Abstract
Diabetes mellitus (DM) and its associated complications are considered one of the major health risks globally. Among numerous complications, diabetic cardiomyopathy (DCM) is characterized by increased accumulation of lipids and reduced glucose utilization following abnormal lipid metabolism in the myocardium along with oxidative stress, myocardial fibrosis, and inflammation that eventually result in cardiac dysfunction. The abnormal metabolism of lipids plays a fundamental role in cardiac lipotoxicity following the occurrence and development of DCM. Recently, it has been revealed that cannabinoid type-2 (CB2) receptors, an essential component of the endocannabinoid system, play a crucial role in the pathogenesis of obesity, hyperlipidemia, and DM. Provided the role of CB2R in regulating the glucolipid metabolic dysfunction and its antioxidant as well as anti-inflammatory activities, we carried out the current study to investigate the protective effects of a selective CB2R agonist, β-caryophyllene (BCP), a natural dietary cannabinoid in the murine model of DCM and elucidated the underlying pharmacological and molecular mechanisms. Mice were fed a high-fat diet for 4 weeks followed by a single intraperitoneal injection of streptozotocin (100 mg/kg) to induce the model of DCM. BCP (50 mg/kg body weight) was given orally for 12 weeks. AM630, a CB2R antagonist, was given 30 min before BCP treatment to demonstrate the CB2R-dependent mechanism of BCP. DCM mice exhibited hyperglycemia, increased serum lactate dehydrogenase, impaired cardiac function, and hypertrophy. In addition, DCM mice showed alternations in serum lipids and increased oxidative stress concomitant to reduced antioxidant defenses and enhanced cardiac lipid accumulation in the diabetic heart. DCM mice also exhibited activation of TLR4/NF-κB/MAPK signaling and triggered the production of inflammatory cytokines and inflammatory enzyme mediators. However, treatment with BCP exerted remarkable protective effects by favorable modulation of the biochemical and molecular parameters, which were altered in DCM mice. Interestingly, pretreatment with AM630 abrogated the protective effects of BCP in DCM mice. Taken together, the findings of the present study demonstrate that BCP possesses the capability to mitigate the progression of DCM by inhibition of lipotoxicity-mediated cardiac oxidative stress and inflammation and favorable modulation of TLR4/NF-κB/MAPK signaling pathways mediating the CB2R-dependent mechanism.
Keywords: cannabinoid type-2 receptors; β-caryophyllene; diabetic cardiomyopathy, lipotoxicity; oxidative stress; inflammation
Diabetes mellitus (DM) is the major predominant chronic metabolic disease and is the fifth main reason of mortality globally.1 DM and its associated complications are considered one of the serious risks to the contemporary society. Among numerous diabetic complications, diabetic cardiomyopathy (DCM) has been gaining enormous attention due to its morbidity and mortality rates.2 The major hallmarks of DCM are the increased accumulation of lipids and reduced glucose utilization as a result of abnormal lipid metabolism in the myocardium, oxidative stress, and inflammation, in addition to structural changes such as myocardial fibrosis and remodeling, which eventually result in cardiac dysfunction.3 The cardiac lipotoxicity caused by abnormal metabolism of lipids plays a fundamental role in the development and progression of DCM.4 Increasing evidence shows that oxidative damage and inflammation are accelerated at the early stage of diabetes, which negatively influence the cardiac function and structure.5,6
Oxidative stress has been shown to play a role in the production of inflammatory cytokines. Consequently, overt generation of free radicals that culminate into oxidative injuries and inflammatory responses are implicated in the pathogenesis of myocardial damage.7 A considerable number of studies reported that type-2 DM provokes many signaling pathways that play an important role in the progression of myocardial inflammation.7 The mitogen-activated protein kinase (MAPK) family, which includes extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, is upregulated with heart failure,8 myocardial hypertrophy,9 insulin resistance,10 and inflammation.11 Additionally, the TLR4-mediated inflammatory response has been shown to play a critical role in DCM pathogenesis.12 Numerous signaling pathways trigger nuclear factor κB (NF-κB), which eventually promotes the overproduction of inflammatory mediators.13
To counter oxidative stress and immunoinflammatory cascades, numerous novel pathways are being explored. One of the recently emerged pharmacological targets for future therapeutics in cardiovascular diseases is the endocannabinoid system, which consists of cannabinoid type-1 and -2 (CB1R and CB2R) receptors.14 Among different elements of the endocannabinoid system, CB2R has attracted enormous attention for its various pharmacological effects in addition to the absence of psychotropic side effects mediated via CB1R.15 The CB2R has been found to be expressed in the left ventricular (LV) fibroblasts of neonates and adults, LV cardiomyocytes, vascular smooth muscle cells, and endothelial cells of larger arteries.16 The CB2R is a class A G protein-coupled receptor (GPCR) that couples predominantly to Gαi/o proteins, leading to inhibition of adenylyl cyclase activity and mediation of MAPK activation.17 A growing amount of evidence has demonstrated that CB2R activation regulates numerous pathophysiological processes18 and is involved in modulating several pathological conditions, including inflammation,19 atherosclerosis,20 diabetes,21 and cardiovascular disease.22 The cannabinoid receptors have been shown to be targeted by endogenous, synthetic, and phytocannabinoid (plant-derived) compounds. In recent years, plant-derived bioactive compounds, commonly known as phytochemicals, have received attention due to their extensive biological activities, pharmacological effects, and therapeutic potential. The phytochemical classes, specifically sesquiterpenes, have attracted much interest due to their potent antioxidant, anti-inflammatory, and hypoglycemic effects and therapeutic benefits by modulating the enzymes, receptors, and signaling pathways disturbed in diabetes and its related complications.23 Among the sesquiterpenes, β-caryophyllene (BCP), a natural bicyclic, non-psychoactive compound predominantly found in various plants such as clove, cinnamon, red pepper, rosemary, hops, basil, black pepper, thyme, and oregano, has attracted attention due to its potent therapeutic and pharmacological properties.24 BCP is accepted to be utilized in flavors and cosmetics according to the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) due to its lower toxicity and favorable scent and taste.24 BCP is considered a safe and nontoxic natural sesquiterpene with a lethal dose (LD50) of 5 g/kg in rodents.25
Among plant-derived agents, BCP has garnered more attention since its recognition as a selective pharmacological agonist of human CB2R with a Ki of 155 nmol/L with negligible affinity for CB1R, which stimulates activation of the Gi/Go subfamily of G-proteins.26 The CB2R-mediated therapeutic benefits of BCP have been demonstrated in various disorders influencing different organs such as liver,27 kidney,28 and brain.29 Moreover, BCP has been shown to have potent antioxidant30 and anti-inflammatory31 properties similar to numerous other phytochemicals. Accumulating evidence reported that BCP possesses a variety of pharmacological effects related to its benefits in metabolic abnormalities such as correction of hyperglycemia and hyperlipidemia and improvement of insulin secretion and sensitivity.32,33 Considering the pleiotropic properties of BCP, it is worthwhile to investigate BCP for its potential benefits in DM and its associated complications and underlying mechanisms. Furthermore, CB2R activation has been shown to alleviate oxidative stress and inflammation associated with diabetes along with accelerating insulin secretion.34 Taking into account the cardiovascular complications associated with type-2 DM and attractive pharmacological activities of an emerging natural CB2R agonist, i.e., BCP, the current study was carried out to investigate the protective effects of BCP against type-2 DM-mediated cardiovascular damage focusing on lipid metabolism abnormalities, oxidative stress, and inflammation, and the underlying mechanisms including CB2R dependency were also investigated.
Materials and Methods
Drugs and Chemicals
β-Caryophyllene (BCP) was obtained from Sigma-Aldrich (St. Louis, MO, USA), and streptozotocin (STZ) was bought from ChemCruz, Santa Cruz, CA, USA. The CB2R antagonist AM630 was obtained from Cayman Chemical Company, Ann Arbor, MI, USA. Trisodium citrate dihydrate, hydrogen peroxide solution, ethanol, xylene, hematoxylin, glutaraldehyde solution, and uranyl acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lead citrate was obtained from Electron Microscopy Sciences, Hatfield, PA, USA.
Animals
Adult, healthy, male C57BL/6 mice, weighing 20–25 g, were used in this study. The animals were bred in the animal research facility of the College of Medicine and Health Sciences, United Arab Emirates University, and were randomized and distributed into different study groups. Approval of the animal experimental protocols was obtained by the Animal Ethics Committee of the United Arab Emirates University, United Arab Emirates. The mice were kept in a pathogen-free plastic cage at a constant temperature of 22 ± 2 °C and a humidity of 50 ± 10% on a 12:12 light/dark cycle and were adapted for 1 week before the beginning of the experiment. The animals had free access to drinking water and were fed with a matching standard rodent diet.
Diabetes Induction and Experimental Design
Murine DCM model was developed by feeding mice with a high-fat diet (HFD: 45% Kcal from fat, no. D12451; Research Diets, New Brunswick, NJ, USA) for 4 weeks with intraperitoneal injection of one dose of STZ at 100 mg/kg/BW [freshly dissolved in 0.1 M citrate buffer with pH 4.5].35 One week following the STZ injection, animals with a fasting blood glucose level of ≥250 mg/dl were defined as diabetic mice and further included in the study and continued to feed with HFD for another 12 weeks.
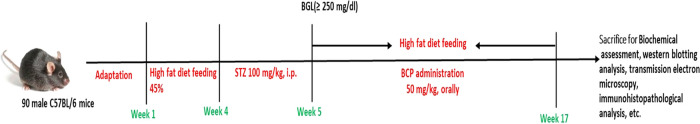
Animals were randomly assigned into six groups, each comprising 15 mice. The groups were as follows Group I: the naïve group received the vehicle (light olive oil) orally for 12 weeks; Group II: the BCP group received BCP (50 mg/kg, orally) for 12 weeks; Group III: the HFD group received the vehicle (light olive oil) orally for 12 weeks; Group IV: the diabetic cardiomyopathy group (DCM) received the vehicle (light olive oil) orally for 12 weeks; Group V: DCM mice received BCP (50 mg/kg, orally) for 12 weeks; and Group VI: DCM mice were treated with AM630 (1.5 mg/kg, orally), a selective CB2R antagonist 30 min prior to the administration of BCP (50 mg/kg, orally) for 12 weeks. BCP was freshly prepared in scientific-grade light olive oil before dosing. The dose of BCP was chosen based on the previous studies including our laboratory studies.36 The schematic representation of the study design and duration is illustrated in Figure 1.
