 20.Gomez H. How heterogeneity drives tumor growth: a computational study. Philos Trans A Math Phys Eng Sci. 2020;378(2171) doi: 10.1098/rsta.2019.0244. [DOI] [PubMed] [Google Scholar]
- 21.Giedt R.J., Koch P.D., Weissleder R. Single cell analysis of drug distribution by intravital imaging. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Jiang K., Dong M., Li C., et al. Unraveling heterogeneity of tumor cells and microenvironment and its clinical implications for triple negative breast cancer. Front Oncol. 2021;11:557477. doi: 10.3389/fonc.2021.557477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas R.M., Haddad G.G. Invited Review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol. 2003;94(5):2068–2083. doi: 10.1152/japplphysiol.01029.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lenz L.S., Faccioni J.L., Bracco P.A., et al. Cancer cell fitness is dynamic. Cancer Res. 2021;81(4):1040–1051. doi: 10.1158/0008-5472.CAN-20-2488. [DOI] [PubMed] [Google Scholar]
- 26.Chittajallu D.R., Florian S., Kohler R.H., et al. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat Methods. 2015;12(6):577–585. doi: 10.1038/nmeth.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano S., Zhang Y., Miwa S., et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle. 2014;13(13):2110–2119. doi: 10.4161/cc.29156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguirre-Ghiso J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goss P.E., Chambers A.F. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10(12):871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 30.Kyle A.H., Baker J.H., Minchinton A.I. Targeting quiescent tumor cells via oxygen and IGF-I supplementation. Cancer Res. 2012;72(3):801–809. doi: 10.1158/0008-5472.CAN-11-3059. [DOI] [PubMed] [Google Scholar]
- 31.Beaumont K.A., Hill D.S., Daignault S.M., et al. Cell cycle phase-specific drug resistance as an escape mechanism of melanoma cells. J Invest Dermatol. 2016;136(7):1479–1489. doi: 10.1016/j.jid.2016.02.805. [DOI] [PubMed] [Google Scholar]
- 32.Granada A.E., Jiménez A., Stewart-Ornstein J., et al. The effects of proliferation status and cell cycle phase on the responses of single cells to chemotherapy. Mol Biol Cell. 2020;31(8):845–857. doi: 10.1091/mbc.E19-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano S., Takehara K., Tazawa H., et al. Cell-cycle-dependent drug-resistant quiescent cancer cells induce tumor angiogenesis after chemotherapy as visualized by real-time FUCCI imaging. Cell Cycle. 2017;16(5):406–414. doi: 10.1080/15384101.2016.1220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dökümcü K., Farahani R.M. Evolution of resistance in cancer: a cell cycle Perspective. Front Oncol. 2019;9:376. doi: 10.3389/fonc.2019.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Fanjul V., Guerrero-López R., Fernández-Varas B., et al. Comparison of colorectal cancer stem cells and oxaliplatin-resistant cells unveils functional similarities. Cells. 2022;11(3):511. doi: 10.3390/cells11030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Rohde L.H., Wu H. Involvement of nucleotide excision and mismatch repair mechanisms in double strand break repair. Curr Genomics. 2009;10(4):250–258. doi: 10.2174/138920209788488544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah M.A., Schwartz G.K. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7(8):2168–2181. [PubMed] [Google Scholar]
- 38.Powathil G. Investigating the development of chemotherapeutic drug resistance in cancer: a multiscale computational study. arXiv preprint arXiv:1407.0865, 2014.
