Abstract
Exosomes are small membrane vesicles containing microRNA, RNA, DNA fragments, and proteins that are transferred from donor cells to recipient cells. Tumor cells release exosomes to reprogram the factors associated with the tumor microenvironment (TME) causing tumor metastasis and immune escape. Emerging evidence revealed that cancer cell-derived exosomes carry immune inhibitory molecule program death ligand 1 (PD-L1) that binds with receptor program death protein 1 (PD-1) and promote tumor progression by escaping immune response. Currently, some FDA-approved monoclonal antibodies are clinically used for cancer treatment by blocking PD-1/PD-L1 interaction. Despite notable treatment outcomes, some patients show poor drug response. Exosomal PD-L1 plays a vital role in lowering the treatment response, showing resistance to PD-1/PD-L1 blockage therapy through recapitulating the effect of cell surface PD-L1. To enhance therapeutic response, inhibition of exosomal PD-L1 is required. Calcium signaling is the central regulator of tumorigenesis and can regulate exosome biogenesis and secretion by modulating Rab GTPase family and membrane fusion factors. Immune checkpoints are also connected with calcium signaling and calcium channel blockers like amlodipine, nifedipine, lercanidipine, diltiazem, and verapamil were also reported to suppress cellular PD-L1 expression. Therefore, to enhance the PD-1/PD-L1 blockage therapy response, the reduction of exosomal PD-L1 secretion from cancer cells is in our therapeutic consideration. In this review, we proposed a therapeutic strategy by targeting calcium signaling to inhibit the expression of PD-L1-containing exosome levels that could reduce the anti-PD-1/PD-L1 therapy resistance and increase the patient's drug response rate.
Keywords: Calcium signaling, CD8+T cells, Exosomal PD-L1, Exosomes biogenesis, Immunosuppression, Immunotherapy
Abbreviations
- ALIX
ASG-2 interacting protein 2
- B7–H1
B7 homolog 1
- CA IX
carbonic anhydrase IX
- CAFs
cancer-associated fibroblasts
- CCBs
calcium channel blockers
- CD274
cluster of differentiation 274
- CDE
caveolin-dependent endocytosis
- CME
clathrin-mediated endocytosis
- COX2
cyclooxygenase-2
- DCs
dendritic cells
- DMA
dimethyl amiloride
- DNA
deoxyribonucleic acid
- EpCAM
epithelial tumor cells express the epithelial cell adhesion molecule
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- ERK
extracellular signal-regulated kinases
- ESCRT
Endosomal Sorting Complex Required for Transport
- FDA
Food and Drug Administration
- GPCRs
G-protein couple receptors
- HER
human epidermal receptor
- HIF
hypoxia inducible factors
- HRS
hepatocyte growth factor-regulated tyrosine kinase substrate
- Hsp
heat shock protein
- IFN-γ
interferon-γ
- IL-6
interleukin-6
- ILV
intra luminal vesicle
- IP3
inositol tri-phosphate
- IRF-1
interferon regulatory factor-1
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITSM
immunoreceptor tyrosine-based switch motif
- iTregs
inducible Tregs
- JAK
Janus kinase
- LAG3
lymphocyte activation gene 3
- mAbs
monoclonal antibodies
- MAPKs
mitogen-activated protein kinases
- MCU
mitochondrial Ca2+ uniporter
- MHC-I
major histocompatibility complex I
- MMP
matrix metalloproteinase
- MVBs
multivesicular bodies
- MYO10
myosin 10
- NK cells
natural killer cells
- nSMase 2
sphingomyelin phosphodiesterase 2
- PD-1
program death protein 1
- PD-L1
program death ligand 1
- RNA
ribonucleic acid
- ROC
receptor-operated calcium channel
- RyR
ryanodine receptor
- SNARE
soluble N-ethylmaleimide-sensitive factor-attachment protein (SNAP) receptor
- STAT
signal transducer and activator of transcription
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor-α
- TSG101
tumor susceptibility gene 101
- VEGF
vascular endothelial growth factor
- VGCC
voltage-gated Ca2+ channels
- VPS4
vacuolar protein sorting-associated protein 4
Introduction
Almost all cells can release a variety of membrane microvesicles and nanovesicles with diversified functional consequences. Microvesicles mainly differ from nanovesicles based on their size and mechanism of generation.1,2 Microvesicles release from the plasma membrane through a shedding or budding process. They are generally larger than 0.2 μm in size and denote microparticles or ectosomes. On the other hand, nanovesicles, including exosomes, are between 30 and 100 nm in size, generate through reverse budding of the peripheral membrane of multivesicular bodies (MVBs) or late endosomes, and are categorized based on endocytic origin3. Exosomes are well-known for transporting a variety of signaling molecules, including nucleic acids, microRNA, functional proteins, and lipids. Due to their cell–cell communication characteristics, numerous studies have explored the role of exosomes in the physiological and pathophysiological processes including tumor metastasis,4 immune modulation,5 and neurodegenerative diseases.6
Emerging evidence reveals that cancer-derived exosomes are the carrier of immunosuppressive proteins like PD-L1 and its receptor PD-1, which are significant immune checkpoint molecules and can stimulate tumor progression via negative regulation of cellular immune responses.7,8 PD-L1 is a type I transmembrane protein of 290 amino acids encoded by the CD274 gene and known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) and containing of immunoglobulin V-like and C-like extracellular domains.9 However, PD-L1 is extensively expressed in numerous cell types, mostly in tumor cells, monocytes, macrophages, NK cells, DCs, and activated CD8+ T cells and in immune-privileged sites such as the brain, cornea, and retina.10 In normal physiological conditions, the activation of the PD-1/PDL1 signaling is closely associated with the initiation of peripheral tolerance, maintenance of CD8+ T cells immune homeostasis, escaping hyperactivation as well as shielding against tissue damage.11 In disease states, PD-L1 transmits a negative signal by interacting with its receptor PD-1 to regulate a series of processes of CD8+ T cell-mediated cellular immune responses, including growth, proliferation, and apoptosis, as well as functional maturation.12 Recent studies have depicted that the activation of the PD-1/PD-L1 signaling pathway can arrest the CD8+ T cell cycle at the G1 phase rather than causing direct apoptosis.13 Furthermore, considering the CD8+ T cells mediating inhibitory effects, the PD-1/PD-L1 signaling pathway can facilitate the down-regulation of CD8+ T cell responses by inducible Tregs (iTregs).14 Metastatic melanoma-derived exosomes contain PD-L1 on their surface and stimulation with interferon-γ (IFN-γ) enhances the amount of PD-L1 on these vesicles and down-regulates anti-tumor response to induce tumor growth.7
In normal cellular homeostasis, cytosolic Ca2+ level control several signaling processes, however disruption of normal Ca2+ is hypothesized to be a reason for enriched proliferation and metastasis induced in numerous cancer.15 Ca2+-dependent proliferation is facilitated by MAPK/calmodulin-dependent signaling, whereas invasion and migration are stimulated via Ca2+-dependent cytoskeleton rearrangement and focal adhesion disassembly.15,16 Although recent studies have identified Ca2+ channels that are amplified with pathophysiological consequences, the influences of Ca2+-dependent effectors are less understood.
Several “Endosomal Sorting complex required for Transport (ESCRT)” and related proteins including HRS, TSG101,17 ALIX, and VPS418 have been associated with exosome release. However, the ESCRTs playing a role in ILV formation on MVBs to modulate exosome release is still unclear.19 Additionally, members of the Rab GTPase family such as Rab5, 7, 11, 27a, 27b, and 35 have also been identified as a regulator of exosome release.20, 21, 22, 23, 24, 25, 26 Recent findings demonstrate that Ca2+-dependent Rab binding protein Munc 13-4 is a crucial factor for Ca2+-dependent membrane fusion. Moreover, they have identified Ca2+, Munc13-4, Rab 11 dependent molecular pathway that underlines increased exosome secretion from cancer cell.27,28 The activation of G-protein coupled receptors (GPCRs) resulting in exosome formation, release, and uptake also represent a potential role of GPCRs in the modulation of exosome biogenesis and function. Activated GPCRs promote the discharge of Ca2+ from the endoplasmic reticulum by the second messenger inositol triphosphate (IP3) and increased intracellular Ca2+ level initiates exosome formation and release.29,30 H+/Na+ and Na+/Ca2+ channels control the intracellular Ca2+ concentration and likely regulate the exosome secretion. Preclinical studies with CT26 tumor-bearing mice have mentioned that blocking these channels using dimethyl amiloride (DMA) inhibits exosome release.31,32 These results suggest that blocking the secretion of exosomes from cancer cells by CCBs might constitute a potential target for cancer therapy. Additionally, L-type Ca2+ channels are expressed and frequently altered in many human cancers and contribute to cancer cell invasion and migration. As a result, controlling the invasion and migration of cancer cells and drastically reducing the number of MYO10 is accomplished by controlling L-type Ca2+ channels through CCBs.33 In this review, we emphasize a novel approach of cancer therapy that involves limiting the release of exosomes from cancer cells that express PD-L1, which has implications on immunotherapeutic methods of cancer treatment.
