Abstract
Fibrin is a promising natural polymer that is widely used for diverse applications, such as hemostatic glue, carrier for drug and cell delivery, and matrix for tissue engineering. Despite the significant advances in the use of fibrin for bioengineering and biomedical applications, some of its characteristics must be improved for suitability for general use. For example, fibrin hydrogels tend to shrink and degrade quickly after polymerization, particularly when they contain embedded cells. In addition, their poor mechanical properties and batch-to-batch variability affect their handling, long-term stability, standardization, and reliability. One of the most widely used approaches to improve their properties has been modification of the structure and composition of fibrin hydrogels. In this review, recent advances in composite fibrin scaffolds, chemically modified fibrin hydrogels, interpenetrated polymer network (IPN) hydrogels composed of fibrin and other synthetic or natural polymers are critically reviewed, focusing on their use for tissue engineering.
Keywords: Fibrin hydrogels in tissue engineering, fibrin-polymer composite scaffolds, PEGylated fibrin hydrogels, natural polymer-fibrin hydrogels, particles encapsulated in fibrin hydrogels
Introduction
Significant advances in molecular and cellular biology over the past two decades have greatly increased our understanding of the role of fibrin in wound healing, blood clotting, fibrinolysis, cellular-matrix interactions, inflammatory response, infection and neoplasia.1,2 Fibrin is composed of fibrinogen, which is a soluble protein derived from the liver. Following tissue damage, fibrinogen is converted to fibrin by thrombin, a clotting enzyme, at the site of bleeding. In 1943, Bailey et al. classified fibrinogen as a fibrous protein with keratin, myosin, and epidermin, based on an X-ray diffraction pattern generated by its coiled-coil structure. 3 It is a 340 -kDa glycoprotein that is normally present in human blood plasma at a concentration of 1.5–4 g/L and is required for several biological functions. 4 The interactive sites of fibrinogen produce fibrin polymerization, which implies a variety of biological functions, including interactions with platelets, leukocytes, fibroblasts, and endothelial cells, as well as binding to thrombin to control the activity of factor XIII (a coagulation protein). 5 Fibrin fibers are the main players because they serve as scaffolds for tissue regeneration and promote both cell migration and tissue ingrowth. In contrast, fibrin is not mechanically strong or stable enough to act as a stand-alone wound repair material. 6 To address this issue, multiple fibrin modifications using natural and synthetic materials with improved stability and medical efficacy are currently available. One strategy consists of combining living cells with polymeric scaffolds made of fibrin and synthetic materials, such as polyglycolic acid (PGA), polylactic acid (PLA), polycaprolactone (PCL), and polyvinyl alcohol (PVA), or natural polymers, such as hyaluronic acid, alginate, or collagen.
Hydrogels are the most common scaffolds used in tissue engineering because of their similar properties to those of the original living tissues, such as water-holding capacity, permeability, and viscoelasticity.7,8 Biocompatibility, biodegradation, affordability, ease of cell migration, and growth while providing mechanical support until new tissue is formed are some of the requirements that a scaffold material must fulfill. Natural materials are especially promising because they present properties similar to the original tissues and organs, such as biocompatibility and mechanical performance almost identical to that of the extracellular matrix (ECM), and also provide specific adhesion sites for cells. 9 Using naturally based hydrogels, researchers have created new scaffolds for cell seeding and growth, tissue regeneration strategies, and novel biomedical products. 10
Owing to the aforementioned properties, fibrin hydrogels have emerged as ingenious scaffolds because of their important role in blood coagulation during natural wound healing. In summary, fibrin properties include controllable and non-toxic degradation, excellent biocompatibility, and minimal inflammation, as it can be obtained from the patient’s own blood. Furthermore, fibrin properties can be tuned by varying the precursor proportions when polymerization is initiated. The ability of the fibrin hydrogel to rapidly gel and control its properties by tuning the fibrinogen concentration makes it ideal for cell encapsulation, cell carriers, and injectable biomaterials for tissue regeneration applications. For this reason, many fibrin-based products have been developed over the years for biomedical procedures as bioadhesives in surgeries for hemostasis, wound closure, or as sealants, which have been extensively analyzed in other review articles.5,11
Despite being frequently used as a biomaterial in numerous clinical and research applications, fibrin has some drawbacks related to its rapid degradation both in vivo and in vitro and its poorly understood shrinkage behavior and poor mechanical properties make it difficult to handle or even unsuitable for other biomedical applications.12,13 To solve these problems, during the past years a new experimental approach has emerged based on the development of improved fibrin scaffolds via fibrin modification and/or combination with other materials. In this article, we present recent advances in this field, their applications, particularly in different tissue engineering scenarios, and a discussion of their future challenges and opportunities.
Fibrinogen basics: Structure, polymerization, acquisition, and commercial use
Fibrinogen structure and fibrin polymerization
Fibrinogen is a glycoprotein secreted from the liver into the blood and is one of the most important components in hemostasis, as it acts as an adhesive protein for platelet aggregation, forming a fibrin clot for blood coagulation when vascular injury occurs. This fibrous (45 nm in length) and 340 KDa dimeric glycoprotein is composed of approximately 132 amino acids. The fibrinogen macromolecule is a symmetrical dimeric protein composed of three domains in which two identical regions (D-domains) are linked by the central E-domain. The hexameric molecule is made up of three pairs of different polypeptide chains known as α, β, and γ (Figure 1(a)). As Weisel et al. report, the nomenclature of the polypeptide chains arises from the designation of the small peptides that are cleaved from fibrinogen by thrombin to yield fibrin as fibrinopeptides A and B and the parent chains, without the fibrinopeptides, as α and β. 14 No peptides were cleaved from their gamma chains using thrombin. The two terminal D-domains are connected to the E-domain through five symmetrical disulfide bridges at the N-terminus by allelic coiled coils.
Figure 1.
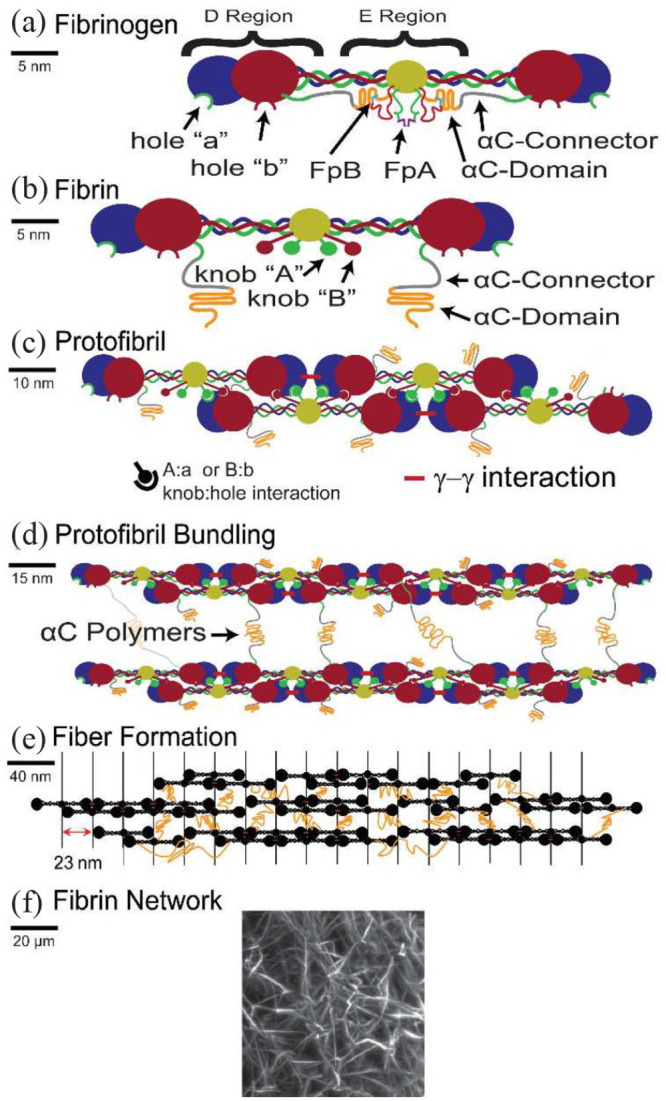
Diagram of the fibrin structure and its polymerization process: (a) Structure of the fibrinogen macromolecule with D-domains and the central E-domain. The domains are linked by three pairs of different polypeptide chains: α, β and γ. (b) Fibrinogen is converted to fibrin monomer by thrombin. It cleaves fibrinopeptides A and B, exposing knobs A and B. (c) Then, the cleaved fibrinopeptides bind to holes a and b, respectively, known as the specific unions A:a and B:b, and form the protofibril molecule. (d) Protofibril bundling is formed by lateral aggregation of protofibrils, owing to interactions of the AC regions, and leading up to thick fibrin fibers. (e) Then, the branching and lateral aggregation of fibers form the fibrin network. (f) Image of a fiber network forming a gel obtained with a light sheet microscope. Reprinted from Belcher et al. 15
Fibrinogen is converted to a fibrin monomer by the cleavage of fibrinopeptides A and B by thrombin (Figure 1(b)). Thrombin is a serine protease with specificity for fibrinopeptides that are formed by the proteolysis of prothrombin by factor Xa in the presence of other factors and phospholipids. Thrombin enzymatically cleaves fibrinopeptide A (FPA) exposing a tripeptide (Gly-Pro-Arg) at the N-terminus of the alfa-chain known as “knob” A that is complementary to a “hole” a in the center region of the other fibrin monomer. 16 This specific union is known as A:a. Fibrinopeptide B cleavage occurs slower than that of FPA and is not necessary for fibrin polymerization, although it plays an important role in lateral aggregation. There is consensus that these electrostatic interactions are the foundation of fibrin polymerization and blood clot formation (Figure 1(c)).
Fibrin polymerization proceeds from highly reactive fibrin monomers (with their FPA released) binding one to another, leading to a self-assembled half-staggered dimer structure. The first stage of polymerization involves the formation of a two-stranded trimer, in which the central region of one fibrin monomer fits the D regions of two different fibrin monomers through the aforementioned knob:hole A interaction. Oligomers grow longitudinally from the two-stranded trimer with the addition of more fibrin monomers until they reach a double-stranded protofibril – 600–800 nm in length. At this stage, the protofibrils begin to aggregate laterally, leading to thick fibrin fibers (Figure 1(d)).
The interaction between monomers occurs in a half-staggered manner, defining the molecular packing in the fibers, giving a periodicity of 22.5 nm, which is exactly half the fibrin monomer length. There is a thermodynamic mechanism that controls the diameter of the fibers, as protofibrils that are newly added to the surface of a fiber must be stretched as their path length increases. Therefore, lateral aggregation stopped when the protofibril stretching energy surpassed the bonding energy.
However, the mechanism underlying the lateral aggregation of protofibrils remains unknown. However, the self-assembly and protofibril origin imply that the interactions in lateral aggregation are weak and cooperative along the axis of the protofibril. Many publications have shown that the following structures and mechanisms besides not being necessary for fibrin polymerization, strongly influence on the final properties and architecture of fibrin clots: knobs ‘B and holes ‘b, the AC regions and the plasma transglutaminase factor XIIIa.17,18
These intermolecular interactions have been demonstrated to promote lateral aggregation. The B:b interactions have been shown to thicken fibrin fibers when compared to fibrinogens, where fibrinopeptides A and B were cleaved to fibrinogen. FPA was cleaved off, suggesting that B:b interactions favor the lateral aggregation of protofibrils. The AC domains enhance the mechanical stability of fibrin clots by promoting lateral aggregation. AC is a fibrinogen extension molecule that interacts with the center of the molecule. FpB cleavage by thrombin action triggers the release of AC, allowing weak intermolecular interactions between them. Specifically, factor XIIIa mechanically and proteolytically stabilizes fibrin clots by forming covalent bonds.
These intermolecular interactions have been demonstrated to promote lateral aggregation. The B:b interactions have been shown to thicken fibrin fibers when compared to fibrinogens, where fibrinopeptides A and B were cleaved to fibrinogen. FPA was cleaved off, suggesting that B:b interactions favor the lateral aggregation of protofibrils. The AC domains enhance the mechanical stability of fibrin clots by promoting lateral aggregation. AC is a fibrinogen extension molecule that interacts with the center of the molecule. FpB cleavage by thrombin action triggers the release of AC, allowing weak intermolecular interactions between them. Specifically, factor XIIIa mechanically and proteolytically stabilizes fibrin clots by forming covalent bonds.
The entire polymerization process, in which numerous proteins and molecules take part, ends with the formation of a 3D fibrous network that appears as an insoluble hydrogel (Figure 1(f)). The mechanical properties, microstructure, and behavior of this fibrin hydrogel depend on many conditions, such as the concentration of the molecules involved in the process or environmental parameters such as temperature or pH.1,14,19
Human fibrinogen acquisition
Human fibrinogen can be obtained in different ways. 20 Fresh frozen plasma (FFP) obtained from patients has extensive use in trauma and transfusions, with a fibrinogen concentration of 1–3 mg/ml.21,22 FFP, which is easily accessible, is the most commonly used source of fibrinogen. To produce FFP, whole blood from a single donor is split into its plasma and cellular components, which are then quickly frozen and stored at 18°C or lower. The contact activation (intrinsic) and tissue factor (extrinsic) clotting system soluble coagulation factors, as well as fibrinogen, were preserved by freezing. 23 Fresh plasma samples can be divided into three types depending on the number of platelets. These include platelet-rich plasma (PRP), platelet-free plasma (PFP), and platelet-poor plasma (PPP), the latter two of which are described as having low platelet concentrations in the literature.24,25 All these types of plasma are produced by sufficiently centrifuging patient blood, but several procedures and techniques have been developed to produce them with enhanced purity at a low cost. 26 The main difference is in the concentration of platelets, which is triggered by the presence of high levels of growth factors. PRP has at least 200 × 10−3 platelets/µL suspended in plasma; however, due to its inconsistent experimental results and poor reproducibility, other alternatives, such as PPP, should be investigated. 27 PPP applications, such as clot formation and wound healing in which platelet-released growth factor concentration is not essential, have recently been discovered and applied in several studies. D’Amico et al. explored the use of factor-decorated fibrin matrices containing platelet-derived growth factor-BB (PDGF-BB) and vascular endothelial growth factor-A (VEGF) together to promote therapeutic arteriogenesis and accelerate wound healing in diabetic mice. This study demonstrated that these matrices, loaded with growth factors and cytokines, stimulated angiogenesis and the formation of new blood vessels in diabetic wounds. 28 PPP is also cost-effective, appropriate for bulk production, and easily translatable, with only minimal regulatory requirements for Food and Drug Administration (FDA) certification.29–31
However, due to the risks associated with using a given blood product, including low total fibrinogen concentrations or considerable fluctuations in concentration across donor packets, FFP is not the optimal supply of fibrinogen. 32 Cryoprecipitation is used to obtain a higher fibrinogen concentration than that in FFP. 33 A cryoprecipitate is a human plasma-derived blood product. It contains factor VIII, von Willebrand factor (vWF), fibrinogen, fibronectin, and factor XIII. Currently, cryoprecipitates are most commonly used to replace fibrinogen in patients with chronic hypofibrinogenemia and hemorrhage. Cryoprecipitation is an excellent fibrinogen source frequently used in the United States and is obtained by thawing FFP, centrifugation, and resuspension of the precipitated proteins in plasma. Each unit of cryoprecipitate contained 200–300 mg of fibrinogen.34–36 Although fibrinogen levels can be adjusted, this can be performed with less accuracy than commercial fibrinogen, and the infusion volume is smaller than that of fresh-frozen plasma. Viral inactivation methods such as methylene blue or psoralen/ultraviolet light treatment are frequently used. However, thawing is necessary before infusion, and ABO compatibility is required. 37 This multi-donor blood product that requires large volumes, cross-matching, and thawing before administration, and is associated with possible pathogen transmission.
Commercial fibrin products
Commercial fibrinogen products are a good way to take advantage of this material for different clinical applications and are available as liquid fibrin glues, sealants, and fibrin patches.38,39 Patches can help blood clot, sealants can form a barrier whether blood is present, and glues can join tissues together. 40 Fibrin glues usually contain a freeze-dried concentrate of clotting proteins such as cryoprecipitate, mainly fibrinogen, factor XIII, fibronectin (sealant), and freeze-dried thrombin. In addition to being used clinically in a variety of surgical specialties, fibrin sealants also play a part in a number of research applications, such as drug delivery, tissue sealing, and hemostasis. 41
The FDA has approved five blood products based on fibrin for topical use. The functional elements of the sealant are fibrinogen and thrombin, which, upon administration, form hemostatic fibrin. FDA-approved products frequently contain these two components; however, the concentrations of fibrinogen and thrombin can be altered to meet specific demands for mechanical strength or to alter the dynamics of sealant polymerization. The blood products approved by the FDA are listed in Table 1 and can be divided into four main groups: 42,43 topical use, absorbable patches, fibrin lyophilization, and a device that produces fibrinogen and thrombin from human plasma.
Table 1.
Fibrin products approved by FDA.
| Product | Company | Principal components | Form | Indication | Ref. |
|---|---|---|---|---|---|
| Topical use | |||||
| ARTISS | Baxter Healthcare Corp. | Fibrinogen (67–106 mg/mL), thrombin (2.5–6.5 IU/mL), CaCl2 (36–44 µmol/mL), factor XIII and aprotinin (2250–3750 KIU/mL) | Spray set | Adhesive for autologous skin grafts and as face-lift for facial rhytidectomy surgeries | Yamamoto and DeJoseph 44 |
| EVICEL | Omrix Biopharmaceutical Ltd. | Fibrinogen (55–85 mg/mL) and thrombin (800–1200 IU/mL) | Spray or syringe | Hemostatic agent for liver, vascular, and general surgeries | Ofikwu et al. 45 |
| FIBRIN SEALAN/Vistaseal | Instituto Grifols/Ethicon | Fibrinogen (80 mg/mL) and thrombin (500 IU/mL) | Spray or in a syringe | Hemostatic agent for vascular surgeries; also approved by the European Medicines Agency (EMA) | Beudert et al. 43 |
| RAPLIXA | The Medicines Co. | Fibrinogen (79 mg/g) and thrombin (699 IU/g) | Spray | Hemostatic agent for general surgeries | McKeage 46 |
| TISSEEL | Baxter Healthcare Corp. | Fibrinogen (67–106 mg/mL), thrombin (400–625 IU/mL), factor XIII (0.6–5 IU/mL) and aprotinin (2250–3750 KIU/mL) | Spray set | Hemostatic agent for cardiopulmonary bypass, splenic injuries, and general injuries, and as sealant in colonic anastomosis | Siedentop et al. 47 |
| Absorbable patches | |||||
| EVARREST | Ethicon | Fibrinogen (8.6 mg/cm2) and thrombin (37.5 U/cm2) | Patch | Hemostatic agent for retroperitoneal, intra-abdominal, pelvic, noncardiac thoracic surgeries and adult liver surgeries | Matonick and Hammond 48 |
| TachoSil | Nycomed Danmark ApS (2019 Ethicon) | Fibrinogen (3.6–7.4 mg/cm2) and thrombin (1.3–2.7 U/cm2) | Patch | Hemostatic agent for cardiovascular and neurological surgeries; also approved by the European Medicines Agency (EMA) | Simo et al. 49 |
| Fibrin lyophilization products | |||||
| RiaSTAP | CSL Behring GmbH | Lyophilized fibrinogen (18–26 mg/mL) | Hemostatic agent for acute bleeding episodes in patients with congenital fibrinogen deficiency | 50 | |
| Fibryna/Fibryga | Octapharma Pharm. Prod. | Lyophilized fibrinogen (120 mg/mL) | Hemostatic agent for treatment of acute bleeding episodes in adults and adolescents with congenital fibrinogen deficiency | Roberts et al. 51 | |
| Device that produces fibrinogen and thrombin from human plasma: | |||||
| Cryoseal | ThermoGenesis | A device to produce fibrinogen and thrombin from human plasma (autologous fibrinogen) | Foams spray and drop tips for application | Adjunct to hemostasis during liver resection | Doria and Vaccino 52 |
The use of these fibrin-based products has significantly reduced pain and the duration of hospital stay by lowering the appearance of postoperative hematomas. 53 Fibrin sealants reduce the cost and time required for these operations.
