Abstract
Ferroptosis is a novel form of regulated cell death characterized by iron-dependent excessive lipid peroxidation. The core organelle involved in ferroptosis is mitochondria. Mitochondria undergoing ferroptosis are distinct from normal mitochondria in terms of morphology, biochemistry, gene expression, and energy metabolism. An increasing number of studies have shown that mitochondria and their associated metabolic pathways mediate ferroptosis in the development and progression of breast cancer. In this review, we discuss the relevant research about ferroptosis in breast cancer and provide a comprehensive summary of mitochondrial regulation in ferroptosis from the perspective of lipid metabolism, oxidative phosphorylation, ion metabolism, glycometabolism, and nucleotide metabolism. We also summarize the application of mitochondrial metabolism-related pathways as ferroptosis treatment targets. Here we provide new insights into the relationship between mitochondria, ferroptosis, and breast cancer treatment.
Keywords: Breast cancer, Cancer treatment, Ferroptosis, Lipid metabolism, Mitochondria, Oxidative phosphorylation
Introduction
Breast cancer is the most common malignancy in women. According to GLOBOCAN2020, there are 2.26 million new cases of breast cancer and more than 680,000 deaths worldwide, which calls for more concern. Despite a large number of funds and staffing invested in breast cancer research, the etiology of breast cancer is not yet fully understood, and treatment resistance and early recurrence remain unresolved. Classical oncology theory considers that cancer cell growth relies not on mitochondrial aerobic respiration but on glycolysis to maintain energy metabolism while studies on the role of mitochondria in breast cancer are still under debate. The term ferroptosis was proposed in 2012 to refer to a novel form of regulated cell death,1 raising the upsurge in the research of mitochondria associated with oxidative stress and iron ion regulation.2 Herein, we focus on mitochondria to elaborate the regulation of ferroptosis by various metabolic pathways involved in mitochondria, conclude the role of mitochondria-related ferroptosis in the disease development and treatment of breast cancer, and shed new light on the relationship between mitochondria and ferroptosis in breast cancer.
Overview of ferroptosis
Iron is an essential trace element that plays a vital role in maintaining metabolisms, such as participation in oxygen transport, DNA biosynthesis, and ATP synthesis.3 Iron is also an essential mediator of regulated cell death. Ferroptosis is characterized by the accumulation of lipid peroxidation resulting from iron ion pooling and is morphologically characterized by the smaller mitochondrial size, decrease or disappearance of mitochondrial cristae, and enhanced mitochondrial membrane density under electron microscopy.4 Ferroptosis is exogenously regulated by the cystine/glutamate transport system (system xc−) and the iron transport system, and endogenously regulated by intracellular antioxidant enzymes such as glutathione peroxidase 4 (GPX4).5,6 Various cellular energy metabolic pathways through mitochondria are involved in the regulation of ferroptosis.
Mitochondria are the primary source of reactive oxygen species (ROS), and about 90% of ROS derived from mitochondria in eukaryotes.7 System xc− on the cell membrane exports intracellular glutamate in exchange for extracellular cystine and oxidizes it to cysteine,8 which in turn forms glutathione (GSH) by combining with glutamate and glycine.9 GSH acts as a reducing agent to form oxidized glutathione (GSSG) via GPX4 and, in turn, reduces lipid peroxides (L-OOH) to the corresponding lipid alcohols (L-OH).10 On the other hand, extracellular ferric ions (Fe3+) were transported into cells via transferrin (TFR) and then reduced to ferrous ions (Fe2+),11 which combine with L-OOH in a Fenton reaction to oxidize Fe2+ to Fe3+ again and generate many hydroxyl radicals (•OH).12 Polyunsaturated fatty acids (PUFAs) on the mitochondrial membrane bind to •OH to generate PUFA radicals (PUFA•). These PUFA• are not stable and rapidly oxidized to PUFA peroxyl radicals (PUFA-OO•) and finally to PUFA hydroperoxides (PUFA-OOH).13 These molecules with peroxidation capacity in the cascade reactions above all belong to ROS.14 The accumulation of toxic lipid reactive oxygen species (L-ROS) destroys the phospholipid bilayers,15 ultimately leading to cell death16,17 (Fig. 1). Ferroptosis has been reported to be associated with neurodegenerative diseases (e.g., Alzheimer's syndrome, Huntington's chorea, etc.), cardiomyopathies, and various cancers.18, 19, 20
Figure 1.
Overview of ferroptosis. The schematic figure shows that the canonical ferroptosis pathway is executed by phospholipid peroxidation associated with oxidative stress and iron ion regulation. Cys, cysteine; Glu, glutamate; Gly, glycine; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, oxidized glutathione; L-OH, lipid alcohols; L-OOH, lipid peroxides; L-ROS, lipid reactive oxygen species; PUFA, polyunsaturated fatty acid; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; SLC40A1, solute carrier family 40 member 1; TFRC, transferrin receptor.
Mitochondria-related signaling pathways involved in ferroptosis
Mitochondria are known as the “powerhouse” of cells. Mitochondria contain high iron levels.21 Ferroptosis leads to significant morphological change and dysfunction in mitochondria.4 As the energy metabolism center of fatty acid oxidation (FAO), tricarboxylic acid cycle (TCA cycle), and electron transfer chain (ETC), mitochondria and their related metabolic pathways play an essential role in ferroptosis (Fig. 2).
Figure 2.
