Abstract
In recent years, significant breakthroughs have been made in the field of gene therapy. Adeno-associated virus (AAV) is one of the most promising gene therapy vectors and a powerful tool for delivering the gene of interest. Among the AAV vectors, AAV serotype 8 (AAV8) has attracted much attention for its efficient and stable gene transfection into specific tissues. Currently, recombinant AAV8 has been widely used in gene therapy research on a variety of diseases, including genetic diseases, cancers, autoimmune diseases, and viral diseases. This paper reviewed the applications and challenges of using AAV8 as a vector for gene therapy, with the aim of providing a valuable resource for those pursuing the application of viral vectors in gene therapy.
Keywords: AAV8, Adeno-associated virus, Gene therapy, Primates, Recombinant, Rodents
Introduction
Gene therapy involves introducing a gene of interest into target cells to replace or repair defective genes and correct genetic disorders. The key to disease treatment lies in gene delivery vectors. Currently, commonly used vectors are delineated into viral vectors and non-viral vectors. Viral vectors account for more than 70% of gene delivery vectors currently used in clinical trials1 and the most successful delivery vectors include retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses (AAVs) (Table 1). Retroviruses are RNA viruses that have the ability to convert RNA into DNA and then integrate the DNA into the host chromosome. Initial studies on retroviruses began in the 1980s when they were first used in clinical trials to treat severe combined immunodeficiency syndrome (SCID-X1).2 Lentivirus, another retrovirus, is derived from the human immunodeficiency virus and is generally the best choice for in vitro treatment of dividing cells. Adenovirus is an unenveloped virus with a large genome of 35 kb but the use of adenovirus is limited because of its many side effects that require a more complex design as a vector. Throughout the history of gene therapy, there have been two major safety incidents where the first was the application of the adenovirus vector led to the induction of a robust immune response and the death of the recipient.3 The second incident was due to a genetic mutation in the retrovirus leading to the patient developing leukemia.4 These two incidences raised the alarm in terms of the application of viral vectors in human gene therapy. Later, Jim Wilson discovered and promoted a safer vector for gene therapy, namely, AAV vector. Compared with other viral vectors, the low AAV viral load needed makes it useful in targeting certain rare diseases. This vector is usually used for non-dividing target cells such as the liver, nervous system, eye, and skeletal muscle cells. The advantage of using this vector is that once it is delivered, the DNA it carries can exist in the free form within the patient's body for an extended period, even a lifetime, and does not lead to potential insertion mutations within the host cell genome, thus, improving the safety of gene therapy. In addition, different AAV serotypes have specific affinities for different tissues and therefore can be exploited in the design of treatment for different target organs. In this review, we highlight the AAV8 serotype, describe its biological properties and discuss its opportunities and challenges in clinical applications.
Table 1.
Viral vector comparison.
| Retrovirus vector | Adenovirus vector | Adeno-associated virus vector | Lentivirus vector | |
|---|---|---|---|---|
| Carrier size | 8.5 kb | 36 kb | 5 kb | 9 kb |
| Nucleic acid type | RNA | DNA | DNA | RNA |
| Target cell type | Dividing cell | Dividing or non-dividing cells | Dividing or non-dividing cells | Dividing or non-dividing cells |
| Gene integration | Random integration | Unconformity | Integration | Integration |
| Exogenous gene expression | Transient expression/stable expression | Transient expression | Stable expression | Stable expression |
| Gene transfection efficiency | High | High | Medium | Medium |
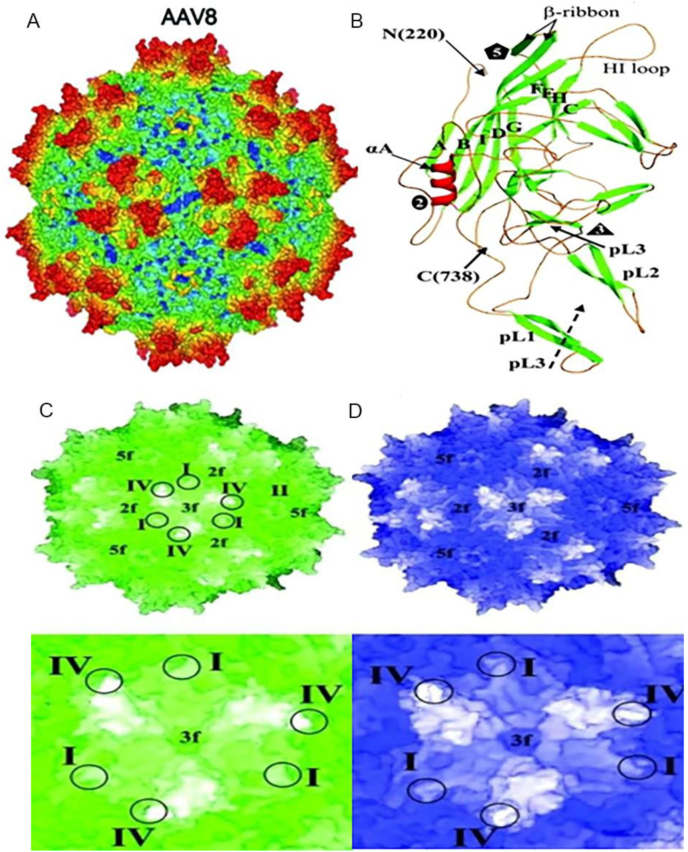
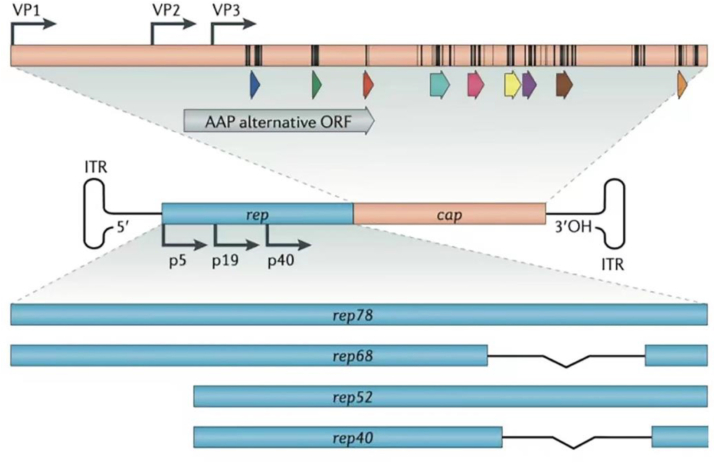
AAV is a single-stranded DNA virus with an overall structure consisting of two parts5,6: an icosahedral protein capsid of approximately 26 nm in diameter and a single-stranded DNA genome of 4.7 kb in length (Fig. 1A). Three types of viral proteins (VP), VP1, VP2, and VP3, are contained in the capsid. The two ends of the AAV genome carry two “T”-shaped inverted terminal repeats (ITRs), and between the ITR sequences is the viral coding region, which contains two genes, Rep and Cap (Fig. 2). The structure of the ordered region of the AAV8 VP monomer consists of the core eight-chain β-barrel motif and the long loop between the chains (Fig. 1B), which includes the HI loop (the loop between βH and βI), αA, and the loop region forming the protrusion around the icosahedral triplet (pL1 to pL3).6 The complex loop between the β-barrel chains forms the surface of the capsid. Variable region I in AAV8 (residues 263 to 271) is known to contain two and five additional amino acids compared to the same region in AAV2 and AAV4, respectively. Variable region II is located at the turn between the β-bands and forms the five-fold axial channel, while variable region IV is in the large loop region between the β-chains βG and βH (called the GH loop).
