Abstract
Adhesion G protein-coupled receptors (aGPCRs) are the second largest diverse group within the GPCR superfamily, which play critical roles in many physiological and pathological processes through cell–cell and cell–extracellular matrix interactions. The adhesion GPCR Adgrg6, also known as GPR126, is one of the better-characterized aGPCRs. GPR126 was previously found to have critical developmental roles in Schwann cell maturation and its mediated myelination in the peripheral nervous system in both zebrafish and mammals. Current studies have extended our understanding of GPR126-mediated roles during development and in human diseases. In this review, we highlighted these recent advances in GPR126 in expression profile, molecular structure, ligand–receptor interactions, and associated physiological and pathological functions in development and diseases.
Keywords: Adhesion G protein-coupled receptor, Function, GPR126/Adgrg6, Ligand, Structure
Introduction
G protein-coupled receptors (GPCRs) are the most studied and largest families of transmembrane proteins, which contain seven α-helical transmembrane regions. The validated GPCRs have been classified into five subfamilies: Rhodopsin, Adhesion, Glutamate, Frizzled/Taste2, and Secretin. Currently, there are at least 33 members of adhesion G protein-coupled receptors (aGPCRs) in the human genome.1 As the second largest subfamily, aGPCRs are mostly recognized as orphan proteins, and a few have recently been identified as critical modulators during development.2,3 aGPCRs have gained increasing interest by virtue of their complex structures. The N-terminal fragment (NTF) of aGPCRs contains multiple domains which have been found in other proteins, including lectin, laminin, cadherin, olfactomedin, immunoglobulin, or thrombospondin domain. The diverse structural domains contribute to the diverse protein–protein interactions to promote the communication between cell and cell or extracellular matrix (ECM). Meanwhile, a large number of exons and various alternatively splicing ways make it complicated to investigate the biological functions of the aGPCRs.4 Most aGPCRs undergo an autoproteolytic process mediated by the GPCR auto-proteolysis-inducing (GAIN) structural domain, resulting in an N-terminal (NTF) and a C-terminal fragment (CTF), respectively. NTF and CTF can attach non-covalently on the cell membrane.5 These aGPCRs have been found to interact with a variety of ligands, ranging from ECM components to membrane-bound proteins and lipids.6 Over the last several decades, numerous studies have focused on the studies of ligands to aGPCRs, in an attempt to uncover the structures and functions of these orphan receptors. A few reviews have highlighted the emerging role of these aGPCRs in cell adhesion, cell migration, and numerous disease implications.7,8 GPR126, known as ADGRG6, is an orphan member of the adhesion GPCR subfamilies. It has been demonstrated with important roles in the Schwann cell myelination in the peripheral nervous system (PNS), and tissue/organ development and diseases. Here, we aim to discuss the current knowledge in GPR126/Adgrg6 structure, ligands, and physiological as well as pathological functions.
Discovery and expression profiles of GPR126
GPR126 is an aGPCR that was first discovered by Fredriksson and co-workers and clustered into VIII aGPCRs by phylogenetic analysis.8,9 The full length of the human GPR126 gene is about 6.8 kb at chromosome 6, including 26 exons and alternative splicing of exon 6 and exon 25, generating 4 protein-coding transcripts. Moriguchi et al first cloned the full-length mouse and human GPR126 cDNAs (known as DREG). They validated the gene expression patterns in mice and identified two cleavage sites in the extracellular region (ECR).10 Meanwhile, Stehlik et al found a highly glycosylated form of GPR126 (VIGR, vascular inducible GPCR) expressed on the endothelial cell surface. They demonstrated its potential roles in the innate immune response and activation of the coagulation system.11
Recent evidence has illustrated that GPR126 is evident in the bone, ceratobranchial, ear, heart, macula, nose, neural crest, pericardium, tail, and nervous system during zebrafish development.12 Meanwhile, GPR126 is found widely distributed in the adult mouse lung, heart, kidney, spleen, skin, bladder, placenta, and brain,13 and high levels of transcripts in the human liver, lung, placenta, kidney, urinary bladder, skin, bone, and brain. The expression of GPR126 in the brain is involved in the cerebral cortex, hippocampus, amygdala, thalamus, hypothalamus, midbrain, cerebellum, pons, and spinal cord. GPR126 is mostly clustered in the excitatory and inhibitory neurons, and microglia based on the human protein atlas database (https://www.proteinatlas.org). The broad distribution and spatio-temporal expression pattern indicate that GPR126 may have a variety of functions in the development and diseases.
Structural characterization of GPR126
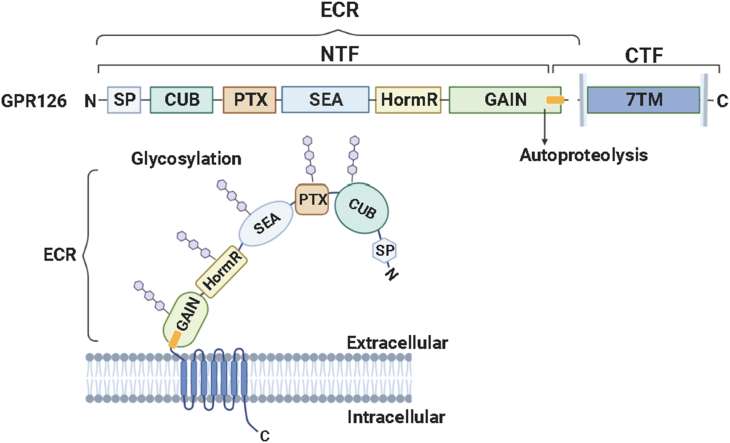
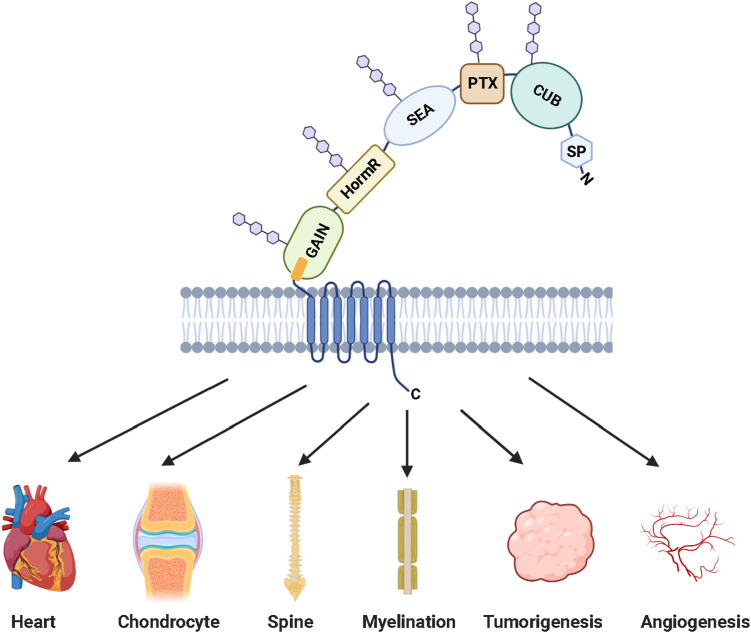
Like most aGPCRs, GPR126 comprises two major components, NTF and CTF. The NTF encompasses most of the protein's ECRs, including five domains: a CUB (complement C1r/C1s, Uegf, Bmp1) domain, a PTX (Pentraxin) domain, a SEA (Sperm protein, Enterokinase, and Agrin) domain, a hormone binding (HormR) domain, and a conserved GAIN domain.14 The CTF is the C-terminal to the GAIN domain's GPCR proteolysis site (GPS), composed of the 7TM domain and an intracellular C-terminal tail. Zebrafish and human GPR126 have experienced high N- and O-linked glycosylation11 (Fig. 1). The structural study of the zebrafish GPR126 indicates glycosylation is found throughout all except the PTX domains of the ECR.14 Human GPR126 contains 26 predicted N-linked glycosylation sites and is reported to undergo N-linked glycosylation in the PTX domain.15 GPR126 is alternatively spliced in exon 6 and exon 25 to produce several isoforms. It has recently been demonstrated that splicing of exon 6 results in a closed conformation of GPR126 ECR compared to an open conformation including the splice site. Meanwhile, the alternative splicing modulates its biological activity in a cAMP signaling pathway.14
Figure 1.