Mitochondria-related signaling pathways in regulating ferroptosis. Mitochondria-related metabolic pathway crosstalk plays an essential role in ferroptosis. Enzymes in lipid metabolism and lipids themselves are involved in ferroptosis regulation, which interacts with the glycolysis and TCA cycle. Intermediate products in the TCA cycle activate and further promote ferroptosis. ETCs in mitochondria drive proton motive force and provide O2•− and thereby influence ferroptosis. In addition, the NADPH as an electron donor during the process of oxidative phosphorylation, glycolysis, and lipid metabolism also takes part in ferroptosis. Besides. Fe3+ and Ca2+ are critical in maintaining mitochondrial membrane stability. Genetic disorders and mtDNA stress could cause iron overload and subsequent ferroptosis. α-KG, α-ketopentane; AMPK, AMP-activated protein kinase; cGAS, cyclic GMP-AMP synthase; CoQ, coenzyme Q; CoQH2, reduced ubiquinone; FBP, fructose 1,6-bisphosphate; FSP1, ferroptosis suppressor protein 1; FtMt, mitochondrial ferritin; GOT1, glutamic-oxaloacetic transaminase 1; GSH, glutathione; GSSG, oxidized glutathione; HK II, anti-hexokinase II; LOX, lipoxygenase; MCU, mitochondrial calcium uniporter; mtDNA, mitochondrial DNA; MUFA, monounsaturated fatty acid; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; O2•−, superoxide; PEP, phosphoenolpyruvate; PFKP, platelet-type phosphofructokinase; PKM2, pyruvate kinase; PUFA, polyunsaturated fatty acid; SCD1, stearoyl coenzyme A desaturase; SOD, superoxide dismutase; STING, stimulator of interferon response cGAMP interactor; TCA, tricarboxylic acid; TFR(C), transferrin; VDAC, voltage-dependent anion-selective channel.
Lipid metabolism regulation in ferroptosis
The synthesis, storage, and degradation of fatty acids are dynamic processes. Fatty acid degradation is initially activated in the cytoplasm to form lipid coenzyme A and then transported into the mitochondria for β-oxidation. Lipid peroxidation in ferroptosis belongs to fatty acid degradation metabolism,22 and mitochondria are the sites providing coenzyme A for lipid peroxidation.23 Stearoyl coenzyme A desaturase (SCD1) is the rate-limiting enzyme that converts saturated fatty acids to monounsaturated fatty acids. Blocking SCD1 reduced the lipid antioxidant coenzyme Q10 in the mitochondrial electron transport chain and increased pro-apoptotic ceramide, thus inducing cellular ferroptosis and apoptosis.24 15-lipoxygenases (15-LOX) is an instrumental proinflammatory lipid peroxidative enzyme which oxidizes polyunsaturated fatty acids,25 and it has been reported to damage the mitochondria directly.26
15-LOX accelerates the induction of ferroptosis by increasing the rate of lipid peroxidation through iron-catalyzed free radical reactions.27 In addition to some enzymes involved in lipid metabolism, certain lipids can directly or indirectly alter the mitochondrial membrane lipid peroxidation level to facilitate or restrain ferroptosis. LOX promotes the synthesis of phosphatidylethanolamine (PE), which could induce ferroptosis, whereas tocopherols and tocotrienols can inhibit LOX and ferroptosis.28 Exogenous monounsaturated fatty acids (MUFA) can inhibit iron death caused by Erastin and RSL3.29 In summary, LOX and its corresponding lipids are crucial in initiating lipid peroxidation in ferroptosis. In contrast, inhibiting fatty acid β-oxidation with the ATP synthesis inhibitor etomoxir or oligomycin restores the sensitivity of renal cancer cells to ferroptosis.30
Oxidative phosphorylation regulation in ferroptosis
Oxidative phosphorylation proceeds in the inner mitochondrial membrane (IMM) of eukaryotic cells, where electrons from ETC complexes I and III on the IMM generate superoxide (O2•−) and are converted to hydrogen peroxide (H2O2).31 H2O2 is a potent oxidizing agent, which oxidizes Fe2+ to Fe3+ and generates hydroxyl radicals to participate in ferroptosis. Inhibition of ETC complexes I–IV decreases the accumulation of lipid peroxides in Erastin-induced ferroptosis.32 Reduced coenzyme II (nicotinamide adenine dinucleotide phosphate, NADPH) is also a critical electron donor in the ETC redox reaction and participates in the pentose phosphate pathway of glycometabolism. Pharmacogenomic studies of compounds with high cell-line-selective lethality reveal that intracellular NADPH abundance could predict cellular sensitivity to ferroptosis inducers. The underlying mechanism of resistance to ferroptosis inducers might be associated with ferroptosis-induced NADPH reduction.33 Consistent with the high-throughput sequencing results, NADPH was also found to assist the ferroptosis regulator ferroptosis suppressor protein 1 (FSP1) to reduce coenzyme Q10 as a radical-trapping antioxidant that prevents lipid peroxidation propagation,34, 35, 36 and NADPH produced from mitochondria may also halt lipid peroxidation and inhibit ferroptosis.37
Ion metabolism regulation in ferroptosis
In mitochondria, iron and calcium are predominant ions involved in ferroptosis. Mitochondria are the center of cellular iron metabolism, and iron ions are imported into mitochondria via the transferrin receptor on the mitochondrial membrane. The ferroptosis inducer Erastin binds to the voltage-dependent anion-selective channel (VDAC) on the mitochondrial membrane, increasing VDAC permeability and mitochondria generate NADPH oxidase 2 (NOX2)-related ROS, which leads to calcium overload,38 and mitochondrial membrane potential increase along with depolarization, and eventually causes ferroptosis due to the loss of mitochondrial membrane stability.39 Ferroptosis can also be mitigated by depletion of serum TRF or knockdown of the mitochondrial transferrin receptor.40 Iron ion plays a pivotal role in heme production, and mitochondrial sideroflexin 2 (SFXN2) is also engaged in heme production. The knockdown of SFXN2 increases intracellular iron and accelerates the process of ferroptosis.41 Mitochondrial heme oxygenase 1 (Hmox1) is significantly up-regulated in murine cardiac cells after Adriamycin treatment, resulting in heme degradation, Fe3+ accumulation, and