The aforementioned properties and use of fibrin as a hemostatic agent in various fields of surgery have drawn attention to this material as a hydrogel in 3D cell culture by tissue engineering researchers.
The use of fibrin hydrogels in tissue engineering: Challenges
Fibrin hydrogels are formed by the polymerization of fibrin monomers, leading to a fibrous structure. Fibrin hydrogels are commonly used in tissue engineering because of their biocompatibility, structural similarity to native tissues, their ability to transport properties, tunable properties, and capacity to deliver drugs and growth factors. The porosity and viscoelastic mechanical properties that mimic the extracellular matrix (ECM), along with the predetermined cell-binding sites in fibrin, result in good cell adhesion, spreading, proliferation and migration. 54 Moreover, specific proteins can bind to fibrin, which plays an active role in interacting with cells. As a fibrous scaffold, it provides a temporal substrate; cells secrete plasminogen and matrix metalloproteinases (MMPs) that mediate fibrinolysis and subsequent degradation of the hydrogel. However, there are still numerous limitations, such as shrinkage of the hydrogel during formation, low mechanical stiffness, batch-to-batch variability, and rapid degradability. Thus, research efforts are required to improve fibrin performance by modifying it with diverse polymers in many different ways and creating new scaffolds for cell seeding.
Fibrin hydrogel degradation
Fibrin hydrogels can be degraded by two different mechanisms, which decrease their effectiveness and chances of successful engraftment in tissue engineering.
First, degradation is caused by cells that are present in fibrin hydrogels; when these cells proliferate, they produce plasmin, which is secreted by a variety of cell types, including endothelial cells.55,56 However, the lifespan of fibrin is limited by rapid fibrinolysis that occurs when plasminogen is present. The use of inhibitors that block the active site of plasmin serine proteases, such as tranexamic acid or epsilon-aminocaproic acid, can help reduce the effect of plasminogen.
The second degradation mechanism is the activation of matrix metalloproteinases (MMPs) through proteolytic and esterolytic reactions, which contribute to the remodeling of the extracellular matrix and serve as a negative feedback mechanism for the matrix degradation response. The use of aprotinin, which inhibits MMPs, prevents the breakdown of fibrin, and promotes the accumulation of extracellular matrices, is a way to reduce this effect.57,58
The rate of fibrin hydrogel degradation can be tuned by the addition of protease inhibitors, such as aminocaproic acid and aprotinin, or by modifying the precursor concentrations of the biomolecules involved in polymerization.59–61 These degradation processes enable proper tissue development as scaffolds are exchanged by cells, and the degradation rate is different for different cell lineages seeded in the scaffold. Different sources of stem cells affect degradation at different rates, which has strong implications for the development of artificial tissues and organs. 62 The rate of hydrogel degradation is crucial for obtaining viable tissues. Controlling this parameter is not always intuitive as it appears to be due to fibrinolysis. The derived products play an important role in the chemical signaling of cells, healing processes, angiogenesis, and stimulation of migration. 63 The rate of hydrogel degradation is crucial for obtaining viable tissues. Controlling this parameter is not always intuitive as it appears to be due to fibrinolysis. The derived products play an important role in the chemical signaling of cells, healing processes, angiogenesis, and stimulation of migration. 64 In most cases, fibrin hydrogels dissolve completely before the tissue is obtained. An increase in hydrogel stability is achieved by adjusting the fibrinogen concentration. 57 Ahmed et al. found that a combination of aprotinin and galardin (MMP inhibitor) hydrogels can last up to 5 weeks, whereas pure fibrin hydrogels degrade completely in 7 days owing to cell activity. 69 In the specific case of retinal pigment epithelium transplantation, it would be interesting to exploit the fast degradation rate of fibrin. 65 For these reasons, fibrinogen-based hydrogels have been used in recent years for different tissue engineering applications, for example, as a scaffold for the culturing of muscle, adipose, cartilaginous, liver, bone, or ocular cells; for wound repair and skin regeneration therapies; or even as a treatment for neurodegenerative diseases and fertility preservation strategies.66,67
Although the use of fibrin could be promising in all tissue regeneration strategies, it could have side effects; for example, in the treatment of cardiac or skin tissue lesions, it might produce undesirable fibrous scars.68,69 Fibrous scars are characterized by the excessive deposition of extracellular matrix components, particularly collagen, which leads to impaired tissue function and limited regenerative potential. The formation of fibrous scars during fibrin-based tissue regeneration can be attributed to several factors. First, the inflammatory response derived from the fibrin degradation products activates fibroblasts, which are responsible for collagen synthesis.70,71 Second, fibrin-derived peptides can induce the expression of profibrotic factors such as transforming growth factor-beta (TGF-β), platelet-derived growth factor, and connective tissue growth factor, further promoting fibrosis.70,72,73 Finally, the mechanical properties of fibrin-based scaffolds, including stiffness and porosity, can influence cellular behavior and tissue remodeling, thereby potentially increasing scar formation.
Fibrin hydrogel contraction
The tendency of fibrin hydrogels to contract, particularly when embedding cells, is one of the aforementioned drawbacks, according to the conclusions of numerous studies.30,74–76 For example, Montero et al. analyzed the contraction behavior of plasma-derived fibrin hydrogels and found that these scaffold contractions in the presence of fibroblasts depend on the fibrinogen concentration and compromise the development of skin tissue culture. 75 They concluded that in fibrin hydrogels, there is a lack of attachment to the culture insert, which causes shrinking in the z-axis and complicates their clinical use and surgical handling. Murphy et al. demonstrated that NaCl and fibrinogen affect hydrogel contraction when cultivating mesenchymal stem cell spheroids. 77 According to Yue et al., this could impede the advancement of customized tissue reconstruction techniques. 78 In recent years, the use of fibrin in combination with synthetic or natural polymers has been investigated as a potential solution to this issue.79–81 Other potential solutions include the use of solid scaffolds and their chemical modifications.
Poor mechanical properties
Compared to other polymers, fibrin exhibits remarkable and distinctive viscoelastic properties that affect its structural, biological, physical, and chemical properties. The mechanical properties of fibrin are critical to its function and determine how it responds in treatments, such as tissue clotting, wound healing, and disease prevention and treatment. Information regarding the structural functions of clot stability is required to understand the role of fibrin hydrogels. The fibrin structure can be described as a branched network in which the mechanical properties are governed by both single fibers and their ensembles. This network also includes changes in the fiber orientation as well as stretching, bending, and buckling. According to Guthold et al., the quantity and configuration of double-stranded, half-staggered protofibrils affect the characteristics of individual fibrin fibers. 82
The stiffness of a fiber can be measured using its Young’s modulus; a higher value indicates a stiffer fiber. The elastic modulus (slope of the stress-strain curve), which indicates how elastic a material is in relation to strain, changes if it is nonlinearly elastic. 82 According to research, uncrosslinked and crosslinked fibrin fibers have Young’s moduli of 1.7 ± 1.3 and 14.5 ± 3.5 MPa, respectively. 83 These values were obtained through laser tweezer flexion experiments, which involved pulling the fibers and measuring their elastic moduli. Similar results were obtained using other independent methods such as stretching experiments, in which the elastic moduli were measured by pulling a bead in the direction of the fiber axis. With this method, the elastic modulus of uncrosslinked fibers was 1.9 ± 1.8 MPa, while that of crosslinked fibers was 11.5 ± 5.1 MPa. Because the fibers are not homogeneous and isotropic, and the experiments are different, these findings are not comparable to those obtained with flexion experiments, but all of these findings provide the basis for understanding the development of clot elasticity.82,83
It has been reported that native heart tissue has a Young’s modulus of up to 67 kPa, and native esophageal tissue has a Young’s modulus of 60 kPa in terms of tissues and hydrogels, when the tensile properties of each scaffold are measured with a uniaxial monotonic material tensile test machine.84,85 In contrast, the typical fibrin hydrogels have Young’s moduli ranging from 0.94 to 6.49 kPa for fibrinogen concentrations of 0.5–3.0 mg/mL. Higher fibrinogen concentrations, such as those present in commercially available fibrin-based adhesives, were measured using a uniaxial monotonic material tensile test machine. Hydrogels that had been developed with 30 and 70 mg/mL of fibrinogen improved in Young’s modulus by 27.5 and 14.6 kPa when they were crosslinked with CaCl2. 84 However, at high fibrinogen concentrations, the cells were unable to spread, proliferate, or ultimately survive.
The Young’s modulus of fibrin is of the same order of magnitude (1–10 MPa) as that of other soft biological fibers with the same stiffness and high extensibility, such as spider silk (Araneus Flag silk) with 3 MPa, myofibrils (sarcomere) with 1 MPa or elastin (bovine ligament) with 1 MPa. 82 However, we observed low extensibility and stiff fibers with higher orders of magnitude such as crosslinked, self-assembled collagen at 5000–7500 MPa, tendon collagen (mammalian tendon) at 160–7500 MPa, or actin at 1800–2500 MPa.
Understanding the mechanical interactions when cells are embedded is difficult because they present mechanical heterogeneities at the microscale and as a function of time. 86 Advances in cartilage tissue repair were achieved by Kim et al. analyzed the chondrogenic differentiation of human adipose-derived stem cells by modifying the fibrinogen and thrombin concentrations, which compromised cell behavior through changes in hydrogel stiffness. 87 Stabilization of fibrin hydrogels by the addition of alginate has also been attempted; however, bone marrow stem cells seem to differentiate and proliferate better in fibrin, highlighting the complex task of improve fibrin in-vitro performance. 88
In addition, fibrin hydrogels, as biomaterials, also influence angiogenesis because their microstructure strongly modifies neovascularization. For example, Tanaka et al. demonstrated that fibrin hydrogels induce macrophages, which opens up several other avenues for anti-inflammatory regenerative therapies. 89 Recently, some research groups have developed strategies for using fibrin as an adhesive between cells to obtain complex tissue constructs. 90
Batch-to-batch variability
Batch-to-batch variation in fibrin hydrogel properties is a significant challenge that can potentially lead to serious consequences if not properly addressed. According to Nair et al., there is a wide range of variation in reproducibility when using different batches of fibrinogen and thrombin, as well as day-to-day variation when using the same batch of fibrinogen. 91 These variations could result from changes in the preparation techniques or fibrinogen sourcing. The swelling of hydrogels from various fibrinogen batches can be studied to identify batch-to-batch variations and understand how hydrogel mechanics change over time. In other words, measuring the amount of water released from the hydrogel at different times is a method for determining the hydrophilicity of the polymer network between the hydrogels. 92 Furthermore, differences in the absolute shear moduli of fibrin hydrogels demonstrate relatively high batch-to-batch variations during polymerization. 93 According to the results of these studies, batch-to-batch variations play a major role in determining the hydrogel fabrication properties and how they change over time. 92
These differences are also notable in the fibrin glues. To create a fibrin clot and induce early hemostasis in the treated area, the glue mimics the final stage of the physiological blood coagulation cascade. In some studies, fibrin glues were prepared from pooled plasma, and the differences in the mechanical and biological properties of the resulting clots were due to batch variabilities.13,94 Remarkably, there are fewer variations between commercially produced fibrin glues that are standardized than between single-donor fibrin glues that suffer from batch-to-batch variability.
Other fibrin limitations
Several studies have reported less-known limitations of fibrin in hydrogels. For example, Gruber et al. indicated that fibrin hydrogel-seeded disk cells do not express aggrecan or chondroitin-6-sulfotransferase, which have crucial functions in tissue engineering. 95 In addition, Demol et al. demonstrated experimentally and computationally that limitations in oxygen mass transfer are responsible for cell density gradients within fibrin hydrogels and concluded that cell culture in fibrin hydrogels can lead to complete anoxia in the carrier center for realistic values of oxygen diffusion and consumption. 96 Moreover, despite the fact that fibrin sealants are effective in oral surgery, they suffer from the following disadvantages 97 : poorly reproducible rheological characteristics; a tendency to trigger the production of coagulation factor inhibitors (especially factor V) related to bovine thrombin, which results in postoperative coagulopathies; and the need for patients to visit the blood bank several days before a surgical procedure. Furthermore, using an infected fibrin clot as a model, Ma et al. demonstrated that the common blood-borne pathogen Staphylococcus epidermidis could affect the mechanical and structural properties of blood clots in in vitro models. 98 Kambic HE et al. added a new limitation of fibrin, and the design of tissue-engineered arrays in innervated fibrin matrices only facilitated the growth of vascular structures with the additional support of fibroblasts and keratinocytes, and their matrices within cells in vitro also showed poor mechanical properties. 99 Finally, Billiet et al. reported low and inhomogeneous mechanical strength, which restricted the porosity and insufficient interconnectivity of the pore distribution, as well as the presence of organic solvent residues, which can pose significant constraints in terms of toxicity risks and carcinogenic effects. 100
To a lesser extent, fibrin hydrogel properties can be varied by changing the thrombin concentration and modifying its mechanical and shrinkage properties. 101 Buffers that are known to be inert, such as HEPES, strongly modify the fibrin microstructure, leading to more transparent hydrogels. 102 Even the salinity of the precursor fibrin hydrogel plays a key role on how the hydrogel will behave. 103 In addition, fibrin hydrogels have been supplemented with proteins to enhance the bioactivity of cells, which also affects fibrin polymerization and the microstructure of the hydrogels. 104
Another drawback is the use of different crosslinking strategies to modify fibrin degradation and mechanical properties because of the cytotoxicity and inflammatory responses of some crosslinkers. It is important to consider specific requirements when selecting an appropriate crosslinking method for fibrin modification. For example, chemical crosslinkers, such as glutaraldehyde, have been explored with the aim to enhance the mechanical properties of fibrin. Glutaraldehyde crosslinking, such as tetranitromethane crosslinking, also improves mechanical strength, allowing for the modulation of mechanical properties and degradation rate, but it has been shown to be cytotoxic and pro-inflammatory.105,106 Moreover, carbodiimide crosslinking also forms stable covalent bonds between fibrin molecules, showing a drastic change in the hydrogel morphology. The swelling rate decreases, degradation increases, and Young’s modulus increases when the concentration of the crosslinker increases. However, growth factors showed lower release, and cytotoxicity assays demonstrated that by increasing the crosslinker concentration, cytotoxicity was observed.107,108 Alternative methods for the enzymatic crosslinking of glutaraldehyde include thrombin, transglutaminase, genipin or horseradish peroxidase (HRP). 105 Transglutaminases with high biocompatibility do not sufficiently crosslink plasma proteins. 105 In contrast, thrombin can crosslink plasma proteins to a satisfactory degree, providing an alternative crosslinker for fibrin hydrogels. Another alternative is the use of genipin as a fibrin crosslinker. For example, Gamboa-Martínez TC et al. demonstrated the cellular viability of an in vitro culture with genipin as a crosslinker. 109 Moreover, the content of primary amino groups in hydrogels crosslinked with genipin did not exceed that of the samples crosslinked with thrombin. 105 Finally, horseradish peroxidase has shown promise for modifying the mechanical properties of fibrin microthreads while preserving their biocompatibility. To optimize crosslinking strategies, parameters such as the crosslinker concentration, reaction time, and temperature must be carefully evaluated. Long-term biocompatibility studies should be conducted to assess the effects of crosslinking on cell behavior, inflammatory responses, and overall tissue integration.
For these reasons, over the past few years, researchers have focused on improving fibrin performance by modifying it with diverse polymers in various ways, which will be discussed below. The goal was to obtain scaffolds with adequate properties for specific biomedical applications without losing the biological properties of fibrin.
Advances in the design of fibrin-based matrices with improved properties
To solve those problems in the wide use of fibrin hydrogels in tissue engineering, some advances were researched and studied that aimed to improve the fibrin hydrogels properties. The first solution is the incorporation of polymer solid composites, where fibrin hydrogels are injected into porous solid scaffolds. The resulting fibrin-polymer solid composite scaffolds have shown great potential in tissue engineering, particularly in cartilage, cardiac tissue, skin, bone, and other tissues, where mechanical stabilization is improved, thereby providing biocompatibility. In addition, the incorporation of synthetic polymers, such as pegylated fibrin and PVA-fibrin hydrogels, among others, have also been researched and studied. These hydrogels provide additional structural support and modulate the biochemical and mechanical properties of the scaffolds. One notable advancement is the incorporation of natural polymers, owing to their compatibility with fibrin hydrogels. Natural polymers, such as collagen, alginate, hyaluronic acid, laminin, elastin, and agarose, have been incorporated into fibrin matrices to enhance specific properties, mimic the native ECM of target tissues, and regulate cell behavior to promote tissue repair and wound healing. Additionally, by incorporating particles into the fibrin matrix, it is possible to introduce specific functionalities such as controlled drug release, improved mechanical properties, and enhanced cell adhesion. These particle-incorporated fibrin hydrogels offer a versatile platform for targeted and localized therapy delivery as well as improved tissue engineering outcomes. These widely studied solutions aim to develop advanced biomaterials that promote tissue repair in damaged or diseased tissues.
Fibrin-polymer solid composite scaffolds
In this section, we discuss previous attempts to incorporate fibrin hydrogels into solid polymeric scaffolds. Synthetic polymeric scaffolds have been used because of their controllable mechanical properties, degradation rates that mimic those of real living tissues, easy handling, production, and low availability in most cases. They are typically obtained as porous scaffolds in the form of sponges and microfibers to culture cells in a 3D structure. Inside these scaffolds, cells proliferate and migrate over the surface of the pores or fibers, similar to a 2D culture; however, these polymers do not permit the development of cells or the expression of genes for differentiation or migration. On the other hand, fibrin is a complex biomaterial with some drawbacks regarding its mechanical properties and long-term stability but with outstanding cell signaling as RGD (arginine–glycine–aspartic acid) motifs that enhance cell adhesion and proliferation.
