Abstract
Study Design.
RNA in situ hybridization (RISH) allows for validation and characterization of the long noncoding (lnc) natural antisense RNA (NAT) Klhl14as in the embryonic murine intervertebral disc (IVD) in the context of loss-of-function mutants for key transcription factors (TFs) in axial skeleton development.
Objective.
Validation of Klhl14as in the developing murine IVD.
Summary of Background Data.
The IVD is a focus of regenerative medicine; however, processes and signaling cascades resulting in the different cell types in a mature IVD still require clarification in most animals including humans. Technological advances increasingly point to implications of lnc NATs in transcription/translation regulation. Transcriptome data generation and analysis identified a protein encoding transcript and related noncoding antisense transcript as downregulated in embryos devoid of key TFs during axial skeleton development. Here, primarily, the antisense transcript is analyzed in this loss-of-function context.
Methods.
4930426D05Rik and 6330403N15Rik were identified as Klhl14as and sense, respectively, two transcripts downregulated in the vertebral column of midgestation Pax1 and Pax9 mutant mouse embryos. RISH on wildtype and mutant embryos for the TF encoding genes Pax1/Pax9, Sox5/Sox6/Sox9, and Bapx1 was used to further analyze Klhl14as in the developing IVD.
Results.
Klhl14as and Klhl14 were the top downregulated transcripts in Pax1−/−; Pax9−/− E12.5 embryos. Our data demonstrate expression of Klhl14as and sense transcripts in the annulus fibrosus (AF) and notochord of the developing IVD. Klhl14as expression in the inner annulus fibrosus (iAF) seems dependent on the TFs Pax1/Pax9, Sox6, Sox9, and Bapx1.
Conclusion.
We are the first to suggest a role for the lncRNA Klhl14as in the developing IVD. Our data link Klhl14as to a previously established gene regulatory network during axial skeleton development and contribute further evidence that lnc NATs are involved in crucial gene regulatory networks in eukaryotic cells.
Level of Evidence:
N/A
Keywords: 4930426D05Rik, 6330403N15Rik, intervertebral disc, IVD, Klhl14 antisense, long noncoding RNA, Pax1, Pax9, Sox5, Sox6, Sox9
Through advances in technology development over the last two decades, our understanding of the genetic make up of an organism and the regulation of its transcriptome has changed dramatically. What was previously labeled as intergenic noncoding “junk” DNA has been shown to contain among other regulatory elements, information for noncoding RNAs with roles in transcription/translation regulation. The implication of the long noncoding (lnc) natural antisense RNA (NAT) Dlx1as in a mild skeletal and neurological phenotype through modulation of the Dlx1 sense transcript level was previously demonstrated.1 Accruing evidence in recent years demonstrates that lncRNAs are not an uncommon regulator of fundamental biological processes in eukaryotic cells, yet the repertoire of molecular mechanisms involved is complex2–6 and one strategy in characterizing a lncRNA is through identifying its cell or tissue-specific localization.
Like many transcription factors (TFs), the paired box TF Pax1 and its paralog Pax9 have pleiotropic roles during development as revealed by spontaneous and engineered mutations.7–10 Spatiotemporally restricted expression initiates as early as E8.5 in the foregut and somites and eventually contributes to palate, teeth, salivary gland epithelium and thymus development, and Pax9 is also expressed in neural crest cell derived tissues. However, both TFs are likely best known for their importance in early patterning of the axial skeleton. Their compensatory roles have been described in tissues of coexpression, yet a functional rescue fails if only one is present (reviewed in11).
Despite pleiotropic roles, specificity of cell differentiation along with tissue and organ development is typically mediated through the impact of TFs on direct and/or indirect downstream target genes. Recently however, new players in the form of lncRNAs are also taking part in the regulation of these fundamental biological processes.2 A longstanding interest in axial skeleton development has led to engineered mutant and wildtype alleles for Pax1,Pax9, the Sox trio (Sox5, Sox6, Sox9), and Bapx1 and resulting mouse lines. Cells isolated from the vertebral column of mid-gestation embryos and sorted according to their TF lineage were subjected to microarray gene expression profiling. Many important downstream target genes regulated by Pax1 and Pax9, the Sox trio, and Bapx1 in vertebral column development were identified and validated in these studies.8,12,13 DNA loci encoding transcripts 4930426D05Rik and 6330403N15Rik were among the top downregulated RNAs in Pax1/Pax9 lineage cells of Pax1−/− Pax9−/− E12.5 embryos (Figure 1A)and within the top 10 downregulated transcripts in Pax1−/−Pax9+/− triple allele mutants.8
Figure 1.