Figure 1.
Schematic presentation of the study protocol.
Assessment of Body Weights and Blood Glucose Concentrations
The body weight (grams) of mice was measured before the initiation of dosing and every 2 weeks and before termination of the experiment. Also, bi-weekly fasting blood glucose concentrations were observed using a glucometer (One Touch Select glucometer).
Biochemical Analysis
After termination of the experiment, the blood samples were collected from the inferior vena cava and were used for biochemical analyses. For serum samples, blood should be allowed to clot at room temperature for at least 15–30 min and then centrifuged at 4000 rpm for 10 min. The serum separated was used for biochemical analyses. The serum concentrations of LDH were analyzed using a VetTest 8008 automatic serum chemistry analyzer (Idexx Laboratories, Hoofddrop, The Netherlands). The serum concentrations of total cholesterol (TC) were examined using a VetTest 8008 automatic serum chemistry analyzer (Idexx Laboratories, Hoofddrop, The Netherlands). The levels of triglycerides (TGs) in mouse serum were assayed using a triglyceride assay kit (ab65336; Abcam, Cambridge, U.K.). The serum levels of high-density lipoprotein cholesterol (HDL-C) in mice were measured using a cholesterol assay kit (ab65390; Abcam, Cambridge, U.K.) exactly as described by the manufacturer.
Low-density lipoprotein cholesterol (LDL-C) levels in serum were assessed using Friedewald’s formula37
Very low-density lipoprotein (VLDL-C) cholesterol was measured using the formula
After euthanizing the animals, the heart was immediately excised from mice of each experimental group and dried on filter papers and directly stored in liquid nitrogen or fixed in 4% paraformaldehyde for further biochemical and histopathological uses.
Hemodynamic Measurements
The noninvasive tail cuff method using a CODA blood pressure system (Kent Scientific Corporation, Torrington, CT, USA) was used to measure the systolic blood pressure (SP), diastolic blood pressure (DP), mean arterial pressure (MAP), and heart rate (HR). The mice were restrained in a plastic tube holder on the heating pad (kept at 37 °C). Each animal should be allowed to acclimatize to the holder for 5 min before the blood pressure measurement. The blood pressure was measured for 10 acclimation cycles followed by 15 measurement cycles. Three days after the training period, formal measurements for the unanesthetized blood pressure and HR were recorded.
Assessment of the Oxidative/Antioxidative Status in the DCM Mouse Heart
The levels of malondialdehyde (MDA) in the heart homogenate were estimated using a commercially available MDA kit (Northwest life science specialties, Vancouver, WA, USA) according to the method described. The reduced glutathione (GSH) contents were examined using commercially available GSH kits (Sigma-Aldrich, St. Louis, MO, USA) according to the method described. The results are expressed as μM MDA and nmoles/ml GSH.
Analysis of Inflammatory Cytokines
The levels of inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in the heart homogenates were obtained by using the enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). The results were expressed as pg/ml.
Oil Red O staining
Myocardial lipid accumulation was determined using an Oil Red O stain kit (ab150678; Abcam, Cambridge, UK). Briefly, heart cryosections (5 μm) were fixed in cold propylene glycol for 5 min; then, slides were kept overnight at room temperature in Oil Red O solution. After differentiation in 85% propylene glycol for one min and rinsing in two changes of distilled water, the frozen sections were counterstained with hematoxylin for one min. Photographs were taken and visualized through a light microscope (BX43, Olympus Co. Ltd, Japan). The lipid droplets were recognized by red staining. The degree of lipid deposits in cardiac tissue was quantitatively measured by using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA).
Transmission Electron Microscopy (TEM)
Following fixation in Karnovsky’s fixative, the heart tissues were dehydrated using ascending ethanol grades and embedded in Epon resin. Ultrathin sections were cut using an ultramicrotome and then stained with uranyl acetate and lead citrate. Finally, the lipid droplets in heart tissues were detected with TEM.
Immunohistochemistry
The heart sections were dewaxed and incubated with citrate buffer at pH (6.1) in a water bath (95 °C) for 40 min to confirm the antigen retrieval. Then, the samples were treated with 0.3% hydrogen peroxide to inhibit endogenous peroxidase activity and stained with VECTASTAIN Elite ABC kits, according to the manufacturer’s protocol supplied with the kit (Vector Laboratories Inc, Burlingame, CA, USA). After blocking with the Vectastain blocking reagent, the sections were incubated overnight with p-NF-κB p65 (1:50) (mouse IgG monoclonal, Cat#136548, Santa Cruz Biotechnology, USA). The sections were then incubated with the biotinylated secondary antibody for 1 h at room temperature and thereafter with the avidin–biotin complex (VECTASTAIN Elite ABC kits) for another 1 h. The sections were then developed with DAB (DAB Kit, Vector Laboratories Inc, Burlingame, CA, USA) for 5 min and counterstained with hematoxylin for 1 min. For detection of the % area of cells immunostained with p-NF-κB p65, the images of selected regions of heart sections were captured by using a digital camera (BX43, Olympus Co. Ltd, Japan). Positive staining (brown) in each section was quantitatively measured by using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA).
Western Blotting
Total protein extracts from frozen heart samples were prepared by homogenizing cardiac tissues in an ice-cold RIPA buffer supplemented with a cocktail of protease and phosphatase inhibitors (Sigma-Aldrich, MO, USA), and the tissue lysates were then centrifuged on a microcentrifuge (14,000 rpm, 30 min, 4 °C). Laemmli loading buffer (Bio-Rad, Hercules, CA, USA) containing 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) was added to the supernatant and then was separated by gel electrophoresis and transferred to PVDF membranes (Amersham Hybond P 0.45, PVDF, GE Healthcare Life Sciences, Germany). The membranes were incubated with the corresponding primary antibody (Table 1) overnight at 4 °C and the appropriate secondary antibodies. Protein blots were developed by using a chemiluminescence West Pico kit obtained from Thermo Fisher Scientific, Waltham, MA, USA. The densitometric analysis of protein bands was done using the ImageJ program (National Institutes of Health, Bethesda, Maryland, USA).
Table 1. Source, Manufacturer, Catalog, and Dilution of Primary Antibodies Used in the Present Study.
| primary antibody | dilution | Cat No | manufacturer | sources of species |
|---|---|---|---|---|
| BNP | 1:500 | ab19645 | Abcam | rabbit |
| p-NF-κB p65 | 1:500 | sc-136548 | Santa Cruz Biotechnology | mouse |
| p-IκBα | 1:500 | 2859 | Cell Signaling Technology | rabbit |
| TLR4 | 1:500 | ab13556 | Abcam | rabbit |
| iNOS | 1:500 | ab3523 | Abcam | rabbit |
| ERK | 1:1000 | ab36991 | Abcam | rabbit |
| p-ERK | 1:500 | sc-7383 | Santa Cruz Biotechnology | mouse |
| JNK | 1:1000 | sc-7345 | Santa Cruz Biotechnology | mouse |
| p-JNK | 1:500 | sc-6254 | Santa Cruz Biotechnology | mouse |
| P38 | 1:1000 | ab31828 | Abcam | rabbit |
| p-p38 | 1:500 | ab47363 | Abcam | rabbit |
| GAPDH | 1:2000 | 97166 | Cell Signaling Technology | mouse |
| β-tubulin | 1:2000 | 2128 | Cell Signaling Technology | rabbit |
| vinculin | 1:2000 | sc-73614 | Santa Cruz Biotechnology | mouse |
Statistical Analysis
Results are reported as mean ± SEM. The nonparametric t-test (Mann–Whitney U test) was used to compare the significance difference between two groups, and analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to compare the mean among various groups. The data were analyzed for statistical significance using GraphPad Prism version 8 (GraphPad Software Boston, MA, USA). Values of P < 0.05 were considered to indicate a statistically significant difference.
Results
Effects of BCP on Body Weight and Blood Glucose Levels in DCM
Figure 2A,B illustrates the alterations in body weights and blood glucose levels throughout the study period. HFD mice demonstrated a remarkable rise in body weight compared to that of naïve mice fed with normal diet, whereas the other groups did not show significant difference in the body weights. In addition, HFD-fed mice had mild hyperglycemia, while DCM mice showed further elevation in fasting blood glucose levels compared to naïve mice. BCP treatment for 12 weeks appeared to decrease fasting blood glucose levels significantly in DCM mice. However, AM630 treatment prior to BCP reversed the effectiveness of BCP on reducing hyperglycemia.
Figure 2.
