Abstract
We investigated the role of the constitutive nitric oxide (NO) in the expression of interferon (IFN) genes in mouse peritoneal macrophages (PM). The treatment of PM with l-arginine-NG-amine (AA), a potent inhibitor of NO-producing enzymes, resulted in a marked accumulation of IFN-α4 mRNA and, to a minor extent, of IFN-β mRNA. In contrast, the expression of IFN-γ mRNA, as well as tumor necrosis factor alpha and interleukin-6 mRNA, was not affected. Furthermore, a remarkable increase in the expression of the IFN regulating factor 1 (IRF-1), but not of IRF-2, mRNA was detected in AA-treated PM. To investigate whether the AA-induced activation of the IFN system correlates with the production and antiviral activity of IFN, the extent of encephalomyocarditis virus (EMCV) replication was monitored in AA-treated PM with respect to control cultures. AA treatment strongly inhibited, in a dose-dependent manner, EMCV yields in PM. Likewise, similar results were obtained by the addition of the NO-scavenger carboxyphenyl-tetramethylimidazoline-oxyl-oxide. In addition, inhibition of NO synthesis by NG-mono-methyl-l-arginine in PM strongly decreased virus replication in coculture of PM and EMCV-infected L929 cells, whereas no antiviral effect was observed in L929 cells alone. Moreover, the AA-mediated antiviral activity was abrogated in the presence of antibody to IFN-α/β, whereas antibody to IFN-γ was completely ineffective. Taken together, these results indicate that low levels of NO, constitutively released by resting PM, negatively regulate the expression and activity of IFN-α/β in PM. We suggest that NO acts as a homeostatic agent in the regulation of IFN pathway expression in macrophages.
Nitric oxide (NO), a free-radical gas, acts as an intra- and extracellular messenger in most mammalian organs (28). Many cell types are capable of producing NO through the enzymatic conversion of l-arginine to l-citrulline by NO synthase (NOS). Three isoforms of NOS have been isolated. NOS 2 was primarily cloned in macrophages, and NOSs 1 and 3 were cloned in brain neuron and endothelial cells, respectively. NOS 1 and 3 activities are dependent on elevated Ca2+ and are generally considered as constitutive. NOS 2 is inducible by a variety of cytokines and bacterial products and does not depend on elevated Ca2+ (8, 28). NO is an important regulator and mediator of a wide range of physiological processes, including blood vessel relaxation, neurotransmission, inflammation, apoptosis, and macrophage-mediated cytotoxicity for microbes and tumor cells (28, 48). Over the past few years, several reports have also demonstrated that NO plays an important role in host defense against virus infections (38, 48). In spite of its beneficial effect in maintaining the immunological homeostasis, NO has also been implicated in disease pathogenesis in a variety of inflammatory syndromes, as well as in viral infections (1, 48). In fact, high levels of NO or NOS 2 expression have been found in inflammatory tissues at or around lesion sites in several systems (10, 13, 38). Moreover, direct evidence of a role for NO in virus-associated pathology has also been provided (1, 22).
Macrophages are generally considered to be important elements in natural resistance against infection (33). These cells are intimately related to the interferon (IFN) system, and the existence of a so-called “IFN-macrophage alliance” has been postulated (33). During viral infection, macrophages are among the first cells in any organ to be exposed to the intruders and are generally considered to be the major producers of alpha/beta IFN (IFN-α/β) soon after infection (33). In addition, several studies performed by our group over the years have revealed that low levels of IFN-β are spontaneously expressed in resting mouse peritoneal macrophages (PM) and are responsible for the natural antiviral state of these cells (5, 35). The IFN-β-mediated antiviral state of freshly harvested PM is progressively lost when these cells are maintained in vitro for a few days (11). In the present study, we provide evidence that endogenous NO, constitutively expressed in PM, acts as a negative regulator of IFN system expression. In fact, neutralization of endogenous NO by using specific NOS inhibitors, results in an increased expression of IFN-α4 and IFN-β mRNA. Moreover, the expression of an IFN-inducible gene, such as IFN regulatory factor 1 (IRF-1), is positively regulated upon NO inhibition. The activation of IFN system induced by NO inhibition correlates with an increased resistance of PM to encephalomyocarditis virus (EMCV) infection. This resistance is abrogated in the presence of antibody to IFN-α/β. Taken together, these results indicate that low levels of NO, constitutively released by resting PM, negatively regulate the expression and activity of IFN-α/β in PM. We suggest that NO acts as a homeostatic agent in the regulation of IFN pathway expression in macrophages.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free 3-week-old Swiss female mice (weight, 20 to 22 g) were purchased from Charles River, Italia, S.P.A. (Milan, Italy) and used within 5 days. Mice were housed in cages at 20°C and had access to food and water ad libitum.
Reagents.