- 39.Yeh A.C., Ramaswamy S. Mechanisms of cancer cell dormancy--another hallmark of cancer? Cancer Res. 2015;75(23):5014–5022. doi: 10.1158/0008-5472.CAN-15-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen B.D., Benada J., Yung P.Y.K., et al. Cancer cells use self-inflicted DNA breaks to evade growth limits imposed by genotoxic stress. Science. 2022;376(6592):476–483. doi: 10.1126/science.abi6378. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Si J., Gan L., et al. Research progress on therapeutic targeting of quiescent cancer cells. Artif Cells Nanomed Biotechnol. 2019;47(1):2810–2819. doi: 10.1080/21691401.2019.1638793. [DOI] [PubMed] [Google Scholar]
- 42.Nik Nabil W.N., Xi Z., Song Z., et al. Towards a framework for better understanding of quiescent cancer cells. Cells. 2021;10(3):562. doi: 10.3390/cells10030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra A., Mishra L., Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–10711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L., Zhang K., He H., et al. The relationship between mesenchymal stem cells and tumor dormancy. Front Cell Dev Biol. 2021:9. doi: 10.3389/fcell.2021.731393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldossary S. Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J. 2019;11:7–15. [Google Scholar]
- 46.Dasari S., Njiki S., Mbemi A., et al. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. 2022;23(3) doi: 10.3390/ijms23031532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo Y., Leverson J.D. New opportunities in chemosensitization and radiosensitization: modulating the DNA-damage response. Expert Rev Anticancer Ther. 2005;5(2):333–342. doi: 10.1586/14737140.5.2.333. [DOI] [PubMed] [Google Scholar]
- 48.Tzamali E., Tzedakis G., Sakkalis V. Modeling how heterogeneity in cell cycle length affects cancer cell growth dynamics in response to treatment. Front Oncol. 2020;10:1552. doi: 10.3389/fonc.2020.01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidya F.U., Sufiyan Chhipa A., Mishra V., et al. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022;5(12) doi: 10.1002/cnr2.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X., Zhang W., Yang W., et al. Acquired resistance for immune checkpoint inhibitors in cancer immunotherapy: challenges and prospects. Aging. 2022;14(2):1048–1064. doi: 10.18632/aging.203833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Zhang H., Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2(2):141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhtarkhavari T., Bahrami A.R., Matin M.M. Downregulation of miR-21 as a promising strategy to overcome drug resistance in cancer. Eur J Pharmacol. 2022;932 doi: 10.1016/j.ejphar.2022.175233. [DOI] [PubMed] [Google Scholar]
- 54.Haider T., Pandey V., Banjare N., et al. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol Rep. 2020;72(5):1125–1151. doi: 10.1007/s43440-020-00138-7. [DOI] [PubMed] [Google Scholar]
- 55.Powathil G.G., Gordon K.E., Hill L.A., et al. Modelling the effects of cell-cycle heterogeneity on the response of a solid tumour to chemotherapy: biological insights from a hybrid multiscale cellular automaton model. J Theor Biol. 2012;308:1–19. doi: 10.1016/j.jtbi.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Cohen A.A., Geva-Zatorsky N., Eden E., et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322(5907):1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- 57.Gascoigne K.E., Taylor S.S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14(2):111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Kreso A., O'Brien C.A., van Galen P., et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339(6119):543–548. doi: 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valent P., Bonnet D., De Maria R., et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12(11):767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 60.Blagosklonny M.V. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20(3):385–391. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 61.Ryl T., Kuchen E.E., Bell E., et al. Cell-cycle position of single MYC-driven cancer cells dictates their susceptibility to a chemotherapeutic drug. Cell Syst. 2017;5(3):237–250. doi: 10.1016/j.cels.2017.07.005. e238. [DOI] [PubMed] [Google Scholar]
- 62.Sun Y., Liu Y., Ma X., et al. The influence of cell cycle regulation on chemotherapy. Int J Mol Sci. 2021;22(13):6923. doi: 10.3390/ijms22136923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheikhi-Mohammareh S., Shiri A., Maleki E.H., et al. Synthesis of various derivatives of [1,3]Selenazolo[4,5-d]pyrimidine and exploitation of these heterocyclic systems as antibacterial, antifungal, and anticancer agents. ChemistrySelect. 2020;5(32):10060–10066. [Google Scholar]
- 64.Mollaei M., Hassan Z.M., Khorshidi F., et al. Chemotherapeutic drugs: cell death- and resistance-related signaling pathways. Are they really as smart as the tumor cells? Transl Oncol. 2021;14(5) doi: 10.1016/j.tranon.2021.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bavi P., Uddin S., Ahmed M., et al. Bortezomib stabilizes mitotic cyclins and prevents cell cycle progression via inhibition of UBE2C in colorectal carcinoma. Am J Pathol. 2011;178(5):2109–2120. doi: 10.1016/j.ajpath.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motwani M., Delohery T.M., Schwartz G.K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999;5(7):1876–1883. [PubMed] [Google Scholar]
