Abstract
Extracellular vesicles (EVs) are nano-size vesicles secreted naturally by all cells into the extracellular space and have been recognized as important cell–cell mediators in multicellular organisms. EVs contain nucleic acids, proteins, lipids, and other cellular components, regulating many basic biological processes and playing an important role in regenerative medicine and diseases. EVs can be traced to their cells of origin and exhibit a similar function. Moreover, EVs demonstrate low immunogenicity, good biocompatibility, and fewer side effects, compared to their parent cells. Mesenchymal stem cells (MSCs) are one of the most important resource cells for EVs, with a great capacity for self-renewal and multipotent differentiation, and play an essential role in stem cell therapy. The mechanism of MSC therapy was thought to be attributed to the differentiation of MSCs after targeted migration, as previously noted. However, emerging evidence shows the previously unknown role of MSC-derived paracrine factors in stem cell therapy. Especially EVs derived from oral tissue MSCs (OMSC-EVs), show more advantages than those of all other MSCs in tissue repair and regeneration, due to their lower invasiveness and easier accessibility for sample collection. Here, we systematically review the biogenesis and biological characteristics of OMSC-EVs, as well as the role of OMSC-EVs in intercellular communication. Furthermore, we discuss the potential therapeutic roles of OMSC-EVs in oral and systemic diseases. We highlight the current challenges and future directions of OMSC-EVs to focus more attention on clinical translation. We aim to provide valuable insights for the explorative clinical application of OMSC-EVs.
Keywords: Disease, Extracellular vesicles, Oral tissue MSC, Therapeutic application, Tissue regeneration
Introduction
Extracellular vesicles (EVs) released by cells of all organisms, such as eukaryotes, prokaryotes, or plants, exert different functions, primarily relying on the properties of the cell origin and the state of the cell at the time of purification.1 One of the most studied EVs is derived from mesenchymal stem cells (MSCs), which possess characteristics that are homogeneous to those of their parental cells. MSCs are a heterogeneous population and exist in a variety of human tissues,2, 3, 4 where they may express different tissue-specific molecular markers.5 Oral tissue MSCs (OMSCs) are a class of MSCs derived from oral tissues, including dental pulp stem cells (DPSCs),6 stem cells from exfoliated deciduous teeth (SHEDs),7 periodontal ligament stem cells (PDLSCs),8 dental follicle progenitor cells (DFPCs),9 stem cells from the apical papilla (SCAPs),10 and gingival mesenchymal stem cells (GMSCs).11,12 They are characterized by great self-renewal and multidirectional differentiation abilities, similar to those of all other tissue-derived MSCs. Of note, OMSCs have remarkable advantages, such as being widely available, easy to obtain, and minimally invasive, and thus have become one of the most promising therapeutic options for tissue regeneration and repair.13 Previously, MSC therapy was believed to be achieved primarily through direct migration to the site after transplantation with the potential for increased proliferation and differentiation. However, in recent years, studies have established that MSC therapy is largely dependent on the paracrine factors of MSCs rather than the MSCs themselves.14 Among the many paracrine factors of MSCs, MSC-derived EVs (MSC-EVs) have started to attract more attention, due to their drug delivery capacity, high specificity, low immunogenicity, good biocompatibility, high stability, and no cytotoxicity, compared to their parental cells.15 In this review, we summarize the biological origin, heterogeneity, and uptake mechanism of EVs, and focus on the role and potential application of OMSC-EVs in local and systemic diseases, in order to provide a valuable reference for the research on EVs, especially OMSC-EVs and their future potential clinical application.
The biogenesis, heterogeneity, and functionality of extracellular vesicles
EVs are membrane-containing vesicles released by all types of cells, which are divided into three main categories according to their biological origin and size (Table 1), including exosomes (EXOs), microvesicles (MVs), and apoptotic extracellular vesicles (ApoEVs).16 Other subpopulations such as supremeres and exomeres have also been recently referred to (Fig. 1).17,18
Table 1.
Characteristics of EVs.
| Characteristics | EXOs | MVs | ApoEVs | Reference |
|---|---|---|---|---|
| Source cells | Living cells | Living cells | Apoptotic cells | 46 |
| Size | ∼50–150 nm | ∼150–1000 nm | ∼500–5000 nm | 19,47,48 |
| Mechanism of biogenesis | Double invagination of the plasma membrane | Plasma membrane budding outward directly | Microtubule spike, apoptopodia, and beaded apoptopodia | 20,49 |
| Cargo | Proteins, mRNA, and miRNA | Proteins, plasmid DNA, mRNA, and miRNA | Organelles, fragments, proteins, nucleic acids | 50, 51, 52 |
| Markers | CD63, CD81, CD9 | Annexin A1, ADP-ribosylation factor 6 | Phosphatidylserine exposure, activated caspase-3 | 28,53 |
| Centrifugal Condition | 100,000–120,000 g | 1000–10,000 g | 500–16,000 g | 54,55 |
Figure 1.
The biogenesis and secretion of EVs. The biogenesis of EXOs involves double invagination of the plasma membrane and the formation of multivesicular bodies (MVBs). Briefly, the plasma membrane first invaginates to form early endosomes (EE), and then EEs undergo the second invagination of the membrane to mature into late endosomes (LE), also called MVB, which includes a large collection of ILVs; lastly, MVBs fuse with the plasma membrane and release ILVs into the extracellular space, which forms EXOs with diameters of ∼30–150 nm; while MVs were produced by the plasma membrane directly budding outward, triggered by the change in cell membrane stiffness and curvature change. Unlike the ApoEV former, the formation is a dynamic process, which is regulated by the morphological steps of apoptotic cell disassembly and contains different forms such as microtubule spike, apoptopodia, and beaded apoptopodia. A variety of molecules, such as MLC, MLCK, and ESCRT, participate in the regulation of EV biogenesis and release. This graph was created with Biorender.com.