RPMI 1640 medium (M. A. Bioproducts, Walkersville, Md.) was supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), and 10% heat-inactivated fetal calf serum (FCS) and used as the cell culture medium. All tissue culture reagents were purchased as endotoxin-free lots, as assessed by the Limulus amebocyte assay. l-Arginine-NG-amine (AA) and NG-mono-methyl-l-arginine (NMMA) were provided by Sigma (Milan, Italy). Carboxyphenyl-tetramethylimidazoline-oxyl-oxide (carboxy-PTIO) was obtained from Calbiochem-Inalco S.P.A. (Milan, Italy). Oxyhemoglobin was kindly provided by B. Bohn (Hôpital Bicêtre, Paris, France).
PM cultures.
PM were harvested by washing the peritoneal cavity with RPMI 1640 containing 10% FCS and then were seeded in plastic dishes. After 2 h, nonadherent cells were removed by two washes with medium. PM were then maintained in culture for 24 h in RPMI 1640 containing 10% FCS. Experiments were undertaken when the cells were firmly adherent to the culture wells after a vigorous washing. More than 95% of the cells stained for nonspecific esterase and were positive in immunofluorescence studies with a rat monoclonal antibody (F4/80) that was specific for mouse macrophages, as previously described (5).
Semiquantitative RNA-PCR.
Total cellular RNA, prepared by the method of Chirgwin et al. (6), was reverse transcribed and amplified as previously described (36), except that the oligonucleotide sense primer was labeled with [γ-32P]ATP and Taq polymerase was neutralized by a TaqStart antibody (Clontech) before use in order to enhance the specificity and sensitivity of the PCR amplification. For IRF-mRNA analysis, cold primers were used.
The amplification program of PCR consisted of an initial denaturation of 3 min at 95°C followed by 30 (for GAPDH [glyceraldehyde-3-phosphate dehydrogenase], IFN-γ, tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6], IRF-1, and IRF-2) to 35 (for IFN-α4 and IFN-β) repeated cycles of denaturation for 40 s at 90°C, primer annealing for 40 s at 62°C, and extension for 60 s at 72°C. A negative control lacking template RNA or reverse transcriptase was included in each experiment. The PCR products were run together with a [γ-32P]ATP-labelled-DNA molecular-weight marker (Marker VI; Boehringer Manheim) on a 5% acrylamide-polyacrylamide (proportion, 29/1) gel. After being dried at 80°C in a dryer, the gel was analyzed with an electronic autoradiography system. Instant Imager (Packard), that allows quantification of labeled PCR products in counts per minute. For RNA quantification, the amount of mRNA corresponding to IFN-β and IFN-α4 present in each sample was normalized to the constitutive stable expression of GAPDH-mRNA. Values are given as the IFN/GAPDH ratio. Gels were then exposed on a photographic plate. For IRF, PCR products were run on agarose gel containing ethidium bromide together with a DNA molecular-weight marker (φX174; Finnzymes).
The sequences for the primers used are as follows: GAPDH sense primer, CCATGGAGAAGGCTGGGG; antisense primer, CAAAGTTGTCATGGATGACC; IFN-α4 sense primer CTCAAAGCCTGTGTGATGC and antisense primer AAGACAGGGCTCTCCAGAC, IFN-β sense primer CCATCCAAGAGATGCTCCAG and antisense primer GTGGAGAGCAGTTGAGGACA, IFN-γ sense primer AACGCTACACACTGCATCTTGG and antisense primer GACTTCAAAGAGTCTGAGG, IRF-1 sense primer CAGAGGAAAGAGAGAAAGTCC and antisense primer CACACGGTGACAGTGCTGG, IRF-2 sense primer CAGTTGAGCATCTTTGGGGC and antisense primer TGGTCATCACTCTCAGTGG, TNF-α sense primer GATCTCAAAGACAACCAACTAGTG and antisense primer CTCCAGCTGGAAGACTCCTCCCAG, and IL-6 sense primer ATGATGGATGCTAACAAACTGG and antisense primer GATGGATTGGATGGTCTTGG.
Primer sense labeling.
Labeling of oligonucleotides sense primers consisted of 45 min of incubation at 37°C of the following mixture prepared for 10 samples: 110 ng of primer sense, 10 U of T4 polynucleotide kinase (New England Biolabs), 25 μCi of [γ-32P]ATP (Amersham), 70 mM Tris-HCl (pH 7.6), 10 mM MgCl2, and 5 mM dithiothreitol, for a final volume of 100 μl. Oligonucleotide was then precipitated by adding 10 μl of 3 M sodium acetate (pH 5.2) and 300 μl of absolute ethanol. After centrifugation at 14,000 rpm for 15 min at 4°C, the supernatant was removed, and 500 μl of 70% ethanol was added. After a further centrifugation in the same conditions, the supernatant was carefully discarded. The oligonucleotide pellet was air dried at room temperature for 10 min, resuspended in water, and dissolved at 37°C for 15 min. At this point the oligonucleotide was ready for use for PCR.
Infection of PM with EMCV and titration of virus yields.