- 67.Damen M.P.F., van Rheenen J., Scheele C.L.G.J. Targeting dormant tumor cells to prevent cancer recurrence. FEBS J. 2021;288(21):6286–6303. doi: 10.1111/febs.15626. [DOI] [PubMed] [Google Scholar]
- 68.O'Farrell P.H. Quiescence: early evolutionary origins and universality do not imply uniformity. Philos Trans R Soc Lond B Biol Sci. 2011;366(1584):3498–3507. doi: 10.1098/rstb.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urbán N., Cheung T.H. Stem cell quiescence: the challenging path to activation. Development. 2021;148(3):dev165084. doi: 10.1242/dev.165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiore A., Spencer V.A., Mori H., et al. laminin-111 and the level of nuclear actin regulate epithelial quiescence via exportin-6. Cell Rep. 2017;19(10):2102–2115. doi: 10.1016/j.celrep.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer S.L., Cappell S.D., Tsai F.C., et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155(2):369–383. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer V.A., Costes S., Inman J.L., et al. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci. 2011;124(Pt 1):123–132. doi: 10.1242/jcs.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valcourt J.R., Lemons J.M., Haley E.M., et al. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11(9):1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumari R., Jat P. Mechanisms of Cellular Senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sebestyén A., Dankó T., Sztankovics D., et al. The role of metabolic ecosystem in cancer progression — metabolic plasticity and mTOR hyperactivity in tumor tissues. Cancer Metastasis Rev. 2021;40(4):989–1033. doi: 10.1007/s10555-021-10006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Considine M.J., Considine J.A. On the language and physiology of dormancy and quiescence in plants. J Exp Bot. 2016;67(11):3189–3203. doi: 10.1093/jxb/erw138. [DOI] [PubMed] [Google Scholar]
- 77.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 78.Rodier F., Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao Z., Ke Z., Gorbunova V., et al. Replicatively senescent cells are arrested in G1 and G2 phases. Aging (Albany NY) 2012;4(6):431–435. doi: 10.18632/aging.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye C., Zhang X., Wan J., et al. Radiation-induced cellular senescence results from a slippage of long-term G2 arrested cells into G1 phase. Cell Cycle (Georgetown, Tex) 2013;12(9):1424–1432. doi: 10.4161/cc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun S., Gresham D. Cellular quiescence in budding yeast. Yeast (Chichester, England) 2021;38(1):12–29. doi: 10.1002/yea.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L., Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohammad K., Dakik P., Medkour Y., et al. Quiescence entry, maintenance, and exit in adult stem cells. Int J Mol Sci. 2019;20(9):2158. doi: 10.3390/ijms20092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu N.Y., Rios A.C., Pal B., et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat Cell Biol. 2017;19(3):164–176. doi: 10.1038/ncb3471. [DOI] [PubMed] [Google Scholar]
- 85.Walter D., Lier A., Geiselhart A., et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 86.Pennycook B.R., Barr A.R. Restriction point regulation at the crossroads between quiescence and cell proliferation. FEBS Lett. 2020;594(13):2046–2060. doi: 10.1002/1873-3468.13867. [DOI] [PubMed] [Google Scholar]
- 87.Parr E. The default state of the cell: quiescence or proliferation? Bioessays. 2012;34(1):36–37. doi: 10.1002/bies.201100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malumbres M., Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1(3):222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 89.Lee S.H., Reed-Newman T., Anant S., et al. Regulatory role of quiescence in the biological function of cancer stem cells. Stem Cell Rev Rep. 2020;16(6):1185–1207. doi: 10.1007/s12015-020-10031-8. [DOI] [PubMed] [Google Scholar]
- 90.Fiore A.P.Z.P., Ribeiro PdF., Bruni-Cardoso A. Sleeping beauty and the microenvironment enchantment: microenvironmental regulation of the proliferation-quiescence decision in normal tissues and in cancer development. Front Cell Dev Biol. 2018;6:59. doi: 10.3389/fcell.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen K., Zhang C., Ling S., et al. The metabolic flexibility of quiescent CSC: implications for chemotherapy resistance. Cell Death Dis. 2021;12(9):835. doi: 10.1038/s41419-021-04116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. 2017;44:72–82. doi: 10.1016/j.semcancer.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Manjili M.H. Tumor dormancy and relapse: from a natural byproduct of evolution to a disease state. Cancer Res. 2017;77(10):2564–2569. doi: 10.1158/0008-5472.CAN-17-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao X.-L., Zhang M., Tang Y.-L., et al. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. Onco Targets Ther. 2017;10:5219–5228. doi: 10.2147/OTT.S140854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu D., Chen S., Tan X., et al. Fra-1 promotes breast cancer chemosensitivity by driving cancer stem cells from dormancy. Cancer Res. 2012;72:3451–3456. doi: 10.1158/0008-5472.CAN-11-2536. [DOI] [PubMed] [Google Scholar]