The synergy between both materials arises from the production of hybrid materials that take advantage of their good properties. Typically, the fabrication of these types of composite scaffolds proceeds by first obtaining a synthetic polymeric scaffold in which a fibrin precursor solution containing cells is infiltrated or poured over the porous scaffold, as shown in Figure 2. The three most common strategies to form fibrin-based hybrid scaffolds are phase-separation freeze-drying scaffold processing, the electrospinning scaffold technique and 3D printed scaffolds with porous, fibrous, and filamentous morphologies. Once the scaffold is produced using one of these strategies (a, b, and c in Figure 2), a fibrin precursor solution containing cells is injected into the solid scaffold and fibrin gelation occurs. 110 Using these procedures, a hybrid material was obtained from fibrin and a synthetic polymeric material.
Figure 2.
Scheme of fibrin-based hybrid scaffold formation in two steps: the fabrication of different solid scaffolds using different strategies (a, b, and c). (a) fibrin precursor solution containing cells is injected into the solid scaffold, and subsequent fibrin gelation occurs. A freeze-drying scaffold processing by phase separation: A polymer with a solvent is frozen to form a freeze-dried scaffold because of solvent sublimation. The resulting scaffold exhibits a porous morphology. (b) Electrospinning scaffold: A polymer with a solvent is dosed using a syringe on a collector drum with a determined electric current entailing solvent evaporation and the formation of a solid polymer fiber mesh. The formed scaffold exhibits a fibrous morphology. (c) 3D printed scaffold: a scaffold is designed in software, and the 3D printer reproduces the design layer by layer using needles loaded with a polymer that acts as bio-ink. The resulting scaffold exhibits a filamentous morphology. The left part of the image was adapted from Roacho-Pérez et al. 110
Applications in cartilage tissue engineering
Fibrin-based hybrid materials have frequently been used as cartilage tissue scaffolds because of their enhanced mechanical properties and promotion of cell differentiation. Lee et al. enhanced cell retention and distribution within a macroporous polyurethane scaffold by combining chondrocytes with a fibrinogen solution. 117 However, the authors used aprotinin to delay fibrin degradation, and the scaffold did not maintain phenotypic conditions after 4 weeks. Recently, Sha’ban et al. discovered that chondrocytes produce more ECM due to the presence of more type II collagen and glycosaminoglycan in poly(lactic-co-glycolic acid) (PLGA) scaffolds with infiltrated fibrin after 3 weeks, compared to PLGA with no fibrin (Figure 3(a)). 111 Li C et al. also used a porous PLGA scaffold filled with fibrin gel, in which chondrocytes maintained a round shape, in contrast to the elongated shape of pure PLGA after 4 weeks. 112 In an in vivo analysis, Sha’ban et al. demonstrated that the hybrid scaffold was still improved to obtain cartilaginous tissue 4 weeks after implantation. 113 The authors suggested the incorporation of cell growth factors and other biomolecules to confer bioactivity to the hybrid system, with the aim of improving its ability to induce chondrocyte differentiation in other cell types.
Figure 3.
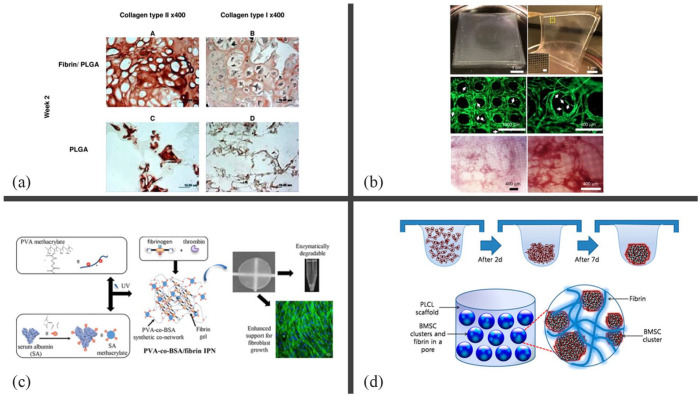
Applications of fibrin-polymer solid composite scaffolds in different tissues engineering. (a) Analysis of in vitro constructs using immunohistochemistry of fibrin/PLGA, which demonstrated strong immunopositivity of collagen type II, and PLGA, with minimal collagen type II expression, after 2 weeks. The immunopositivity of both constructs for collagen type I was moderate. Reprinted from Sha’ban et al. 111 (b) Micropillar array of poly (dimethylsiloxane) (PDMS) at square mesh (5 × 5 cm) (top images) with poured fibrin gel, which guided organization of the cells and produced angiogenic sprouts (white arrows) during 2 weeks of in vitro culture (middle images), and demonstrated a high degree of vascularization after 2 weeks of subcutaneous implantation in SCID-Beige mice (bottom images). Reprinted from Song et al. 114 (c) Research of scaffolds of polyvinyl alcohol (PVA) with serum albumin (SA), as a degradation agent by enzymes, at different concentrations. PVA and SA were previously methacrylated before being copolymerized into PVA-SA scaffolds via free radical copolymerization. Finally, fibrin gel with fibroblasts was infiltrated and polymerized by thrombin, resulting in enzymatically degradable IPNs. It promotes cell growth and mimics the physiological microenvironment of tissues. Reprinted from Bidault et al. 115 (d) Design for PLCL-fibrin scaffold fabrication in cartilage using stromal cells from rabbit bone marrow. Clusters of cells were aggregated using a hanging drop method and added inside the scaffolds using fibrin-gel infiltration. Reprinted from Lee et al. 116
A PLGA scaffold filled with fibrin sponges (obtained by freeze-drying) seems to increase water absorption and was characterized by Wei et al. to observe adipose-derived stem cell differentiation promoting cartilage regeneration in vivo. 117 Sukri NM et al. seeded rabbit bone marrow mesenchymal stem cells (BMSCs) on a PLGA/fibrin scaffold, in which the cells produced chondrogenic matrices but lacked signaling factors to induce chondrogenesis in vitro. 118 These investigations demonstrate the promising use of fibrin as a mesenchymal cell carrier to promote chondrogenesis in a hybrid scaffold with appropriate mechanical stability as a cartilage scaffold. Therefore, Lee et al. recently developed a hybrid poly (lactide-co-caprolactone)/fibrin scaffold in which fibrin was used to disperse BMSCs aggregates to achieve mature cartilaginous tissues. 116 Cell aggregation in combination with hybrid scaffolds improved chondrogenesis in vivo and cartilage-specific genes such as sulfated glycosaminoglycan (sGAG), collagen, and lacunae.
As previously discussed, tissue engineering also faces the problem of contraction of the scaffold during the culture of anatomically shaped structures. Cells tend to contract hydrogel scaffolds, especially when they are embedded inside, owing to proliferation, migration and cell mediated degradation.119,120 To address this, Visscher et al. designed a 3D printed PCL cage around a fibrin hydrogel that completely inhibited matrix contraction after 28 days in the presence of chondrocytes, perichondrocytes, and adipose-derived stem cells. 121 Setayeshmehr et al. lyophilized a mixture of devitalized costal cartilage matrix and aminated PVA, which was crosslinked in different ways, to embed a fibrin adipose-derived mesenchymal stromal cell (ASC) hydrogel. 122 Combining these materials allows cells to differentiate and prevents cell-mediated contractions. Research results and developments suggest the use of a combination of materials with the intention of fulfilling all properties and requirements for a specific tissue culture. Recently, a hybrid scaffold composed of a 3D-printed PCL lattice structure was coated with fibrin and ECM, which resulted in enhanced cell viability. 123
Applications in cardiac tissue engineering
Fibrin-based hybrid materials are frequently used in cardiovascular tissue engineering. For example, Pankajakshan et al. and Gundy et al. enhanced the expansion, proliferation, and survival of human umbilical vein endothelial cells and human coronary artery smooth muscle cells, respectively, using combinations of a solvent-cast PCL scaffold and a warp-knit PLA textile with a fibrin hydrogel, respectively.124,125 Generally, the tissues to be regenerated in vitro tend to be very complex, which means that the current design of scaffolds is a complicated task that requires a combination of different materials. Each type of material has its disadvantages. Hybridization plays an important role in balancing the properties of the hybrid material to approximate those of the original organ or tissue. Microvascular meshes with functional blood vessels have been obtained by Song et al. using a micropillar array of polydimethylsiloxane (PDMS) in which they poured a fibrin gel and this combined patterned scaffold guided the cell organization and prevented the matrix from shrinkage (Figure 3(b)). 114 Recently, multiscale hybrid scaffolds made of fibrin hydrogels, electrospun PCL fibers, and alginate hydrogels have been mechanically conditioned to support different types of cells in co-cultures to promote cardiovascular tissue and blood-vessel formation. 126
Applications in skin tissue engineering
Scaffolds for skin tissue can be created by combining fibrin and synthetic polymers using a variety of methods, such as electrospinning or physical blending.127,128 Studies using these scaffolds for wound healing demonstrated that a nanofiber wound dressing containing fibrin can be prepared using a coaxial electrospinning technique, which was tested in animal models. 129 The coaxial structure of the nanofibers allows for the encapsulation and controlled release of fibrin, which promotes angiogenesis and accelerates wound healing. The nanofiber dressing exhibited excellent biocompatibility and demonstrated enhanced wound healing capabilities, including increased cell migration and proliferation, as well as improved vascularization at the wound site. 130 Bidault et al. developed and tested a scaffold containing fibrin and PVA for the growth of fibroblast. 131 PVA was combined with a fibrin hydrogel and crosslinked by free-radical polymerization inside the scaffold after modification with methacrylate groups. According to this study, the scaffold had excellent mechanical properties such as tensile strength and elasticity because of the synergistic interactions between fibrin and PVA. In addition, these scaffolds provide an environment conducive to fibroblast growth, which may be helpful in wound healing. The self-supporting nature of scaffolds allows them to be easily handled and placed on complex wounds. Another approach is to add different PVA of serum albumin (SA) at different concentrations to allow the material to be degraded by enzymes (Figure 3(c)). 115 The PVA–SA co-networks were synthesized by free radical copolymerization of polyvinyl alcohol (PVAm) and serum albumin (SAm), both previously modified with methacrylate functions. The fibrin gel was then added to the solution and polymerized using CaCl2 and thrombin. This study demonstrated that by adjusting the crosslinking density and composition of IPN, the degradation rate of the biomaterial could be controlled.
Another polymer frequently used to create scaffolds into which polymerized fibrin solution is injected is poly(lactide-co-glycolide) (PLGA). Fibrous PLGA/fibrin scaffolds have demonstrated potential as substitutes for the skin. When combined with fibrin, PLGA, a biodegradable polymer, provides benefits, such as improved mechanical properties, controlled degradation, and support for cell adhesion and proliferation. PLGA/fibrin scaffolds have shown potential as skin substitutes. 132 Fibrin increased tensile strength while decreasing elongation at break. Direct and indirect 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl-2H-tetrazolium bromide (MTT) assays were performed on the scaffolds, and the incorporation of fibrin improved cell adhesion and viability. Therefore, PLGA/fibrin is a promising material for use as a skin substitute. PLGA/fibrin scaffolds have also been used in wound healing models. In particular, a bilayer scaffold comprising an electrospun PLGA/fibrin membrane and a fibrin hydrogel layer was investigated. To produce a skin substitute, Bastidas et al. developed an electrospun membrane and fibrin hydrogel layer on a rat skin model. Keratinocytes were grown on electrospun membranes, and fibroblasts were grown in a fibrin hydrogel layer. The study showed that the scaffolds induced collagen deposition, granulation tissue growth, and epithelial tissue remodeling. The fibrous structure of the scaffold closely resembled the architecture of native skin, facilitating dermal cell infiltration and promoting the formation of new tissue. 133
Another interesting approach involves the healing effect of a fibrin-based scaffold loaded with platelet lysates in full-thickness skin wounds. 134 This study focused on treating full-thickness skin wounds with a fibrin-based scaffold loaded with a platelet lysate. Platelet-derived growth factors and cytokines, which have strong regenerative properties, are present in platelet lysates. Platelet lysates can potentially be used to treat diabetic foot ulcers because they accelerate reepithelialization and increase collagen deposition, thereby improving wound healing.
Furthermore, poly(ether)urethane-polydimethylsiloxane (PU-PDMS) scaffolds can be combined with fibrin to control the delivery of proangiogenic growth factors for the formation of new blood vessels. 135 The controlled release of proangiogenic growth factors from the scaffold improves vascularization in the surrounding tissue, resulting in better wound healing. The composite scaffold enabled targeted angiogenic stimulation and the formation of functional vasculature within the regenerated tissue by precisely regulating the release kinetics of these growth factors.
These scaffolds provide a biomimetic microenvironment that closely resembles the natural composition and architecture of skin. They promote cell adhesion, migration, and proliferation, while providing mechanical support and regulating the release of bioactive factors.
Applications in bone tissue engineering
Fibrin offers various scaffolding options for bone tissue engineering. Moreover, synthetic polymers incorporated into scaffolds, such as PLGA, polycaprolactone (PCL), or PVA, support cellular processes and have favorable mechanical properties that contribute to cellular viability. Studies on bone repair in scaffolds with interconnected pore structures that mimic the native bone microenvironment and promote cell infiltration and nutrient diffusion have been the main focus of the current literature. The incorporation of fibrous or nanofibrous structures improves cell adhesion and proliferation, and fibrin creates a biocompatible and biodegradable matrix. The scaffolds are typically made with PCL, PVA, and PLGA, among other materials, and are produced using the methods mentioned before, then, the fibrin precursor solution containing cells will be infiltrated or poured over the porous scaffold.136–139 For instance, Lee et al created an engineered cartilage scaffold complex with cells. 117 By using a gel-pressing technique, they engineered poly (lactide-co-caprolactone) (PLCL) scaffolds, and rabbit bone marrow stromal cells (BMSCs) clusters were added using fibrin-gel infiltration (Figure 3(d)). For up to 8 weeks, they implanted the scaffold into nude mice in order to differentiate chondrocytes, maintain their phenotypes, and increase glycosaminoglycan (GAG) production. All results show that the fibrin-based scaffold successfully repaired segmental bone defects, highlighting its potential as a clinically relevant approach for bone defect treatment. A 3D freeze-dried scaffold made of PVA and fibrin was developed for application in bone repair. The creation of platelet-rich fibrin (PRF)-loaded nano-biphasic calcium phosphate (nBCP)/PVA composites was researched. 140 This demonstrates how low-temperature Robocasting, a form of additive 3D printing, can be used to produce 3D printed BCP/PVA/PRF scaffolds with desired internal structures and bioactive factors to enhance segmental bone repair.
Another approach for bone repair is the use of biodegradable scaffolds. Mesenchymal stem cells and fibrin glue were injected into a biodegradable tricalcium phosphate (TCP) scaffold developed by Yamada et al. for bone repair. The scaffold acts as a carrier for mesenchymal stem cells (MSCs) and facilitates targeted delivery to damaged tissue. 141 The combination of MSCs, fibrin glue, and biodegradable scaffolds improved bone regeneration.
The combination of fibrin with other natural polymers in an injected solution is a novel approach. The use of alginate or collagen, for example, in combination with fibrin, improves the gelation properties, encapsulation, controlled release, or mechanical properties to improve the scaffold’s viability and regeneration.13,142 For example, fibrin-alginate hydrogels can be injected into a poly-ε-caprolactone (PCL) scaffold to treat bone defects. 143 The scaffold has proangiogenic properties because it promotes blood vessel formation and nutrient supply. The mechanical support, bioactivity, and controlled-release characteristics were provided by a combination of PCL, fibrin, and alginate. Additionally, Zhou et al. created alginate-fibrin microbeads to be injected into scaffolds for bone tissue engineering to promote the fast release of stem cells. 144 This study emphasized the importance of cell release kinetics from the scaffold and its influence on cell viability and functionality.
These microbeads had excellent mechanical properties that were comparable to those of natural bone, was biocompatible, allowed the controlled release of bioactive molecules, permitted osteoconductivity and osteoinductivity, and allowed the scaffold to be gradually replaced by new bone. 145 Kohli et al. used calcium phosphate to investigate the potential of composite scaffolds made of fibrin, alginate, and calcium phosphate for bone tissue engineering applications. The combination of the proangiogenic and osteogenic properties of these scaffolds provides an approach for promoting bone regeneration. 146
Other applications in tissue engineering
The possibility of increasing the range of applications of different blends of materials and cell types has led to the development of new hybrid materials that can be used for different cell culture lineages. For instance, a mixture of PCL and calcium monophosphide (CaP) can be used to 3D print lattice scaffolds in which a cell-laden fibrin hydrogel can infiltrate, promoting the proliferation and differentiation of mesenchymal stem cells to the osteogenic lineage. 147 Hokugo et al. incorporated PGA fibers into a fibrin hydrogel and lyophilized the entire construct. The addition of fibers suppresses cell-mediated contraction and does not affect fibroblast viability, showing promise for applications in skin or soft tissue engineering. 148 Using fibrin hydrogels as cell carriers is interesting when homogenizing cell cultures and ensuring cell proliferation in a urethral scaffold that mimics the biomechanical properties of native tissue using blends of PCL and PLCL. 149 The incorporation of a Smart Matrix on the surface of a plasma-polymerized PDMS membrane led to promising results in the preparation of a product for pressure sore treatment or as a dermal scaffold. 150
In renal tissue-engineered models, a PGA electrospun construct was filled with a fibrin precursor solution and gelled inside podocytes and glomerular endothelial cells, modifying its mechanical properties and increasing the long-term proliferation rates of cells. 151 A similar hybrid material made of wet-spun PCL fibers was used to culture human osteosarcoma to produce a hybrid scaffold for hard-tissue engineering. 152 These examples demonstrate versatility in terms of developing or regenerating different tissues and organs, paving the way for personalized regenerative medicine.
Synthetic polymers—fibrin hydrogels
As a different strategy, some authors have modified the properties of fibrin hydrogels. Two strategies can be envisioned for introducing synthetically functionalized polymers during fibrin polymerization. In one case, the synthetic polymer forms a network apart from that formed by fibrin, leading to the formation of an interpenetrating polymer network (IPN), in which both networks coexist simultaneously. In another case, the synthetic polymer interacts with fibrinogen or fibrin monomers to modify the native fibrin network, for example, by polyethylene glycol (PEGylation).
Typically, fibrin participates in the formation of an IPN by forming one of the networks via thrombin activation, whereas the synthetic polymer bears acrylate groups to form its own network via UV-activated polymerization (Figure 4(b)). Polyethylene glycol (PEG) and polyvinyl acetate (PVA) were used to enhance the mechanical properties of IPN, rehydration ratios, and stability. However, the use of UV light and acrylates in combination with cells is a concern in terms of cell viability, and in some cases, excess acrylates inhibit fibrin polymerization.131,153 An IPN based on enzymatic activation (thrombin) and Michael-addition crosslinking between a thiol and an amphiphilic block copolymer (Tetronic T904) was developed by Zhang et al. for the controlled release of non-viral genes. 154 The degradation ratio was controlled by modulating the T904/fibrinogen ratio, which in turn modulated the transfection level.