After a 24-h culture, PM were infected with EMCV in RPMI 1640 supplemented with 2% inactivated FCS at a multiplicity of infection (MOI) of 1. After 1 h of adsorption the cells were washed twice with medium and then incubated in RPMI 1640 containing 2% FCS. Cell supernatants were harvested 48 h later, clarified by centrifugation, and stored at −80°C. The titers of virus yields were determined as previously described (5).
Coculture of EMCV-infected L929 cells and PM.
Confluent L929 cells were infected by EMCV (MOI = 10−5) in RPMI with 2% FCS. After 1 h of adsorption, the cells were washed with Hanks balanced salt solution, trypsinized and resuspended in RPMI with 2% FCS. Infected L929 cells (L-EMCV) (3 × 105 cells/ml) were added to 24-h cultures of PM. After 24 h of incubation, duplicate samples of L-EMCV or L-EMCV plus PM were scraped off with a rubber policeman, harvested with culture fluids, pooled, and stored at −80°C. The samples were frozen and thawed once, sonicated for 2 min at 47 kHz in an ultrasonic cleaner (Bransonic B-1200 E1), clarified by centrifugation (1,250 × g, 10 min), and titrated for intra- and extracellular EMCV yields as described above. In parallel, cocultures of L929 cells and PM were performed as a control for cytotoxic activity of PM against uninfected cells and evaluated by a photometric method (16).
IFN assay.
The biological IFN assay was based on the protection of L929 cells against the cytopathic effect of vesicular stomatitis virus (VSV). Serial twofold dilutions of the supernatants were transferred to 24-h semiconfluent monolayers of L929 cells in a 96-well plate. Supernatants were removed 24 h later, and VSV was added to each well (80 infectious particles/well). After 48 h the cytopathic effect was evaluated by a photometric method (16). IFN titers were adjusted to a laboratory standard IFN preparation that was calibrated against an international standard for IFN-β.
Antibodies to mouse IFNs.
Antiserum to mouse IFN-γ was provided by HyCult Biotechnology (Uden, The Netherlands). The origin of the partially purified sheep immunoglobulin to mouse IFN-α/β (neutralizing titer of 6.4 × 106 against 4 U of mouse IFN-α/β) has been described in detail elsewhere (5).
Titration of NO.
NO release was assayed by nitrites (NO2−) titration: 100-μl samples in duplicate in a 96-well microtiter plate (Falcon) were reacted with 200 μl of the Griess reagent prepared by mixing equal volumes of sulfanilamide (1% in 1.2 N HCl) and N-(1-naphtyl)ethylenediamine (0.3% in H2O) (Sigma). After 30 min of shaking in the dark, the absorbance at 550 nm was measured, and the nitrite concentration was determined by using a curve calibrated with NaNO2 standards.
Statistical analysis.
Statistical analysis of data was performed by using the Kruskall-Wallis test.
RESULTS
Effect of NO synthesis inhibition on the expression of IFN and IFN-inducible genes.
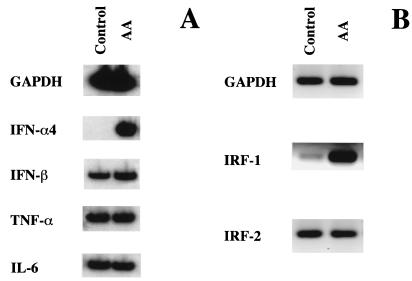
In preliminary experiments, we measured the level of NO in PM cultures. As detected by nitrite titration, a low level of NO (i.e., 0.8 ± 0.1 μM) was found in the cell supernatants after 24 h of culture. To investigate whether this low secretion of NO could have some role in the regulation of IFN gene expression, the steady-state levels of IFN transcripts were analyzed by RNA-PCR in cultures treated with a specific NO inhibitor (i.e., AA). As shown in Fig. 1A, control PM did not express detectable levels of IFN-α4 mRNA, but this transcript markedly accumulated after AA treatment. In contrast, the same treatment resulted in only a modest increase in IFN-β mRNA expression that was constitutively expressed in control PM. Quantification of the PCR products shown in Fig. 1A revealed that the steady-state levels of IFN-β mRNA was slightly increased (fold increase, 2.2) after AA treatment (IFN-β/GAPDH control PM, 2.6; AA-treated PM, 5.2), whereas a marked induction (fold increase, 42) of IFN-α4 was observed under the same experimental conditions (IFN-α4/GAPDH control PM, 0.031; AA-treated PM, 1.3). To investigate whether the effect of AA was specific for IFN-α/β, we also analyzed the expression of IFN-γ, TNF-α, and IL-6 mRNA in AA-treated cultures. No IFN-γ mRNA was detected with or without AA treatment (data not shown). In addition, as shown in Fig. 1A, no changes in the expression of TNF-α and IL-6 mRNAs were detected in the presence of AA. Experiments were then performed to establish whether other factors involved in the regulation of IFN gene expression were also modulated by the inhibition of NO synthesis. We thus analyzed the expression of IRF-1 and -2 that act as activator and repressor, respectively, of the transcription of target genes involved in the biological response to IFN (34). As shown in Fig. 1B, a strong accumulation of IRF-1 mRNA was observed in PM cultured in the presence of AA, whereas the expression of IRF-2 mRNA was not affected.
