Abstract
Background:
Screening people living with HIV for hepatitis B virus (HBV) co-infection is recommended in resource-rich settings to optimize HIV antiretroviral therapy (ART) and mitigate HBV-related liver disease. This review examines the need, feasibility, and impact of screening for HBV in resource-limited settings (RLS).
Methods:
We searched 6 databases to identify peer-reviewed publications between 2007 and 2013 addressing (1) HIV/HBV co-infection frequency in sub-Saharan Africa (SSA); (2) performance of hepatitis B surface antigen (HBsAg) rapid strip assays (RSAs) in RLS; (3) impact of HBV co-infection on morbidity, mortality, or liver disease progression; and/or (4) impact of HBV-suppressive antiretroviral medications as part of ART on at least one of 5 outcomes (mortality, morbidity, HIV transmission, retention in HIV care, or quality of life). We rated the quality of individual articles and summarized the body of evidence and expected impact of each intervention per outcome addressed.
Results:
Of 3940 identified studies, 85 were included in the review: 55 addressed HIV/HBV co-infection frequency; 6 described HBsAg RSA performance; and 24 addressed the impact of HIV/HBV co-infection and ART. HIV/HBV frequency in sub-Saharan Africa varied from 0% to > 28.4%. RSA performance in RLS showed good, although variable, sensitivity and specificity. Quality of studies ranged from strong to weak. Overall quality of evidence for the impact of HIV/HBV co-infection and ART on morbidity and mortality was fair and good to fair, respectively.
Conclusions:
Combined, the body of evidence reviewed suggests that HBsAg screening among people living with HIV could have substantial impact on preventing morbidity and mortality among HIV/HBV co-infected individuals in RLS.
Keywords: hepatitis B virus, resource-limited setting, hepatitis B surface antigen, rapid strip assay, HIV/HBV co-infection
BACKGROUND
Globally, an estimated 5%–10% of people living with HIV (PLHIV) are co-infected with hepatitis B virus (HBV). HIV and HBV share common risk factors, and many generalized HIV epidemics occur in populations with higher HBV prevalence, leading to an increased risk for HBV co-infection (HIV/HBV co-infection).1,2 People who inject drugs and men who have sex with men are also at higher risk for HIV/HBV co-infection.3,4 In resource-rich settings, persons with HIV/HBV co-infection have been shown to have poorer health outcomes.5–7 Studies have shown that HBV is a leading cause of non-AIDS–related deaths among PLHIV in settings where HIV-suppressive antiretroviral therapy (ART) is widely available8,9; this is likely in part because PLHIV on ART are living long enough to develop liver disease, including HBV-related cirrhosis and hepatocellular carcinoma (HCC).
Several studies strongly suggest that HIV co-infection increases HBV replication, leading to higher levels of detectable virus, accelerated cirrhosis, and increased likeli-hood of developing HCC.3,8,10 Mortality among HIV/HBV co-infected persons is substantially higher than among HIV mono-infected persons. In the US-based Multicenter AIDS Cohort Study, those with HIV/HBV co-infection were shown to be over 8 times more likely to die from liver disease than those with HIV mono-infection and approaching 19 times more likely to die from liver disease than the HBV mono-infected participants.5 The impact of HBV on HIV disease progression is less clear,6,11–15 although it is postulated that HBV infection may potentially lead to a blunted immune response in patients receiving ART and increase patient susceptibility to ART-related liver toxicity.16
Potential complications of HIV treatment in the presence of HBV co-infection include (1) HBV-associated hepatic inflammation during immune recovery, and (2) the development of HBV resistance with rebound HBV viremia when lamivudine (3TC) or emtricitabine (FTC) is used without a second HBV-suppressive agent such as tenofovir (TDF).17–19
Most resource-rich settings have adopted HIV treatment guidelines that call for HBV screening of PLHIV and early initiation of ART containing at least 2 antiretroviral agents able to suppress both HIV and HBV [ie, the US Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents (http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/0/)]. As a result, HBV screening among PLHIV has been extensively implemented in resource-rich settings, and there is widespread treatment of HIV/HBV co-infected persons with ART regimens containing TDF and 3TC or FTC.
In contrast, the World Health Organization (WHO) 2013 Consolidated Guidelines for Antiretroviral Therapy (http://www.who.int/hiv/pub/guidelines/arv2013/en/) targeted for use in resource-limited settings (RLS) in low- and middle-income countries, defines HBV screening as “desired” but not recommended as a part of the initial ART workup and when switching HIV/HBV co-infected persons to second-line ART. Early initiation of ART among HIV/HBV co-infected persons regardless of CD4 count is only recommended when there is evidence of severe chronic liver disease. However, assessment of liver disease is difficult in RLS, with limited availability of liver biopsy and non-invasive tests for fibrosis, resulting in most patients being diagnosed after they develop decompensated cirrhosis. For those patients who are diagnosed with HIV/HBV co-infection, the 2013 WHO guidelines do specifically recommend the use of ART containing at least 2 agents with HBV-suppressive activity for known HIV/HBV co-infection and recommend first-line treatment of all HIV/HBV co-infected adults with TDF + 3TC (or FTC) + efavirenz.
At the country level, a number of national guidelines in RLS now recommend HBV serologic screening for PLHIV as a part of the initial HIV workup and when switching to second-line ART, at the minimum testing for hepatitis B surface antigen (HBsAg), a marker of current HBV infection.20 However, because of resource constraints and other barriers, implementation of HBV screening varies across and within countries and is not generally done on a routine basis in RLS. Furthermore, the majority of HIV clinics in RLS do not have access to laboratory-based HBV serology. Because current HIV testing algorithms in RLS rely on rapid point-of-care (POC) HIV testing, POC rapid HBsAg tests would likely be the most accessible mechanism to screen for HBV infection in the majority of clinics. The following evidence review aims to help guide countries considering implementation of HBV screening using HBsAg rapid strip assays (RSAs) to improve HIV outcomes among PLHIV co-infected with HBV. This article is one of the 12 articles in this supplement addressing care and support interventions offered to PLHIV in RLS.
METHODS
As described in the introductory article to this supplement, we conducted a literature search of 6 medical databases to identify appropriate peer-reviewed publications: Medline, Embase, Global Health, CINAHL, SOCA, and African Index Medicus. The initial search focused on studies relevant to understanding the impact of HBV screening, particularly HBsAg screening, on HIV patient outcomes that were published between January 1995 and July 2013. To ensure the largest possible yield of data, eligible studies containing relevant data on the impact of HBV (or HBsAg) screening on HIV outcomes, publications must have (1) included persons diagnosed with HIV/HBV co-infection; (2) reported the results of HBV screening of PLHIV using HBsAg or other markers of current HBV infection; (3) been conducted in RLS; and (4) reported at least one of the 5 outcomes of interest (mortality, morbidity, HIV transmission, retention in HIV care, or quality of life) or a costing or cost-effectiveness analysis. A more detailed description of the overall search terms and geographic filters used can be found in the introduction to this supplement.21 Search terms related to HBV screening are shown in Table 1 of this article.
TABLE 1.
HBV Screening Literature Search Terms
| Hepatitis B Screening-Specific Search Terms | |
|---|---|
| Hepatitis B | Hepatitis B surface antigen |
| Hepatitis B virus | Screening |
| HBV | Serology |
| HBsAg | Hepatitis B serology |
| HB Ab | Anti-HBs |
| HBeAg | HBV envelope Ag |
| Co-infection | Antiretroviral |
| Liver disease | ARV |
| Chronic liver disease | Treatment |
| Chronic active hepatitis | Therapy |
| Liver fibrosis | Antiretroviral therapy |
| Cirrhosis | ART |
| End-stage liver disease | ART initiation |
| Hepatocellular carcinoma | Tenofovir |
Titles and abstracts from the search results were examined for relevance to the stated intervention. Finding no direct data or specific evidence regarding the impact of HBV screening on the key outcomes of interest, we revised the literature review strategy to identify publications providing indirect evidence for the benefits of HBV screening and knowledge of HBV infection status among PLHIV.