EXOs, also called small extracellular vesicles (sEVs), are one group of EVs that originate from endosomes with diameters of around 30–150 nm.19 The biogenesis and development of EXOs have been extensively reviewed elsewhere20, 21, 22 and it mainly involves double invagination of the plasma membrane and the formation of multivesicular bodies (MVBs) containing abundant intraluminal vesicles (ILVs).23 It is known that the cellular protein network plays a crucial role in the biogenesis and secretion of EXOs, including endosomal sorting complexes required for transport (ESCRTs), and tetraspanins such as CD63, CD81, and CD9.23, 24, 25 Functionally, EXOs carry many bioactive molecules and play an important role in intercellular communication, signal transduction, regulation of immune response, antigen presentation, and epigenetic reprogramming of recipient cells, and it depends largely on the physiological and pathological conditions of the tissues or cells of origin and the surface receptors of the recipient cells.21 MVs are small membrane sacs produced by exuding from the cell surface with a size of 150–1000 nm.26 It is generally believed that the biogenesis of MVs is a multistep process, including the transport of cellular cargoes to the plasma membrane, the rearrangement of membrane lipids, and the mechanical contraction-mediated blebbing extrusion.27,28 MVs have been widely detected in various biological fluids such as peripheral blood, urine, and ascites, and play an important role in various diseases, including tumor invasion and metastasis, inflammation, and tissue regeneration by promoting horizontal transfer of bioactive molecules such as proteins, RNA, and microRNA.29, 30, 31 ApoEVs are the largest type of EV produced by cells when they were undergoing apoptosis, typically having a size greater than 1 μm. ApoEV formation is a dynamic process, which is regulated by the morphological steps of apoptotic cell disassembly, controlled by protein kinases such as Rho-associated protein kinase 1 (ROCK1) and MLCK, the membrane channel PANX1.32 ApoEVs are “small, sealed sacs” that were previously considered garbage bags. Until recently, their ability to deliver useful materials, such as self-antigens, to neighboring or distant receptor cells has come to light. ApoEVs can not only promote efficient removal of cell debris by surrounding phagocytes, but also carry biomolecules, including protein, microRNA, and DNA, to regulate cell-to-cell communication.33,34
EVs show great heterogeneity in cargo and functionality according to their sources of cells and tissues, and even the method of purification. It is generally believed that EVs obtained by differential centrifugation contain a heterogeneous mixture of all types of EVs and non-vesicular compartments and even some levels of soluble proteins.35 Ultracentrifugation (UC) and size-exclusion chromatography (SEC) appeared to give a very high better purity of EVs.36 Indeed, subtle molecular differences at the level of a single EV may lead to significant changes in the biological function of EVs.37 It becomes increasingly critical to develop a novel technology to analyze EVs at a single-molecule level to be able to identify the heterogeneity of the EV population. Recently, advances in novel fluorescence microscopy techniques make it possible to detect EVs at the single-molecule level.38 More isolation methods have been reported, such as the combined enrichment method,39 modern approaches based on size, charge, and affinity,40 and apoplastic washing fluid.41 Additionally, nanoflow cytometry or advanced flow cytometry has been used to differentiate EVs by recognizing the different surface markers labeled with fluorophores.42,43 A single EV flow cytometry analysis (FCA)44 is enabled by target-initiated engineering (TIE) of DNA nanostructures on individual EVs, which employs a conformation-switchable DNA probe to bind to the surface marker of the EV, triggering the engineering of a DNA nanostructure by a hybridization chain reaction (HCR).45 HCR products not only enlarge the overall size of a single EV to be beyond 500 nm, but can also bind to multiple fluorophores to amplify the signal from the marker molecules located on the limited area of the EV surface, both enabling visualization of a single EV in a conventional flow cytometer and greatly simplifying the measurement of multiple markers on the same EV. However, it is still challenging to isolate the entire population of EVs using current methods as only certain known surface markers are available for EV detection.44
The mechanisms of EVs in intercellular communication
Recent studies highlight that EVs released by cells, acting as paracrine factors, interact with the recipient cell by migrating to distant sites and reaching the target cells.56 The topological structure of EVs is similar to that of cells, and functional communication between EVs and cells can involve different types of interactions, including fusion of the EV-plasma membrane, endocytosis uptake, and binding of EVs to the cell surface via the ligand-receptor without delivery of the contents (Fig. 2).57
Figure 2.
The mechanism of EV uptake. EVs can participate in cellular communication through molecule transport or signal transfer between cells. EVs can enter recipient cells by direct membrane fusion. Alternatively, EVs can also be internalized by macropinocytosis and clathrin and caveolin-mediated endocytosis. Otherwise, some larger EVs, such as apoEVs, can also be taken up by target cells through phagocytosis. This graph was created with Biorender.com.
The direct fusion of the EV membrane with the lipid bilayer of the plasma membrane is one way to communicate with neighborhood cells, which is regulated by several proteins, such as soluble receptor for the N-ethylmaleimide-sensitive factor attachment protein (SNARE), Rab and Sec1/Munc18-like proteins (SM),57 although it maybe not be the main route for EVs entering cells under physiological conditions.58,59 Endocytosis is considered one of the main pathways for the uptake of EVs, including clathrin and caveolin-mediated endocytosis, macropinocytosis, and phagocytosis.60 Clathrin-mediated endocytosis is achieved by clathrin-coated vesicles that deform the membrane into bubbles and break off. In general, clathrin and caveola-mediated endocytosis play a role in EV uptake, but the precise mechanism of this pathway remains to be further investigated.60 Macropinocytosis is another endocytosis uptake pathway, and the mechanism is similar to phagocytosis, but it does not require direct contact with internalizing substances, rather relies on the GTPase RAC1, actin, and cholesterol, and requires Na+/H+ exchange activity.61, 62, 63 These studies at least suggest that macropinocytosis may be a secondary pathway for the internalization of EV or an uptake mechanism for certain cell types. Phagocytosis is a receptor-ligand-mediated endocytosis event. When EVs bind to the cell membrane by the corresponding receptor and ligand, downstream signal transduction can be initiated, leading to aggregation of actin at the uptake site. Actin contraction causes the plasma membrane to protrude and form pseudopodia to envelop EVs or other extracellular molecules, and then the pseudopodia fuse to form bubbles. In the cytoplasm, the dynein at the neck of the bubbles contracts and breaks off the bubbles to form phagosomes. This process occurs primarily during the uptake of large EVs, such as ApoEVs, which are mainly carried out by specialized cells such as macrophages and dendritic cells.60,64 Phosphatidylserine (PS) exposure is the characteristic surface structure of ApoEVs, which differs from the other two types of EV, and PS can recruit phagocytes and promote the phagocytosis of ApoEVs.65
The superiority of OMSC-EVs and their biological function in health
Compared to MSCs from other tissues, OMSCs have greater advantages of wide source, easy access, and being minimally invasive, together with their excellent proliferation and differentiation abilities,66,67 making them a strong regenerative and therapeutic potential for promising application prospects.68,69 Due to the origin of the neural crest, OMSCs have considerable potential for neurogenic differentiation, thereby OMSC-EVs have unique advantages in the prevention and treatment of neurotrophic and peripheral neurosystemic diseases.70, 71, 72, 73 Furthermore, OMSC-EVs have stronger immunomodulatory abilities.74,75 DPSC-EXOs have been reported to inhibit differentiation of CD4+ T cells in Th17 to reduce the secretion of pro-inflammatory cytokines IL-17 and TNF-α, but promote differentiation in Tregs to increase the release of anti-inflammatory cytokines IL-10 and TGF-β, compared to BMSC-EXOs.76 Even though different tissue-derived MSCs have similar stem cell characteristics, their relative differences in cytokine profiles can give them unique biological significance.77 For example, OMSCs possess a unique cytokine profile and therefore have more significant advantages than BMSCs in proliferation, osteogenesis, and odontogenesis.66,77,78 Especially in the aspect of tooth and periodontal tissue regeneration, OMSCs have significant advantages over other MSCs, such as PDLSCs in periodontal regeneration79 and DPSCs in dental pulp regeneration.80 Therefore, we focused on OMSC-EVs in this review and discussed their biological functions in health and disease.