FIG. 1.
Expression of IFN genes (A) and IFN regulatory genes (B) in AA-treated PM. PM (107 cells) were seeded in 90-mm plastic Petri dishes as described in Materials and Methods. After a 24-h incubation, PM cultures were washed and treated with AA at a concentration of 1,000 μM. Total cellular RNA was then extracted 6 h later and processed for semiquantitative reverse transcriptase PCR. Results are representative of three different experiments.
Effect of NO inhibitors on the replication of EMCV in PM.
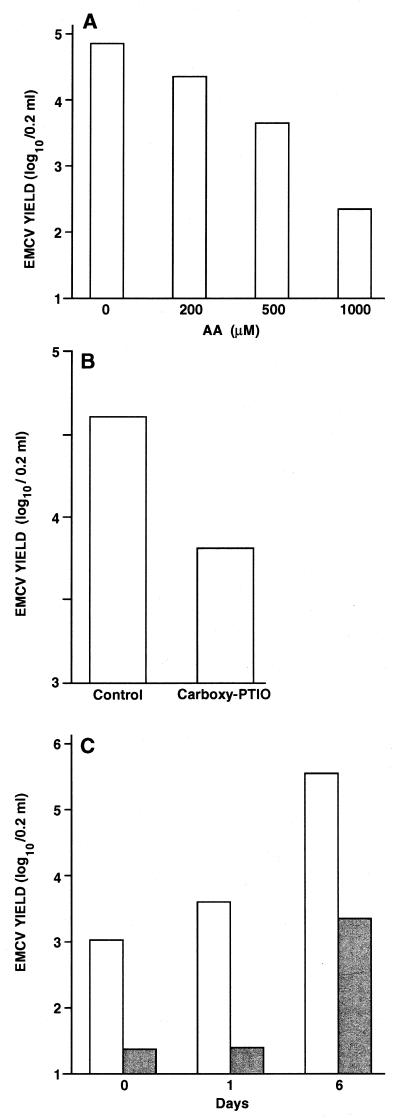
We then carried out a series of experiments to determine whether AA treatment resulted in some secretion of IFN in the culture medium. In spite of the remarkable effect of AA on the expression of IFN-α4 and IFN-β mRNA expression, we failed to detect any IFN-α/β secretion in supernatants of AA-treated PM (data not shown). Since undetectable levels of IFN-α/β are sufficient to induce an antiviral state in macrophages (5, 12, 35), we investigated the effect of AA on the replication of EMCV. As shown in Fig. 2A, AA exerted an antiviral activity in a dose-dependent manner, with maximal activity at a concentration of 1,000 μM. This AA dose did not result in any toxic effect on cell viability (data not shown). Similar antiviral activity was obtained in the presence of a different NO inhibitor, the carboxy-PTIO (Fig. 2B), a scavenger molecule that oxidizes NO to NO2− in a stoichiometric manner and completely abolishes its biological activity (29). We have previously shown that resident PM harvested from different strains of mice are naturally resistant to the infection by several animal viruses (5). This natural antiviral state of PM is largely mediated by IFN-β production and is acquired during mouse development (14). In addition, the resistance of PM to viral infection is progressively lost during in vitro culture (35). We thus carried out experiments in which PM were treated with AA and infected with EMCV at different times of in vitro culture. As shown in Fig. 2C, AA exerted a strong antiviral activity on PM independently of the time of culture. In all experiments, no variation of the low constitutive production of NO in PM was observed upon virus infection (data not shown).
FIG. 2.
Inhibition of EMCV replication in cultured mouse PM treated with NO inhibitors. (A) Peritoneal macrophages (106/well) were seeded in 24-well cluster plates as described in Materials and Methods. After a 24-h incubation and a vigorous washing, PM cultures were infected with EMCV at an MOI of 1. After viral adsorption, incubation proceeded for 48 h in the presence of various concentrations of AA, and virus yields were then determined as described in Materials and Methods. Values represent the mean of three different experiments, and each experiment contained duplicate samples per condition. Statistical analysis by Kruskall-Wallis test gave the following results: 0 versus 500 μM AA, P < 0.006; 0 versus 1,000 μM AA, P < 0.004. (B) Experiment was performed as for panel A in the presence of 30 μM carboxy-PTIO. Values represent a mean of three different experiments, and each experiment contained duplicate samples per condition. A statistical analysis by Kruskall-Wallis test gave the following result: carboxy-PTIO versus control, P < 0.02. (C) Experiment was performed as for panel A in the presence of 1,000 μM AA at different times of in vitro culture before infection (0, 1, or 6 days).