The revised literature review criteria for inclusion required that a publication address 1 or more of the following: (1) current understanding of existing HBV prevalence rates in Sub-Saharan Africa (SSA); (2) performance of HBsAg RSAs; (3) impact of HBV co-infection on morbidity and mortality; and (4) impact of HBV-suppressive antiretroviral medications, such as TDF or combination TDF + 3TC, as part of ART. For category 1, we restricted selection of studies reporting rates of HIV/HBV co-infection to those conducted between January 2005 and July 2013, inclusive. This inclusion criterion was applied to reflect more recent ART regimens and expanded ART coverage that might impact HIV/HBV co-infection. Selection of studies based on performance of HBV screening methods focused on HBsAg RSA, as RSA implementation would be far more feasible in RLS clinics than laboratory-based HBV serology. For categories 3 and 4, the search criteria were expanded to include publications conducted in resource-rich settings or high-income countries because of the limited number of identified studies that had been conducted in low- and middle-income countries.
Publications meeting the search inclusion criteria, with the exception of those that focused solely on HIV/HBV frequencies, were reviewed in their entirety and abstracted and summarized on the basis of study design (eg, randomized control trial, cohort study, case series), comparison group(s), number of participants/samples, and assessment of impact on the outcome(s) of interest [expressed as Hazard Ratios, Odds Ratios, or Relative Risk, and the respective 95% Confidence Intervals (CIs) if available]. For each study, these characteristics and results were used to rate internal and external validity as good, fair, or poor. An assessment of the overall quality of each study was made on the basis of study design, number of participants, and internal and external validity and rated as strong, medium, or weak, as described in the introductory article of this supplement.21 Quantitative synthesis of the publication results was not attempted because of the heterogeneity of study populations and samples, study designs, and outcomes. Data from all eligible studies were grouped according to outcome, and the quality of the entire body of evidence for that outcome was rated as good, fair, or poor. The expected impact by outcome was rated as high, moderate, low, or uncertain based on the magnitude of effect demonstrated in individual studies and the quality of the body of evidence across the studies. The quality of studies and surveys reporting on frequency of HIV/HBV co-infection was not rated or entered into the standard rating grid (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A655) because of space constraints and the need to prioritize rating those clinical studies whose outcomes would most directly inform decision making about the potential health impact of interventions that address HBV infection among PLHIV. Studies on the frequency of co-infection presented in this article represent findings from peer-reviewed publications using standard methodology for estimating the frequency of current or chronic HBV infection and/or describing the frequency of occult HBV infection in SSA.
RESULTS
Revised search criteria generated 3940 abstracts that were screened against the revised inclusion criteria. A total of 136 studies were considered for full-text review. After examining the full texts, 55 were excluded and the remaining 81 studies were included as part of this systematic review (Fig. 1).
FIGURE 1.
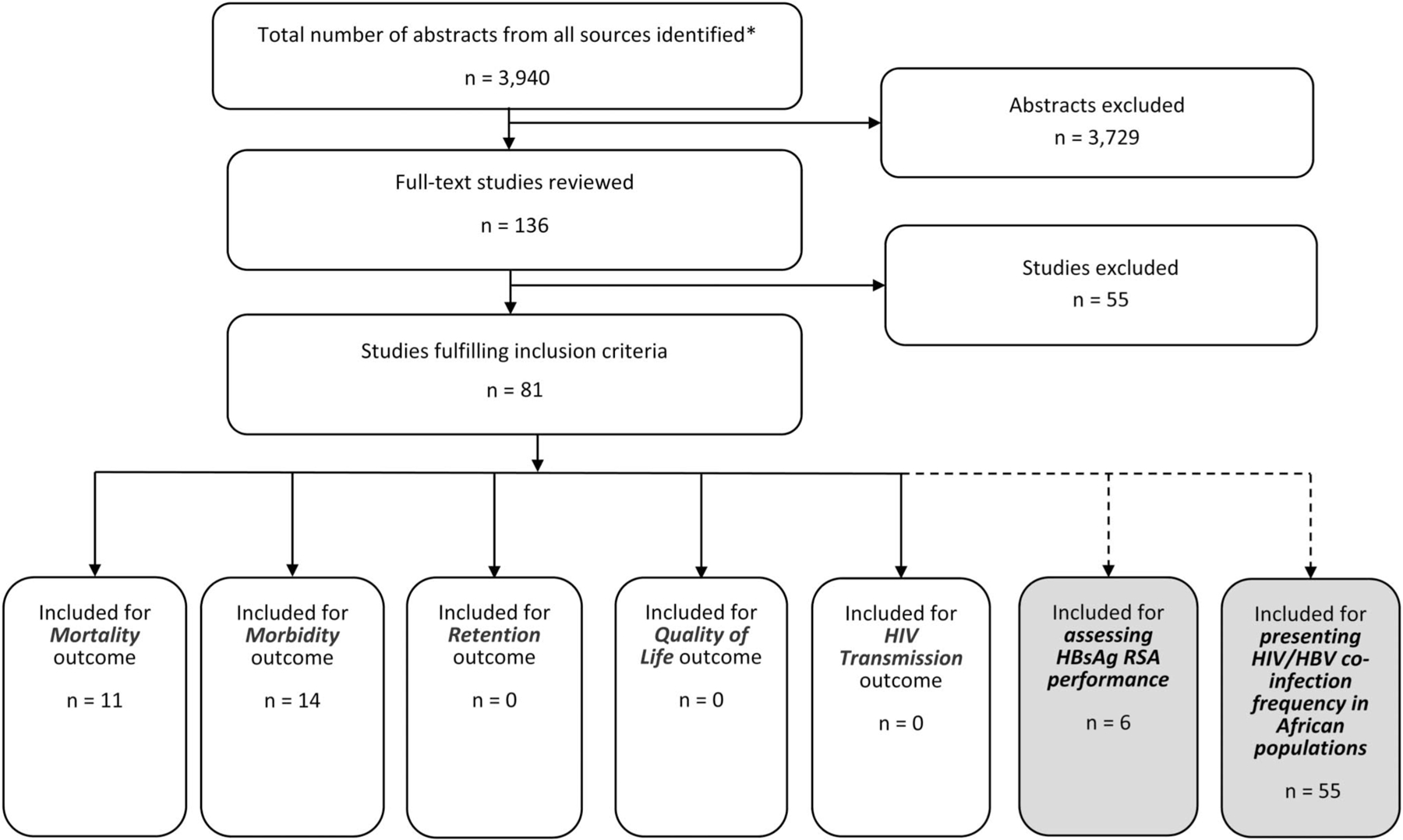
Study flow diagram. *Duplicate citations removed.
The revised search strategy identified 31 publications relevant to outcomes of interest: 25 examined the morbidity and mortality of HIV/HBV co-infected persons5–9,15,22–40; most of these addressed the impact of ART containing HBV suppressive antiviral agents (eg, TDF and/or 3TC) on HBV related markers and outcomes; and 6 reported on the performance of HBsAg RSA41–46 (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A655).) In addition, we also identified 49 peer-reviewed publications meeting the inclusion criteria that reported HBV serology results among PLHIV in SSA.23,34,37,39,42,47–90 Of these, 14 articles presented co-infection data from Nigeria and 8 presented co-infection data from South Africa. Serology studies from only 16 countries in Africa met criteria for inclusion in this review. Six additional articles described the prevalence of occult HBV infection among PLHIV,91–96 defined as the presence of detected HBV virus and isolated IgG antibody to hepatitis B core antigen in persons who are HBsAg-negative.