A schematic diagram of GPR126 protein structure consisting of an N-terminal fragment (NTF) and a C-terminal fragment (CTF). The NTF encompasses most of the protein's large extracellular regions (ECRs) and is composed of newly identified five domains: a CUB (complement C1r/C1s, Uegf, Bmp1) domain, a PTX (Pentraxin) domain, a SEA (Sperm protein, Enterokinase, and Agrin) domain, a hormone binding (HormR) domain, and a conserved GPCR autoproteolysis-inducing (GAIN) domain. The CTF is C-terminal to the GAIN domain's GPCR proteolysis site (GPS), and composed of the 7TM domain and an intracellular C-terminal tail. N- and O-linked glycosylation are indicated as blue hexagons.
The CUB domain is most conserved in the ECR of GPR126, especially the calcium-coordinating site. Importantly, the calcium coordination aligns the CUB domain neighboring to the HormR domain, thus making a closed conformation of GPR126 ECR. The calcium-binding pocket is critical for GPR126 function in both PNS myelination and ear development in zebrafish.14 The CUB and PTX domains have a critical role in the interaction with type IV collagen, facilitating the understanding of its mechanism of GPR126 activation.16
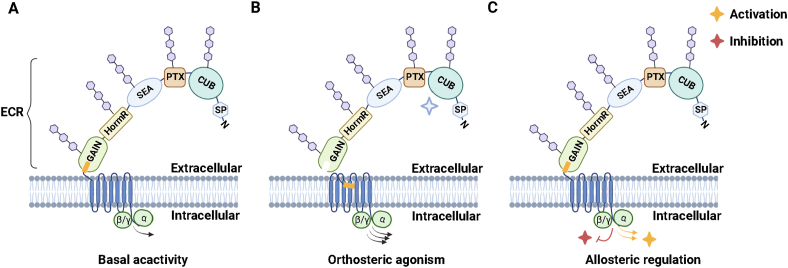
A previous study indicated that GPR126 contains a furin-mediated cleavage site between PTX and HormR domain.10 This furin-cleavage site in the GPR126 ECR has been newly identified as the SEA domain.14 Similar to GAIN domain autoproteolysis, the cleaved SEA domain remains intact by noncovalent interdomain contact. The GPS within the GAIN domain cleaves GPR126 into an NTF and a CTF. Moreover, a short Stachel sequence within the GPS motif functions as a tethered agonist to trigger GPR126 activation.5 This leading mode of adhesion GPCR activation is recognized as orthosteric agonism (tethered-peptide agonism) (Fig. 2). Upon NTF cleavage and dissociation, the hydrophobic tethered-peptide agonist residues are exposed to the aqueous extracellular environment. The hydrophobic properties drive the tethered-peptide agonist to rapidly bind intramolecularly to its orthosteric pocket within the 7TM.7 Although the tethered agonist activation mode does not account for all GPR126 modulations, it facilitates a better understanding of endogenous Stachel sequence-induced GPR126 activation. Overall, the structural studies of GPR126 strongly demonstrate that GPR126 could be a promising drug target when mechanistic details on the regulation have been uncovered.
Figure 2.
Models of GPR126 activation. (A) GPR126 functions via a variety of G protein signaling. The N-terminal subdomains are indicated in multiple colored modules to reflect the potential activities. (B) In the orthosteric agonism model of activation, the ligand–receptor interactions trigger the dissociation of NTF and CTF, resulting in the exposure of the tethered peptide to facilitate its binding to an orthosteric site within the 7TM. (C) In the allosteric activation model, various ligands bind to diverse domains of the receptor to activate or inhibit the GPR126-mediated signaling.
Ligand–receptor interaction of GPR126
Except for the aforementioned tethered peptide-mediated orthosteric agonism model of GPR126 activation, GPR126 modulation has been demonstrated in another allosteric regulation mode (Fig. 2), which has been known as a tunable model. In the allosteric model, ligands interact with the N-terminal domains of GPR126 to maintain the receptor conformation, thus triggering the downstream signaling. It has been known that aGPCRs interact with various ligands including hormones, pheromones, lipids, photons, and free proteins in the physiological process. The main components of the ECM are proteoglycans and fibrous proteins, such as collagens, fibronectins, and laminins. The long ECR in the NTF of GPR126 has been demonstrated to interact with various ligands, including the ECM components collagen IV and laminin-211, and the prion protein. In addition, two steroid hormones, progesterone, and 17-hydroxyprogesterone are reported to bind to the CTF of GPR126 and initiate the downstream Gi coupling.17 We mainly summarized those identified ligands interacting with the NTF and CTF of GPR126, respectively.
Ligand type IV collagen binds to the NTF of GPR126
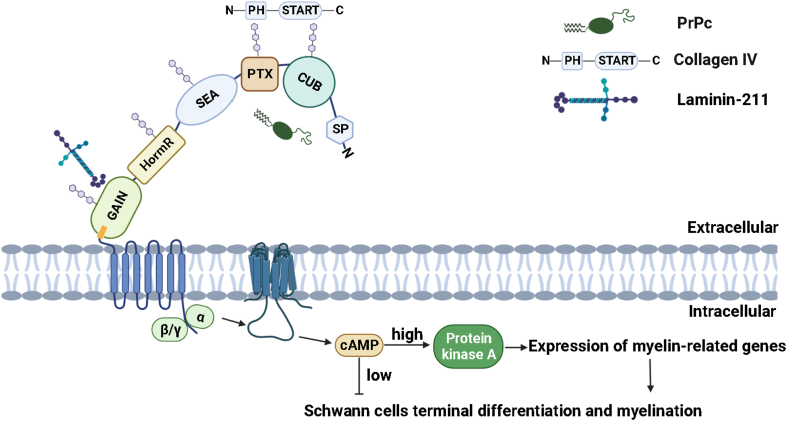
Type IV collagen is composed of an N-terminal 7S domain, a triple helix forming collagen domain, and a C-terminal non-collagenous NC1 domain.18 It is an important component of the basement membranes surrounding Schwann cells (SC).19,20 Type IV collagen mediates the cell interaction between Schwann cells and the basement membrane to initiate Schwann cell differentiation and peripheral axonal growth. Schwann cells secrete type IV collagen, laminin, and other components to form a continuous basal lamina.21,22 A recent study reveals that type IV collagen acts as an endogenous ligand for GPR126 to regulate myelination.16 Unlike GPR56 binding to type III collagen, the CUB and PTX domains in the NTF of GPR126 have a high affinity and specificity binding to type IV collagen (Fig. 3). Type IV collagen binds to the N-terminal region, thus antagonizing the N-terminal repression of the receptor signaling activity, mediating the activation of GPR126 signaling. In a proposed action model, the binding initially activates only Gs, accompanied by a transient rise of cAMP, and followed by PKA phosphorylation of the receptor forming a feedback loop. The continuously increased Gs-coupled signaling may associate with the attenuation of Gi-mediated signaling. It would explain the observation of a transient increase and a reduction to the constant level of cAMP at the immediate and late stage of type IV collagen supplementation.16 It has been demonstrated that type IV collagen acts as an autocrine signal mediating GPR126 to promote Schwann cell myelination, confirming that the proper formation of the basal lamina is essential for Schwann cell myelination. In addition, the binding of type IV collagen to the NTF of GPR126 illustrates the function of GPR126 independent of the 7TM domain and essential for heart development.23
Figure 3.