Figure 1.
AAV8 virus structure. (A) Schematic representation of the AAV8 virus particle. (B) AAV8 VP3 ribbon diagram. Comparison between the (C) AAV8 and (D) AAV2 capsid structures. The top represents the shell surface topology, the bottom represents the triple symmetry axis region, and the circles represent the positions of variable regions I, II, and IV.
Figure 2.
AAV genome structure. The AAV genome consists of two parts: rep and cap genes; the ITR is located at both ends. These two genes encode all non-structural (rep78, rep68, rep52, rep40) and structural proteins (VP1, VP2, VP3) for replication regulation and capsid structure respectively.
AAVs are classified as new serotypes or variants based on serological reactions and amino acid homology. AAV8 was isolated from rhesus monkey tissue and is highly homologous to other AAVs, with the highest structural similarity of 83% between AAV8 and AAV2 capsids.5 Crystal structure analysis demonstrated that AAV8 and AAV2 viruses have different capsid surface topologies (Fig. 1C, D). These differences lie in residues involved in controlling transduction efficiency and antibody recognition. In addition, the bulge around the triple symmetry axis and the bulge region between the duplex and quintuple symmetry axes has resulted in different receptor recognition regions. The primary receptor for some AAV serotypes is a specific glycan motif. The primary receptor of AAV2 is the heparan sulfate proteoglycan and AAV2 utilizes this glycan for cellular recognition under the action of heparin sulfate affinity. On the other hand, AAV8 has no affinity for heparan sulfate, and the 37/67-kDa laminin receptor (LamR) is considered as the host cellular receptor for AAV8.7 Variation in the sequence and spatial conformation of the AAV8 capsid protein means that the cell receptor bound by AAV8 and the virus particles' nuclear transport process after entering the cell are also different. Together, these variations lead to significant differences in vector infectivity and cell transfection efficiency.8
Tissue distribution of AAV8 vector
To a large extent, the safety of the vector is influenced by the distribution of AAV in vivo. Numerous experimental data have shown that recombinant AAV (rAAV) can be detected in the circulatory system in addition to infecting multiple cells in specific tissues depending on the route of administration of the vector. Following intra-retinal or intra-vitreal injections of rAAV8 vector administered into the choroid of primates, the presence of the vector was detected in the atrial fluid, anterior chamber, and blood. Detection of the vector in the peripheral organs by quantitative PCR revealed the presence of rAAV8 in the spleen and liver, with lower copy numbers in lymphoid organs.9 Lieshout et al reported that the AAV8 vector was detected in all major tissues and blood examined 2 weeks after mice tail vein injection, with the highest levels in the liver and the lowest in the brain.10 In addition, 3 months after intramuscular injection of the vector into rhesus monkeys, expression in both muscle and liver was detectable, albeit higher levels were observed in the liver and correlated positively with the concentration of the vector.11 Taken together, these findings reflect the hepatophilic nature of AAV8.
Tissue transfection rate of the AAV8 vector
AAV8 has a broad tissue tropism including the brain, liver, heart, retina, lung, and muscle cells (Table 2). Serotypes such as AAV2, AAV5, AAV8, and AAV9 are more frequently used to transfect liver cells, and studies have shown that AAV8 and AAV9 both have a strong affinity for liver cells, with AAV8 being the most hepatophilic. In mice, dogs, or primates, rAAV8 can efficiently and stably transfect hepatocytes via the peripheral vein, portal vein, or intraperitoneal injection. It has been demonstrated that in the liver, AAV8-mediated expression of target genes was nearly 10-to-100-fold higher than that of other serotypes.12 Nakai et al.13 found that all hepatocytes were able to convert the incoming single-stranded vector genome into double-stranded DNA. This allowed stable transduction of the rAAV8 vector, achieving nearly 100% transfection rate at doses up to 7.2 × 1012 (vg/mouse) by portal vein injection. The transfection did not lead to any hepatotoxicity and the process was able to deliver 2.5-fold more DNA into the liver when compared to intramuscular administration. One-week-old infant monkeys injected intravenously with AAV8 vectors had a 98% hepatocyte transduction rate within 7 days of administration. In addition, cells of multiple organs can be transduced with extremely high efficiency after peripheral intravenous injection at increasing vector doses. In comparison, the number of hepatocytes transduced with the rAAV1 vector at the same dose was 24%,14 whereas saturation was reached when rAAV2 was administered using a vector dose of 3.0 × 1011 vg/mouse resulting in a transduction efficiency of about 10% total hepatocytes.15 A comparative study of rAAV8 injections via the tail vein and portal vein in mice found similar hepatic transduction efficiencies and no significant differences in transgene expression between the two injection routes, irrespective of the vector dose.14 Sarkar et al16 reported that at a dose of 1.0 × 1011 vg/mouse, the rAAV8 vector expressing canine factor VIII (cFVIII) was equally effective when injected as either a portal or caudal injection route in a background of hemophiliac mice, which resulted in normal levels of factor VIII (FVIII). When AAV8 and other serotype vectors were delivered at the same dose (3 × 1010 vg/mouse), AAV8 transfected the liver most efficiently and achieved high levels of FVIII expression compared to the other serotypes (AAV2, AAV5, and AAV7). Cells transfected with the rAAV8 vector expressing cFVIII maintained more than 10% FVIII activity over a period of up to 12 weeks.17
Table 2.
