Abstract
Normal aging is linked to various endocrine gland changes, including changes in the adrenal glands. Aging is linked to alterations of the hypothalamic-pituitary-adrenal (HPA) axis, including an increase in cortisol levels, a disruption of the negative cortisol feedback, and attenuation of cortisol’s diurnal pattern. In addition, secretion of aldosterone and adrenal androgens [dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS)] from the adrenal cortex decreases with aging. In this review, we describe normal adrenal function, the adrenal response to stress and immunomodulation in aging individuals as well as the effects of adrenal aging on body composition, metabolic profile, bone health and cognition.
Keywords: Adrenal aging, adrenal glands, stress, immunosenescence
Introduction
Aging is characterized by a gradual decline in all organ function. It has been previously hypothesized that hormonal changes occurring with aging may contribute to the age-related phenotypes that older individuals display[1]. Aging affects several endocrine systems including the growth hormone, the reproductive and the adrenal axis[1]. While some endocrine changes occurring with aging are well defined (such as the decline of estradiol levels in women after menopause), other age-related endocrine changes are not as clearly understood.
Among all endocrine axes that are affected by aging, the adrenal axis appears to be the most complex mainly due to the multiple hormones produced by the different adrenal zones and the essential role of adrenal glands in human body homeostasis. Aging appears to affect the secretion of all adrenal cortical hormones including cortisol, aldosterone, and adrenal androgens[2–4]. Aging can impact the diurnal secretion of cortisol and the adrenal response to stress and immune stimuli[2]. As humans age, they are often required to respond to such stimuli to survive, and the hypothalamic-pituitary-adrenal (HPA) axis plays a critical role in this stress response and immunomodulation[5, 6]. As changes in the adrenal gland and the global HPA axis can have a significant impact on human function and survival, this narrative review aims to present an overview of normal adrenal physiology and the effect of aging on adrenal function.
Methods
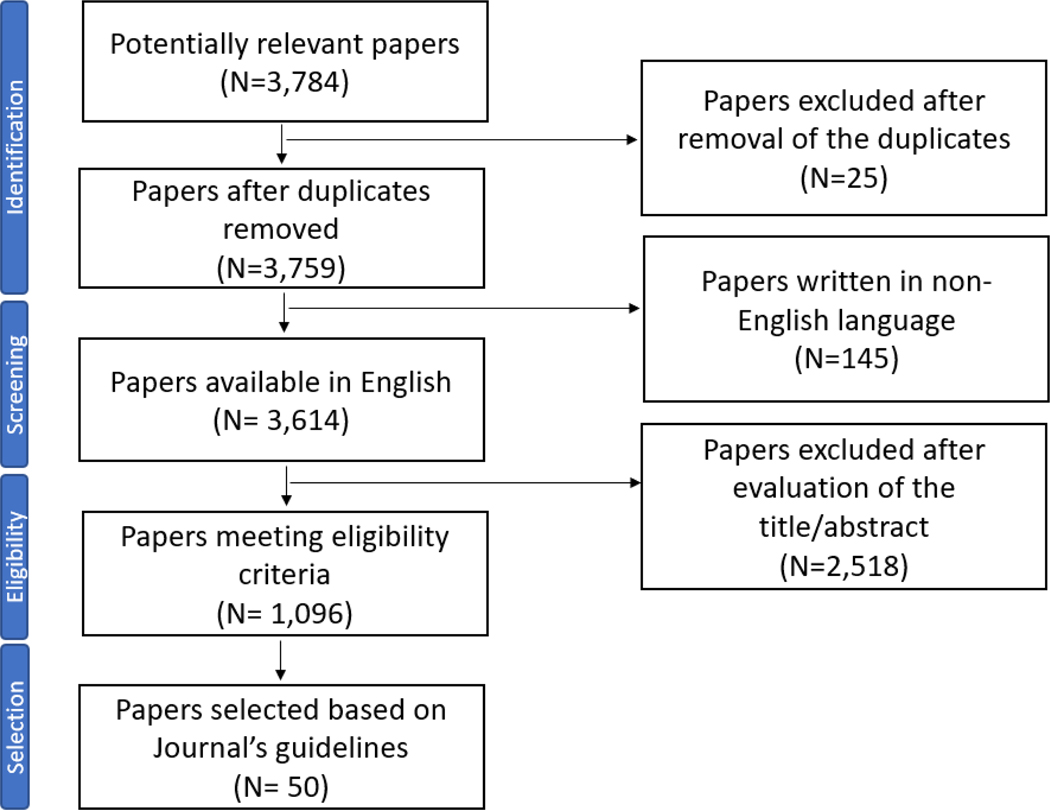
A multiple step strategy was utilized to conduct this narrative review as shown in Figure 1. Identification of relevant articles was conducted through August 2022 by utilizing the Pubmed database and applying the following query (Q1): adrenal [MeSH] OR cortisol [MeSH] OR DHEAS [MeSH] OR Aldosterone [MeSH] AND aging [MeSH]. To focus our search in the respective sections of the this review, we also queried the database using the query (Q1) in combination with the following keywords: Q1 AND stress [All Fields] resulting in 653 published articles; Q1 AND immunosenescence [All Fields] resulting in 28 papers, Q1 AND body composition [All Fields] resulting in 99 published papers, Q1 AND OR bone density [All Fields] resulting in 55 published articles; Q1 AND cognitive function [All Fields] resulting in 168 published articles; Q1 AND cognition [All Fields] resulting in 224 published articles; Q1 AND adrenal nodules [All Fields] resulting in 9 published articles; and finally, Q1 AND inflammation [All Fields] resulting in 7 published articles. Screening of all published articles was conducted with the Rayyan Software [7]. After duplicates were removed, 3,759 published articles were identified. Most articles were published in the years 1990–2010 (N =1,754), followed by articles published before the year 1990 (N=1,502) and articles published in the years 2010–2022 (N=503). Next, 124 papers were removed from the pipeline as the abstract and full text were not written in English. Eligibility was then assessed using the following criteria: publications focusing on: 1) The normal adrenal and HPA function; (2) Adrenal changes with aging; (3) The HPA axis response in stress and immunosenescence; and (4) HPA changes with aging: effects on body composition, metabolic profiles, bone density and cognition. A total of 1,096 published articles met criteria for inclusion in this review study and a subset of representative articles (n=50) were cited in this narrative review to comply with the Journal’s guidelines for authors.
