Abstract
In response to the social inequities that exist in health care, the NIH-funded Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) recently formed a diversity committee to examine systemic racism and implicit bias in the care and research of eosinophilic gastrointestinal diseases (EGIDs). Herein, we describe our process, highlighting milestones and issues addressed since the committee’s inception, which we hope will inspire other researchers to enhance diversity, equity, inclusion, and accessibility (DEIA) in their fields. Our journey began by establishing mission and vision statements to define the purpose of the committee. Regular discussion of diversity-related topics was incorporated into existing meetings and web-based materials were shared. This was followed by educational initiatives, including establishing a library of relevant publications and a speaker series to address DEIA topics. We then established a research agenda focused on the following actionable items: (1) to define what is known about the demographics of EGIDs by systematic review of population-based studies; (2) to develop a practical tool for reporting participant demographics to reduce bias in EGID literature; (3) to examine health disparities in the care of individuals with eosinophilic esophagitis who present to the emergency department with an esophageal food impaction; (4) to examine how access to a gastroenterologist affects the conclusions of published research examining the prevalence of pediatric eosinophilic esophagitis; and (5) to develop a model for examining the dimensions of diversity, and provide a framework for CEGIR’s ongoing projects and data capture. In addition to promoting consciousness of DEIA, this initiative has fostered inclusivity among CEGIR members and will continue to inspire positive changes in EGID care and research.
Keywords: eosinophilic esophagitis, eosinophilic gastrointestinal diseases, equity, diversity
Plain language summary
Diversity in Eosinophilic Gastrointestinal Disease Research
To address systemic bias in patient care and research in eosinophilic gastrointestinal diseases, the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) recently formed a diversity committee. The CEGIR diversity committee has defined its purpose through mission and vision statements and developed structured educational and research initiatives to enhance diversity, equity, inclusivity, and accessibility (DEIA) in all CEGIR activities. Here, we share the process of formation of our diversity committee, highlighting milestones achieved and summarizing future directions. We hope that this report will serve as a guide and an inspiration for other researchers to enhance DEIA in their fields.
Introduction
In 2014, the Consortium for Eosinophilic Gastrointestinal Disease Researchers (CEGIR) initiated its studies supported by funding from an NIH U54 grant [Rare Diseases Clinical Research Network (RDCRN), National Center for Advancing Translational Science (NCATS), National Institute of Allergy and Infectious Diseases (NIAID), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)]. Now in its second funding cycle, CEGIR is composed of 18 US clinical sites and 130 members. CEGIR is dedicated to improving the lives of individuals with eosinophilic gastrointestinal diseases (EGIDs) through innovative research, clinical expertise, and education via collaborations between scientists, health care providers, patients, and patient advocacy groups (PAGs). CEGIR focuses its research on identifying the natural history of rare EGIDs with an emphasis on eosinophilic esophagitis (EoE), eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis, and on establishing clinical outcome metrics for therapeutic trials. Importantly, this funding mechanism requires involvement of PAGs in all research projects. CEGIR enjoys the participation of five PAGs: the American Partnership for Eosinophilic Disorders, the Campaign Urging Research for Eosinophilic Disease, the Eosinophilic Family Coalition, AuSEE, Inc. and EOS Network.
EGIDs are a relatively newly described group of disorders, and little is known about their demographic characteristics. EoE is the most common EGID, with a prevalence of 34.4 cases/100,000 inhabitants. 1 However, most data on EGIDs has been collected in the United States and Europe, limiting the generalizability of these assessments. EoE has conventionally been regarded as a disease that predominantly affects White males, and much less is known about the demographics of non-EoE EGIDs. CEGIR investigators have challenged this assumption and hypothesize the actual disease burden of EoE is more heterogeneous between Black and White patients. Considering that the median delay in diagnosis of EoE is 6 years, early recognition and treatment is crucial to prevent progressive features such as esophageal fibrosis and strictures. 2 For Black and other non-White populations, this delay may be even greater due to perceived demographic differences suggested by prior epidemiologic studies. The efforts of the diversity committee to interrogate disparities relating to DEIA are essential for CEGIR’s efforts to continuously improve care for patients with EGIDs.
Inspired by past and recent world events highlighting racial disparities in the United States, including its healthcare system, CEGIR members established the CEGIR diversity committee in August 2020. A major reason to create a diversity committee was to promote awareness of issues related to DEIA in CEGIR-associated research. The committee’s highly interactive group, composed of investigators, trainees, research coordinators, research administrators, and patient advocates, developed its infrastructure and a resultant set of deliverables through transparent and honest conversations about relevant and meaningful issues related to DEIA in research. The diversity committee meets twice per month and is composed of 34 members from the CEGIR membership. Here, we recount milestones and issues addressed since the committee’s inception.