HIV/HBV Co-Infection Frequency in SSA
The reviewed publications suggest that HIV/HBV co-infection rates differ greatly across SSA, varying from 0% to over 28.4% with a median co-infection rate of 7.8% (Fig. 2).23,34,37,39,42,47–90 However, very few of these reported rates come from population-based surveillance. The median HIV/HBV co-infection rate among pregnant women was 3.8% (0%–13%) and was 7.4% (1.2%–7.8%) among children aged 18 months to 17 years. Overall, West African countries seem to have the highest co-infection rates (median: 11.5%), Southern African countries have the second highest (median: 5.4%), and East African countries the lowest (median: 4.1%). However, there is also wide variability within countries in each region (Fig. 2).
FIGURE 2.
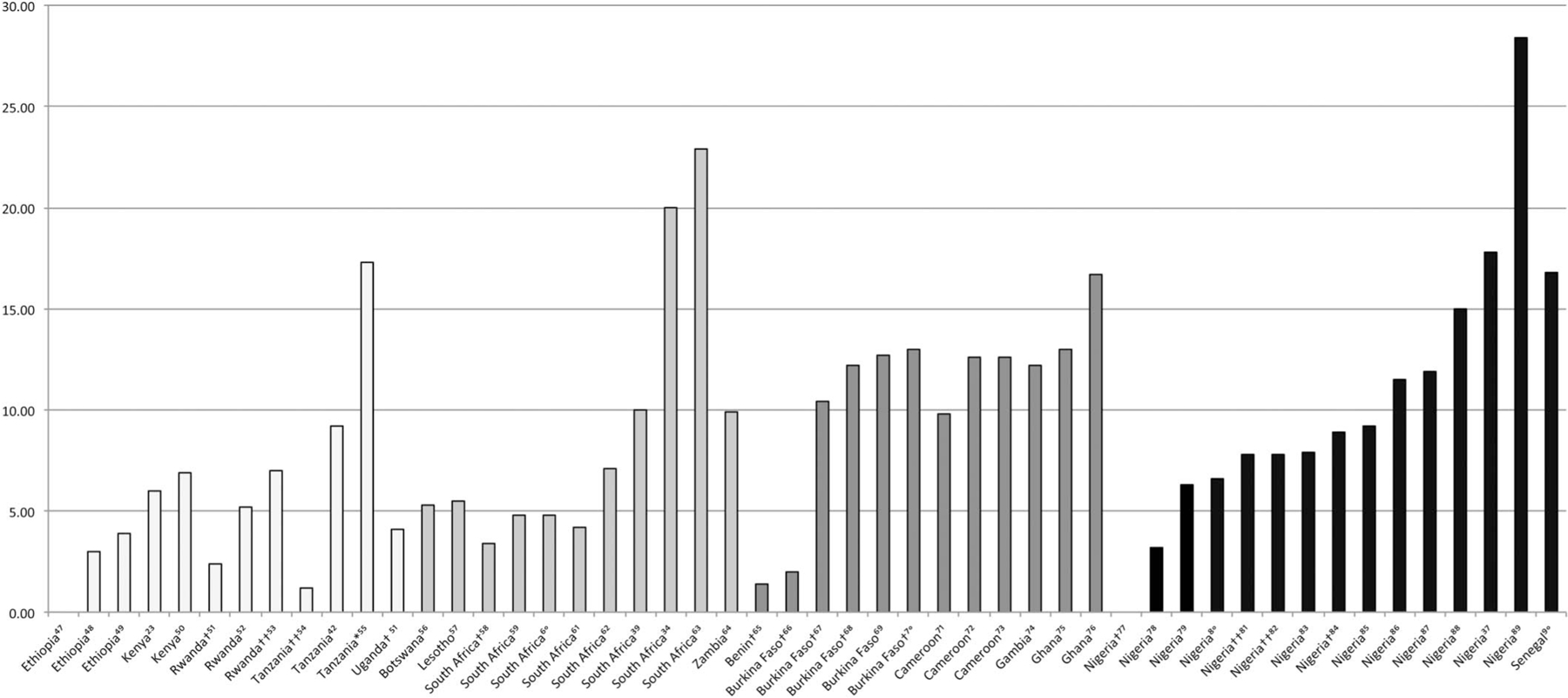
. Reported HIV/HBV co-infection frequencies in East, West, and Southern Africa. †Pregnant women. ††Children and adolescents. *Includes patients recently hospitalized presenting to a tertiary hospital; might not be representative. Co-infection is defined as the % of HIV patients in the study who also have HBV. These studies were not done using a systematic population-based or risk-based surveillance method. Additionally, the sample size and methodologies varied greatly. The studies included in the graph are from 2005 on.
Occult HBV co-infection with HIV also varied greatly across the continent. The identified studies, mostly from South Africa and Western Africa, reported occult HBV rates from 10% to 33%.91–96
Performance of the HBV Rapid Strip Assay
Six studies assessed the performance of 21 HBsAg RSA diagnostics,41–46 4 in clinics in RLS in SSA.41,42,44,46 The Scheiblauer et al45 study had the most comprehensive assay performance analysis, although limited in its direct applicability to the use of RSA as a POC diagnostic in RLS. This study showed high sensitivity (94.5%–99.3%) and specificity (96.4%–99.5%) among the 19 RSAs tested when performed on a panel of 146 HBsAg-positive clinical samples representative of various HBV genotypes, subtypes, and countries of origin. An HBsAg dilution series ranging from 0.031 to 4.0 IU/mL created from an 8-member International Consortium for Blood Safety HBsAg panel demonstrated that all 19 RSA exhibited poor (> 1 IU/mL) detection sensitivity; 13 RSAs failed to detect HBsAg concentrations of 4 IU/mL; and 5 RSAs failed to detect HBsAg concentration as high as 20 IU/mL in nondiluted panel members. Another study, conducted in the National AIDS Control Organisation-National Reference Laboratory in Kolkata, India, evaluated 3 commercially available HBsAg RSA (J. Mitra: New Delhi, India SPAN: Surat, India Stnadard: Haryana, India), all showing sensitivity and specificity of 100% on 100 characterized serum samples.43
Three of the remaining 4 studies were conducted in a RLS (Uganda, Tanzania and Malawi) and showed variability in RSA performance. The first, in Uganda, found similar specificity of the tested RSA (Cortez Rapidtest; Cortez Diagnostics, Calabasas, CA) to an enzyme immunoassay (EIA) (Siemens Centaur EIA platform; Siemens Diagnostics, Tarrytown, NY) (95.8% and 97.9%, respectively) but a much lower sensitivity (43.5% and 77.4%, respectively) using HBV DNA results as the gold standard.46 They observed that the best performance of the RSA was in individuals with high HBV viral load (VL) and the poorest performance in individuals who seroconverted to detectable antibody to hepatitis B envelope antigen (HBeAg) or had a low VL, indicating that RSA diagnostics alone may not be sufficient to achieve improved patient care in the African setting. In a second study conducted in a clinic in rural Tanzania, the Alere Determine HBsAg RSA (Alere Ind., MA) demonstrated a 96% sensitivity (95% CI: 82.8% to 99.6%) and 100% specificity (95% CI: 98.9% to 100%) when compared with an EIA reference test [Murex HBsAg 3.0 (Abbott Diagnostics, Wiesbaden, Germany)].42 In a third study by Nyirenda et al,44 in an RLS clinic in Malawi, the Abbott Determine HBsAg RSA (Abbott Laboratories, Abbott Park, IL) performed poorly with a sensitivity of 56% and specificity of 69% when compared with an EIA reference test (BioKit, Barcelona, Spain) conducted in a laboratory in Royal Liverpool University on frozen aliquots from the original patient blood draws. The final study reviewed was a follow on to the Malawi study by the same group and tested samples from PLHIV in Malawi but in a controlled laboratory in the United Kingdom.41 In this setting, the RSA used (Determine Inverness Medical; Japan Co., Ltd., Tokyo, Japan) showed a specificity of 100% (95% CI: 93% to 100%) and sensitivity of 100% (95% CI: 86% to 100%) compared with the same EIA reference test used in Nyirenda et al. Authors cited possible user error in local operational execution or unexpected technical issues within the clinic setting in Malawi contributing to RSA results in the Nyirenda et al study.