- 96.Nathanson D.A., Gini B., Mottahedeh J., et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343(6166):72–76. doi: 10.1126/science.1241328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimizu T., Sugihara E., Yamaguchi-Iwai S., et al. IGF2 preserves osteosarcoma cell survival by creating an autophagic state of dormancy that protects cells against chemotherapeutic stress. Cancer Res. 2014;74(22):6531–6541. doi: 10.1158/0008-5472.CAN-14-0914. [DOI] [PubMed] [Google Scholar]
- 98.Almog N., Ma L., Schwager C., et al. Consensus micro RNAs governing the switch of dormant tumors to the fast-growing angiogenic phenotype. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ameri K., Maltepe E. HIGD1A-mediated dormancy and tumor survival. Mol Cell Oncol. 2015;2(4) doi: 10.1080/23723556.2015.1030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyu T., Jia N., Wang J., et al. Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics. 2013;8(12):1330–1346. doi: 10.4161/epi.26675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fluegen G., Avivar-Valderas A., Wang Y., et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. 2017;19(2):120–132. doi: 10.1038/ncb3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma S.V., Lee D.Y., Li B., et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Der Vartanian A., Quétin M., Michineau S., et al. PAX3 confers functional heterogeneity in skeletal muscle stem cell responses to environmental stress. Cell Stem Cell. 2019;24(6):958–973. doi: 10.1016/j.stem.2019.03.019. e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scaramozza A., Park D., Kollu S., et al. Lineage tracing reveals a subset of reserve muscle stem cells capable of clonal expansion under stress. Cell Stem Cell. 2019;24(6):944–957. doi: 10.1016/j.stem.2019.03.020. e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu Z., Pestell T.G., Lisanti M.P., Pestell R.G. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 107.Aramini B., Masciale V., Grisendi G., et al. Dissecting tumor growth: the role of cancer stem cells in drug resistance and recurrence. Cancers (Basel) 2022;14(4):976. doi: 10.3390/cancers14040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dallas N.A., Xia L., Fan F., et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69(5):1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levina V., Marrangoni A.M., DeMarco R., et al. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clarke M.F., Dick J.E., Dirks P.B., et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 111.Shigdar S., Li Y., Bhattacharya S., et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345(2):271–278. doi: 10.1016/j.canlet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 112.Beyes S., Bediaga N.G., Zippo A. An Epigenetic Perspective on intra-tumour heterogeneity: novel insights and new challenges from multiple fields. Cancers (Basel) 2021;13(19):4969. doi: 10.3390/cancers13194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dalerba P., Cho R.W., Clarke M.F. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 114.Trosko J.E. The concept of “cancer stem cells” in the context of classic carcinogenesis hypotheses and experimental findings. Life (Basel) 2021;11(12):1308. doi: 10.3390/life11121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 116.Al-Hajj M., Wicha M.S., Benito-Hernandez A., et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collins A.T., Berry P.A., Hyde C., et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 118.Eramo A., Lotti F., Sette G., et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 119.Hermann P.C., Huber S.L., Herrler T., et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Ma S., Chan K.W., Lee T.K., et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6(7):1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 121.O’Brien C.A., Pollett A., Gallinger S., et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 122.Prince M.E., Sivanandan R., Kaczorowski A., et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh S.K., Hawkins C., Clarke I.D., et al. Identification of human brain tumor initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 124.Takaishi S., Okumura T., Tu S., et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang S., Balch C., Chan M.W., et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cirillo N., Wu C., Prime S.S. Heterogeneity of cancer stem cells in tumorigenesis, metastasis, and resistance to antineoplastic treatment of head and neck tumours. Cells. 2021;10(11):3068. doi: 10.3390/cells10113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015;31:84–88. doi: 10.1016/j.semcancer.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 128.Koff J.L., Ramachandiran S., Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. Int J Mol Sci. 2015;16(2):2942–2955. doi: 10.3390/ijms16022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Safa A.R., Pollok K.E. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel) 2011;3(2):1639–1671. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang K.D., Ling M.T. Targeting drug-resistant prostate cancer with dual PI3K/mTOR inhibition. Curr Med Chem. 2014;21(26):3048–3056. doi: 10.2174/0929867321666140414100127. [DOI] [PubMed] [Google Scholar]