The affinity of AAV serotypes to cell in various tissues and organs.
| Tissue affinity | Serotype |
|---|---|
| CNS | AAV1, AAV2, AAV5, AAV8, AAV9 |
| Heart | AAV1, AAV8, AAV9 |
| Liver | AAV7, AAV8, AAV9 |
| Pancreas | AAV8 |
| Photoreceptor cell | AAV2, AAV5, AAV8 |
| Retinal pigment epithelium | AAV1, AAV2, AAV4, AAV5, AAV8 |
| Skeletal muscle | AAV1, AAV6, AAV7, AAV8, AAV9 |
AAV8 vectors can also transfect cells in other tissues, including muscle, heart, pancreas, and brain. In newborn dogs, a single jugular injection of AAV8 presents extensive and persistent systemic transduction in skeletal and myocardial muscle, and this expression persists in the heart for at least one year.18 In a report on the transfection of heart cells, AAV8 carrying the green fluorescent protein (GFP) gene was subjected to hind limb intramuscular injection and GFP expression levels in the heart were significantly better than when the AAV1 and AAV2 vectors were used. Nonetheless, while GFP expression was selectively retained in muscle and heart cells, green fluorescence was decreased or lost in liver cells, most likely due to hepatocyte cell division and degradation which eventually led to the dilution or loss of the vector DNA.19
Pancreas can also be efficiently transfected by rAAV8. With in vivo transduction of pancreatic cells, rAAV8 carrying the LacZ reporter gene is expressed mainly in pancreatic follicular cells and significant levels of expression were observed 1 week after the injection. The transduction efficiency was at least 10-fold higher than that of rAAV2,20 and rAAV8 was also detected in the pancreatic duct and blood vessels, while intra-pancreatic ductal injection of rAAV8 mediated gene transfer to almost every β cell.21
In addition, rAAV8 can efficiently transduce the brain. Stereotactic brain injections were performed to compare the transfection efficiency of different serotypes in different regions of the brain. The transgenic expression of rAAV8 in glial cells was significant.22 Moreover, after intramuscular injection of rAAV8, effective and stable transduction was found in the white matter, dorsal root ganglion neurons, and peripheral nerves of the spinal cord of neonatal mice. In particular, more neurons were transduced in the lower part of the spinal cord than in the upper part.23
AAV8 vector specificity in terms of gender
The tissue distribution and expression of the rAAV8 vector have been shown to correlate with the gender of the recipient. After targeted delivery of rAAV8 into the liver, transgene expression in male mice was 5-to-13-fold higher than that in female mice and the expressed protein reacted positively with androgens,24 but this gender difference is reversed in non-human primates (NHPs).25 In mice and NHPs, the recombinant vector copy number was essentially the same over the first 3 days and began to decrease within 4 weeks, which correlated with the transcript level or mRNA half-life. Aimee et al26 injected AAV8-PCSK9 into male and female C57BL/6 mice to investigate potential differences in PCSK9 (proprotein convertase subtilisin/kexin type 9) expression and the development of hypercholesterolemia between male and female mice. The PCSK9 protein plays a role in cholesterol and fatty acid metabolism. At the same dose of 3 × 1010 vector genomes, total cholesterol and PCSK9 levels were lower in female mice than in male mice. It was proposed that the different tissue targeting of AAV8 in male and female mice led to the production of PCSK9 mRNA in tissues other than the liver. In addition, low doses of AAV8 were primarily targeted to the liver of male mice, in contrast to a threefold increase in the vector dose to female mice to achieve similar levels of expression as seen in the male mice, highlighting the importance of appropriate doses of the virus to induce the correct phenotype in males and females. In contrast, the distribution of serotypes was greater in females than in males, and the transduction of AAV8 into the female gonads was significant.27
Immunogenicity of the AAV8 vector
The presence of pre-existing neutralizing antibodies (NAb) against AAV8 is one of the major obstacles to the success of AAV8-mediated gene therapy. Even low titers of anti-AAV8 NAb have been shown to reduce the efficiency of transgene expression in clinical and non-clinical studies. Patients with detectable pre-existing NAbs are excluded from current gene therapy trials to ensure critical assessment of NAbs for successful gene therapy. AAV7 and AAV8 were isolated from non-human primates; hence, the degree of seropositive antibodies against these viruses is lower in humans. Previous studies have shown wide variation in the prevalence of anti-AAV8 Nab with most studies reporting a range of 30%–50%.28, 29, 30 The considerable variation in the reported prevalence of anti-AAV8-NAb across the different studies may reflect study population differences as well as a lack of standardization in the testing protocols.31 Within the general population, NAb prevalence increases with age, however, there is little geographic variation.32 Boutin et al reported that anti-AAV8 IgG antibody was detected in 38% of the population tested, which was significantly lower than antibody levels towards AAV2 (72%), and the titer of the antibodies was low.33 A study undertaken in the United Kingdom on testing pre-existing immunity to AAV8 in patients with hemophilia type A showed that 23% of patients had antibodies against AAV8 and 38% of patients had inhibitors of AAV8 cellular transduction.34
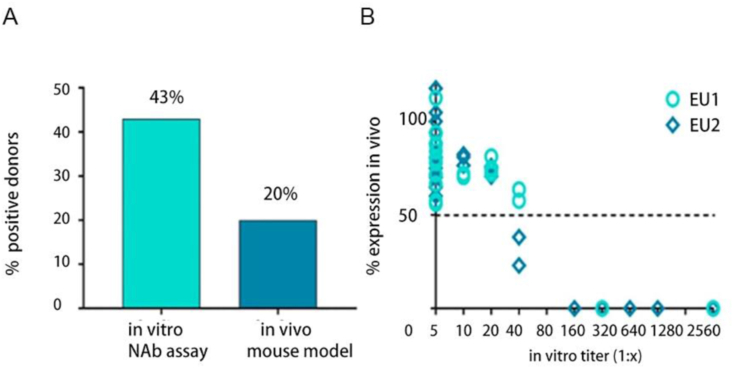
NAb against AAV can be detected not only in humans but also in animals. Kruzik et al30 compared the neutralizing antibody profiles of mice in vivo and in vitro and found that the prevalence of anti-AAV8-NAb was only 20% in vivo, whereas in vitro assays detected a 43% prevalence of NAb (Fig. 3A) with positive titers at ≥ 1:80 (Fig. 3B) for both in vivo and in vitro assays. Using an in vitro NAb assay, Sun et al35 found anti-AAV8 NAb in 69.57% of rhesus donors. Stable expression of anti-AAV8-NAb was also found in the liver of pigs, with an overall NAb prevalence of 62.5% and with low titers.36 In hemophilic dogs treated with AAV8 vectors, NAb against AAV8 was not detected initially, however, after administration of AAV8 and proteasome inhibitors, AAV8 NAb titers in dogs peaked in week 2 but began to decrease in week 8.37
Figure 3.