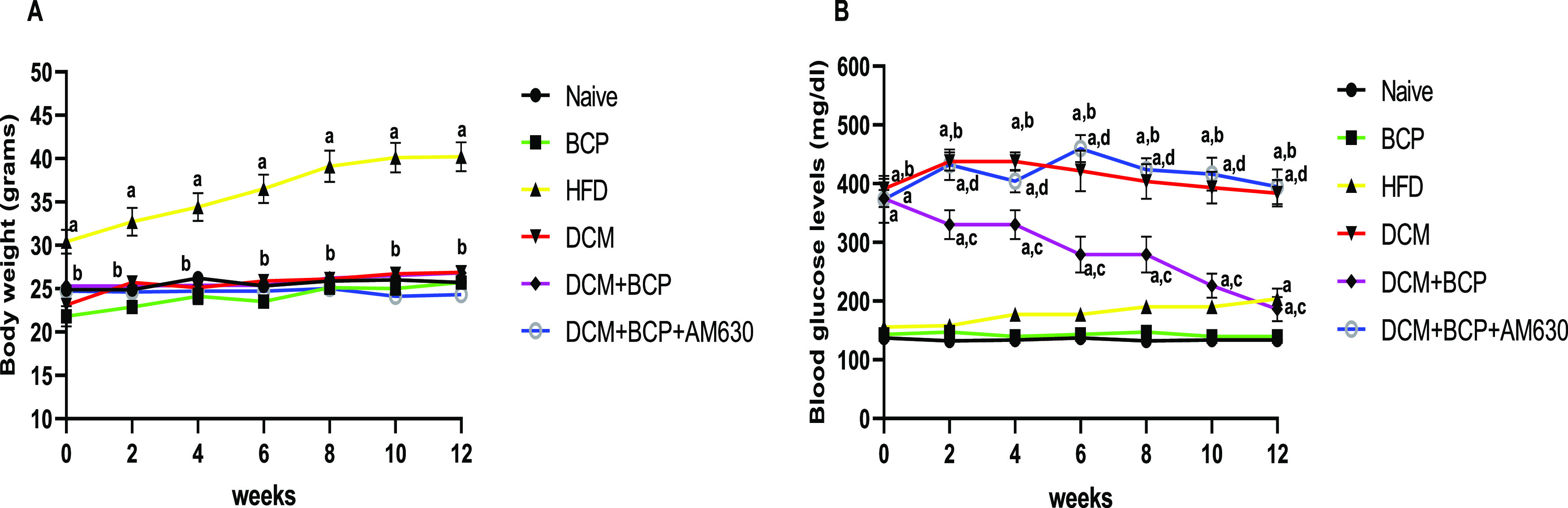
Effects of BCP on body weight and blood glucose levels in DCM mice. (A) Body weight. (B) Blood glucose. Data are expressed as mean ± SEM, n = 8–10. aP < 0.05 compared to naive, bP < 0.05 compared to HFD, cP < 0.05 compared to DCM, and dP < 0.05 compared to DCM + BCP.
Effects of BCP on Myocardial Injury, Cardiac Function, and Hypertrophy in DCM
Serum LDH and hemodynamic parameters were estimated to evaluate the myocardial injury and cardiac function of the DCM mice. The serum level of LDH was significantly elevated in DCM mice compared to naïve mice, indicating significant myocardial damage. However, BCP administration inhibited the injury-induced LDH release into the serum as indicated by a remarkable reduction in cardiac injury marker enzymes in the serum compared to DCM mice (Figure 3A). Interestingly, the protective effects of BCP on LDH release were significantly blocked by prior treatment with AM630 to the DCM mice as depicted in Figure 3A. As shown in Figure 3B–E, HFD mice and DCM mice revealed a remarkable elevation in systolic, diastolic, and mean arterial pressure, while a significant decrease in the heart rate compared to the naïve group was observed. BCP treatment significantly restored the systolic, diastolic, and mean arterial pressure without showing significant difference in the heart rate compared to DCM mice. However, AM630 administration prior to treatment with BCP has no significant alteration in the hemodynamic parameters of DCM mice.
Figure 3.
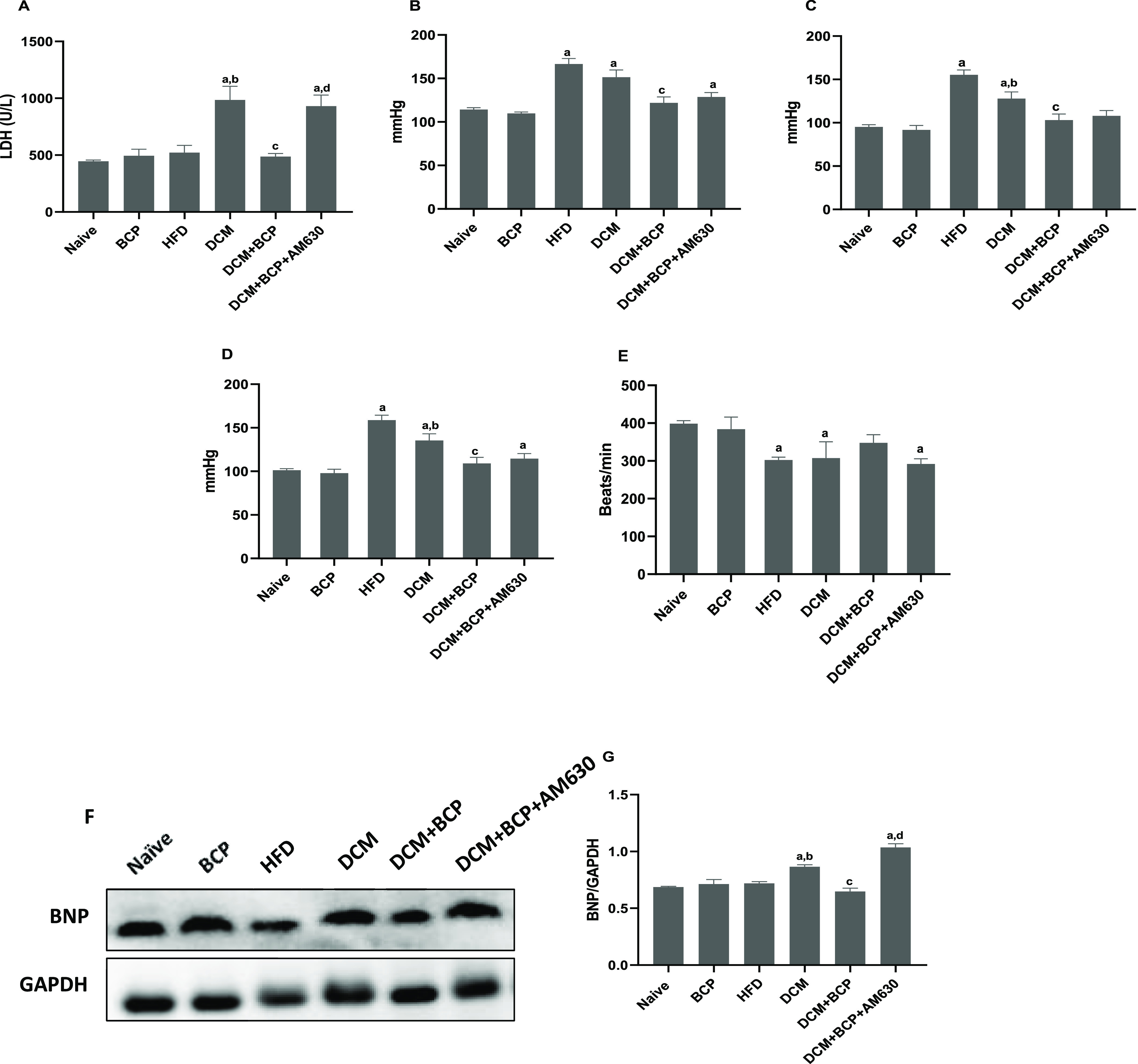
Effects of BCP on the cardiac function, myocardial injury, and hypertrophy in DCM mice. (A) Serum marker of myocardial injury; LDH. n = 6. (B) Systolic blood pressure. (C) Diastolic blood pressure. (D) Mean arterial pressure. (E) Heart rate. n = 6–8. (F) Representative image of western blot analysis of BNP. (G) Densitometric analysis of the myocardial protein expression of BNP. n = 3. Data are expressed as mean ± SEM. aP < 0.05 compared to naive, bP < 0.05 compared to HFD, cP < 0.05 compared to DCM, and dP < 0.05 compared to DCM + BCP. LDH, lactate dehydrogenase and BNP, brain natriuretic peptide.
Cardiac hypertrophy is also one of the characteristic features of DCM. Hence, the effect of BCP oral administration on myocardial hypertrophy was assessed. We examined the protein expression of the myocardial hypertrophy marker brain natriuretic peptide (BNP) by western blot (Figure 3F,G). The results revealed that BCP treatment markedly attenuated diabetes-stimulated BNP. Interestingly, prior administration of AM630 abrogated this protective effect of BCP. These findings suggested that BCP ameliorates type-2 diabetes-induced myocardial damage and hypertrophy by activating CB2R.
Effects of BCP on Myocardial Lipid Metabolism in DCM
Abnormalities in fatty acid metabolism in cardiac tissue are one of the underlying reasons of DCM.38 Excessive lipid intake causes lipid accumulation in cardiomyocytes, which are stored in the cardiomyocyte cytosol as lipid droplets. Thus, myocardial lipid accumulation is one of the major features of DCM.38 As depicted in Figure 4A–E, HFD and DCM mice demonstrated atherogenic lipid profiles featured by a remarkable elevation of TGs levels, TC, LDL-C, HDL-C, and VLDL-C when compared to the naïve mice. Treatment of the DCM mice with BCP showed significant reversal of these lipid profile derangements except for HDL-C. Administration of AM630 before BCP treatment reversed the positive effect of BCP on these lipid parameters except for TC and HDL-C.
Figure 4.