CEGIR diversity committee infrastructure milestones
Development of mission and vision statements created synergy of purpose
To lay the foundation of the diversity committee, several initial discussions were held during our virtual meetings that led to drafting, reviewing, and establishing the following mission and vision statements:
Mission statement
We will develop an understanding of cultural, ethnic, and racial diversity and structural racism, particularly as it pertains to underrepresented populations of patients with EGIDs; strive to examine systemic and implicit biases; identify associated barriers to our research; and integrate the knowledge into our research studies and educational initiatives, so that we can better serve all patients with EGIDs.
Vision statement
CEGIR actively promotes a culture of diversity and inclusivity in its membership, in its innovative research studies, and its educational initiatives.
These two statements form the guiding principles of the diversity committee as well as CEGIR’s general membership and are read by different CEGIR members at the beginning of each of the consortium-wide, bi-weekly meetings and at our annual meeting. In essence, these statements serve as a constant reminder of our commitment to prioritizing the research landscape of DEIA within CEGIR.
Creation of a diversity library and speaker series formed a common knowledge base
Since CEGIR members possessed various knowledge levels on the impact of DEIA on research, an educational platform was developed in which an easily accessible reference library was created using Sciwheel 3 and a schedule of speakers was established. The reference library is populated by CEGIR diversity committee members and provides a rich up-to-date resource for our own knowledge and future publications, particularly with regard to establishing common and correct terminology for DEIA-related topics. Scheduled speakers present and engage in our virtual meetings on areas of DEIA research and discuss their experiences, data, and opinions while encouraging interactive discussions among CEGIR members. In addition, information on DEIA-themed workshops, webinars, conferences, and seminars are continuously shared with CEGIR members. These steps not only enhance CEGIR members’ knowledge of DEIA in research but also facilitate conversations on sensitive topics and learning new ways to approach research through a DEIA lens. Invited experts encourage CEGIR members to recognize biases, deficiencies, and identify potential solutions through various scenarios. Consistent messaging and development of a rigorous knowledge base promotes CEGIR’s efforts to carry out its mission and vision statements.
Establishing a research agenda instilled purpose
To translate learned DEIA concepts into action, a research agenda was established to accrue novel data that would impact issues related to DEIA in EGID research. This element proved critical to the success of the CEGIR diversity committee because it was developed by all members and provided a common shared framework to change the research landscape as currently established. Three areas were chosen based on current knowledge and needs. First, a systematic review of the literature was performed to understand the demographics of EGIDs. Second, efforts were dedicated to developing a reference tool that would allow CEGIR researchers to assess whether studies were conducted, and demographics were reported, in accordance with current guidance in the AMA manual of style. 4 Finally, an agenda was developed to understand clinical observations that may or may not be relevant to disparity in the care of EGIDs. Each of these projects will be described in further detail in the next sections.
Communication platforms promoted sense of community
Prior to the COVID-19 pandemic, CEGIR held an in-person annual meeting and would host ad hoc live meetings at various national academic society meetings. Since the diversity committee was created during the pandemic, all meetings have been virtual. Virtual meetings enabled a robust means of regular communication and included regular and ad hoc virtual meetings at times when most members could attend. Members live in all US time zones; therefore, meeting times are rotated between early morning and evening to accommodate all participating members. Second, during our bi-weekly general CEGIR meetings of the principal investigators, research coordinators, NIH representatives and PAGs, a 5–10 min educational presentation is scheduled that includes updates on work and presentations of pivotal DEIA publications in the field of gastroenterology, allergy, and nutrition. Third, committee members established the ‘Diversity Corner’ 5 on the CEGIR website that summarizes the educational presentations and is available to all CEGIR members and other interested constituents. In total, these elements allow the diversity committee to have a community as well as inform others of its important products.
Importantly, a point of emphasis of the diversity committee was to be proactively inclusive in its membership, involving CEGIR members early and late in their career development, members from all ethnic and racial backgrounds, research coordinators, and our PAGs. This leverages the extensive expertise of the CEGIR group in conducting research, which can be applied towards DEIA research and educational initiatives. This type of organization promoted ongoing and close collaborative interactions among investigators at all stages of their career development and PAGs. PAGs were instrumental in bringing new perspectives, ideas, and expertise to the discussions, as their experience stemming from interactions with patients is complementary to that of the investigators.
Taken together, the development of mission and vision statements, creation of an educational platform, establishment of a research agenda, and formation of viable communication systems provided a robust infrastructure to address DEIA within the CEGIR diversity committee and to establish and integrate DEIA into the fabric of all CEGIR interactions, research, and educational endeavors.