Figure 1:
Flowchart of the review search results
(i). Normal adrenal and HPA function
(A). The HPA axis and response to stress:
The adrenal glands are essential endocrine organs that function under the regulatory control of hypothalamic corticotropin releasing hormone (CRH) that stimulates the secretion of adrenocorticotrophic hormone (ACTH) in the anterior pituitary[8]. Under the control of ACTH, adrenal glands secrete glucocorticoids (i.e. cortisol) in relatively high amounts (10–20 mg/day). Glucocorticoids act via the glucocorticoid receptor that is expressed throughout the body resulting in diverse actions, with roles in glucose regulation, adipocyte differentiation, osteoblast function, and blood pressure control[9], while glucocorticoid excess can lead to hypertension, insulin resistance, osteoporosis and immunosuppression[10]. Apart from glucocorticoids, the adrenal cortex is responsible for secretion of mineralocorticoids (i.e., aldosterone). Mineralocorticoids are secreted in lower amounts than the glucocorticoids (100–105 mcg/day) under the control of potassium, angiotensin II and ACTH (to a lesser extent)[11]. Finally, adrenal androgens (dehydroepiandrosterone [DHEA] and its sulfated ester [DHEAS]) are the most abundant steroids secreted from the adrenal glands. DHEA is a precursor of sex steroids with the potential for estrogenic or androgenic effects based on peripheral conversion in target tissues. Although adrenal steroids are a principal component of circulating androgens in women, their contribution in men is much smaller due to gonadal androgen production[8].
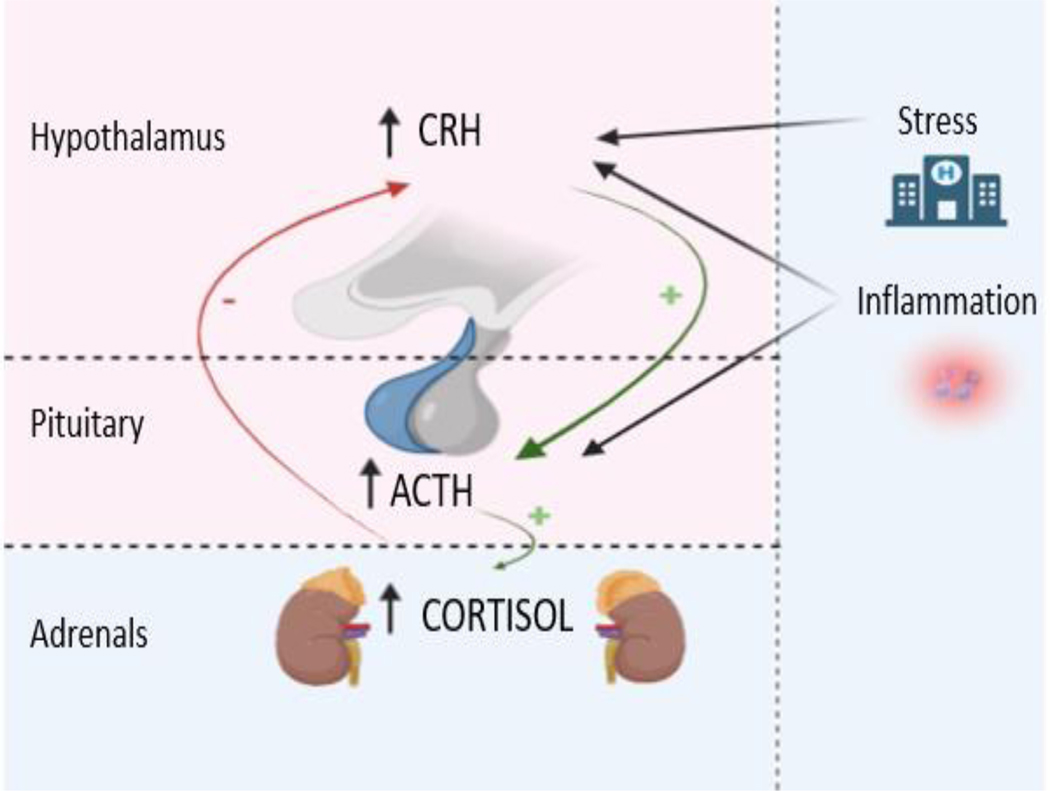
Stress is commonly defined as a state of perceived threat to homeostasis. Several principal components of the response to stress are located in the hypothalamus (paraventricular nucleus), the anterior pituitary gland and the adrenal glands, i.e., the HPA axis. Modification of the glucocorticoid receptor expression with gene knockout has revealed its essential role in survival as well as other functions throughout the body, including immunomodulation [12]. This is consistent with the fact that glucocorticoids are a critical component of the stress response and display anti-inflammatory/immunosuppressive actions[8]. Physical stress increases CRH, ACTH and cortisol secretion through hypothalamic mediated processes, with increased HPA axis activity being documented in response to stressful stimuli, including surgery, significant trauma, and acute illness (Figure 2)[13]. That was demonstrated in a study by Boonen et al that reported 83% higher cortisol levels in the patients with critical illness compared with controls[13]. In contrast to the increase in circulating cortisol demonstrated in the acute stress response, cortisol levels in chronic and neuropsychiatric diseases are less well defined. Cortisol levels are positively correlated with the symptom severity experienced by patients with chronic malignancies[14], and individuals with chronic depression and other neuropsychiatric diseases demonstrate higher cortisol levels[15].