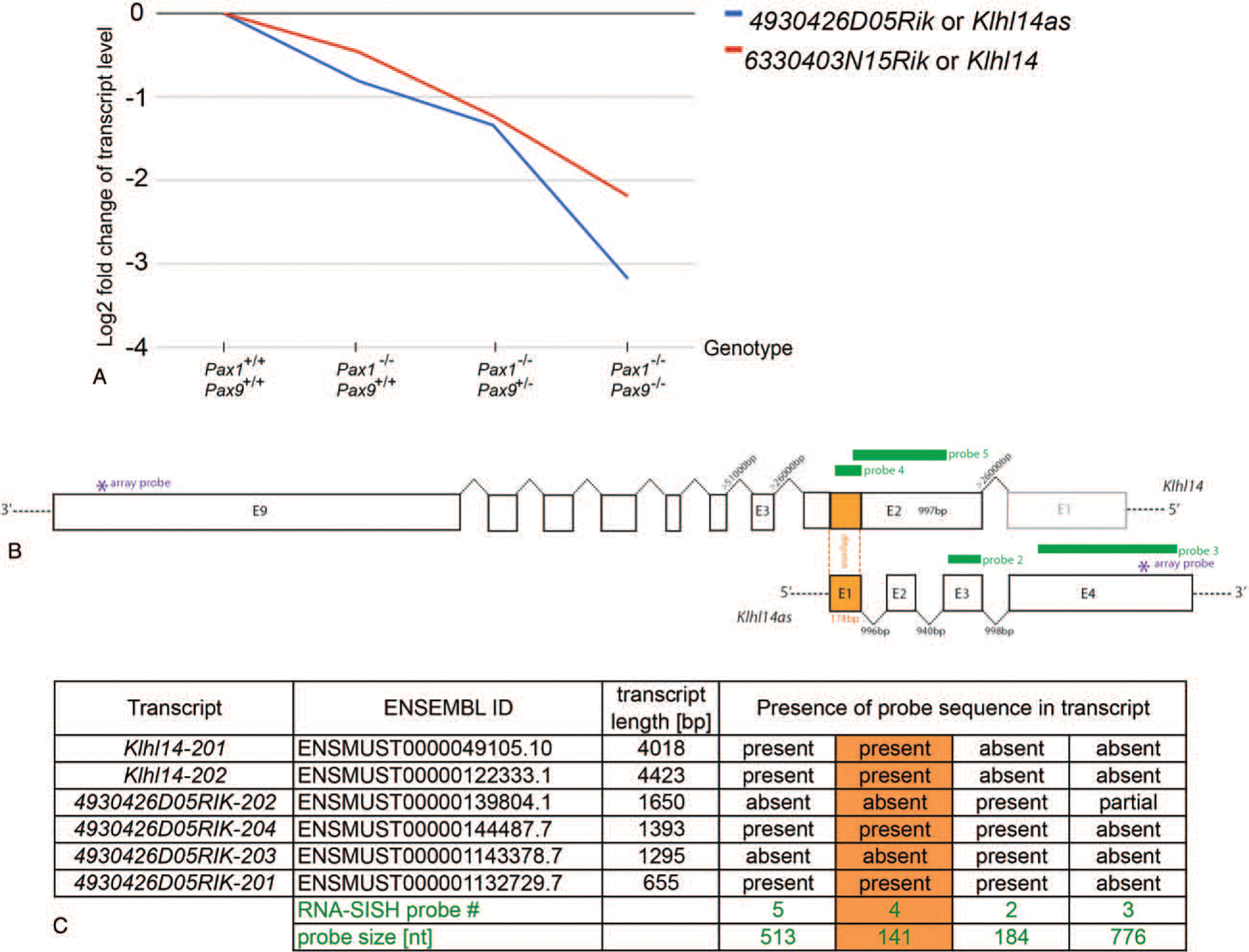
(A) Log2 fold change in transcript levels obtained from microarray transcriptome profiling of Pax1 and Pax9 lineage cells isolated from the vertebral column of loss-of-function mouse embryos in reference to the wildtype.8 Klhl14 antisense (blue) and sense (red) transcript levels correlate with the successive loss of functional Pax alleles. (B) Schematic aligning the genomic DNA of the Klhl14 sense and antisense locus. Sense/antisense overlap is colored in orange; RISH probes are shown in green. The asterix indicates the location of the oligonucleotide probe of the Illumina array. (C) All known transcripts of Klhl14as and Klhl14 are listed in respect to the RISH probes used in this study. bp indicates base pairs; E, exon.