Research projects conducted and milestones achieved
Challenging assumptions about the demographics of EGIDs
Since EGIDs are a relatively newly described group of disorders, little is known about their demographic characteristics. EoE, the most common of the EGIDs, has traditionally been regarded as a disease that predominantly affects White males, and much less is known about the demographics of non-EoE EGIDs. While existing studies of EGIDs frequently describe the sample characteristics, most single or multi-center retrospective studies are not population-based, and are, therefore, subject to selection bias. In addition, many population-based studies do not report the denominator for the reference population from which the EGID population was selected. This selection bias likely extends to participation in clinical trials and may perpetuate stereotypes that contribute to diagnostic disparities in marginalized populations. Consequently, the diversity committee conducted a systematic review of population-based studies of EGIDs to identify the actual demographics of EGIDs. Knowledge acquired will inform the research community to address potential provider biases, enhance recruitment goals for diverse patient populations in clinical trials, ensure proper allocation of resources, reduce diagnostic delay, identify various phenotypes of EGIDs, and tailor therapies accordingly.
As a secondary objective, we analyzed the reporting methods employed by the articles included in the systematic review. We focused on comparisons, organization, and terminology to generate a checklist of best practices for reporting sex, gender, race, and ethnicity based on recent recommendations from the AMA manual of style. 4 We identified several common reporting practices which may contribute to implicit bias. CEGIR members can now use this abbreviated checklist (Table 1) as they prepare their own manuscripts and encourage others to adhere to best practices through the peer review process. This manuscript has been submitted for review. By using appropriate and culturally sensitive reporting practices we can enhance our understanding of how racial, ethnic, and socioeconomic factors influence EGID care to promote a culture of inclusivity in EGID research.
Table 1.
CEGIR checklist for reporting demographics.
| Category | Recommendation |
|---|---|
| Reporting | Report both male and female categories when indicating sex. |
| Report race and ethnicity categories. | |
| Delineate the specific type of multiracial and multiethnic groups to the extent possible. | |
| Methods | Do not use the nonspecific group label ‘other’ for a convenience grouping or label unless it was a prespecified formal category in a database or research instrument. In such cases, define and report ‘other’ groups. |
| Avoid study design and statistical comparisons of White versus ‘non-White’ groups. | |
| Formatting | List categories for race and ethnicity in alphabetical order in text and tables. |
| Avoid merging race and ethnicity with a virgule as ‘race/ethnicity’ as a virgule often signifies ‘and/or’. | |
| The names of races, ethnicities, and tribes should be capitalized. | |
| Do not hyphenate combinations of proper adjectives derived from geographic entities when used as racial or ethnic descriptors (e.g. Asian American, African American). | |
| Avoid abbreviations of categories for race and ethnicity unless necessary because of space constraints. | |
| Terminology | Use the terms male and female when describing the sex of human participants or other sex-related biological or physiological factors. |
| Do not confuse the terms sex and gender. Gender comprises the social, environmental, cultural, and behavioral factors and choices that influence a person’s self-identity and health. | |
| Do not use the general term ‘minorities’ when describing groups or populations because it is vague and implies a hierarchy among groups. | |
| Use a modifier when using the word ‘minority’ (e.g. racial and ethnic minority groups or individuals) and do not use the term as a stand-alone noun. | |
| Avoid the term mixed race unless specifically used in data collection. | |
| Avoid collective reference to racial and ethnic minority groups as ‘non-White’. | |
| Do not use racial and ethnic terms in the noun form (e.g. avoid Asians, Blacks, etc.). The adjectival form is preferred (Asian women, Black patients, etc.) | |
| Do not use the term Caucasian unless referring specifically to people from the Caucasus region in Eurasia. | |
| Do not use the terms African American or Black interchangeably unless both terms were formally used in the study. | |
| American Indian or Alaska Native are preferred to Native American. The term Indigenous is also acceptable. | |
| Latinx and Latine are gender-inclusive or nonbinary terms for people of Latin American culture or ethnic identity in the United States. |
Source: Adapted from the AMA manual of style.
CEGIR, consortium of eosinophilic gastrointestinal disease researchers.
Investigating potential racial and ethnic disparities in the diagnosis of EoE
During the bi-monthly discussions related to the current evidence that identified EoE as a disease that primarily affects White males,6–10 one of the CEGIR investigators presented single-center data suggesting that Black patients presenting to the emergency department with an esophageal food impaction were less likely to receive a diagnostic upper endoscopy with esophageal biopsies as compared to White patients. EoE is known to be one of the leading causes of esophageal food impactions in children and adults.11,12 Since EoE is the most common cause of esophageal food impactions, this finding is critical to help identify barriers to diagnosis and treating patients expediently.