Ligands in the extracellular matrix interact with the N-terminal fragment to activate GPR126 signaling. The activation enhances Gs-mediated signaling via the accumulation of cAMP to drive SC terminal differentiation and myelination.
As the major component in the basement membrane, type IV collagen is known to associate with laminin via nidogen/entactin to form supramolecular sheet-like structures. The cell-surface proteins including β1-integrins and dystroglycans facilitate the deposition of laminin polymer via site-specific interactions.24 The integrins α1β1, α2β1, α10β1, and α11β1 are classified into the β1 containing collagen receptors. Whereas, integrins α3β1, α6β1, α7β1, and α6β4 belong to laminin receptors mediating cell adhesion and migration to the basement membranes.25 It is reported that folded type IV collagen can bind α1β1-integrin and α2β1-integrin, whereas denatured type IV collagen can bind αvβ3-integrin.26 Whether the integrins bridge the interaction between GPR126 and type IV collagen remains unknown. In a recent preprinted manuscript, GPR126 was found to co-immunoprecipitate with α3β1-integrin, but not with the α1β1-integrin.27 Meanwhile, GPR126 was demonstrated to interact with Lrp1 and α3β1-integrin, facilitating the GPR126-mediated functions in cell migration.27 In future studies, the question of whether GPR126 interaction with Lrp1 and α3β1-integrin has an important role in Schwann cell myelination needs to be further explored.
Ligand laminin-211 interacts with the NTF of GPR126
Similar to type IV collagen, laminin is a major component of the basal lamina, consisting of one α-, one β-, and one γ-chain.28 It plays an essential role in the process of Schwann cell proliferation and migration, which are essential for Schwann cell-mediated radial sorting and myelination of peripheral axons. Laminin-211 is one of the heterotrimeric proteins, composed of the α2, β1, and γ1 chains.29 Laminin-211 is required for Schwann cell myelination. Via binding to integrin, laminin-211 can effectively assemble basement membranes and mediate cell adhesion for efficient myelination.30 Laminin-211 regulates the neuregulin signaling to inhibit the protein kinase A activation in the myelination.31 In the PNS, Schwann cells radially sort axons wrapping an axonal segment to form myelin sheaths, a process that requires the involvement of GPR126. In addition to type IV collagen, GPR126 also physically interacts with the SC-released ligand laminins. It is reported that laminin-211 binds to a special domain in the NTF region of GPR126 (Fig. 3)32,33. Both laminin-211 and the NTF region of GPR126 are essential for axon sorting in SC development. The specific binding does not behave as an agonist in the classical manner of ligand–receptor interactions. Given the Stachel-mediated activation mode for GPR126, laminin-211 acts in a way of stabilizing the noncovalent association between the NTF and CTF, preventing CTF-coupled Gs signaling and cAMP accumulation. Therefore, the interaction of GPR126 with laminin-211 is involved in Schwann cell differentiation and myelination, mediated by ensuring appropriate levels of cAMP to regulate the early and late stages of Schwann cell development.
Prion protein is an agonistic ligand of GPR126
Prion protein, a cell-surface glycoprotein, is expressed at relatively high levels in the peripheral and central nervous systems. Within the central nervous system, the prion protein is highly expressed in neurons and astrocytes.34 Prion proteins mainly exist in two distinct isoforms: PrPC, a normal cellular isoform, and PrPSc, a misfolding disease-associated isoform. Prion diseases are known for the conversion of benign PrPC isoform into an abnormally aggregated PrPSc isoform. The accelerated aggregation and deposition of the PrPSc isoform drive severe neuropathological changes, including neuronal loss and gliosis.34,35 Previous studies suggest that the PrPC isoform is a critical axonal modulator in myelin maintenance in the PNS.36 The deficiency of PrPC in mouse sciatic nerve can result in decreased cAMP levels, suggesting that PrPC may modulate the downstream signaling through a G protein-coupled receptor. A recent study revealed that PrPC maintains myelin homeostasis via the interaction of its terminal flexible tail with GPR126.37 The flexible tail of PrPC comprises a cAMP-inducing domain appearing in type IV collagen, an identified agonist of Gpr126, and induces the GPR126-mediated cAMP activation (Fig. 3). Meanwhile, the deletion of GPR126 in mice causes prominent hypomyelination, and the phenotype of deficiency in Prnp is contrarily moderate and late-onset, which may be compensated by the interaction with type IV collagen and/or laminin-211. Given the conserved homology region between type IV collagen and PrPC, they might share a similar activation mode in GPR126 activation, whereas the interaction between laminin-211 and GPR126 might exhibit a specific activation mode. PrPC is highly expressed in neuron and non-neuronal cells and its misfolding form causes neurodegenerative prion disease in the central nervous system. Although the expression of GPR126 in the central nervous system has not been identified, this interaction of GPR126 and PrPC raises the question of whether GPR126 has a critical role in prion diseases within the CNS.