Emerging evidence supports that MSC-induced paracrine signaling is important to maintain tissue homeostasis.81,82 EVs, as a component of the MSC secretome, participate in the transfer of proteins and genetic material, and the regulation of various physiological processes.82 It is known that EVs secreted by SCAP, which are derived from the apical papilla of incompletely developed teeth, play an important role in root formation and development.83,84 OMSC-EVs may have a positive effect on odontogenic differentiation and dentin-pulp formation. PIWI-interacting RNAs (piRNAs) are a new class of small ncRNAs, which form RNA-induced silencing complexes by binding to the P-element-induced wimpy testis protein family (PIWI), one of the argonautes, contributing to maintaining the functional integrity of stem cells.85 Compared to the piRNA expression profiles of SCAP-EXOs with BMSC-EXOs, the higher expression of piRNAs in SCAP-EXOs activated the MAPK signaling pathway, resulting in odonto-/osteogenic differentiation.86 It has been suggested that dentin-pulp formation involves complex epithelial–mesenchymal interactions between Hertwig epithelial root sheath cells (HERS) and dental cells (DPC), and EXOs secreted by HERS (ELVs-H1) containing the Wnt3a protein, can up-regulate the expression of β-catenin and activate Wnt/β-catenin signaling pathway in DPC to induce angiogenesis, odontoblast differentiation, and neural differentiation of dental MSCs.87 The beneficial effect of OMSC-EVs in inducing odontogenic differentiation of stem cells suggests that OMSC-EVs may play an important role in the formation of pulp and dentin, and also provides a valuable theory for pulp and dentin regeneration.88,89 DPSC-EXOs promote odontogenic differentiation through transporting miR-27a-5p to down-regulate the latent TGF-β-binding protein 1 (LTBP1), one of the inhibitory molecules of TGFβ1 signaling, to activate TGFβ1/Smad signaling pathway.88 DPSC-EXOs also induce odontogenic differentiation through the p38/MAPK pathway, which increases the expression of key regulators such as DMP1 and DPP.89 In addition, the balance between bone formation of osteoblasts and bone resorption of osteoclasts is crucial for the development of oral and facial bones. Studies exploring the communication between OMSC and osteoclasts found that miR-3-4p from OMSC-EXOs is an important downstream factor of GATA4 that directly targets BMP-3 and NFATc1 in activated T cells to regulate the functions of OMSCs and osteoclasts,90 which plays a key role in the development of oral facial bone.
The role of OMSC-EVs in the regeneration
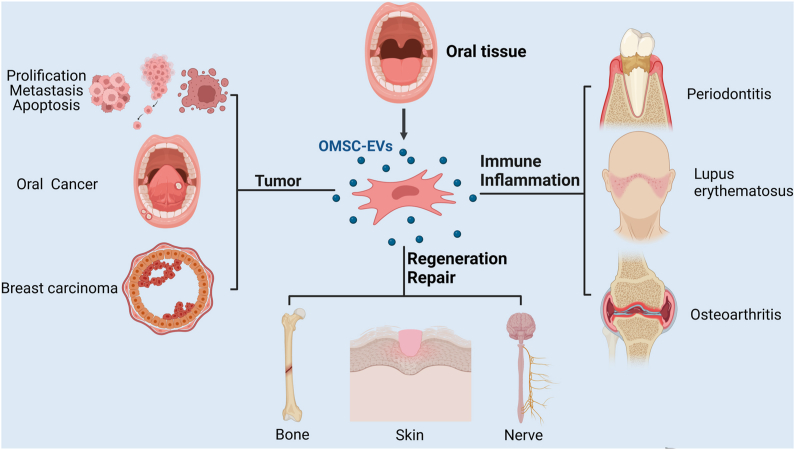
Effective tissue repair and regeneration after injury are crucial for maintaining the physiological functions of tissues. Here, we focus on the role and current application of OMSC-EVs in various tissue regeneration (Fig. 3).
Figure 3.
Role of oral MSC-EVs in disease and tissue regeneration. EVs have a potential therapeutic role in the regeneration of tissue injuries such as bone, nerve, and skin soft tissue injuries, and in the inflammatory repair of immune-inflammatory diseases such as osteoarthritis, systemic lupus erythematosus, and periodontitis. Furthermore, EVs are associated with tumor cell proliferation, migration, and invasion, so they may have potential applications for tumor diagnosis and targeted therapy. This graph was created with Biorender.com.
The role of OMSC-EVs in periodontal bone remodeling
OMSC-EVs have prominent advantages in periodontal bone regeneration.83,91 Periodontal bone defects generally include inflammatory bone loss caused by periodontitis and mechanical bone loss caused by trauma or surgery. MVs obtained from DFPCs have been reported to activate the phospholipase C (PLC)/protein kinase C (PKC)/mitogen-activated protein kinase (MAPK) pathway in DFPCs to promote alveolar bone regeneration.92 Healthy PDLSC-EXOs can inhibit the overactivation of classical Wnt signal in an inflammatory environment to maintain the osteogenic capacity of endogenous stem cells and promote periodontal bone regeneration.93 SHED-EXOs have been shown to activate the BMP/Smad and Wnt/β-catenin signaling pathways by transporting Wnt3a and BMP2 mRNA and proteins to recipient cells, effectively promoting osteogenic differentiation of PDLSCs in a dose-dependent manner,94 and also promote periodontal bone regeneration by promoting neovascularization and new bone formation through the AMPK signaling pathway.95 In addition, SHED-EXOs also promote bone formation and inhibit lipogenesis, which up-regulated the expression of Runx2 and p-Smad5 and decreased the expression of Pparγ.96 EV derived from inflammatory DPSCs (iDPSC-EVs) transport the limb development membrane protein 1 (LMBR1)-targeting miR-758-5p to promote PDLSCs osteogenic and odontogenic differentiation via BMP signaling.97 The increased EXOs produced by TNF-α-pretreated GMSCs up-regulated the expression of CD73, which induced anti-inflammatory M2 macrophages and inhibit inflammation and osteoclast activity to prevent periodontal bone loss.98 EVs are also often combined with materials for bone tissue repair after injury. EVs derived from GMSCs and DPSCs combined with materials can improve the expression of osteogenic markers RUNX2, vascular endothelial growth factor (VEGFA), and osteopontin (OPN) and promote the repair of bone defects in rats.99,100
The role of OMSC-EVs in tooth regeneration
SCAP-EXOs have been suggested to be a potential therapeutic approach for the regeneration of dentine–pulp complex and bone regeneration. SCAP generated during early tooth root development and SCAP-EXOs were found to promote the regeneration of dentine–pulp complex by inducing specific odontogenesis and osteogenesis by activating the mitogen-activated protein kinase (MAPK) signaling pathway.86,101 Furthermore, SCAP-EXOs can promote Rho-GTPase Cdc42-mediated angiogenesis, providing essential blood supply and nutrients to support tissue regeneration.102 In bioengineered tooth regeneration, EXOs derived from human DPSC (hDPSC) aggregates promoted the tooth regeneration process by up-regulating the odontogenic and angiogenic capacity of hDPSCs, leading to functional tooth regeneration, supporting ongoing root development in humans.103 The regenerative effects of DPSC-EV on jawbone defects suggested that JB-MSC could efficiently uptake DPSC-EVs, which in turn significantly promoted the expression of osteogenic genes, such as runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and osteocalcin (OCN), and the osteogenic differentiation capacity of JB-MSCs.104 Similar beneficial effects for bone regeneration were observed in rat calvarial bone defects.100 ELVs-H1 can also be combined with biomaterials such as collagen to form a sustained release system, which is beneficial to prolong the biological activity of ELV and highly biocompatible ELV-H1 collagen provides a microenvironment suitable for odontoblast differentiation.87
The role of OMSC-EVs in soft tissue regeneration