These preliminary findings led the diversity committee to develop a hypothesis-driven study that addressed this concern through a retrospective analysis of eight geographically dispersed CEGIR sites. Because emergency food impaction represents an unequivocal symptom of esophageal dysfunction, the study is designed to identify health disparities among patients presenting to the emergency department with food impaction. We hypothesized that some individuals may be less likely to have endoscopic biopsies and referral to gastroenterology after an esophageal food impaction. A data query will be performed of each site’s electronic medical records using a common clinical research form, and data analysis will be centralized. Results from the study will allow dissection of potential racial or ethnic disparities during the initial stage of an EoE diagnostic evaluation and in those whose disease is poorly controlled.
Examining access to care in EoE diagnosis
In a large, US-based, database study of children enrolled in Medicaid, McGowan et al. 13 determined that EoE was less prevalent among children living in rural areas. The odds of being diagnosed with EoE were lower in rural areas and in areas with more neighborhood poverty. The authors recommended exploration of unique environmental factors that may be protective for the development of EoE in these populations, as EoE is a food/environmental antigen-driven disease. They also suggested the alternate possibility of under-diagnosis of EoE in resource-poor settings. The diversity committee hypothesized this discrepancy was attributable to a disparity in access to care. 13 To receive a diagnosis of EoE, a child must be referred to and evaluated by a pediatric gastroenterologist and undergo a diagnostic upper endoscopy with esophageal biopsies under anesthesia. 14 This requires access to pediatric subspecialty care and a tertiary care center with anesthesia capabilities. The diversity committee examined the distribution of pediatric gastroenterologists in the United States and found that most were in urban communities; therefore, we speculated that the lower prevalence of rural pediatric EoE patients likely resulted from underdiagnosis due to decreased provider access.
The findings were further investigated in a subsequent collaboration with McGowan and colleagues in a study that re-analyzed their previously published data to examine the distance to a pediatric gastroenterology provider. 15 When adjusting for distance to provider, there was no longer an independent, inverse association between living in a rural area and EoE diagnosis. These findings suggest that diagnostic disparities for EoE among children residing in rural areas can be attributed to a lack of access to pediatric gastroenterology subspecialty care. Furthermore, while distance to provider attenuated the association between urban/rural status and EoE diagnosis, this was not observed for neighborhood-level poverty. Children living in areas with higher neighborhood-level poverty continued to be less likely to be diagnosed with EoE. Together, these findings provide important public health information as they suggest that improving access to subspecialty care in various parts of the US may support more equitable diagnostic evaluations for EoE.
Creation of a platform to assess diversity in all CEGIR research on EGIDs
To directly improve CEGIR’s research, the committee developed a tool to assess whether DEIA issues were considered during the development and execution of a research project. The committee took advantage of our PAGs’ expertise and resources related to understanding how diversity can be addressed in the workplace. A PAG member presented an approach to understand the different dimensions of diversity in the workplace termed the Diversity Wheel. This platform was developed in 1990 by Marilyn Loden and Judy Rosener 16 and consisted of a framework for thinking about the different dimensions of diversity within individuals and institutions. Depicted as concentric circles, this Diversity Wheel has been used in many ways to encourage thinking about values, beliefs, and dimensions of identity for people and organizations. The wheel represents the various dimensions of diversity, namely internal, external, and organizational dimensions. Internal dimensions are central to an individual’s experience, have sustained influence on one’s life, and are often the dimensions with which one closely identifies. External dimensions, while critically important in determining one’s identity, have a greater degree of control or self-determination to change. Organizational dimensions contribute to how one experiences their time within an organization, or for our purposes, in clinical research.
In October 2021, diversity committee members and other interested CEGIR members met in a virtual workshop to generate a CEGIR Diversity Wheel to help enhance DEIA in CEGIR investigator clinical trials. Workshop members represented the diversity that exists within CEGIR and were comprised of PAGs, clinical research coordinators, principal investigators, and trainees. Together they applied the CEGIR Diversity Wheel to develop a checklist of DEIA items to incorporate into EoE/EGID clinical trial design and implementation.
An additional task of the committee has been the improvement of data capture in our natural history study, including redesigning our case report forms to collect various social determinants of health including socioeconomics, insurance status, proximity to pollution via geocoding, and access to medical foods among other factors. Together, the CEGIR Diversity Wheel and recent improvements in our data capture have led to changes in CEGIR’s standard operating procedures for all clinical trials and research studies. These efforts will ensure more equity in CEGIR research and more broadly impact all CEGIR members as they address DEIA in their research activities.