Figure 4.
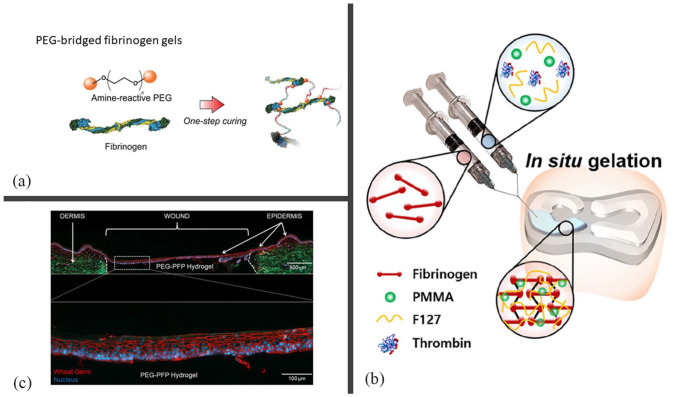
Modification of fibrin hydrogels by synthetic polymers. (a) Process of the formation of a PEGylated hydrogel. First, PEG is functionalized to react in the presence of the amine groups of a protein. Then, the di-functional PEG is added to the fibrinogen leading to a new type of fibrin hydrogel (reprinted from Roberts et al. 51 ). (b) Scheme of an IPN formation of fibrinogen with Pluronic® F-127 (F127) and poly(methy)methacrylate (PMMA) polymers, thanks to thrombin. The hydrogel is formed by a dual-syringe system which dispenses a Fb/F127/PMMA solution and thrombin and fills a meniscal region, (reprinted from An et al. 155 ). (c) The top part shows a polyethylene glycol-platelet free plasma hydrogel treated 4 mm ex vivo explant that was cultured for 14 days. It is stained with wheat germ (red) to visualize the plasma membrane and counterstained with DAPI for nuclei (blue). At the bottom, an enlarged epidermis formed over the PEG-PFP hydrogel is shown (reprinted from Stone et al. 29 ).
Acylate-bearing PEG is used extensively to obtain IPNs in combination with bovine serum albumin (BSA) modified with methacrylate groups (mBSA). Kuten Pella O et al. combined PEGylated albumin and fibrinogen to obtain hydrogels for drug delivery systems, in which it was possible to control the mechanical properties, biocompatibility, and drug release by controlling the PEG, fibrinogen, and albumin content, respectively. 156 Similarly, mPVA and mBSA were combined to obtain IPNs in the presence of fibrin, leading to more stable gels in terms of mechanical properties and degradation, whereas cell viability was enhanced owing to the presence of BSA in comparison with the aforementioned studies on mPVA. 115 As these PEG-BSA IPNs modify the hydrogel microporosity, they could hamper the cellular infiltration required to obtain cultured 3D functional tissues in-vitro; therefore, Gsib et al. proposed further research on obtaining modified fibrin hydrogels with controlled porosity. 157 IPNs modified by these methods are promising because their mechanical properties and biodegradability can be easily tuned by changing their composition, which makes them a good material choice for tissue engineering applications. 158
To avoid these complications in IPN hydrogels, scientists have identified alternative ways to obtain fibrin-based IPNs using different strategies. Recently, Loebel et al. used a combination of supramolecular and covalent networks to enhance the mechanical properties of hydrogels while promoting the proliferation and spreading of cells by means of the dynamic nature of the supramolecular network. 159 As in previous studies, the covalent network was composed of PEGDA and fibrinogen, whereas the supramolecular network was made of hyaluronic acid functionalized with either cyclodextrin or adamantane. Gsib et al. developed an IPN; however, instead of forming both networks simultaneously, they performed it in a sequential manner. They first obtained a hydrogel composed of PEG-BSA, as in previous studies, and lyophilized it to obtain a microporous construct that was subsequently filled with the hydrogel precursor solution to develop a fibrin network in the pores of the previous one. 160 Thus, they avoided the aforementioned porosity modifications while obtaining an enhanced fibrin-based hydrogel.
As real examples for applicability Recently, injectable hydrogel systems based on fibrin have been developed for meniscal regeneration using two different approaches to improve the properties of fibrin. Unlike previous examples, Kim et al. incorporated polyethylene oxide (PEO) or Pluronic F127 (P-F127) as an injectable hydrogel into a fibrinogen solution to form a semi-IPN in the defect area. The incorporation of free PEO in the fibrin hydrogel improved the mechanical properties and apparently enhanced the tissue regeneration and the quality of this regenerated tissue after 12 weeks in rabbits. 161 In contrast, the incorporation of P-F127 and polymethyl methacrylate (PMMA) microbeads into fibrin formulations seems to enhance the mechanical properties, degradation, and tissue regeneration ratios, but particularly improves shear stress to facilitate hydrogel administration in damaged areas. 155 How ever, free PEO, P-F127, and PMMA microbeads were released from the hydrogel with unknown effects when used in vivo. Therefore, further studies are required to confirm these findings.
PEGylated—fibrin hydrogels
PEG is frequently used to obtain modified hydrogels with enhanced cell-culture properties. Basically, difunctionalized PEG is added to a fibrin precursor solution that reacts with native fibrinogen, leading to a new type of fibrin hydrogel. This process is known as PEGylation, and the material is referred to in the literature as a PEGylated fibrin scaffold (Figure 4(a)). Generally, PEG is functionalized with benzotriazole carbonate, succinimidyl glutarate, and acrylate groups to react in the presence of the amine groups of the protein, as in the case of fibrinogen, leading to stable urethane (carbamate), amide, or Michael addition bond, respectively.162,163 These modifications of native fibrinogen before fibrin polymerization increase the long-term stability, mechanical response, and hydrophilicity of fibrin hydrogels. Different cell types, regenerative approaches, and strategies have been tested using PEGylated fibrin hydrogels, which appear to promote cell migration and proliferation without affecting cell viability, or even improve it in some cases.164,165 For example, Figure 4(c) shows re-epithelialization of a 4 mm ex vivo explant into the skin, showing an epidermis completely formed over a wound with a polyethylene glycol-platelet-free plasma (PEG-PFP) hydrogel. Typically, a 1:10 (PEG:fibrin) molar ratio is used for fibrin modification, as this was found to be the best formulation without causing a delay in the gel time. 163 Typically, bifunctional PEGs with an average molecular weight of 3400 Da are used to better control the PEG/fibrinogen ratio. As PEG-fibrin can be modified prior to gelation it can be placed in wounds or injected into damaged areas acting as a vehicle for cells or growth factors.166–168 PEG-fibrin and PEG-PFP hydrogels have been used to heal burn wounds in pigs, preventing wound contraction and reducing the number of neutrophils and macrophages. 30 The same authors investigated wound healing strategies in vitro to test biomaterials on discarded human skin. They found that PEG-PFP hydrogels re-epithelialized the wound area faster and enhanced keratinocyte proliferation, migration, and differentiation compared to collagen and PEG-fibrin hydrogels.
As confirmed by Shpichka et al. using small-angle X-ray scattering (SAXS), the addition of bifunctional PEG to fibrinogen increases the oligomeric species at the beginning of polymerization, thus modifying the hydrogel structure and behavior. 169 Commonly, fibrin hydrogels modified by PEGylation have a microporous structure instead of the native fibrous structure, which is dependent on the fibrinogen:PEG ratio, and increases the storage modulus, transparency of the hydrogel, and vascularization in wounds for full-thickness skin regeneration. 31 Furthermore, Shpichka et al. suggested that the anchoring of PEG to different sites of fibrin molecules could increase cell migration owing to the masking effect of RGD moieties. Gorkun et al. related the formation of capillary- and tubular-like structures of cells inside the hydrogel to the change in microstructure and the aforementioned masking effect. 170 PEGylation also increases the long-term stability of fibrin hydrogels by reducing their degradation rates, thus opening new strategies in ovarian tissue engineering. 171
Because PEGylation is simple to perform, new strategies to understand and improve fibrin hydrogel properties have emerged in recent years. Recently, Pal et al. synthesized bi- or tetra-functionalized biodegradable crosslinkers based on PEG and polypropylene glycol (PPG) for blood plasma modification, which enhanced the mechanical properties and shortened the gel time without compromising the toxicity of plasma-based hydrogels. 172 In addition, Leon-Valdivieso et al. have functionalized PEG with peptides that react specifically to “a” and “b” knobs introducing defects during the fibrin polymerization inside the fibrin fibers. Through this modification, the authors softened the mechanical properties of the gel and studied its effect on fibroblast migration and colonization. 153
PVA—fibrin hydrogels
Polyvinyl alcohol (PVA) is a synthetic polymer belonging to a family of polyvinyl compounds. It is formed by polymerizing vinyl acetate and hydrolyzing it to remove the acetate groups, resulting in polyvinyl alcohol. PVA is a water-soluble polymer with excellent film-forming and adhesive properties. 127
PVA combined with fibrin improves the properties of fibrin hydrogels for wound healing, regeneration, and tissue engineering for several reasons. PVA is a synthetic polymer known and used for excellent mechanical properties including tensile strength and elasticity. When combined with fibrin, which is a natural biopolymer with low mechanical strength, PVA improves the overall mechanical stability of the composite material. This is particularly important for wound dressings and scaffolds because it provides structural integrity and supports healing tissue. 173 Another reason is moisture retention; PVA has hydrophilic properties and maintains a moist environment, which provides a favorable microenvironment for cell migration, proliferation, and tissue regeneration. 174 The biocompatibility and synergistic effects of PVA-based wound dressing materials, which lead to improved cell adhesion, proliferation, and tissue regeneration, combined with the previously mentioned advantages of fibrin, provide a versatile platform for wound healing and tissue engineering applications. Although fibrin contributes to the biocompatibility and biological activity of the composite, PVA improves the mechanical stability, moisture retention, and controlled drug release properties of the material. 173
The synthetic fibrin hydrogel formation of PVA was performed using chemical methods. Xu et al. investigated the effects of adding freeze-dried granule-lyophilized platelet-rich fibrin (G-L PRF) to a PVA hydrogel for wound healing applications. 175 The results demonstrated that the combination of PRF and PVA enhanced cell proliferation, angiogenesis, and wound closure. The incorporation of PRF provided a favorable microenvironment for cell adhesion, migration, and proliferation, whereas the PVA hydrogel acted as a scaffold, providing mechanical stability and facilitating controlled drug release.
When fibrin and PVA are combined, chemical crosslinking can be used to create a stable hydrogel. This method involves the use of crosslinking agents to covalently bond the fibrin and PVA networks. Epichlorohydrin, boric acid, aldehydes, and heavy metal compounds that form complexes with PVA molecules are examples of commonly used crosslinkers. 128 Emulsion template-based fabrication of fibrin/PVA scaffolds for skin tissue engineering was studied. 176 Fibrin and PVA were combined in an emulsion in which one phase was dispersed as droplets within the other phase. Subsequently, glutaraldehyde was used to crosslink the emulsion to create a scaffold structure with interconnected pores, which can be used for skin tissue engineering applications. These results demonstrated that the fibrin/PVA hydrogels possessed interconnected porous structures that were favorable for cell infiltration and nutrient diffusion. Fibrin hydrogels used for skin tissue engineering applications incorporate fibrin to promote cell adhesion and proliferation.
Other synthetic—fibrin hydrogels
Hydrogels made of methacrylated fibrin (MA-fibrin) are an alternative method for synthesizing synthetically modified fibrin hydrogels. Methacrylate groups are added to fibrin molecules to produce methacrylated fibrin. With this modification, fibrin can be crosslinked via photopolymerization to create a stable hydrogel. An innovative biomedical hydrogel was created by Haneen et al. for 3D cell culture or as a biodegradable delivery matrix for in vivo implantation.176,177 Methacrylic anhydride (MAA) was used to denature methacrylate fibrinogen in solution through light-activated free-radical polymerization in the presence of macromolecular crosslinking polymers. The study showed that in 3D cultures of human dermal fibroblasts, hydrogels offer a biocompatible environment that supports cell adhesion, proliferation, and differentiation. Hydrogels also have the potential to act as carriers of therapeutic agents with controlled release, opening up opportunities for precise delivery in tissue engineering and regenerative medicine.
Furthermore, fibrin can be modified by poly(N-isopropylacrylamide) (PNIPAAm), resulting in the formation of a nanogel. 178 PNIPAAm is a thermoresponsive polymer that undergoes a reversible phase transition near the body temperature. “Smart” hydrogels that can go through gel-sol transitions in response to temperature changes can be made by incorporating PNIPAAm into fibrin hydrogels. Owing to its high drug-loading capacity, the authors developed a nanogel system that could deliver two different therapeutic agents: one for promoting angiogenesis and tissue regeneration and the other for inhibiting fibrosis. As a result of the incorporation of PNIPAAm into fibrin hydrogels, this method simultaneously promotes tissue repair and reduces fibrosis.
Another interesting approach involves the use of polyacrylic acid (PAA)- modified fibrin hydrogels. PAA is an electroresponsive polymer that can be incorporated into fibrin hydrogels to introduce electricity-dependent swelling and drug release properties. Because of its increased electrical sensitivity, PAA-modified can to convert environmental stimuli, such as electrical energy, into mechanical forces. This allows for the creation of mechanically stimulating hydrogel-based smart devices that are electrosensitive and biocompatible. 179 Based on these findings, the hydrogel functions as a mechanical pump that guides the alignment of smooth muscle cells under electrical stimulation and facilitates their infiltration and distribution throughout the structure.
Natural polymers—fibrin hydrogels
In recent years, new approaches have been developed to improve fibrin properties by combining fibrin with natural polymers.
Fibrin-collagen hydrogels
Collagen is a major component of the extracellular matrix (ECM) in many tissues and organs and plays a key role in tissue development and function. 180 This material exhibits low immunogenicity, porosity, high permeability, biocompatibility, and biodegradability. However, its poor mechanical properties require crosslinking or modification with natural or synthetic polymers or inorganic materials. Collagen-based materials show higher cell adhesion and proliferation in vitro and are widely combined with fibrin matrices for tissue engineering applications. Earlier studies demonstrated that fibrin-collagen composite networks displayed extra stiffness and durability, improved elasticity, and permissive endothelial network formation in vitro and in vivo, providing support for their use instead of purified collagen and fibrin. 181
For example, graphs showing the variation in the elastic modulus of the hydrogels (Figure 5(a)) show a fibrin network with enhanced mechanical properties in the presence of collagen. Several studies have concluded that the addition of collagen to fibrin improves its mechanical stability. Several studies, such as those conducted by Nilforoushzadeh et al., concluded that adding collagen to fibrin improves mechanical stability. 182 These authors transplanted pre-vascularized hydrogels into five human subjects with diabetic wounds and concluded that fibrin–collagen hydrogels were suitable for skin organotypic cell culture with an increase in skin thickness and density in the vascular beds. Patel et al. adapted fibrin scaffolds, type I collagen hydrogels, and type I collagen fibrin matrices to develop self-manufactured vascular tissue rings and accommodate human fibroblasts to create adventitia vessels. 183 They concluded that a combination of collagen and fibrin was the ideal solution for creating and strengthening engineered adventitial vessels because it exhibited the best overall combination of tensile strength (the most important function for adventitia), cellularity, collagen content, and collagen fiber maturity. Another example is the use of collagen-fibrin hydrogels for cardiac tissue engineering using human iPSC-derived cardiomyocytes. The solution was a balance between the concentration of fibrin, which is associated with increased compaction, and that of collagen, which is associated with decreased compaction. At present, some chemical modifications of collagen are being investigated to improve fibrin cell adhesion and migration and reduce the rapid degradation rate in vivo, for example, collagen was crosslinked with polyethylene glycol ether tetrasuccinimidyl glutarate (4S-StarPEG), chitosan-crosslinked collagen sponges or structural protein collagen modified with genipin, a natural aglycone, that resulted in mechanically stronger hydrogels.109,184,185
Figure 5.
Modification of fibrin hydrogels with natural polymers. (a) Elastic modulus graph of collagen-fibrin mixed hydrogels at different collagen concentrations, adapted from Coradin et al. 186 (b) Proliferation assay of fibroblasts seeded inside plasma and 10% of oxidized alginate hydrogels after (left) 48 h and (right) 7 days, reprinted from Sanz-Horta et al. 187 (c) Hematoxylin & eosin staining of cross-section, of an implantation after 12 days in a mouse, of an hydrogel of fibrin-hyaluronic acid containing red blood cells (represented by red arrows), adapted from Hinsenkamp et al. 188 The presence of fibrin enhances the formation of blood vessels, and the infiltration of cells and extracellular. The use of fibrin was also found to support the biological process of matrix remodeling. (d) Elastin-fibrin hydrogel preparation: Elastin-N3 with plasma and AmchaFibrin is mixed to Elastin-Cyclo with NaCl and CaCl2 at 37°C, adapted from Stojic et al. 189
Fibrin-alginate hydrogels
Alginate is another common polymer that is combined with fibrin hydrogels. 190 It is a biomaterial commonly used in the food industry, pharmacology, and tissue engineering because of its capacity to form physical hydrogels with divalent cationic elements such as Ca2+ and Ba2+. In addition, alginate can form covalent hydrogels through the chemical modification of carboxylic acid or hydroxyl groups, adding new functional groups to react with a crosslinker. Some studies have investigated physical IPNs to combine the desirable adhesion and stimulatory characteristics of fibrin with the tunable mechanical properties of alginate. Vorwald et al. tested and corroborated its efficacy by examining capillary network formation with entrapped co-cultures of mesenchymal stromal cells and endothelial cells; 191 and Shikanov et al. improved the in vitro growth of ovarian follicles using the dynamic cell-responsive mechanical properties of IPNs and provided a fundamental tool for investigation of follicle maturation. 192 In addition, alginate can form covalent hydrogels through the chemical modification of carboxylic acid or hydroxyl groups, adding new functional groups to react with a crosslinker. The most common chemical modification is alginate oxidation. For example, Sanz-Horta et al. developed fibrin-alginate hydrogels using modified alginate dialdehyde (ADA) to produce natural hydrogels incorporating human primary fibroblasts. 187 They demonstrated that fibrin-ADA matrices presented mechanical enhancement compared to fibrin matrices because of the inhibition of fibrin polymerization in the presence of ADA. They also investigated the calcium chloride concentration in the final hydrogel microstructure, which increased the fiber diameter. These results indicated the potential of using these types of hydrogels in tissue engineering (Figure 5(b)). Additionally, Zhou et al. fabricated oxidized alginate-fibrin microbeads encapsulating human umbilical cord mesenchymal stem cells (hUCMSCs) for cell release and observed fast-degradable alginate-fibrin microbeads with excellent proliferation, osteodifferentiation, and bone mineral synthesis. 144 They proposed the delivery of stem cells inside fibrin-ADA matrices to promote bone tissue regeneration. Another example of using chemically modified alginate with fibrin is an investigation in which the authors prepared a triple modification of alginate hydrogels by fibrin blending, iron nanoparticle (Fe-NP) embedding, and serum protein coating (SPC) to improve endothelial cell viability and proliferation. 193 This effect was attributed to the accumulation of agglomerated Fe-NPs and serum proteins along the fibrin fibers as a novel strategy for the modification of various hydrogel-based biomaterials and biomaterial coatings.