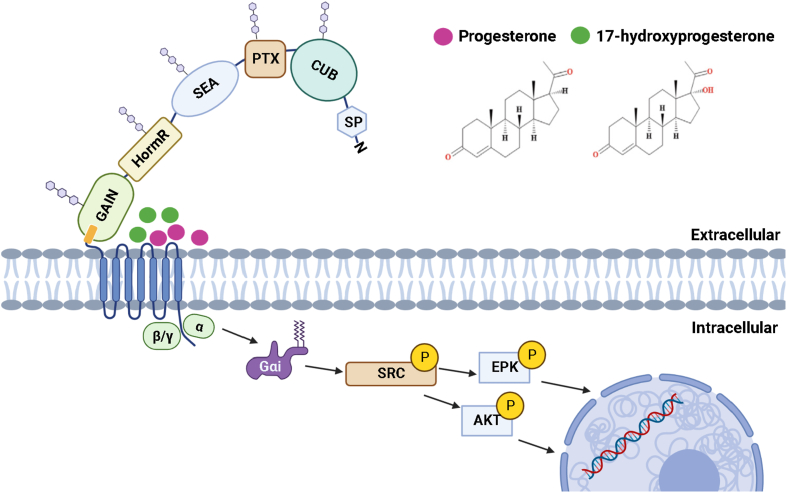
Ligands progesterone and 17-hydroxyprogesterone bind to the CTF of GPR126
Aside from the endogenous ligands binding to the NTF of GPR126, a few studies revealed the direct interacting partners with the CTF of GPR126. The C-terminal fragment of GPR126 contains a 7TM bundle, which is fundamental for GPCRs mediating signaling transduction. Most steroid hormones, including estrogens, glucocorticoids, progestogens, and androgens, function as signaling molecules via binding to their intracellular nuclear receptors. Among them, progesterone and 17-hydroxyprogesterone are two critical steroid hormones in the maintenance of the reproductive system. In a recent study, progesterone and 17-hydroxyprogesterone are found to sufficiently bind to the CTF of GPR12617 (Fig. 4). The steroid core of progesterone locates in a flat surface, a perpendicular direction to the TM5, and an angle of ∼60° orientation from the TM3. On the contrary, the modeled 17-hydroxyprogesterone assumed a conformation perpendicular to that of progesterone. In the simulated model, progesterone and 17-hydroxyprogesterone share nine common contacting residues in the ligand binding pocket of GPR126. With the two mutations located in the 7TM domain of CTF, the R1057Q mutant, rather than V769E, reduces the signaling potency and efficiency of progesterone.17 Compared to the previously reported ligand binding pocket of GPR97,38 GPR126 shares homologous residues in contact with steroid hormones. Given the conserved residues among the adhesion GPCR subfamily, it is worth investigating whether other steroid hormones function as ligands for aGPCRs. Meanwhile, the biological function study reveals that progesterone-induced GPR126 activation selectively acts through Gi-mediated SRC signaling pathways to enhance ripple-negative breast cancer cell growth and tumorigenesis.
Figure 4.
Ligands interact with the C-terminal fragment to activate GPR126 signaling. The binding activates GPR126 and triggers the downstream Gi-mediated signaling pathway.
Together, the ligand–receptor interaction studies of GPR126 accelerate the functional analysis of GPR126 in the physiological and pathological processes. A detailed understanding of GPR126 structure and function is valuable for cell signaling and molecular recognition, as well as drug discovery because of their fundamental roles in health and disease.
The physiological role of GPR126: essential for myelination and glial cell development
In the nervous system, myelination is a complicated process that initiates from the postnatal period and maintains adulthood. Myelin is the fatty membrane full of cholesterol and phospholipid in glial cells, wrapping around the axons of neurons to allow for rapid long-distance conduction of nerve impulses.39 The major compositions of myelin vary with proteolipid protein and myelin basic protein as the primary proteins in the CNS and with myelin protein zero as the dominant protein of PNS myelin.40 Moreover, the process of myelination is mediated by oligodendrocytes in the CNS or Schwann cells in the PNS. In the CNS, oligodendrocytes contact the axonal membrane and initiate myelination with the molecular changes to reorganize the cytoskeletal elements.41 Mature oligodendrocytes regulate myelination by wrapping the neuronal axons and furnishing trophic support to axons.42,43 In the PNS, Schwann cells are the main mediators for myelination. Immature SCs first surround multiple axons and undergo a radial axonal sorting process. Then, immature SCs extend the process into axon bundles and envelop an axon segment. As SCs mature, the pro-myelinating SCs wrap around the axon to set up the myelin sheath. Contrarily, the non-myelinating SCs ensheath the “Remak bundle” of nonmyelinated axons, composed of multiple small-caliber axons.33,44 The G protein-coupled receptor GPR126 has been reported to drive SCs-mediated myelination via elevating cAMP levels.45 With the mutation of GPR126 in zebrafish, SCs fail to express the transcription factor Oct6 and maintain myelination. The addition of cAMP can further restore the deficits.45 In another study, GPR126 is necessary for Schwann cells to induce krox20 expression. The activity of krox20 at the onset of myelination is dependent on GPR126 signaling. After the myelination is initiated, the krox20 expression is sustained. Myelination can be no longer dependent on GPR126-mediated signaling by elevation of cAMP.46 Overall, the role of GPR126 signaling is required for Schwann cells-initiated myelination but is not essential in the maturation of myelin. The deficiency of GPR126 results in delayed axonal sorting by Schwann cells in mice.47,48 Schwann cells are observed arrested at the promyelinating stage and non-myelinating Schwann cells are scarce in the GPR126−/− mice.47 The defects of myelination in GPR126 deficient cultures can be revived by raising cAMP levels and activation of protein kinase A. The cAMP levels in GPR126 conditional mutant sciatic nerves are significantly reduced and regulated via both Gs- and Gi-, but not Gq-coupled GPR126 signals.48 In addition, GPR126 undergoes autoproteolytic cleavage into an NTF and a transmembrane CTF. The NTF is required for axon sorting, whereas the CTF is necessary for wrapping the axonal segment to produce myelin via cAMP elevation. The dual roles of two fragments in GPR126 reflect domain-specific impacts on Schwann cell development, which are regulated by the binding of GPR126 to laminin-211.32 Aside from the role of the interaction between GPR126 and lamin-211 in the early and late stages of Schwann cell development, it is reported that the flexible tail of the prion protein PrPC triggered a dose-dependent elevation of cAMP levels in primary Schwann cells.37 The effect of the PrPC-mediated GPR126 activation is essential for myelin homeostasis. Above all, the extracellular endogenous ligands for GPR126, type IV collagen, laminin-211, and the prion protein (PrPc) interact with GPR126 to trigger cAMP signaling and mediate biological function in Schwann cells through Gs coupling. With the increasing understanding of the functions and mechanisms of GPR126 in the Schwan cells, it can deepen our understanding of the pathological roles of GPR126 in nerve repair and allow for novel therapeutic breakthroughs in human disorders (Fig. 5).
Figure 5.
Roles of GPR126 in physiology and pathophysiology. GPR126 plays diverse roles in the development and human diseases via multiple mechanisms.