finally induced ferroptosis. Reversely, mitochondria-targeted antioxidant MitoTEMPO significantly rescued Adriamycin-induced cardiomyopathy.42 Mitochondrial ferritin (FtMt) could regulate iron redistribution between mitochondria and cytoplasm, maintaining mitochondrial iron homeostasis.43 Once the ferrous iron levels were increased, the production of ROS generated, resulting in cellular macromolecule damage and finally triggering cell death, especially in some oxygen-dependent tissues such as the brain.44 Iron and calcium (Ca2+) in mitochondria also crosstalk with each other through ROS signaling.45 VDAC permeability increases during ferroptosis, and the iron overload leads to excess Ca2+ entry into mitochondria, resulting in mitochondrial Ca2+ overload and disrupting mitochondrial function.32 This is observed in neurodegenerative diseases such as Huntington's disease, Parkinson's disease, and Alzheimer's disease,46 addressing the crucial role of ferroptosis. In contrast, the calcium overload also engaged in cellular antioxidant defense and ROS generation,47 disturbing the mitochondrial iron and reactive oxygen homeostasis.48 What's more, Ca2+ chelators reduce mitochondrial iron uptake and suppress either glutamine-induced or cysteine-deprivation-induced ferroptosis.1,49 Overall, the imbalance between iron and calcium was the key factor of disease,50 indicating the potential role of their crosstalk in the development of ferroptosis.
Glycometabolism regulation in ferroptosis
Glycolysis is the first step of glycometabolism, and the TCA cycle is the third stage of aerobic respiration in eukaryotes. Warburg effect suggests that cancer cells metabolize most of their glucose via glycolysis by dysregulating mitochondrial function, while the ability of glycolysis is reduced when ferroptosis occurs.1 RSL3 inhibited glycolysis by decreasing the production of three kinases in glycolysis: anti-hexokinase II (HKII), platelet-type phosphofructokinase (PFKP), and pyruvate kinase (PKM2).51 This may be due to the increased permeability of VDAC, which also increases the entry of most metabolic raw materials, such as glutamate52 and ATP53 into the mitochondria. α-ketopentane (α-KG), an essential intermediate metabolite in the TCA cycle54 links the TCA cycle to ferroptosis. α-KG can replace glutamine as a reducing agent in the oxidation reaction,55 causing lipid ROS accumulation and ferroptosis. In addition, the downstream metabolites of α-KG in the TCA cycle, such as succinate, fumarate, and malate, can all be substitutes for glutamine in ferroptosis.32 In contrast, the upstream metabolites of α-KG are impacted more by glucose than by glutamine.32 In addition to α-KG, acetyl coenzyme A in the TCA cycle also affects ferroptosis. Lipid ROS levels can be inhibited by induction or simulation of energy stress through the cellular energy sensor AMP-activated protein kinase (AMPK) activation, which diminishes PUFA synthesis.56
Nucleotide metabolism regulation in ferroptosis
Mitochondria also contain a small amount of mitochondrial DNA (mtDNA). mtDNA depletion syndrome is a recessive genetic disorder caused by gene mutations, one subtype of which is the deoxyribonucleoside activating enzyme (DGUOK) mutation. Iron overload and the subsequent ferroptosis were found in patients with DGUOK mutation, which could lead to severe liver failure.57 Apart from genetic disorders, zalcitabine, a nucleotide analog used as an antiviral drug, can activate the cGAS-STING1 pathway by inducing mtDNA stress, and cause autophagosome formation, leading to autophagy-dependent ferroptosis via lipid peroxidation.58
Mitochondria-related pathways regulate ferroptosis in breast cancer
The effect of ferroptosis on the tumorigenesis of breast cancer
The growth of cancer cells depends on abnormal energy metabolism. Cancer cells are under persistent metabolic disturbance, such as oxidative stress caused by redox reactions of iron ions and thiol. Thus they are more likely to evade ferroptosis than normal cells.59 Normal breast epithelial cells lack α6β4 integrin, which inhibits the extracellular matrix (ECM), thereby maintaining cells susceptive to ferroptosis because of GPX4 suppression. However, breast cancer cells express α6β4 integrin, protecting them from ferroptosis.60
Different susceptibilities to ferroptosis according to subtypes of breast cancer
Breast cancer can be divided into subtypes based on hormone receptors and human epidermal growth factor receptor 2 (HER2) status. Triple-negative breast cancer (TNBC) cell lines (MDA-MB-231, MDA-MB-468, MDA-MB-157) were more sensitive to RSL3 than other subtypes.4 This may be interpreted that TNBC is more susceptible to cystine starvation, and system xc− also depends on cystine/glutamate metabolism. Thus low cystine levels are more likely to induce ferroptosis in TNBC.61 Further preclinical and clinical studies revealed that the positive regulator of ferroptosis, acyl-coenzyme A synthetase long-chain family member 4 (ASCL4), has higher expression in TNBC than other subtypes,62 therefore, enhancing cellular sensitivity to ferroptosis.4 Corresponding to this, ROS levels were significantly higher in TNBC than in hormone receptor-positive breast cancers, which originate mainly from mitochondria.63 Iron deficiency activates the Notch pathway, triggering epithelial–mesenchymal transition (EMT) in mouse TNBC cells. CD44-mediated cellular iron endocytosis also increases iron-dependent demethylase activity, triggering EMT, and further facilitating cancer cell metastasis.64 In addition, ferroptosis resistance is a feature of metastatic breast cancer cells, and GPX4 knockdown inhibited the tumorigenic and metastatic activity of TNBC cell line 4T1 in 27-hydroxycholesterol-resistant mice.65 Tumor-associated macrophages modulate human hepatic leukemia factor and transactivated gamma glutamyltransferase 1 to promote ferroptosis resistance, resulting in TNBC proliferation, metastasis, and cisplatin resistance.66 In general, inducing ferroptosis in breast cancer, especially in TNBC, offers a new idea for future treatment.