Mortality
Although the systematic review did not identify studies that directly attributed HIV clinical outcomes to the provision of HBV testing, it did identify 7 studies conducted in resource-rich settings characterizing the association of HBV co-infection with mortality among PLHIV.5–9,15,22 All found an increased risk of death in HIV/HBV co-infected subjects. Among these studies, Thio et al might be most generalizable to RLS. That study found much higher rates of liver-related mortality among HIV/HBV co-infected persons, especially those with lower nadir CD4 counts (relative risk: 11.6 at 100 cells/μL vs 6.8 at 250 cells/μL).5 Although this study reported that liver-related mortality was greater after the advent of ART, data were from a period before the use of TDF, when ART regimens may have been more hepatotoxic and were usually initiated at much greater levels of immunosuppression. Also important to note is that overall AIDS-related mortality was notably decreasing at this time, allowing PLHIV to live long enough to develop HBV-related liver disease. A follow-up study of the same cohort, published in 2009, indicated that overall mortality was higher in HIV/HBV co-infection and was mainly attributed to liver disease despite HBV-active ART.
The Chun et al6 study, conducted in the post-ART era, showed a significant impact of HBV-HIV co-infection on HIV outcomes, including nearly a doubling of the risk of AIDS-related death and the Weber et al9 study found a strong association between immunodeficiency and liver-related death. The Salmon-Ceron8,22 mortality studies, which focused on HCV and HBV co-infection, when taken together showed a relatively high but stable proportion of HBV-related deaths from 2000 to 2005, suggesting a potentially protective effect of dually active ART.
Four studies conducted in RLS, 1 randomized controlled trial (RCT) and 3 cohort studies, containing mortality data were available for review.23–26 The RCT from South Africa, like studies in the West, suggested a higher mortality among those HIV/HBV co-infected than among HIV mono-infected (17% vs 11%, P = 0.04), median, 24.7 months of follow-up.25 In contrast, the 3 cohort studies did not suggest differences in mortality between HIV populations served in RLS. This could be explained by the relatively short period of follow-up time, particularly in the cohort studies from Malawi26 and South Africa24 with less than 2 years of follow-up, as well as known and unknown study population differences that could influence or mitigate liver disease progression (baseline liver disease, alcohol consumption, gender, HBV genotype). Given that HBV suppression was initially achieved (or assumed to be achieved) in at least some of the patients in these studies, some partial treatment of HBV and slowed progression of HBV liver disease may have occurred during the short time frame, before HBV resistance to 3TC developed.
Morbidity
Five studies, including 4 cohort studies27,28,30,31 and 1 RCT,29 conducted in resource-rich settings and 9 studies32–40 conducted in RLS provided good evidence on the effect of ART containing HBV-suppressive agents on various measures that correlate with long-term clinical outcomes, such as HBV DNA level, HBeAg seroconversion, and liver fibrosis. Evidence from the reviewed studies consistently reported high rates of HBV DNA suppression in the setting of TDF + 3TC/FTC-containing ART regimens,27–31,33,40 increased rates of HBeAg seroconversion, and complete clearance of HBsAg among a small fraction of patients; of these, 2 reported improvements in liver fibrosis.27,30 In contrast, in settings where TDF was not used, HBV-related measures were mixed and 3TC-only ART did not seem to decrease progression of liver fibrosis.32 A subanalysis of a trial of structured treatment interruptions in resource-rich settings also noted that HIV/HBV co-infected patients have increased rates of CD4 decline when ART is stopped, suggesting that HIV/HBV co-infected patients may be at increased risk for poor outcomes when ART adherence or retention are poor or when second-line ART regimens do not contain TDF as the primary agent responsible for sustained HBV suppression without development of HBV resistance to therapy.29
One cohort study reported that 17% of ART-naive HIV-infected Nigerian patients known to be positive for HBsAg had significant fibrosis or cirrhosis (as measured by transient elastography) and were 5 times more likely to have significant liver fibrosis than those with HIV mono-infection.35 This indicates relatively high rates of liver disease in HIV/HBV co-infected patients not yet enrolled in ART. CD4 recovery and HIV VL suppression in 3 mortality studies conducted in RLS did not differ between HIV/HBV co-infected and HIV mono-infected patients.23,24,26 Although articles that exclusively focused on hepatotoxicity based on liver enzyme evaluations were not included in this review, some studies included in this review found liver enzymes were higher among HIV/HBV co-infected persons but did not seem to affect overall outcomes.
The review identified no costing or cost-effectiveness studies meeting inclusion criteria.
DISCUSSION
Given the lack of direct data or evidence about the impact of HBsAg screening on PLHIV outcomes, we reviewed studies in which the aggregate findings could be used to determine if routine HBV or HBsAg screening should be considered within a country context, and particularly RLS such as SSA. Although data gaps exist, examining (1) HIV/HBV co-infection rates in SSA, (2) the performance of HBsAg RSA, (3) the morbidity and mortality in HIV/HBV co-infection, and (4) the potential role of HBV-suppressive ART regimens in mitigating the negative impact of HBV co-infection, allowed the reviewers to put forward the following programmatic considerations for national HIV programs contemplating implementation of HBV screening programs.
Programmatic Considerations
Our review showed that there is a general lack of good-quality prevalence data on HIV/HBV co-infection for most countries in SSA. Accurate prevalence estimates are fundamentally important to inform country decisions about whether to prioritize routine HBV screening in general and in special populations, including PLHIV and pregnant women. Despite the gaps in the available data, it is clear that a number of areas within SSA have a moderate-to-high HBV frequency of infection among PLHIV and among the general populations. Given the large HBV burdens in these countries, obtaining standardized and representative prevalence estimates should be a high public health priority. Toward this end, countries could consider the inclusion of HBV testing in regular population-based surveys (eg, AIDS Indicator Surveys or Demographic Health Surveys), especially in countries with suspected moderate-to-high population rates of chronic HBV or where population-based data are not available. Consideration could also be given to collecting and analyzing other quality data to verify the accuracy of estimates across regions and populations in the country. Good-quality data on HIV/HBV co-infection rates will help the country in various ways, including (1) decisions on whether HBV screening should be a priority intervention; (2) choice and speed of country-wide transition to first-line HIV regimens containing combination TDF + 3TC/FTC; and (3) plans for selection of second-line HIV treatment that may be needed to maintain HBV suppression, still including TDF + 3TC/FTC for HBV co-infected PLHIV.