The skin, as the first major defence barrier in the body, is prone to damage due to acute or chronic injuries after trauma, burns, or diabetes. Currently, studies on skin regeneration focus on accelerating wound healing after injury, and MSC-EVs have been considered to have good potential in repairing skin injury.105 SCAP-EXOs promote the formation of Cdc42-dependent filaments in endothelial cells to promote cell migration by transporting Cdc42 to recipient cells, which then accelerate angiogenesis and wound healing of mouse palatal gingival soft tissue.102 HDPSC-EVs were also shown to have the ability to promote angiogenesis without being influenced by the periodontal inflammatory environment, providing a possible source of EVs that can be obtained from extracted teeth due to periodontitis.106 Compared to skin wounds, gingival wounds heal faster and GMSCs have been found to rely on the Fas/Fas-associated phosphatase-1 (Fap-1)/caveolin-1 (Cav-1) mechanism to generate EV with high expression of IL-1RA, leading to the promotion of the healing of gingival wounds in mice. Local application of GMSC-EVs with high expression of IL-1RA can also promote wound healing of the skin, further demonstrating the beneficial effect of GMSC-EVs on the repair of soft tissue injury.107 Furthermore, chronic, delayed, or even non-healing wounds often occur in patients with systemic diseases such as diabetes due to high glycemic load, impaired angiogenesis, and unbalanced cytokine profiles, which can lead to the risk of gangrene, amputation, and even death. Fortunately, the combination treatment of GMSC-EXO with hydrogel shows the potential to address the problem, which has been identified to effectively promote skin wound healing in diabetic rats by promoting collagen reepithelialization, deposition, and remodeling.108 These findings highlight the role of GMSC-EXOs in wound healing and provide a novel non-invasive method with practical value for skin repair using OMSC-EVs.
The role of OMSC-EVs in neural tissue regeneration
OMSCs appear to have a more prominent potential for neural regeneration due to their origin in the neural crest and the expression of some neural progenitor cells and mature cell markers. It is known to promote the repair and regeneration of nerve injuries, but the specific underlying mechanism has not been fully elucidated. Recent studies have shown that paracrine factors of OMSC, especially EVs, play an important role in nerve regeneration and have great therapeutic potential.109 In a mouse model of sciatic nerve injury, GMSC-derived EVs (GMSC-EVs) up-regulate the protein level of C-Jun, Notch1, glial fibrillary acid protein (GFAP), and the sex-determined region Y-box 2 (SOX2), which are the characteristic genes for dedifferentiation, and promote the proliferation and migration of Schwann cells to promote regeneration and repair of injured nerves.110 Similarly, GMSC-EVs were also found to promote nerve regeneration and functional recovery by increasing the proliferation of rat dorsal root ganglion cells.111 MSCs stimulated by inflammatory cytokines like TNF-α tend to secrete a large number of soluble mediators to exert their immunosuppressive potential. For instance, EXOs derived from TNF-α-stimulated GMSCs enhanced the protective effects of RGC and microglia and reduced neuroinflammation by inhibiting microglial activation through MEG3/miR-21a-5p axis.112 SHED-EXOs can also reduce neuroinflammation by altering microglia polarization.113 Furthermore, SHED-EXOs grown on three-dimensional laminin-coated alginate microcarriers have been found to become a potential treatment for Parkinson's disease, which can inhibit the apoptosis of dopaminergic neurons to protect nerves.114
The role of OMSC-EVs in immune disease
MSCs exert an immunomodulatory effect by interacting with the innate and adaptive immune systems. EVs, as one of the important paracrine factors of MSCs, play a crucial role in a variety of immune and inflammatory diseases. OMSC-EVs also exhibit good immunomodulatory properties, which play a role in immune and inflammation-related diseases. Compared to BMSCs and DPSCs-derived EXO immunomodulatory capacity, DPSC-EXOs show stronger inhibition of CD4+ T cell differentiation in Th17 cells to reduce the release of pro-inflammatory cytokines, while promoting CD4+ T cell differentiation in Tregs to increase the release of anti-inflammatory factors, thus exerting immunomodulatory function.76 GMSC-EXOs can reduce the release of inflammatory cytokines, inhibit lipid accumulation, and promote the polarization of pro-inflammatory macrophages into anti-inflammatory phenotypes in a high-lipid microenvironment.115 Therefore, OMSC-EVs have promise in the treatment of various inflammatory diseases.
MSC-derived secretomes also show great potential to treat systemic immune-inflammatory diseases as a novel alternative treatment. It has been shown that hPDLSC-derived EXOs/MVs (hPDLSCs-EMVs) obtained from relapse-retreat-multiple sclerosis patients or healthy donors have been shown to improve experimental autoimmune encephalomyelitis through anti-inflammatory and immunosuppressive effects and reverse disease progression by restoring tissue integrity through remyelination in the spinal cord.116 Studies have shown that intravenous injection of DPSC-EXOs can reduce cerebral edema, cerebral infarction, and nerve injury induced by cerebral ischemia/reperfusion (I/R) in mice, which inhibits inflammatory responses through box 1 protein of the high mobility group (HMGB1)/toll-like receptor 4 (TLR4)/myeloid differentiation protein 88 (MyD88)/nuclear factor kappa B (NF-κB) pathway to improve neuroinflammation after cerebral I/R injury.117 Sonoda S et al found that intravenous injection of SHED-EV could improve the phenotype similar to systemic lupus erythematosus of MRL/lpr mice, which is achieved by improving the hematopoietic niche and immunomodulatory function of BMSCs to promote telomerase activity.118 EVs generated by miR-140-5p-overexpressed DPSCs can exert anti-apoptotic effects, which induce a significant reduction in IL-1β-induced chondrocyte apoptosis to improve knee osteoarthritis in rat osteoarthritis. These results suggest that the combination of genetic engineering of DPSCs with transgenic DPSC-derived EVs can be a novel strategy for the treatment of osteoarthritis.119 Furthermore, DPSC-EXOs can inhibit the production of reactive oxygen species (ROS) and reduce the polarization of M1 macrophages through the ROS-MAPK-NFκB-P65 signaling pathway, becoming a potential treatment for spinal cord injury.120
In addition to systemic diseases, OMSC-EVs also have great therapeutic potential for oral inflammation-related diseases. In the rat pulp exposure model, DPSC-EVs, enriched with miR125a-3p, can promote the polarization of M2 macrophages through TLR and NFκB signals to inhibit the pulpitis response. Furthermore, those EVs were found to promote macrophage release of BMP2 and activate the BMP pathway to facilitate the dentin formation of DPSCs.121 Some studies have proven that EVs derived from bacterial lipopolysaccharide (LPS) treated with DFPC also inhibit inflammation to promote periodontitis healing because they have an antioxidant effect and can inhibit intracellular ROS, reducing the ratio of the NF–B receptor activator of NFκB ligand (RANKL)/osteoprotegerin (OPG) of PDLSCs by inhibiting the ROS/c-Jun N-terminal kinase (JNK) signal under inflammatory conditions, and promote the polarization of M2-type macrophages through ROS/extracellular signal-regulated kinase (ERK) signaling.122 However, EVs produced by LPS-treated PDLSCs have been reported to encourage inflammatory progression by inducing M1-type macrophage polarization.77 Unlike LPS-treated PDLSCs, healthy PDLSC-EVs can decrease NF-κB activity through the TLR4 signaling pathway to inhibit inflammation, suggesting that PDLSC-EVs may be used to treat chronic periodontitis.123 PDLSC-EXOs also alleviate the inflammatory microenvironment through the Th17/Treg/miR-155–5p/SIRT1 regulatory network and enhance the ability of PDLSCs to promote angiogenesis by regulating EXO-mediated VEGFA transfer.124 These results indicate that the biological composition and function of OMSC-EVs are closely related to the parental cells from which they are derived, which can be not only a potential therapeutic tool but also a potential therapeutic target for periodontitis to some extent. In a rat model of periodontal disease, GMSC-EVs also significantly improve the regeneration of damaged periodontal tissue by reducing pro-inflammatory cytokines secreted by monocytes/macrophages and T cells, which inhibit T cell activation and induce Treg formation.125 In conclusion, OMSC-EVs can inhibit inflammation and promote disease recovery in a variety of ways, which is a potential treatment for diseases related to immune inflammation.