Targeting mitochondria-specific regulation to induce ferroptosis in breast cancer
There are various therapies according to breast cancer molecular subtypes, including endocrine therapy, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. The current targets for ferroptosis in breast cancer treatment mainly focus on critical regulators of endogenous and exogenous regulatory pathways: inhibition of system xc−, inhibition of GPX4, inhibition of Fe3+, or inhibition of upstream regulatory factors of ferroptosis such as p53.67 There are relatively few treatments targeting mitochondria, leaving a vast space for research. In particular, regulating various metabolic pathways involved in mitochondria may become a new direction for breast cancer treatment in the future (Fig. 3).
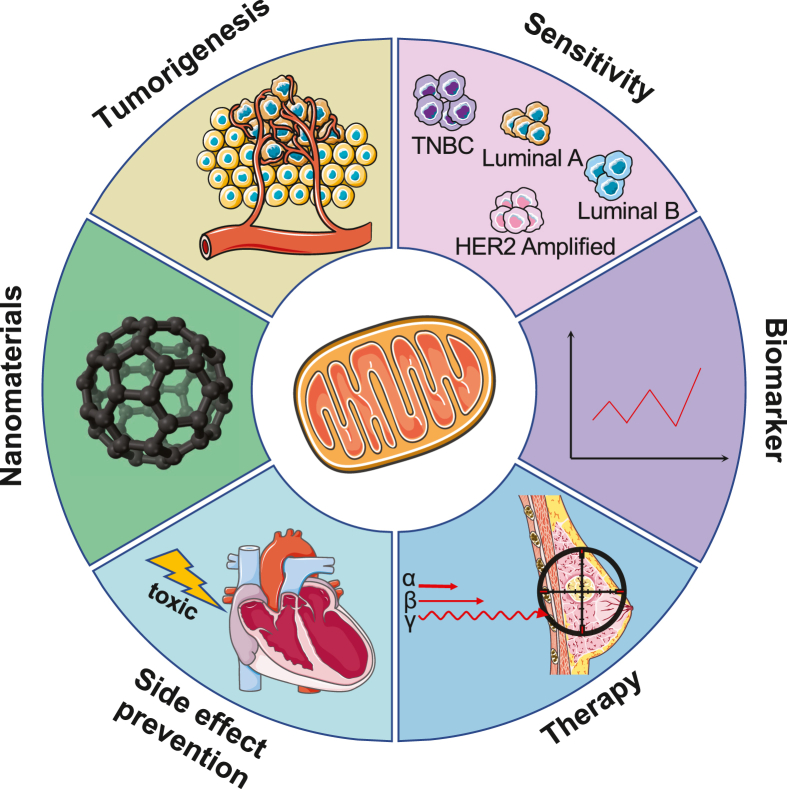
Figure 3.
Applications of targeting mitochondrial metabolism in ferroptosis on the diagnosis and treatment of breast cancer. Mitochondria-specific regulations have a pivotal impact on virtually all aspects linked to the diagnosis and treatment of breast cancer: encompassing malignant transformation, breast cancer heterogeneity, biomarker monitoring, response to treatment, anticancer side effect surveillance, and nanomaterials research and development. HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Lipid metabolism as therapy targets
Lipid metabolism is the most critical mitochondrial metabolic pathway in ferroptosis. Therefore, there is a wide range of applications in breast cancer diagnosis and treatment targeting the regulation of mitochondrial lipid metabolism. Natural substance DMOCPTL, a derivative of parthenolide, was highly lethal to TNBC cells, exhibiting 15 times more than its parent compound PTL. DMOCPTL directly binds GPX4 and induces GPX4 ubiquitination, leading to mitochondria-mediated apoptosis by regulating early growth response 1.68 It has also been reported that SCD1 expression can be used as a biomarker for breast cancer recurrence.69 Herceptin, a HER2-targeted drug for breast cancer, increased mitochondrial ROS levels in rat cardiomyocytes and decreased GPX4 expression, inducing ferroptosis. The addition of the ferroptosis inhibitor ferrostatin-1 reversed the elevation of ROS level.70 This finding suggests that ferroptosis inhibitors could be used to prevent cardiotoxicity in HER2+ breast cancer targeted therapy.