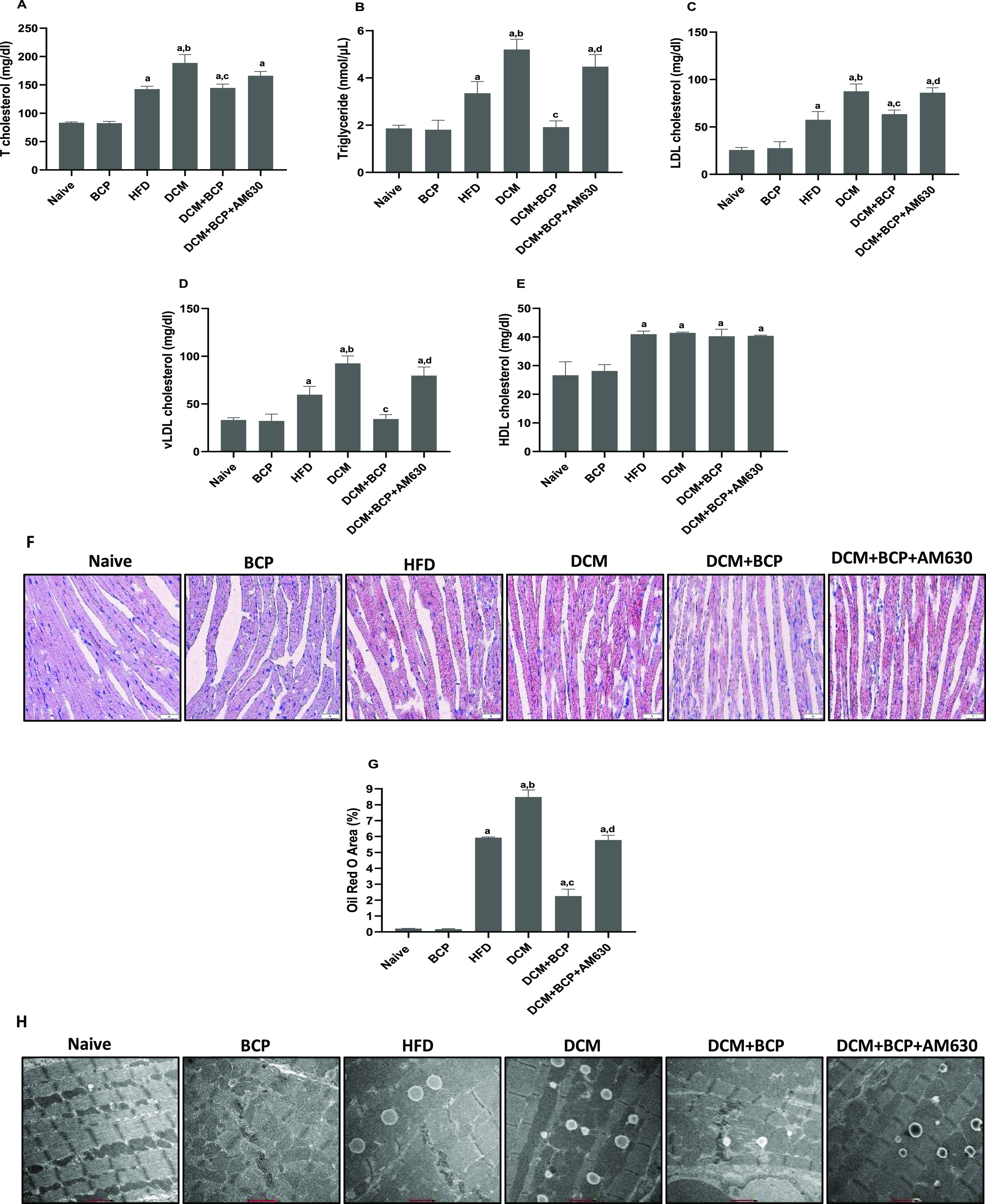
Effects of BCP on lipid metabolism in DCM mice. Serum levels of (A) TC, (B) TGs, (C) LDL-C, (D) VLDL-C, and (E) HDL-C. n = 6. (F) Lipid droplet accumulation in myocardium by Oil red O staining (40×). (G) Semiquantitative analysis of Oil red O staining to evaluate the lipid accumulation. n = 3. (H) Electron micrographs of lipid droplets in cardiomyocytes (11,500×). n = 3. Data are expressed as mean ± SEM. aP < 0.05 compared to naive, bP < 0.05 compared to HFD, cP < 0.05 compared to DCM, and dP < 0.05 compared to DCM + BCP. TC, total cholesterol; TGs, triglycerides; LDL-C, low-density lipoprotein; VLDL-C, very low-density lipoprotein; HDL-C, high-density lipoprotein.
Besides, Oil Red O staining was used for staining lipid droplets in cardiac tissues to assess the degree of myocardial lipid accumulation (Figure 4F,G). The results demonstrated a remarkable elevation in myocardial lipid accumulation of HFD-fed mice, compared to naïve mice. DCM mice displayed more increase in myocardial lipid accumulation. Treating DCM mice with BCP significantly reduced the level of lipid accumulation in the cardiac tissues. However, AM630 administration prior to BCP treatment removed the protective effects of BCP. The degree of lipid accumulation in the myocardium was further proved by transmission electron microscopy (Figure 4H). The results demonstrate a remarkable lipid droplet in the myocardium of HFD-fed mice and further rise in DCM mice. BCP treatment significantly reduced lipid droplets, which was further abrogated by AM630, a CB2R antagonist. Taken together, these data reveal that BCP can attenuate the abnormal lipid metabolism in cardiomyocytes induced by T2DM in a CB2R-dependent mechanism.
Effects of BCP on Cardiac Oxidative Damage in DCM
HFD mice showed a significant increase in cardiac oxidative stress, as manifested by a remarkable elevation of the lipid peroxidation product MDA along with a significant reduction of GSH. The DCM group mice further showed enhanced myocardial oxidative stress, which was entirely inhibited by BCP oral treatment. Giving AM630 ahead of BCP treatment markedly alleviated the antioxidant effects of BCP (Figure 5A,B). Overall, these findings indicate that BCP treatment inhibited oxidative damage in DCM mice mediating activation of CB2R.
Figure 5.
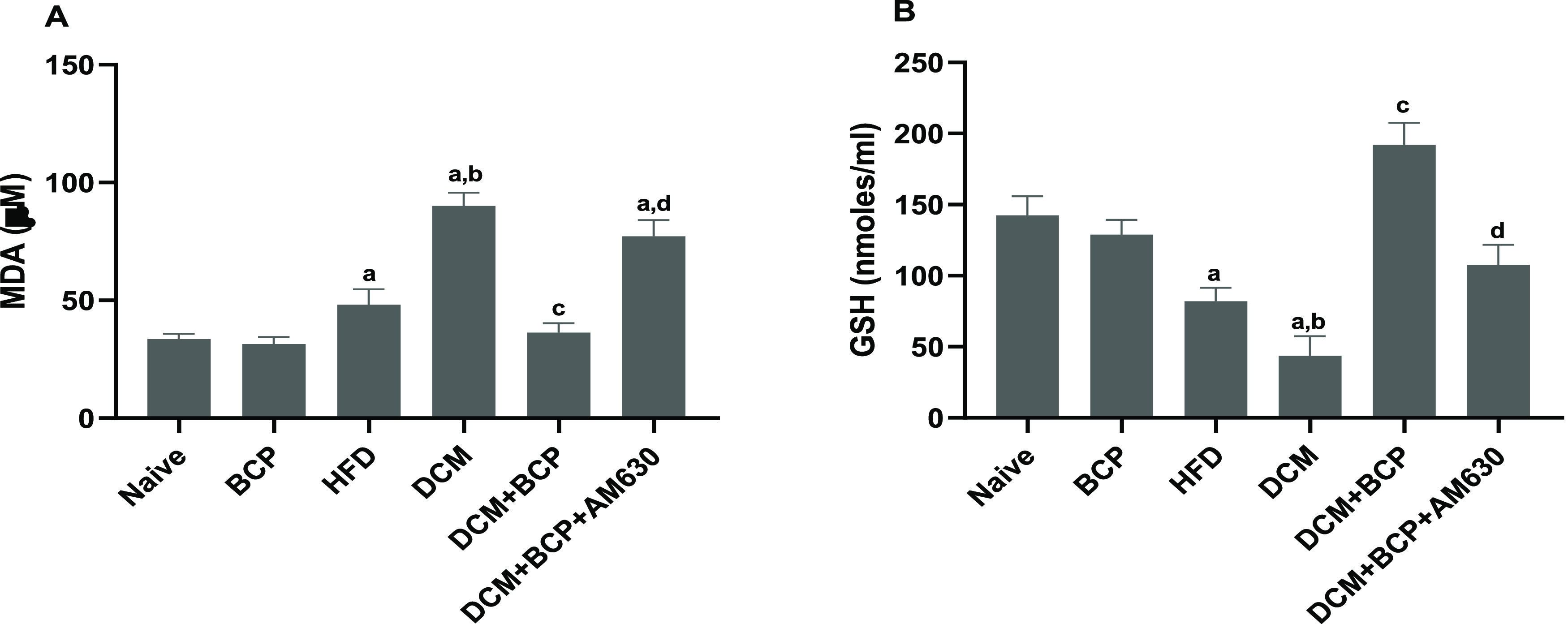
Effects of BCP on oxidative stress markers in DCM mice. (A) MDA. (B) GSH. Data are expressed as mean ± SEM, n = 6. aP < 0.05 compared to naive, bP < 0.05 to HFD, cP < 0.05 to DCM, and dP < 0.05 to DCM + BCP. MDA, malondialdehyde and GSH, reduced glutathione.
Effects of BCP on Cardiac Inflammation in DCM
Next, to examine the inflammation in DCM mice, the proinflammatory cytokine levels (i.e., TNF-α, IL-6, and IL-1β) in heart tissues were evaluated. As shown in Figure 6A–C, the cardiac levels of TNF-α, IL-6, and IL-1β were markedly elevated in HFD mice in comparison with the naïve group and further increased in DCM mice. Treating DCM mice with BCP significantly decreased the production of proinflammatory cytokines, an effect that was abrogated by prior administration of AM630.
Figure 6.