Fibrin—hyaluronic hydrogels
Another natural polymer commonly used in the modification of fibrin is hyaluronic acid (HA). 194 Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan found primarily in the extracellular matrix. HA is widely used in tissue engineering owing to its low immunogenicity and biodegradability. Figure 5(c) shows the histological image of an in vivo remodeling-injected hydrogel of fibrin and hyaluronic acid after 12 weeks. HA permits the remodeling process and fibrin permits blood vessel formation, showing red blood cells in the histological cross-section. In other words, hyaluronic-fibrin hydrogels have a positive effect on tissue remodeling.
By combining it with fibrin, an interpenetrating polymer network (IPN) hydrogel can be developed for biomedical applications. For instance, Zhang et al. prepared this IPN using an orthogonal disulfide crosslinking reaction to strengthen the mechanical properties of a fibrin gel and improve its applicability as a hydrogel for tissue engineering applications. 195 They modified by combining thiol-derivatized HA with thrombin and 2-dithiopyridyl-modified HA with fibrinogen and then mixing the obtained liquid formulations with thrombin in Dulbecco’s Modified Eagle Medium (DMEM). The high hydrophilicity of HA prevents compaction of the fibrin network, whereas fibrin provides an adhesive environment for in situ-encapsulated cells. Another approach is the use of hyaluronic acid–tyramine (HA–Tyr) for IPN generation to improve the mechanical properties of fibrin hydrogels. 76 This HA network was formed through the coupling of tyramine moieties using horseradish peroxidase (HRP) and different concentrations of hydrogen peroxide (H2O2) to modify the degree of crosslinking of HA–Tyr. The resulting IPN hydrogel maintained structural support and shape stability, and permitted cell proliferation and capillary formation. In addition to mechanical stabilization and cell proliferation, HA induces mesenchymal stem cell (MSC) osteogenesis in vitro and provides a sufficiently nonadhesive surface that allows cells to move more freely. These interesting characteristics of HA can improve fibrin matrices, 196 which also stimulate appropriate differentiation and articular cartilage regeneration of MSC. Snyder et al. modified hyaluronic acid with methacrylic anhydride (MA) to form a hyaluronic–MA polymer (HA-MA) which reinforced the fibrin hydrogel and improved its mechanical properties. 197 These hydrogels permit bone marrow-derived MSC proliferation and early chondrogenesis as alternative methods for regenerating tissues in osteoarthritis therapy. Another study demonstrated that HA-fibrin IPNs permit the generation of dermoepidermal skin substitutes for tissue engineering. Montero et al. developed HA-fibrin hydrogels that improved the poor mechanical properties of fibrin, enhanced the mechanical properties of fibrin matrices, and allowed primary human fibroblast (hFB) proliferation and primary human keratinocyte (hKC) differentiation. 198 In these studies, HA-fibrin hydrogels were formed from plasma-derived fibrin and thiolated hyaluronic acid (HA-SH) crosslinked with poly(ethylene glycol) diacrylate (plasma/HA-SH-PEGDA).
Fibrin-elastin hydrogels
Some authors have used elastin, another commonly used natural polymer, to reinforce fibrin matrices. Elastin is a vital protein component of the ECM, which is present in many mammalian tissues. It prevents skin contractures, improves scar quality, and enhances elasticity. 199 For example, one study combined plasma-derived fibrin was combined with an elastin-like recombinant (ELR) network to achieve better mechanical properties. 131 (Figure 5(d)); these types of IPN also improved elasticity and increased hKCs proliferation compared to fibrin hydrogel substitutes. ELR-fibrin IPN has also been used in cardiovascular tissue engineering applications, providing a pore-elastic hydrogel that could be used as a heart valve substitute. 200 Tissue analysis revealed the production of collagen I and III, which are fundamental proteins in cardiovascular constructs. Another study combined collagen I and III with IPN fibrin-elastin as a new artificial connective matrix used as an artificial tympanic membrane in rabbits. 201 The results of this study will enable future clinical studies.
Agarose-fibrin hydrogels
Agarose is another natural polymer commonly found in fibrin hydrogels. Agarose is a linear polysaccharide composed of repeating units of agarobiose; its neutral charge, low gelling temperature, and the formation of stable gels with large pore sizes make agarose a good scaffold for containing cells. 202 Agarose is used in multiple hydrogel scaffolds for tissue engineering combined with other polymers such as fibrin, allowing successful tissue fabrication of different biological substitutes with promising ex vivo and in vivo results. There are multiple potential clinical applications of fibrin-agarose hydrogels, including the repair of damaged human organs such as the cornea, bone, and skin. Using a nanostructuring technique, Ionescu et al. bioengineered human corneal stroma composed of fibrin at different agarose concentrations. 203 These new nanostructured corneal constructs were viscoelastic and transparent, similar to native corneas ex vivo. In particular, nanostructured constructs of fibrin with 0.1% agarose exhibited rheological behavior similar to that of native corneas. Their results suggested that this construct may be an effective candidate for the creation of an artificial cornea. It has been demonstrated that agarose is biocompatible and allows for the growth of epithelial and stromal cells, increasing its use in human transplantation. Therefore, a novel substitute for human skin derived from fibrin-agarose biomaterials was developed in this study. The purpose of this study was to isolate and expand dermal fibroblasts and epithelial keratinocytes in fibrin-agarose scaffolds to create a full-thickness human skin construct, which was then grafted onto immunodeficient nude mice to study its functional characteristics. 204 According to these results, artificial skin from fibrin-agarose was biocompatible and had appropriate biomechanical properties, suggesting that these tissues might be able to recreate native skin. The addition of agarose resulted in a significant improvement in biomechanical properties compared to fibrin hydrogels. These interesting characteristics of fibrin-agarose hydrogels stimulate appropriate tissue regeneration.
Martin-Piedra et al. developed nanostructured fibrin-agarose biomaterials to produce bone substitutes with and without adipose-derived mesenchymal stem cells in immunodeficient animal models, improving the morphofunctional aspects of their maxillofacial structures. 205 A preliminary analysis determined that these cellular substitutes could improve the density of the regenerated tissue and provide isolated islands of bone and cartilage. There are preliminary signs that cellular fibrin-agarose substitutes are useful for the treatment of severely critical mandibular bone defects.
Laminin-fibrin hydrogels
The combination of laminin and fibrin hydrogels has emerged as a promising approach for regeneration across different tissues. Laminin is an ECM glycoprotein used to enhance the regenerative properties of fibrin hydrogels. It promotes cell adhesion, migration, and tissue regeneration within the hydrogel by mimicking the natural extracellular matrix environment. By involving cell surface receptors, laminin activates signaling pathways that regulate cellular behavior.206,207 When combined with fibrin, it creates a supportive and bioactive environment for tissue engineering applications, primarily for regenerative processes.
Salazar et al. combined laminin and nidogen, formerly known as entactin, which is an important component of the basement membrane in skeletal muscles, with fibrin in hydrogels. They used these natural hydrogels to regenerate the soft palate and showed that they improved tissue regeneration and reduced fibrosis compared with fibrin hydrogels alone. 208 They also showed that after 56 days of wounding, collagen was deposited and myofibers were formed. Growth factors are delivered by laminin isoforms incorporated into hydrogel platforms to promote osteogenesis and neural growth, both of which are important for tissue regeneration. Several studies have used the laminin-fibrin combination for skeletal muscle regeneration, which is challenging owing to its limited intrinsic regenerative capacity. Furthermore, the fibrin-laminin hydrogel combination promotes the growth of mesenchymal stem cell (MSC) spheroids, providing a supportive environment for MSC spheroids, aiding tissue repair, and promoting functional recovery following trauma to skeletal muscle. 209 Another approach is to incorporate laminin-111, a specific isoform of laminin, into fibrin hydrogels, resulting in highly fibrous scaffolds with progressively thinner interlaced fibers that improve muscle regeneration following trauma by facilitating cellular adhesion, migration, and differentiation.103,210 Fibrin hydrogels containing laminin-111 have also been used to regenerate salivary glands. They facilitate the formation of salivary cell clusters. 211 This combination enhances the organization and differentiation of the parotid gland cells, thereby allowing for the formation of functional tissue structures. Another approach involves the conjugation of laminin-1 peptides with fibrin hydrogels to regenerate irradiated mouse submandibular glands. Nam et al. showed that adding lamnin-1 to fibrin hydrogels promoted the growth of parotid gland cell clusters containing lumens, which aided in the regeneration of salivary gland tissue. 212
In the context of neural regeneration, fibrin hydrogels combined with laminin have shown promising results in guiding the differentiation of human induced pluripotent stem cells (hiPSCs) into mixed dorsal/ventral spinal neuron identities. The fibrin hydrogel provides a three-dimensional environment that mimics the neural tissue, and the presence of laminin directs the differentiation process, enabling the generation of diverse spinal neuron populations. 213
Tri-component hydrogels
In this series of studies, there were several combinations of natural polymers with fibrin-alginate matrices. Figure 6(a) shows the hydrogel injection of collagen, HA, and fibrin into intervertebral disks to regenerate damaged disk tissues. For example, Montalbano et al. developed a tri-component hydrogel using collagen, alginate, and fibrin (CAF) as functional extracellular matrix analogs. 214 CAF combine the excellent biological properties of collagen as an extracellular matrix structural protein, fibrin as a natural regulator of hemostasis and tissue repair, and alginate for controlled protein release and improvement of structural behavior. In this study, CAF showed thermoresponsive crosslinking capacity, cytocompatibility, and good cell proliferation and viability in human mesenchymal stem cells (hMSCs), L929 murine fibroblasts, and pancreatic MIN6 cells (Figure 6(b)). Additionally, Devi et al. explored a novel wound-dressing material composed of alginate, chitosan, and fibrin attached by ionic bonding between the amine groups of chitosan and the carboxyl groups of alginate. 215 Chitosan is a natural polymer that tends to form aggregates in acid conditions, it is known in the wound management field for its hemostatic properties, stimulate cell proliferation and natural healing in tissues, and it is particularly useful for wound treatment. 216 The alginate-chitosan-fibrin matrix exhibited improved mechanical properties and was structurally stable owing to the strong ionic bonding between the amine groups of chitosan and the carboxyl groups of alginate. The composite was selected and applied to the clinical wounds of dogs to determine its efficacy as a wound dressing material. This study is currently in progress.
Figure 6.
Modification of fibrin hydrogels with tri-natural-component hydrogels: (a) scheme of incorporation of collagen-HA-fibrin hydrogels with articular chondrocyte cells into intervertebral disks to regenerate damaged disk tissues, reprinted from Gansau et al. 217 and (b) proliferation assay thanks to Live/dead staining of murine fibroblasts inside of collagen, alginate and fibrin (CAF) hydrogels at different collagen concentrations, adapted from Montalbano et al. 214
Sawadkar et al. combined elastin with collagen and fibrin to create 3D porous scaffolds for tissue regeneration. 218 Human adipose-derived seeded into natural scaffolds composed of these polymers (collagen, fibrin, and elastin) to improve their ability to restore adipose tissue function. They found that natural polymers mimicking the ECM seeded with stem cells affected adipogenesis in vitro and in vivo, opening avenues for the design of 3D grafts for soft tissue repair.
Incorporation of particles in fibrin hydrogels
This section describes different approaches to address aspects related to tissue engineering and regenerative medicine in fibrin hydrogels by incorporating particles that provide hydrogels with properties that add value to this type of application.
Several efforts have been made to deliver biomolecules, such as growth factors or small molecules, from particles incorporated into fibrin hydrogels to prepare controlled delivery systems. Specifically, systems based on chitosan nanoparticles loaded with recombinant human epidermal growth factor (rhEGF) were prepared by Mou et al. to promote cell proliferation and accelerate wound healing in fibrin hydrogels. Nanoparticles prepared by ionotropic gelation were incorporated into a fibrin gel matrix during polymerization. Release studies showed that the fibrin gel loaded with rhEGF-chitosan nanoparticles achieved a more sustained release of rhEGF than either chitosan nanoparticles or an unloaded fibrin gel, and the release rate was controlled by altering the fibrinogen and thrombin contents in this delivery system. 219 Similarly, Alonso et al. prepared nanocapsules of hyaluronic acid (HA) loaded with two different drugs: dexamethasone and a galectin-3 inhibitor. The particles were incorporated into a hydrogel formed in situ based on fibrin and hyaluronic acid, which, containing 30% (v/v) nanocapsules, showed the capacity to control the release of the encapsulated drug for 72 h. In vivo results showed good suppression of inflammatory joints, where the gelation time, rheological properties, and porosity of the system could be adjusted using different parameters. 220 Other efforts have focused on the in vitro evaluation of local antibiotic delivery via fibrin hydrogels by incorporating ciprofloxacin and loading the antibiotic during gelation of the fibrin hydrogel. Fibrin hydrogels tested in vitro demonstrated a promising local antibiotic delivery system for dental use because of their ability to control ciprofloxacin release while maintaining drug efficacy. 221
Other authors have focused on the incorporation of magnetic particles, for example, in the preparation of 3D magnetic biomaterials by encapsulating magnetic nanoparticles and human hyaline chondrocytes within fibrin-agarose hydrogels, with potential applications as articular hyaline cartilage-like tissues. This system improved the rheological properties of hydrogels by incorporating magnetic iron oxide nanoparticles and controlled their swelling capacity without affecting their biocompatibility and expression of collagen type II. 222 In a similar study, magnetic iron oxide nanoparticles were used to improve the mechanical properties and hydrogel crosslinking process, serving as nucleation sites for the attachment of the fibrin polymer via the indirect attraction of fibrinogen through the attached nanoparticles. Because of this attraction, the monomers condense into the nuclei of the dense phase. By the end of the polymerization process, the nuclei (knots) of the dense phase crosslink the fibrin threads, enhancing their mechanical properties. 223 Kazaryan et al. evaluated the influence of non-magnetic iron oxide nanoparticles on fibrin gel formation and its structure, accelerating the rate of fibrin gel formation by activating thrombin. This increase in thrombin concentration or its activation with iron oxide nanoparticles decreases the contribution of the diffusive mode and increases the contribution of the exponent of the power-law function, thereby increasing the complexity of the fibrin gel structure. 224
Fibrin hydrogels incorporating graphene-oxide-based nanocomposites have been prepared using sol-gel methods to produce scaffolds with bone regeneration properties. The nanocomposite with graphene oxide enhanced the porous architecture of the scaffold and mimicked the natural ECM of bone tissue by providing structural integrity, controlled degradation, and osteoinductive potential. In addition, the in vivo results showed that the bone-healing potential of the fabricated scaffolds. 225 The dynamic formation of fibrin fibers for the assembly of reduced graphene oxide (rGO) to prepare multifunctional fibrin-rGO conductive carbon-based bionanocomposites, CBNCs (F-G CBNCs), in a rapid, mild, and one-pot manner was performed by Fu et al. These systems exhibit good formability, flexibility, mechanical strength, stability, adhesiveness to different surfaces, electrochemical activity, and a high immobilization ability for enzymes and nanoparticles. The F-G CBNCs show great potential as a versatile conductive platform to incorporate functional enzymes and PtNPs to develop electrochemical catalysis and sensing applications. 226
Other examples of particles incorporated into fibrin hydrogels include those in the study by Jayakumar et al., who added alginate nanobeads interspersed in a fibrin network to develop a biomimetic bioresorbable injectable system using a dual syringe apparatus (Figure 7(A)). Hydrogels developed using simple nature-inspired crosslinking chemistry exhibit mechanical strength suitable for tissue-engineering constructs for the regeneration of soft tissues. In terms of cytocompatibility, they have good attachment, proliferation, and infiltration within the hydrogel, similar to fibrin (Figure 7(B)). Other authors have incorporated ferulic acid-loaded silica microspheres for antimicrobial wound dressing applications into biomimetic scaffolds based on a fibrin/chitosan/keratin hybrid. The developed hybrid scaffolds showed good thermal, porosity, compression, and water uptake properties; effective antimicrobial activity against common wound pathogens; and great potential for soft tissue engineering applications, particularly for the treatment of chronic and infected wounds. 227
Figure 7.
(A) Scheme and characteristics of interspersion of alginate nanobeads into fibrin hydrogel for soft tissue engineering and (B) Stained mesenchymal stem cells (red): with alginate nanobeads alone at 6 h (a) and 48 h (d), into fibrin composite with alginate nanobeads at 6 h (b) and 48 h (e), and into fibrin network at 6 h (c) and 48 h (f). Scale bar: 5 μm. Reprinted from Deepthi et al. 228
Conclusions and future perspectives
Significant advances in molecular and cellular biology in recent decades have expanded our understanding of the role of fibrin in relevant physiopathological processes, such as wound healing, blood clotting, fibrinolysis, cellular-matrix interactions, inflammatory response, angiogenesis, neoplasia, and tissue remodeling. These properties have made fibrin one of the most interesting materials for bioengineering and biomedical applications, some of which (as sealants, biological adhesives, or hemostats in surgeries; see Table 1) have commercial clinical applications. In addition, some of their properties such as biocompatibility, biodegradability, easy and controllable polymerization, promotion of cell adhesion and growth, and ease of modification have made fibrin hydrogels one of the most investigated and used scaffolds in tissue engineering. However, fibrin hydrogels have multiple limitations, such as early degradation of the hydrogel by plasminogen and metalloproteinases secreted by the cells, low Young’s modulus of the scaffolds, rapid contraction of the scaffolds, and batch-to-batch variability. Therefore, new experimental strategies have emerged in recent years to combine the excellent properties of fibrin with improvements in hydrogel characteristics. We have focused on methods such as combining fibrin hydrogels with solid composite scaffolds, preparing chemically modified fibrin hydrogels, generating interpenetrated polymer networks, and incorporating particles in a wide range of different and new approaches.