- 131.Zang F., Wei X., Leng X., et al. C-FLIP(L) contributes to TRAIL resistance in HER2-positive breast cancer. Biochem Biophys Res Commun. 2014;450(1):267–273. doi: 10.1016/j.bbrc.2014.05.106. [DOI] [PubMed] [Google Scholar]
- 132.Dembinski J.L., Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26(7):611–623. doi: 10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin W.C., Rajbhandari N., Liu C., et al. Dormant cancer cells contribute to residual disease in a model of reversible pancreatic cancer. Cancer Res. 2013;73(6):1821–1830. doi: 10.1158/0008-5472.CAN-12-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao M.Q., Choi Y.P., Kang S., et al. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29(18):2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 135.Roesch A., Fukunaga-Kalabis M., Schmidt E.C., et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141(4):583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zeuner A., Francescangeli F., Contavalli P., et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-XL inhibition in non-small cell lung cancer. Cell Death Differ. 2014;21(12):1877–1888. doi: 10.1038/cdd.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holtz M.S., Forman S.J., Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19(6):1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 138.Ebinger S., Ozdemir E.Z., Ziegenhain C., et al. Characterization of rare, dormant, and therapy-resistant cells in acute lymphoblastic leukemia. Cancer Cell. 2016;30(6):849–862. doi: 10.1016/j.ccell.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J., Li Y., Yu T.S., et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lan X., Jorg D.J., Cavalli F.M.G., et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017;549(7671):227–232. doi: 10.1038/nature23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liang D.H., Choi D.S., Ensor J.E., et al. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016;376(2):249–258. doi: 10.1016/j.canlet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kabraji S., Sole X., Huang Y., et al. AKT1(low) quiescent cancer cells persist after neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res. 2017;19(1):88. doi: 10.1186/s13058-017-0877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Angelis M.L., Francescangeli F., La Torre F., et al. Stem cell plasticity and dormancy in the development of cancer therapy resistance. Front Oncol. 2019;9:626. doi: 10.3389/fonc.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puig I., Tenbaum S.P., Chicote I., et al. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest. 2018;128(9):3887–3905. doi: 10.1172/JCI96393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tu S.M., Estecio M.R., Lin S.H., et al. Stem cell theory of cancer: rude awakening or bad dream from cancer dormancy? Cancers (Basel) 2022;14(3):655. doi: 10.3390/cancers14030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bajaj J., Diaz E., Reya T. Stem cells in cancer initiation and progression. J Cell Biol. 2020;219(1) doi: 10.1083/jcb.201911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirata N., Yamada S., Shoda T., et al. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 148.Safa A.R. Drug and apoptosis resistance in cancer stem cells: a puzzle with many pieces. Cancer Drug Resist. 2022;5(4):850–872. doi: 10.20517/cdr.2022.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manic G., Sistigu A., Corradi F., et al. Replication stress response in cancer stem cells as a target for chemotherapy. Semin Cancer Biol. 2018;53:31–41. doi: 10.1016/j.semcancer.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Maugeri-Sacca M., Bartucci M., De Maria R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11(8):1627–1636. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]
- 151.Moreira H., Szyjka A., Grzesik J., et al. Celastrol and resveratrol modulate SIRT genes expression and exert anticancer activity in colon cancer cells and cancer stem-like cells. Cancers (Basel) 2022;14(6):1372. doi: 10.3390/cancers14061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mandal P.K., Blanpain C., Rossi D.J. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12(3):198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 153.Vitale I., Manic G., De Maria R., et al. DNA damage in stem cells. Mol Cell. 2017;66(3):306–319. doi: 10.1016/j.molcel.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 154.Bao S., Wu Q., McLendon R.E., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 155.Facchino S., Abdouh M., Chatoo W., et al. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30(30):10096–10111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen W., Dong J., Haiech J., et al. Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells Int. 2016;2016 doi: 10.1155/2016/1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Banerjee S., Nomura A., Sangwan V., et al. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20(9):2388–2399. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lim Y.C., Roberts T.L., Day B.W., et al. A role for homologous recombination and abnormal cell-cycle progression in radioresistance of glioma-initiating cells. Mol Cancer Ther. 2012;11(9):1863. doi: 10.1158/1535-7163.MCT-11-1044. [DOI] [PubMed] [Google Scholar]