Interestingly, sequence alignment revealed these two transcripts as a sense/antisense pair. In the meantime, 6330403N15Rik has been annotated as Kelch-like protein 14 (Klhl14) and described in a yeast-two-hybrid screen as a protein interactor of torsinA (Printor).14 At the time, no information was available about the locus 4930426D05Rik, and only very recently, a name change was suggested to Klhl14 antisense (Klhl14as) RNA15 based on the antisense overlap with Klhl14. Klhl14as was identified as a locus with the highest fold-change to be specifically enriched in the thyroid bud at E10.5.16 At least four alternative transcripts of Klhl14as are currently known, listed in ENSEMBL and found differentially expressed in adult tissues as characterized by qRT-PCR and RNA in situ hybridization (RISH) in the adult mouse.15 Klhl14as RNA was found to be highly expressed in a cell-type specific manner, present in the spleen, thyroid, kidney, ovary, testis, and brain, detected in the lung only by qRT-PCR and described as absent from heart and liver.15 The focus here lies on a possible role of Klhl14as in axial skeleton development, mostly that of the intervertebral disc (IVD). To validate previous microarray findings,8 expression of the different transcripts was characterized through RISH with probes corresponding to each of the four exons of the Klhl14as RNA as well as probes detecting different exons of the Klh14 sense transcript in wildtype. To further investigate the role of this lncRNA, the analysis of Klhl14as was expanded to loss-of-function mutants for crucial TFs during axial skeleton development such as Pax1, Pax9, Sox6, Sox9, and Bapx1 mutant embryos.8,12,13,17,18
MATERIALS AND METHODS
Mouse Lines and Ethic Statement
Animal procedures were performed adhering to guidelines of the Institutional Animal Care and Use Committee (IACUC) as set by the National Advisory Committee for Laboratory Animal Research (NACLAR). The relevant IACUC protocols No: 110689 and 110648 were reviewed and approved before any animal work. All mice in this study were housed and maintained under IACUC and NACLAR guidelines. Midgestation embryos of various allele combinations of Bapx1, Col2a1-Cre, Pax1, Pax9, Sox5, Sox6, Sox9 were generated through timed matings of wildtype or genetically modified loci of respective mouse lines described previously and made available to the public.8,12,13,19,20 E0.5 refers to the morning a vaginal plug was observed. Embryos of timed matings for the outbred strain CD1 were used for expression validation in the wildtype.
RISH and Histology
RISH was carried out as described21 on 7uM sections of 4% (w/v) PFA fixed and paraffin-embedded mouse embryos with digoxigenin (DIG) labeled RNA probes generated from PCR products with a T7 promoter sequence included in the reverse primer to allow for the generation of an antisense transcript and a T3 promoter sequence included in the forward primer to allow for the generation of a sense transcript. Primer details are summarized in Table 1. Qualitative gene expression was assessed through alkaline phosphatase (AP) driven conversion of Nitro Blue Tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate.22,23 For Sox6, Sox9, Bapx1 and sense/as of probe 4 (Figure 1), the Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA) was used for signal amplification. For histology, Mallory tetrachrome staining was performed as described.21,24 Results were documented on a Motic BA310 compound microscope with a Moticam1.3MP (Carolina Biological Supply Company, Burlington, NC).
TABLE 1.