One interesting alternative is the infiltration of fibrin hydrogels into solid polymeric scaffolds owing to the excellent cell signaling and proliferation properties arising from fibrin and the controllable mechanical properties, degradation rates, easy handling, production, and affordable availability of synthetic polymers. These hybrid scaffolds can be created by infiltrating a fibrin precursor solution into a synthetic polymeric scaffold created by freeze-drying using phase separation, electrospinning, or 3D printing techniques. The use of PLGA scaffolds filled with fibrin gel or the 3D-printing of a PCL cage surrounding a fibrin hydrogel as cartilage tissue scaffolding are two examples of the many applications of these types of hybrid scaffolds in tissue engineering. In cartilage tissue engineering, hybrid scaffolds promote chondrocyte differentiation, ECM production, and cartilage regeneration in vivo. In cardiac tissue engineering, the combination of fibrin hydrogels and synthetic polymeric scaffolds enhances cell spreading, proliferation, and survival. Similarly, the incorporation of PGA fibers into fibrin hydrogels has shown promise in skin and soft tissue engineering by suppressing cell-mediated contraction without affecting fibroblast viability. Scaffolds of PCL, PVA, and PLGA with fibrin precursors and cells have been used to create a solution for bone repair. This approach modifies the mechanical properties of the scaffold and improves the long-term cell proliferation rates, offering potential advancements in the development of bone tissue models. Additionally, wet-spun poly(-caprolactone) (PCL) fibers combined with fibrin have been used to culture human osteosarcoma cells to create hybrid scaffolds for hard-tissue engineering. Other interesting strategies include combining PCL and calcium monophosphide scaffolds with fibrin to favor osteogenesis, developing a urethral scaffold with fibrin using PCL and PLCL, or studying renal tissue-engineered models using PGA electrospun constructs filled with fibrin. Although fibrin-polymer composite scaffolds have shown promising results, there are still some limitations. On the one hand, it is necessary to find a solution to the poor mechanical stability of fibrin, but, on the other hand, the mechanical improvement of the scaffold could inhibit, even drastically, the degradation rate (highly dependent on the local environment). 229 It is clearly necessary to optimize the balance between the mechanical properties and biological properties of the scaffold to control the degradation rate of the composite scaffold. Another limitation is the difficulty in sterilizing the solid scaffolds: it could promote bacterial contamination and, with different sterilization techniques, it could suffer degeneration due to high temperature and pressure. 230 Some researchers have suggested the incorporation of growth factors and other biomolecules to enhance the bioactivity of hybrid scaffolds, leading to better cell differentiation and tissue regeneration.231,232
Another alternative for increasing the long-term stability, improving the mechanical response, and controlling the biodegradability of fibrin hydrogels is to modify their properties by introducing synthetic functionalized polymers during fibrin polymerization, resulting in an interpenetrated polymer network or a modified native fibrin network, as in the case of PEGylation, making them suitable for tissue engineering applications. PEG -fibrin hydrogels have been used by multiple researchers to treat burn wounds and promote wound healing in vivo, improve vascularization, stimulate cell migration, and lengthen the stability of fibrin hydrogels. Although these modified fibrin hydrogels show promise for various applications in tissue engineering, further research is needed to evaluate their effects on cells, both in vitro and in vivo, because there are several limitations associated with the use of synthetically modified fibrin hydrogels. The main drawback is the limited control over the degradation rate in the resulting PEGylated fibrin hydrogels: the degradation rate of pegylated fibrin hydrogels is typically slower than that of the unmodified fibrin hydrogels and may be more difficult to control, which limits its use in some tissue applications requiring high mechanical strength. 233 The limited cell adhesion and proliferation is also a limitation, while fibrin hydrogels are generally biocompatible and support cell adhesion and proliferation, PEGylation can reduce these properties and negatively affect the cell behaviorDisadvantages include limited cell adhesion and proliferation, whereas fibrin hydrogels are generally biocompatible and support cell adhesion and proliferation, PEGylation can reduce these properties and negatively affect cell behavior.234,235 Further research is required to optimize these parameters and evaluate the long-term stability and functionality of tissue-engineered constructs.
Another approach involves the combination of PVA and fibrin hydrogels, which offer several advantages in wound healing, tissue engineering, and regenerative medicine. The incorporation of PVA also improves the mechanical stability of the hydrogel, providing tensile strength and elasticity to the composite material. Additionally, biocompatibility of PVA, combined with the benefits of fibrin, enhances cell adhesion, proliferation, and tissue regeneration. The controlled drug-release properties of PVA further add versatility to the composite material, allowing for targeted therapeutic delivery. However, there are drawbacks to consider; the slow biodegradability of PVA can hinder natural tissue remodeling, and its synthetic nature may induce an immune response. 236
Alternative methods include the use of methacrylated fibrin (MA-fibrin) or fibrin modification with polymers such as poly(N-isopropylacrylamide) (PNIPAAm) or poly(acrylic acid) (PAA). Methacrylation allows for photopolymerization-based crosslinking, creating a stable hydrogel suitable for 3D cell culture and the controlled release of therapeutic agents. PNIPAAm exhibits thermoresponsive properties, enabling gel-sol transitions in response to temperature changes. The PAA modification imparts electrical responsiveness, allowing for electrosensitive swelling and drug release.
Another interesting approach is to modify the fibrin network with natural polymers such as alginate, collagen, or elastin to improve the mechanical properties (contraction, Young’s modulus, and degradation) of fibrin for different tissue applications. For example, collagen hydrogels demonstrate higher cell adhesion and proliferation in vitro, as well as improved mechanical stability, giving collagen-fibrin hydrogels an advantage in tissue applications such as diabetic wounds and vascular and cardiac tissues. The combination of fibrin and collagen has demonstrated increased stiffness and durability, improved elasticity, and permissiveness for endothelial network formation, both in vitro and in vivo, making it a suitable material for tissue engineering applications. However, the mechanical strength of hydrogels is an important limitation, as it can be influenced by the ratio of fibrin to collagen and the crosslinking method used. Although some studies have reported that the addition of collagen to fibrin hydrogels can improve their mechanical properties, there is still room for improvement to achieve optimal stiffness and elasticity for different tissue types. 237 Additionally, some drawbacks have been reported in the application of these types of hydrogels, such as the low mechanical strength and low osteogenicity of collagen, limiting their application in bone regeneration 238 ; thrombogenic properties and in vivocalcification in cardiovascular repair 238 ; and the lack of revascularization in multilayered tissues. 239
The combination of fibrin and alginate has also shown promise in promoting the growth of ovarian follicles and bone tissue regeneration. The addition of modified alginate dialdehyde to fibrin resulted in the mechanical enhancement of hydrogels and the potential for its use in tissue engineering applications incorporating human primary fibroblasts. However, it is important to consider the limited stability and rapid degradation. Alginate is susceptible to rapid degradation by different alginate lyases, G or M block–specific lyases, which can cause a loss of mechanical stability and degradation of encapsulated bioactive molecules.192,240 Another natural polymer commonly used in fibrin modification is hyaluronic acid (HA), which allows the generation of dermoepidermal skin substitutes, fibroblast proliferation, and keratinocyte differentiation. However, the mechanical properties of HA-fibrin hydrogels are highly dependent on their composition and crosslinking density. For example, hyaluronic acid-fibrin hydrogels may not be strong enough for load-bearing applications such as cartilage repair. 235 Another limitation is hyaluronidase, which exists in cells and serum and degrades hyaluronate, the salt form of hyaluronic acid. 241 Furthermore, elastin, a vital protein component of the ECM that prevents skin contractures, improves scar quality, increases elasticity, and can be used to reinforce fibrin matrices. Agarose is another common natural polymer found alongside fibrin hydrogels, and nanostructured fibrin-agarose biomaterials have been developed as bone substitutes. The combination of laminin and fibrin hydrogels is another promising approach to tissue regeneration. Laminin enhances cell adhesion, migration, and tissue regeneration, and this combination has shown positive outcomes in skeletal muscle, salivary gland, and neural tissue regeneration. Laminin isoforms incorporated into the hydrogel platform deliver growth factors that promote osteogenesis in skeletal muscle tissues and facilitate the differentiation of stem cells into spinal neuronal populations in neural tissues. Finally, in this series of studies, several combinations of natural polymers with fibrin scaffolds, including collagen, alginate, and fibrin, were used with thermoresponsive crosslinking capacity for the proliferation of mesenchymal stem cells, alginate, chitosan, and fibrin for wound treatment, and elastin with collagen and fibrin to create 3D porous hydrogels for tissue regeneration. These modifications provide an opportunity to develop interpenetrating polymer network hydrogels for various biomedical applications, such as tissue engineering, drug delivery, and wound healing.
Furthermore, other natural components, such as elastin, agarose, and even scaffolds with thermoresponsive crosslinking capacity, have been used in different studies (e.g. wound treatment, bone substitutes), and improvements over fibrin-only hydrogels have been reported. Clearly, this variety of possible modifications and combinations provides many opportunities to develop interpenetrating polymer network hydrogels for various biomedical applications, such as tissue engineering, drug delivery, and wound healing. This approach is likely to be widely explored in the future.
Finally, incorporating particles into fibrin hydrogels has become a promising approach for tissue engineering and regenerative medicine. Different types of particles such as chitosan nanoparticles, hyaluronic acid nanocapsules, magnetic nanoparticles, graphene oxide-based nanocomposites, and silica microspheres have been incorporated into fibrin hydrogels to provide controlled delivery of bioactive molecules, improve mechanical properties, enhance biocompatibility, and promote tissue regeneration. Moreover, the properties of these hydrogels can be tuned by varying parameters such as the concentrations of particles, fibrinogen, thrombin, and gelation time. However, this approach has certain limitations. First, the incorporation of the particles may affect the porosity and degradation rate of the hydrogel. For instance, the addition of hyaluronic acid-loaded nanocapsules to a fibrin-hyaluronic acid hydrogel controlled the release of the encapsulated drug but also reduced the porosity of the hydrogel. 228 Moreover, the cytotoxicity of some particles and the potential immune responses that they may induce should be considered. Furthermore, optimization of the incorporation of particles into fibrin hydrogels requires a thorough understanding of the physicochemical properties of the particles and their interactions with fibrinogen and thrombin for potential clinical applications.224,228 In summary, the incorporation of particles into fibrin hydrogels provides a versatile and promising approach for the development of advanced tissue engineering and regenerative medicine applications.
In the future, fibrin will become a highly interesting biomaterial, particularly for the development of new experimental strategies in tissue engineering. However, it currently has limitations that make it a complex material, particularly if hydrogels are obtained by plasma polymerization, which temporarily entraps many of its components in the hydrogel and is challenging to handle. Fortunately, as we have demonstrated in this article, different experimental approaches based on the use of scaffolds formed from modified fibrin and hybrid scaffolds containing fibrin and other natural and artificial polymers are being explored. Although the reported results are promising, at least two factors need to be considered: (1) Most of these results have been obtained in in vitro experiments, and in some cases, the products have been transplanted into animals, mainly mice, which are far from clinical use. (2) Given the different structural and functional characteristics of the diverse types of tissues for which tissue engineering strategies are being developed, it seems clear that ad hoc solutions must be developed for each application.
However, the products and approaches described in this article have increasing applications in the economically important field of testing pharmaceutical, cosmetic, and chemical products for human use. For ethical, regulatory, and efficacy reasons, these industries require the development of products that are more robust and similar to human tissues than the existing ones. Therefore, they need to adapt to new technologies such as 3D bioprinting and tissues/organs-on-a-chip, which will open new challenges and opportunities.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Programa de Actividades de I + D entre Grupos de Investigación de la Comunidad de Madrid, S2018/BAA-4480, Biopieltec-CM, Programa Estatal de I + D + i Orientada a los Retos de la Sociedad, RTI2018-101627-B-I00, Proyectos de Generación de Conocimiento 2021, PID2021-126523OB-I00, Proyectos en colaboración público-privada 2021, CPP2021-008396, LOLICOMB Project, PID2020-116439GB-I00 and Cátedra Fundación Ramón Areces. Grant PID2021-126523OB-I00 funded by MCIN/AEI/ 10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe.” Grant CPP2021-008396 funded by MCIN/AEI/ 10.13039/501100011033 and by the European Union “NextGenerationEU/PRTR.”
ORCID iDs: Ana Matesanz  https://orcid.org/0000-0001-7135-9422
https://orcid.org/0000-0001-7135-9422
Diego Velasco  https://orcid.org/0000-0002-1531-1595
https://orcid.org/0000-0002-1531-1595
References
- 1. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005; 3: 1894–1904. [DOI] [PubMed] [Google Scholar]
- 2. Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2006; 936: 355–367. [DOI] [PubMed] [Google Scholar]
- 3. Bailey K, Astbury WT, Rudall KM. Fibrinogen and fibrin as members of the keratin-myosin group. Nature 1943; 151: 716–717. [Google Scholar]
- 4. Weisel JW. Fibrinogen and fibrin. Advances in protein chemistry, 70, 247–299. [DOI] [PubMed] [Google Scholar]
- 5. Bayer IS. Advances in fibrin-based materials in wound repair: a review. Molecules 2022; 27: 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem 2017; 82: 405–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mantha S, Pillai S, Khayambashi P, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019; 12: 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract 2013; 2013: 316–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosso F, Marino G, Giordano A, et al. Smart materials as scaffolds for tissue engineering. J Cell Physiol 2005; 203: 465–470. [DOI] [PubMed] [Google Scholar]
- 10. Reddy MSB, Ponnamma D, Choudhary R, et al. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021; 13: 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng K, Gu Q, Zhou D, et al. Recent progress in surgical adhesives for biomedical applications. Smart Mater Med 2022; 3: 41–65. [Google Scholar]
- 12. Barsotti MC, Felice F, Balbarini A, et al. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem 2011; 58: 301–310. [DOI] [PubMed] [Google Scholar]
- 13. Noori A, Ashrafi SJ, Vaez-Ghaemi R, et al. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomedicine 2017; 12: 4937–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weisel JW, Medved L. The structure and function of the αC domains of fibrinogen. Ann N Y Acad Sci 2001; 936: 312–327. [DOI] [PubMed] [Google Scholar]
- 15. Belcher HA, Litwa K, Guthold M, et al. The applicability of current turbidimetric approaches for analyzing fibrin fibers and other filamentous networks. Biomolecules 2022; 12: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crawley JT, Zanardelli S, Chion CK, et al. The central role of thrombin in hemostasis. J Thromb Haemost 2007; 5 Suppl 1: 95–101. [DOI] [PubMed] [Google Scholar]
- 17. Duval C, Profumo A, Aprile A, et al. Fibrinogen αC-regions are not directly involved in fibrin polymerization as evidenced by a “Double-Detroit” recombinant fibrinogen mutant and knobs-mimic peptides. J Thromb Haemost 2020; 18: 802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Litvinov RI, Gorkun OV, Galanakis DK, et al. Polymerization of fibrin: direct observation and quantification of individual B:b knob-hole interactions. Blood 2007; 109: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bucay I, O'Brien Et, 3rd, Wulfe SD, et al. Physical determinants of fibrinolysis in single fibrin fibers. PLoS One 2015; 10: e0116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenger-Eriksen C, Christiansen K, Laurie J, et al. Fibrinogen concentrate and cryoprecipitate but not fresh frozen plasma correct low fibrinogen concentrations following in vitro haemodilution. Thromb Res 2013; 131: e210–e213. [DOI] [PubMed] [Google Scholar]
- 21. Roberts JR. Roberts and Hedges’ clinical procedures in emergency medicine and acute care E-book. Amsterdam: Elsevier Health Sciences, 2017. [Google Scholar]
- 22. Meyer MA, Ostrowski SR, Windeløv NA, et al. Fibrinogen concentrates for bleeding trauma patients: what is the evidence? Vox Sang 2011; 101: 185–190. [DOI] [PubMed] [Google Scholar]
- 23. Leung WW-F. Centrifugal separations in biotechnology. Oxford: Butterworth-Heinemann, 2020. [Google Scholar]
- 24. Aurora A, Wrice N, Walters TJ, et al. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss. Acta Biomater 2018; 65: 150–162. [DOI] [PubMed] [Google Scholar]
- 25. Chellini F, Tani A, Zecchi-Orlandini S, et al. Influence of platelet-rich and platelet-poor plasma on endogenous mechanisms of skeletal muscle repair/regeneration. Int J Mol Sci 2019; 20: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 2014; 7: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 2001; 10: 225–228. [DOI] [PubMed] [Google Scholar]
- 28. D’Amico R, Malucelli C, Uccelli A, et al. Therapeutic arteriogenesis by factor-decorated fibrin matrices promotes wound healing in diabetic mice. J Tissue Eng 2022; 13: 20417314221119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone R, Wall JT, Natesan S, et al. PEG-plasma hydrogels increase epithelialization using a human ex vivo skin model. Int J Mol Sci 2018; 19: 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burmeister DM, Roy DC, Becerra SC, et al. In situ delivery of fibrin-based hydrogels prevents contraction and reduces inflammation. J Burn Care Res 2018; 39: 40–53. [DOI] [PubMed] [Google Scholar]