The physiological role of GPR126: critical for tissue/organ development
aGPCRs are crucial mediators of conserved developmental processes. The adhesion GPR126 has been demonstrated a key role in Schwann cell maturation and inner ear morphogenesis in the zebrafish.45 Aside from the role of Schwann cell development, GPR126 plays a critical role in the development of various tissues/organs, including the heart, sciatic nerve, cartilage, and vascular. Loss of GPR126 impedes embryonic development, resulting in organ malformation and severe mid-gestation embryo lethality.13,49 Cardia development undergoes several important steps including septation and the formation of the outflow tract. A very low GPR126LacZ expression and a pronounced thinning of the myocardial wall in the myocardial tissue from homozygous mutants were observed. In a current study, the endocardium-specific deletion of GPR126 does not affect cardiac development, and the lethality in GPR126-deficient mice is not recovered by the expression of GPR126 in the endocardium. The inactivation of GPR126 in the placenta results in embryonic death.50 It is presumed that the heart abnormalities in GPR126-deficient mice are associated with placental effects. Meanwhile, the analysis of LacZ expression in GPR126tm1a mice suggests the expression pattern of GPR126 in the endothelium of vessels, including the endocardium. An increase of fluid shear stress or intraluminal pressure in endocardial cells up-regulates the GPR126 expression, which indicates the role of GPR126 in mechano-dependent signaling.49,51,52 The GPR126-mediated mechano-dependent signal transduction may be associated with the homeostasis of papillary collecting ducts and urothelium or ureter and bladder, where the expression of GPR126 is confirmed.49 Moreover, GPR126 is observed with a high expression in chondrocytes in multiple cartilage tissues but decreased in the later stage of cartilage development. The ossification of the spine is observed delayed in the developmental process due to the deletion of GPR126 in zebrafish.53 Meanwhile, mutations of the three single-nucleotide polymorphisms including the allele C of rs9403380, allele G of rs6570507, and allele A of rs7774095 in the GPR126 locus have been reported to be associated with AIS in humans.53, 54, 55 In addition, a reduced inclusion of exon6 in GPR126 caused by a genetic variant (rs41289839 G > A) is involved in cartilage development in adolescent idiopathic scoliosis (AIS) patients.56 This evidence indicates the important role of exon6 in GPR126 for cartilage development. Given the wide expression profile of GPR126 and its significant roles in cell and organ development, it is undoubtedly that mutations of GPR126 can cause severe deficits in cell biological functions and be associated with various disease conditions.
The pathological role of GPR126: closely related to degenerative joint disorders
The maturation of a healthy and functional spine requires the integration of musculoskeletal tissues, including bone, cartilage, muscle, and the PNS. The spine is composed of a series of segmented bony vertebral bodies by fibrocartilaginous joints named the intervertebral discs (IVDs), which are important for lateral and rotational flexibility as well as cushioning loading of the spinal column.57 GPR126 is previously demonstrated to be unnecessary for the overall morphology of IVDs. Specific deletion of GPR126 in the adult IVDs shows an endplate-oriented herniation.58 Alterations of ECM composition and inflammatory signaling have shown critical mechanical properties in the progression of disc degeneration and osteoarthritis.59 Mechanical properties are altered in the gene and protein expression of typical extracellular matrix and collagen. RNA-Seq analysis reveals pro-inflammatory signaling pathways, including suppressor of cytokine signaling (SOCS) and STAT3 signaling, are involved in the IVDs of GPR126-specific mutant mice. In contrast, systemic inhibition of STAT3 activation is found to alleviate the pathogenesis of endplate-oriented dis degeneration.58 On the other hand, the deletion of GPR126 in the osteoblast lineage leads to a decrease in body length and bone mass with delayed osteoblast cell differentiation and mineralization. Osteoblast differentiation is positively regulated by the interaction of GPR126 with type IV collagen, but not laminin-211. Although Wnt/β-catenin signaling is an important signaling pathway to regulate osteoblast differentiation. The type IV-collagen triggered GPR126 activation in regulating osteoblast differentiation is mediated by stimulating AMP signaling, rather than Wnt/β-catenin signaling.60 Thus, GPR126 is a promising therapeutic target for degenerative joint disorders.
The pathological role of GPR126: associated with adolescent idiopathic scoliosis
Adolescent idiopathic scoliosis (AIS) is a serious structural deformity of the pediatric spine and skeletal disease with a prevalence of 2%–4%.57,61 AIS is considered a complicated disease by virtue of genetic and environmental factors. Genetic factors are accounted for a critical role in the etiology of AIS. Recent evidence has revealed that a few candidate genes have been suggested in correlation with the etiopathogenesis of AIS, including matrilin 1 (MATN1),62 tryptophan hydroxylase 1 (TPH1),63 melatonin receptor 1B (MTNR1B),64 estrogen receptor 1 (ESR1) and 2 (ESR2).65,66 The genome-wide association studies (GWAS) have advanced the genetic research of AIS and extended our understanding of the etiology. Based on GWAS data, GPR126 is strongly correlated with the occurrence of AIS.53,55,67 In addition, it is noteworthy that the genetic role of GPR126 in the pathogenesis of AIS has been successfully validated by other clinical studies, including the southern and northern Chinese Han population as well as European ancestry.53, 54, 55,68 It is known that GPR126 is highly expressed in the cartilage of humans and chondrocytes of the embryonic mouse.66 Abnormal limb posture and skeletal growth are found in the Gpr126-null mice.13 Loss of GPR126 accelerates cell apoptosis of chondrocytes in the ribs and vertebrae and increases the expression of Gal3st4, a gene encoding Galactose-3-O-sulfotransferase 4.69 An SNP of GPR126, rs6570507, is reported to be associated with the height in European populations.70 Moreover, GPR126 is known to be essential for mammalian myelination, a process that can be restored by elevating cAMP levels with forskolin.45 The ultrastructural changes in nerve fibers and muscle spindles from AIS patients reveal the membranous bodies in myelinated nerve fibers. Moreover, increases in lipid droplets and glycogen particles are found in the intrafusal muscle fiber, indicating the abnormal metabolism of the muscle system in the AIS.71 Thus, whether the role of GPR126 in myelination contributes to the etiology of AIS warrants further investigation. The functions of both chondrocytes and Schwann cells are known to be related to mechanical signals. Yap and Taz are important mediators for mechanical signals in peripheral myelination. Previous studies suggested that laminin-211, a basal lamina component, may activate Yap and Taz via GPR126-mediated mechanical stimulation in a positive feedback loop by virtue of the ligand–receptor interaction between laminin-211 and GPR126.32,72 Because of the dysfunction of Gi-dependent signaling occurring in the AIS cells, the Gi-coupled mechano-dependent signaling induced by GPR126 may be involved in the AIS.49 Moreover, GPR126 is found to regulate the biomechanical effects of tendons and stimulate CREB signaling-regulated genes in cartilaginous tissues to maintain spine alignment.57 A genetic variant in the alternative splicing site results in a decreased inclusion of exon6 in GPR126,14 correlated to cartilage development in the AIS population.56 Thus, with the advances in the etiology and pathogenesis of AIS, the findings of GRP126 would open up novel therapeutic strategies for human scoliosis.
The pathological role of GPR126: critical for Schwann cell function during peripheral nerve injury
Peripheral nerve injury is relatively common and unique in its ability to regenerate after nerve injury. In the PNS, Schwann cells are a major type of glial cells. Myelinated SCs envelop axons with myelin sheaths to stimulate action potential propagation. Terminal SCs (tSCs), a population of non-myelinated SCs, can maintain and restore the neuromuscular junction (NMJ) in muscle. GPR126 is an aGPCR that is essential for myelin formation in Schwann cells. Meanwhile, GPR126 is involved in the repair process following nerve crush injury, particularly in the terminal Schwann cell at the NMJ following peripheral nerve injury. The absence of Gpr126 impairs the NMJ in the hind limbs of aged mice rather than young adult mice. An inducible SC-specific knockout of GPR126 results in delayed remyelination after nerve-crush injury and delayed NMJ reinnervation followed by reduced extension of tSC cytoplasmic processes.73,74 During the peripheral nerve repair following injury, the repaired SCs secrete various chemokines and recruit infiltrating macrophages to the injury sites. The infiltrated blood-derived macrophages play important roles in myelin debris clearance in the Wallerian degeneration and axonal regeneration process.75 Immune responses can be induced by the specific knockout of GPR126 in SCs. The selective deficiency of GPR126 results in reduced chemokines expression, including CCL2, CCL3, CXCL10, and TNF, and a decreased macrophage recruitment to the peripheral nerve injury sites. Superior cervical ganglion 10 (SCG10) levels rapidly decrease in distal axons following injury and are maintained during axon regeneration.76 The length and number of SCG10+ axons are reduced in the injured sciatic nerves of the GPR126 conditional knockout mice.74 Together, these findings indicate that GPR126 is essential for the expression of the chemokines by repaired SCs to recruit peripheral macrophages for proper axon regeneration following nerve injury.