Biogenesis and functional relevance of exosomes in cancer
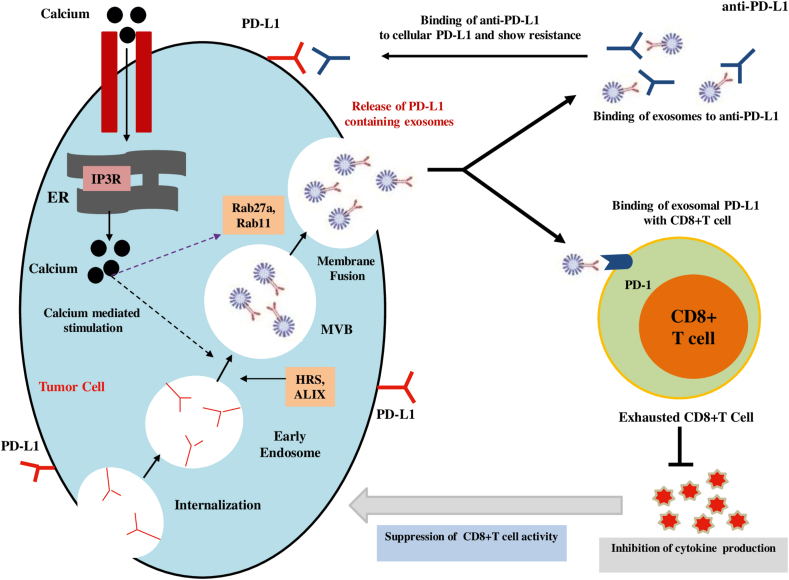
The biogenesis of exosomes is regulated by the endosomal system. Early endosomes mature into the late endosomes or MVBs, necessary for the endosomal membrane to invigilate to produce ILVs in the lumen of the organelles.3 The ESCRT complex plays a crucial role in this processing by recognizing ubiquitinated membrane proteins and promoting their internalization into the MVBs. Finally, the MVBs fuse with the cellular membrane to release exosomes into the extracellular space.31,34 Exosome release can be triggered by hypoxia, which is frequently present in malignancies and is positively regulated by the Rab GTPases 27a and 27b.31 (Fig. 1).
Figure 1.
Role of calcium signaling in exosomal PD-L1 secretion and cancer progression. Intracellular calcium stimulates biogenesis factors and Rab family proteins to increase the secretion of PD-L1-positive exosomes from cancer cells. Exosomes carrying PD-L1 are responsible for limiting the effectiveness of anti-PD-1/PD-L1 therapy through binding to antibodies. Additionally, exosomes containing PD-L1 play a role in the suppression of immunity by reducing CD8+ T cell-mediated activities through inhibiting cytokine production.
Exosomes contain a fundamental set of identical proteins which are released from different kinds of cells including members of the ESCRT complex (TSG101, Alix), members of the tetraspanin family (CD9, CD63, CD81, and CD82), and heat shock proteins (Hsp 60, Hsp 70, Hsp 90).35 Apart from these, exosomes also bring some specific proteins. Melanoma cells secrete exosomes carrying the tumor-associate antigen Mart-1.36 Exosomes from epithelial tumor cells express the epithelial cell adhesion molecule (EpCAM).37 Gastric cancer, breast cancer, or pancreatic cancer cell-derived exosomes contain the members of the human epidermal receptor (HER) family.31,38
The uptake of exosomes in the recipient cell takes place in a non-random process in association with transmembrane proteins. Recent evidence describes that the tetraspanin-integrin complex plays a role noticeably in pursuing the binding of exosomes to recipient cells.39 Moreover, a pro-inflammatory environment causes the expression of ICAM-1 as a receptor molecule on the membrane surface, which enhances the adhesion of exosomes to the recipient cells.40 Exosomes can directly release their cargo to the cytoplasm of recipient cells through the fusion with the cell membrane. Cellular internalization of exosomes can occur by following several mechanisms such as phagocytosis, macropinocytosis, Clathrin-mediated endocytosis (CME), Caveolin-dependent endocytosis (CDE), and plasma membrane fusion.41 Additional studies are required to elucidate how exosomes are heading for the target cells in cancer and whether the cell-specific composition of exosomes results in organotropism connected with metastatic cancer.
Exosome-mediated transfer of proteins between cancer cells can lead to drug resistance. Exosomes from two docetaxel-resistant prostate cancer cell lines can deliberate chemoresistance to non-resistant prostate cancer cell lines via exosome-mediated transfer of drug transporter, MDR-1.42 Acquired drug resistance by exosome-mediated transfer of proteins can be recognized to the decreased intracellular concentration of chemotherapeutic agents, epithelial–mesenchymal transition (EMT), altered expression of oncogenes or tumor suppressor genes, enriched DNA damage repair, autophagy, and highly acidic microenvironment of tumors.43,44 These observations characterize a new paradigm of how malignant cells may raise their tumorigenic potential and develop drug resistance via exosomes.
Prostate and mesothelioma cancer cell-derived exosomes carry TGF-β1 protein, which is transmitted to recipient cells in a biologically active form and TGF-β1 containing exosomes can cause the differentiation of fibroblasts to myofibroblasts as a key source of tumor angiogenesis and matrix remodeling proteins.45,46 Moreover, cancer cells expressing exosomes contain membrane-bound EGFR to endothelial cells that activate the autocrine VEGF/VEGFR-2 pathway to lead to tumor angiogenesis.47,48 Additionally, CD105-positive exosomes contribute to forming a pre-metastatic niche by enhancing MMP2, MMP9, and VEGFR1.48 Similarly, exosomes from fibroblasts stimulate an autocrine Wnt-signaling pathway in breast cancer cells to lead to migration, invasion, and metastasis.49 Collectively, these studies have indicated that tumor-derived exosomes play an influential role in operating the tumor microenvironment (TME) for the benefit of cancer cells.
Cancer cell-derived exosomes control fundamental functional features of the lymphoid components of the TME and exert direct influence in immunosuppression to lead to tumor progression. Exosomes from gastric cancer cells (especially MKN-45 and MKN-28) changed gene expression and cytokine secretion patterns of CD8+ T cells to lead to an immunosuppressive condition for metastatic niche formation in the lung.50 Moreover, exosomes from other cancer cell lines facilitate IL-6 production in MDSCs through the activation of the Toll-like receptor 2 by the Hsp 72 which elicits an immunosuppressive effect.32 It has been demonstrated that the exosomes secreted from hepatocellular carcinoma promoted the expansion of the TIM-1+ regulatory B cell (Breg) population by expressing IL-10, and inhibiting CD8+ T cells proliferation, TNF-α, and IFN-γ production to suppress anti-tumor immunity.51 The expansion of regulatory T (Treg) cells is stimulated by the tumor cell-derived exosomes that cause immunosuppression by impairing the function of anti-tumorigenic CD8+ T cells.51,52
Elsner et al53 demonstrated that exosomes from hepatocellular carcinoma cell lines and pancreatic cancer cells carry Hsp 70 that can directly up-regulate active natural killer (NK) cells, and consequently, NK cells induce apoptosis in tumors through granzyme B. Moreover, exosomes from tumor cells can transmit antigens to antigen-presenting dendritic cells through MHC-I molecules, and dendritic cells activate cytotoxic T lymphocytes and evoke an anti-tumor response, and suppress tumor growth in vivo.54 Nonetheless, there is still an ongoing debate about whether cancer cells containing exosomes are an adequate mode of vaccination against tumor cells.
Biology of exosomal PD-L1
One of the mechanisms by which tumor cells induce immune evasion is the stimulation of surface expression of PD-L1 that interacts with PD-1 on effector T cells, suppressing their activation. To interfere with this pathological basis, anti-PD-1/PD-L1 mAbs have been developed; however, the overall response is limited.7,55 Accumulating evidence recommends that exosomes are an important source of extra-tumoral PD-L1 and may be one molecular process contributing to PD-1 antibody treatment resistance.
Biogenesis and expression of exosomal PD-L1
Exosomal PD-L1 performs as the crossroads of inflammation and tumor progression, therefore illuminating the responsible factors as well as mechanisms that lead to its biogenesis and release is needed to intensely understand the inflammatory response of tumor cells during malignant evolution. Poggio et al56 reported that exosomal PD-L1 is generated from the plasma membrane, rather than from the endoplasmic reticulum or Golgi apparatus. Similarly, PD-L1 internalized through the plasma membrane might be a source of PD-L1 in exosomes (Fig. 1). Evidence recommends that PD-L1 is distributed amongst diverse cellular compartments, which have been deeply connected with immunotherapy failure.57 However, the precise molecular mechanisms that dictate exosomal PD-L1 biogenesis and PD-L1 distribution are not completely elucidated.