Figure 2:
Hypothalamic-pituitary-adrenal (HPA) axis response to stress and inflammation.
As a response to stress CRH is produced at the hypothalamic level stimulating ACTH production in the pituitary, that in turn leads to increase release of cortisol from the adrenal glands. The HPA axis is also activated in response to inflammation both at the hypothalamic or the pituitary level. (Figure created in biorender.com)
(B). The role of the HPA axis in immunomodulation:
Glucocorticoids have a role in responding to and modulating the immune response. Proinflammatory cytokines, including tumor necrosis factor 1 (TNF1), interleukins 1 and 6 (IL-1 and IL-6), and leukemia inhibitory factor (LIF, a IL-6 cytokine member) activate the HPA axis via directly increasing ACTH or by augmenting the CRH effect in the anterior pituitary[16]. On the other hand, glucocorticoids also demonstrate inhibitory immune effects, as demonstrated by the development of highly potent glucocorticoid therapies that are used for treating a variety of autoimmune and inflammatory diseases. The glucocorticoid-induced inhibitory immune effects are mediated via multiple mechanisms with most of the glucocorticoid associated anti-inflammatory effects linked to gene suppression associated with the glucocorticoid receptor. The activated glucocorticoid receptor can affect multiple pro-inflammatory transcription factors, such as the activator protein 1 and the nuclear factor kappa B (NF-kB) [17]. Glucocorticoids have also direct actions on both T and B lymphocytes, by inhibiting the action of NF-kB[18], inhibiting macrophage phagocytosis, suppressing monocyte differentiation into macrophages and reducing local inflammation by blocking the inflammatory effects of histamine and plasminogen activators [19]. Finally, glucocorticoids suppress the production of multiple interleukins that serve as immunomodulatory chemokines, including IL2 [20] and interferon gamma (INFγ), by underregulating the STAT-1 expression[21, 22].
(C). Intraadrenal regulation of steroidogenesis:
While a detailed discussion of intraadrenal regulation of adrenal function is beyond the scope of this review, it is now appreciated that locally produced hormones, neuropeptides, and cytokines act in a paracrine fashion and modulate adrenal cortisol production[23]. ACTH-independent regulation of cortisol secretion was suggested with observations of ACTH and glucocorticoid dissociation in sepsis, surgery, malignancy and depression and has been demonstrated in studies using intact isolated adrenals or in vivo models[24]. There remains much to be understood regarding the mechanisms of ACTH-independent cortisol secretion, but in the future comprehensive discussion of the HPA axis and the stress response will need to incorporate accumulating evidence about non-ACTH mechanisms modulating cortisol secretion.
(ii). Adrenal changes with aging:
Aging is defined as progressive decline in biological functions over time. Among other physiologic functions that decline during aging, changes in adrenal function may occur with the passage of time (Table 1).
Table 1:
Changes of the Hypothalamic-pituitary adrenal axis and adrenal hormone production with aging.
| Changes with Aging | |
|---|---|
| Cortisol production | ↑ |
| HPA axis diurnal variation | ↓ |
| Cortisol negative feedback | ↓ |
| Aldosterone production | ↓ |
| DHEA(S) production | ↓ |
A). Changes in cortisol secretion and response to stress with aging:
Increasing age is linked to higher cortisol secretion with a flat diurnal cortisol pattern and a disruption of the HPA axis negative feedback loop [2]. With age, cortisol exhibits an attenuated awakening response and a more gradual decline later in the day, resulting in a higher evening nadir [2]. In a study of 24-hour profiles of plasma cortisol from healthy individuals (ages 18–83 years), mean cortisol levels in both sexes increased by 20–50% between 20–80 years of age. Aging led to a progressive increase in the nocturnal cortisol nadir level in both sexes. This study demonstrated that with older age the diurnal pattern of cortisol secretion was preserved but peak amplitude and quiescent periods of cortisol secretion were reduced [25]. Similarly, aging was shown to increase basal HPA-axis activity and decrease diurnal variation in a study of healthy subjects (23–85 years of age). There were significant age-associated increases in minimum and mean cortisol plasma concentrations and shortening of the evening cortisol quiescent period, suggesting progressively impaired circadian function with higher basal activity and a flattening of the diurnal amplitude of the HPA axis with increasing age [26].