The role of OMSC-EVs in tumor progression and their therapeutic potential
EVs are nanoscale messengers and play a role in intercellular communication not only in normal tissues but also in tumor tissue, coordinating all kinds of autocrine and paracrine functions to change the tumor microenvironment, thus facilitating tumor growth and progression.126,127 Previous studies have demonstrated MSCs-EVs and their roles in the regulation of the tumor microenvironment and the potential application prospect in the treatment of tumors.128 Cancer stem cells-derived EXOs (CSC-EXOs) also contain stem cell-specific proteins, regulatory miRNA, and survival factors that contribute to maintaining tumor heterogeneity and altering tumor progression.129 Compared to MSCs-EVs that are derived from other tissues, OMSCs have unique neural crest origin and show many unique stem-like characteristics,130 indicating that they have potential application value for tumor progression and treatment, although few studies on OMSC-EVs in tumors are available up to now.
Recent studies have shown that EVs derived from oral leukoplakia MSCs (LK-MSCs) and oral cancer MSCs (CA-MSCs) can transport miR-8485 to promote tumor cell proliferation, migration, and invasion instead of the normal oral mucosa (N-MSCs). However, inhibiting the release of EVs using GW4869, a commonly used exosome secretion inhibitor, attenuates the pro-tumoral effect of LK-MSC and EVs derived from CA-MSCs.131 Taken together, LK-MSC-derived EXO can be involved in the recurrence and malignant transformation of oral leukoplakia, and miR-8485 may be a potential marker of disease progression and a potential target for clinical intervention. Interestingly, due to their inherent ability to deliver biomolecules, EVs can be used as unique vectors for the intracellular transport of various therapeutic materials, such as miRNA and proteins, to cancer tissues.132 Compared to traditional liposome agents, EVs exhibit relatively satisfactory tissue tolerance and greater specificity for cancer targeting, and cancer cells can ingest and internalize more EVs than normal cells.133 OMSC-EVs have good drug-loading capability and tumor-targeting properties. For example, EXOs, which are derived from hDPSCs cultivated with gemcitabine (GCB), significantly inhibited the cell growth of pancreatic carcinoma cell lines in vitro.134 OMSC-EVs also have the additional advantages of being less invasive, easy to access, and rapid to expand, which are favored in the treatment of tumors.135 DPSCs are considered valuable sources for EV production due to their great potential for multidirectional differentiation and reproductive activity. MiR-34a is highly methylated in several cancers, leading to tumor cell apoptosis. Some researchers established DPSCs engineered with miR-34a and then obtained DPSC-EXOs that are abundant in miR-34a to evaluate the role of engineered EXOs in the treatment and prognosis of breast cancer. The results showed that DPSC-EXOs engineered with miR-34a enriched with miR-34a induced apoptosis of breast cancer cells in a dose-dependent manner, thus reducing the migration and invasion of cancer cells,136 indicating the role of OMSC-EV in tumor development and migration, thus may provide a potential application value for tumor diagnosis and treatment.
Conclusions and perspectives
To date, EVs have become emerging mediators in cell–cell communication, having a variety of physiological functions, such as participating in intercellular information transport and regulating the physiological and pathological processes of distal cells in health and disease. Although increasing investigations have been conducted in the field of EV biology, the biological characteristics, molecular composition, function, targeting, and uptake mechanisms of EVs are still not completely elucidated to some extent. EVs have been emphasized to play a role in the transfer of biological molecules such as proteins, lipids, and nucleic acids, and are expected to be used as a new cell-free therapy in various diseases, such as immune diseases, bone and tooth regeneration, neurological diseases, wound healing, etc. Among all MSC-EVs, OMSC-EVs show great application prospects in many local and systemic diseases. Although there is not enough evidence to fully evaluate and compare specific molecular differences, biological functions, and therapeutic applications between OMSC-EVs and other different types of MSC-EVs, many studies revealed that OMSC-EVs had stronger immunomodulatory, anti-apoptotic, and pro-regeneration characteristics compared to other MSC-EVs. However, it should be noted that a more accurate comparison between these studies in MSC-EVs is needed, as the methodology for the purification, characterization, and evaluation of the function of EVs varies greatly from study to study. Despite relatively few studies on OMSC-derived ApoEVs, the role of OMSC-derived EXOs and other EVs in local or systemic diseases has been widely studied, suggesting the potential role of OMSC-EVs in the development and treatment of diseases. There is no doubt that the research and application of OMSC-EVs still have limitations and unsolved problems that are similar to other studies of MSC-EVs at present, such as the lack of the gold standard for the separation and quantification of OMSC-EVs, and the standardized methods for the storage, transportation, and mass production of OMSC-EVs. Furthermore, the nomenclature of OMSC-EVs and the molecular and functional variation among EVs secreted from tissue-specific origin cells under normal and disease statutes are still unresolved questions. This is now being addressed by constantly updated guidelines on extraocular vesicle research, and the minimal requirements for studies of extracellular vesicles (MISEV).137 Furthermore, the exploration of appropriate EV dose application methods, the proper treatment of their parental cells, and efficient EV purification methods are also needed to achieve the desired effect. Using further investigation of these questions, we hope to gain deeper insight into the biology of OMSC-EVs shortly to accelerate the transformation of OMSC-EVs into clinical applications in a rational way.