Exosomal marker HRS and PD-L1 co-localize with CD63, representing that PD-L1 is enveloped in exosomes and the pre-exosomal PD-L1 is localized in the subcellular structure.56,58 Therefore, the knockdown of HRS in the WM9 cell line also led to a decrease in PD-L1 expression in exosomes but retained expression in the parent cell apparently due to shrank exosome formation.7 The ESCRT-associated protein ALIX has been identified to directly regulate the loading of PD-L1 onto exosomes from the endosomal lumen in basal-like breast cancer cells. Deficiency of ALIX in HCC1954 cells resulted in retained tumor cell-surface PD-L1 expression but reduced exosomal PD-L1 level.59 Furthermore, knockout of the Rab family protein Rab27a and nSMase 2 in PC3 cells inhibit exosome secretion and dramatically down-regulate the coexpression of CD63 and PD-L1 in sucrose fractionation.56 PD-L1 may be sorted into exosomes from the plasma membrane, yet additional points remain to be clarified including how other mechanisms of endosome maturation and exosome release stimulate exosomal PD-L1 biogenesis.
Poggio et al56 and Monypenny et al59 noted that experimental deletion of members of the ESCRT complex and accessory proteins down-regulates exosomal PD-L1 secretion and up-regulates PD-L1 expression at the cell surface. Kim et al60 found that the amount of exosomal PD-L1 in the culture supernatant of lung cancer cell lines characterized the amount of PD-L1 expression on the cell surface, while the plenty of exosomal PD-L1 isolated from plasma of non-small-cell lung cancer patients interrelated with tumor PD-L1 positivity. Undoubtedly, one of the major complications of immunotherapy is differential PD-L1 expression among tumor types, and there may have an association between the exosomal membrane and the amount of PD-L1 expressed at the cell surface.59,61 Understanding the nature of the association between the exosomal membrane and the amount of PD-L1 expressed at the cell surface and the responsible factors that may lead to a PD-L1 alteration from the cell surface to the exosome membrane is required in further study.
Numerous human melanoma cell lines revealed that the expression level of exosomal PD-L1 is higher in metastatic tumors compared to primary tumors indicating that the PD-L1 distribution at the cellular level and the release of exosomal PD-L1 may be linked with the range of metastatic capacity of tumor cells.7 In circulation, other forms of extracellular PD-L1 are also found; circulating PD-L1 microvesicles or its membrane-free soluble forms have been identified.62,63 Further investigation is required to identify the biological consequence of other forms of soluble PD-L1 and the molecular mechanisms that lead to their biogenesis and secretion to advance immunotherapy efficacy.
Regulation of exosomal PD-L1
Moon et al64 revealed that when CD8+ T lymphocytes release IFN-γ, PD-L1 is triggered by attaching to the interferon-gamma receptor. This activates JAK/STAT signaling, which then activates IRF-1, further increasing PD-L1 production on tumor cells. The promotional effect of IFN-γ worked similarly for exosomal PD-L1. IFN-γ has been proven not to enhance the number of vesicles from release but exhibit higher binding affinity to PD-1, and thus exosomal PD-L1 is stimulated by IFN-γ7,56. Tumor cells not only contain PD-L1 molecules directly on the cell membrane but also impede the CD8+ T cell activation to accomplish immune escape. Most importantly, tumor cells with high malignancy inhibit antitumor immunity by secreting PD-L1 enriched exosomes directly to the TME or through draining lymph nodes to distant places, targeting diverse effector T cells. However, once the exosomal PD-L1 is inhibited, the body may develop sustained robust anti-tumor immunity. Recent findings support the statement that PD-L1 release is partly facilitated by cytokine induction, likewise growth of exosomal PD-L1 release by IFN-α, IFN-γ, and TNF-α in melanoma and glioblastoma cells.56,62 Additional cytokines, such as TGF-β1 and IL-17, have also been linked with PD-L1 expression; however, their impact on exosomal PD-L1 is not fully clarified.65
Tumor immune escape partly results from adaptive tumor responses by the influence of a variety of factors. Micro-environmental factors including alterations in pH, nutrients, and oxygen concentration are closely associated with immunosuppression. Concerning these issues, tumor hypoxia has been recognized as an influential driving force of immune escape and tumor progression.66 The response to hypoxia is mediated by hypoxia-inducible factors (HIF) in low oxygen concentrations, which encourages the stabilization as well as nuclear translocation of HIF-1α to form the HIF1α/β complex which associates with STAT3 to stimulate PD-L1 expression.66,67 Hypoxia plays a crucial role in increasing exosome secretion in a HIF-1α-dependent manner.68 Moreover, hypoxia-mediated exosome release has been operated by Rab27a, which has also been associated with exosomal PD-L1 biogenesis.21,68 Consequently, hypoxia can promote exosomal PD-L1 release, and inhibiting the response to hypoxia by targeting HIF-1α and STAT3 can be a promising approach in both PD-L1 and exosome inhibition.
Along with TME factors, therapeutic interventions can also stimulate exosomal PD-L1 biogenesis and secretion. Zhang et al69 describe that the chemotherapeutic agent 5-fluorouracil (5-FU) enhances the expression of exosomal PD-L1 via the miR-940/Cbl-b/STAT5a axis in patients with advanced gastric cancer. Dosset et al70 highlighted that immunocyte-derived cytokines may rise PD-L1 expression after chemotherapy in colon cancer. Similarly, in a syngeneic model of prostate cancer, p300/CBP inhibition by a small molecule p300/CBP inhibitor intensely increased the efficacy of PD-L1 blockade treatment by hindering both the intrinsic and IFN-γ induced PD-L1 expression.71
Exosomal PD-L1 on CD8+ T cell dysfunction
Considering the tumor immune escape, CD8+ T cells have built up an exhausted phenotype owing to continuous antigen-mediated activation, inhibitory receptor signaling, metabolic dysfunction, and other microenvironmental factors.72 The application of immune checkpoint inhibitors is a promising approach to treatment for advanced tumors, mainly because inhibiting the PD-L1/PD-1 axis strengthens CD8+ T cell-mediated response which includes direct cytotoxic activity against tumor cells.73 Upon binding to PD-L1, PD-1 goes through a conformational change that leads to the phosphorylation of the immunoreceptor tyrosine-based inhibitory motif (ITIM) and the immunoreceptor tyrosine-based switch motif (ITSM), enhancing the enrollment of cytoplasmatic SHP-1 and SHP-2 protein tyrosine phosphatases.61 Afterward, SHP-1/2 inhibits the phosphorylation of intracellular mediators of the PI3K/AKT/mTOR and MAPK signaling pathways and consequently terminates CD8+ T cell activation61,74 (Fig. 1). However, CD8+ T cells target and induce tumor cell apoptosis through cytotoxic activity. Tumor cell-derived exosomes with high levels of PD-L1, COX-2, CTLA-4, CD15s, or CD44v3, can induce apoptosis in activated CD8+ T cells.75 Chen et al and Poggio et al7,56 depict that cancer cell secreting exosomes with PD-L1 inhibit CD8+ T cells to facilitate the progression of melanoma in vitro and in vivo, and additionally down-regulate CD8+ T cells activity in draining lymph nodes in prostate cancer. Moreover, exosomal PD-L1 from other cancer cell lines, such as colon (RKO) and lung (HCC827), has parallel inhibitory functions in CD8+ T cell activation.58
To describe the molecular basis of exosomal PD-L1 operated functional inhibition of CD8+ T cells, recent findings demonstrate that glioblastoma-derived exosomal PD-L1 blocks TCR-mediated T cell activation.76 Throughout the CD8+ T cell maturation, any failure in the TCR-CD3 complex may lead the defects in CD8+ T cell function and immunosuppressive clinical symptoms. The CD3-ζ chain in CD8+ T cells stimulates the signal transduction of the TCR-CD3 complex,77 as well as down-regulation of the CD3-ζ chain in CD8+ T cells, affects the cytotoxic functions.78 Theodoraki et al79 revealed that exosomal PD-L1 suppresses the CD8+ effector T cell-mediated activity. The amounts of PD-L1 in circulating exosomes were positively associated with the knack of exosomal PD-L1 to suppress the CD8+ effector T cell activation. Furthermore, the contribution of the PD-L1-mediated pathway in the negative regulation of surface expression of CD69 (a T cell activation marker) in activated CD8+ T cells after coincubation with exosomal PD-L1 has been confirmed as well. Similarly, exosomal PD-L1 suppresses CD3/CD28-operated CD8+ T cell activation signaling cascades. Researchers have found that exosomal PD-L1 significantly down-regulates CD3/CD28-induced ERK phosphorylation and NF-κB activation.58 In addition, melanoma cells release exosomal PD-L1 that can inhibit cytokine production and CD8+ T cell-mediated cytotoxicity by inhibiting the expression of GzmB and the production of IL-2 and TNF. IL-2 can augment CTL activation and survival through the Janus kinase 1 (JAK1)/JAK3-STAT5 pathway.7 These findings suggest that PD-L1 in exosomes effectively terminates CD8+ T cell activation, inhibits CD8+ T cell effector functions, down-regulates the number of CD8+ T cells, and induces immunosuppression in the TME. Hence, further research is required to elucidate the contribution of additional inhibitory ligands in extracellular vesicles and how they associate with PD-L1 function.