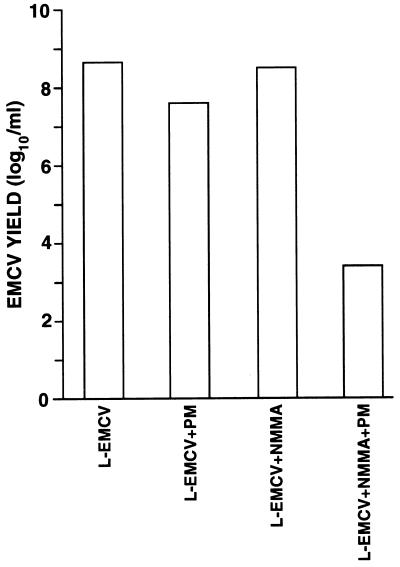
PM treated with NMMA confer an antiviral state to EMCV when cocultured with L929 cells.
As shown in Fig. 3, the treatment with NMMA, a specific inhibitor of NO synthesis, did not result in any inhibition of EMCV replication in L929 mouse cells. Similar results were also obtained by using NG-nitro-l-arginine or the oxyhemoglobin (300 μM), a potent NO scavenger (data not shown), as NOS inhibitors. These results indicated that NOS inhibition did not induce IFN production in any type of cells, suggesting that a specific pattern of regulation may occur in macrophages. The cocultivation of L929 cells with freshly harvested PM resulted in a slight reduction of EMCV yield compared to control cultures. Previous studies had shown that the capability of PM to transfer an antiviral state to L929 cells was due to the production of low level of IFN (35). Interestingly, when the coculture was performed in the presence of NMMA there was a much stronger reduction of EMCV yield.
FIG. 3.
Inhibition of EMCV replication in L929 cells cocultured with NMMA-treated PM. PM were previously cultivated in medium alone for 24 h. L929 cells, infected with EMCV at an MOI of 10−5, were then added to PM cultures as described in Materials and Methods at a macrophage/target cell ratio of 1. Virus yields were determined after 24 h of infection in the presence of 100 μM NMMA. Statistical analysis by Kruskall-Wallis test gave the following results: L-EMCV versus L-EMCV + PM, P < 0.05; L-EMCV + NMMA versus L-EMCV + PM + NMMA, P < 0.05; L-EMCV + PM versus L-EMCV + PM + NMMA, P < 0.05.
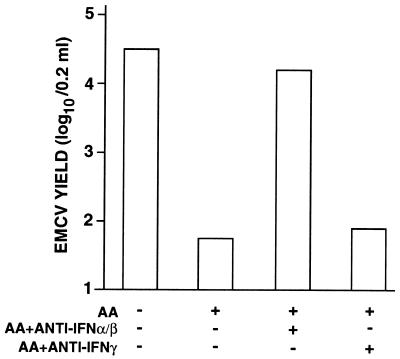
The AA-induced antiviral state of resident PM is abolished by antibody to IFN-α/β.
In order to establish whether the antiviral effect exhibited by AA in PM was mediated by IFN, we carried out experiments in the presence of specific antibodies to IFNs. As shown in Fig. 4, the addition of antibody to IFN-α/β completely abolished the AA-induced antiviral effect. In contrast, antibody to IFN-γ was ineffective.
FIG. 4.
Inhibition of AA-induced antiviral activity by anti-IFN-α/β serum. PM (106/well) were seeded in 24-well cluster plates as described in Materials and Methods. After a 24-h incubation and a vigorous washing, PM cultures were infected with EMCV at an MOI of 1. After viral adsorption, incubation proceeded for 48 h in the presence of 1,000 μM AA and 4,000 neutralizing units of anti-IFN-α/β serum or 600 neutralizing units of anti-IFN-γ serum. Virus yields were then determined as described in Materials and Methods. Values represent a mean of three different experiments, and each experiment contained duplicate samples per condition. Statistical analysis by Kruskall-Wallis test gave the following results: AA versus control, P < 0.004; AA + antibody to IFN-α/β versus AA, P < 0.004.
DISCUSSION
It has been extensively described that NO can act as a positive and/or negative regulator of the cytokine network (30). In this regard, interactions between the IFN system and NO pathway have also been clearly established. In particular, several reports demonstrated that the NO pathway can be positively regulated by both IFN-α/β (39, 46, 47) and γ-IFN (41, 47) in murine macrophages. However, it appears that a pretreatment of cells with IFN-α and/or IFN-β counteracts the induction of NO production in activated macrophages (7, 26). In contrast, only a few reports have described the capacity of NO to regulate IFNs expression. Ito and coworkers (19, 20) described the inhibitory effect of various NO-releasing agents on the NK cell activity of nonadherent human peripheral blood mononuclear cells against cytomegalovirus or varicella-zoster virus-infected fibroblasts. This inhibitory effect was related to a reduced production of IFN-α by CD16− HLA-DR+ cells (20). A number of studies also showed that NO, endogenously produced or exogenously supplied, inhibited IFN-γ production in stimulated splenocytes or T cells (4, 42). Furthermore, pulmonary leukocytes from NOS2−/− knockout mice infected with influenza virus have been shown to produce higher levels of IFN-γ than wild-type cells (22).