Furthermore, the effect of aging on the HPA-mediated response to stress is not completely understood. In a metanalysis comparing younger (average age of 28 years) with older subjects (average age of 69 years), a greater cortisol response to a stimulatory test or less decline after a suppression test was shown in older subjects[5]. Interestingly, other studies have shown similar cortisol profiles in younger and older subjects after stress[27]. Furthermore, aging affects the HPA axis sensitivity to the negative feedback of cortisol secretion. To study this negative feedback, Boscaro and colleagues [28] studied the ACTH and cortisol suppressibility by an infusion of hydrocortisone in healthy older men (65 – 99 years old) and young men (18 – 26 years old). Despite a similar cortisol response in both study groups, the older group displayed an insignificant decline of ACTH levels during the first 15 minutes after hydrocortisone infusion, whereas the young controls demonstrated a marked decrease in ACTH levels within 15 minutes. In contrast, older subjects exhibited a significant decline from 15 to 60 minutes after hydrocortisone infusion compared to a less pronounced decline in younger subjects. In addition, studies comparing the diurnal rhythm of serum cortisol secretion before and after dexamethasone administration demonstrated that older subjected were more likely not to respond (serum cortisol levels > 5 mcg/dL the morning following dexamethasone administration)[29], compared to healthy young controls, highlighting the role of aging in impaired HPA sensitivity to negative cortisol feedback. It is possible that the failure of cortisol to suppress with dexamethasone in some older adults could in part be attributed to the increasing prevalence of autonomous cortisol-producing adenomas with increasing age. Mild cortisol secretion is seen in 50% of patients with adrenal cortical adenomas and fails to suppress with dexamethasone [30]. Recognizing such patients among the aging population is crucial, as mild autonomous cortisol secretion has been linked to cardiovascular disease, diabetes, osteopenia, osteoporosis, and overall mortality [30, 31].
Finally, there is likely an interaction between sex and aging on adrenal function. While several studies focusing on animals have shown that cortisol levels are higher in females compared to males after HPA axis stimulation, these data are not consistent in humans[32, 33]. In aging human populations, exposure to a stress task has shown higher ACTH/cortisol in males [34, 35], while other studies demonstrated higher cortisol response in females[36]. In contrast, studies that included individuals from all age groups showed no sex differences in cortisol response to a stress task among young adults and children[37]. Most recently, in a study by Lanfear et al., hair cortisol concentrations were higher in aging males compared to females[38], while a study by Dettenborn et al. suggested that men display higher cortisol levels compared to women, regardless of their age[39]. These sex differences in production and excretion of cortisol and its metabolites have been linked to sex differences in 11 beta hydroxysteroid dehydrogenase (11β-HSD) activity in females compared to men[40]. However, the precise mechanism of this sex dimorphism is unknown. Additionally, the effect of sex on the association of cortisol awaking response and DNA damage (evaluated by telomere length) was recently studied[41]. Telomere length decreases with aging and is a marker of cellular aging and senescence. While higher levels of cortisol awaking response were associated with longer telomere length in males, the opposite was observed in females, suggesting that the response to stress and its consequences in cellular aging differs between sexes[41]. More studies on the effect of sex in adrenal aging and cellular senescence are required to fully understand the biological and cellular pathways controlling those differences.
B). Changes in aldosterone secretion:
Aldosterone secretion from the adrenal cortex declines with aging in both sexes [3]. When urinary plasma aldosterone levels were studied in healthy older subjects (>50 years of age) compared to younger individuals (<30 years of age), in an upright position, older individuals were noted to have lower plasma aldosterone concentration compared to younger subjects that were studied both before and after sodium depletion [3]. The secretion rates of aldosterone in aging subjects appears to be lower than those of young subjects mainly due to a decrease in the RAAS activity [42]. On the other hand, while an endogenous decrease in aldosterone secretion is observed with aging, the incidence of aldosterone-producing cell clusters increases in the same population and these may manifest as secondary hypertension[43].
C). Changes in adrenal androgen secretion: Changes in DHEAS
DHEA and DHEAS secretion decreases profoundly during aging[2]. DHEA(S) levels appear to be low in infancy and childhood, rise during adrenarche and reach their peak in the third decade, after which a decline of 1–2% per year is noted. As a result by the age of 70–80 years, levels are 20–30% of the lifetime peak concentration[4]. Interestingly, despite the numerous studies showing the positive effects of DHEAS on inflammation, sexual function, body composition, insulin resistance, bone metabolism, physical strength, and cognitive function[44], clinical trial data have not showed any benefits of DHEA therapy for any those outcomes[45].
D). Changes in catecholamine production with aging
Studies in mice have shown that aging leads to an increase in dopamine levels but a decline in epinephrine production in the mouse adrenal gland[46]. Similarly, human studies suggest that basal secretion, response to acute stress and clearance of epinephrine are all lower with increasing age[47]. Investigations suggest that the mechanism of reduced stress-induced epinephrine release is due to changes in calcium channel function at a cellular level[48]. This decrease in catecholamine production appears to be protective for aging individuals, as higher sympathetic activation (measured by urinary catecholamine excretion) has been linked to higher mortality and functional decline in healthy aging individuals[49].
(iii). The HPA axis and immunosenescence
Immunological changes observed during aging are known as immunosenescence. As already discussed, over-activation of the HPA axis can affect the susceptibility to infectious diseases, and in contrast, blunted HPA axis responses are associated with autoimmune states [50]. Thus, it has been suggested that changes in HPA axis and overall adrenal function that can occur in older individuals may be related to the decline in immune responses seen with aging.