Exosomal PD-L1 in acquired resistance to anti-PD-1/PD-L1 therapy
The clinical significance of plasma circulating exosomal PD-L1 has been shown in patients with a wide variety of malignancies. There is outsized evidence that indicates the significant role of exosomal PD-L1 in the low patient response rate of anti-PD-L1/PD-1 therapy (Fig. 1). Most importantly, the abundance of circulating exosomal PD-L1 before treatment is responsible for resulting in lesser clinical response.7,79,80 Several preclinical studies indicated that the inhibition of exosomal PD-L1 secretion with GW4869, a selective inhibitor of nSMase 2, and GW4869 with ferroptosis inducer (Fe3+), or knocking down Rab27a and Smpd3 in tumor cells enhanced the efficiency of anti-PD-1 therapy.56,58,81 Likewise, FDA-approved drugs sulfisoxazole and macitentan are capable of reducing exosomal PD-L1 levels from breast cancer cells and xenograft models by targeting endothelin receptor A (ETA), and effectively reinvigorate exhausted CD8+ T cells and thus elicit robust antitumor effects in combination with anti-PD-1 antibody.82,83 Additionally, by decreasing the binding of PD-1 with exosomal PD-L1, macitentan enhanced CD8+ T cell-mediated killing activity.83 In another study, inhibition of exosomal PD-L1 level from the colorectal MC38 model can negatively regulate tumor growth and enhance the survival rate by PD-L1 blockage treatment.56 These findings indicated that the exosomal PD-L1 mediated resistance to current anti-PD-L1/PD-1 therapy, and several pharmaceutical agents have the capability to regulate the therapeutic resistance through improving antitumor efficacy. However, the detailed molecular mechanism underlying the therapeutic resistance of exosomal PD-L1 is still unknown.
Intracellular calcium in exosome formation and secretion
Calcium is a ubiquitous intracellular messenger in eukaryotic cells and plays a regulatory role from the origin of cells to cell death. The constricted regulation of Ca2+ homeostasis is a very sophisticated process, which is crucial for diverse cellular processes such as energy metabolism, cell signaling, and cell motility.84 From the perspective of the evolutionary process, some approaches had been augmented to control the Ca2+ homeostasis that includes the compartmentalization in organelles, such as endoplasmic reticulum, mitochondria, nucleus, and a number of ion channels and pumps playing crucial roles in this complex network.85, 86, 87 Mainly membrane-associated proteins are directly involved in calcium homeostasis: IP3R, the Sarco/endoplasmic reticulum and plasma membrane Ca2+-ATPases, ryanodine receptor (RyR), the store- and receptor-operated calcium channels (SOC and ROC), likewise ORAI and transient receptor potential channel (TRP), calcium release-activated calcium channel (CRAC), voltage-gated Ca2+ channels (VGCC), mitochondrial calcium channels, purinergic ionotropic receptors, the exchangers, such as the Na+/Ca2+ exchanger (NCX), and the mitochondrial Ca2+ uniporter (MCU).16,87, 88, 89, 90 The well-established issue is that the interruption at the cellular Ca2+ homeostasis is directly associated with the augmented free radical production, mitochondrial permeabilization, and numerous types of cell death,15,91 which may lead to tissue toxicity and various diseases including cancer.
In almost all cell types, exosome release is likely a regulated process where the intracellular Ca2+ concentration is essential to persuade this regulated secretion process. Savina et al29 showed that via binding with the receptor, transferrin increases exosome release in a calcium-dependent manner, which organizes a physiological stimulus for exosome secretion in K562 cells. The Rab family of small GTPases functions in the regulation of vesicle trafficking among diverse compartments along endo-lysosomal as well as secretory pathways. This report demonstrated that K562 cells have comparatively more massive extents of Rab 11 than the other 60 members of this protein family.92 Therefore, Rab11 plays a key role in exosome secretion by regulating the linking between endocytic, recycling, and secretory pathways of exosome release.22 Similarly, another group reported that treatment of transfected cells with a cytosolic calcium concentration increasing agent like monensin produced a marked widening of the MVBs and regulated exosome secretion in Rab11 overexpressing cells.23 This result suggests convergent action of Rab11 and calcium is responsible for generating larger MVBs.
Munc 13-4 is a significant Ca2+-dependent Rab binding protein expressed in numerous cells and tissues with secretory functions to regulate the trafficking of Rab 11-positive vesicles. It is categorized by the existence of two C2-domains at the N and C terminus and two central Munc-homology domains (MHC).27,93 The significance of these domains in the collaboration of Munc13-4 with SNARE proteins for stimulating membrane fusion has been described recently.94 Additionally, Munc13-4 is considered a key regulator of vesicle tethering and membrane fusion via promoting the Ca2+-stimulated fusion of VAMP8-containing liposomes with liposome-positive exocytic or endosomal Q-SNAREs and straightly correlated with late endosomal SNARE complexes.95 Messenger et al28 indicate that the acute raise of Ca2+ concentration in cancer cells stimulated a fivefold enhancement of CD63+, CD9+, and ALIX+ exosome secretion that was reduced by Munc13-4 knockdown. It was worth noting that Munc13-4 controlled MVB maturation and produced MVBs competent for exosome release by a Rab11-positive trafficking pathway.
Extracellular vesicles like exosomes and microvesicles are released by almost all cells into the extracellular space in both normal physiological and disease conditions. Exosomes are significantly responsible for cellular communications through the trafficking of cytosolic components and membrane proteins, from donor cells to recipient cells. However, the exosome-mediated transmission of such factors is associated with cancer development and progression.96 During cancer conditions, cells secrete extensive amounts of exosomes, and higher intracellular Ca2+ is responsible for tumor progression and metastasis, but the principle of this mechanism is unknown. Most importantly, elevated level of Ca2+ positively regulates exosome biogenesis and secretion machinery and finally lead to an excess amount of exosome release from cells (Fig. 1). Therefore, the discovery of approaches aiming at suppressing exosome biogenesis and secretion from tumor cells by targeting intracellular Ca2+ level may have important therapeutic implications.97
Pharmacological perspectives for inhibiting exosome biogenesis and secretion
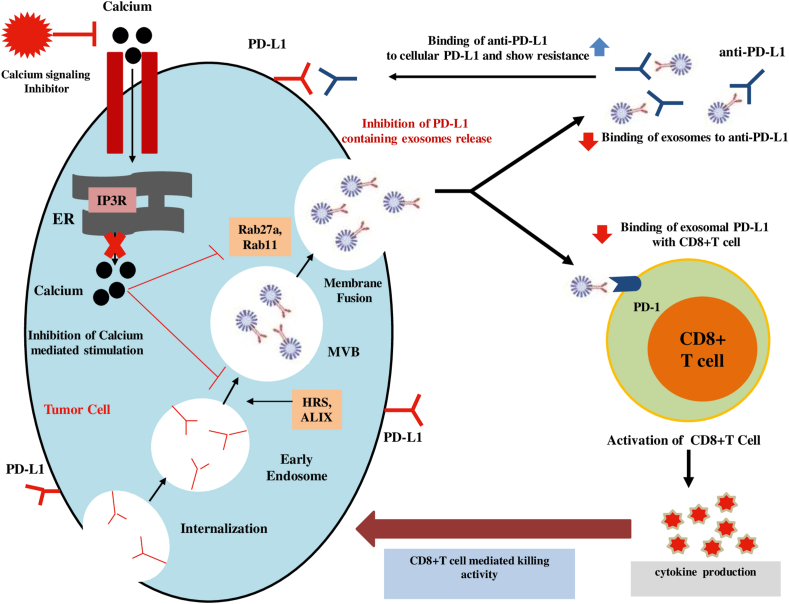
Inhibition of the generation and secretion of tumor-derived exosomes has risen as a novel and remarkable therapeutic approach for the advancement of anticancer drugs.2 Many findings have already depicted that the suppression of exosome secretion can be accomplished by antibodies,98 chemical inhibitors,99 or genetic manipulation100 that can enhance therapeutic efficacy in the management of metastatic cancer. The effective approaches to reducing exosome secretion are targeting molecules by numerous pharmacological agents which are responsible for their formation, packaging, and release of them (Fig. 2). Identification and development of a new inhibitor of exosome secretion as a novel anti-cancer therapeutic agent by using drug repositioning approach, a process of finding new indication of existing FDA-approved drugs, can reduce risk of cytotoxicity and safety because these drugs have already passed toxicity and safety tests in humans.101,102 For instance, the FDA-approved drug dimethyl amiloride (DMA) has been found to suppress exosome secretion by targeting H+/Na+ and Na+/Ca2+ channels and eliminate the exosome-mediated immune suppressive effects, and enhance anti-tumor immunity as a chemotherapeutic agent.29,32 It is obvious that ESCRT machinery is pivotal to exosome biogenesis, cargo sorting, and release. Other ESCRT-independent pathways, such as lipid-mediated and tetraspanin-mediated, also play a crucial role. However, several pharmacologically active compounds tipifarnib, neticonazole, climbazole, ketoconazole, triademenol, manumycin A, and nexinhibs showed exosome inhibitory effects by decreasing the protein expression involved in both ESCRT-dependent exosome biogenesis and transport (Rab27A).55,99,101,103 Additionally, exosome inhibitors targeting ESCTR-independent N-sphingomyelinase (nSMase), GW4869,104 and spiroepoxide105 have the ability to block the secretion of the exosomes.