In the present study, we provide evidence that low levels of endogenous NO, constitutively produced by PM, exert a negative control on the expression of IFN-α and, to a lesser extent, of IFN-β mRNA in PM. This correlates with a downregulation of IFN-α/β production and activity as shown by the antiviral effect of NOS inhibitors on PM and its inhibition by an anti-IFN-α/β serum. Our results suggest that the inhibitory effect of NO was specific for IFN-α/β. In fact, NO inhibition did not induce any upregulation of IFN-γ mRNA expression (data not shown). Similarly, the antiviral activity induced upon NOS inhibition was not related to IFN-γ production since antibody to IFN-γ did not affect this activity. Furthermore, we failed to detect any modification of the steady-state levels of some proinflammatory cytokine mRNA, such as TNF-α and IL-6. Our results also indicate that IFN-β and IFN-α4 mRNA expression is affected differently by NO. In this regard, it is of interest to mention that although IFN-β and IFN-α4 promoters show striking sequence similarities, they also exhibit differences in their transcriptional regulation. In fact, it has been reported that different pathways mediate virus inducibility of the human IFN-α1 and IFN-β genes (27). Moreover, it has been shown that the transcription factor NF-κB is involved in the transcriptional regulation of IFN-β but not of IFN-α genes (25, 43). On the other hand, the IFN-α1 promoter contains at least one novel virus-responsive element, the “TG sequence,” that differs from PRDI and NF-κB binding sequences responsible for IFN-β promoter induction (27).
IRF-1 is a transcriptional factor implicated in the positive regulation of both IFN-α (3, 9, 32) and IFN-β (9, 18, 32) gene expression. IRF-2 was identified as a suppressor of the activity of IRF-1 on IFN-β promoter (18). On the other hand, IRF-1 is involved in the positive control of the promoter expression of iNOS (NOS 2), the inducible form of NOS (31). Interestingly, macrophages from mice with a targeted disruption of IRF-1 produce little or no NO and synthesize barely detectable levels of iNOS mRNA in response to stimulation (21). In the present study, we show that the inhibition of constitutive NO synthesis results in the specific upmodulation of mRNA for IRF-1 but not for IRF-2 in PM. In light of these results, we suggest that the IFN mRNA accumulation observed upon NOS inhibition could be due, at least in part, to the transcriptional activity of IRF-1. Further studies are needed to define the role of this transcription factor in the NO-mediated regulation of IFN upregulation, as well as the involvement of additional factors.
In recent years, the importance of NO in the host antiviral defense, as well as in the pathogenesis of various viral diseases has been increasingly recognized (1, 48). Our study here represents the first example of a negative regulation of IFN-α/β by endogenous NO in macrophages. A physiologic role for such a pathway could be to limit not only the basal level of IFN synthesis but also the induced high level of IFN production during the response to pathogens. This regulatory loop could be beneficial in preventing toxic effects due to an excess of IFN. In this regard, trehalose dimycolate (TDM), a glycolipid from Mycobacterium tuberculosis, injected in mice induces a high level of IFN in PM (15, 16), and this production was shown to be also enhanced upon in vitro treatment with inhibitors of NO (data not shown). Of particular interest is the fact that TDM-treated PM are also stimulated to produce substantial level of NO (17), which raises the possibility that NO-mediated negative regulation of IFN pathway occurs even during an inducible high output of NO. In this regard, inhibitors of NOSs have been used extensively to limit the damages provoked by a high production of NO in virus-infected animals (1, 2, 22–24, 40, 44). Therefore, a side effect of these treatments could be the activation of the IFN response. This could result in the decrease of virus spread but also in some deleterious toxicity of the overproduced IFN and thus suggests that the use of NO inhibitors as therapeutic agents should be contemplated cautiously.
In conclusion, in the light of our data, we can envisage the following pathway. (i) In physiological conditions, the constitutive NO released by resting macrophages maintains at a low level the expression of IFN-α/β genes. (ii) During the response to pathogens and inflammatory process leading to high production of NO, the same regulation pathway takes place to control the overproduction of IFN, thus avoiding its detrimental effects. Therefore, NO appears as a homeostatic agent for the regulation of IFN-mediated immune responses. The discovery of this regulatory loop between IFN and NO in macrophages adds novel insights to our understanding of the regulation of macrophage functions in the immune response to environmental stimuli. A perspective of particular interest will be to investigate the relevance of this pathway in human monocytes, which have been shown to express both the inducible and constitutive isoforms of NOS (37, 45).
ACKNOWLEDGMENTS
We thank Sabrina Tocchio, Istituto Superiore di Sanità, Rome, Italy, for excellent editorial assistance. We are indebted to Stefano Belli for help with the statistical analysis. We also thank Stefania Mochi, Laboratory of Virology, Istituto Superiore di Sanità, for oligonucleotide synthesis.
E.G. was the recipient of a fellowship from the Istituto Superiore di Sanità.