The CEGIR diversity committee journey: challenges and future directions
We acknowledge that to rectify existing disparities in the clinical care of EGIDs we must address inclusivity in our research. This will increase the generalizability of EGID research findings and identify areas for improving care in all patients. CEGIR’s efforts to promote DEIA are summarized in Figure 1 and key metrics are noted in Table 2. The successful establishment of a viable virtual platform by the diversity committee has facilitated addressing several important issues and improved our understanding of DEIA in EGID research. This has included an assessment of the diversity within CEGIR membership (Table 2). In the future, we plan to continue current studies and pursue new directions stemming from recent results. We are hopeful that the identification of gaps in knowledge and care will lead to future funding opportunities, as this is a major obstacle to supporting the investigators and infrastructure for these endeavors. By implementing and disseminating the results of the above projects through publications, we have the potential to shape several aspects of EGID patient care, including timely diagnosis and management and enhancing diversity in clinical research participation. The primary goal of the diversity committee is to align CEGIR membership, research studies, and educational initiatives with the clear mission statement that is read aloud at every meeting. We aim to continue our endeavors through promotion of opportunities for underserved populations and underrepresented groups as diverse learners through a career enhancement core, development of patient and provider education initiatives, and dedication of CEGIR pilot funds for DEIA-related research studies.
Figure 1.
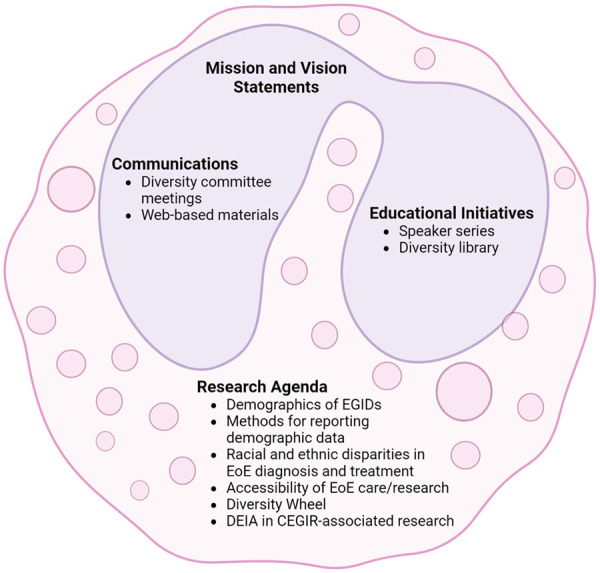
Summary of CEGIR’s efforts to enhance DEIA in EGIDs.
Source: Created with biorender.com.
CEGIR, consortium of eosinophilic gastrointestinal disease researchers; DEIA, diversity, equity, inclusion, and accessibility; EGIDs, eosinophilic gastrointestinal diseases.
Table 2.
CEGIR metrics.
| Category | Metric | Total |
|---|---|---|
| CEGIR member demographics a | Demographics | n (%) |
| Age | ||
| Under 18 | 0 (0) | |
| 18–24 | 6 (9.7) | |
| 25–34 | 8 (12.9) | |
| 35–44 | 20 (32.3) | |
| 45–54 | 15 (24.2) | |
| 55–64 | 9 (14.5) | |
| 65–74 | 4 (6.4) | |
| 75 or older | 0 (0) | |
| Sex | ||
| Female | 43 (69.4) | |
| Male | 19 (30.6) | |
| Intersex | 0 (0) | |
| Gender | ||
| Man | 18 (29.0) | |
| Woman | 43 (69.4) | |
| Non-binary | 0 (0) | |
| Transgender | 0 (0) | |
| Not reported | 1 (1.6) | |
| Race | ||
| American Indian or Alaska Native | 0 (0) | |
| Asian | 6 (9.7) | |
| Black or African American | 3 (4.8) | |
| Native Hawaiian or other Pacific Islander | 0 (0) | |
| White | 45 (72.6) | |
| Prefer not to say | 1 (1.6) | |
| Other b | 6 (9.7) | |
| Not reported | 1 (1.6) | |
| Ethnicity | ||
| Hispanic, Latino/Latina/Latinx or of Spanish origin | 2 (3.2) | |
| Not-Hispanic, Latino/Latina/Latinx or of Spanish Origin | 57 (92.0) | |
| Other c | 1 (1.6) | |
| Not reported | 2 (3.2) | |
| Place of birth | ||
| United States | 51 (82.3) | |
| Other | 8 (12.9) | |
| Not reported | 3 (4.8) | |
| Number of states represented | 18 | |
| Number of countries represented | 3 | |
| CEGIR diversity committee | Diversity committee meeting attendance (range) | 6–15 |
| Diversity committee members | 34 | |
| Educational initiatives | References added to CEGIR diversity library | 164 |
| CEGIR all-call presentations on diversity | 7 | |
| Research agenda | CEGIR pilot projects on diversity | 3 |
| CEGIR members participating in development of Diversity Wheel | 36 | |
| CEGIR scholar work on diversity | 1 | |
| Publications related to diversity topics | 2 | |
| Ongoing clinical studies reviewed using Diversity Wheel | 2 | |
Not all members of CEGIR represented.