The pathological role of GPR126: aberrant GPR126 signaling regulates tumorigenesis and angiogenesis
GPR126 is a newly-defined member of aGPCRs that is critical for the normal development of various tissues and organs. It is not surprising that GPR126 plays an important role in tumor progression, including breast cancer,17 bladder cancer,77, 78, 79 and colorectal cancer.80 GPR126 mutation and copy number variation are associated with tumor aggressiveness and poor patient survival. The protein-coding sequences account for less than 2% of the total genomic regions. Some non-coding regions with high mutation frequencies affect cancer development. GPR126 is identified with a high prevalence of a hotspot of noncoding somatic mutations in intron 6 of GPR126 in breast cancer.81 GPR126 is highly expressed in breast cancer tissues and is associated with a shorter overall survival rate. In a triple-negative breast cancer model, it is demonstrated that progesterone binds to GPR126 and triggers its activation via Gi-coupled-SRC downstream pathway to promote cancer cell growth.17 Moreover, a profound high expression of GPR126 is found in most colorectal cancer cell lines and colorectal tumor tissues. GPR126 deficiency can suppress colorectal cancer cell viability and colony formation by regulating the expression of histone deacetylase 2 and GLI2.80 Currently, whole-genome sequencing reveals that GPR126 enhancer mutations in the noncoding genomic regions are associated with urothelial bladder carcinoma development. The somatic mutations in the enhancer region are two hotspots of a single nucleotide, Chr. 6: 142,706,206 G > A transition and Chr. 6: 142,706,209 C > T transition, in intron 6 of GPR126 gene.78,79 GPR126 enhancer mutations exhibit higher expression levels of GPR126 compared to no these mutations in UBC tumors78.79. Meanwhile, GPR126 enhancer mutations are significantly correlated with older patients and have a much worse prognosis than those without mutations.79
Angiogenesis, a process of new blood vessel formation, is important for development, wound healing, and tumor progression. GPR126 is found an important role in angiogenesis by the regulation of endothelial cell proliferation, migration, and tube formation. The deletion of the GPR126 gene in the mouse retina inhibits the hypoxia-induced retinal neovascularization and results in defects in intersegmental vessel formation during zebrafish embryogenesis.82 Given the pivotal role of the VEGF pathway in the modulation of angiogenesis, GPR126 is found to regulate angiogenesis by modulating the expression of VEGFR2. The mechanistic study demonstrates that GPR126 regulates VEGFR2 expression by targeting STAT5 and GATA2 through the cAMP-activated PKA-CREB signaling pathway.82 The physiological and pathological roles of GPR126 in angiogenesis further extend the knowledge of GPCRs in vascular development and tumor biology.
Conclusions and perspectives
In this review, we mainly focused on the current studies of GPR126 in the expression profile, ligand–receptor interactions and associated signaling transduction, and its physiological and pathological roles in the development and human diseases. A thorough understanding of GPR126 would also pave the way to uncovering its new roles in the pathogenesis of human disorders. Despite several remarkable progress in recent years, the different variants and verified ligands of GPR126 remain further illustrated in the development and human disorders. Meanwhile, in virtue of the diverse roles of aGPCRs on health and disease, aGPCRs gain more interest in pharmacological intervention and candidate drug targets. However, currently approved drugs targeting aGPCRs are still very limited. The complex domain structures and ligand–receptor interactions of aGPCRs exhibit potentially diverse approaches, including strategies of small molecule- and macromolecule-based modulators to interrupt the interactions and modulate the downstream signaling activities. For the chemical-synthesized compounds, the small molecule antagonist of GPR56 and GPR114 have been investigated through a high-throughput activity screening assay.83 In the future, with the aid of aGPCR structural characterization, the most effective small molecule candidates can be discovered with preferentially and specifically binding to the CTF or NTF to regulate specific aGPCR functions. Meanwhile, the tethered agonist activation mode has been identified for the GPR56, GPR64, GPR126, GPR133, GPR110, GPR116, and LPHN1.84 Based on the Stachel peptide-mediated signaling, the possible utility of peptide-based modulators is worthwhile to investigate and develop the specific agonists or antagonists docked into the binding pockets of aGPCRs. Finally, other plausible approaches to developing recombinant monobodies targeting specific extracellular domains provide a proof-of-concept framework for future therapeutic strategies. Thus, it is necessary to accelerate the current research findings transforming into clinical studies and to make breakthroughs of orphan aGPCRs into potential drug targets in the future.
Author contributions
Q. L prepared the draft manuscript and figures. A. H, M. L, J. W, Q. Y, M. C, X. C, Y. Q, Y. Q, and Y. L contributed to the revision. H. C and Q. C conceived the idea and supervised the entire project including the manuscript preparation and revision. All authors read and approved the final manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32200778), the Natural Science Foundation of Jiangsu Province, China (No. BK20220494), Suzhou Medical and Health Technology Innovation Project (China) (No. SKY2022107), startup fund of Soochow University (China) (No. NH21500221, NH21500122), the Clinical Research Center of Neurological Disease in The Second Affiliated Hospital of Soochow University, China (No. ND2022A04 to Qifei Cong), and the Nantong Municipal Health and Family Planning Commission (China) (No. QA2021017 to Xin Chu).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Hengxiang Cui, Email: hxcui@ion.ac.cn.
Qifei Cong, Email: qfcong@suda.edu.cn.