REFERENCES
- 1.Akaike T, Suga M, Maeda H. Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. PSEBM (Proc Soc Exp Biol Med) 1998;217:64–73. doi: 10.3181/00379727-217-44206. [DOI] [PubMed] [Google Scholar]
- 2.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au W-C, Su Y, Raj N B, Pitha P M. Virus-mediated induction of interferon A gene requires cooperation between multiple binding factors in the interferon-α promoter region. J Biol Chem. 1993;268:24032–24040. [PubMed] [Google Scholar]
- 4.Bauer H, Jung T, Tsikas D, Stichtenoth D O, Frölich J C, Neumann C. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology. 1997;90:205–211. doi: 10.1046/j.1365-2567.1997.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belardelli F, Vignaux F, Proietti E, Gresser I. Injection of mice with antibody to interferon renders peritoneal macrophages permissive for vesicular stomatitis virus and encephalomyocarditis virus. Proc Natl Acad Sci USA. 1984;81:602–606. doi: 10.1073/pnas.81.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirgwin J M, Przybyla A E, MacDonald R J, Rytter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 7.Deguchi M, Inaba K, Muramatsu S. Counteracting effect of interferon-gamma-induced production of nitric oxide which is suppressive for antibody response. Immunol Lett. 1995;45:157–162. doi: 10.1016/0165-2478(94)00246-n. [DOI] [PubMed] [Google Scholar]
- 8.Forstermann U, Boissel J P, Kleinert H. Expressional control of the “constitutive” isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 9.Fujita T, Kimura Y, Miyamoto M, Barsoumian E L, Taniguchi T. Induction of endogenous IFN-α and IFN-α genes by a regulatory transcription factor, IRF-1. Nature. 1989;337:270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 10.Gendelman H E, Zheng J, Coulter C L, Ghorpade A, Che M, Thylin M, Rubocki R, Persidsky Y, Hahn F, Reinhard J, Jr, Swindells S. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998;178:1000–1007. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- 11.Gessani S, Dieffenbach C W, Conti L, Di Marzio P, Wilson K L, Belardelli F. Selective alteration of the turnover of interferon β mRNA in peritoneal macrophages from LPS-hyporesponsive mice and its role in the defective expression of spontaneous interferon. Virology. 1993;193:507–509. doi: 10.1006/viro.1993.1154. [DOI] [PubMed] [Google Scholar]
- 12.Gessani S, Puddu P, Varano B, Borghi P, Conti L, Fantuzzi L, Belardelli F. Induction of beta interferon by human immunodeficiency virus type 1 and its gp120 protein in human monocytes-macrophages: role of beta interferon in restriction of virus replication. J Virol. 1994;68:1983–1986. doi: 10.1128/jvi.68.3.1983-1986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannoni G, Miller R F, Heales S J, Land J M, Harrison M J, Thompson E J. Elevated cerebrospinal fluid and serum nitrate and nitrite levels in patients with central nervous system complications of HIV-1 infection: a correlation with blood-brain-barrier dysfunction. J Neurol Sci. 1998;156:53–58. doi: 10.1016/s0022-510x(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 14.Gresser I, Maury C, Kaido T, Bandu M T, Tovey M G, Maunoury M T, Fantuzzi L, Gessani S, Greco G, Belardelli F. The essential role of endogenous IFN alpha/beta in the anti-metastatic action of sensitized T lymphocytes in mice injected with Friend erythroleukemia cells. Int J Cancer. 1995;63:726–731. doi: 10.1002/ijc.2910630520. [DOI] [PubMed] [Google Scholar]
- 15.Guillemard E, Geniteau-Legendre M, Mabboux B, Poilane I, Kergot R, Lemaire G, Petit J F, Labarre C, Quero A M. Antiviral action of trehalose dimycolate against EMC virus: role of macrophages and interferon α/β. Antiviral Res. 1993;22:201–213. doi: 10.1016/0166-3542(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 16.Guillemard E, Geniteau-Legendre M, Kergot R, Lemaire G, Petit J F, Labarre C, Quero A M. Role of TDM-induced interferon α/β in the restriction of EMC virus growth in vivo and in peritoneal macrophage cultures. Antiviral Res. 1995;28:175–189. doi: 10.1016/0166-3542(95)00047-p. [DOI] [PubMed] [Google Scholar]
- 17.Guillemard E, Geniteau-Legendre M, Kergot R, Lemaire G, Gessani S, Labarre C, Quero A-M. Simultaneous Production of IFN-γ, IFN-α/β and Nitric Oxide in Peritoneal Macrophages from TDM-Treated Mice. J Biol Regul Homeostatic Agents. 1998;12:67–75. [PubMed] [Google Scholar]