Figure 2.
Proposed model of calcium signaling inhibitor-mediated enhancement of cancer immunotherapy. Calcium signaling inhibitors can inhibits the biogenesis and secretion of PD-L1-positive exosomes from cancer cells. Suppression of exosome secretion leads to the reduction of the interaction of exosomal PD-L1 with anti-PD-L1 antibodies and PD-1 on CD8+ T cells. Consequently, anti-PD-L1 get a chance to bind the PD-L1 on tumor cell and augments the activation of CD8+ T cells. Finally, calcium signaling inhibitor-mediated reinvigoration of exhausted CD8+ T cells can lead to tumor cell killing and improve the overall anti-PDL1-related immune checkpoint blockage.
Further possible options for suppressing exosome secretion are the use of store-operated calcium channel blocking agent ketotifen,106 proton pump inhibitors (PPIs),107 and simvastatin.108 Ketotifen was also presented to reduce the expression of several proteins which are associated with increased invasiveness of cancer cells and could decrease exosome release by controlling intra-cellular calcium levels.109 PPIs are extensively prescribed to mitigate gastric acid that impairs the secretion of acidic vesicles and vesicle-like structures from tumor cells through inhibiting vacuolar H+-ATPase-driven efflux pumps, and finally enhance the efficacy of chemotherapy.110
FDA-approved oral drugs sulfisoxazole and macitentan are capable of inhibiting the biogenesis and secretion of exosomes from breast cancer cells through interference with endothelin receptor A, a member of the G-protein coupled receptor (GPCR) family.83,102 Most importantly, both drugs have shown significant anti-tumor and anti-metastatic effects in breast cancer xenograft mouse model, and the reduced expression of proteins contributed to exosome formation and release and induced lysosomal degradation of multivesicular endosomes.55,83,102 Acute myeloblastic leukemia patients develop anti-cancer drug resistance due to altered drug targets, drug inactivation, reduced drug accumulation in the cancer cells, or alteration in the drug metabolic pathway and thus do not respond to treatment and experience cancer relapse. U937 cells showed resistance against the cytotoxic effect of the PEGylated liposomal doxorubicin drug through with PEGylated liposomal doxorubicin-containing chemotherapy regimens and found that the combination therapy significantly lowered the required concentrations of PEGylated liposomal doxorubicin as well as the drug side effects. Recently, one report showed that protein exchanger carbonic anhydrase IX (CA IX) plays a role in tumor pH regulation and overexpresses many cancer types. CA IX was found to be high in exosomes isolated from the plasma of prostate cancer patients suggesting that CA IX overexpression is also involved in exosome biogenesis and secretion. Therefore, CA IX inhibitor SLC-0111 is in a clinical trial. Cannabidiol, a phytocannabinoid extracted from Cannabis sativa, not only has analgesic and anti-inflammatory actions but also showed chemo-preventive actions. 5 μM cannabidiol can also block exosome secretion by 50% from prostate cancer cell PC3, hepatocellular carcinoma HEPG2, and breast adenocarcinoma MDA-MB-231.111,112 Leblanc et al113 characterized a small pharmacological inhibitor targeting the PDZ2 domain of syntenin. The chemical compound is non-toxic to MCF-7 breast carcinoma cells and can impair cell proliferation, migration, and primary sphere formation and decreased the amount of exosomal syntenin, ALIX, and syndecan 4 (PDZ partner of syntenin), and thus affected exosome composition and activity.
The risk of cytotoxicity can be reduced by employing the drug repositioning technique to identify and develop new inhibitors of exosome secretion as novel anti-cancer therapeutic agents because these medications have already passed toxicity and safety tests in humans.101,102 Therefore, the efficacy of the drug-repositioning strategy for preventing exosome secretion could provide a remarkable and safe approach for developing the immune inhibitory functions of exosomal-PD-L1 and therapeutic indications of metastatic cancer. Finally, designing appropriate clinical trials for the inhibition of exosomes to target cancers that have developed drug resistance is demanded.
Regulation of tumor microenvironment by calcium signaling
During tumor progression, cancer cells form a specific evolutionary process, that in conjugation with immune cells, extracellular matrix, and vascular endothelial cells, adapt to these changes. Together these components lead to tumor niche formation, which is called TME. As a crucial part of the ion channel, calcium channels play a significant role in tumorigenesis through the modification of the physic–chemical properties of TME.114 Immune responses are significant components in TME, which are inhibited during tumor formation, and calcium signaling is one of the responsible factors. Calcium has been designated as a significant messenger for T lymphocytes, which forms a complex with calmodulin called CaM. CaM's affinity for CaM's targets, such as the CaMK (Ca2+/CaM-dependent Ser-Thr kinase) family, is what activates calcium. Functional modulations of these kinases have been proven to be interconnected in the immune-suppressive nature in TME.115 Recent studies demonstrated that the regulation of calcium signaling on CD8+ T cells was mediated by CRAC channel STIM1 and STIM2,116 and IP3.117 Moreover, calcium signaling has been pointed out to regulate macrophage recruitment118 and NK cells.119 A detailed study on the molecular basis of the remodeling of immune response by calcium signaling could help improve the efficacy of immunotherapies as well as provide new insight into disease progression.
Excessive vascular growth is a vital sign of cancer, which support cancer cells to survive in TME. Many studies have described that calcium channels have a regulatory role in angiogenesis in different types of cancer. For instance, TRPV4 was released in excess amounts on tumor-derived endothelial cells and enhanced the angiogenic properties in breast cancer. However, TTRPV4-mediated calcium entry was responsible for the migration of endothelial cells, thus playing a regulatory role in angiogenesis.120 Hypoxia is an essential phenomenon in TME for the continual division and proliferation of cancer cells. In this process, calcium signaling has reported a broad interconnection with hypoxia-inducible factor (HIF). Several studies have described that HIF-1 is controlled by calcium signaling in different approaches, likewise (a) transcription of HIF-1α,121 (b) steadiness of HIF-1α (such as STIM1 in hepatocarcinoma,122 TRPM8 in prostate cancer,123 TRPC6 in glioma124), and (c) nuclear translocation of HIF-1α (such as TRPC5 in breast cancer125). Therefore, future research direction should focus on the modulation of both calcium signaling and HIF-1 pathways to control cancer progression.
Cancer-associated fibroblasts (CAFs) are the major element of the TME and take part in complex bidirectional association with tumor cells. Considering the broad contribution of CAFs towards cancer progression, proliferation, migration, genetic instability, and chemoresistance are associated with calcium signaling in CAFs and cancer.126 For example, an in vitro study aiming at the interaction between prostate cancer cells and CAFs pointed out that resveratrol activates the TRPA1 calcium channel in CAFs and leads to calcium influx and release of VEGF and HGF. Therefore, TRPA1 activation significantly down-regulates prostate cancer cell death. However, the use of TRPA1 antagonist expressively inhibited the calcium influx, VEGF, and HGF emission and obstructed the protective effects on prostate cancer.127 Plenty of studies have revealed the diverse role of calcium signaling in promoting tumorigenesis, as well as in the development of CAF features. There are still many unexplored issues of calcium signaling in CAF cancer progression.
Regulation of immune cells and immune checkpoints by calcium signaling
The transport of Ca2+ to the cytosol has paramount importance for immunoreceptor signaling, regulation of differentiation and activation of lymphocyte, antibody and cytokine secretion, and cytotoxicity.128 Several well-characterized and inter-connected mechanisms are elucidated to increase the cytosolic free Ca2+ concentrations: release of ER Ca2+ stores and store-operated Ca2+ entry via plasma membrane channel.128,129 Previously, Revy et al130 noted that naïve T cell TCR recognition of MHC molecules on dendritic cells triggers small Ca2+ responses which are crucial for their survival. Surh et al131 mentioned that Cav1.4 is a significant homeostasis regulator for naïve CD4+ and CD8+ T cells which represents that this channel modulates the signal required for their survival through connecting TCR signaling with self-peptide MHC molecules and IL-7R signaling after IL-7 exposure. Consequently, Omilusik et al132 hypothesized that the interactions of TCR with self-antigen induce naïve T cells to open Cav1.4 channel. They found that Ca2+ influx from outside the cell through Cav1.4 channel is responsible for inducing a signaling cascade and contributes to the filling of intracellular Ca2+ stores critical for TCR survival. Moreover, the effect of Ca2+ on facilitating CD3 phosphorylation is mostly due to the charge of ion, as highlighted by replacing Ca2+ with non-physiological ion that showed the same feedback effect. The regulatory pathway of Ca2+ has a positive feedback effect on amplifying CD3 phosphorylation that eventually enhances T-cell sensitivity to foreign antigens.133 Collectively, these studies describe the role of the Ca2+ channel in lymphocyte physiology through the regulation of intracellular Ca2+ storage, antigen receptor signal transduction, and effector function.