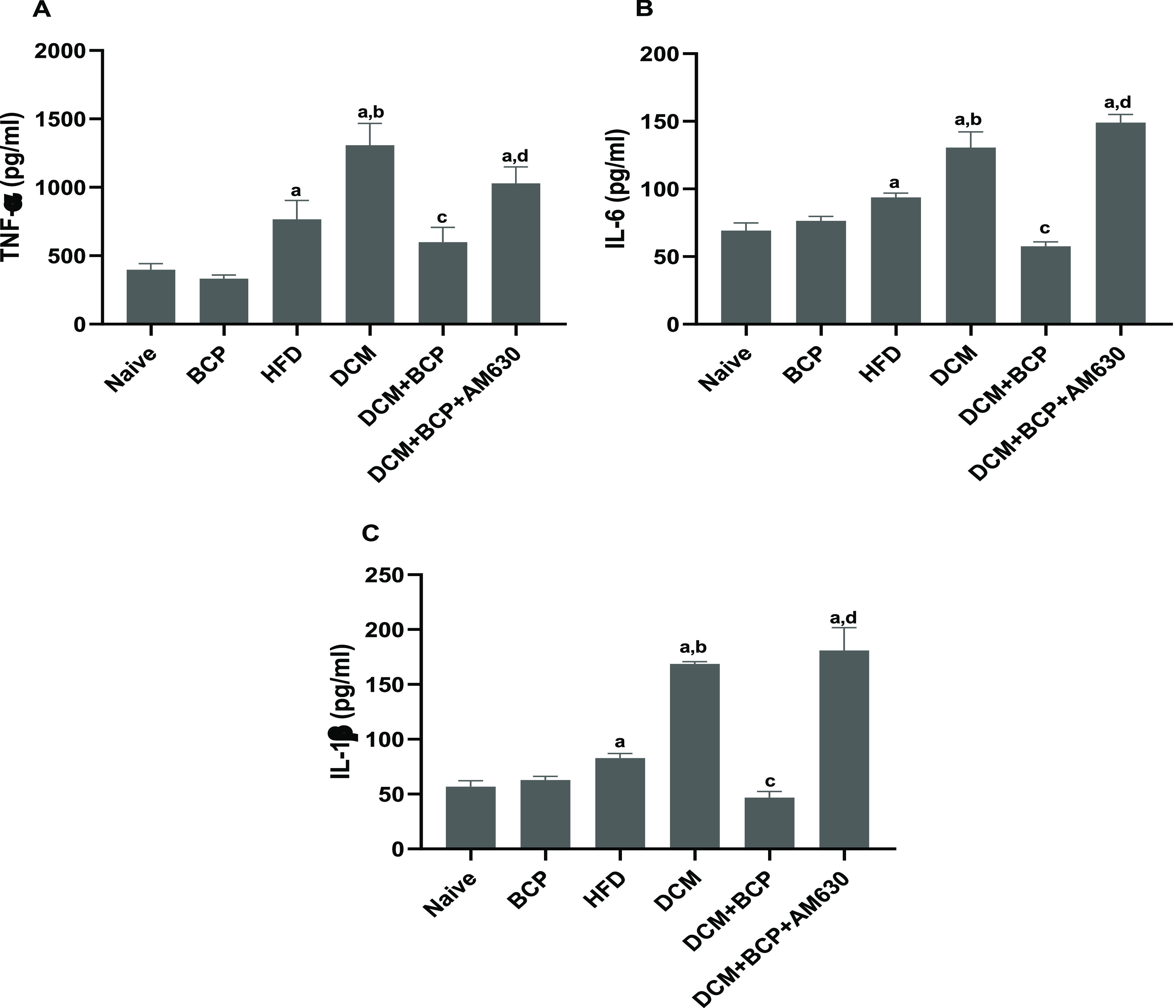
Effects of BCP on proinflammatory markers in DCM mice (A) TNF-α, (B) IL-6, and (C) IL-1β. Data are expressed as mean ± SEM, n = 6. aP < 0.05 compared to naive, bP < 0.05 compared to HFD, cP < 0.05 compared to DCM, and dP < 0.05 compared to DCM+BCP. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; and IL-1β, interleukin-1β.
Effects of BCP on Proinflammatory Mediator Levels and TLR4/NF-κB/MAPK Signaling in Cardiac Tissue of DCM
Western blot data showed that the expression of inflammatory mediators involving iNOS, TLR4, p-NF-κB p65, and p-IκBα was significantly increased in DCM mice when compared to the naïve group. Furthermore, the protein expression in heart tissue and densitometric analysis of MAPK signaling components showed a remarkable increase in the MAPK signaling cascade, particularly the expression levels of p38 MAPK and JNK in the myocardium of HFD mice with a further increase in DCM mice compared to naïve mice. BCP treatment, however, significantly downregulated the expression of p38 MAPK and JNK compared to DCM. There was no change in the ERK activation in the DCM heart tissues compared to the naïve group; BCP treatment of DCM mice had no significant effect on ERK activation. Prior administration of AM630 significantly reversed the positive effects of BCP in DCM (Figure 7A–D).
Figure 7.
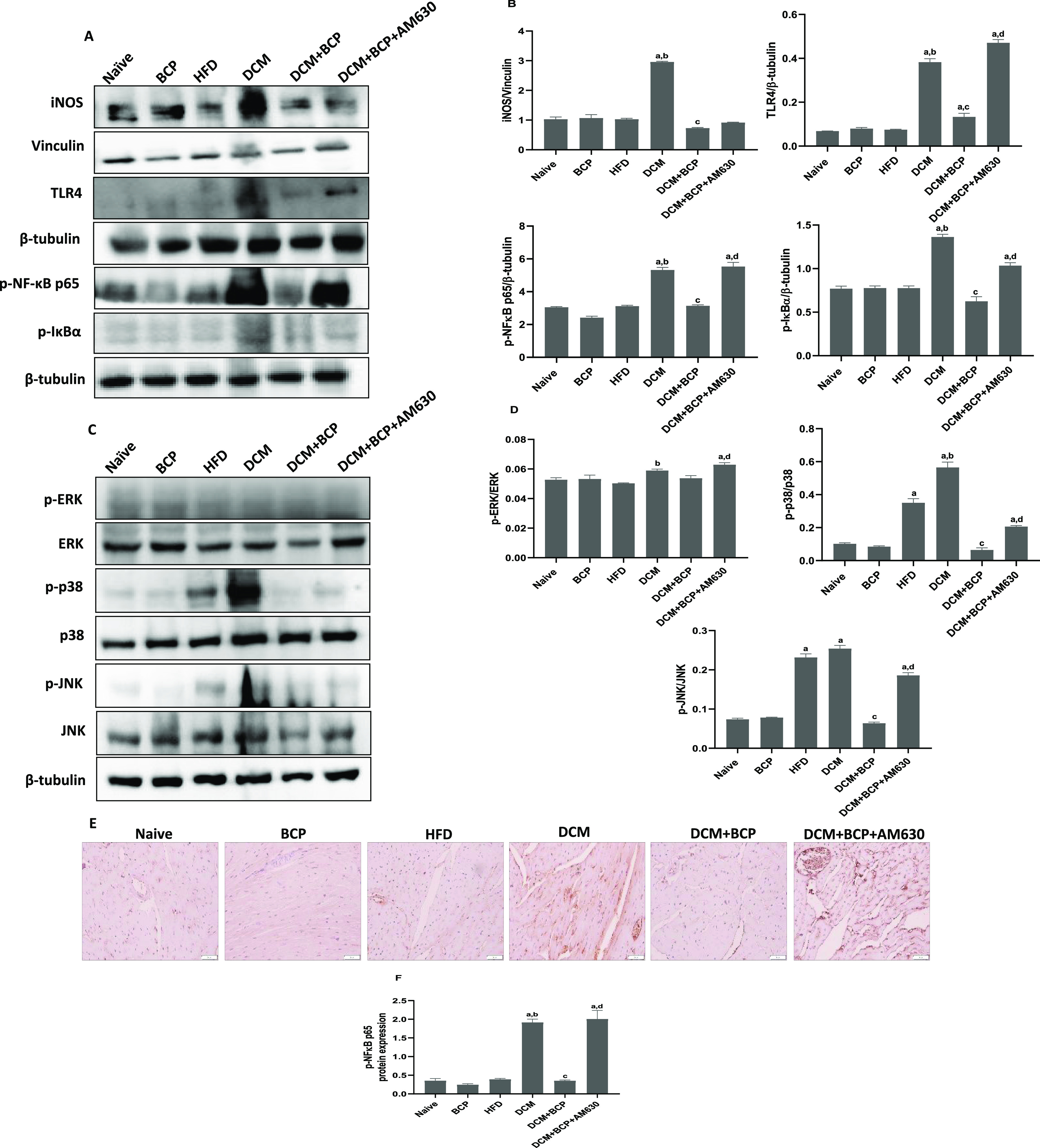
Effects of BCP on inflammatory mediators and NF-κB/MAPK signaling proteins. (A–D) Western blotting analysis on the expressions and densitometric analysis of inflammatory mediators and NF-κB/MAPK signaling proteins. (E, F) Immunohistochemistry analysis for the expressions and densitometry of p-NF-κB-p65 (40× magnification). Data are expressed as mean ± SEM, aP < 0.05 compared to naive, bP < 0.05 compared to HFD, cP < 0.05 compared to DCM, and dP < 0.05 compared to DCM + BCP.
Supporting the results of protein expression data, immunohistochemistry study (Figure 7E,F) demonstrates the increased expression of p-NF-κB p65 in myocardial tissue of DCM mice. Nevertheless, BCP treatment to DCM mice resulted in significant reduction in the expression of p-NF-κB p65. However, AM630 notably abolished the positive effect of BCP in DCM. The data demonstrated that BCP by CB2R activation prevents T2DM-induced myocardial inflammation through attenuation of TLR4/NF-κB/MAPK signaling pathways.
Discussion
Diabetes and diabetes-associated complications are a major health risk globally. Among numerous complications, DCM is characterized by increased accumulation of lipids and reduced glucose utilization following abnormal lipid metabolism in the myocardium along with oxidative stress, myocardial fibrosis, and inflammation and altered cellular signaling that eventually results in cardiac dysfunction. BCP, a naturally available selective agonist of CB2R, is recognized to exhibit numerous biological properties and pharmacological effects; however, the protective role of BCP against DCM-mediated myocardial injury and underlying mechanisms remains to be studied.