PCR-Primer Sequences for RISH Probe Template Generation
| Locus | Forward 5′->3′ | Reverse 5′->3′ | Probe Size, bp | Target Sequence |
|---|---|---|---|---|
| Klhl14as ex1 overlap | TACTGCATGCGGGTCTCACG | GTGGAAGATGTGCTGTTGCT | 141 | NM_001271580.1 |
| Klhl14as ex3 | AGGGACCCTCTTGCCCGT | GATGGGAAAGTGAGTGGAGAGG | 184 | NM_001271580.1 |
| Klhl14as ex4 | CTTCAAGCGCTCTGTGCAAT | TTGAATGCCTCCAACTCCCA | 776 | NM_001271580.1 |
| Klhl14 ex2 overlap | TACTGCATGCGGGTCTCACG | GTGGAAGATGTGCTGTTGCT | 141 | NM_001271580.1 |
| Klh14 ex2 | GCTCGCAGTACTTCCGATCA | GCACGCATCTCCTCGAAGTT | 513 | NM_001081403.1 |
| Pax1 ex2 | AGACGTACGGCGAAGTGAAC | TGAGTGCCCATCTTAGTGCC | 520 | XM_006498911.3 |
| Pax9 ex2 | TCGCATCGTGGAATTAGCCC | GATGCCCAGAATGTCCGTGA | 532 | NM_011041.3 |
| Sox6 | GACACCGGGACTTGGAAACT | G CTTATCAGCAGCAGCG TTC | 1391 | NM_001277327.1 |
| Sox9 | CCAGCAAGAACAAGCCACAC | TCAGCTCTGTCACCATAGCTTTT | 1261 | NM_011448.4 |
| Bapx1 | CCGAATTCCAATTAACCCTCACTAAAGG | CCAAGCTTGTAATACGACTCACTATAGGGC | 2400 | B7K4 plasmid |
| Col2a1 | CCATTG CGA ACC CAAAGGAC | CACCATTGTGTAGGA CACGC | 329 | NM_031163.3 |
Database Search and Sequence Alignment
NCBI nucleotide and NCBI BLAST software (www.ncbi.nlm.nih.gov) was used with the sequences NM_001271580.1 (Klhl14as lncRNA), XM_006525822.2 (Klhl14 sense transcript variant 1), and NC_000084.6 (Mus musculus strain C57BL/6J chromosome 18, GRCm38.p4C57BL/6J) to identify exon-intron borders and sequence overlaps. The ENSEMBL release 90 from August 2017 (www.ensembl.org) was used for further details of the four Klhl14as lncRNA transcripts available to date: 4930426D05Rik-201 (ENSMUST00000132729.7) 655 nucleotides (nt), 4930426D05Rik-202 (ENSMUST00000139804.1) 1650nt, 4930426D05Rik-203 (ENSMUST00000143378.7) 1295nt and 4930426D05Rik-204 (ENSMUST00000144487.7) 1393nt. Two Klhl14 transcripts were listed in the ENSEMBL release 90: The Klhl14–201 transcript (ENSMUST00000049105.10) with a length of 4018nt is translated into 630 amino acids (AAs) and the Klhl14–202 transcript, which contains an additional nonprotein coding upstream exon, has a transcript length of 4423nt and is also translated into 630 AAs. Microarray data and array probe sequence was available from Supplement 8 of 8 and was located in exon4 of Klhl14as and exon9 of Klhl14, respectively.
RESULTS
Sense/Antisense Relation of Klhl14 and RISH Probes
Klhl14as and sense transcripts were 3.18 and 2.19 Log2 fold downregulated in Pax1/Pax9 lineage cells from the axial skeleton of Pax1−/−; Pax9−/− E12.5 mutant mouse embryos8 compared with those with wildtype alleles, respectively (Figure 1A). This led us to further investigate this lnc NAT during IVD development. A total of four alternatively spliced transcripts have been identified for Klhl14as, all sharing exon3. Two of these transcripts, 4930426D05Rik-201 and 4930426D05Rik-204, can act as antisense transcript to Klhl14 through the overlap in exon1 (Figure1B, C).NCBIBLAST analysis revealed that exon1 of the Klhl14as lncRNA (NM_001271580.1) shares a 174 bp overlap with exon2 of the Klhl14 sense transcript variant 1 (XM_006525822.2) or Klhl14–202, including an upstream in-frame stop codon at position 150 of Klhl14as. Aside from this overlap, the remaining four exons of Klhl14as are located in the >20kb intron2 sequence of Klhl14. This is only the second largest intron of Klhl14 after intron3, which spans >50kb (NC_000084.6) (Figure 1B).