Conflict of interests
The authors declare that they have no conflict of interests.
Funding
This study was supported by the National Key R&D Program of China (No. 2022YFC2504200), the National Natural Science Foundation of China (No. 82270960), and the Science & Technology Development Talent Project of Jilin Financial Department, Jilin, China (No. JCSZ2021893-35) to AZ.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Paul T. Sharpe, Email: paul.sharpe@kcl.ac.uk.
Hongchen Sun, Email: hcsun@jlu.edu.cn.
Zhengwen An, Email: wenny_an@jlu.edu.cn.
References
- 1.Greening D.W., Simpson R.J. Understanding extracellular vesicle diversity–current status. Expert Rev Proteomics. 2018;15(11):887–910. doi: 10.1080/14789450.2018.1537788. [DOI] [PubMed] [Google Scholar]
- 2.Gao Q., Wang L., Wang S., et al. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front Cell Dev Biol. 2022;9 doi: 10.3389/fcell.2021.787118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez A.M., Elabd C., Amri E.Z., et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87(1):125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.El Omar R., Beroud J., Stoltz J.F., et al. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng B Rev. 2014;20(5):523–544. doi: 10.1089/ten.TEB.2013.0664. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 6.Gronthos S., Mankani M., Brahim J., et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura M., Gronthos S., Zhao M., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo B.M., Miura M., Gronthos S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 9.Bi R., Lyu P., Song Y., et al. Function of dental follicle progenitor/stem cells and their potential in regenerative medicine: from mechanisms to applications. Biomolecules. 2021;11(7):997. doi: 10.3390/biom11070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J., Fan W., Deng Q., et al. Stem cells from the apical papilla: a promising source for stem cell-based therapy. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roato I., Chinigò G., Genova T., et al. Oral cavity as a source of mesenchymal stem cells useful for regenerative medicine in dentistry. Biomedicines. 2021;9(9):1085. doi: 10.3390/biomedicines9091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du L., Yang P., Ge S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J Dent Sci. 2016;11(3):304–314. doi: 10.1016/j.jds.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrige M., Frank E., Herardot E., et al. The future of regenerative medicine: cell therapy using pluripotent stem cells and acellular therapies based on extracellular vesicles. Cells. 2021;10(2):240. doi: 10.3390/cells10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cell. 2017;35(4):851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 15.Wei W., Ao Q., Wang X., et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Théry C., Witwer K.W., Aikawa E., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018):a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand S., Samuel M., Mathivanan S. Exomeres: a new member of extracellular vesicles family. Subcell Biochem. 2021;97:89–97. doi: 10.1007/978-3-030-67171-6_5. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Jeppesen D.K., Higginbotham J.N., et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol. 2021;23(12):1240–1254. doi: 10.1038/s41556-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juan T., Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Vidal M. Exosomes: revisiting their role as garbage bags. Traffic. 2019;20(11):815–828. doi: 10.1111/tra.12687. [DOI] [PubMed] [Google Scholar]
- 22.Wei D., Zhan W., Gao Y., et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31(2):157–177. doi: 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 24.Bissig C., Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014;24(1):19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 25.D'Souza-Schorey C., Schorey J.S. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018;62(2):125–133. doi: 10.1042/EBC20170078. [DOI] [PubMed] [Google Scholar]
- 26.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Tricarico C., Clancy J., D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8(4):220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muralidharan-Chari V., Clancy J., Plou C., et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muralidharan-Chari V., Clancy J.W., Sedgwick A., et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biancone L., Bruno S., Deregibus M.C., et al. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27(8):3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 31.Xie L., Mao M., Zhou L., et al. Spheroid mesenchymal stem cells and mesenchymal stem cell-derived microvesicles: two potential therapeutic strategies. Stem Cell Dev. 2016;25(3):203–213. doi: 10.1089/scd.2015.0278. [DOI] [PubMed] [Google Scholar]
- 32.Atkin-Smith G.K., Poon I.K.H. Disassembly of the dying: mechanisms and functions. Trends Cell Biol. 2017;27(2):151–162. doi: 10.1016/j.tcb.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Li M., Liao L., Tian W. Extracellular vesicles derived from apoptotic cells: an essential link between death and regeneration. Front Cell Dev Biol. 2020;8:573511. doi: 10.3389/fcell.2020.573511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller M., Bekeredjian-Ding I., Heyder P., et al. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15(1):183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Freitas D., Kim H.S., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidhom K., Obi P.O., Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):6466. doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bordanaba-Florit G., Royo F., Kruglik S.G., et al. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat Protoc. 2021;16(7):3163–3185. doi: 10.1038/s41596-021-00551-z. [DOI] [PubMed] [Google Scholar]
- 38.Lee K., Fraser K., Ghaddar B., et al. Multiplexed profiling of single extracellular vesicles. ACS Nano. 2018;12(1):494–503. doi: 10.1021/acsnano.7b07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stam J., Bartel S., Bischoff R., et al. Isolation of extracellular vesicles with combined enrichment methods. J Chromatogr, B: Anal Technol Biomed Life Sci. 2021;1169:122604. doi: 10.1016/j.jchromb.2021.122604. [DOI] [PubMed] [Google Scholar]
- 40.Liangsupree T., Multia E., Riekkola M.L. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Wang S., Cai Q., et al. Effective methods for isolation and purification of extracellular vesicles from plants. J Integr Plant Biol. 2021;63(12):2020–2030. doi: 10.1111/jipb.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biggs C.N., Siddiqui K.M., Al-Zahrani A.A., et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget. 2016;7(8):8839–8849. doi: 10.18632/oncotarget.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padda R.S., Deng F.K., Brett S.I., et al. Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma. Prostate. 2019;79(6):592–603. doi: 10.1002/pros.23764. [DOI] [PubMed] [Google Scholar]