Comparison between the outcome of the Nab assay when performed in vivo and in vitro.
Application of AAV8 in disease treatment
In recent years, with the advancements in molecular biology, vector technology and transgenic technology have led to the opening of new avenues of gene therapy for disease treatment. Studies have shown that rAAV vectors are safe, are able to transfect a wide range of host cells, and can effectively mediate long-term stable expression of exogenous genes in a variety of cells without expressing any viral protein genes. Several AAV8 programs currently in the clinical phase are summarized in Table 3. The use of AAV8 as a gene therapy vector has been reported in mouse models for the long-term correction of hemophilia A, familial hypercholesterolemia, and type II glycogen storage disease.38, 39, 40 In addition, AAV8 liver-directed gene therapy has been successfully validated in a canine model.5 Chen et al also reported the successful targeting of this vector in the mouse pancreas.41
Table 3.
Clinical AAV8 project in progess.
| Adaptation disease | Transgene | AAV subtype | Clinical stage | Company |
|---|---|---|---|---|
| Familial Hypercholesterolea | LDLR | AAV8 | Phase I, II | University of Pennsyluania |
| GSD1a | G6PC | AAV8 | Phase I, II | Ultragenyx |
| Haemophilia A | FVIII | AAV8 | Phase I, II | Shire |
| Haemophilia B | FIX | AAV8 | Phase I, II | Shire |
| Achromatopsia | CNGB3 | AAV8 | Phase I, II | MeiraGTx |
| Tay-Sachs disease | HEXA and HEXB | AAVrh8 | Phase I | Axovant Sciences |
| AIDS | VRC07 | AAV8 | Phase I | NIH |
| OTC | OTC | AAV8 | Phase I, II | Ultragenyx |
| Retinitis Pigmentosa | RLBP1 | AAV8 | Phase I, II | Novartis |
| Crigler-Najjar-Syndrome | UGT1A1 | AAV8 | Phase I, II | Audentes |
| Pompe Disease | GAA | AAV8 | Phase I, II | Amicus |
| Myotubular myapathy | MTM1 | rAAV8 | Phase II | Audentes |
| MPS-VI | ARSB | AAV8 | Phase I, II | Fondazione Telethon |
| Wet-AMD | Anti-VEGF antibody | AAV8 | Phase I | Regenxbio |
| Congenital Retinoschisis | RS1 | AAV8 | Phase I, II | NEI |
GSD1a, Glycogen Storage Disease Type 1a Disease; AIDS, Acquired Immune Deficiency Syndrome; OTC, Ornithine Transcarbamylase Deficiency; MPS-VI, Mucopolysaccharidosis Type VI; Wet-AMD, Wet age-related macular degeneration.
Hemophilia
Hemophilia is a genetic bleeding disorder in which patients develop severe clotting abnormalities due to a lack of specific clotting factors in the blood. Achieving 1%–5% of normal blood clotting factor activity can stop spontaneous bleeding, making hemophilia suitable for gene therapy. The high efficiency of AAV8 transduction in the liver is attributed to its uncoating ability whereby AAV8 enters cells and rapidly decapsulates and converts the single strand of DNA into a biologically active double-stranded molecular form, which enables the stable expression of foreign genes.42 Mao et al injected AAV8 carrying the FVIII gene into mice with impaired FVIII activity and found that FVIII activity in the plasma of the mice was detectable and hemophilia A symptoms were eliminated.17 Jiang et al compared the transfection rates of different serotypes AAV2, 5, 6, and 8 carrying the functional coagulation FVIII gene in mouse liver and AAV8 had superior rates compared to the other serotypes. More importantly, hemophilic dogs receiving rAAV8-F injections attained therapeutic amounts of FVIII expression during a 3-year observation period, and no antibodies or other toxic reactions against the transgene expression products were produced.43 In hemophilia B gene therapy, serum levels of canine factor IX (cFIX) reached 10%–26% of normal levels and were maintained for more than one year in hemophilic dogs treated with AAV8-cFIX via portal vein injection, and no hepatotoxicity was observed.44 Nathwani et al.45 investigated the expression of the liver-specific human coagulation factor IX (hFIX) regulated by self-complementary AAV8 carrying the liver-specific promoter LPI in non-human primates and showed that the expression of hFIX in the serum of rhesus monkeys reached 22% of typical values after peripheral intravenous injection of 1 × 1012 vg/kg of scAAV8-LP1-hFIX. The presence of the recombinant AAV8 vector and hFIX expression was maintained for at least 9 months, showing good results in the treatment of hemophilia.