Based on electronic survey of CEGIR members, n = 62.
Other = Indian
Other = Australian.
CEGIR, consortium of eosinophilic gastrointestinal disease researchers.
Acknowledgments
None.
Footnotes
ORCID iD: Benjamin L. Wright  https://orcid.org/0000-0002-7586-4722
https://orcid.org/0000-0002-7586-4722
Contributor Information
Mirna Chehade, Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1198, New York, NY 10029, USA.
Glenn Furuta, Digestive Health Institute, Section of Pediatric Gastroenterology, Hepatology and Nutrition, Children’s Hospital of Colorado, Auroral, CO, USA; Gastrointestinal Eosinophilic Disease Program, Mucosal Inflammation Program, University of Colorado School of Medicine, Aurora, CO, USA.
Amy Klion, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
J Pablo Abonia, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Seema Aceves, University of California San Diego, Rady Children’s Hospital of San Diego, San Diego, CA, USA.
Paroma Bose, Riley Hospital for Children, Indiana University, Indianapolis, IN, USA.
Margaret H. Collins, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA
Carla Davis, Baylor College of Medicine, Houston, TX, USA.
Evan S. Dellon, University of North Carolina School of Medicine, Chapel Hill, NC, USA
Grant Eickel, Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Gary Falk, University of Pennsylvania, Philadelphia, PA, USA.
Sandeep Gupta, Riley Hospital for Children, Indiana University, Indianapolis, IN, USA.
Girish Hiremath, Vanderbilt University Medical Center, Nashville, TN, USA.
Amari Howard, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Elizabeth T. Jensen, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Susamita Kesh, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Paneez Khoury, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Kendra Kocher, Digestive Health Institute, Section of Pediatric Gastroenterology, Hepatology and Nutrition, Children’s Hospital of Colorado, Auroral, CO, USA; Gastrointestinal Eosinophilic Disease Program, Mucosal Inflammation Program, University of Colorado School of Medicine, Aurora, CO, USA.
Ellyn Kodroff, Campaign Urging Research for Eosinophilic Disease, Lincolnshire, IL, USA.
Shay Kyle, Campaign Urging Research for Eosinophilic Disease, Lincolnshire, IL, USA.
NaDea Mak, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Dawn McCoy, American Partnership for Eosinophilic Disorders, Atlanta, GA, USA.
Pooja Mehta, Digestive Health Institute, Section of Pediatric Gastroenterology, Hepatology and Nutrition, Children’s Hospital of Colorado, Auroral, CO, USA; Gastrointestinal Eosinophilic Disease Program, Mucosal Inflammation Program, University of Colorado School of Medicine, Aurora, CO, USA.
Paul Menard-Katcher, University of Colorado School of Medicine, Anchutz Medical Campus, Aurora, CO, USA.
Vincent Mukkada, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Ally Paliana, Campaign Urging Research for Eosinophilic Disease, Lincolnshire, IL, USA.
Marc Rothenberg, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Kathleen Sable, American Partnership for Eosinophilic Disorders, Atlanta, GA, USA.
Cara Schmitt, American Partnership for Eosinophilic Disorders, Atlanta, GA, USA.
Melissa Scott, Eosinophilic Family Coalition, Cincinnati, OH, USA.
Jonathan Spergel, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Mary Jo Strobel, American Partnership for Eosinophilic Disorders, Atlanta, GA, USA.
Joshua B. Wechsler, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Guang-Yu Yang, Northwestern University, Evanston, IL, USA.
Amy Zicarelli, Eosinophilic Family Coalition, Cincinnati, OH, USA.
Amanda B. Muir, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Benjamin L. Wright, Division of Allergy, Asthma, and Clinical Immunology, Department of Medicine, Mayo Clinic Arizona, Scottsdale, AZ, USA; Section of Allergy and Immunology, Division of Pulmonology, Phoenix Children’s Hospital, Phoenix, AZ, USA.
Dominique D. Bailey, Columbia University Vagelos College of Physicians and Surgeons, New York-Presbyterian Morgan Stanley Children’s Hospital, New York, NY, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Mirna Chehade: Conceptualization; Data curation; Writing – original draft.