- 44.Shen W., Guo K., Adkins G.B., et al. A single extracellular vesicle (EV) flow cytometry approach to reveal EV heterogeneity. Angew Chem Int Ed Engl. 2018;57(48):15675–15680. doi: 10.1002/anie.201806901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dirks R.M., Pierce N.A. Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A. 2004;101(43):15275–15278. doi: 10.1073/pnas.0407024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akers J.C., Gonda D., Kim R., et al. Biogenesis of extracellular vesicles (EV):exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro Oncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratajczak M.Z., Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia. 2020;34(12):3126–3135. doi: 10.1038/s41375-020-01041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battistelli M., Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology. 2020;9(1):21. doi: 10.3390/biology9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkin-Smith G.K., Tixeira R., Paone S., et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pathan M., Fonseka P., Chitti S.V., et al. Vesiclepedia 2019:a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–D519. doi: 10.1093/nar/gky1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeppesen D.K., Fenix A.M., Franklin J.L., et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koppers-Lalic D., Hackenberg M., Bijnsdorp I.V., et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Poon I.K.H., Parkes M.A.F., Jiang L., et al. Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles. 2019;8(1) doi: 10.1080/20013078.2019.1608786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kowal J., Arras G., Colombo M., et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurunathan S., Kang M.H., Jeyaraj M., et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitt J.M., Kroemer G., Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:10. doi: 10.3402/jev.v3.24641. 3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parolini I., Federici C., Raggi C., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathieu M., Martin-Jaular L., Lavieu G., et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 60.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 61.Kerr M.C., Teasdale R.D. Defining macropinocytosis. Traffic. 2009;10(4):364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 62.Costa Verdera H., Gitz-Francois J.J., Schiffelers R.M., et al. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Contr Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Sanz-Moreno V., Gadea G., Ahn J., et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 64.Swanson J.A. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9(8):639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Chen X., Gueydan C., et al. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28(1):9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunimatsu R., Nakajima K., Awada T., et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow–derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501(1):193–198. doi: 10.1016/j.bbrc.2018.04.213. [DOI] [PubMed] [Google Scholar]
- 67.Hara K., Yamada Y., Nakamura S., et al. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow–derived mesenchymal stem cells for mineralized tissue-forming cell biology. J Endod. 2011;37(12):1647–1652. doi: 10.1016/j.joen.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 68.Gholami L., Nooshabadi V.T., Shahabi S., et al. Extracellular vesicles in bone and periodontal regeneration: current and potential therapeutic applications. Cell Biosci. 2021;11:16. doi: 10.1186/s13578-020-00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dave J.R., Tomar G.B. Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Crit Rev Biomed Eng. 2018;46(5):429–468. doi: 10.1615/CritRevBiomedEng.2018027342. [DOI] [PubMed] [Google Scholar]
- 70.Kim D., Lee A.E., Xu Q., et al. Gingiva-derived mesenchymal stem cells: potential application in tissue engineering and regenerative medicine - a comprehensive review. Front Immunol. 2021;12:667221. doi: 10.3389/fimmu.2021.667221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma Y., Shobha K., Sundeep M., et al. Neural basis of dental pulp stem cells and its potential application in Parkinson's disease. CNS Neurol Disord: Drug Targets. 2022;21(1):62–76. doi: 10.2174/1871527320666210311122921. [DOI] [PubMed] [Google Scholar]
- 72.Anoop M., Datta I. Stem cells derived from human exfoliated deciduous teeth (SHED) in neuronal disorders: a review. Curr Stem Cell Res Ther. 2021;16(5):535–550. doi: 10.2174/1574888X16666201221151512. [DOI] [PubMed] [Google Scholar]
- 73.Venugopal C., K S., Rai K.S., et al. Neuroprotection by human dental pulp mesenchymal stem cells: from billions to nano. Curr Gene Ther. 2018;18(5):307–323. doi: 10.2174/1566523218666180913152615. [DOI] [PubMed] [Google Scholar]
- 74.Andrukhov O., Behm C., Blufstein A., et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cell. 2019;11(9):604–617. doi: 10.4252/wjsc.v11.i9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z., Jiang C.M., An S., et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20(1):25–34. doi: 10.1111/odi.12086. [DOI] [PubMed] [Google Scholar]
- 76.Ji L., Bao L., Gu Z., et al. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol Res. 2019;67(4):432–442. doi: 10.1007/s12026-019-09088-6. [DOI] [PubMed] [Google Scholar]
- 77.Yamada Y., Nakamura-Yamada S., Umemura-Kubota E., et al. Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy. Int J Mol Sci. 2019;20(23):5900. doi: 10.3390/ijms20235900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang C.M., Shin M.K., Jeon M., et al. Distinctive cytokine profiles of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. J Dent Sci. 2022;17(1):276–283. doi: 10.1016/j.jds.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeda H., Tomokiyo A., Fujii S., et al. Promise of periodontal ligament stem cells in regeneration of periodontium. Stem Cell Res Ther. 2011;2(4):33. doi: 10.1186/scrt74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Z., Shen Z., Zhan P., et al. Functional dental pulp regeneration: basic research and clinical translation. Int J Mol Sci. 2021;22(16):8991. doi: 10.3390/ijms22168991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kichenbrand C., Velot E., Menu P., et al. Dental pulp stem cell-derived conditioned medium: an attractive alternative for regenerative therapy. Tissue Eng B Rev. 2019;25(1):78–88. doi: 10.1089/ten.TEB.2018.0168. [DOI] [PubMed] [Google Scholar]
- 82.Bar J.K., Lis-Nawara A., Grelewski P.G. Dental pulp stem cell-derived secretome and its regenerative potential. Int J Mol Sci. 2021;22(21):12018. doi: 10.3390/ijms222112018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu C., Li Y., Han G. Advances of mesenchymal stem cells released extracellular vesicles in periodontal bone remodeling. DNA Cell Biol. 2022;41(11):935–950. doi: 10.1089/dna.2022.0359. [DOI] [PubMed] [Google Scholar]
- 84.Tziafas D., Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36(5):781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Anzelon T.A., Chowdhury S., Hughes S.M., et al. Structural basis for PiRNA targeting. Nature. 2021;597(7875):285–289. doi: 10.1038/s41586-021-03856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang A., Liu J., Zhuang X., et al. Identification and comparison of PiRNA expression profiles of exosomes derived from human stem cells from the apical papilla and bone marrow mesenchymal stem cells. Stem Cell Dev. 2020;29(8):511–520. doi: 10.1089/scd.2019.0277. [DOI] [PubMed] [Google Scholar]
- 87.Zhang S., Yang Y., Jia S., et al. Exosome-like vesicles derived from Hertwig's epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics. 2020;10(13):5914–5931. doi: 10.7150/thno.43156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu X., Zhong Y., Kong Y., et al. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther. 2019;10:170. doi: 10.1186/s13287-019-1278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C.C., Narayanan R., Alapati S., et al. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–115. doi: 10.1016/j.biomaterials.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo S., Gu J., Ma J., et al. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics. 2021;11(17):8379–8395. doi: 10.7150/thno.58052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shoushrah S.H., Transfeld J.L., Tonk C.H., et al. Sinking our teeth in getting dental stem cells to clinics for bone regeneration. Int J Mol Sci. 2021;22(12):6387. doi: 10.3390/ijms22126387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yi G., Zhang S., Ma Y., et al. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res Ther. 2022;13:41. doi: 10.1186/s13287-022-02721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei F., Li M., Lin T., et al. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022;141:333–343. doi: 10.1016/j.actbio.2021.12.035. [DOI] [PubMed] [Google Scholar]
- 94.Wang M., Li J., Ye Y., et al. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111:1–11. doi: 10.1016/j.diff.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Wu J., Chen L., Wang R., et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. 2019;5(7):3561–3571. doi: 10.1021/acsbiomaterials.9b00607. [DOI] [PubMed] [Google Scholar]