Cancer
Cancers are multifactorial diseases resulting from changes in gene regulation triggered by genetic and environmental factors. Potential applications of gene therapy include gene silencing, inhibition of tumor angiogenesis, immunomodulation, cell suicide, inactivation of oncogenes, and expression of oncology drug-related genes. An increasing number of studies have shown that recombinant AAV vectors carrying exogenous genes (e.g., anti-angiogenic genes) can be stably expressed in vivo for a long period to counteract pro-tumor growth factors. Zhou et al46 used rAAV8 carrying the TRAIL (TNF-related apoptosis-inducing ligand) gene to induce apoptosis in colon cancer HCT116 cells, and the recombinant virus-infected colon cancer cells exhibited increased expression of caspase-3 and caspase-8. This indicated that AAV8-TRAIL could activate the caspase pathway and promote apoptosis in colon cancer cells HCT116, thus effectively inhibiting the growth of cancer cells in vitro without any significant effect on normal human hepatocytes. In addition, the AAV8-mediated vasohibin gene (which encodes the angiogenesis inhibitor) achieved stable expression in muscle tissue to resist tumor angiogenesis, maximize tumor cell apoptosis, and effectively limit the growth of tumor cells.
AAV8 has also been used to transfect antitumor genes such as cytokines and microRNAs into the human liver to treat hepatocellular carcinoma (HCC). Delivery of AAV8-bearing IL-15 or IL-12 gene to HCC mouse models significantly inhibited tumor growth and improved survival of tumor-bearing mice.47,48 miR-26-bearing AAV8 was injected intravenously into HCC mouse models and showed high expression of miR-26 in both hepatocytes and HCC cells with good suppression of tumor cells.49 AAV8 has also been shown to target pancreatic tissue for potential pancreatic ductal adenocarcinoma (PDAC) treatment in preclinical animal models.50 The capsid-optimized AAV8 YF (tyrosine–phenylalanine) mutant vector enhanced gene transfection efficiency in both normal and diseased pancreatic tissues and was more effective in transducing pancreatic cells via the intraperitoneal route compared to intravenous injection.41 This suggests that transperitoneal delivery of the AAV8 capsid double mutant is a promising method for pancreatic gene delivery.
Systemic lupus erythematosus (SLE)
SLE is an autoimmune disorder caused by the immune system attacking its tissues, leading to widespread inflammation and tissue damage in affected organs. SLE is prevalent in young women and often leads to multi-organ damage and flare-ups and remissions in SLE patients that can last for years or even decades.51,52 Treatment of SLE primarily uses glucocorticoids and immunosuppressants; however, these drugs are known to cause many side effects. Researchers have tried to develop better treatment strategies based on findings from basic and clinical studies. Ye et al.53 injected AAV8 vectors expressing the fusion proteins CTLA4-lg or CD40-lg into newborn NZB/NZW mice before the onset of lupus and found that the treatment effectively delayed and reduced autoantibody and proteinuria production, and no kidney injury occurred. The mechanism may involve the inhibition of CD4+ T cell activation following which they are converted from initial T cells to memory T cells. This inhibition has been shown to improve in terms of efficacy when the two fusion carriers are co-injected.53
Acquired immune deficiency syndrome (AIDS)
HIV prevention and treatment can be initiated with the induction of broadly neutralizing antibodies. Casazza et al54 proposed AAV virus-mediated DNA encoding potent broadly neutralizing anti-HIV antibodies as a new powerful tool for the treatment of AIDS and other infectious diseases. In their study, a recombinant double cis–trans AAV8 vector, VRC07, carrying the gene encoding an anti-HIV monoclonal antibody that was isolated from the blood of HIV patients, was administered to eight HIV-infected adults to express a broad-spectrum neutralizing antibody (bNAb). This bNAb may be able to block the infection of human cells by both HIV-1 and HIV-2. The results showed that the subjects developed mild local adverse reactions at the highest dose of the vector (2.5 × 1012 vg/kg), but this reaction subsided after 1–2 weeks. To a certain extent, AAV8-VRC07 injection was considered safe and well tolerated. Delivery of antibody genes based on AAV vectors allows for sustained and safe levels of antibodies in the blood.
Diabetes
Type 1 diabetes is caused by an inability to produce insulin in the body. Previously, it was shown that the AAV8 vector carrying the modified and codon-optimized human insulin gene was injected into the mouse liver after which the mouse was able to secrete insulin, thereby ameliorating streptozotocin (STZ)-induced diabetes.55 On this basis, a biosafety mechanism, which combined a Tet-Off switch and the AAV8-insulin vector, was developed. The results showed that insulin secretion following AAV8 gene therapy could be reversibly shut down with doxycycline in diabetic mice induced with STZ and that insulin levels could be restored after discontinuation of doxycycline.56 Manzoor et al57 found that AAV8-bearing IL-35 cytokine selectively targets β cells in non-obese diabetic (NOD) mice and prevents the development of diabetes. With a transfection of 2.5 × 1010 and 10 × 1010 recombinant vector particles, 60% and 79%, respectively, of NOD female mice remained non-diabetic, and a reduction in the number of CD4+ and CD8+ T cells infiltrating the islets was also observed.
Adverse effects of AAV8 in animal and human experiments
AAV vector gene therapy carries certain potential risks, including immunogenic, genotoxic, and hepatotoxic risks as well as risks arising from insertional mutations and transgene variability. Adverse reactions have been reported following the use of AAV8-based gene therapy in clinical trials (Table 4). Astellas Pharma Inc. conducted clinical trials on its AAV gene therapy candidate AT132 in patients with X-linked myotubular myopathy (XLMTM). Unfortunately, four boys died as a result of sepsis and gastrointestinal bleeding.58 The fourth patient died after a period of treatment with a high dose (3.5 × 1014 vg/kg) of an AAV8-based therapy. In the same year, another clinical trial with AAV8-mediated gene therapy was called off by the Food and Drug Administration USA when a subject with late-onset Pompe disease developed peripheral sensory neuropathy adverse events after treatment with AT845.59
Table 4.
Adverse effects of AAV8 gene therapy in clinical trials.
| Gene therapy | Vector | Dosage | Injection route | Adverse events | Indication |
|---|---|---|---|---|---|
| AT132 | AAV8 | 3 × 1014 vg/kg | IV | Liver failure | XLMTM |
| AT845 | AAV8 | 6 × 1013 vg/kg | IV | Peripheral sensory neuropathy | LOPD |
| BIIB112 | AAV8 | / | SR | Clinical primary Endpoint not achieved | XLRP |
| AAV-cFVIII | AAV8 | 3 × 1014 vg/dog | IV | Liver failure | Hemophilia A |
| AAV8-VRC07 | AAV8 | 2.5 × 1012 vg/kg | IM | Local reactogenicity | AIDS |
XLMTM, X-linked motubulary myopathy; LOPD, Late-onset Pompe disease; XLRP, x-linked retinitis pigmentosa; AIDS, Acquired Immune Deficiency Syndrome.