Our review also indicated that the HBsAg RSAs examined show good sensitivity and specificity rates with high levels of HBV viremia, indicating that HBsAg RSAs may be a viable option for screening PLHIV at the clinic level when EIA is not feasible. However, of concern was that performance decreased at lower VL levels of HBV, likely representative of late resolving phase or chronic carriers, and varied according to the test used. National programs considering implementation of rapid testing should be urged to validate the performance of each manufacturer-specific HBsAg RSA under consideration for use in their setting to select the most sensitive and specific option and understand the limitations of each assay for detecting and identifying all HBV-infected individuals. Appropriate training of staff performing assays and maintenance of proficiency will be key to achieving the level of sensitivity and specificity reported by the manufacturer. All HBsAg tests should be validated against standards. Internationally recognized assays and panels and operator training and performance evaluation should be instituted to maintain accurate testing (Table 2).
TABLE 2.
Summary of Evidence
| Overall Quality of Evidence |
Impact of the Intervention |
Evidence From Economic Evaluation |
||||
|---|---|---|---|---|---|---|
| Studies (No. Studies Addressing Each Outcome and References) | Overall Quality of the Body of Evidence (For All Studies Addressing Each Outcome) (1 = Good; 2 = Fair; 3 = Poor) (Score and Narrative) | Expected Impact of the Intervention*† (Based on the Main Findings From Good-Quality Studies Addressing the Intervention) (1 = High; 2 = Moderate; 3 = Low; 4 = Uncertain) | Studies (No. Studies With Cost-Effectiveness Data Addressing Each Outcome) | Quality of Evidence From Economic Evaluation (Summary Assessment) | Comments | |
| Mortality | ||||||
| Studies conducted in resource-rich settings (High-income countries) | 7 (5–9,15,22) | 2 | 2 | 0 | None | This literature provides a snapshot of the evidence that HBV co-infection has deleterious effects/impact on HIV+ individuals. None of the 7 studies, however, are representative of the general population in SSA. Several also do not provide substantial information about the effect of TDF-containing ART on overall mortality |
| Studies conducted in RLS (Low- and middle-income) | 4 (23–26) | 2 | 2 | 0 | None | Relatively short follow-up and partial treatment of HBV may account for some studies showing no differences in mortality. Genotypes also likely different than those in Western Studies |
| Mortality | ||||||
| Studies conducted in resource-rich settings (High-income) | 5 (27–31) | 1 | 1 | 0 | None | These studies show the importance of understanding HBV status among PLHIV because co-managing HBV improves patient outcomes, reducing morbidity because of liver-related disease |
| Studies conducted in RLS (Low- and middle-income) | 9 (32–40) | 2–3 | 1–2 | 0 | None | One study reported delayed CD4 recovery (others did not). One study reported high prevalence (17%) of liver fibrosis in ART-naïve co-infected persons. Minimal overall relationship to traditional markers of hepatoxicity |
| Performance of RSA | 6 (41–46) | 2–3 | 1 | 0 | None | These studies provide information on the reliability (specificity and sensitivity) of many of the RSA diagnostics on the market for HBsAb. This review points out the limits of current knowledge of the application of such assays as POC diagnostics within-country settings and resource-constrained environment |
| HIV/HBV co-infection frequency in SSA | 55 | 2–3 | N/A | 0 | N/A | Would benefit from better quality, population-based or representative data to drive decision making. Many studies not likely representative of the population recommend standardized surveillance, possibly as a part of AIDS indicator surveys, DHS surveys etc |
The assessment of the expected impact of the intervention was based on published evidence. Additional considerations that would inform implementation decisions would have to take into account the cost-effectiveness information and country-specific contextual considerations.
The expected impact of the intervention was rated as: High = Intervention expected to have a high impact on the outcome, Moderate = Likely to have a moderate impact on the outcome, Low = Intervention expected to have a low impact on the outcome, and Uncertain = Available information is not adequate to assess estimated impact on the outcome.
Evidence on the negative impact of untreated HBV co-infection on HIV morbidity and mortality and the successful suppression of HBV infection in the setting of HIV infection and the ability of HBsAg RSA to accurately identify HBV co-infected PLHIV were used as proxies to assess the anticipated expected benefit of knowing HBV co-infection status and appropriately managing the HBV co-infection.
Despite limited quality information on the frequency of HIV/HBV co-infection, the literature reviewed from both resource-rich settings and RLS, particularly in SSA, indicated a clear impact of HIV on the progression of HBV-related morbidity and mortality, as well as a potential role for ART composed of TDF + 3TC/FTC in mitigating the progression of liver disease among HIV/HBV co-infected persons. The findings suggest that it is reasonable for HIV programs in RLS to expedite transitioning all PLHIV to first-line ART regimens composed of TDF + 3TC/FTC + efavirenz, particularly in SSA where background population HBV infection prevalence is estimated to be moderate-to-high. This strategy would ensure that HIV/HBV co-infected persons receive concurrent HBV- and HIV-suppressive therapy. If this approach is not feasible, HBV screening, at a minimum screening for HBsAg, and prioritization of TDF-containing regimens for HIV/HBV co-infected persons should be strongly considered. In all cases, the review results suggest that HBV screening should be provided for patients without documented HBV infection status before transitioning them to second-line HIV-suppressive ART, particularly from first-line regimens containing TDF. It also suggests that all HBV co-infected persons should be maintained on TDF + 3TC/FTC or, at a minimum, on TDF to prevent resurgence of HBV viral replication, liver flare, and CD4 decline, in keeping with current WHO recommendations. Although the incremental health benefit and cost-effectiveness of treating HIV/HBV co-infected patients with CD4 > 500 has not been shown, it may be reasonable to infer that there might be some degree of reduction in risk of cirrhosis and/or HCC among patients with higher CD4 who start treatment on a TDF-based regimen earlier, particularly among those with high HBV VL or who test positive for HBeAg (those at the highest risk for HBV liver disease).
Limitations
Although HIV/HBV co-infection rates presented in this review may provide reasonable estimates and variations across SSA, it is important to consider that most of these studies were not conducted using standardized population-based surveillance methodologies, testing methodologies were not always validated, and data were available from only 16 countries in SSA. Therefore, estimates may not be fully representative of population prevalence. Despite the overall good performance of HBsAg RSA found in this review, there are clear limitations. There were only a small number of studies conducted and even fewer studies executed in the field using RSA as POC diagnostics because they might be deployed in primary RLS clinics. A further limitation includes the inability of HBsAg RSAs to identify low HBV VL. Before deployment of this technology in any country, a decision on the appropriate RSA will require validation of the POC assays against a standard and training and proficiency testing of HBsAg RSA operators. Regarding estimating treatment benefits, most of the available studies had relatively small numbers and short follow-up periods, and would therefore not have been expected to have sufficient power to measure potential long-term benefits of HBV treatment in preventing HCC or cirrhosis among PLHIV, the 2 most serious sequelae of HBV infection.
Gaps and Future Directions
Additional research is needed to more clearly define rates of HBV infection among different populations in SSA, including PLHIV, and the full extent of HBV-associated morbidity and mortality among PLHIV in the new ART era. Resources allowing countries without quality data are encouraged to gather epidemiologic data on HBV and HIV/HBV co-infection rates, especially within the context of large HIV-focused and other population surveillance studies. Such research and enhanced surveillance could inform future decision making about resource allocation for HBV screening and treatment not only through an HIV-specific lens, but also in consideration of the potential need for HBV treatment more broadly and in comparison with the other pressing health needs. HBV testing might be reasonably integrated into large cohort studies and demographic surveillance systems that have been established to study HIV in SSA and other global regions, minimizing the cost of de novo or focused hepatitis B studies and surveillance. The outputs could provide much-needed population-based HBV infection estimates, identify locales and subpopulations at highest risk, and provide useful data on the natural history of HBV in Africa. Existing samples or planned specimen collections could be tested for HBsAg, HBeAg, and HBV DNA at a relatively lower cost. Populations from SSA should also be prioritized in future studies to characterize the risk/benefit ratio of ART for HBV-related HCC because previous research has not addressed the issue well. Because momentum builds to do more to address the major global health problem of viral hepatitis, particularly impacting SSA, integration of HBV testing and treatment within existing HIV treatment platforms can benefit PLHIV.