In the present study, animals with T2DM-associated cardiac complication were treated with 50 mg of BCP/kg orally for 12 weeks. BCP mitigated DCM-mediated metabolic and lipidemic alternations, myocardial injury, hypertrophy, and oxidative stress and inflammation in the heart tissues. Furthermore, BCP ameliorated DCM-induced inflammatory signaling cascades via favorable modulation of the TLR4/NF-κB/MAPK signaling pathway in a CB2R-dependent manner. To the best of our knowledge, this study is the first to systematically report the protective effects of BCP against DCM and associated myocardial dysfunction in mice by mitigating abnormal lipid metabolism-induced oxidative stress and inflammation mediating activation of CB2R.
Generally, sustained and over-intake of high-fat diet for a long duration causes body weight gain due to accumulations of saturated fat in the body. However, administration of streptozotocin leads to uncontrolled hyperglycemia, which eventually results in weight reduction of diabetic animals.39 In the present study, only HFD mice showed weight gain, while other groups did not show a remarkable alternation in body weights. Glycemic control, i.e., bringing blood glucose to the normal level, is a main priority for diabetes treatment because it might relieve metabolic abnormalities and inhibit the progression of diabetic complications.40 In the current study, BCP treatment significantly reduced hyperglycemia in DCM mice after 12 weeks of treatment. The observed antihyperglycemic effect of BCP is supported by the results of previous studies in vitro32 and in vivo41 studies. This antihyperglycemic effect of BCP may be partly supported by the enhancement of insulin release from pancreatic β cells,32 increase of glucose uptake and utilization,42 inhibition of hepatic gluconeogenesis,21 and modulation of carbohydrate metabolism.43 The antihyperglycemic activity of BCP was abolished by prior treatment with the CB2R antagonist AM630 that has obviously indicated the CB2R-related mechanism beyond the antihyperglycemic effect of BCP.
It has been well known that T2DM patients are vulnerable to myocardial dysfunction and cardiovascular diseases.44 The assessment of biomarkers of the cardiac function provides important information about cardiac functioning, and biomarkers such as LDH and BNP have been regarded as some of the standard biomarkers for the detection of cardiac injury and hypertrophy, respectively.45 The raised levels of LDH and BNP in the DCM mice in the present study reaffirmed cardiac injury and hypertrophy. The findings detected in the present study are in agreement with earlier studies.46 However, DCM mice that received BCP treatment for 12 weeks showed significant improvement in cardiac function as evidenced by the remarkable decrease in LDH levels as well as downregulation of the BNP expression compared to DCM mice. The restoration of LDH and BNP demonstrates the membrane stabilizing effect on cardiomyocytes and further demonstrates the cardioprotective potential of BCP. The protective effects of BCP were significantly reversed by prior treatment with AM630 that reveals a CB2R-dependent mechanism of BCP.
We further investigated the effect of BCP on hemodynamics in DCM mice. The results display remarkable elevation in the systolic, diastolic, and mean arterial pressure, whereas a decrease in heart rate was observed in the DCM mice model. The altered hemodynamic parameters are suggestive of cardiac insufficiency in the DCM mice. BCP treatment for 12 weeks significantly restored the alterations in hemodynamic parameters, although AM630 treatment given before BCP did not alter the hemodynamic parameters in DCM mice. These observations are in harmony with earlier studies displaying the cardioprotective effects of BCP.47 The data suggested that BCP can correct altered cardiac dysfunction, hypertrophy, and myocardial injury in DCM mice.
Hyperlipidemia has been well identified to raise the risk of cardiovascular disorders.48 In the current study, DCM mice display elevated levels of serum TC, TGs, LDL-C, VLDL-C, and HDL-C. These observations of the present study are in line with the previous studies.49,50 Therefore, modulating lipid metabolic alterations is crucial otherwise that has the potential to result in cardiovascular diseases. In this study, BCP treatment significantly improved the lipid parameter derangement in DCM mice that demonstrates the hypolipidemic effect of BCP. The positive effect of BCP on abnormal lipid profiles can be reasonably ascribed to its insulinotropic effects. The hypolipidemic and hypocholesterolemic properties of BCP are in accordance with earlier studies.41,51 The favorable modulation of enzymes involved in cholesterol metabolism has been attributed to the cholesterol lowering effects of BCP.51 Interestingly, prior administration of the CB2R antagonist, AM630 remarkably reversed the antihyperlipidemic effect of BCP and further reveals CB2R-dependent actions of BCP.
Cardiac lipid metabolism disorder is also a major reason of cardiac oxidative stress and inflammatory in type-2 diabetes. It has been demonstrated that type-2 diabetes leads to increased blood glucose, insulin resistance, and hyperinsulinemia, and these pathologic alternations lead to a reduction in the utilization of glucose and an elevation in the availability of fatty acids.52 The heart is not a main organ for lipid storage, but hyperlipidemic conditions in obese and diabetic patients cause extreme cardiac uptake of fatty acids, and a part of the fatty acids are transformed into TGs and accumulated in cardiac myocytes.52 Following lipid accumulation in cardiomyocytes, the heart produces toxic lipid intermediates including diglycerides, neuropterin, and reactive oxygen species (ROS), eventually resulting in cardiac inflammation, fibrosis, and sequalae.53 The present study reveals that myocardial lipid accumulation significantly increased in DCM mice, and BCP treatment has produced significant reduction in the degree of myocardial lipid accumulation, whereas prior administration of AM630 before BCP reversed the positive effects of BCP that clearly reveals the CB2R-dependent mechanism underlying the antilipotoxic effect of BCP. These findings clearly demonstrate that BCP has potential to diminish abnormalities in lipid metabolism in DCM mice by activating CB2R.
The coincidence of the hyperglycemic and hyperlipidemic status is correlated with elevated ROS release, which promotes oxidative stress and contributes to the development and progression of DCM and further cardiovascular disorders.54 Augmented ROS production along with the reduced antioxidant defense mechanism encourages oxidative injury in the diabetic heart.5 In harmony with earlier studies, the DCM mouse heart showed an increase in the MDA level, coupled with a reduced GSH level.5,50 Excessive lipid peroxidation disrupts the phospholipid bilayer integrity and causes inactivation of membrane-bound enzymes and receptors, resulting in enhanced cell permeability and apoptosis. Additionally, ROS can lessen the antioxidant activity at the cellular level by stimulating oxidation of the antioxidant enzymes.55 Thus, maintaining cellular redox homeostasis provides an efficient approach to mitigate oxidative damage in numerous disorders. Herein, BCP amplified the antioxidant status and mitigated oxidative damage in the DCM mouse heart, evidenced by a reduced level of MDA and increased level of GSH. In agreement, BCP showed to attenuate cyclophosphamide56 and doxorubicin-mediated myocardial injury57 via repressing lipid peroxidation and amelioration of the endogenous antioxidant system. Moreover, the antioxidant potential and free radical scavenging activity of BCP might be linked to the noticed alleviation of oxidative injury in DCM mice.58 The positive effects of BCP were mitigated by treatment with AM630, evidently revealing the CB2R-related mechanism beyond the antioxidant activity of BCP. These results exhibited that BCP can lessen cardiac injury in DCM mice by alleviating oxidative stress via a CB2R-dependent mechanism.
The oxidative stress and inflammatory process are closely connected to further progression of myocardial damage under T2DM.7 DCM mice in the current study displayed an elevation in cardiac levels of proinflammatory cytokines and mediators including TNF-α, IL-6, IL-1β, and iNOS. These proinflammatory mediators have a significant impact in diabetic cardiovascular diseases, and earlier findings described a positive connection among their levels, oxidative damage, and diminished function of the left ventricle in experimental DCM.5,59 TNF-α has been involved in myocardial dysfunction and remodeling. Giving TNF-α in vivo caused myocardial inflammation and dysfunction.60 Also, treating diabetic animals with the anti-TNF-α monoclonal antibody repressed the cardiac inflammatory response and fibrosis.61 Hereafter, approaches to decrease proinflammatory cytokine release could provide fruitful cardioprotective effects in diabetic patients.62 Treating DCM mice with BCP in the present study demonstrates a remarkable decrease in the cardiac proinflammatory cytokine and mediator levels (TNF-α, IL-6, IL-1β, and iNOS). Particularly, the efficiency of BCP as an anti-inflammatory compound is at least partial because of its capacity to suppress the main proinflammatory mediators such as TNF-α, IL-1β, IL-6, iNOS, NF-κB, cyclooxygenase 1 (COX-1), and cyclooxygenase 2 (COX-2).26,41 Correspondingly, BCP inhibited cardiac inflammation in doxorubicin-induced rats.57 Also, BCP reduced diet-induced inflammation in rats via the stimulation of PPAR-γ, probably through receptor cross-talk between PPAR-γ receptor systems and CB2R systems.41 Interestingly, prior treatment with AM630 significantly blocked the positive effect of BCP, which has clearly revealed the CB2R-related mechanism beyond the anti-inflammatory activities of BCP. These findings corroborate our theory that BCP suppresses the production of proinflammatory cytokines in the heart of DCM mice via activating CB2R.
Moreover, a long-term sustained low-grade inflammatory state has been regarded as an alternative important contributing factor promoting numerous signaling involving TLRs, MAPKs, and NF-κB in the pathophysiology of DCM.13 Toll-like receptors (TLRs) are the main elements of the innate immune system, and examining TLR signaling provides valuable data regarding inflammation.63 In an activated state, TLR signaling is implemented though myeloid differentiation factor 88 (MyD88), which activates in turn IL-1β receptor-associated kinases (IRAKs) and tumor necrosis factor-α receptor-associated factor 6 (TRAF6), which then stimulates phosphorylation and degradation of IκBα via activation of IKK (IκB kinases) that stimulate NF-κB release and generation of proinflammatory cytokines.63 In the current study, the protein expression level of TLR4 was remarkably overexpressed with enhanced p-NF-κB p65 and p-IκBα expression in the heart of DCM mice. Moreover, immunohistochemical study proved the activation of p-NF-κB p65 in the heart of DCM mice. The stimulation of TLR4/NF-κB signaling to boost inflammatory conditions and myocardial damage has been described by many investigators.12 Earlier, Bagul et al.64 detected the stimulation of proinflammatory transcription factor i.e., NF-κB, in the diabetic heart. In response to activated TLR4/NF-κB, proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 were markedly triggered concomitant to reduced anti-inflammatory cytokines such as IL-10 in mice with T2DM, implying the key role of inflammatory events in the pathophysiology of myocardial injury.65 The findings of the study revealed that BCP treatment significantly reduced the expression levels of TLR4, p-NF-κB p65, and p-IκBα, revealing prevention of TLR4/NF-κB signaling.