References
- 1.Bassilana F., Nash M., Ludwig M.G. Adhesion G protein-coupled receptors: opportunities for drug discovery. Nat Rev Drug Discov. 2019;18(11):869–884. doi: 10.1038/s41573-019-0039-y. [DOI] [PubMed] [Google Scholar]
- 2.Langenhan T., Piao X., Monk K.R. Adhesion G protein-coupled receptors in nervous system development and disease. Nat Rev Neurosci. 2016;17(9):550–561. doi: 10.1038/nrn.2016.86. [DOI] [PubMed] [Google Scholar]
- 3.Hamann J., Aust G., Araç D., et al. International union of basic and clinical pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev. 2015;67(2):338–367. doi: 10.1124/pr.114.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnadóttir T.K., Geirardsdóttir K., Ingemansson M., et al. Identification of novel splice variants of Adhesion G protein-coupled receptors. Gene. 2007;387(1–2):38–48. doi: 10.1016/j.gene.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 5.Liebscher I., Schön J., Petersen S., et al. A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 2014;9(6):2018–2026. doi: 10.1016/j.celrep.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lala T., Hall R.A. Adhesion G protein-coupled receptors: structure, signaling, physiology, and pathophysiology. Physiol Rev. 2022;102(4):1587–1624. doi: 10.1152/physrev.00027.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vizurraga A., Adhikari R., Yeung J., et al. Mechanisms of adhesion G protein-coupled receptor activation. J Biol Chem. 2020;295(41):14065–14083. doi: 10.1074/jbc.REV120.007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosa M., Noel T., Harris M., et al. Emerging roles of adhesion G protein-coupled receptors. Biochem Soc Trans. 2021;49(4):1695–1709. doi: 10.1042/BST20201144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredriksson R., Gloriam D.E.I., Höglund P.J., et al. There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochem Biophys Res Commun. 2003;301(3):725–734. doi: 10.1016/s0006-291x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 10.Moriguchi T., Haraguchi K., Ueda N., et al. DREG, a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Gene Cell. 2004;9(6):549–560. doi: 10.1111/j.1356-9597.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 11.Stehlik C., Kroismayr R., Dorfleutner A., et al. VIGR–a novel inducible adhesion family G-protein coupled receptor in endothelial cells. FEBS Lett. 2004;569(1–3):149–155. doi: 10.1016/j.febslet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Patra C., Monk K.R., Engel F.B. The multiple signaling modalities of adhesion G protein-coupled receptor GPR126 in development. Receptors Clin Investig. 2014;1(3):79. doi: 10.14800/rci.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waller-Evans H., Prömel S., Langenhan T., et al. The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0014047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon K., Cunningham R.L., Riback J.A., et al. Structural basis for adhesion G protein-coupled receptor Gpr126 function. Nat Commun. 2020;11:194. doi: 10.1038/s41467-019-14040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristiansen T.Z., Bunkenborg J., Gronborg M., et al. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3(7):715–728. doi: 10.1074/mcp.M400015-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Paavola K.J., Sidik H., Zuchero J.B., et al. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal. 2014;7(338):ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An W., Lin H., Ma L., et al. Progesterone activates GPR126 to promote breast cancer development via the Gi pathway. Proc Natl Acad Sci U S A. 2022;119(15) doi: 10.1073/pnas.2117004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown K.L., Cummings C.F., Vanacore R.M., et al. Building collagen IV smart scaffolds on the outside of cells. Protein Sci. 2017;26(11):2151–2161. doi: 10.1002/pro.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernousov M.A., Yu W.M., Chen Z.L., et al. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56(14):1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 20.Chen P., Cescon M., Bonaldo P. The role of collagens in peripheral nerve myelination and function. Mol Neurobiol. 2015;52(1):216–225. doi: 10.1007/s12035-014-8862-y. [DOI] [PubMed] [Google Scholar]
- 21.Bunge M.B., Bunge R.P. Linkage between Schwann cell extracellular matrix production and ensheathment function. Ann N Y Acad Sci. 1986;486:241–247. doi: 10.1111/j.1749-6632.1986.tb48077.x. [DOI] [PubMed] [Google Scholar]
- 22.Yurchenco P.D., Amenta P.S., Patton B.L. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22(7):521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Patra C., van Amerongen M.J., Ghosh S., et al. Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc Natl Acad Sci U S A. 2013;110(42):16898–16903. doi: 10.1073/pnas.1304837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 25.Schnittert J., Bansal R., Storm G., et al. Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev. 2018;129:37–53. doi: 10.1016/j.addr.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Xu J., Rodriguez D., Petitclerc E., et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154(5):1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakogiannos N., Scalise A.A., Martini E., et al. Dual role of brain endothelial Gpr126 in blood-brain barrier development and ischemic stroke. bioRxiv. 2022 doi: 10.1101/2022.09.09.507316. [DOI] [Google Scholar]
- 28.Li Z.Z., Han W.J., Sun Z.C., et al. Extracellular matrix protein laminin β1 regulates pain sensitivity and anxiodepression-like behaviors in mice. J Clin Invest. 2021;131(15) doi: 10.1172/JCI146323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feltri M.L., Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10(2):128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- 30.McKee K.K., Yang D.H., Patel R., et al. Schwann cell myelination requires integration of laminin activities. J Cell Sci. 2012;125(Pt 19):4609–4619. doi: 10.1242/jcs.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghidinelli M., Poitelon Y., Shin Y.K., et al. Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLoS Biol. 2017;15(6) doi: 10.1371/journal.pbio.2001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen S.C., Luo R., Liebscher I., et al. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron. 2015;85(4):755–769. doi: 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta P., Piao X. Adhesion G-protein coupled receptors and extracellular matrix proteins: roles in myelination and glial cell development. Dev Dyn. 2017;246(4):275–284. doi: 10.1002/dvdy.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watts J.C., Bourkas M.E.C., Arshad H. The function of the cellular prion protein in health and disease. Acta Neuropathol. 2018;135(2):159–178. doi: 10.1007/s00401-017-1790-y. [DOI] [PubMed] [Google Scholar]
- 35.Castle A.R., Gill A.C. Physiological functions of the cellular prion protein. Front Mol Biosci. 2017;4:19. doi: 10.3389/fmolb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremer J., Baumann F., Tiberi C., et al. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13(3):310–318. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- 37.Küffer A., Lakkaraju A.K.K., Mogha A., et al. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536(7617):464–468. doi: 10.1038/nature19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ping Y.Q., Mao C., Xiao P., et al. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature. 2021;589(7843):620–626. doi: 10.1038/s41586-020-03083-w. [DOI] [PubMed] [Google Scholar]
- 39.Fields R.D., Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298(5593):556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S.S., Zhang Z., Zhu T.B., et al. Myelin injury in the central nervous system and Alzheimer's disease. Brain Res Bull. 2018;140:162–168. doi: 10.1016/j.brainresbull.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Nave K.A., Werner H.B. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 42.Fünfschilling U., Supplie L.M., Mahad D., et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes E.G., Appel B. The cell biology of CNS myelination. Curr Opin Neurobiol. 2016;39:93–100. doi: 10.1016/j.conb.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbert A.L., Monk K.R. Advances in myelinating glial cell development. Curr Opin Neurobiol. 2017;42:53–60. doi: 10.1016/j.conb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monk K.R., Naylor S.G., Glenn T.D., et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325(5946):1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glenn T.D., Talbot W.S. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013;140(15):3167–3175. doi: 10.1242/dev.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monk K.R., Oshima K., Jörs S., et al. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138(13):2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mogha A., Benesh A.E., Patra C., et al. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33(46):17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musa G., Cazorla-Vázquez S., van Amerongen M.J., et al. Gpr126 (Adgrg6) is expressed in cell types known to be exposed to mechanical stimuli. Ann N Y Acad Sci. 2019;1456(1):96–108. doi: 10.1111/nyas.14135. [DOI] [PubMed] [Google Scholar]