Countries may also be able to identify more synergistic and cost-effective ways to better characterize HBV infection burden in their generalized populations and strengthen HBV testing, care and treatment, as well as prevention by building on the infrastructure and capacities established for HIV programs. Implementation research and demonstration projects related to HBV testing and treatment should be contemplated to begin to provide data on feasibility, acceptability, cost, and logistical requirements of HBV treatment in different contexts and populations in SSA.
Better data on HBV treatment costs and logistics for HBV mono-infection will be critical to inform decision making about whether to prioritize treatment for different populations, not only within but also beyond demonstration projects. The currently recommended first-line antiviral agents for HBV treatment—either oral TDF or entecavir (ETV)—are both highly effective as monotherapy against all HBV genotypes, with very low rates of serious adverse events, less laboratory monitoring requirements than ART, and minimal resistance occurring during long-term therapy of 5 years or more among treatment-naive patients (1.2% resistant with ETV97 and 0% with TDF98). Furthermore, the recommended doses of TDF 300 mg or ETV 0.5 mg daily formulations for HBV treatment are available as low-cost generic tablets for HIV treatment in SSA. However, simplified protocols for initiating and following patients with HBV mono-infection in RLS are still under development.
CONCLUSIONS
This summary of evidence may be useful to national HIV/AIDS programs considering implementing HBV screening programs. Continued research is needed to quantify the effectiveness of screening and identify best practices for implementation of a screening program.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gail Bang and Emily Weyant of the Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, GA, for their work in generating the initial list of abstracts used for this literature review.
Supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Department of State, Office of the US Global AIDS Coordinator and Health Diplomacy, the US Agency for International Development, the US Centers for Disease Control and Prevention, as well as supported through a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the US Department of Defense.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The authors have no conflicts of interest to disclose.
The views in this article are those of the authors and should not be construed to represent the positions of the US Department of State’s Office of the US Global AIDS Coordinator and Health Diplomacy, the US Centers for Disease Control and Prevention, the US Agency for International Development, the US Department of Defense or the US Federal Government.
REFERENCES
- 1.Garfein RS, Vlahov D, Galai N, et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996;86:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis 2007;7:402–409. [DOI] [PubMed] [Google Scholar]
- 3.Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606. [DOI] [PubMed] [Google Scholar]
- 4.van Houdt R, Bruisten SM, Speksnijder AG, et al. Unexpectedly high proportion of drug users and men having sex with men who develop chronic hepatitis B infection. J Hepatol 2012;57:529–533. [DOI] [PubMed] [Google Scholar]
- 5.Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360:1921–1926. [DOI] [PubMed] [Google Scholar]
- 6.Chun HM, Roediger MP, Hullsiek KH, et al. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J Infect Dis 2012;205:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teira R, Group VS. Hepatitis-B virus infection predicts mortality of HIV and hepatitis C virus coinfected patients. AIDS 2013;27:845–848. [DOI] [PubMed] [Google Scholar]
- 8.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol 2005;42:799–805. [DOI] [PubMed] [Google Scholar]
- 9.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–1641. [DOI] [PubMed] [Google Scholar]
- 10.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. J Hepatol 2009;49(5 suppl):S138–S145. [DOI] [PubMed] [Google Scholar]
- 11.Scharschmidt BF, Held MJ, Hollander HH, et al. Hepatitis B in patients with HIV infection: relationship to AIDS and patient survival. Ann Intern Med 1992;117:837–838. [DOI] [PubMed] [Google Scholar]
- 12.Sinicco A, Raiteri R, Sciandra M, et al. Coinfection and superinfection of hepatitis B virus in patients infected with human immunodeficiency virus: no evidence of faster progression to AIDS. Scand J Infect Dis 1997;29:111–115. [DOI] [PubMed] [Google Scholar]
- 13.Solomon RE, VanRaden M, Kaslow RA, et al. Association of hepatitis B surface antigen and core antibody with acquisition and manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Am J Public Health 1990;80:1475–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ockenga J, Tillmann HL, Trautwein C, et al. Hepatitis B and C in HIV-infected patients. Prevalence and prognostic value. J Hepatol 1997;27: 18–24. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann CJ, Seaberg EC, Young S, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS 2009;23:1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wandeler G, Gsponer T, Bihl F, et al. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis 2013;208:1454–1458. [DOI] [PubMed] [Google Scholar]
- 17.Bessesen M, Ives D, Condreay L, et al. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin Infect Dis 1999;28:1032–1035. [DOI] [PubMed] [Google Scholar]
- 18.Pillay D, Cane PA, Ratcliffe D, et al. Evolution of lamivudine-resistant hepatitis B virus and HIV-1 in co-infected individuals: an analysis of the CAESAR study. CAESAR co-ordinating committee. AIDS 2000;14: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 19.Bellini C, Keiser O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–18. [DOI] [PubMed] [Google Scholar]
- 20.Easterbrook P, Sands A, Harmanci H. Challenges and priorities in the management of HIV/HBV and HIV/HCV coinfection in resource-limited settings. Semin Liver Dis 2012;32:147–157. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JE, Hamm TE, Forhan S, et al. The impact of HIV care and support interventions on key outcomes in low and middle-income countries: A literature review–introduction. J Acquir Immune Defic Syndr 2015;68(suppl 3):S253–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmon-Ceron D, Rosenthal E, Lewden C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalite 2005 study. J Hepatol 2009;50:736–745. [DOI] [PubMed] [Google Scholar]
- 23.Day SL, Odem-Davis K, Mandaliya KN, et al. Prevalence, clinical and virologic outcomes of hepatitis B virus co-infection in HIV-1 positive Kenyan women on antiretroviral therapy. PLoS One 2013;8:e59346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann CJ, Charalambous S, Martin DJ, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis 2008;47:1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews GV, Manzini P, Hu Z, et al. Impact of lamivudine on HIV and hepatitis B virus-related outcomes in HIV/hepatitis B virus individuals in a randomized clinical trial of antiretroviral therapy in southern Africa. AIDS 2011;25:1727–1735. [DOI] [PubMed] [Google Scholar]
- 26.Moore E, Beadsworth MB, Chaponda M, et al. Favourable one-year ART outcomes in adult Malawians with hepatitis B and C co-infection. J Infect 2010;61:155–163. [DOI] [PubMed] [Google Scholar]