Oxidative phosphorylation as therapy targets
Ferroptosis regulated by mitochondrial oxidative phosphorylation has less application in breast cancer treatment, which is just used as a biomarker for efficacy assessment. Manganese superoxide dismutase 2 (SOD2) is responsible for depleting oxygen radicals generated in ETC and converting them to H2O2.71 SOD2 expression is significantly elevated in advanced and invasive breast cancers and can be used as a biomarker of breast cancer progression.72 Breast cancer radiotherapy increases iron accumulation and ROS generation via increasing lysosomal membrane permeability. At the same time, overexpression of SOD2 can decrease cellular ROS levels, autophagy, and cell death, resulting in poor efficacy of radiotherapy.73 MtDNA deletion and mutations have been demonstrated in various cancers, including breast cancer.74 However, it remains unclear how to utilize the alteration in mtDNA in cancer therapy. MtDNA mutation frequency increases with aging like nuclear DNA, and mtDNA deletions and mutations are mainly related to respiratory chain dysfunction. Thus, mtDNA is also considered an indicator of oxidative damage. An analysis of lipid ROS and DNA in blood samples from breast cancer patients revealed higher levels of oxidative stress and damage compared to healthy controls and a higher level of SOD2, suggesting that SOD2 may protect mtDNA from ROS damage in breast cancer.75 It illustrates that the bidirectional role of SOD2 in breast cancer is dual considering the application of SOD2 in oxidative phosphorylation-regulated ferroptosis in breast cancer: SOD2 expression may protect mtDNA in breast cancer patients, and it also needs to be noted that SOD2 overexpression has the potential to affect the efficacy of breast cancer radiotherapy. But in general, there is no doubt about the role of SOD2 as an inhibitor of ferroptosis in breast cancer treatment.76 Overall, free radicals are directly generated in oxidative phosphorylation, which is an essential intermediate in ferroptosis induction. Therefore, increasing the specificity and sensitivity of mitochondrial oxidative phosphorylation-regulated ferroptosis to target tumor cells precisely is a challenging problem that remains to be solved.
Ion metabolism as therapy targets
The ions involved in regulating mitochondrial ferroptosis are mainly iron and calcium. Calcium is primarily used as a chelator to inhibit ferroptosis, thus, is mostly applied in neuropathy to protect cells from ferroptosis rather than to promote ferroptosis. The ion-related breast cancer treatment is mainly focused on iron-related targeted drugs. The newly discovered iron-sulfur proteins CISD1 and CISD2 have the function of promoting breast cancer proliferation.77 Knocking down CISD1 and CISD2 or targeting breast cancer mitochondria with the mitogen derivative MAD-28 can disrupt iron-sulfur proteins, increase iron accumulation in mitochondria, and inhibit breast cancer cell growth.78 MAD-28 is also highly selective, with iron accumulation in mitochondria occurring only in breast cancer cells. In contrast, normal breast cells are not affected by MAD-28, suggesting that MAD-28 may be a potential targeting agent for the induction of ferroptosis and anticancer therapy.
Nanomaterials as therapy targets
With the development of pharmacological technologies, there are many applications of new nanomaterials in breast cancer treatment due to their high efficiency and safety, which also includes the use of mitochondria-specific nanomaterials loaded with ferroptosis drugs. Sorafenib is an oral multi-kinase inhibitor with antitumor efficacy. Studies have shown that sorafenib induces ferroptosis and the depletion of intracellular iron stores is associated with sorafenib resistance.79 Mitochondrial membrane-anchored oxidation/reduction response and Fenton-Reaction-Accelerable magnetic nanophotosensitizer complex self-assemblies loading sorafenib (CSO-SS-Cy7-Hex/SPION/Srfn) generates lipid peroxide burst, enhancing the ferroptosis-inducing therapeutic efficacy of sorafenib by 18-fold in breast cancer cells.80 Another ferroptosis drug, simvastatin, achieved high efficacy through a feat of new nanomaterials as well. Simvastatin inhibits the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, which regulates the ab initio metabolism of cholesterol by magnetic nanoparticles Fe3O4@PCBMA, thereby down-regulating the mevalonate metabolic pathway and the GPX4 pathway, inducing ferroptosis in TNBC cells without liver or kidney toxicity. These two pathways are also related to mitochondrial glycometabolism and lipid metabolism.81 A Cu-tetra (4-carboxyphenyl) porphyrin chloride (Fe(III)) (Cu-TCPP(Fe)) metal–organic framework (MOF)-based nanosystem was reported to be integrated with Au NPs and loaded with RSL3, which impedes GSH biosynthesis by disrupting the pentose phosphate pathway of mitochondrial glycometabolism, affecting a variety of enzymes involved in ferroptosis, ultimately enhancing the function of RSL3.82 Most of these ferroptosis nanomaterials targeting mitochondria are still under research, but the high-efficiency ferroptosis induced by the win–win cooperation of new nanomaterials and ferroptosis is the dawn for the future treatment of breast cancer, especially TNBC.