- 31. Natesan S, Stone R, Coronado RE, et al. PEGylated platelet-free blood plasma-based hydrogels for full-thickness wound regeneration. Adv Wound Care 2019; 8: 323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isbister JP, Shander A, Spahn DR, et al. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev 2011; 25: 89–101. [DOI] [PubMed] [Google Scholar]
- 33. Yang L, Stanworth S, Baglin T. Cryoprecipitate: an outmoded treatment? Transfus Med 2012; 22: 315–320. [DOI] [PubMed] [Google Scholar]
- 34. Collins PW, Solomon C, Sutor K, et al. Theoretical modelling of fibrinogen supplementation with therapeutic plasma, cryoprecipitate, or fibrinogen concentrate. Br J Anaesth 2014; 113: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong H, Curry N. Do we need cryoprecipitate in the era of fibrinogen concentrate and other specific factor replacement options? ISBT Sci Ser 2018; 13: 23–28. [Google Scholar]
- 36. Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014; 54: 1389–1405. [DOI] [PubMed] [Google Scholar]
- 37. Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus 2012; 10: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brennan M. Fibrin glue. Blood Rev 1991; 5: 240–244. [DOI] [PubMed] [Google Scholar]
- 39. Barton B, Moore EE, Pearce WH. Fibrin glue as a biologic vascular patch–a comparative study. J Surg Res 1986; 40: 510–513. [DOI] [PubMed] [Google Scholar]
- 40. Mintz PD, Mayers L, Avery N, et al. Fibrin sealant: clinical use and the development of the University of Virginia Tissue Adhesive Center. Ann Clin Lab Sci 2001; 31: 108–118. [PubMed] [Google Scholar]
- 41. Spotnitz WD. Fibrin sealant: the only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg 2014; 2014: 203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson MR. Fibrin sealants in surgical practice: an overview. Am J Surg 2001; 182: 1S–7S. [DOI] [PubMed] [Google Scholar]
- 43. Beudert M, Gutmann M, Lühmann T, et al. Fibrin sealants: challenges and solutions. ACS Biomater Sci Eng 2022; 8: 2220–2231. [DOI] [PubMed] [Google Scholar]
- 44. Yamamoto KT, DeJoseph LM. Efficacy and safety of artiss fibrin tissue sealant use in rhytidectomy: a review of 120 cases. Surg J 2017; 3: e69–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ofikwu GI, Sarhan M, Ahmed L. EVICEL glue-induced small bowel obstruction after laparoscopic gastric bypass. Surg Laparosc Endosc Percutan Tech 2013; 23: e38–e40. [DOI] [PubMed] [Google Scholar]
- 46. McKeage K. Raplixa™: a review in improving surgical haemostasis. Clin Drug Investig 2015; 35: 519–524. [DOI] [PubMed] [Google Scholar]
- 47. Siedentop KH, Park JJ, Shah AN, et al. Safety and efficacy of currently available fibrin tissue adhesives. Am J Otolaryngol 2001; 22: 230–235. [DOI] [PubMed] [Google Scholar]
- 48. Matonick JP, Hammond J. Hemostatic efficacy of EVARREST™, fibrin sealant patch vs. TachoSil® in a heparinized swine spleen incision model. J Invest Surg 2014; 27: 360–365. [DOI] [PubMed] [Google Scholar]
- 49. Simo KA, Hanna EM, Imagawa DK, et al. Hemostatic agents in hepatobiliary and pancreas surgery: a review of the literature and critical evaluation of a novel carrier-bound fibrin sealant (TachoSil). ISRN Surg 2012; 2012: 729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lavine G. (2009). HHS action plan to prevent healthcare-associated infections. American Journal of Health-System Pharmacy 2009; 66: 428–430. [DOI] [PubMed] [Google Scholar]
- 51. Roberts IV, Bukhary D, Valdivieso CYL, et al. Fibrin matrices as (injectable) biomaterials: formation, clinical use, and molecular engineering. Macromol Biosci 2020; 20: e1900283. [DOI] [PubMed] [Google Scholar]
- 52. Doria C, Vaccino S. Topical hemostasis: a valuable adjunct to control bleeding in the operating room, with a special focus on thrombin and fibrin sealants. Expert Opin Biol Ther 2009; 9: 243–247. [DOI] [PubMed] [Google Scholar]
- 53. Bolhari B, Meraji N, Ghorbanzadeh A, et al. Applications of fibrin-based products in Endodontics: a literature review. Dent Hypotheses 2019; 10: 85. [Google Scholar]
- 54. Tan J, Li L, Wang H, et al. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mater Sci Eng C 2021; 121: 111749. [DOI] [PubMed] [Google Scholar]
- 55. Kupcsik L, Alini M, Stoddart MJ. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng Part A 2009; 15: 2309–2313. [DOI] [PubMed] [Google Scholar]
- 56. Demol J, Eyckmans J, Roberts SJ, et al. Does tranexamic acid stabilised fibrin support the osteogenic differentiation of human periosteum derived cells? Eur Cell Mater 2011; 21: 272–285. [DOI] [PubMed] [Google Scholar]
- 57. Ahmed TA, Griffith M, Hincke M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng 2007; 13: 1469–1477. [DOI] [PubMed] [Google Scholar]
- 58. Lee E, Vaughan DE, Parikh SH, et al. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ Res 1996; 78: 44–49. [DOI] [PubMed] [Google Scholar]
- 59. Chauhan S, Kumar BA, Rao BH, et al. Efficacy of aprotinin, epsilon aminocaproic acid, or combination in cyanotic heart disease. Ann Thorac Surg 2000; 70: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 60. Chauhan S, Gharde P, Bisoi A, et al. A comparison of aminocaproic acid and tranexamic acid in adult cardiac surgery. Ann Card Anaesth 2004; 7: 40. [PubMed] [Google Scholar]
- 61. Park CH, Oh JH, Jung H-M, et al. Effects of the incorporation of ε-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater 2017; 61: 134–143. [DOI] [PubMed] [Google Scholar]
- 62. Chaires-Rosas CP, Ambriz X, Montesinos JJ, et al. Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton’s jelly cultured in a fibrin hydrogel. J Tissue Eng 2019; 10: 2041731419840622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yakovlev S, Medved L. Effect of fibrinogen, fibrin, and fibrin degradation products on transendothelial migration of leukocytes. Thromb Res 2018; 162: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eyrich D, Brandl F, Appel B, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007; 28: 55–65. [DOI] [PubMed] [Google Scholar]
- 65. Gandhi JK, Manzar Z, Bachman LA, et al. Fibrin hydrogels as a xenofree and rapidly degradable support for transplantation of retinal pigment epithelium monolayers. Acta Biomater 2018; 67: 134–146. [DOI] [PubMed] [Google Scholar]
- 66. Nazari B, Kazemi M, Kamyab A, et al. Fibrin hydrogel as a scaffold for differentiation of induced pluripotent stem cells into oligodendrocytes. J Biomed Mater Res Part B Appl Biomater 2020; 108: 192–200. [DOI] [PubMed] [Google Scholar]
- 67. Chiti MC, Dolmans MM, Donnez J, et al. Fibrin in reproductive tissue engineering: a review on its application as a biomaterial for fertility preservation. Ann Biomed Eng 2017; 45: 1650–1663. [DOI] [PubMed] [Google Scholar]
- 68. Robinson S, Parigoris E, Chang J, et al. Contracting scars from fibrin drops. Integr Biol 2022; 14: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Melly L, Grosso A, Stanciu Pop C, et al. Fibrin hydrogels promote scar formation and prevent therapeutic angiogenesis in the heart. J Tissue Eng Regen Med 2020; 14: 1513–1523. [DOI] [PubMed] [Google Scholar]
- 70. Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013; 304: C216–C225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen 2007; 15: S46–S53. [DOI] [PubMed] [Google Scholar]
- 72. Keane TJ, Horejs C-M, Stevens MM., Scarring vs. Functional healing: matrix-based strategies to regulate tissue repair. Adv Drug Deliv Rev 2018; 129: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leask A, Parapuram SK, Shi-wen X, et al. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal 2009; 3: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tuan T-L, Song A, Chang S, et al. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res 1996; 223: 127–134. [DOI] [PubMed] [Google Scholar]
- 75. Montero A, Acosta S, Hernández R, et al. Contraction of fibrin-derived matrices and its implications for in vitro human skin bioengineering. J Biomed Mater Res A 2021; 109: 500–514. [DOI] [PubMed] [Google Scholar]
- 76. Lee F, Kurisawa M. Formation and stability of interpenetrating polymer network hydrogels consisting of fibrin and hyaluronic acid for tissue engineering. Acta Biomater 2013; 9: 5143–5152. [DOI] [PubMed] [Google Scholar]
- 77. Murphy KC, Whitehead J, Zhou D, et al. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater 2017; 64: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yue H, Pathak JL, Zou R, et al. Fabrication of chondrocytes/chondrocyte-microtissues laden fibrin gel auricular scaffold for microtia reconstruction. J Biomater Appl 2021; 35: 838–848. [DOI] [PubMed] [Google Scholar]
- 79. Baniasadi H, Mashayekhan S, Fadaoddini S, et al. Design, fabrication and characterization of oxidized alginate-gelatin hydrogels for muscle tissue engineering applications. J Biomater Appl 2016; 31: 152–161. [DOI] [PubMed] [Google Scholar]
- 80. Aguilar-Ayala FJ, Aguilar-Pérez FJ, Nic-Can GI, et al. A molecular view on biomaterials and dental stem cells interactions: literature review. Appl Sci 2022; 12: 5815. [Google Scholar]
- 81. Geer DJ, Swartz DD, Andreadis ST. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng 2002; 8: 787–798. [DOI] [PubMed] [Google Scholar]
- 82. Guthold M, Liu W, Sparks EA, et al. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys 2007; 49: 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Collet J-P, Shuman H, Ledger RE, et al. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci 2005; 102: 9133–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Linnes MP, Ratner BD, Giachelli CM. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 2007; 28: 5298–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sierra DH, Eberhardt AW, Lemons JE. Failure characteristics of multiple-component fibrin-based adhesives. J Biomed Mater Res 2002; 59(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 86. Juliar BA, Keating MT, Kong YP, et al. Sprouting angiogenesis induces significant mechanical heterogeneities and ECM stiffening across length scales in fibrin hydrogels. Biomaterials 2018; 162: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim J-S, Kim TH, Kang DL, et al. Chondrogenic differentiation of human ASCs by stiffness control in 3D fibrin hydrogel. Biochem Biophys Res Commun 2020; 522: 213–219. [DOI] [PubMed] [Google Scholar]
- 88. Ho ST, Cool SM, Hui JH, et al. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2010; 31: 38–47. [DOI] [PubMed] [Google Scholar]
- 89. Tanaka R, Saito Y, Fujiwara Y, et al. Preparation of fibrin hydrogels to promote the recruitment of anti-inflammatory macrophages. Acta Biomater 2019; 89: 152–165. [DOI] [PubMed] [Google Scholar]
- 90. Deller RC, Richardson T, Richardson R, et al. Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation. Nat Commun 2019; 10: 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nair CH, Shah GA, Dhall DP. Effect of temperature, pH and ionic strength and composition on fibrin network structure and its development. Thromb Res 1986; 42: 809–816. [DOI] [PubMed] [Google Scholar]
- 92. Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods 2016; 13: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Namani R, Wood MD, Sakiyama-Elbert SE, et al. Anisotropic mechanical properties of magnetically aligned fibrin gels measured by magnetic resonance elastography. J Biomech 2009; 42: 2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Montana M, Tabélé C, Curti C, et al. Organic glues or fibrin glues from pooled plasma: efficacy, safety and potential as scaffold delivery systems. J Pharm Pharm Sci 2012; 15: 124–140. [DOI] [PubMed] [Google Scholar]
- 95. Gruber HE, Leslie K, Ingram J, et al. Cell-based tissue engineering for the intervertebral disc: in vitro studies of human disc cell gene expression and matrix production within selected cell carriers. Spine J 2004; 4: 44–55. [DOI] [PubMed] [Google Scholar]
- 96. Demol J, Lambrechts D, Geris L, et al. Towards a quantitative understanding of oxygen tension and cell density evolution in fibrin hydrogels. Biomaterials 2011; 32: 107–118. [DOI] [PubMed] [Google Scholar]
- 97. Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 521–528. [DOI] [PubMed] [Google Scholar]
- 98. Ma TM, VanEpps JS, Solomon MJ. Structure, mechanics, and instability of fibrin clot infected with Staphylococcus epidermidis. Biophys J 2017; 113: 2100–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kambic H, Murabayashi S, Nose Y. Biomaterials in artificial organs. Chem Eng News 1986; 64: 30–48. [Google Scholar]
- 100. Billiet T, Vandenhaute M, Schelfhout J, et al. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012; 33: 6020–6041. [DOI] [PubMed] [Google Scholar]
- 101. Rowe SL, Lee S, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater 2007; 3: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kurniawan NA, van Kempen THS, Sonneveld S, et al. Buffers strongly modulate fibrin self-assembly into fibrous networks. Langmuir 2017; 33: 6342–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jarrell DK, Vanderslice EJ, Lennon ML, et al. Increasing salinity of fibrinogen solvent generates stable fibrin hydrogels for cell delivery or tissue engineering. PLoS One 2021; 16: e0239242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marcinczyk M, Elmashhady H, Talovic M, et al. Laminin-111 enriched fibrin hydrogels for skeletal muscle regeneration. Biomaterials 2017; 141: 233–242. [DOI] [PubMed] [Google Scholar]
- 105. Bode T, Höltje K, Leal-Marin S, et al. Evaluation and implementation of biocompatible methods for the cross-linking of plasma proteins. Curr Dir Biomed Eng 2021; 7: 187–190. [Google Scholar]
- 106. Furlan M, Beck EA. Cross-linking of human fibrinogen with glutaraldehyde and tetranitromethane. Thromb Res 1975; 7: 827–838. [DOI] [PubMed] [Google Scholar]
- 107. Karimi F, Biazar E, Heidari-Keshel S, et al. Platelet-rich fibrin (PRF) gel modified by a carbodiimide crosslinker for tissue regeneration. RSC Adv 2022; 12: 13472–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Grasman JM, Page RL, Dominko T, et al. Crosslinking strategies facilitate tunable structural properties of fibrin microthreads. Acta Biomater 2012; 8: 4020–4030. [DOI] [PubMed] [Google Scholar]
- 109. Gamboa-Martínez TC, Luque-Guillén V, González-García C, et al. Crosslinked fibrin gels for tissue engineering: two approaches to improve their properties. J Biomed Mater Res A 2015; 103: 614–621. [DOI] [PubMed] [Google Scholar]
- 110. Roacho-Pérez JA, Garza-Treviño EN, Moncada-Saucedo NK, et al. Artificial scaffolds in cardiac tissue engineering. Life 2022; 12: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sha’ban M, Kim SH, Idrus RB, et al. Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: an in vitro study. J Orthop Surg 2008; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li C, Wang L, Yang Z, et al. A viscoelastic chitosan-modified three-dimensional porous poly(L-Lactide-co-ε-caprolactone) scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed 2012; 23: 405–424. [DOI] [PubMed] [Google Scholar]
- 113. Munirah S, Kim SH, Ruszymah BHI, et al. The use of fibrin and poly(lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cell Mater 2008; 15: 41–52. [DOI] [PubMed] [Google Scholar]
- 114. Song W, Chiu A, Wang LH, et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat Commun 2019; 10: 4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bidault L, Deneufchatel M, Hindié M, et al. Fibrin-based interpenetrating polymer network biomaterials with tunable biodegradability. Polymer 2015; 62: 19–27. [Google Scholar]
- 116. Lee S, Lee K, Kim SH, et al. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017; 9: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wei Y, Hu H, Wang H, et al. Cartilage regeneration of adipose-derived stem cells in a hybrid scaffold from fibrin-modified PLGA. Cell Transplant 2009; 18: 159–170. [DOI] [PubMed] [Google Scholar]
- 118. Sukri NM, Sha’ban M, Radzi MAA, et al. The feasibility of using human primary chondrocytes derived from osteoarthritic patients overexpressed with SOX9 seeded on PLGA-fibrin hybrid scaffolds for cartilage engineering. Malays J Fundam Appl Sci 2022; 18: 304–322. [Google Scholar]
- 119. Zhang Q, Wang P, Fang X, et al. Collagen gel contraction assays: from modelling wound healing to quantifying cellular interactions with three-dimensional extracellular matrices. Eur J Cell Biol 2022; 101: 151253. [DOI] [PubMed] [Google Scholar]
- 120. Helary C, Bataille I, Abed A, et al. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010; 31: 481–490. [DOI] [PubMed] [Google Scholar]
- 121. Visscher DO, Bos EJ, Peeters M, et al. Cartilage tissue engineering: preventing tissue scaffold contraction using a 3D-Printed polymeric cage. Tissue Eng Part C Methods 2016; 22: 573–584. [DOI] [PubMed] [Google Scholar]
- 122. Setayeshmehr M, Esfandiari E, Hashemibeni B, et al. Chondrogenesis of human adipose-derived mesenchymal stromal cells on the [devitalized costal cartilage matrix/poly(vinyl alcohol)/fibrin] hybrid scaffolds. Eur Polym J 2019; 118: 528–541. [Google Scholar]
- 123. Honarvar A, Karbasi S, Hashemibeni B, et al. Effects of cartilage acellular solubilised ECM on physicomechanical and biological properties of polycaprolactone/fibrin hybrid scaffold fabricated by 3D-printing and salt-leaching methods. Mater Technol 2022; 37: 204–212. [Google Scholar]
- 124. Pankajakshan D, Philipose LP, Palakkal M, et al. Development of a fibrin composite-coated poly(ε-caprolactone) scaffold for potential vascular tissue engineering applications. J Biomed Mater Res Part B Appl Biomater 2008; 87B: 570–579. [DOI] [PubMed] [Google Scholar]
- 125. Gundy S, Manning G, O’Connell E, et al. Human coronary artery smooth muscle cell response to a novel PLA textile/fibrin gel composite scaffold. Acta Biomater 2008; 4: 1734–1744. [DOI] [PubMed] [Google Scholar]
- 126. Kook Y-M, Hwang S, Kim H, et al. Cardiovascular tissue regeneration system based on multiscale scaffolds comprising double-layered hydrogels and fibers. Sci Rep 2020; 10: 20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jiang S, Liu S, Feng W. PVA hydrogel properties for biomedical application. J Mech Behav Biomed Mater 2011; 4: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 128. Wang M, Bai J, Shao K, et al. Poly(vinyl alcohol) hydrogels: the old and new functional materials. Int J Polym Sci 2021; 2021: 1–16. [Google Scholar]
- 129. Hoseini FS, Taherian R, Atashi A. Manufacturing and properties of poly vinyl alcohol/fibrin nanocomposite used for wound dressing. Adv Appl NanoBio-Technol 2021; 2: 6–12. [Google Scholar]
- 130. Tavakoli M, Mirhaj M, Salehi S, et al. Coaxial electrospun angiogenic nanofiber wound dressing containing advanced platelet rich-fibrin. Int J Biol Macromol 2022; 222: 1605–1618. [DOI] [PubMed] [Google Scholar]
- 131. Bidault L, Deneufchatel M, Vancaeyzeele C, et al. Self-supported fibrin-polyvinyl alcohol interpenetrating polymer networks: an easily handled and rehydratable biomaterial. Biomacromolecules 2013; 14: 3870–3879. [DOI] [PubMed] [Google Scholar]
- 132. Bastidas JG, Maurmann N, da Silveira MR, et al. Development of fibrous PLGA/fibrin scaffolds as a potential skin substitute. Biomed Mater 2020; 15: 055014. [DOI] [PubMed] [Google Scholar]
- 133. Bastidas JG, Maurmann N, Oliveira L, et al. Bilayer scaffold from PLGA/fibrin electrospun membrane and fibrin hydrogel layer supports wound healing in vivo. Biomed Mater 2023; 18: 025020. [DOI] [PubMed] [Google Scholar]
- 134. Losi P, Briganti E, Sanguinetti E, et al. Healing effect of a fibrin-based scaffold loaded with platelet lysate in full-thickness skin wounds. J Bioact Compat Polym 2015; 30: 222–237. [Google Scholar]
- 135. Losi P, Briganti E, Magera A, et al. Tissue response to poly(ether)urethane-polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 2010; 31: 5336–5344. [DOI] [PubMed] [Google Scholar]
- 136. Osathanon T, Linnes ML, Rajachar RM, et al. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials 2008; 29: 4091–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Al-Maawi S, Dohle E, Lim J, et al. Biologization of Pcl-Mesh using platelet rich fibrin (Prf) enhances its regenerative potential in vitro. Int J Mol Sci 2021; 22: 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gentile P, Chiono V, Carmagnola I, et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci 2014; 15: 3640–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Liu J, Chen G, Xu H, et al. Pre-vascularization in fibrin gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater 2018; 10: 827–839. [Google Scholar]