- 27.Boyd A, Lasnier E, Molina JM, et al. Liver fibrosis changes in HIV-HBV-coinfected patients: clinical, biochemical and histological effect of long-term tenofovir disoproxil fumarate use. Antivir Ther 2010;15:963–974. [DOI] [PubMed] [Google Scholar]
- 28.de Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immune-deficiency virus and hepatitis B virus. Gastroenterology 2010;139: 1934–1941. [DOI] [PubMed] [Google Scholar]
- 29.Dore GJ, Soriano V, Rockstroh J, et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010;24:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Carbonero L, Teixeira T, Poveda E, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS 2011;25:73–79. [DOI] [PubMed] [Google Scholar]
- 31.Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, et al. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J Infec Dis 2012;206:974–980. [DOI] [PubMed] [Google Scholar]
- 32.Appiah L, Foster G, Cozzi LA, et al. The burden of liver disease in HIV/HBV co-infected patients accessing antireroviral therapy in Ghana: The HEPIK study. HIV Med 2012;13:43. [Google Scholar]
- 33.Avihingsanon A, Matthews GV, Lewin SR, et al. Assessment of HBV flare in a randomized clinical trial in HIV/HBV coinfected subjects initiating HBV-active antiretroviral therapy in Thailand. AIDS Res Ther 2012;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayuk J, Mphahlele J, Bessong P. Hepatitis B virus in HIV-infected patients in northeastern South Africa: prevalence, exposure, protection and response to HAART. S Afr Med J 2013;103:330–333. [DOI] [PubMed] [Google Scholar]
- 35.Firnhaber C, Chen CY, Evans D, et al. Prevalence of hepatitis B virus (HBV) co-infection in HBV serologically-negative South African HIV patients and retrospective evaluation of the clinical course of mono-and co-infection. Int J Infect Dis 2012;16:e268–e272. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins C, Agbaji O, Ugoagwu P, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis 2013;57:e189–e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladep NG, Agaba PA, Agbaji O, et al. Rates and impact of hepatitis on human immunodeficiency virus infection in a large African cohort. World J Gastroenterol 2013;19:1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews GV, Ali RJ, Avihingsanon A, et al. Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals. PLoS One 2013;8:e61297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayaphi SH, Roussow TM, Masemola DP, et al. HBV/HIV co-infection: the dynamics of HBV in South African patients with AIDS. S Afr Med J 2012;102(3 pt 1):157–162. [DOI] [PubMed] [Google Scholar]
- 40.Mendes-Correa MC, Pinho JR, Gomes-Gouvea MS, et al. Predictors of HBeAg status and hepatitis B viraemia in HIV-infected patients with chronic hepatitis B in the HAART era in Brazil. BMC Infect Dis 2011; 11:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies J, van Oosterhout JJ, Nyirenda M, et al. Reliability of rapid testing for hepatitis B in a region of high HIV endemicity. Trans R Soc Trop Med Hyg 2010;104:162–164. [DOI] [PubMed] [Google Scholar]
- 42.Franzeck FC, Ngwale R, Msongole B, et al. Viral hepatitis and rapid diagnostic test based screening for HBsAg in HIV-infected patients in rural Tanzania. PLoS One 2013;8:e58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maity S, Nandi S, Biswas S, et al. Performance and diagnostic usefulness of commercially available enzyme linked immunosorbent assay and rapid kits for detection of HIV, HBV and HCV in India. Virol J 2012;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyirenda M, Beadsworth MB, Stephany P, et al. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. J Infect 2008;57:72–77. [DOI] [PubMed] [Google Scholar]
- 45.Scheiblauer H, El-Nageh M, Diaz S, et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang 2010;98(3 pt 2):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seremba E, Ocama P, Opio CK, et al. Validity of the rapid strip assay test for detecting HBsAg in patients admitted to hospital in Uganda. J Med Virol 2010;82:1334–1340. [DOI] [PubMed] [Google Scholar]
- 47.Ramos JM, Toro C, Reyes F, et al. Seroprevalence of HIV-1, HBV, HTLV-1 and Treponema pallidum among pregnant women in a rural hospital in Southern Ethiopia. J Clin Microbiol 2011;51:83–85. [DOI] [PubMed] [Google Scholar]
- 48.Manyazewal T, Sisay Z, Biadgilign S, et al. Hepatitis B and hepatitis C virus infections among antiretroviral-naive and -experienced HIV co-infected adults in Addis Abba, Ethiopia. Int J Infect Dis 2012;16:96. [DOI] [PubMed] [Google Scholar]
- 49.Shimelis T, Torben W, Medhin G, et al. Hepatitis B virus infection among people attending the voluntary counselling and testing centre and anti-retroviral therapy clinic of St Paul’s General Specialised Hospital, Addis Ababa, Ethiopia. Sex Transm Infect 2008;84:37–41. [DOI] [PubMed] [Google Scholar]
- 50.Harania RS, Karuru J, Nelson M, Stebbing J. HIV, hepatitis B and hepatitis C coinfection in Kenya. AIDS 2008;22:1221–1222. [DOI] [PubMed] [Google Scholar]
- 51.Pirillo MF, Bassani L, Germinario EA, et al. Seroprevalence of hepatitis B and C viruses among HIV-infected pregnant women in Uganda and Rwanda. J Med Virol 2007;79:1797–1801. [DOI] [PubMed] [Google Scholar]
- 52.Rusine J, Ondoa P, Asiimwe-Kateera B, et al. High seroprevalence of HBV and HCV infection in HIV-infected adults in Kigali, Rwanda. PLoS One 2013;8:e63303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mutwa PR, Boer KR, Rusine JB, et al. Hepatitis B virus prevalence and vaccine response in HIV-infected children and adolescents on combination antiretroviral therapy in Kigali, Rwanda. Pediatr Infect Dis J 2013; 32:246–251. [DOI] [PubMed] [Google Scholar]
- 54.Telatela SP, Matee MI, Munubhi EK. Seroprevalence of hepatitis B and C viral co-infections among children infected with human immunode-ficiency virus attending the paediatric HIV care and treatment center at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. BMC Public Health 2007;7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagu TJ, Bakari M, Matee M, Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health 2008;8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel P, Davis S, Tolle M, et al. Prevalence of hepatitis B and hepatitis C coinfections in an adult HIV centre population in Gaborone, Botswana. Am J Trop Med Hyg 2011;85:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabenau HF, Lennemann T, Kircher C, et al. Prevalence- and gender-specific immune response to opportunistic infections in HIV-infected patients in Lesotho. Sex Transm Dis 2010;37:454–459. [DOI] [PubMed] [Google Scholar]
- 58.Andersson MI, Maponga TG, Ijaz S, et al. Cross sectional analysis of the prevalence and character of hepatitis B virus infection in HIV-infected and HIV-uninfected pregnant woemn in the Western Cape, South Africa. J Hepatol 2012;56:S33. [Google Scholar]
- 59.Di Bisceglie AM, Maskew M, Schulze D, et al. HIV-HBV coinfection among South African patients receiving antiretroviral therapy. Antivir Ther 2010;15(3 pt B):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Firnhaber C, Reyneke A, Schulze D, et al. The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J 2008;98:541–544. [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann CJ, Dayal D, Cheyip M, et al. Prevalence and associations with hepatitis B and hepatitis C infection among HIV-infected adults in South Africa. Int J STD AIDS 2012;23:e10–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyles TH, Cohen K. The prevalence of hepatitis B infection in a rural South African HIV clinic. S Afr Med J 2011;101:470–471. [PubMed] [Google Scholar]