On the contrary, the mitogen-activated protein kinase (MAPK) pathway mediated by ERK, JNK, and p38 has been implicated in the regulation of various cellular events such as insulin resistance10 and inflammation.66 Stimulation of JNK and p38 MAPK protein kinases has a vital function in the generation of inflammatory responses.66 Another MAPK protein kinase such as ERK becomes phosphorylated and activated by MEK, and activated ERK1/2 has a critical effect in inflammation since NF-κB has been known as its downstream target and recognized to promote IL-1β signaling.67 The enhancement of p38 MAPK and JNK is proved from the overexpression of p38 MAPK and JNK proteins in the heart of DCM mice, while treating DCM mice with BCP has attenuated the DCM-mediated stimulation of p38 MAPK and JNK pathways indicated by the downregulated protein expression of p38 MAPK and JNK. However, prior treatment with AM630 markedly reversed the protective effects of BCP in DCM mice, obviously revealing the functional CB2R beyond the suppression of the TLR4/NF-κB/MAPK signaling pathway.
Conclusions
The present study findings demonstrate that BCP produced remarkable cardioprotection via its modulatory effects on diabetes mellitus-associated metabolic derangements, lipidemia, and cardiac dysfunction. Also, BCP mitigated cardiac lipotoxicity and suppressed diabetic cardiomyopathy by alleviating oxidative stress and favorably modulating TLR4/NF-κB/MAPK signaling pathway-dependent pathways in the event of inflammation. The CB2R-dependent actions of BCP have been attributed to mediate its protective effect, and the CB2R-selective agonism is believed to provide a safe alternative over other cannabinoids being devoid of psychotropic effects. BCP in numerous other studies has been shown to mitigate oxidative stress and hyperactivation of immunoinflammatory cascades. Corroborating its protective effects in other cardiovascular diseases as well as its abundance in edible plants and dietary availability, BCP may be a promising agent to be developed as a nutraceutical as well as pharmaceutical compound for diabetic cardiomyopathy with a pharmacological rationale of being an activator of CB2R. Provided its safety, wider availability, accessibility, and dietary bioavailability, BCP could be considered for future clinical studies to manage cardiomyopathy and other cardiovascular complications associated with diabetes mellitus. The observations of the present study demonstrate that CB2R agonists can be a first-in-class medication for preventing cardiomyopathy in diabetic conditions. Moreover, regulatory toxicology and human studies are suggested to translate the findings and establish further safety, efficacy, and pharmacokinetics.
Acknowledgments
The authors are thankful to the College of Graduate Studies and Assistant Dean for Research and Graduate Studies College of Medicine and Health Sciences for the award of the PhD fellowship to Mrs Hebaallah M Hashiesh. The authors are thankful to the United Arab Emirates University for the award of the research grants (#12R104, 12M121) to Dr. Shreesh Ojha.
Data Availability Statement
The data used to support the findings of this study are already incorporated in the results section.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.3c00027.
Nonsentence format listing the contents of the material (PDF)
Author Contributions
conceptualization, S.O.; methodology, S.O. and H.M.H.; formal analysis, H.M.H. and M.F.N.M.; investigation, H.M.H., treatment protocol and animal surgery, H.M.H., S.A., and M.F.N.M., imaging studies, D.S. and S.M., original draft preparation, H.M.H.; scheme drawn, N.K.J.; review and editing, B.S., S.O., and N.K.J.; supervision, S.O.; and funding acquisition, S.O.
The authors declare no competing financial interest.
Notes
The study was approved by the Animal Ethics Committee of United Arab Emirates University, UAE.
Supplementary Material
References
- Shaw J. E.; Sicree R. A.; Zimmet P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Poornima I. G.; Parikh P.; Shannon R. P. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ. Res. 2006, 98, 596–605. 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- Feng W.; Lei T.; Wang Y.; Feng R.; Yuan J.; Shen X.; Wu Y.; Gao J.; Ding W.; Lu Z. GCN2 deficiency ameliorates cardiac dysfunction in diabetic mice by reducing lipotoxicity and oxidative stress. Free Radicals Biol. Med. 2019, 130, 128–139. 10.1016/j.freeradbiomed.2018.10.445. [DOI] [PubMed] [Google Scholar]
- Kocabaş U.; Yılmaz Ö.; Kurtoğlu V. Diabetic cardiomyopathy: acute and reversible left ventricular systolic dysfunction due to cardiotoxicity of hyperglycaemic hyperosmolar state-a case report†. Eur. Heart J.-Case Rep. 2019, 3, ytz049 10.1093/ehjcr/ytz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R.; Cao Y.; Wang C.; Xu L.; Zhang X.; Deng Y.; Li F.; Wang S. Taohuajing reduces oxidative stress and inflammation in diabetic cardiomyopathy through the sirtuin 1/nucleotide-binding oligomerization domain-like receptor protein 3 pathway. BMC Complementary Med. Ther. 2021, 21, 78 10.1186/s12906-021-03218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A. A.; Reza M. I.; Shafiq M.; Kumariya S.; Katekar R.; Hanif K.; Gayen J. R. Cissus quadrangularis extract mitigates diabetic cardiomyopathy by inhibiting RAAS activation, inflammation and oxidative stress. Biomarkers 2022, 27, 743–752. 10.1080/1354750x.2022.2107703. [DOI] [PubMed] [Google Scholar]
- Oguntibeju O. O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol., Pathophysiol. Pharmacol. 2019, 11, 45–63. [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Wang Y.; Zhao Y.; Peng K.; Li W.; Wang Y.; Zhang J.; Zhou S.; Liu Q.; Li X.; et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes 2014, 63, 3497–3511. 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Kang L.; Li C.; Wang X.; Sun C.; Li Q.; Liu R.; Wang J. Resveratrol Ameliorates Diabetes-Induced Cardiac Dysfunction Through AT1R-ERK/p38 MAPK Signaling Pathway. Cardiovasc. Toxicol. 2016, 16, 130–137. 10.1007/s12012-015-9321-3. [DOI] [PubMed] [Google Scholar]
- Nandipati K. C.; Subramanian S.; Agrawal D. K. Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell. Biochem. 2017, 426, 27–45. 10.1007/s11010-016-2878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo G.; Ren X.; Qian X.; Ye P.; Luo J.; Gao X.; Zhang J.; Chen S. Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy. J. Cell. Physiol. 2019, 234, 1925–1936. 10.1002/jcp.27070. [DOI] [PubMed] [Google Scholar]
- Yu L.; Feng Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediators Inflammation 2018, 2018, 9874109 10.1155/2018/9874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryavanshi S. V.; Kulkarni Y. A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P.; Rajesh M.; Pan H.; Patel V.; Mukhopadhyay B.; Bátkai S.; Gao B.; Haskó G.; Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radicals Biol. Med. 2010, 48, 457–467. 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl S.-L. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim. Biophys. Acta, Mol. Cell Res. 2020, 1867, 118462 10.1016/j.bbamcr.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Howlett A. C.Cannabinoid Receptor Signaling. In Handbook of Experimental Pharmacology; Springer, 2005; pp 53–79. [DOI] [PubMed] [Google Scholar]
- Aghazadeh Tabrizi M.; Baraldi P. G.; Borea P. A.; Varani K. Medicinal Chemistry, Pharmacology, and Potential Therapeutic Benefits of Cannabinoid CB2 Receptor Agonists. Chem. Rev. 2016, 116, 519–560. 10.1021/acs.chemrev.5b00411. [DOI] [PubMed] [Google Scholar]
- Turcotte C.; Blanchet M. R.; Laviolette M.; Flamand N. The CB(2) receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F.; Mach F.; Vuilleumier N.; Montecucco F. Cannabinoid receptor type 2 activation in atherosclerosis and acute cardiovascular diseases. Curr. Med. Chem. 2014, 21, 4046–4058. 10.2174/0929867321666140915141332. [DOI] [PubMed] [Google Scholar]
- Basha R. H.; Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 116, 1469–1479. 10.1016/j.acthis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Steffens S.; Pacher P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323. 10.1111/j.1476-5381.2012.02042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiesh H. M.; Meeran M. F. N.; Sharma C.; Sadek B.; Kaabi J. A.; Ojha S. K. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients 2020, 12, 2963 10.3390/nu12102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C.; Al Kaabi J. M.; Nurulain S. M.; Goyal S. N.; Kamal M. A.; Ojha S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. 10.2174/1381612822666160311115226. [DOI] [PubMed] [Google Scholar]
- Francomano F.; Caruso A.; Barbarossa A.; Fazio A.; La Torre C.; Ceramella J.; Mallamaci R.; Saturnino C.; Iacopetta D.; Sinicropi M. S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420 10.3390/app9245420. [DOI] [Google Scholar]
- Gertsch J.; Leonti M.; Raduner S.; Racz I.; Chen J. Z.; Xie X. Q.; Altmann K. H.; Karsak M.; Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 9099–9104. 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikubo R.; Kai K.; Tsuji-Naito K.; Akagawa M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. 10.1002/mnfr.201600197. [DOI] [PubMed] [Google Scholar]