Over the past decade, immune checkpoint blockade therapies have made a remarkable promise in the management of metastatic cancers. Antibody drugs, like anti-PD-1 and anti-PD-L1, and antagonists targeting immune checkpoints, exhibit advantages such as extensive applicability through cancer types and long-lasting clinical response when treatment is effective.134 Remarkably, immune checkpoints are also connected with calcium signaling. Voltage-gated calcium ion trans-membrane channel subunits CACNA1E and CACNA1A are highly connected with tumor immunity and immune checkpoint expression regulation during tumorigenesis.135,136 Reduced cell GSH levels caused an increase in PD-L1 expression when glutamine was scarce by reducing SERCA activity, which triggers the calcium/NF-kB signaling cascade. As a result, T cell antitumor activity was reduced when glutamine metabolism was inhibited in immunocompetent mouse tumors.137 Additionally, suppression of proprotein convertases blocked proteolytic maturation of the Notch precursor, impaired calcium/NFAT and NF-κB signaling, and up-regulated ERK activation that lead to inhibition of PD-L1 expression.138 Transcription factor NFATc1 is activated by calcium signaling and TCR. NFATc1 is stimulated by targeting PD-1 in CD4+ and CD8+ T cells in tumor-bearing mice and connected with increased antitumor cytotoxic responses.139 Likewise, calcium fluctuations of CD8+ T cells are augmented by targeting the immune checkpoint LAG3 with a novel antibody TSR-033.140
CaMK1D-mediated treatment showed resistance to anti-PD-1/PD-L1. In those cancer types of anti-PD-1/PD-L1 treatment, CaMK1D is up-regulated by CTL and then down-regulated by the activation and functions of caspase-3, caspase-6, and caspase-7 and finally leads to treatment failure.141 Furthermore, the deficiency of TRAF3 hinders the auto-reactive B cell energy by enhancing calcium influx in response to BCR stimulation, which is responsible for autoimmune exhibitions and lymphoid organ disorders.142 The expression of CCR5 stimulates calcium signaling and thus elevates regulatory T cell differentiation and migration to inflammation sites and recent studies suggested that there has profound coaction between CCR5 and immune checkpoints function.143,144
Therapeutic approaches targeting calcium signaling and immune checkpoints
Immune checkpoint blockade therapy using antibodies to block receptor–ligand interaction has become a promising anti-cancer therapy for numerous cancer types. For example, the efficacy and survival rates of patients who are treated with nivolumab are recovered more than the patients cured with conventional chemotherapy.145,146 However, like other anti-cancer therapies immune checkpoint blockade therapy also faces great challenges, such as a low overall response rate. Therefore, recent findings have described some reasons behind the low patient response rate to immunotherapy. Firstly, there may be other factors or mechanisms responsible for immunosuppression in tumors. Secondly, the PD-1 antagonists showed signs of immunotherapy resistance and did not provide the elongated immune response, but reduced the efficacy of treatment.58,147 The existing strategy of immunotherapy can only object and block the PD-1/PD-L1 on the surface of tumor cells, which releases the exosomal PD-L1 to escape the immune response through binding to PD-1 on T cells to obstruct CD8+ T cell activation.58 Therefore, anti-exosomal PD-L1 therapy by using exosomal PD-L1 inhibitors may create systemic and robust anti-tumor immunity as well as may overcome the resistance of existing anti-PD-1/PD-L1 therapy.
Throughout this review, it has been presented that enhanced calcium signaling is largely connected with tumorigenesis by promoting malignant behaviors of cancer cells, contributes to numerous oncogenes and oncogenic signaling, exosome biogenesis and release, and suppression of immune response by regulating immune checkpoints. These observations altogether recommend that inhibition of calcium signaling can suppress the exosomal PD-L1 expression by impairing exosome secretion from cancer cells and that may be a promising strategy for cancer treatment by using immune checkpoint blockade therapy (Fig. 2). Therefore, all kinds of calcium-permeable ion channels or pumps seem to be natural targets. The application of calcium channel blockers in clinical practice has a long background. For instance, nifedipine is a first-line agent for hypertension.148 Subsequently, nifedipine can down-regulate colorectal cancer proliferation and metastasis by inhibiting the NFAT2 activation and nuclear translocation by reducing the calcium influx. Additionally, nifedipine can activate tumor immune response by repressing the expression of PD-L1 and PD-1.149 Another calcium channel blocker amlodipine can deplete PD-L1 expression and calpain-dependent stabilization of PD-L1 protein by impairing calcium flux and finally enhance CD8+ T cell-mediated immunity.150 Dihydropyridine calcium channel blockers (lercanidipine and nicardipine) and non-dihydropyridine calcium channel blockers (diltiazem and verapamil) suppress PD-L1 expression from the cytoplasm and cell surface by inhibiting the phosphorylation of STAT1, which is considered a key transcription factor of PD-L1.151,152 When cancer cells are infiltrated by CD8+ T cells, the PD-L1 expression is stimulated by IFN-γ, released by CD8+ T cells. However, lercanidipine can reduce the IFN-γ-induced PD-L1 transcription and finally enhance the CD8+ T cell-mediated killing activity.
Tumor cell surface PD-L1 and exosomal PD-L1 have the same topological structure and biological functions, which suggests that to regulate tumorigenesis, targeting exosomal PD-L1 would be an effective approach.7 Therefore, the leading strategy for targeting exosomal PD-L1 is to eliminate exosomes, including inhibition of exosome biogenesis and secretion. There are many methods regarding the suppression of exosome formation and secretion. Among them, targeting the calcium signaling or calcium channel by using calcium channel blockers will be the effective approach, because intracellular calcium level and calcium signaling are intensely connected with exosome biology, immune checkpoint regulation, and cancer progression. For example, ketotifen and simvastatin have been found to impair the secretion of exosomes.106,108 However, we need more research to investigate the use of other calcium channel blockers (amlodipine, nifedipine, lercanidipine, diltiazem, and verapamil) in the regulation of exosome secretion and exosomal PD-L1 expression as these pharmacological agents have already shown the inhibitory functions on cellular PD-L1 expression. Attention should be paid to observing the cytotoxicity of exosome inhibitors to normal cells. The combination of inhibition of exosome secretion and anti-PD-L1 antibody therapy could be more effective because exosomal PD-L1 characterizes a striking therapeutic target in immunotherapy resistance.
Author contributions
MRA, MMR, and ZL contributed equally to the conceptualization, writing, editing, and finalizing of the final version of the manuscript. ZL critically reviewed the final version of the manuscript.
Conflict of interests
The authors declared no conflict of interests.