Targeting on ferroptosis to reverse drug resistance in breast cancer
Overcoming drug resistance is also a daunting challenge in breast cancer treatment. In addition to inducing ferroptosis in breast cancer treatment, ferroptosis has also been shown to correlate with drug resistance to breast cancer. Ferroptosis biomarkers could be applied to predict drug sensitivity in breast cancer. A cell death index model involving ferroptosis-related genes could predict resistance to the standard adjuvant chemotherapy regimen in breast cancer. Patients with a higher cell death index might be more sensitive to palbociclib.83 GPX4 and ACSL4 expression has also been demonstrated to be a predictive factor for pathological complete response in breast cancer neoadjuvant chemotherapy. As ACSL4 increased and GPX 4 decreased, pathological complete response rates rose, indicating that ACSL4 inducer and/or GPX4 inhibitor might promote neoadjuvant chemotherapy efficacy.84 Apart from its prognostic value, ferroptosis also has been proven to defeat drug resistance. For example, the inhibition of GPX4 has been proven to reverse gefitinib resistance in TNBC,85 and divalent metal transporter 1 inhibition also induces ferroptosis and reverses multidrug resistance in breast tumors.86 Fibroblast growth factor receptor 4 (FGFR4) is one of the FGFR family and was reported to co-expressed with HER-2 in breast cancer.50 It was reported that m6A hypomethylation mediates FGFR4 up-regulation, and then accelerates cystine uptake and Fe2+ efflux, resulting in ferroptosis resistance in recalcitrant HER2-positive breast cancer. The application of FGFR4 inhibitor roblitinib could remarkably restore trastuzumab sensitivity in recalcitrant HER2-positive breast cancer.87 These findings hold hope for developing novel therapeutics for drug resistance problems in traditional chemotherapy, targeted therapy, and maybe immunotherapy in the future.
Challenges in targeting ferroptosis in breast cancer
In addition to these applications, targeting ferroptosis is not without challenges to making it fully harnessed. First, up to now, there are few treatments targeting mitochondria and their related metabolic pathways due to their imperfect efficiency. The nanomaterials we discussed above could provide appreciable activity. Combining ferroptosis therapy with immunotherapy or other targeted therapy may be another resolution. Furthermore, will long-time use of ferroptosis therapy cause physiological change? If these ferroptosis inducers become available, the toxicity and side effect in humans remains a major hurdle. Based on existing studies, it seems that ferroptosis treatments might cause toxicities involved in the kidney, liver, and central nervous system.88,89 Hence, the optimized dose and drug schedule need to be discovered cautiously. Finally, the development of predictive biomarkers still needs to be resolved. Ways such as testing biomarkers in patients' body fluids will be more convenient and cheaper. With the resolution of these issues, there will be a brighter future for ferroptosis-targeted therapies.
Conclusions
The process of ferroptosis is complex, involving the metabolism of almost every organelle and various molecules in the cell, and the regulatory network is even more intricate. Mitochondria, as the hub of signal transduction, play an essential role in glycometabolism, lipid metabolism, oxidative phosphorylation, and ion regulation for ferroptosis. However, the specific mechanisms of mitochondria in various tumor-related metabolisms in breast cancer are not yet clear. How the contact, crosstalk, and signaling regulatory networks between mitochondria and other organelles are carried out. A more in-depth understanding of mitochondria-specific governess in ferroptosis will advance our fundamental discovery of this cell death pathway as well as identify new therapeutic opportunities to target ferroptosis in breast cancer.
Author contributions
Conception and design: Huijuan Dai; Administrative support: Aijun Sun and Xiaonan Sheng; Manuscript writing: Xinrui Dong, Ye Li, and Weihang Zhou. Manuscript figures were drawn by Xinrui Dong and Ye Li. The final approval of the manuscript was done by all authors. Xinrui Dong and Ye Li contributed equally to this research.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This study was sponsored and funded by the Shanghai Sailing Program (China) (No. 22YF1424500 to Xiaonan Sheng) and the Science and Technology Commission of Huaian Municipality, Jiangsu, China (No. HAB202209 to Aijun Sun).
Acknowledgements
We are grateful to Prof. Shuheng Jiang (State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China) for his critical suggestion for our review.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2023.03.019.
Contributor Information
Aijun Sun, Email: huaiansunaijun@163.com.