- Picciolo G.; Pallio G.; Altavilla D.; Vaccaro M.; Oteri G.; Irrera N.; Squadrito F. β-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164 10.3390/biomedicines8060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Ma W.; Du J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. 10.1016/j.biopha.2018.03.168. [DOI] [PubMed] [Google Scholar]
- Calleja M. A.; Vieites J. M.; Montero-Meléndez T.; Torres M. I.; Faus M. J.; Gil A.; Suárez A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. 10.1017/s0007114512001298. [DOI] [PubMed] [Google Scholar]
- Alberti T. B.; Barbosa W. L. R.; Vieira J. L. F.; Raposo N. R. B.; Dutra R. C. (−)-β-Caryophyllene, a CB2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 691 10.3390/ijms18040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijun W.; Zhen Y.; Ying G.; Yanfang W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. 10.1016/j.bbrc.2013.11.136. [DOI] [PubMed] [Google Scholar]
- Kumawat V. S.; Kaur G. Cannabinoid 2 receptor agonist and L-arginine combination attenuates diabetic cardiomyopathy in rats via NF-κβ inhibition. Can. J. Physiol. Pharmacol. 2022, 100, 259–271. 10.1139/cjpp-2021-0046. [DOI] [PubMed] [Google Scholar]
- Kumawat V. S.; Kaur G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628 10.1016/j.ejphar.2019.172628. [DOI] [PubMed] [Google Scholar]
- Wu X.; Huang L.; Zhou X.; Liu J. Curcumin protects cardiomyopathy damage through inhibiting the production of reactive oxygen species in type 2 diabetic mice. Biochem. Biophys. Res. Commun. 2020, 530, 15–21. 10.1016/j.bbrc.2020.05.053. [DOI] [PubMed] [Google Scholar]
- Franco-Arroyo N. N.; Viveros-Paredes J. M.; Zepeda-Morales A. S. M.; Roldán E.; Márquez-Aguirre A. L.; Zepeda-Nuño J. S.; Velázquez-Juárez G.; Fafutis-Morris M.; López-Roa R. I. β-Caryophyllene, a Dietary Cannabinoid, Protects Against Metabolic and Immune Dysregulation in a Diet-Induced Obesity Mouse Model. J. Med. Food 2022, 25, 993–1002. 10.1089/jmf.2021.0166. [DOI] [PubMed] [Google Scholar]
- Friedewald W. T.; Levy R. I.; Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- Kuhlmann J.; Neumann-Haefelin C.; Belz U.; Kalisch J.; Juretschke H. P.; Stein M.; Kleinschmidt E.; Kramer W.; Herling A. W. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes 2003, 52, 138–144. 10.2337/diabetes.52.1.138. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Kong X. Q. Bixin ameliorates high fat diet-induced cardiac injury in mice through inflammation and oxidative stress suppression. Biomed. Pharmacother. 2017, 89, 991–1004. 10.1016/j.biopha.2017.02.052. [DOI] [PubMed] [Google Scholar]
- Grossman A.; Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc. Diabetol. 2017, 16, 3 10.1186/s12933-016-0485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef D. A.; El-Fayoumi H. M.; Mahmoud M. F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem.-Biol. Interact. 2019, 297, 16–24. 10.1016/j.cbi.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Geddo F.; Scandiffio R.; Antoniotti S.; Cottone E.; Querio G.; Maffei M. E.; Bovolin P.; Gallo M. P. PipeNig-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients 2019, 11, 2788 10.3390/nu11112788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha R. H.; Sankaranarayanan C. Protective role of β-caryophyllene, a sesquiterpene lactone on plasma and tissue glycoprotein components in streptozotocin-induced hyperglycemic rats. J. Acute Med. 2015, 5, 9–14. 10.1016/j.jacme.2015.02.001. [DOI] [Google Scholar]
- Ernande L.; Audureau E.; Jellis C. L.; Bergerot C.; Henegar C.; Sawaki D.; Czibik G.; Volpi C.; Canoui-Poitrine F.; Thibault H.; et al. Clinical Implications of Echocardiographic Phenotypes of Patients With Diabetes Mellitus. J. Am. Coll. Cardiol. 2017, 70, 1704–1716. 10.1016/j.jacc.2017.07.792. [DOI] [PubMed] [Google Scholar]
- Aydin S.; Ugur K.; Aydin S.; Sahin İ.; Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc. Health Risk Manage. 2019, 15, 1–10. 10.2147/vhrm.s166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Guo Z.; Wang Y.; Geng J.; Han S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309. 10.1002/ddr.21495. [DOI] [PubMed] [Google Scholar]
- Meeran M. F. N.; Laham F.; Azimullah S.; Sharma C.; Al Kaabi A. J.; Tariq S.; Adeghate E.; Goyal S. N.; Ojha S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates β-adrenergic agonist-induced myocardial injury in a cannabinoid receptor-2 dependent and independent manner. Free Radicals Biol. Med. 2021, 167, 348–366. 10.1016/j.freeradbiomed.2021.01.046. [DOI] [PubMed] [Google Scholar]
- Burkhardt R. Hyperlipidemia and cardiovascular disease: new insights on lipoprotein(a). Curr. Opin. Lipidol. 2019, 30, 260–261. 10.1097/mol.0000000000000594. [DOI] [PubMed] [Google Scholar]
- Yan M.; Li L.; Wang Q.; Shao X.; Luo Q.; Liu S.; Li Y.; Wang D.; Zhang Y.; Diao H.; et al. The Chinese herbal medicine Fufang Zhenzhu Tiaozhi protects against diabetic cardiomyopathy by alleviating cardiac lipotoxicity-induced oxidative stress and NLRP3-dependent inflammasome activation. Biomed. Pharmacother. 2022, 148, 112709 10.1016/j.biopha.2022.112709. [DOI] [PubMed] [Google Scholar]
- Lu Q.; Zheng R.; Zhu P.; Bian J.; Liu Z.; Du J. Hinokinin alleviates high fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2021, 137, 111361 10.1016/j.biopha.2021.111361. [DOI] [PubMed] [Google Scholar]
- Baldissera M. D.; Souza C. F.; Grando T. H.; Doleski P. H.; Boligon A. A.; Stefani L. M.; Monteiro S. G. Hypolipidemic effect of β-caryophyllene to treat hyperlipidemic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 215–223. 10.1007/s00210-016-1326-3. [DOI] [PubMed] [Google Scholar]
- Haffar T.; Bérubé-Simard F.; Bousette N. Impaired fatty acid oxidation as a cause for lipotoxicity in cardiomyocytes. Biochem. Biophys. Res. Commun. 2015, 468, 73–78. 10.1016/j.bbrc.2015.10.162. [DOI] [PubMed] [Google Scholar]
- Palomer X.; Salvadó L.; Barroso E.; Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. 10.1016/j.ijcard.2013.07.150. [DOI] [PubMed] [Google Scholar]
- Al-Trad B.; Alkhateeb H.; Alsmadi W.; Al-Zoubi M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. Life Sci. 2019, 216, 183–188. 10.1016/j.lfs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Wilson A. J.; Gill E. K.; Abudalo R. A.; Edgar K. S.; Watson C. J.; Grieve D. J. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. 10.1136/heartjnl-2017-311448. [DOI] [PubMed] [Google Scholar]
- Younis N. S. β-Caryophyllene Ameliorates Cyclophosphamide Induced Cardiac Injury: The Association of TLR4/NFκB and Nrf2/HO1/NQO1 Pathways. J. Cardiovasc. Dev. Dis. 2022, 9, 133 10.3390/jcdd9050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeran M. F. N.; Al Taee H.; Azimullah S.; Tariq S.; Adeghate E.; Ojha S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB(2)) receptors in rats. Chem. Biol. Interact. 2019, 304, 158–167. 10.1016/j.cbi.2019.02.028. [DOI] [PubMed] [Google Scholar]
- Calleja M. A.; Vieites J. M.; Montero-Meterdez T.; Torres M. I.; Faus M. J.; Gil A.; Suárez A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2012, 109, 394–401. 10.1017/S0007114512001298. [DOI] [PubMed] [Google Scholar]
- Jin Q.; Zhu Q.; Wang K.; Chen M.; Li X. Allisartan isoproxil attenuates oxidative stress and inflammation through the SIRT1/Nrf2/NF-κB signalling pathway in diabetic cardiomyopathy rats. Mol. Med. Rep. 2021, 23, 215 10.3892/mmr.2021.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B.; Kribbs S. B.; Clubb F. J. Jr.; Michael L. H.; Didenko V. V.; Hornsby P. J.; Seta Y.; Oral H.; Spinale F. G.; Mann D. L. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998, 97, 1382–1391. 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- Westermann D.; Linthout S.; Dhayat S.; Dhayat N.; Schmidt A.; Noutsias M.; Song X. Y.; Spillmann F.; Riad A.; Schultheiss H. P.; Tschöpe C. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500–507. 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- Abukhalil M. H.; Althunibat O. Y.; Aladaileh S. H.; Al-Amarat W.; Obeidat H. M.; Al-khawalde A. A. A.; Hussein O. E.; Alfwuaires M. A.; Algefare A. I.; Alanazi K. M.; et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410 10.1016/j.biopha.2021.111410. [DOI] [PubMed] [Google Scholar]
- El-Zayat S. R.; Sibaii H.; Mannaa F. A. Toll-like receptors activation, signaling, and targeting: an overview. Bull. Natl. Res. Cent. 2019, 43, 187 10.1186/s42269-019-0227-2. [DOI] [Google Scholar]
- Bagul P. K.; Deepthi N.; Sultana R.; Banerjee S. K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. 10.1016/j.jnutbio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Tian J.; Zhao Y.; Wang L.; Li L. Role of TLR4/MyD88/NF-κB signaling in heart and liver-related complications in a rat model of type 2 diabetes mellitus. J. Int. Med. Res. 2021, 49, 300060521997590 10.1177/0300060521997590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubaidi F. F.; Zainalabidin S.; Taib I. S.; Hamid Z. A.; Anuar N. N. M.; Jalil J.; Mohd Nor N. A.; Budin S. B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582 10.3390/ijms23158582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y.; Ilyosbek S.; Lee B. H.; Yi K. Y.; Jung Y. S. A novel urotensin II receptor antagonist, KR-36676, prevents ABCA1 repression via ERK/IL-1β pathway. Eur. J. Pharmacol. 2017, 803, 174–178. 10.1016/j.ejphar.2017.03.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are already incorporated in the results section.