Funding
The work was supported by the National Institutes of Health (No. R01 CA266579 to Zhiguo Li) and partially supported by the UK CARES Career Development Program (No. P30 ES026529) and the American Cancer Society (No. IRG 19-140-31).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Andaloussi S.E., Mäger I., Breakefield X.O., et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 3.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plebanek M.P., Angeloni N.L., Vinokour E., et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8:1319. doi: 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura K., Hohjoh H., Fukuoka M., et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9:17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gui Y., Liu H., Zhang L., et al. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G., Huang A.C., Zhang W., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie F., Xu M., Lu J., et al. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18:146. doi: 10.1186/s12943-019-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L., Chen S., Yang L., et al. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco L.M., Salinas V.H., Brown K.E., et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardhan K., Anagnostou T., Boussiotis V.A. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamimura N., Wolf A.M., Iwai Y. Development of cancer immunotherapy targeting the PD-1 pathway. J Nippon Med Sch. 2019;86(1):10–14. doi: 10.1272/jnms.JNMS.2019_86-2. [DOI] [PubMed] [Google Scholar]
- 13.Patsoukis N., Brown J., Petkova V., et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S.A., Niyazi H.E., Hong W., et al. Effect of EBI3 on radiation-induced immunosuppression of cervical cancer HeLa cells by regulating Treg cells through PD-1/PD-L1 pathway. Tumour Biol. 2017;39(3) doi: 10.1177/1010428317692237. [DOI] [PubMed] [Google Scholar]
- 15.Prevarskaya N., Skryma R., Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11(8):609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 16.Déliot N., Constantin B. Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848(10):2512–2522. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Colombo M., Moita C., van Niel G., et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 18.Jackson C.E., Scruggs B.S., Schaffer J.E., et al. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys J. 2017;113(6):1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abels E.R., Breakefield X.O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webber J.P., Spary L.K., Sanders A.J., et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowski M., Carmo N.B., Krumeich S., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 22.Savina A., Vidal M., Colombo M.I. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115(Pt 12):2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 23.Savina A., Fader C.M., Damiani M.T., et al. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 24.Gorji-Bahri G., Moghimi H.R., Hashemi A. RAB5A is associated with genes involved in exosome secretion: integration of bioinformatics analysis and experimental validation. J Cell Biochem. 2021;122(3–4):425–441. doi: 10.1002/jcb.29871. [DOI] [PubMed] [Google Scholar]
- 25.Park D.J., Yun W.S., Kim W.C., et al. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J Nanobiotechnol. 2020;18:178. doi: 10.1186/s12951-020-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu C., Morohashi Y., Yoshimura S.I., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J.L., He J., Ramadass M., et al. Munc13-4 is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J Biol Chem. 2016;291(7):3423–3438. doi: 10.1074/jbc.M115.705871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messenger S.W., Woo S.S., Sun Z., et al. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217(8):2877–2890. doi: 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariel S., Marcelo F., Michel V., et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 30.Isola A.L., Chen S. Exosomes: the link between GPCR activation and metastatic potential? Front Genet. 2016;7:56. doi: 10.3389/fgene.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahlert C., Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmin F., Ladoire S., Mignot G., et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacquemet G., Baghirov H., Georgiadou M., et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun. 2016;7:13297. doi: 10.1038/ncomms13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henne W.M., Stenmark H., Emr S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harbor Perspect Biol. 2013;5(9) doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mears R., Craven R.A., Hanrahan S., et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4(12):4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 37.Runz S., Keller S., Rupp C., et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107(3):563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 38.Ciravolo V., Huber V., Ghedini G.C., et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 39.Nazarenko I., Rana S., Baumann A., et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu M., Martin-Jaular L., Lavieu G., et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 41.Jadli A.S., Ballasy N., Edalat P., et al. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467:77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- 42.Mansoori B., Mohammadi A., Davudian S., et al. The different mechanisms of cancer drug resistance: a brief review. Adv Pharmaceut Bull. 2017;7(3):339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mostafazadeh M., Samadi N., Kahroba H., et al. Potential roles and prognostic significance of exosomes in cancer drug resistance. Cell Biosci. 2021;11:1. doi: 10.1186/s13578-020-00515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q., Yang Z., Nie Y., et al. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Wu Q., Zhou L., Lv D., et al. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. doi: 10.1186/s13045-019-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li I., Nabet B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song W., Yan D., Wei T., et al. Tumor-derived extracellular vesicles in angiogenesis. Biomed Pharmacother. 2018;102:1203–1208. doi: 10.1016/j.biopha.2018.03.148. [DOI] [PubMed] [Google Scholar]
- 48.Guo Y., Ji X., Liu J., et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39. doi: 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schubert A., Boutros M. Extracellular vesicles and oncogenic signaling. Mol Oncol. 2021;15(1):3–26. doi: 10.1002/1878-0261.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J., Wu S., Zheng X., et al. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep. 2020;10:14749. doi: 10.1038/s41598-020-71573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kugeratski F.G., Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021;288(1):10–35. doi: 10.1111/febs.15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wada J., Onishi H., Suzuki H., et al. Surface-bound TGF-beta1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions. Anticancer Res. 2010;30(9):3747–3757. [PubMed] [Google Scholar]
- 53.Elsner L., Muppala V., Gehrmann M., et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179(8):5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 54.Wolfers J., Lozier A., Raposo G., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 55.Yin Z., Yu M., Ma T., et al. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: a key role of exosomal PD-L1. J Immunother Cancer. 2021;9(1) doi: 10.1136/jitc-2020-001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poggio M., Hu T., Pai C.C., et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y., Zhang P., Wang Y., et al. The biogenesis, biology, and clinical significance of exosomal PD-L1 in cancer. Front Immunol. 2020;11:604. doi: 10.3389/fimmu.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y., Li C.W., Chan L.C., et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monypenny J., Milewicz H., Flores-Borja F., et al. ALIX regulates tumor-mediated immunosuppression by controlling EGFR activity and PD-L1 presentation. Cell Rep. 2018;24(3):630–641. doi: 10.1016/j.celrep.2018.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim D.H., Kim H., Choi Y.J., et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13. doi: 10.1038/s12276-019-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daassi D., Mahoney K.M., Freeman G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20(4):209–215. doi: 10.1038/s41577-019-0264-y. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X., Lang J. Soluble PD-1 and PD-L1:predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–97682. doi: 10.18632/oncotarget.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moon J.W., Kong S.K., Kim B.S., et al. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-18132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong P., Xiong Y., Yue J., et al. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. doi: 10.3389/fonc.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Fan Y., Che X., et al. 5-FU-induced up regulation of exosomal PD-L1 causes immune suppression in advanced gastric cancer patients. Front Oncol. 2020;10:492. doi: 10.3389/fonc.2020.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dosset M., Vargas T.R., Lagrange A., et al. PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. OncoImmunology. 2018;7(6) doi: 10.1080/2162402X.2018.1433981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J., He D., Cheng L., et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene. 2020;39(19):3939–3951. doi: 10.1038/s41388-020-1270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salmaninejad A., Valilou S.F., Shabgah A.G., et al. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234(10):16824–16837. doi: 10.1002/jcp.28358. [DOI] [PubMed] [Google Scholar]
- 75.Theodoraki M.N., Hoffmann T.K., Whiteside T.L. Separation of plasma-derived exosomes into CD3(+) and CD3(-) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol. 2018;192(3):271–283. doi: 10.1111/cei.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ricklefs F.L., Alayo Q., Krenzlin H., et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4(3):eaar2766. doi: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klibi J., Niki T., Riedel A., et al. Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 78.Taylor D.D., Gerçel-Taylor C., Lyons K.S., et al. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–5119. [PubMed] [Google Scholar]