Glenn Furuta: Conceptualization; Data curation; Writing – review & editing.
Amy Klion: Conceptualization; Data curation; Writing – review & editing.
J Pablo Abonia: Conceptualization; Data curation.
Seema Aceves: Conceptualization; Data curation.
Paroma Bose: Conceptualization; Data curation.
Margaret H. Collins: Conceptualization; Data curation; Writing – review & editing.
Carla Davis: Conceptualization; Data curation; Writing – review & editing.
Evan S. Dellon: Conceptualization; Data curation; Writing – review & editing.
Grant Eickel: Conceptualization; Data curation; Writing – review & editing.
Gary Falk: Conceptualization; Data curation; Writing – review & editing.
Sandeep Gupta: Conceptualization; Data curation.
Girish Hiremath: Conceptualization; Data curation.
Amari Howard: Conceptualization; Data curation.
Elizabeth T. Jensen: Conceptualization; Data curation; Writing – review & editing.
Susamita Kesh: Conceptualization; Data curation.
Paneez Khoury: Conceptualization; Data curation; Writing – review & editing.
Kendra Kocher: Conceptualization; Data curation.
Ellyn Kodroff: Conceptualization; Data curation.
Shay Kyle: Conceptualization; Data curation.
NaDea Mak: Conceptualization; Data curation.
Dawn McCoy: Conceptualization; Data curation.
Pooja Mehta: Conceptualization; Data curation.
Paul Menard-Katcher: Conceptualization; Data curation; Writing – review & editing.
Vincent Mukkada: Conceptualization; Data curation.
Ally Paliana: Conceptualization; Data curation.
Marc Rothenberg: Conceptualization; Data curation.
Kathleen Sable: Conceptualization; Data curation.
Cara Schmitt: Conceptualization; Data curation.
Melissa Scott: Conceptualization; Data curation.
Jonathan Spergel: Conceptualization; Data curation.
Mary Jo Strobel: Conceptualization; Data curation.
Joshua B. Wechsler: Conceptualization; Data curation.
Guang-Yu Yang: Conceptualization; Data curation.
Amy Zicarelli: Conceptualization; Data curation.
Amanda B. Muir: Conceptualization; Data curation.
Benjamin L. Wright: Conceptualization; Data curation; Writing – review & editing.
Dominique D. Bailey: Conceptualization; Data curation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC). As a member of the RDCRN, CEGIR is also supported by its Data Management and Coordinating Center (DMCC) (U2CTR002818). Funding support for the DMCC is provided by the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Neurological Disorders and Stroke (NINDS). This study was funded in part by the Division of Intramural Research of NIAID.
MC received consultant fees from Regeneron, Adare/Ellodi, AstraZeneca, Sanofi, Bristol Myers Squibb, Recludix Pharma, Allakos, Shire/Takeda, Phathom, and received research funding from Regeneron, Allakos, Shire/Takeda, AstraZeneca, Adare/Ellodi, Danone. GFuruta serves as CMO for EnteroTrack and received research funding from Arena/Pfizer, Holoclara, and NIH. JPA received payment or honoraria for lectures from Takeda Global Research and Development, participated on a Data Safety Monitoring Board for OctaPharma USA, Inc., and received grants or contracts from Cures Within Reach and Celgene. SA received consultant fees for Regeneron, AstraZeneca, Bristol Meyers Squibb, research funding from Implicit Biosciences, is co-inventor of oral viscous budesonide UCSD patent Takeda license, and received educational speaker fees from Sanofi/Genzyme, Regeneron. MHC received research funding from AstraZeneca, Ception, GlaxoSithKline, Meritage Pharma Inc., Receptos/Celgene/BMS, Regeneron Pharmaceuticals and Shire, a Takeda company, and is a consultant for Allakos, Arena Pharmaceuticals, AstraZeneca, Calypso Biotech, EsoCap Biotech, GlaxoSmithKline, Receptos/Celgene/BMS, Regeneron Pharmaceuticals, Robarts Clinical Trials Inc./Alimentiv, Inc. and Shire, a Takeda company. ESD received research funding from Adare/Ellodi, Allakos, Arena, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, Shire/Takeda, consultant fees from Abbott, Abbvie, Adare/ Ellodi, Aimmune, Akesobio, Alfasigma, ALK, Allakos, Amgen, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, Eupraxia, Ferring, GSK, Gossamer Bio, Holoclara, Invea, Landos, LucidDx, Morphic, Nexstone Immunology, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE, Upstream Bio, and educational grants from Allakos, Holoclara, Invea. GFalk received consultant fees for Adare/Ellodi, Allakos, Bristol Myers Squibb/Celgene, Lucid, Nexstone, Phathom, Regeneron/Sanofi, Shire/Takeda, Upstream