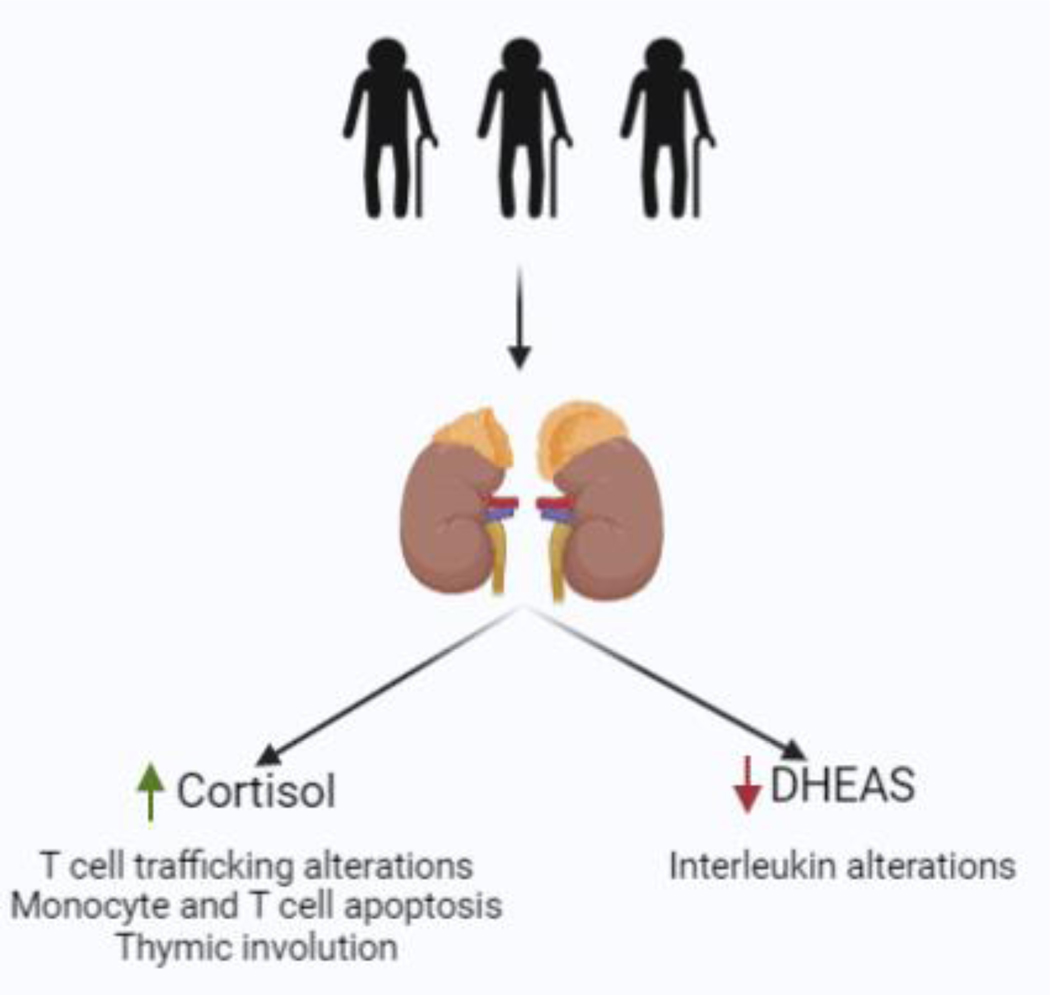
Several mechanisms have been suggested by which adrenal aging may have immunomodulatory effects. Elevated cortisol levels that occur with aging have been shown to alter T-cell trafficking, and exogenous glucocorticoids can induce apoptosis of both monocytes and T-cells[6]. Those data are also supported by the fact that healthy older patients who demonstrate increased HPA axis activation also have significantly lower T-cell proliferation[51]. In addition, the thymic involution that is a common consequence of aging has also been observed after glucocorticoid treatment[6], suggesting that the age-related rise in cortisol could be playing a role in the weakened immune response that is observed after the thymic involution.
Furthermore, DHEA is an important immunomodulator, although the mechanisms by which DHEA may modulate several immune responses are not completely understood. DHEAS has been shown to stimulate IL2 production from CD4+ cells, suggesting of an immunostimulatory effect of this hormone[52]. Importantly, it has been hypothesized that the immunomodulation occurring during aging is the result of the countereffects of cortisol and DHEAS on the expression levels of the receptor for activate C Kinase 1 (RACK-1), an important scaffold protein for immune function[53]. In summary, as shown in Figure 3 the age-related increase in cortisol in combination with the decrease in DHEA(S) appear to contribute to the immunosenescence observed in older subjects and may partly explain the increased risk for infection and inflammatory diseases that is observed with aging.
Figure 3:
The effect of adrenal function changes with aging on immunomodulation
Elevated cortisol levels that occur with aging have been shown to alter T-cell trafficking, monocyte and T cell apoptosis and thymic involution, whereas the decrease that is seen the DHEAS levels with aging can lead to altered secretion of interleukins, including IL-2 and IL-6 (Figure created in biorender.com)
Finally, as aging is linked to menopause in females, changes in the immune response can be linked to hypothalamic/gonadal changes that occur in the post-menopausal state. It is known that menopause is associated with multiple changes in the immune response. Interestingly, menopausal hormone therapy has been shown to rescue the decrease in B-cell production and the CD4/CD8 ratio[54, 55] and reduce the increased levels of IL-6 in postmenopausal women[56]. In contrast, other studies have not shown a beneficial effect of menopausal hormone therapy on immunosenescence [57]. Thus, given the lack of studies in this field, further investigation is required to understand the mechanisms of immunosenescence and the role of hypothalamic, adrenal and gonadal hormones on those biological pathways.
(iv). HPA changes with aging: effects on body composition, metabolic changes, bode density and cognition
Body composition and metabolic changes:
Loss of lean muscle mass and increased visceral adiposity are observed with aging. It is possible that hypercortisolemia observed in the aging population could be associated with the body composition changes[58] and adverse metabolic effects, including insulin resistance and diabetes[59]. In addition, decline in the levels of DHEAS may contribute to body composition and metabolic changes. In animal models DHEAS can stimulate lipolysis by increasing PPARγ and adiponectin levels while leptin levels decrease contributing to overall improvement of insulin sensitivity[60]. In addition, lower DHEAS levels in postmenopausal women have been linked to worsening cardiovascular outcomes[61] with other studies not finding an association[62]. Importantly, DHEA/S have been shown to prevent the progression of the atherosclerotic plaque and have a direct effect on cardiomyocytes and vascular smooth muscle cell remodeling[63]. These data suggest a potential adrenal component to the metabolic and cardiovascular consequences of aging[63]. Further studies are required to explore the role of adrenal hormones as potential therapeutic agents for the cardiovascular and metabolic diseases that accompany aging.