- 96.Wei J., Song Y., Du Z., et al. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol. 2020;51(4):455–466. doi: 10.1007/s10735-020-09896-3. [DOI] [PubMed] [Google Scholar]
- 97.Yan C., Li N., Xiao T., et al. Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med. 2022;20(1):208. doi: 10.1186/s12967-022-03412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakao Y., Fukuda T., Zhang Q., et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diomede F., Gugliandolo A., Cardelli P., et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 2018;9(1):104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imanishi Y., Hata M., Matsukawa R., et al. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm Regen. 2021;41(1):12. doi: 10.1186/s41232-021-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuang X., Ji L., Jiang H., et al. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cell Int. 2020;2020:5816723. doi: 10.1155/2020/5816723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y., Zhuang X., Yu S., et al. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res Ther. 2021;12(1):76. doi: 10.1186/s13287-021-02151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo H., Li B., Wu M., et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials. 2021;279:121223. doi: 10.1016/j.biomaterials.2021.121223. [DOI] [PubMed] [Google Scholar]
- 104.Lee A.E., Choi J.G., Shi S.H., et al. DPSC-derived extracellular vesicles promote rat jawbone regeneration. J Dent Res. 2023;102(3):313–321. doi: 10.1177/00220345221133716. [DOI] [PubMed] [Google Scholar]
- 105.Hade M.D., Suire C.N., Mossell J., et al. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–2125. doi: 10.1002/med.21918. [DOI] [PubMed] [Google Scholar]
- 106.Zhou H., Li X., Wu R.X., et al. Periodontitis-compromised dental pulp stem cells secrete extracellular vesicles carrying miRNA-378a promote local angiogenesis by targeting Sufu to activate the Hedgehog/Gli1 signalling. Cell Prolif. 2021;54(5) doi: 10.1111/cpr.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kou X., Xu X., Chen C., et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10(432):eaai8524. doi: 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi Q., Qian Z., Liu D., et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gugliandolo A., Mazzon E. Dental mesenchymal stem cell secretome: an intriguing approach for neuroprotection and neuroregeneration. Int J Mol Sci. 2021;23(1):456. doi: 10.3390/ijms23010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mao Q., Nguyen P.D., Shanti R.M., et al. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. 2019;25(11–12):887–900. doi: 10.1089/ten.TEA.2018.0176. [DOI] [PubMed] [Google Scholar]
- 111.Rao F., Zhang D., Fang T., et al. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cell Int. 2019;2019:2546367. doi: 10.1155/2019/2546367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu Z., Wen Y., Jiang N., et al. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. doi: 10.1016/j.biomaterials.2022.121484. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Yang Y.Y., Ren J.L., et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8:198. doi: 10.1186/s13287-017-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jarmalavičiūtė A., Tunaitis V., Pivoraitė U., et al. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy. 2015;17(7):932–939. doi: 10.1016/j.jcyt.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y., Wang Z., Shi B., et al. Effect of gingival mesenchymal stem cell-derived exosomes on inflammatory macrophages in a high-lipid microenvironment. Int Immunopharm. 2021;94:107455. doi: 10.1016/j.intimp.2021.107455. [DOI] [PubMed] [Google Scholar]
- 116.Rajan T.S., Giacoppo S., Diomede F., et al. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci Rep. 2016;6:38743. doi: 10.1038/srep38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S., Luo L., He Y., et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54(8) doi: 10.1111/cpr.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sonoda S., Murata S., Kato H., et al. Targeting of deciduous tooth pulp stem cell-derived extracellular vesicles on telomerase-mediated stem cell niche and immune regulation in systemic lupus erythematosus. J Immunol. 2021;206(12):3053–3063. doi: 10.4049/jimmunol.2001312. [DOI] [PubMed] [Google Scholar]
- 119.Lin T., Wu N., Wang L., et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR-140-5p-overexpressing human dental pulp stem cells. Int J Mol Med. 2021;47(3):7. doi: 10.3892/ijmm.2020.4840. [DOI] [PubMed] [Google Scholar]
- 120.Liu C., Hu F., Jiao G., et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J Nanobiotechnol. 2022;20:65. doi: 10.1186/s12951-022-01273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng J., Kong Y., Hu X., et al. microRNA-enriched small extracellular vesicles possess odonto-immunomodulatory properties for modulating the immune response of macrophages and promoting odontogenesis. Stem Cell Res Ther. 2020;11:517. doi: 10.1186/s13287-020-02039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang Y., Liu Q., Liu L., et al. Lipopolysaccharide-preconditioned dental follicle stem cells derived small extracellular vesicles treating periodontitis via reactive oxygen species/mitogen-activated protein kinase signaling-mediated antioxidant effect. Int J Nanomed. 2022;17:799–819. doi: 10.2147/IJN.S350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Čebatariūnienė A., Kriaučiūnaitė K., Prunskaitė J., et al. Extracellular vesicles suppress basal and lipopolysaccharide-induced NFκB activity in human periodontal ligament stem cells. Stem Cell Dev. 2019;28(15):1037–1049. doi: 10.1089/scd.2019.0021. [DOI] [PubMed] [Google Scholar]
- 124.Zheng Y., Dong C., Yang J., et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234(11):20662–20674. doi: 10.1002/jcp.28671. [DOI] [PubMed] [Google Scholar]
- 125.Zarubova J., Hasani-Sadrabadi M.M., Dashtimoghadam E., et al. Engineered delivery of dental stem-cell-derived extracellular vesicles for periodontal tissue regeneration. Adv Healthc Mater. 2022;11(12) doi: 10.1002/adhm.202102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meng W., Hao Y., He C., et al. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. 2019;18:57. doi: 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Au Yeung C.L., Co N.N., Tsuruga T., et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sharma A. Role of stem cell derived exosomes in tumor biology. Int J Cancer. 2018;142(6):1086–1092. doi: 10.1002/ijc.31089. [DOI] [PubMed] [Google Scholar]
- 130.Xu X., Chen C., Akiyama K., et al. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92(9):825–832. doi: 10.1177/0022034513497961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W., Han Y., Zhao Z., et al. Oral mucosal mesenchymal stem cell-derived exosomes: a potential therapeutic target in oral premalignant lesions. Int J Oncol. 2019;54(5):1567–1578. doi: 10.3892/ijo.2019.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Antimisiaris S.G., Mourtas S., Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10(4):218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu M., Zhao X., Xing H., et al. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm. 2018;550(1–2):100–113. doi: 10.1016/j.ijpharm.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 134.Klimova D., Jakubechova J., Altanerova U., et al. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Mol Cell Probes. 2023;67:101894. doi: 10.1016/j.mcp.2023.101894. [DOI] [PubMed] [Google Scholar]
- 135.Coccè V., Franzè S., Brini A.T., et al. In vitro anticancer activity of extracellular vesicles (EVs) secreted by gingival mesenchymal stromal cells primed with paclitaxel. Pharmaceutics. 2019;11(2):61. doi: 10.3390/pharmaceutics11020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vakhshiteh F., Rahmani S., Ostad S.N., et al. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871. doi: 10.1016/j.lfs.2020.118871. [DOI] [PubMed] [Google Scholar]
- 137.Witwer K.W., Goberdhan D.C., O'Driscoll L., et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10(14) doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]