- 50.Torregrosa-Carrión R., Piñeiro-Sabarís R., Siguero-Álvarez M., et al. Adhesion G protein-coupled receptor Gpr126/Adgrg6 is essential for placental development. Sci Adv. 2021;7(46) doi: 10.1126/sciadv.abj5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson M., Karlsson L., Svensson P.A., et al. Differential global gene expression response patterns of human endothelium exposed to shear stress and intraluminal pressure. J Vasc Res. 2005;42(5):441–452. doi: 10.1159/000087983. [DOI] [PubMed] [Google Scholar]
- 52.White S.J., Hayes E.M., Lehoux S., et al. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol. 2011;226(11):2841–2848. doi: 10.1002/jcp.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kou I., Takahashi Y., Johnson T.A., et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 54.Qin X., Xu L., Xia C., et al. Genetic variant of GPR126 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Spine. 2017;42(19):E1098–E1103. doi: 10.1097/BRS.0000000000002123. [DOI] [PubMed] [Google Scholar]
- 55.Xu J.F., Yang G.H., Pan X.H., et al. Association of GPR126 gene polymorphism with adolescent idiopathic scoliosis in Chinese populations. Genomics. 2015;105(2):101–107. doi: 10.1016/j.ygeno.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Xu E., Shao W., Jiang H., et al. A genetic variant in GPR126 causing a decreased inclusion of exon 6 is associated with cartilage development in adolescent idiopathic scoliosis population. BioMed Res Int. 2019;2019 doi: 10.1155/2019/4678969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Z., Hussien A.A., Wang Y., et al. An adhesion G protein-coupled receptor is required in cartilaginous and dense connective tissues to maintain spine alignment. Elife. 2021;10 doi: 10.7554/eLife.67781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Easson G.W.D., Zhao J., et al. Dysregulation of STAT3 signaling is associated with endplate-oriented herniations of the intervertebral disc in Adgrg6 mutant mice. PLoS Genet. 2019;15(10) doi: 10.1371/journal.pgen.1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen Q.T., Jacobsen T.D., Chahine N.O. Effects of inflammation on multiscale biomechanical properties of cartilaginous cells and tissues. ACS Biomater Sci Eng. 2017;3(11):2644–2656. doi: 10.1021/acsbiomaterials.6b00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun P., He L., Jia K., et al. Regulation of body length and bone mass by Gpr126/Adgrg6. Sci Adv. 2020;6(12) doi: 10.1126/sciadv.aaz0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Z., Xu L., Qiu Y. Current progress in genetic research of adolescent idiopathic scoliosis. Ann Transl Med. 2015;3(Suppl 1):S19. doi: 10.3978/j.issn.2305-5839.2015.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z., Tang N.L.S., Cao X., et al. Promoter polymorphism of matrilin-1 gene predisposes to adolescent idiopathic scoliosis in a Chinese population. Eur J Hum Genet. 2009;17(4):525–532. doi: 10.1038/ejhg.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H., Wu Z., Zhuang Q., et al. Association study of tryptophan hydroxylase 1 and arylalkylamine N-acetyltransferase polymorphisms with adolescent idiopathic scoliosis in Han Chinese. Spine. 2008;33(20):2199–2203. doi: 10.1097/BRS.0b013e31817c03f9. [DOI] [PubMed] [Google Scholar]
- 64.Qiu X.S., Tang N.L., Yeung H.Y., et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine. 2007;32(16):1748–1753. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- 65.Wu J., Qiu Y., Zhang L., et al. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine. 2006;31(10):1131–1136. doi: 10.1097/01.brs.0000216603.91330.6f. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H.Q., Lu S.J., Tang M.X., et al. Association of estrogen receptor beta gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine. 2009;34(8):760–764. doi: 10.1097/BRS.0b013e31818ad5ac. [DOI] [PubMed] [Google Scholar]
- 67.Kou I., Watanabe K., Takahashi Y., et al. A multi-ethnic meta-analysis confirms the association of rs6570507 with adolescent idiopathic scoliosis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-29011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G., Liu S., Lin M., et al. Genetic polymorphisms of GPR126 are functionally associated with PUMC classifications of adolescent idiopathic scoliosis in a Northern Han population. J Cell Mol Med. 2018;22(3):1964–1971. doi: 10.1111/jcmm.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karner C.M., Long F., Solnica-Krezel L., et al. Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum Mol Genet. 2015;24(15):4365–4373. doi: 10.1093/hmg/ddv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soranzo N., Rivadeneira F., Chinappen-Horsley U., et al. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009;5(4) doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Low W.D., Chew E.C., Kung L.S., et al. Ultrastructures of nerve fibers and muscle spindles in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 1983;174:217–221. [PubMed] [Google Scholar]
- 72.Poitelon Y., Lopez-Anido C., Catignas K., et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat Neurosci. 2016;19(7):879–887. doi: 10.1038/nn.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jablonka-Shariff A., Lu C.Y., Campbell K., et al. Gpr126/Adgrg6 contributes to the terminal Schwann cell response at the neuromuscular junction following peripheral nerve injury. Glia. 2020;68(6):1182–1200. doi: 10.1002/glia.23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mogha A., Harty B.L., Carlin D., et al. Gpr126/Adgrg6 has Schwann cell autonomous and nonautonomous functions in peripheral nerve injury and repair. J Neurosci. 2016;36(49):12351–12367. doi: 10.1523/JNEUROSCI.3854-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen P., Piao X., Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130(5):605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- 76.Shin J.E., Geisler S., DiAntonio A. Dynamic regulation of SCG10 in regenerating axons after injury. Exp Neurol. 2014;252:1–11. doi: 10.1016/j.expneurol.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vacher S., Suybeng V., Girard E., et al. Genomic instability signature of palindromic non-coding somatic mutations in bladder cancer. Cancers. 2020;12(10):2882. doi: 10.3390/cancers12102882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garinet S., Pignot G., Vacher S., et al. High prevalence of a hotspot of noncoding somatic mutations in intron 6 of GPR126 in bladder cancer. Mol Cancer Res. 2019;17(2):469–475. doi: 10.1158/1541-7786.MCR-18-0363. [DOI] [PubMed] [Google Scholar]
- 79.Wu S., Ou T., Xing N., et al. Whole-genome sequencing identifies ADGRG6 enhancer mutations and FRS2 duplications as angiogenesis-related drivers in bladder cancer. Nat Commun. 2019;10:720. doi: 10.1038/s41467-019-08576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui H., Yu W., Yu M., et al. GPR126 regulates colorectal cancer cell proliferation by mediating HDAC2 and GLI2 expression. Cancer Sci. 2021;112(5):1798–1810. doi: 10.1111/cas.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nik-Zainal S., Davies H., Staaf J., et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui H., Wang Y., Huang H., et al. GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling. J Biol Chem. 2014;289(50):34871–34885. doi: 10.1074/jbc.M114.571000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoveken H.M., Bahr L.L., Anders M.W., et al. Dihydromunduletone is a small-molecule selective adhesion G protein-coupled receptor antagonist. Mol Pharmacol. 2016;90(3):214–224. doi: 10.1124/mol.116.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Folts C.J., Giera S., Li T., et al. Adhesion G protein-coupled receptors as drug targets for neurological diseases. Trends Pharmacol Sci. 2019;40(4):278–293. doi: 10.1016/j.tips.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]