Bone density:
Aging has been associated with a decrease in bone mass and is a risk factor for osteopenia, osteoporosis and fractures [64]. The presence of glucocorticoid receptors in bone cells result in susceptibility to cortisol excess and higher cortisol levels during aging may inhibit new bone formation via osteoblast stimulation and osteocytes apoptosis as well as suppression of new osteoblast formation [65]. In addition, the decrease in DHEAS occurring with aging has been linked to osteoporosis. In a 25 year longitudinal study of 1003 subjects, it was shown that higher baseline DHEAS levels were linked to lower bone loss in a 15-year follow up time [66]. These data were similar to results of a prior study reporting a link between low serum DHEAS levels and a higher risk of non-vertebral fractures in aging men[67]. Hence, the role of cortisol and DHEAS changes with aging may have a direct effect on bone mineral density decline and the mechanisms of this process warrant additional studies.
Cognition:
Changes of neurocognitive function in older people have previously been associated with HPA axis alterations that become evident with age. High cortisol release after stress has been linked to cognitive impairment [68] A study of 1337 adults over 60 years of age demonstrated that higher salivary cortisol was associated with lower cognitive function. Cortisol appeared to modulate the interaction of inflammatory markers and cognition in older individuals. For example, the slope of the association between increasing IL-6 and poorer cognition was steeper in individuals with lower cortisol levels [69]. Importantly, a recent systematic review and metanalysis demonstrated that higher nighttime control was associated with worse cognitive ability, while a larger diurnal drop and higher cortisol awaking response were associated with better cognition[70]. Consistent with these findings, a flatter diurnal cortisol slope has been linked to poor emotional and physical health [71, 72]. Thus, preliminary evidence suggests cortisol levels in aging adults can affect cognitive function.
Conclusions
Aging is a pervasive process to which the adrenal glands are not immune. Changes with aging have been demonstrated in the three cortical adrenal zones. As discussed in this review, aging has been associated with higher mean cortisol levels and reduced sensitivity to negative cortisol feedback as well as reductions in aldosterone and DHEA(S) secretion. Given the importance of adrenal function in maintaining physiologic homeostasis and stress response, altered HPA axis function with aging may contribute to age-related changes in metabolic and immune function. Further study into these complex processes is warranted to understand mechanisms driving changes to adrenal function with aging and to explore how healthy aging can be promoted by further understanding of the impact of these changes.
Highlights.
Aging is linked to higher mean cortisol levels.
During aging, the sensitivity of the hypothalamic-pituitary-adrenal (HPA) axis to negative cortisol feedback decreases.
Aging is associated with reduced aldosterone and DHEA(S) secretion.
Altered HPA axis function with aging may contribute to age-related changes in metabolic and immune function.
Funding
Dr. Caitlin Colling was supported by T32DK007028 and Dr. Dichtel was supported by K23DK113230.
Footnotes
Provenance and peer review
This article was commissioned and was externally peer reviewed.
Declaration of competing interest
Dr. Dichtel has received drug donation from Pfizer (recent), research support from Perspectum Ltd. (current) and research support/drug donation from Lumos Pharma (current), all per investigator-initiated requests. Dr. Dichtel is a Mass General Brigham Innovation Fellow hosted by Third Rock Ventures a venture capital firm. She remains full-time at MGH during the period of this educational program (anticipated 10/1/2022 – 9/30/2024). Dr. Dichtel’s financial interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamberts SW, van den Beld AW, and van der Lely AJ, The endocrinology of aging. Science, 1997. 278(5337): p. 419–24. [DOI] [PubMed] [Google Scholar]
- 2.Piazza JR, et al. , Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci, 2010. 65(5): p. 513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegstad R, et al. , Aging and aldosterone. Am J Med, 1983. 74(3): p. 442–8. [DOI] [PubMed] [Google Scholar]
- 4.Ravaglia G, et al. , The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab, 1996. 81(3): p. 1173–8. [DOI] [PubMed] [Google Scholar]
- 5.Otte C, et al. , A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology, 2005. 30(1): p. 80–91. [DOI] [PubMed] [Google Scholar]
- 6.Bauer ME, Stress, glucocorticoids and ageing of the immune system. Stress, 2005. 8(1): p. 69–83. [DOI] [PubMed] [Google Scholar]
- 7.Ouzzani M, et al. , Rayyan-a web and mobile app for systematic reviews. Syst Rev, 2016. 5(1): p. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlomo Melmed KSP, Reed Larsen P. and Henry M. Kronenberg, 13th Edition, Williams Textbook of Endocrinology. 2015. [Google Scholar]
- 9.Oakley RH and Cidlowski JA, The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol, 2013. 132(5): p. 1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasir M, Goyal A, and Sonthalia S, Corticosteroid Adverse Effects, in StatPearls. 2022: Treasure Island (FL). [PubMed] [Google Scholar]