RISH probes reflecting sense and antisense transcripts were designed in exons of the above-mentioned sequences based on genomic DNA sequences available from the NCBI nucleotide and ENSEMBL software. The strongest signal was observed with probe2 detecting exon3 from position 343 to 526 of NM_001271580.1 (Figure 1B, C).
Klhl14as lncRNA Expression in the Wildtype Embryo
To evaluate a potential role of Klhl14as in IVD development, RISH was conducted on serial sagittal (Figure 2A–E) or dorsoventral (dv) sections (Figure 2F–J) of CD1 wildtype mouse embryos. Of the seven probes we designed, probe1-T7, probe2-T7, and probe4-T7 are shown here for the Klhl14as lncRNA transcript and probe4-T3 and probe5-T7 were used to detect the Klhl14 sense transcript. All probes showed a similar expression pattern, however, as Klhl14as exon3 is shared by all known transcripts of the lncRNA, probe2-T7 representing exon3 of the Klhl14as was chosen for most of our analysis. Sense and antisense expression were similar, as shown for the overlap between Klhl14as exon1 (Figure 2G,L,Q) and Klhl14 exon2 (Figure 2F,K,P), providing a possibility to act through an antisense mechanism. Overall, both sense and antisense transcripts showed segmental expression in the axial skeleton with expression in the developing IVDof E12.5 embryos (Figure 2A,F,G). At E12.5, the notochord is still present as a rod-like structure (Figure 2J). In mouse, notochordal cells will eventually become restricted to the IVD anlagen and give rise to the nucleus pulposus (NP).25 Both sense and antisense transcript expression indicate cellular heterogeneity in the notochord at E12.5 (Figure 2A,F,G). Although Pax1 and Pax9 were absent from the notochord (Figure 2B,C,U,V), other key TFs in axial skeleton development such as Sox6 (Figure 2D,Y,DD,II) and Bapx1 (Figure 2E,X,CC,HH) were transcribed. To focus on Klhl14as in the nascent IVD, cross-sections were carefully selected on the basis of morphological criteria, as cells are more condensed in the iAF in areas of the forming IVD (Figure 2P–R,T,FF–JJ)compared with