- 79.Theodoraki M.N., Yerneni S.S., Hoffmann T.K., et al. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J., Zeng H., Zhang H., et al. The role of exosomal PD-L1 in tumor immunotherapy. Transl Oncol. 2021;14(5):101047. doi: 10.1016/j.tranon.2021.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang G., Xie L., Li B., et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat Commun. 2021;12:5733. doi: 10.1038/s41467-021-25990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin J.M., Lee C.H., Son S., et al. Sulfisoxazole elicits robust antitumour immune response along with immune checkpoint therapy by inhibiting exosomal PD-L1. Adv Sci. 2022;9(5) doi: 10.1002/advs.202103245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee C.H., Bae J.H., Choe E.J., et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics. 2022;12(5):1971–1987. doi: 10.7150/thno.68864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monteith G.R., McAndrew D., Faddy H.M., et al. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7(7):519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 85.Saris N.E., Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochem Mosc. 2005;70(2):187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 86.Capiod T. Cell proliferation, calcium influx and calcium channels. Biochimie. 2011;93(12):2075–2079. doi: 10.1016/j.biochi.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Marchi S., Pinton P. Alterations of calcium homeostasis in cancer cells. Curr Opin Pharmacol. 2016;29:1–6. doi: 10.1016/j.coph.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007;74:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 89.Prakriya M., Lewis R.S. Store-operated calcium channels. Physiol Rev. 2015;95(4):1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cui C., Yang J., Fu L., et al. Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: a potential target for cancer treatment. Br J Pharmacol. 2019;176(9):1190–1205. doi: 10.1111/bph.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orrenius S., Gogvadze V., Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 92.Zerial M., McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 93.Tan Y., Luo X., Lv W., et al. Tumor-derived exosomal components: the multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis. 2021;12(6):547. doi: 10.1038/s41419-021-03825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boswell K.L., James D.J., Esquibel J.M., et al. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol. 2012;197(2):301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woo S.S., James D.J., Martin T.F.J. Munc13-4 functions as a Ca2+ sensor for homotypic secretory granule fusion to generate endosomal exocytic vacuoles. Mol Biol Cell. 2017;28(6):792–808. doi: 10.1091/mbc.E16-08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Catalano M., O'Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1) doi: 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishida-Aoki N., Tominaga N., Takeshita F., et al. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther. 2017;25(1):181–191. doi: 10.1016/j.ymthe.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Datta A., Kim H., Lal M., et al. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017;408:73–81. doi: 10.1016/j.canlet.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bobrie A., Krumeich S., Reyal F., et al. Rab27a supports exosome-dependent and-independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 101.Datta A., Kim H., McGee L., et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep. 2018;8:8161. doi: 10.1038/s41598-018-26411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Im E.J., Lee C.H., Moon P.G., et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. 2019;10:1387. doi: 10.1038/s41467-019-09387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson J.L., Ramadass M., He J., et al. Identification of neutrophil exocytosis inhibitors (nexinhibs), small molecule inhibitors of neutrophil exocytosis and inflammation. J Biol Chem. 2016;291(50):25965–25982. doi: 10.1074/jbc.M116.741884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lallemand T., Rouahi M., Swiader A., et al. nSMase2 (type 2-neutral sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe-/- mice. Arterioscler Thromb Vasc Biol. 2018;38(7):1479–1492. doi: 10.1161/ATVBAHA.118.311208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takahashi A., Okada R., Nagao K., et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan F.M., Saleh E., Alawadhi H., et al. Inhibition of exosome release by ketotifen enhances sensitivity of cancer cells to doxorubicin. Cancer Biol Ther. 2018;19(1):25–33. doi: 10.1080/15384047.2017.1394544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye L., Zhu Z., Chen X., et al. The importance of exosomal PD-L1 in cancer progression and its potential as a therapeutic target. Cells. 2021;10(11):3247. doi: 10.3390/cells10113247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kulshreshtha A., Singh S., Ahmad M., et al. Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions. Sci Rep. 2019;9:16373. doi: 10.1038/s41598-019-52765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim H.J., Park M.K., Kim S.Y., et al. Novel suppressive effects of ketotifen on migration and invasion of MDA-MB-231 and HT-1080 cancer cells. Biomol Ther (Seoul) 2014;22(6):540–546. doi: 10.4062/biomolther.2014.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spugnini E.P., Citro G., Fais S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010;29(1):44. doi: 10.1186/1756-9966-29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kosgodage U.S., Mould R., Henley A.B., et al. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889. doi: 10.3389/fphar.2018.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kosgodage U.S., Matewele P., Awamaria B., et al. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front Cell Infect Microbiol. 2019;9:324. doi: 10.3389/fcimb.2019.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leblanc R., Kashyap R., Barral K., et al. Pharmacological inhibition of syntenin PDZ2 domain impairs breast cancer cell activities and exosome loading with syndecan and EpCAM cargo. J Extracell Vesicles. 2020;10(2) doi: 10.1002/jev2.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu L., Lian W., Zhao L. Calcium signaling in cancer progression and therapy. FEBS J. 2021;288(21):6187–6205. doi: 10.1111/febs.16133. [DOI] [PubMed] [Google Scholar]
- 115.Racioppi L., Nelson E.R., Huang W., et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat Commun. 2019;10:2450. doi: 10.1038/s41467-019-10424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weidinger C., Shaw P.J., Feske S. STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol Med. 2013;5(9):1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim K.D., Bae S., Capece T., et al. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat Commun. 2017;8:15365. doi: 10.1038/ncomms15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang S.H., Zhu L.L., Zhang M., et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut. 2019;68(11):1994–2006. doi: 10.1136/gutjnl-2018-317479. [DOI] [PubMed] [Google Scholar]
- 119.Maul-Pavicic A., Chiang S.C.C., Rensing-Ehl A., et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A. 2011;108(8):3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fiorio Pla A., Ong H.L., Cheng K.T., et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31(2):200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Azimi I., Milevskiy M.J.G., Kaemmerer E., et al. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J Cell Sci. 2017;130(14):2292–2305. doi: 10.1242/jcs.196659. [DOI] [PubMed] [Google Scholar]
- 122.Li Y., Guo B., Xie Q., et al. STIM1 mediates hypoxia-driven hepatocarcinogenesis via interaction with HIF-1. Cell Rep. 2015;12(3):388–395. doi: 10.1016/j.celrep.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 123.Yu S., Xu Z., Zou C., et al. Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2-independent and RACK1-mediated mechanism of HIF-1α stabilization. J Pathol. 2014;234(4):514–525. doi: 10.1002/path.4413. [DOI] [PubMed] [Google Scholar]
- 124.Li S., Wang J., Wei Y., et al. Crucial role of TRPC6 in maintaining the stability of HIF-1α in glioma cells under hypoxia. J Cell Sci. 2015;128(17):3317–3329. doi: 10.1242/jcs.173161. [DOI] [PubMed] [Google Scholar]
- 125.Zhu Y., Pan Q., Meng H., et al. Enhancement of vascular endothelial growth factor release in long-term drug-treated breast cancer via transient receptor potential channel 5-Ca2+-hypoxia-inducible factor 1α pathway. Pharmacol Res. 2015;93:36–42. doi: 10.1016/j.phrs.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 126.Sadras F., Monteith G.R., Roberts-Thomson S.J. An emerging role for calcium signaling in cancer-associated fibroblasts. Int J Mol Sci. 2021;22(21):11366. doi: 10.3390/ijms222111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vancauwenberghe E., Noyer L., Derouiche S., et al. Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF) Mol Carcinog. 2017;56(8):1851–1867. doi: 10.1002/mc.22642. [DOI] [PubMed] [Google Scholar]
- 128.Trebak M., Kinet J.P. Calcium signalling in T cells. Nat Rev Immunol. 2019;19(3):154–169. doi: 10.1038/s41577-018-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang P.C., Jafri M.S. Ca2+ signaling in T lymphocytes: the interplay of the endoplasmic reticulum, mitochondria, membrane potential, and CRAC channels on transcription factor activation. Heliyon. 2020;6(3) doi: 10.1016/j.heliyon.2020.e03526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Revy P., Sospedra M., Barbour B., et al. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2(10):925–931. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 131.Surh C.D., Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17(3):183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Omilusik K., Priatel J., Chen X., et al. The CaV1.4 calcium channel is a critical regulator of T cell receptor signaling and naive T cell homeostasis. Immunity. 2011;35(3):349–360. doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 133.Shi X., Bi Y., Yang W., et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493(7430):111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 134.He X., Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheng Q.J., Tian W.Y., Dou X.G., et al. Programmed death 1, ligand 1 and 2 correlated genes and their association with mutation, immune infiltration and clinical outcomes of hepatocellular carcinoma. World J Gastrointest Oncol. 2020;12(11):1255–1271. doi: 10.4251/wjgo.v12.i11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang X., Dong Y. CACNA1C is a prognostic predictor for patients with ovarian cancer. J Ovarian Res. 2021;14:88. doi: 10.1186/s13048-021-00830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Byun J.K., Park M., Lee S., et al. Inhibition of glutamine utilization synergizes with immune checkpoint inhibitor to promote antitumor immunity. Mol Cell. 2020;80(4):592–606.e8. doi: 10.1016/j.molcel.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 138.Tomé M., Pappalardo A., Soulet F., et al. Inactivation of proprotein convertases in T cells inhibits PD-1 expression and creates a favorable immune microenvironment in colorectal cancer. Cancer Res. 2019;79(19):5008–5021. doi: 10.1158/0008-5472.CAN-19-0086. [DOI] [PubMed] [Google Scholar]
- 139.Heim L., Friedrich J., Engelhardt M., et al. NFATc1 promotes antitumoral effector functions and memory CD8+ T-cell differentiation during non-small cell lung cancer development. Cancer Res. 2018;78(13):3619–3633. doi: 10.1158/0008-5472.CAN-17-3297. [DOI] [PubMed] [Google Scholar]
- 140.Sullivan M.R., Ugolini G.S., Sarkar S., et al. Quantifying the efficacy of checkpoint inhibitors on CD8+ cytotoxic T cells for immunotherapeutic applications via single-cell interaction. Cell Death Dis. 2020;11(11):979. doi: 10.1038/s41419-020-03173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Volpin V., Michels T., Sorrentino A., et al. CAMK1D triggers immune resistance of human tumor cells refractory to anti-PD-L1 treatment. Cancer Immunol Res. 2020;8(9):1163–1179. doi: 10.1158/2326-6066.CIR-19-0608. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z., Krinsky A., Woolaver R.A., et al. TRAF3 acts as a checkpoint of B cell receptor signaling to control antibody class switch recombination and anergy. J Immunol. 2020;205(3):830–841. doi: 10.4049/jimmunol.2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiao X., Nawab O., Patel T., et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res. 2019;79(19):4801–4807. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Escandon Brehm J., Bedogni B. Blockade of CCR5 in melanoma: an alternative immune checkpoint modulator. Exp Dermatol. 2020;29(2):196. doi: 10.1111/exd.14065. [DOI] [PubMed] [Google Scholar]
- 145.Sharma P., Allison J. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ferrari R., Pavasini R., Camici P.G., et al. Anti-anginal drugs-beliefs and evidence: systematic review covering 50 years of medical treatment. Eur Heart J. 2019;40(2):190–194. doi: 10.1093/eurheartj/ehy504. [DOI] [PubMed] [Google Scholar]
- 149.Wu L., Lin W., Liao Q., et al. Calcium channel blocker nifedipine suppresses colorectal cancer progression and immune escape by preventing NFAT2 nuclear translocation. Cell Rep. 2020;33(13):108582. doi: 10.1016/j.celrep.2020.108582. [DOI] [PubMed] [Google Scholar]
- 150.Li C., Yao H., Wang H., et al. Repurposing screen identifies amlodipine as an inducer of PD-L1 degradation and antitumor immunity. Oncogene. 2021;40(6):1128–1146. doi: 10.1038/s41388-020-01592-6. [DOI] [PubMed] [Google Scholar]
- 151.Pan X., Li R., Guo H., et al. Dihydropyridine calcium channel blockers suppress the transcription of PD-L1 by inhibiting the activation of STAT1. Front Pharmacol. 2021;11:539261. doi: 10.3389/fphar.2020.539261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alam M.R., Kim J.I., Lee C.H., et al. Characterization of immune checkpoint inhibition efficacy through inhibition of exosome secretion by calcium channel blockers in melanoma. Yakhak Hoeji. 2021;65(4):313–320. [Google Scholar]