- 11.Nanba K, Vaidya A, and Rainey WE, Aging and Adrenal Aldosterone Production. Hypertension, 2018. 71(2): p. 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaides NC, Chrousos G, and Kino T, Glucocorticoid Receptor, in Endotext, Feingold KR, et al. , Editors. 2000: South Dartmouth (MA). [Google Scholar]
- 13.Boonen E, et al. , Reduced cortisol metabolism during critical illness. N Engl J Med, 2013. 368(16): p. 1477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundstrom S. and Furst CJ, Symptoms in advanced cancer: relationship to endogenous cortisol levels. Palliat Med, 2003. 17(6): p. 503–8. [DOI] [PubMed] [Google Scholar]
- 15.Genis-Mendoza AD, et al. , Increased Levels of Cortisol in Individuals With Suicide Attempt and Its Relation With the Number of Suicide Attempts and Depression. Front Psychiatry, 2022. 13: p. 912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrousos GP, The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med, 1995. 332(20): p. 1351–62. [DOI] [PubMed] [Google Scholar]
- 17.Desmet SJ and De Bosscher K, Glucocorticoid receptors: finding the middle ground. J Clin Invest, 2017. 127(4): p. 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay LI and Cidlowski JA, Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev, 1999. 20(4): p. 435–59. [DOI] [PubMed] [Google Scholar]
- 19.Peers SH and Flower RJ, The role of lipocortin in corticosteroid actions. Am Rev Respir Dis, 1990. 141(2 Pt 2): p. S18–21. [PubMed] [Google Scholar]
- 20.Paliogianni F, et al. , Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J Clin Invest, 1993. 91(4): p. 1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, et al. , Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol, 2003. 170(9): p. 4833–9. [DOI] [PubMed] [Google Scholar]
- 22.De Benedetti F, et al. , Targeting interferon-gamma in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol, 2021. 17(11): p. 678–691. [DOI] [PubMed] [Google Scholar]
- 23.Ehrhart-Bornstein M, et al. , Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev, 1998. 19(2): p. 101–43. [DOI] [PubMed] [Google Scholar]
- 24.Bornstein SR, et al. , Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab, 2008. 19(5): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 25.Van Cauter E, Leproult R, and Kupfer DJ, Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab, 1996. 81(7): p. 2468–73. [DOI] [PubMed] [Google Scholar]
- 26.Deuschle M, et al. , With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci, 1997. 61(22): p. 2239–46. [DOI] [PubMed] [Google Scholar]
- 27.Kudielka BM, et al. , Psychosocial stress and HPA functioning: no evidence for a reduced resilience in healthy elderly men. Stress, 2000. 3(3): p. 229–40. [DOI] [PubMed] [Google Scholar]
- 28.Boscaro M, et al. , Age-related changes in glucocorticoid fast feedback inhibition of adrenocorticotropin in man. J Clin Endocrinol Metab, 1998. 83(4): p. 1380–3. [DOI] [PubMed] [Google Scholar]
- 29.Carroll BJ, et al. , A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry, 1981. 38(1): p. 15–22. [DOI] [PubMed] [Google Scholar]
- 30.Reimondo G, et al. , Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J Clin Endocrinol Metab, 2020. 105(4). [DOI] [PubMed] [Google Scholar]
- 31.Elhassan YS, et al. , Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann Intern Med, 2019. 171(2): p. 107–116. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura S, et al. , Sex-differences in adrenocortical responsiveness during development in rats. Steroids, 2003. 68(5): p. 439–45. [DOI] [PubMed] [Google Scholar]
- 33.Kudielka BM and Kirschbaum C, Sex differences in HPA axis responses to stress: a review. Biol Psychol, 2005. 69(1): p. 113–32. [DOI] [PubMed] [Google Scholar]
- 34.Kudielka BM, et al. , Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab, 1998. 83(5): p. 1756–61. [DOI] [PubMed] [Google Scholar]
- 35.Traustadottir T, Bosch PR, and Matt KS, Gender differences in cardiovascular and hypothalamic-pituitary-adrenal axis responses to psychological stress in healthy older adult men and women. Stress, 2003. 6(2): p. 133–40. [DOI] [PubMed] [Google Scholar]
- 36.Seeman TE, et al. , Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology, 2001. 26(3): p. 225–40. [DOI] [PubMed] [Google Scholar]
- 37.Kudielka BM, et al. , HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology, 2004. 29(1): p. 83–98. [DOI] [PubMed] [Google Scholar]
- 38.Lanfear JH, et al. , Hair cortisol measurement in older adults: Influence of demographic and physiological factors and correlation with perceived stress. Steroids, 2020. 163: p. 108712. [DOI] [PubMed] [Google Scholar]
- 39.Dettenborn L, et al. , The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress, 2012. 15(6): p. 578–88. [DOI] [PubMed] [Google Scholar]
- 40.Raven PW and Taylor NF, Sex differences in the human metabolism of cortisol. Endocr Res, 1996. 22(4): p. 751–5. [DOI] [PubMed] [Google Scholar]
- 41.Thomas N, et al. , Influence of cortisol awakening response on telomere length: Trends for males and females. Eur J Neurosci, 2022. 55(9–10): p. 2794–2803. [DOI] [PubMed] [Google Scholar]
- 42.Mulatero P, et al. , Primary Aldosteronism in the Elderly. J Clin Endocrinol Metab, 2020. 105(7). [DOI] [PubMed] [Google Scholar]
- 43.Pauzi FA and Azizan EA, Functional Characteristic and Significance of Aldosterone-Producing Cell Clusters in Primary Aldosteronism and Age-Related Hypertension. Front Endocrinol (Lausanne), 2021. 12: p. 631848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales AJ, et al. , Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab, 1994. 78(6): p. 1360–7. [DOI] [PubMed] [Google Scholar]