- 63.Lukhwareni A, Burnett RJ, Selabe SG, et al. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol 2009;81:406–412. [DOI] [PubMed] [Google Scholar]
- 64.Kapembwa KC, Goldman JD, Lakhi S, et al. HIV, Hepatitis B, and Hepatitis C in Zambia. J Glob Infect Dis 2011;3:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Paschale M, Manco MT, Belvisi L, et al. Prevalence of markers of hepatitis B virus infection or vaccination in HBsAg-negative subjects. Blood Transfus 2012;10:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sangare L, Sombie R, Combassere AW, et al. Antenatal transmission of hepatitis B virus in an area of HIV moderate prevalence, Burkina Faso [in French]. Bull Soc Pathol Exot 2009;102:226–229. [PubMed] [Google Scholar]
- 67.Ilboudo D, Karou D, Nadembega WM, et al. Prevalence of human herpes virus-8 and hepatitis B virus among HIV seropositive pregnant women enrolled in the Mother-to-Child HIV Transmission Prevention Program at Saint Camille Medical Centre in Burkina Faso. Pak J Biol Sci 2007; 10:2831–2837. [DOI] [PubMed] [Google Scholar]
- 68.Ilboudo D, Simpore J, Ouermi D, et al. Towards the complete eradication of mother-to-child HIV/HBV coinfection at Saint Camille Medical Centre in Burkina Faso, Africa. Braz J Infect Dis 2010;14:219–224. [DOI] [PubMed] [Google Scholar]
- 69.Bado G, Penot P, N’Diaye MD, et al. Hepatitis B seroprevalence in HIV-infected patients consulting in a public day care unit in Bobo Dioulasso, Burkina Faso. Med Mal Infect 2013;43:202–207. [DOI] [PubMed] [Google Scholar]
- 70.Ouermi D, Simpore J, Belem AM, et al. Co-infection of Toxoplasma gondii with HBV in HIV-infected and uninfected pregnant women in Burkina Faso. Pak J Biol Sci 2009;12:1188–1193. [DOI] [PubMed] [Google Scholar]
- 71.Kouanfack C, Aghokeng AF, Mondain AM, et al. Lamivudine-resistant HBV infection in HIV-positive patients receiving antiretroviral therapy in a public routine clinic in Cameroon. Antivir Ther 2012;17:321–326. [DOI] [PubMed] [Google Scholar]
- 72.Zoufaly A, Onyoh EF, Tih PM, et al. High prevalence of hepatitis B and syphilis co-infections among HIV patients initiating antiretroviral therapy in the north-west region of Cameroon. Intl J STD AIDS 2012;23: 435–438. [DOI] [PubMed] [Google Scholar]
- 73.Onyoh EF. High Prevalence of Hepatitis B and syphilis co-infection among newly diagnosed HIV patients in the northwest region of Cameroon. Trop Med Int Health 2012;17:12. [Google Scholar]
- 74.Jobarteh M, Malfroy M, Peterson I, et al. Seroprevalence of hepatitis B and C virus in HIV-1 and HIV-2 infected Gambians. Virol J 2010;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sagoe KW, Agyei AA, Ziga F, et al. Prevalence and impact of hepatitis B and C virus co-infections in antiretroviral treatment naive patients with HIV infection at a major treatment center in Ghana. J Med Virol 2012; 84:6–10. [DOI] [PubMed] [Google Scholar]
- 76.Geretti AM, Patel M, Sarfo FS, et al. Detection of highly prevalent hepatitis B virus coinfection among HIV-seropositive persons in Ghana. J Clin Microbiol 2010;48:3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obi RK, Nwanebu FC, Ohalete CN, et al. A prospective study of three blood-borne viral pathogens among pregnant women attending ante-natal care in Owerri, Nigeria. J Public Health Epidemiol 2012;4:226–229. [Google Scholar]
- 78.Salami T, Babatope I, Adewuyi G, et al. Hepatitis B and HIV co-infection-experience in a rural/suburban health center in Nigeria. J Microbiol Biotechnol Res 2012;2:841–844. [Google Scholar]
- 79.Okocha EC, Oguejiofor OC, Odenigbo CU, et al. Prevalence of hepatitis B surface antigen seropositivity among HIV-infected and non-infected individuals in Nnewi, Nigeria. Niger Med J 2012;53:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adekunle AE, Oladimeji AA, Temi AP, et al. Baseline CD4+ T lymphocyte cell counts, hepatitis B and C viruses seropositivity in adults with Human Immunodeficiency Virus infection at a tertiary hospital in Nigeria. Pan Afr Med J 2011;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadoh AE, Sadoh WE. Some laboratory features of HIV infected Nigerian children co-infected with heaptitis B and C. Ann Biomed Sci 2012;11:29–39. [Google Scholar]
- 82.Anigilaje EA, Olutola A. Prevalence and Clinical and Immunoviralogical Profile of Human Immunodeficiency Virus-Hepatitis B Coinfection among Children in an Antiretroviral Therapy Programme in Benue State, Nigeria. ISRN Pediatr 2013;2013:932697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tremeau-Bravard A, Ogbukagu IC, Ticao CJ, et al. Seroprevalence of hepatitis B and C infection among the HIV-positive population in Abuja, Nigeria. Afr Health Sci 2012;12:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adesina O, Oladokun A, Akinyemi O, et al. Human immuno-deficiency virus and hepatitis B virus coinfection in pregnancy at the University College Hospital, Ibadan. Afr J Med Med Sci 2010;39:305–310. [PubMed] [Google Scholar]
- 85.Lesi OA, Kehinde MO, Oguh DN, et al. Hepatitis B and C virus infection in Nigerian patients with HIV/AIDS. Niger Postgrad Med J 2007;14: 129–133. [PubMed] [Google Scholar]
- 86.Adewole OO, Anteyi E, Ajuwon Z, et al. Hepatitis B and C virus co-infection in Nigerian patients with HIV infection. J Infect Dev Ctries 2009;3:369–375. [DOI] [PubMed] [Google Scholar]
- 87.Otegbayo JA, Taiwo BO, Akingbola TS, et al. Prevalence of hepatitis B and C seropositivity in a Nigerian cohort of HIV-infected patients. Ann Hepatol 2008;7:152–156. [PubMed] [Google Scholar]
- 88.Olokoba A, Olokoba L, Salawu F, et al. Hepatitis B virus and human immunodeficiency virus co-infection in North-Eastern Nigeria. Int J Trop Med 2008;34:73–75. [Google Scholar]
- 89.Balogun TM, Emmanuel S, Ojerinde EF. HIV, Hepatitis B and C viruses’ coinfection among patients in a Nigerian tertiary hospital. Pan Afr Med J 2012;12:100. [PMC free article] [PubMed] [Google Scholar]
- 90.Diop-Ndiaye H, Toure-Kane C, Etard JF, et al. Hepatitis B, C seroprevalence and delta viruses in HIV-1 Senegalese patients at HAART initiation (retrospective study). J Med Virol 2008;80:1332–1336. [DOI] [PubMed] [Google Scholar]
- 91.Garrido C, Trevino A, Bautista J, et al. High rate of chronic hepatitis B, overt and occult, in a virological survey of hepatitis viruses and human retroviruses in Ghana. Antivir Ther 2011;15:1029–1034. [Google Scholar]
- 92.N’Dri-Yoman T, Anglaret X, Messou E, et al. Occult HBV infection in untreated HIV-infected adults in Cote d’Ivoire. Antivi Ther 2010;15: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 93.Araujo NM, Branco-Vieira M, Silva AC, et al. Occult hepatitis B virus infection in HIV-infected patients: Evaluation of biochemical, virological and molecular parameters. Hepatol Res 2008;38:1194–1203. [DOI] [PubMed] [Google Scholar]
- 94.Barth RE, Huijgen Q, Tempelman HA, et al. Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol 2011;83:929–934. [DOI] [PubMed] [Google Scholar]
- 95.Mphahlele MJ, Lukhwareni A, Burnett RJ, et al. High risk of occult hepatitis B virus infection in HIV-positive patients from South Africa. J Clin Microbiol 2006;35:14–20. [DOI] [PubMed] [Google Scholar]
- 96.Bell TG, Makondo E, Martinson NA, et al. Hepatitis B virus infection in human immunodeficiency virus infected southern African adults: occult or overt–that is the question. PLoS One 2012;7:e45750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009;49:1503–1514 [DOI] [PubMed] [Google Scholar]
- 98.Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology 2014;59:432–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