Huijuan Dai, Email: daihuijuan1988@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., Kang R., Kroemer G., et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan A.R., Miyazawa M., Hashimoto K., et al. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41(3):274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doll S., Proneth B., Tyurina Y.Y., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockwell B.R., Friedmann Angeli J.P., Bayir H., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang D., Kroemer G. Ferroptosis. Curr Biol. 2020;30(21):R1292–R1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 7.Handy D.E., Loscalzo J. Redox regulation of mitochondrial function. Antioxidants Redox Signal. 2012;16(11):1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato H., Tamba M., Ishii T., et al. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274(17):11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 9.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 10.Seiler A., Schneider M., Förster H., et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metabol. 2008;8(3):237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.de Jong G., van Dijk J.P., van Eijk H.G. The biology of transferrin. Clin Chim Acta. 1990;190(1–2):1–46. doi: 10.1016/0009-8981(90)90278-z. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z., Zhao P., Wang H., et al. Biomedicine meets Fenton chemistry. Chem Rev. 2021;121(4):1981–2019. doi: 10.1021/acs.chemrev.0c00977. [DOI] [PubMed] [Google Scholar]
- 13.Conrad M., Pratt D.A. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15(12):1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 14.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Z., Song J., Yung B.C., et al. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30(12) doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su L.J., Zhang J.H., Gomez H., et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao W.D., Pang P., Zhou X.T., et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28(5):1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X., Cai Z., Wang H., et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 2020;127(4):486–501. doi: 10.1161/CIRCRESAHA.120.316509. [DOI] [PubMed] [Google Scholar]
- 20.Stockwell B.R., Jiang X., Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30(6):478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lill R., Freibert S.A. Mechanisms of mitochondrial iron-sulfur protein biogenesis. Annu Rev Biochem. 2020;89:471–499. doi: 10.1146/annurev-biochem-013118-111540. [DOI] [PubMed] [Google Scholar]
- 22.Speijer D. Oxygen radicals shaping evolution: why fatty acid catabolism leads to peroxisomes while neurons do without it: FADH₂/NADH flux ratios determining mitochondrial radical formation were crucial for the eukaryotic invention of peroxisomes and catabolic tissue differentiation. Bioessays. 2011;33(2):88–94. doi: 10.1002/bies.201000097. [DOI] [PubMed] [Google Scholar]
- 23.Hunt M.C., Tillander V., Alexson S.E.H. Regulation of peroxisomal lipid metabolism: the role of acyl-CoA and coenzyme A metabolizing enzymes. Biochimie. 2014;98:45–55. doi: 10.1016/j.biochi.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Tesfay L., Paul B.T., Konstorum A., et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79(20):5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brash A.R. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 26.Pallast S., Arai K., Wang X., et al. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111(3):882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoyanovsky D.A., Tyurina Y.Y., Shrivastava I., et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–161. doi: 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagan V.E., Mao G., Qu F., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magtanong L., Ko P.J., To M., et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol. 2019;26(3):420–432.e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miess H., Dankworth B., Gouw A.M., et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene. 2018;37(40):5435–5450. doi: 10.1038/s41388-018-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao M., Yi J., Zhu J., et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73(2):354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada K., Hayano M., Pagano N., et al. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem Biol. 2016;23(2):225–235. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bersuker K., Hendricks J.M., Li Z., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doll S., Freitas F.P., Shah R., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 36.Kanzok S.M., Fechner A., Bauer H., et al. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291(5504):643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 37.Wu H., Wang F., Ta N., et al. The multifaceted regulation of mitochondria in ferroptosis. Life. 2021;11(3):222. doi: 10.3390/life11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douda D.N., Khan M.A., Grasemann H., et al. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112(9):2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeHart D.N., Fang D., Heslop K., et al. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem Pharmacol. 2018;148:155–162. doi: 10.1016/j.bcp.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao M., Monian P., Quadri N., et al. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mon E.E., Wei F.Y., Ahmad R.N.R., et al. Regulation of mitochondrial iron homeostasis by sideroflexin 2. J Physiol Sci. 2019;69(2):359–373. doi: 10.1007/s12576-018-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X., Wang H., Han D., et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao G., Chang Y.Z. Mitochondrial ferritin in the regulation of brain iron homeostasis and neurodegenerative diseases. Front Pharmacol. 2014;5:19. doi: 10.3389/fphar.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zecca L., Youdim M.B.H., Riederer P., et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 45.Gleitze S., Paula-Lima A., Núñez M.T., et al. The calcium–iron connection in ferroptosis-mediated neuronal death. Free Radic Biol Med. 2021;175:28–41. doi: 10.1016/j.freeradbiomed.2021.08.231. [DOI] [PubMed] [Google Scholar]
- 46.Núñez M.T., Hidalgo C. Noxious iron-calcium connections in neurodegeneration. Front Neurosci. 2019;13:48. doi: 10.3389/fnins.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Görlach A., Bertram K., Hudecova S., et al. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipper C.H., Karmi O., Sohn Y.S., et al. Structure of the human monomeric NEET protein MiNT and its role in regulating iron and reactive oxygen species in cancer cells. Proc Natl Acad Sci U S A. 2018;115(2):272–277. doi: 10.1073/pnas.1715842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Do Van B., Gouel F., Jonneaux A., et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Santambrogio P., Ripamonti M., Paolizzi C., et al. Harmful iron-calcium relationship in pantothenate kinase associated neurodegeneration. Int J Mol Sci. 2020;21(10):3664. doi: 10.3390/ijms21103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Lu S., He C., et al. RSL3 induced autophagic death in glioma cells via causing glycolysis dysfunction. Biochem Biophys Res Commun. 2019;518(3):590–597. doi: 10.1016/j.bbrc.2019.08.096. [DOI] [PubMed] [Google Scholar]