In addition, CD8+ T cell proliferation and liver-related adverse events (associated with liver injury) were observed in AAV8-mediated gene therapy for hemophilia. Introduction of the recombinant AAV8 vector led to clonal expansion of hepatocytes in a dog model of hemophilia A.60 Administration of AAV8 or AAV9 vectors expressing cFVIII (AAV-cFVIII) showed promise whereby the expression of FVIII increased from 1.9% to 11.3% of the normal FVIII levels. However, the long-term follow-up of nearly 10 years after treatment revealed that the therapeutic gene fragment carried by the AAV vector was partially integrated into the genome of dog liver cells, which presented a risk of carcinogenesis. Therefore, long-term monitoring is critical to support the development, clinical trials, and application of AAV gene therapy.
Non-clinical evaluation concerns for AAV8
Selection of experimental animals
When considering the selection of experimental animals, AAV8-targeted hepatic gene transfer has yielded good results in non-human primates. In addition, many studies have also been performed on animals of different species, including rats, mice, cats, and dogs. Since many inherited metabolic disorders manifest at a very early age, early intervention and treatment are particularly critical. In particular, the biological characteristics of young non-human primates are very similar to those of humans, and young monkeys provide a good model for studying the validity, stability, and safety of AAV gene transfer but are less stable compared to adolescent animals.13 In another example, in a mouse model of lethal propionic acidemia, an effective long-term rescue was achieved by injecting the rAAV8 vector at the neonatal stage. In a canine model of type Ia glycogen storage disease, a single injection of the AAV2/8 vector into 1-day-old dogs achieved significant biochemical correction of the deficient enzymes.61 Certain immune diseases require animal models based on specific immune characteristics, such as SLE mentioned above, and NZZB/NZW mice exhibit clinical and immunological characteristics most similar to those of human SLE.53 Therefore, the choice of animal species as the disease model should be determined on a case-by-case basis.
Tissue assays for biodistribution
Greig et al62 evaluated the safety of the rAAV8 vector for the treatment of familial hypercholesterolemia in wild-type rhesus and heterozygous macaques. Throughout the study, the vector was concentrated in the target organ, i.e., the liver, and the gene copy number was 2 logs higher than in other tissues. Aspartate transaminase (AST) and alanine transaminase (ALT) levels were measured to monitor target organ toxicity. Except for abnormally elevated baseline transaminase levels, the elevated levels were only transient, and other parameters reflecting liver pathology, such as bilirubin, were within normal limits throughout the study.63 In addition, after 28 days of vector administration, animals were necropsied and lymphocytes isolated from the liver, spleen, and bone marrow exhibited no T cell responses.62 In a separate study, observation of lung tissue from mice after vector injection revealed a reduction in mononuclear cell infiltration and alveolar cell tissue hyperplasia,64 indicating that the animals did not show any significant signs of discomfort after vector infusion. Biodistribution studies confirmed the high level and stable targeting of AAV8 to liver tissue.
Relatively low immunogenicity
AAV 8 infection only targets some primates but not humans. As a result, antibodies to the AAV8 coat protein are almost non-existent in human serum, making AAV8 a promising potential vector for gene therapy. The transfection efficiency of the AAV8 vector is significantly higher than that of other serotypes of AAV.12,65 Meanwhile, the persistence of the AAV8 coat protein in hepatocytes after liver-targeted delivery is significantly shorter than that of AAV2, which can reduce the possibility of triggering an immune response. Therefore, the use of novel AAVs found in some primates as vectors for human gene therapy to avoid inducing an immune response is feasible and effective. Importantly, the vector's ability to rapidly de-coat and release its genome upon cell entry allows for therapeutic transgene expression using lower doses of the vector.
Discussion and conclusions
AAV8 vectors are valued for their high hepatophilic effect and relatively low immunogenicity. Pre-existing immunity is a major challenge for gene therapy, and these pre-existing antibodies can interfere with virus–cell interactions and severely hinder gene therapy outcomes. Therefore, in terms of improving efficacy and safety, gene therapy with AAV vectors should be target-specific to alleviate toxic effects on non-target cells. The presence of NAb can be overcome by immunosuppression, plasma replacement, increasing the vector dose, or introducing an empty coat.66 For example, Hurlbut et al67 studied the effect of AAV8 pre-existing immunity on rAAV8 transfection and transgene expression using mice and primates as models. The issue of pre-existing AAV8 antibodies was partially resolved by increasing the AAV8 vector dose by a factor of 10. The introduction of an empty coat shell can protect the AAV vector from neutralization by acting as a decoy for anti-AAV antibodies, and a correlation between antibody titers and the dose of the empty coat shell was noted. In addition, exosomes, which are membrane-bound extracellular vesicles, can wrap AAVs for improved cellular transduction and reduced host antibody neutralization.68
Clinical trials with AAV-mediated gene therapy are increasing every year, providing strong support towards further validating the safety and efficacy of the vector, while continuous technology innovation will help develop novel vectors. Long-term monitoring still needs to be refined to achieve higher transduction efficiency, lower immune response, and predictable treatment outcomes. Therefore, it is critical to take full advantage of the promise of AAV gene therapy and to overcome the current challenges to facilitate the application of the rAAV8 system for research on human diseases.
Author contributions
Liyuan Zhao: Writing - Original draft preparation.
Naping Tang: Writing- Reviewing.
Zixuan Yang, Minhui Zheng, Lei Shi, Mengyun Gu, Gang Liu, Feng Miao, Yan Chang, Fanghua Huang: Visualization, Investigation.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Fanghua Huang, Email: huangfh@cde.org.cn.
Naping Tang, Email: napingtang@innostar.cn.