Bio, and research support from Adare/Ellodi, Allakos, Arena/Pfizer, Bristol Myers Squibb/Celgene, Lucid, Nexteos, Regeneron/Sanofi, Shire/Takeda. SG is a Consultant/DSMB member/author for Adare, BMS, QOL, Takeda, MedScape, PVI, ViaSkin, and UpToDate, and received research support from Allakos, Ellodi, AstraZeneca. ETJ received consulting fees for Regeneron Pharmaceuticals and Jazz Pharmaceuticals. PK received funding from APFED. DM has received past honoraria from GSK for awareness activities unrelated to this publication and/or disease state. MR is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Nextstone One, Bristol Myers Squibb, Astra Zeneca, Ellodi Pharma, GlaxoSmith Kline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first seven listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. MR is an inventor of patents owned by Cincinnati Children’s Hospital. JS received grant support from Regeneron/Sanofi, Novartis, NIH, FARE, consulting fees from Regeneron, Sanofi, Alladapt, Readysetfood, ARS Pharmacy, and royalties from Uptodate. ABM received research funding from Morphic and served on medical advisory board for Bristol Myers Squib and Nextone Immunology. VM received consultant fees from Allakos, Regeneron, Sanofi, and Shire/Takeda, and served on an adjudication board for Alladapt. CD received research funding from DBV Technologies, Food Allergy Research and Education, Allergenis, Regeneron Pharmaceuticals, Pfizer, and consultant or advisory board fees for Aimmune Therapeutics. JBW has received consultant fees from Allakos, Ellodi, Regeneron, Sanofi/Genzyme, AstraZeneca, and Invea Therapeutics, and clinical trial/research funding from Allakos, Invea Therapeutics, and Sanofi-Regeneron. BLW, DDB, AK, GE, GH, KK, EK, SKyle, PM, PM-K, CS, MJS, G-YY, KS, NM, SKesh, PB, MS, AZ, AP, AH, and GF have no conflicts of interest to declare.
Availability of data and materials: Not applicable
References
- 1. Navarro P, Arias A, Arias-Gonzalez L, et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019; 49: 1116–1125. [DOI] [PubMed] [Google Scholar]
- 2. Bredenoord AJ, Patel K, Schoepfer AM, et al. Disease burden and unmet need in eosinophilic esophagitis. Am J Gastroenterol 2022; 117: 1231–1241. [DOI] [PubMed] [Google Scholar]
- 3. Sciwheel. Reference manager & generator, https://sciwheel.com (2000, accessed 27 July 2022).
- 4. Christiansen S, Iverson C, Livingston EH, et al. AMA manual of style: a guide for authors and editors, https://www.amamanualofstyle.com/ (2020, accessed 26 July 2022).
- 5. CEGIR. Diversity corner, https://www1.rarediseasesnetwork.org/cms/cegir/News-Spotlights-Events (2021, accessed 27 July 2022).
- 6. Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009; 48: 30–36. [DOI] [PubMed] [Google Scholar]
- 7. Franciosi JP, Tam V, Liacouras CA, et al. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2009; 7: 415–419. [DOI] [PubMed] [Google Scholar]
- 8. Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125: 1660–1669. [DOI] [PubMed] [Google Scholar]
- 9. Katzka DA. Demographic data and symptoms of eosinophilic esophagitis in adults. Gastrointest Endosc Clin N Am 2008; 18: 25–32; viii. [DOI] [PubMed] [Google Scholar]
- 10. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20.e26; quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 11. Hurtado CW, Furuta GT, Kramer RE. Etiology of esophageal food impactions in children. J Pediatr Gastroenterol Nutr 2011; 52: 43–46. [DOI] [PubMed] [Google Scholar]
- 12. Lenz CJ, Leggett C, Katzka DA, et al. Food impaction: etiology over 35 years and association with eosinophilic esophagitis. Dis Esophagus 2019; 32: doy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGowan EC, Keller JP, Dellon ES, et al. Prevalence and geographic distribution of pediatric eosinophilic esophagitis in the 2012 US Medicaid population. J Allergy Clin Immunol Pract 2020; 8: 2796–2798.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 2018; 155: 1022–1033.e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGowan EC, Keller JP, Muir AB, et al. Distance to pediatric gastroenterology providers is associated with decreased diagnosis of eosinophilic esophagitis in rural populations. J Allergy Clin Immunol Pract 2021; 9: 4489–4492.e4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loden M, Rosener JB. Workforce America!: managing employee diversity as a vital resource. Homewood, Ill: Business One Irwin, 1991. [Google Scholar]