- 159.Yuan M., Eberhart C.G., Kai M. RNA binding protein RBM14 promotes radio-resistance in glioblastoma by regulating DNA repair and cell differentiation. Oncotarget. 2014;5(9):2820–2826. doi: 10.18632/oncotarget.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Desai A., Webb B., Gerson S.L. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother Oncol. 2014;110(3):538–545. doi: 10.1016/j.radonc.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Janzen D.M., Tiourin E., Salehi J.A., et al. An apoptosis-enhancing drug overcomes platinum resistance in a tumour-initiating subpopulation of ovarian cancer. Nat Commun. 2015;6:7956. doi: 10.1038/ncomms8956. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.Karimi-Busheri F., Rasouli-Nia A., Mackey J.R., et al. Senescence evasion by MCF-7 human breast tumor-initiating cells. Breast Cancer Res. 2010;12(3):R31. doi: 10.1186/bcr2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Venere M., Hamerlik P., Wu Q., et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ. 2014;21(2):258–269. doi: 10.1038/cdd.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ffrench B., Gasch C., Hokamp K., et al. CD10(-)/ALDH(-) cells are the sole cisplatin-resistant component of a novel ovarian cancer stem cell hierarchy. Cell Death Dis. 2017;8(10):e3128. doi: 10.1038/cddis.2017.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu W.K., Wang Z., Fong C.C., et al. Chemoresistant lung cancer stem cells display high DNA repair capability to remove cisplatin-induced DNA damage. Br J Pharmacol. 2017;174(4):302–313. doi: 10.1111/bph.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheung T.H., Rando T.A. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferreira C.G., Tolis C., Giaccone G. p53 and chemosensitivity. Ann Oncol. 1999;10(9):1011–1021. doi: 10.1023/a:1008361818480. [DOI] [PubMed] [Google Scholar]
- 168.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crea F., Nur Saidy N.R., Collins C.C., et al. The epigenetic/noncoding origin of tumor dormancy. Trends Mol Med. 2015;21(4):206–211. doi: 10.1016/j.molmed.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 170.Lagadec C., Meignan S., Adriaenssens E., et al. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28(18):1960–1970. doi: 10.1038/onc.2009.61. [DOI] [PubMed] [Google Scholar]
- 171.Huang X., Gan G., Wang X., et al. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15(7):1258–1279. doi: 10.1080/15548627.2019.1580105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moeckel S., LaFrance K., Wetsch J., et al. ATF4 contributes to autophagy and survival in sunitinib treated brain tumor initiating cells (BTICs) Oncotarget. 2019;10(3):368–382. doi: 10.18632/oncotarget.26569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ojha R., Leli N.M., Onorati A., et al. ER translocation of the MAPK pathway drives therapy resistance in BRAF-mutant melanoma. Cancer Discov. 2019;9(3):396–415. doi: 10.1158/2159-8290.CD-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ou J., Peng Y., Yang W., et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat Commun. 2019;10(1):1078. doi: 10.1038/s41467-019-08902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ghaffari S. Lysosomal regulation of metabolism in quiescent hematopoietic stem cells: more than just autophagy. Cell Stem Cell. 2021;28(3):374–377. doi: 10.1016/j.stem.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nazio F., Bordi M., Cianfanelli V., et al. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702. doi: 10.1038/s41418-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rothe K., Porter V., Jiang X. Current outlook on autophagy in human leukemia: foe in cancer stem cells and drug resistance, friend in new therapeutic interventions. Int J Mol Sci. 2019;20(3):461. doi: 10.3390/ijms20030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smith A.G., Macleod K.F. Autophagy, cancer stem cells and drug resistance. J Pathol. 2019;247(5):708–718. doi: 10.1002/path.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jo H., Jia Y., Subramanian K.K., et al. Cancer cell-derived clusterin modulates the phosphatidylinositol 3’-kinase-Akt pathway through attenuation of insulin-like growth factor 1 during serum deprivation. Mol Cell Biol. 2008;28(13):4285–4299. doi: 10.1128/MCB.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang X., Lee J., Xie C. Autophagy regulation on cancer stem cell maintenance, metastasis, and therapy resistance. Cancers (Basel) 2022;14(2):381. doi: 10.3390/cancers14020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harper K.L., Sosa M.S., Entenberg D., et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature. 2016;540(7634):588–592. doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hosseini H., Obradovic M.M.S., Hoffmann M., et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lawson D.A., Bhakta N.R., Kessenbrock K., et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Werner-Klein M., Scheitler S., Hoffmann M., et al. Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat Commun. 2018;9(1):595. doi: 10.1038/s41467-017-02674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seely K.D., Morgan A.D., Hagenstein L.D., et al. Bacterial involvement in progression and metastasis of colorectal neoplasia. Cancers (Basel). 