- 140. Song Y, Lin K, He S, et al. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. Int J Nanomedicine 2018; 13: 505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yamada Y, Boo JS, Ozawa R, et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg 2003; 31: 27–33. [DOI] [PubMed] [Google Scholar]
- 142. Gassling V, Hedderich J, Açil Y, et al. Comparison of platelet rich fibrin and collagen as osteoblast-seeded scaffolds for bone tissue engineering applications. Clin Oral Implants Res 2013; 24: 320–328. [DOI] [PubMed] [Google Scholar]
- 143. Ren J, Kohli N, Sharma V, et al. Poly-ε-caprolactone/fibrin-alginate scaffold: a new pro-angiogenic composite biomaterial for the treatment of bone defects. Polymers 2021; 13: 3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011; 32: 7503–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jeong J, Kim JH, Shim JH, et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res 2019; 23: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kohli N, Sharma V, Orera A, et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J Tissue Eng 2021; 12: 20417314211005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schantz J-T, Brandwood A, Hutmacher DW, et al. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J Mater Sci Mater Med 2005; 16: 807–819. [DOI] [PubMed] [Google Scholar]
- 148. Hokugo A, Takamoto T, Tabata Y. Preparation of hybrid scaffold from fibrin and biodegradable polymer fiber. Biomaterials 2006; 27: 61–67. [DOI] [PubMed] [Google Scholar]
- 149. Zhang K, Fu Q, Yoo J, et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater 2017; 50: 154–164. [DOI] [PubMed] [Google Scholar]
- 150. Sharma V, Kohli N, Moulding D, et al. Design of a novel Two-Component hybrid dermal scaffold for the treatment of pressure sores. Macromol Biosci 2017; 17: 185. DOI: 10.1002/mabi.201700185 [DOI] [PubMed] [Google Scholar]
- 151. Tuffin J, Burke M, Richardson T, et al. A composite hydrogel scaffold permits self-organization and matrix deposition by cocultured human glomerular cells. Adv Healthc Mater 2019; 8: e1900698. [DOI] [PubMed] [Google Scholar]
- 152. Malikmammadov E, Tanir TE, Kiziltay A, et al. Preparation and characterization of poly(ε-caprolactone) scaffolds modified with cell-loaded fibrin gel. Int J Biol Macromol 2019; 125: 683–689. [DOI] [PubMed] [Google Scholar]
- 153. Akpalo E, Bidault L, Boissière M, et al. Fibrin-polyethylene oxide interpenetrating polymer networks: new self-supported biomaterials combining the properties of both protein gel and synthetic polymer. Acta Biomater 2011; 7: 2418–2427. [DOI] [PubMed] [Google Scholar]
- 154. Zhang J, Sen A, Cho E, et al. Poloxamine/fibrin hybrid hydrogels for non-viral gene delivery. J Tissue Eng Regen Med 2017; 11: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. An YH, Kim J-A, Yim H-G, et al. Meniscus regeneration with injectable pluronic/PMMA-reinforced fibrin hydrogels in a rabbit segmental meniscectomy model. J Tissue Eng 2021; 12: 20417314211050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kuten Pella O, Hornyák I, Horváthy D, et al. Albumin as a biomaterial and therapeutic agent in Regenerative Medicine. Int J Mol Sci 2022; 23: 10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gsib O, Duval J-L, Goczkowski M, et al. Evaluation of fibrin-based interpenetrating polymer networks as potential biomaterials for tissue engineering. Nanomater 2017; 7: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Deneufchâtel M, Larreta-Garde V, Fichet O. Polyethylene glycol-albumin/fibrin interpenetrating polymer networks with adaptable enzymatic degradation for tissue engineering applications. Polym Degrad Stab 2018; 152: 218–227. [Google Scholar]
- 159. Loebel C, Ayoub A, Galarraga JH, et al. Tailoring supramolecular guest-host hydrogel viscoelasticity with covalent fibrinogen double networks. J Mater Chem B 2019; 7: 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gsib O, Eggermont LJ, Egles C, et al. Engineering a macroporous fibrin-based sequential interpenetrating polymer network for dermal tissue engineering. Biomater Sci 2020; 8: 7106–7116. [DOI] [PubMed] [Google Scholar]
- 161. Kim J-A, An YH, Yim H-G, et al. Injectable fibrin/polyethylene oxide semi-IPN hydrogel for a segmental meniscal defect regeneration. Am J Sports Med 2021; 49: 1538–1550. [DOI] [PubMed] [Google Scholar]
- 162. Barker TH, Fuller GM, Klinger MM, et al. Modification of fibrinogen with poly(ethylene glycol) and its effects on fibrin clot characteristics. J Biomed Mater Res 2001; 56: 529–535. [DOI] [PubMed] [Google Scholar]
- 163. Zhang G, Wang X, Wang Z, et al. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue engineering 2006; 12: 9–19. [DOI] [PubMed] [Google Scholar]
- 164. Natesan S, Zhang G, Baer DG, et al. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A 2011; 17: 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Galler KM, Cavender AC, Koeklue U, et al. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen Med 2011; 6: 191–200. [DOI] [PubMed] [Google Scholar]
- 166. Zhang G, Hu Q, Braunlin EA, et al. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A 2008; 14: 1025–1036. [DOI] [PubMed] [Google Scholar]
- 167. Thomas H, Cowin AJ, Mills SJ. The importance of pericytes in healing: wounds and other pathologies. Int J Mol Sci 2017; 18: 1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ricles LM, Hsieh P-L, Dana N, et al. Therapeutic assessment of mesenchymal stem cells delivered within a PEGylated fibrin gel following an ischemic injury. Biomaterials 2016; 102: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Shpichka AI, Konarev PV, Efremov YM, et al. Digging deeper: structural background of PEGylated fibrin gels in cell migration and lumenogenesis. RSC Adv 2020; 10: 4190–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gorkun AA, Shpichka AI, Zurina IM, et al. Angiogenic potential of spheroids from umbilical cord and adipose-derived multipotent mesenchymal stromal cells within fibrin gel. Biomed Mater 2018; 13: 044108. [DOI] [PubMed] [Google Scholar]
- 171. Dadashzadeh A, Moghassemi S, Amorim CA. Evaluation of PEGylated fibrin as a three-dimensional biodegradable scaffold for ovarian tissue engineering. Mater Today Chem 2021; 22: 100626. [Google Scholar]
- 172. Pal A, Tripathi K, Pathak C, et al. Plasma-based fast-gelling biohybrid gels for biomedical applications. Sci Rep 2019; 9: 10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kumar A, Han SS. PVA-based hydrogels for tissue engineering: a review. Int J Polym Mater Polym Biomater 2017; 66: 159–182. [Google Scholar]
- 174. Jin SG. Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem Asian J 2022; 17: e202200595. [DOI] [PubMed] [Google Scholar]
- 175. Xu F, Zou D, Dai T, et al. Effects of incorporation of granule-lyophilised platelet-rich fibrin into polyvinyl alcohol hydrogel on wound healing. Sci Rep 2018; 8: 14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou G, Zhu J, Inverarity C, et al. Fabrication of fibrin/polyvinyl alcohol scaffolds for skin tissue engineering via emulsion templating. Polymers 2023; 15: 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Simaan-Yameen H, Bar-Am O, Saar G, et al. Methacrylated fibrinogen hydrogels for 3D cell culture and delivery. Acta Biomater 2023; 164: 94–110. [DOI] [PubMed] [Google Scholar]
- 178. Mihalko E, Huang K, Sproul E, et al. Targeted treatment of ischemic and fibrotic complications of myocardial infarction using a dual-delivery microgel therapeutic. ACS Nano 2018; 12: 7826–7837. [DOI] [PubMed] [Google Scholar]
- 179. Rahimi N, Molin DG, Cleij TJ, et al. Electrosensitive polyacrylic acid/fibrin hydrogel facilitates cell seeding and alignment. Biomacromolecules 2012; 13: 1448–1457. [DOI] [PubMed] [Google Scholar]
- 180. Cen L, Liu W, Cui L, et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res 2008; 63: 492–496. [DOI] [PubMed] [Google Scholar]
- 181. Kim OV, Litvinov RI, Chen J, et al. Compression-induced structural and mechanical changes of fibrin-collagen composites. Matrix Biol 2017; 60-61: 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nilforoushzadeh MA, Sisakht MM, Amirkhani MA, et al. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: a clinical study for diabetic wound healing. J Tissue Eng Regen Med 2020; 14: 424–440. [DOI] [PubMed] [Google Scholar]
- 183. Patel B, Xu Z, Pinnock CB, et al. Self-assembled collagen-fibrin hydrogel reinforces tissue engineered adventitia vessels seeded with human fibroblasts. Sci Rep 2018; 8: 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Seyedhassantehrani N, Li Y, Yao L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr Biol 2016; 8: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wang W, Lin S, Xiao Y, et al. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 2008; 82: 190–204. [DOI] [PubMed] [Google Scholar]
- 186. Coradin T, Wang K, Law T, et al. Type I collagen-fibrin mixed hydrogels: preparation, properties and biomedical applications. Gels 2020; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sanz-Horta R, Matesanz A, Jorcano JL, et al. Preparation and characterization of plasma-derived fibrin hydrogels modified by alginate di-Aldehyde. Int J Mol Sci 2022; 23: 4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hinsenkamp A, Fülöp Hricisák L, et al. Application of injectable, crosslinked, fibrin-containing hyaluronic acid scaffolds for in vivo remodeling. J Funct Biomater 2022; 13: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Stojic M, Ródenas-Rochina J, López-Donaire ML, et al. Elastin-plasma hybrid hydrogels for skin tissue engineering. Polymers 2021; 13: 2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012; 37: 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Vorwald CE, Gonzalez-Fernandez T, Joshee S, et al. Tunable fibrin-alginate interpenetrating network hydrogels to support cell spreading and network formation. Acta Biomater 2020; 108: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Shikanov A, Xu M, Woodruff TK, et al. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009; 30: 5476–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Richter A, Li Y, Rehbock C, et al. Triple modification of alginate hydrogels by fibrin blending, iron nanoparticle embedding, and serum protein-coating synergistically promotes strong endothelialization. Adv Mater Interfaces 2021; 8: 2002015. [Google Scholar]
- 194. Abatangelo G, Vindigni V, Avruscio G, et al. Hyaluronic acid: redefining its role. Cells 2020; 9: 1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhang Y, Heher P, Hilborn J, et al. Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biomater 2016; 38: 23–32. [DOI] [PubMed] [Google Scholar]
- 196. Park JS, Yang HN, Woo DG, et al. Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials 2011; 32: 1495–1507. [DOI] [PubMed] [Google Scholar]
- 197. Snyder TN, Madhavan K, Intrator M, et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng 2014; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Montero A, Atienza C, Elvira C, et al. Hyaluronic acid-fibrin hydrogels show improved mechanical stability in dermo-epidermal skin substitutes. Mater Sci Eng C 2021; 128: 112352. [DOI] [PubMed] [Google Scholar]
- 199. Rnjak J, Wise SG, Mithieux SM, et al. Severe burn injuries and the role of elastin in the design of dermal substitutes. Tissue Eng Part B Rev 2011; 17: 81–91. [DOI] [PubMed] [Google Scholar]
- 200. Gonzalez de, Torre I, Weber M, Quintanilla L, et al. Hybrid elastin-like recombinamer-fibrin gels: physical characterization and in vitro evaluation for cardiovascular tissue engineering applications. Biomater Sci 2016; 4: 1361–1370. [DOI] [PubMed] [Google Scholar]
- 201. Bonzon N, Carrat X, Deminiére C, et al. New artificial connective matrix made of fibrin monomers, elastin peptides and type I + III collagens: structural study, biocompatibility and use as tympanic membranes in rabbit. Biomaterials 1995; 16: 881–885. [DOI] [PubMed] [Google Scholar]
- 202. Zarrintaj P, Manouchehri S, Ahmadi Z, et al. Agarose-based biomaterials for tissue engineering. Carbohydr Polym 2018; 187: 66–84. [DOI] [PubMed] [Google Scholar]
- 203. Ionescu AM, Alaminos M, de la Cruz Cardona J, et al. Investigating a novel nanostructured fibrin-agarose biomaterial for human cornea tissue engineering: rheological properties. J Mech Behav Biomed Mater 2011; 4: 1963–1973. [DOI] [PubMed] [Google Scholar]
- 204. Carriel V, Garzón I, Jiménez J-M, et al. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. CTO 2012; 196: 1–12. [DOI] [PubMed] [Google Scholar]
- 205. Martin-Piedra M-A, Gironés-Camarasa B, España-López A, et al. Usefulness of a nanostructured fibrin-agarose bone substitute in a model of severely critical mandible bone defect. Polymers 2021; 13: 3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Guldager Kring Rasmussen D, Karsdal MA. Chapter 29 - Laminins. In: Karsdal MA. (ed.) Biochemistry of collagens, laminins and elastin. Cambridge, MA: Academic Press 2016, pp.163–196. [Google Scholar]
- 207. Jury M, Matthiesen I, Rasti Boroojeni F, et al. Bioorthogonally cross-linked hyaluronan-laminin hydrogels for 3D neuronal cell culture and biofabrication. Adv Healthc Mater 2022; 11: e2102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Rosero Salazar DH, van Rheden REM, van Hulzen M, et al. Fibrin with laminin-nidogen reduces fibrosis and improves soft palate regeneration following palatal injury. Biomolecules 2021; 11: 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Genovese P, Patel A, Ziemkiewicz N, et al. Co-delivery of fibrin-laminin hydrogel with mesenchymal stem cell spheroids supports skeletal muscle regeneration following trauma. J Tissue Eng Regen Med 2021; 15: 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ziemkiewicz N, Hilliard GM, Dunn AJ, et al. Laminin-111-Enriched fibrin hydrogels enhance functional muscle regeneration following trauma. Tissue Eng Part A 2022; 28: 297–311. [DOI] [PubMed] [Google Scholar]
- 211. Nam K, Jones JP, Lei P, et al. Laminin-111 peptides conjugated to fibrin hydrogels promote formation of lumen containing parotid gland cell clusters. Biomacromolecules 2016; 17: 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Nam K, Dos Santos HT, Maslow F, et al. Laminin-1 peptides conjugated to fibrin hydrogels promote salivary gland regeneration in irradiated mouse submandibular glands. Front Bioeng Biotechnol 2021; 9: 729180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Edgar JM, Robinson M, Willerth SM. Fibrin hydrogels induce mixed dorsal/ventral spinal neuron identities during differentiation of human induced pluripotent stem cells. Acta Biomater 2017; 51: 237–245. [DOI] [PubMed] [Google Scholar]
- 214. Montalbano G, Toumpaniari S, Popov A, et al. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater Sci Eng C 2018; 91: 236–246. [DOI] [PubMed] [Google Scholar]
- 215. Pandima Devi M, Sekar M, Chamundeswari M, et al. A novel wound dressing material — fibrin–chitosan–sodium alginate composite sheet. Bull Mater Sci 2012; 35: 1157–1163. [Google Scholar]
- 216. Matica MA, Aachmann FL, Tøndervik A, et al. Chitosan as a wound dressing starting material: Antimicrobial Properties and mode of action. Int J Mol Sci 2019; 20: 5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Gansau J, Buckley CT. Incorporation of collagen and hyaluronic acid to enhance the bioactivity of fibrin-based hydrogels for nucleus pulposus regeneration. J Funct Biomater 2018; 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sawadkar P, Mandakhbayar N, Patel KD, et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J Tissue Eng 2021; 12: 20417314211019238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhou W, Zhao M, Zhao Y, et al. A fibrin gel loaded with chitosan nanoparticles for local delivery of rhEGF: preparation and in vitro release studies. J Mater Sci Mater Med 2011; 22: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 220. Storozhylova N, Crecente-Campo J, Cabaleiro D, et al. An in situ hyaluronic acid-fibrin hydrogel containing drug-loaded nanocapsules for intra-articular treatment of inflammatory joint diseases. Regen Eng Transl Med 2020; 6: 201–216. [Google Scholar]
- 221. Chotitumnavee J, Parakaw T, Srisatjaluk RL, et al. In vitro evaluation of local antibiotic delivery via fibrin hydrogel. J Dent Sci 2019; 14: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bonhome-Espinosa AB, Campos F, Durand-Herrera D, et al. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J Mech Behav Biomed Mater 2020; 104: 103619. [DOI] [PubMed] [Google Scholar]
- 223. Bonhome-Espinosa AB, Campos F, Rodriguez IA, et al. Effect of particle concentration on the microstructural and macromechanical properties of biocompatible magnetic hydrogels. Soft Matter 2017; 13: 2928–2941. [DOI] [PubMed] [Google Scholar]
- 224. Kirichenko MN, Chaikov LL, Krivokhizha SV, et al. Effect of iron oxide nanoparticles on fibrin gel formation and its fractal dimension. J Chem Phys 2019; 150: 155103. [DOI] [PubMed] [Google Scholar]
- 225. Pathmanapan S, Periyathambi P, Anandasadagopan SK. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair - in vitro and in vivo study. Nanomed 2020; 29: 102251. [DOI] [PubMed] [Google Scholar]
- 226. Zhang L, Liu Z, Xie Q, et al. Bio-inspired assembly of reduced graphene oxide by fibrin fiber to prepare multi-functional conductive bio-nanocomposites as versatile electrochemical platforms. Carbon N Y 2019; 153: 504–512. [Google Scholar]
- 227. Sivakumar S, Murali R, Arathanaikotti D, et al. Ferulic acid loaded microspheres reinforced in 3D hybrid scaffold for antimicrobial wound dressing. Int J Biol Macromol 2021; 177: 463–473. [DOI] [PubMed] [Google Scholar]
- 228. Deepthi S, Jayakumar R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact Mater 2018; 3: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Motealleh A, Çelebi-Saltik B, Ermis N, et al. 3D printing of step-gradient nanocomposite hydrogels for controlled cell migration. Biofabrication 2019; 11: 045015. [DOI] [PubMed] [Google Scholar]
- 230. Holy CE, Cheng C, Davies JE, et al. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 2001; 22: 25–31. [DOI] [PubMed] [Google Scholar]
- 231. Almeida HV, Eswaramoorthy R, Cunniffe GM, et al. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater 2016; 36: 55–62. [DOI] [PubMed] [Google Scholar]
- 232. Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 2004; 32: 477–486. [DOI] [PubMed] [Google Scholar]
- 233. Ho T-C, Chang C-C, Chan H-P, et al. Hydrogels: Properties and applications in Biomedicine. Molecules 2022; 27: 2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Das S, Das D. Rational design of peptide-based smart hydrogels for therapeutic applications. Front Chem 2021; 9: 770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Liu Y, Meng H, Konst S, et al. Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl Mater Interfaces 2014; 6: 16982–16992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Alexandre N, Ribeiro J, Gärtner A, et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting In vitro and in vivo studies. J Biomed Mater Res A 2014; 102: 4262–4275. [DOI] [PubMed] [Google Scholar]
- 237. Zhu J, Li Z, Zou Y, et al. Advanced application of collagen-based biomaterials in tissue repair and restoration. J Leather Sci Eng 2022; 4: 30. [Google Scholar]
- 238. Ferreira AM, Gentile P, Chiono V, et al. Collagen for bone tissue regeneration. Acta Biomater 2012; 8: 3191–3200. [DOI] [PubMed] [Google Scholar]
- 239. Hong S, Jung BY, Hwang C. Multilayered engineered tissue sheets for vascularized tissue regeneration. Tissue Eng Regen Med 2017; 14: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zhu B, Chen M, Yin H, et al. Enzymatic hydrolysis of alginate to produce oligosaccharides by a new purified endo-type alginate lyase. Mar Drugs 2016; 14: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev 2001; 101: 1869–1879. [DOI] [PubMed] [Google Scholar]