- 45.Elraiyah T, et al. , Clinical review: The benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab, 2014. 99(10): p. 3536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amano A, et al. , Age-related changes of dopamine, noradrenaline and adrenaline in adrenal glands of mice. Geriatr Gerontol Int, 2013. 13(2): p. 490–6. [DOI] [PubMed] [Google Scholar]
- 47.Seals DR and Esler MD, Human ageing and the sympathoadrenal system. J Physiol, 2000. 528(Pt 3): p. 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elhamdani A, Palfrey CH, and Artalejo CR, Ageing changes the cellular basis of the “fight-or-flight” response in human adrenal chromaffin cells. Neurobiol Aging, 2002. 23(2): p. 287–93. [DOI] [PubMed] [Google Scholar]
- 49.Reuben DB, et al. , High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci, 2000. 55(10): p. M618–24. [DOI] [PubMed] [Google Scholar]
- 50.Sternberg EM, Neuroendocrine regulation of autoimmune/inflammatory disease. J Endocrinol, 2001. 169(3): p. 429–35. [DOI] [PubMed] [Google Scholar]
- 51.Luz C DF, Scapini E, Collaziol D, Preissler T, Cruz I, and ME B, Psychological and nutritional correlates of T-cell function in the healthy elderly. Stress 5:80., 2002. [Google Scholar]
- 52.Dillon JS, Dehydroepiandrosterone, dehydroepiandrosterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr Drug Targets Inflamm Allergy, 2005. 4(3): p. 377–85. [DOI] [PubMed] [Google Scholar]
- 53.Buoso E, et al. , Opposing effects of cortisol and dehydroepiandrosterone on the expression of the receptor for Activated C Kinase 1: implications in immunosenescence. Exp Gerontol, 2011. 46(11): p. 877–83. [DOI] [PubMed] [Google Scholar]
- 54.Engelmann F, et al. , Impact of Estrogen Therapy on Lymphocyte Homeostasis and the Response to Seasonal Influenza Vaccine in Post-Menopausal Women. PLoS One, 2016. 11(2): p. e0149045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter VR, et al. , Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol, 2001. 36(2): p. 311–26. [DOI] [PubMed] [Google Scholar]
- 56.Saucedo R, et al. , Transdermal estradiol in menopausal women depresses interleukin-6 without affecting other markers of immune response. Gynecol Obstet Invest, 2002. 53(2): p. 114–7. [DOI] [PubMed] [Google Scholar]
- 57.Yang JH, et al. , Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T-cell subpopulation or interferon-gamma production in postmenopausal women. Fertil Steril, 2000. 74(2): p. 261–7. [DOI] [PubMed] [Google Scholar]
- 58.Waters DL, et al. , Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol A Biol Sci Med Sci, 2008. 63(5): p. 536–41. [DOI] [PubMed] [Google Scholar]
- 59.Cefalu WT, et al. , Contribution of visceral fat mass to the insulin resistance of aging. Metabolism, 1995. 44(7): p. 954–9. [DOI] [PubMed] [Google Scholar]
- 60.Teixeira CJ, Veras K, and de Oliveira Carvalho CR, Dehydroepiandrosterone on metabolism and the cardiovascular system in the postmenopausal period. J Mol Med (Berl), 2020. 98(1): p. 39–57. [DOI] [PubMed] [Google Scholar]
- 61.Shufelt C, et al. , DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health--National Heart, Lung, and Blood Institute (NHLBI)sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab, 2010. 95(11): p. 4985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boxer RS, et al. , Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing, 2010. 39(4): p. 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannella P, et al. , Dehydroepiandrosterone and Cardiovascular Disease. Vitam Horm, 2018. 108: p. 333–353. [DOI] [PubMed] [Google Scholar]
- 64.Perry HM 3rd, The endocrinology of aging. Clin Chem, 1999. 45(8 Pt 2): p. 1369–76. [PubMed] [Google Scholar]
- 65.Gonzalez Rodriguez E, et al. , High Evening Cortisol Level Is Associated With Low TBS and Increased Prevalent Vertebral Fractures: OsteoLaus Study. J Clin Endocrinol Metab, 2017. 102(7): p. 2628–2636. [DOI] [PubMed] [Google Scholar]
- 66.Ghebre MA, et al. , Association between DHEAS and bone loss in postmenopausal women: a 15-year longitudinal population-based study. Calcif Tissue Int, 2011. 89(4): p. 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohlsson C, et al. , Low Serum DHEAS Predicts Increased Fracture Risk in Older Men: The MrOS Sweden Study. J Bone Miner Res, 2017. 32(8): p. 1607–1614. [DOI] [PubMed] [Google Scholar]
- 68.de Souza-Talarico JN, et al. , Cortisol reactivity to a psychosocial stressor significantly increases the risk of developing Cognitive Impairment no Dementia five years later. Psychoneuroendocrinology, 2020. 115: p. 104601. [DOI] [PubMed] [Google Scholar]
- 69.Stebbins RC, et al. , Immune function, cortisol, and cognitive decline & dementia in an aging latino population. Psychoneuroendocrinology, 2021. 133: p. 105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner M, et al. , Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: individual participant meta-analysis of five cohorts. Sci Rep, 2019. 9(1): p. 4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adam EK, et al. , Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 2017. 83: p. 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho RTH, et al. , Diurnal Cortisol Slope Mediates the Association Between Affect and Memory Retrieval in Older Adults With Mild Cognitive Impairment: A Path-Analytical Study. Front Aging Neurosci, 2020. 12: p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]