- 18.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Watanabe M, Kamiya H, Sakurai M. Inhibition of natural killer (NK) cell activity against varicella-zoster virus (VZV)-infected fibroblasts and lymphocyte activation in response to VZV antigen by nitric oxide-releasing agents. Clin Exp Immunol. 1996;106:40–44. doi: 10.1046/j.1365-2249.1996.d01-807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Watanabe M, Kamiya H, Sakurai M. Inhibition of natural killer cell activity against cytomegalovirus-infected fibroblasts by nitric oxide-releasing agents. Cell Immunol. 1996;174:13–18. doi: 10.1006/cimm.1996.0288. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitanoshapiro J, Le J, Koh S I, Kimura T, Green S J, Mak T W, Taniguchi T, Vilcek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 22.Karupiah G, Chen J H, Mahalingam S, Nathan C F, MacMicking J D. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreil T R, Eibl M M. Nitric oxide and viral infection: no antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 24.Lane T E, Paoletti A D, Paoletti D, Buchmeier M J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenardo M J, Fang C-M, Maniatis T, Baltimore D. The involvement of NF-κB in β-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989;57:287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- 26.Lòpez-Collazo E, Hortelano S, Rojas A, Bosca L. Triggering of peritoneal macrophages with IFN-α/β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-κB activation. J Immunol. 1998;160:2889–2895. [PubMed] [Google Scholar]
- 27.MacDonald N J, Kuhl D, Maguire D, Naf D, Gallant P, Goswammy A, Hug H, Büeler H, Chaturvedi M, de la Fuente J, Weissman C. Different pathways mediate virus inducibility of the human IFN-α1 and IFN-β genes. Cell. 1990;60:767–779. doi: 10.1016/0092-8674(90)90091-r. [DOI] [PubMed] [Google Scholar]
- 28.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H, Akaike T, Yoshida M, Suga M. Multiple function of nitric oxide in pathophysiology and microbiology: analysis by a new nitric oxide scavenger. J Leukoc Biol. 1994;56:588–592. doi: 10.1002/jlb.56.5.588. [DOI] [PubMed] [Google Scholar]
- 30.Marcinkiewicz J. Regulation of cytokine production by eicosanoids and nitric oxide. Arch Immunol Ther Exp. 1997;45:163–167. [PubMed] [Google Scholar]
- 31.Martin E, Nathan C, Xie Q-W. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1 that specifically binds to IFN-β gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 33.Mogensen S C, Virelizier J L. The interferon-macrophage alliance. Interferon. 1987;8:55–84. [PubMed] [Google Scholar]
- 34.Nguyen H, Hiscott J, Pitha P M. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 35.Proietti E, Gessani S, Belardelli F, Gresser I. Mouse peritoneal cells confer an antiviral state on mouse cell monolayers: role of interferon. J Virol. 1986;57:456–463. doi: 10.1128/jvi.57.2.456-463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf S F, Belardelli F, Gessani S. IL-12 induces IFN-γ expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 37.Reiling N, Ulmer A J, Duchrow M, Ernst M, Flad H-D, Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur J Immunol. 1994;24:1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- 38.Reiss C S, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riches D W H. Expression of interferon-β during the triggering phase of macrophage cytocidal activation. J Biol Chem. 1991;266:24785–24792. [PubMed] [Google Scholar]
- 40.Rose J W, Hill K E, Wada Y, Kurtz C I B, Tsunoda I, Fujinami R S, Cross A H. Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler’s virus infection. J Neuroimmunol. 1998;81:82–89. doi: 10.1016/s0165-5728(97)00162-8. [DOI] [PubMed] [Google Scholar]
- 41.Stuehr D J, Marletta M A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987;139:518–525. [PubMed] [Google Scholar]
- 42.Taylor-Robinson A, Liew F Y, Severn A, Xu D, McSorley S J, Garside P, Padron J, Phillips R S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 43.Visvanathan K V, Goodbourn S. Double-stranded RNA activates binding of NF-κβ to an inducible element in the human β-interferon promoter. EMBO J. 1989;8:1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W Z, Matsumori A, Yamada T, Shioi T, Okada I, Matsui S, Sato Y, Suzuki H, Shiota K, Sasayama S. Beneficial effect of amlodipine in a murine model of congestive heart failure induced by viral myocarditis. A possible mechanism through inhibition of nitric oxide production. Circulation. 1997;95:245–251. doi: 10.1161/01.cir.95.1.245. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg J B, Misukonis M A, Shami P J, Mason S N, Sauls D L, Dittman W A, Wood E R, Smith G K, McDonald B, Bachus K E, Haney A F, Granger D L. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 46.Zhang X, Alley E W, Russel S W, Morrison D C. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect Immun. 1994;62:33–40. doi: 10.1128/iai.62.1.33-40.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou A, Chen Z, Rummage J A, Jiang H, Kolosov M, Kolosova I, Stewart C H, Leu R W. Exogenous interferon-γ induces endogenous synthesis of interferon-α and -β by murine macrophages for induction of nitric oxide synthase. J Interferon Cytokine Res. 1995;15:897–904. doi: 10.1089/jir.1995.15.897. [DOI] [PubMed] [Google Scholar]
- 48.Zidek Z, Masek K. Erratic behavior of nitric oxide within the immune system: illustrative review of conflicting data and their immunopharmacological aspects. Int J Immunopharmacol. 1998;20:319–343. doi: 10.1016/s0192-0561(98)00036-8. [DOI] [PubMed] [Google Scholar]