References
- 1.Ginn S.L., Amaya A.K., Alexander I.E., et al. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20(5) doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 2.Fischer A., Hacein-Bey-Abina S. Gene therapy for severe combined immunodeficiencies and beyond. J Exp Med. 2020;217(2) doi: 10.1084/jem.20190607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raper S.E., Chirmule N., Lee F.S., et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metabol. 2003;80(1–2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., et al. LMO2- associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Calcedo R., Nichols T.C., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105(8):3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 6.Nam H.J., Lane M.D., Padron E., et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol. 2007;81(22):12260–12271. doi: 10.1128/JVI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akache B., Grimm D., Pandey K., et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80(19):9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mietzsch M., Barnes C., Hull J.A., et al. Comparative analysis of the capsid structures of AAVrh.10, AAVrh.39, and AAV8. J Virol. 2020;94(6) doi: 10.1128/JVI.01769-19. :e01769-e01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seitz I.P., Michalakis S., Wilhelm B., et al. Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman Primates. Invest Ophthalmol Vis Sci. 2017;58(13):5792–5801. doi: 10.1167/iovs.17-22473. [DOI] [PubMed] [Google Scholar]
- 10.Chen V.P., Gao Y., Geng L., et al. Systemic safety of a recombinant AAV8 vector for human cocaine hydrolase gene therapy: a good laboratory practice preclinical study in mice. Hum Gene Ther. 2020;31(1–2):70–79. doi: 10.1089/hum.2019.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greig J.A., Peng H., Ohlstein J., et al. Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao G.P., Alvira M.R., Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Bell P., Lin J., et al. AAV8-mediated hepatic gene transfer in infant Rhesus monkeys (Macaca mulatta) Mol Ther. 2011;19(11):2012–2020. doi: 10.1038/mt.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakai H., Fuess S., Storm T.A., et al. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79(1):214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao W., Pei X., Cui C., et al. Superior human hepatocyte transduction with adeno-associated virus vector serotype 7. Gene Ther. 2019;26(12):504–514. doi: 10.1038/s41434-019-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar R., Tetreault R., Gao G., et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103(4):1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 17.Mao J.H., Shen Y., Wang Q., et al. Optimized AAV package and experimental application of recombinant AAV8/hFⅧ for gene therapy on hemophilia A mice. Zhonghua Xue Ye Xue Za Zhi. 2020;41(1):34–39. doi: 10.3760/cma.j.issn.0253-2727.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan X., Yue Y., Zhang K., et al. Long-term robust myocardial transduction of the dog heart from a peripheral vein by adeno-associated virus serotype-8. Hum Gene Ther. 2013;24(6):584–594. doi: 10.1089/hum.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Zhu T., Qiao C., et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23(3):321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 20.Wang A.Y., Peng P.D., Ehrhardt A., et al. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15(4):405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 21.Ramzy A., Tudurí E., Glavas M.M., et al. AAV8 Ins1-Cre can produce efficient β-cell recombination but requires consideration of off-target effects. Sci Rep. 2020;10 doi: 10.1038/s41598-020-67136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aschauer D.F., Kreuz S., Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayers J.I., Fromholt S., Sinyavskaya O., et al. Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease-modifying effect in ALS mice. Mol Ther. 2015;23(1):53–62. doi: 10.1038/mt.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidoff A.M., Ng C.C., Zhou J., et al. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102(2):480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- 25.Nietupski J.B., Hurlbut G.D., Ziegler R.J., et al. Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman Primates despite low hepatic expression. Mol Ther. 2011;19(11):1999–2011. doi: 10.1038/mt.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vozenilek A.E., Blackburn C.M.R., Schilke R.M., et al. AAV8-mediated overexpression of mPCSK9 in liver differs between male and female mice. Atherosclerosis. 2018;278:66–72. doi: 10.1016/j.atherosclerosis.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pañeda A., Vanrell L., Mauleon I., et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20(8):908–917. doi: 10.1089/hum.2009.031. [DOI] [PubMed] [Google Scholar]
- 28.Aronson S.J., Veron P., Collaud F., et al. Prevalence and relevance of pre-existing anti-adeno-associated virus immunity in the context of gene therapy for crigler-najjar syndrome. Hum Gene Ther. 2019;30(10):1297–1305. doi: 10.1089/hum.2019.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruzik A., Fetahagic D., Hartlieb B., et al. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol Ther Methods Clin Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruzik A., Koppensteiner H., Fetahagic D., et al. Detection of biologically relevant low-titer neutralizing antibodies against adeno-associated virus require sensitive in vitro assays. Hum Gene Ther Methods. 2019;30(2):35–43. doi: 10.1089/hgtb.2018.263. [DOI] [PubMed] [Google Scholar]
- 31.Louis Jeune V., Joergensen J.A., Hajjar R.J., et al. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. 2013;24(2):59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimuro J., Mizukami H., Shima M., et al. The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J Med Virol. 2014;86(11):1990–1997. doi: 10.1002/jmv.23818. [DOI] [PubMed] [Google Scholar]
- 33.Boutin S., Monteilhet V., Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 34.Stanford S., Pink R., Creagh D., et al. Adenovirus-associated antibodies in UK cohort of hemophilia patients: a seroprevalence study of the presence of adenovirus-associated virus vector-serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res Pract Thromb Haemost. 2019;3(2):261–267. doi: 10.1002/rth2.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L., Tu L., Gao G., et al. Assessment of a passive immunity mouse model to quantitatively analyze the impact of neutralizing antibodies on adeno-associated virus-mediated gene transfer. J Immunol Methods. 2013;387(1–2):114–120. doi: 10.1016/j.jim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Watano R., Ohmori T., Hishikawa S., et al. Utility of microminipigs for evaluating liver-mediated gene expression in the presence of neutralizing antibody against vector capsid. Gene Ther. 2020;27(9):427–434. doi: 10.1038/s41434-020-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J., Shao W., Chen X., et al. An observational study from long-term AAV re-administration in two hemophilia dogs. Mol Ther Methods Clin Dev. 2018;10:257–267. doi: 10.1016/j.omtm.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lengler J., Coulibaly S., Gruber B., et al. Development of an in vitro biopotency assay for an AAV8 hemophilia B gene therapy vector suitable for clinical product release. Mol Ther Methods Clin Dev. 2020;17:581–588. doi: 10.1016/j.omtm.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Muthuramu I., Somanathan S., et al. Developing a second-generation clinical candidate AAV vector for gene therapy of familial hypercholesterolemia. Mol Ther Methods Clin Dev. 2021;22:1–10. doi: 10.1016/j.omtm.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han S.O., Gheorghiu D., Li S., et al. Minimum effective dose to achieve biochemical correction with adeno-associated virus vector-mediated gene therapy in mice with Pompe disease. Hum Gene Ther. 2022;33(9–10):492–498. doi: 10.1089/hum.2021.252. [DOI] [PubMed] [Google Scholar]