2022;14(4):1019. doi: 10.3390/cancers14041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weidenfeld K., Barkan D. EMT and stemness in tumor dormancy and outgrowth: are they intertwined processes? Front Oncol. 2018;8:381. doi: 10.3389/fonc.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 189.Pantel K., Brakenhoff R.H. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 190.Dasgupta A., Lim A.R., Ghajar C.M. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11(1):40–61. doi: 10.1002/1878-0261.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hen O., Barkan D. Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Semin Cancer Biol. 2020;60:157–165. doi: 10.1016/j.semcancer.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 192.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 193.Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12(6):425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 194.Ohashi S., Natsuizaka M., Naganuma S., et al. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 2011;71(21):6836–6847. doi: 10.1158/0008-5472.CAN-11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uramoto H., Iwata T., Onitsuka T., et al. Epithelial-mesenchymal transition in EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res. 2010;30(7):2513–2517. [PubMed] [Google Scholar]
- 196.Xie M., Zhang L., He C.S., et al. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib-acquired resistant lung cancer cells. J Cell Biochem. 2012;113(5):1501–1513. doi: 10.1002/jcb.24019. [DOI] [PubMed] [Google Scholar]
- 197.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 198.Zhang H., Steed A., Co M., et al. Cancer stem cells, epithelial-mesenchymal transition, ATP and their roles in drug resistance in cancer. Cancer Drug Resist. 2021;4:684–709. doi: 10.20517/cdr.2021.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Derksen P.W., Liu X., Saridin F., et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10(5):437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 200.Giancotti F.G. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kallergi G., Papadaki M.A., Politaki E., et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13(3):R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mego M., Mani S.A., Lee B.N., et al. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2012;130(4):808–816. doi: 10.1002/ijc.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee J.M., Dedhar S., Kalluri R., et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Aktas B., Tewes M., Fehm T., et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.LeBleu V.S., O’Connell J.T., Gonzalez Herrera K.N., et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Byers L.A., Diao L., Wang J., et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19(1):279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yu M., Bardia A., Wittner B.S., et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Carlson P., Dasgupta A., Grzelak C.A., et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat Cell Biol. 2019;21(2):238–250. doi: 10.1038/s41556-018-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ogba N., Manning N.G., Bliesner B.S., et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16(6):489. doi: 10.1186/s13058-014-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aguirre-Ghiso J.A., Sosa M.S. Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Annu Rev Cancer Biol. 2018;2(1):377–393. [Google Scholar]
- 212.Polzer B., Klein C.A. Metastasis awakening: the challenges of targeting minimal residual cancer. Nat Med. 2013;19(3):274–275. doi: 10.1038/nm.3121. [DOI] [PubMed] [Google Scholar]
- 213.Ghajar C.M. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Holohan C., Van Schaeybroeck S., Longley D.B., et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 215.Abderrahman B., Jordan V.C. Rethinking extended adjuvant antiestrogen therapy to increase survivorship in breast cancer. JAMA Oncol. 2018;4(1):15–16. doi: 10.1001/jamaoncol.2017.3510. [DOI] [PubMed] [Google Scholar]
- 216.Recasens A., Munoz L. Targeting cancer cell dormancy. Trends Pharmacol Sci. 2019;40(2):128–141. doi: 10.1016/j.tips.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 217.García-Prat L., Martínez-Vicente M., Perdiguero E., et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 218.Kudlova N., De Sanctis J.B., Hajduch M. Cellular senescence: molecular targets, biomarkers, and senolytic drugs. Int J Mol Sci. 2022;23(8):4168. doi: 10.3390/ijms23084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Filippi-Chiela E.C., Silva MmBe, Thomé M.P., et al. Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage. Autophagy. 2015;11(7):1099–1113. doi: 10.1080/15548627.2015.1009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Essers M.A., Trumpp A. Targeting leukemic stem cells by breaking their dormancy. Mol Oncol. 2010;4(5):443–450. doi: 10.1016/j.molonc.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saito Y., Uchida N., Tanaka S., et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28(3):275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vanner R.J., Remke M., Gallo M., et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26(1):33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]