- 52.Gincel D., Silberberg S.D., Shoshan-Barmatz V. Modulation of the voltage-dependent anion channel (VDAC) by Glutamate1. J Bioenerg Biomembr. 2000;32(6):571–583. doi: 10.1023/a:1005670527340. [DOI] [PubMed] [Google Scholar]
- 53.Rostovtseva T., Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72(5):1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang W., Suzuki M., Saito T., et al. Emerging role of TCA cycle-related enzymes in human diseases. Int J Mol Sci. 2021;22(23):13057. doi: 10.3390/ijms222313057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeBerardinis R.J., Mancuso A., Daikhin E., et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H., Zandkarimi F., Zhang Y., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22(2):225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo J., Duan L., He X., et al. A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK mutant mtDNA depletion syndrome. Adv Sci. 2021;8(10):2004680. doi: 10.1002/advs.202004680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C., Zhang Y., Liu J., et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17(4):948–960. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyokuni S., Ito F., Yamashita K., et al. Iron and thiol redox signaling in cancer: an exquisite balance to escape ferroptosis. Free Radic Biol Med. 2017;108:610–626. doi: 10.1016/j.freeradbiomed.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Brown C.W., Amante J.J., Goel H.L., et al. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol. 2017;216(12):4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang F., Xiao Y., Ding J.H., et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metabol. 2023;35(1):84–100.e8. doi: 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Dattilo M.A., Benzo Y., Herrera L.M., et al. Regulatory mechanisms leading to differential Acyl-CoA synthetase 4 expression in breast cancer cells. Sci Rep. 2019;9:10324. doi: 10.1038/s41598-019-46776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarmiento-Salinas F.L., Delgado-Magallón A., Montes-Alvarado J.B., et al. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front Oncol. 2019;9:480. doi: 10.3389/fonc.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Müller S., Sindikubwabo F., Cañeque T., et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12(10):929–938. doi: 10.1038/s41557-020-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W., Chakraborty B., Safi R., et al. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021;12:5103. doi: 10.1038/s41467-021-25354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H., Yang P., Wang J., et al. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol. 2022;15:2. doi: 10.1186/s13045-021-01223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z., Chen L., Chen C., et al. Targeting ferroptosis in breast cancer. Biomark Res. 2020;8:58. doi: 10.1186/s40364-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding Y., Chen X., Liu C., et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J Hematol Oncol. 2021;14:19. doi: 10.1186/s13045-020-01016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luis G., Godfroid A., Nishiumi S., et al. Tumor resistance to ferroptosis driven by stearoyl-CoA desaturase-1 (SCD1) in cancer cells and fatty acid binding protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43:102006. doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L., Wang H., Yu S., et al. Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int J Mol Med. 2022;49(2):17. doi: 10.3892/ijmm.2021.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacMillan-Crow L.A., Thompson J.A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366(1):82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 72.Hart P.C., Mao M., de Abreu A.L.P., et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma S., Fu X., Liu L., et al. Iron-dependent autophagic cell death induced by radiation in MDA-MB-231 breast cancer cells. Front Cell Dev Biol. 2021;9:723801. doi: 10.3389/fcell.2021.723801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu C.W., Yin P.H., Hung W.Y., et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes Chromosomes Cancer. 2005;44(1):19–28. doi: 10.1002/gcc.20213. [DOI] [PubMed] [Google Scholar]
- 75.Nie H., Chen G., He J., et al. Mitochondrial common deletion is elevated in blood of breast cancer patients mediated by oxidative stress. Mitochondrion. 2016;26:104–112. doi: 10.1016/j.mito.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sui S., Xu S., Pang D. Emerging role of ferroptosis in breast cancer: new dawn for overcoming tumor progression. Pharmacol Ther. 2022;232:107992. doi: 10.1016/j.pharmthera.2021.107992. [DOI] [PubMed] [Google Scholar]
- 77.Sohn Y.S., Tamir S., Song L., et al. NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci U S A. 2013;110(36):14676–14681. doi: 10.1073/pnas.1313198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai F., Morcos F., Sohn Y.S., et al. The Fe-S cluster-containing NEET proteins mitoNEET and NAF-1 as chemotherapeutic targets in breast cancer. Proc Natl Acad Sci U S A. 2015;112(12):3698–3703. doi: 10.1073/pnas.1502960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Louandre C., Ezzoukhry Z., Godin C., et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 80.Sang M., Luo R., Bai Y., et al. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics. 2019;9(21):6209–6223. doi: 10.7150/thno.36283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao X., Xie R., Cao Y., et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnol. 2021;19:311. doi: 10.1186/s12951-021-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li K., Lin C., Li M., et al. Multienzyme-like reactivity cooperatively impairs glutathione peroxidase 4 and ferroptosis suppressor protein 1 pathways in triple-negative breast cancer for sensitized ferroptosis therapy. ACS Nano. 2022;16(2):2381–2398. doi: 10.1021/acsnano.1c08664. [DOI] [PubMed] [Google Scholar]
- 83.Zou Y., Xie J., Zheng S., et al. Leveraging diverse cell-death patterns to predict the prognosis and drug sensitivity of triple-negative breast cancer patients after surgery. Int J Surg. 2022;107:106936. doi: 10.1016/j.ijsu.2022.106936. [DOI] [PubMed] [Google Scholar]
- 84.Sha R., Xu Y., Yuan C., et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine. 2021;71:103560. doi: 10.1016/j.ebiom.2021.103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song X., Wang X., Liu Z., et al. Role of GPX4-mediated ferroptosis in the sensitivity of triple negative breast cancer cells to gefitinib. Front Oncol. 2020;10:597434. doi: 10.3389/fonc.2020.597434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turcu A.L., Versini A., Khene N., et al. DMT1 inhibitors kill cancer stem cells by blocking lysosomal iron translocation. Chemistry. 2020;26(33):7369–7373. doi: 10.1002/chem.202000159. [DOI] [PubMed] [Google Scholar]
- 87.Zou Y., Zheng S., Xie X., et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat Commun. 2022;13:2672. doi: 10.1038/s41467-022-30217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eaton J.K., Furst L., Ruberto R.A., et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16(5):497–506. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou Y., Schreiber S.L. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem Biol. 2020;27(4):463–471. doi: 10.1016/j.chembiol.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.