- 41.Chen M., Maeng K., Nawab A., et al. Efficient gene delivery and expression in pancreas and pancreatic tumors by capsid-optimized AAV8 vectors. Hum Gene Ther Methods. 2017;28(1):49–59. doi: 10.1089/hgtb.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenney R.M., Bell C.L., Wilson J.M. AAV8 capsid variable regions at the two-fold symmetry axis contribute to high liver transduction by mediating nuclear entry and capsid uncoating. Virology. 2014;454–455:227–236. doi: 10.1016/j.virol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H., Lillicrap D., Patarroyo-White S., et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108(1):107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 44.Shao W., Sun J., Chen X., et al. Chimeric mice engrafted with canine hepatocytes exhibits similar AAV transduction efficiency to hemophilia B dog. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.815317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathwani A.C., Gray J.T., McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109(4):1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou B., Qiao H., Tong L., et al. Apoptosis-inducing effect of recombinant adeno-associated virus serotype 8 with TRAIL gene on human colon cancer cell line. Chin J Gen Surg. 2006;(4):281–283. [Google Scholar]
- 47.Lo C.H., Chang C.M., Tang S.W., et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med. 2010;12(5):423–434. doi: 10.1002/jgm.1452. [DOI] [PubMed] [Google Scholar]
- 48.Chang C.M., Lo C.H., Shih Y.M., et al. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Hum Gene Ther. 2010;21(5):611–621. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 49.Yin L., Keeler G.D., Zhang Y., et al. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther. 2021;28(7–8):422–434. doi: 10.1038/s41434-020-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W., Li H., Liu X., et al. Precise and efficient silencing of mutant KrasG12D by CRISPR-CasRx controls pancreatic cancer progression. Theranostics. 2020;10(25):11507–11519. doi: 10.7150/thno.46642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kósa F., Kunovszki P., Gimesi-Országh J., et al. High risk of depression, anxiety, and an unfavorable complex comorbidity profile is associated with SLE: a nationwide patient-level study. Arthritis Res Ther. 2022;24:116. doi: 10.1186/s13075-022-02799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang P., Zhang X., Chen S., et al. A novel serum tsRNA for diagnosis and prediction of nephritis in SLE. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye X., Zhu T., Bastacky S., et al. Prevention and reversal of lupus in NZB/NZW mice by costimulatory blockade with adeno-associated virus-mediated gene transfer. Arthritis Rheum. 2005;52(12):3975–3986. doi: 10.1002/art.21417. [DOI] [PubMed] [Google Scholar]
- 54.Casazza J.P., Cale E.M., Narpala S., et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med. 2022;28(5):1022–1030. doi: 10.1038/s41591-022-01762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gan S.U., Notaridou M., Fu Z.Y., et al. Correction of murine diabetic hyperglycaemia with a single systemic administration of an AAV2/8 vector containing a novel codon optimized human insulin gene. Curr Gene Ther. 2016;16(1):65–72. doi: 10.2174/1566523216666160122113958. [DOI] [PubMed] [Google Scholar]
- 56.Gan S.U., Fu Z., Sia K.C., et al. Development of a liver-specific Tet-off AAV8 vector for improved safety of insulin gene therapy for diabetes. J Gene Med. 2019;21(1) doi: 10.1002/jgm.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manzoor F., Johnson M.C., Li C., et al. β-cell-specific IL-35 therapy suppresses ongoing autoimmune diabetes in NOD mice. Eur J Immunol. 2017;47(1):144–154. doi: 10.1002/eji.201646493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Philippidis A. Fourth boy dies in clinical trial of Astellas' AT132. Hum Gene Ther. 2021;32(19–20):1008–1010. doi: 10.1089/hum.2021.29182.bfs. [DOI] [PubMed] [Google Scholar]
- 59.Astellas’ Pompe disease gene therapy placed on hold by FDA. Cell Gene Therapy. 2022 https://www.fiercebiotech.com/biotech/astellas-gene-therapy-strategy-faces-fresh-setback-fda-puts-pompe-disease-drug-hold [Google Scholar]
- 60.Nguyen G.N., Everett J.K., Kafle S., et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat Biotechnol. 2021;39(1):47–55. doi: 10.1038/s41587-020-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang H.R., Gjorgjieva M., Smith S.N., et al. Pathogenesis of hepatic tumors following gene therapy in murine and canine models of glycogen storage disease. Mol Ther Methods Clin Dev. 2019;15:383–391. doi: 10.1016/j.omtm.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greig J.A., Limberis M.P., Bell P., et al. Non-clinical study examining AAV8.TBG.hLDLR vector-associated toxicity in chow-fed wild-type and LDLR+/−Rhesus macaques. Hum Gene Ther Clin Dev. 2017;28(1):39–50. doi: 10.1089/humc.2017.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho K.J., Bass C.E., Kroemer A.H.K., et al. Optimized adeno-associated virus 8 produces hepatocyte-specific Cre-mediated recombination without toxicity or affecting liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G412–G419. doi: 10.1152/ajpgi.00590.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greig J.A., Limberis M.P., Bell P., et al. Nonclinical pharmacology/toxicology study of AAV8.TBG.mLDLR and AAV8.TBG.hLDLR in a mouse model of homozygous familial hypercholesterolemia. Hum Gene Ther Clin Dev. 2017;28(1):28–38. doi: 10.1089/humc.2017.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broekman M.L.D., Comer L.A., Hyman B.T., et al. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or-2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138(2):501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 66.Gross D.A., Tedesco N., Leborgne C., et al. Overcoming the challenges imposed by humoral immunity to AAV vectors to achieve safe and efficient gene transfer in seropositive patients. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.857276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurlbut G.D., Ziegler R.J., Nietupski J.B., et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther. 2010;18(11):1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B., Li Z., Huang S., et al. AAV-containing exosomes as a novel vector for improved gene delivery to lung cancer cells. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.707607. [DOI] [PMC free article] [PubMed] [Google Scholar]