cells in sections closer to (Figure 2S) or within the vertebral body. Klhl14as transcripts were present throughout the iAF (Figure 2M, R), similar to Pax1 (Figure 2AA,FF), while Pax9 is more dorsally restricted (Figure 2BB,GG). Probes reflecting the overlap between Klhl14 sense and antisense transcripts showed a more limited expression in the AF (Figure 2P,Q), closer resembling that of Bapx1 (Figure 2HH). Bapx1, Sox6, and Sox9 were present in the AF and notochord (Figure 2X–Z,HH–JJ).
Figure 2.
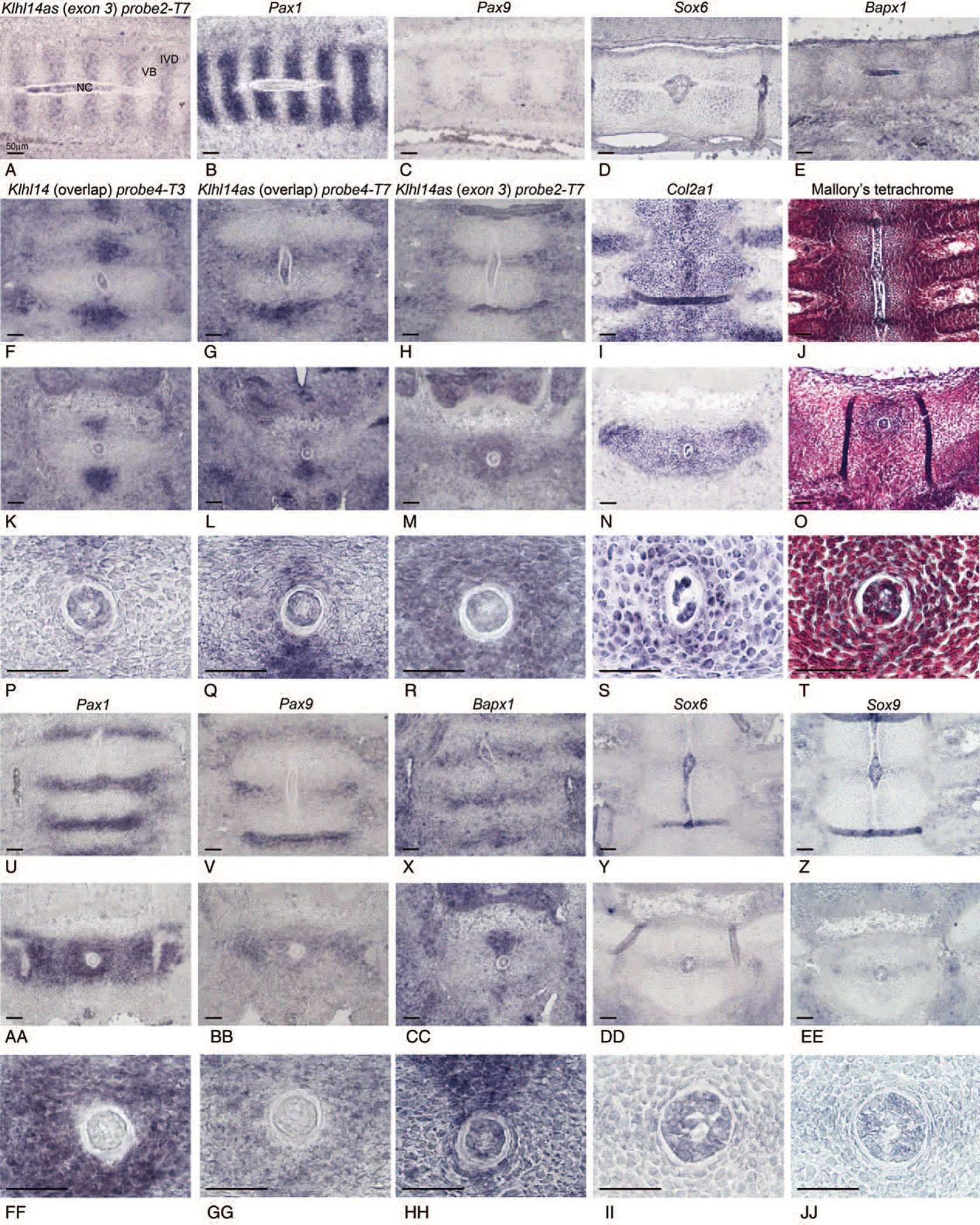
Expression of Klhl14as and sense transcripts shown by RISH in comparison to key transcription factors in the developing axial skeleton of E12.5 mouse embryos with focus on the IVD as shown at 10x and 40x magnification in sagittal sections (A–E) and dorsoventral sections (F–JJ). Mallory tetrachrome staining was used for histological reference (J,O,T). Scale bar reflects 50μm. NC indicates notochord; VB, vertebral body.
Klhl14as lncRNA Expression in Embryos, Devoid of Key Skeletogenic Transcription Factors
We further analyzed Klhl14as transcripts via probe2 in mouse mutant embryos of TFs relevant to axial skeleton development (Figures 1B,C and 3). A decrease in functional Pax alleles (Figure 3A–J) was accompanied by a reduction in Klhl14as-expressing cells in the iAF, while in the notochord, naturally devoid of Pax1 and Pax9 transcripts (Figure 2U,V), Klhl14as seemed unaffected (Figure 3A–J). A more extensive reduction of Klhl14as was observed in the iAF of Bapx1 and Sox6 mutants (Figure 3K–N). Sox9-ablation is early embryonic lethal; therefore, a conditional Sox9 mutant (Sox9c) where Sox9 is functionally ablated through a Col2a1 driven Cre-recombinase20 was used. Col2a1 is expressed throughout the developing vertebral column as shown at E12.5 (Figure 2N,S) and remains present in the caudal iAF at E15.5 (Figure 3S,T). Despite strong expression in the skin of E15.5 Sox9c embryos, Klhl14as is absent from the iAF of caudal IVDs (Figure 3O,P) yet remains in the notochord, as also seen in Sox6 mutants (Figure 3M, N).
Figure 3.
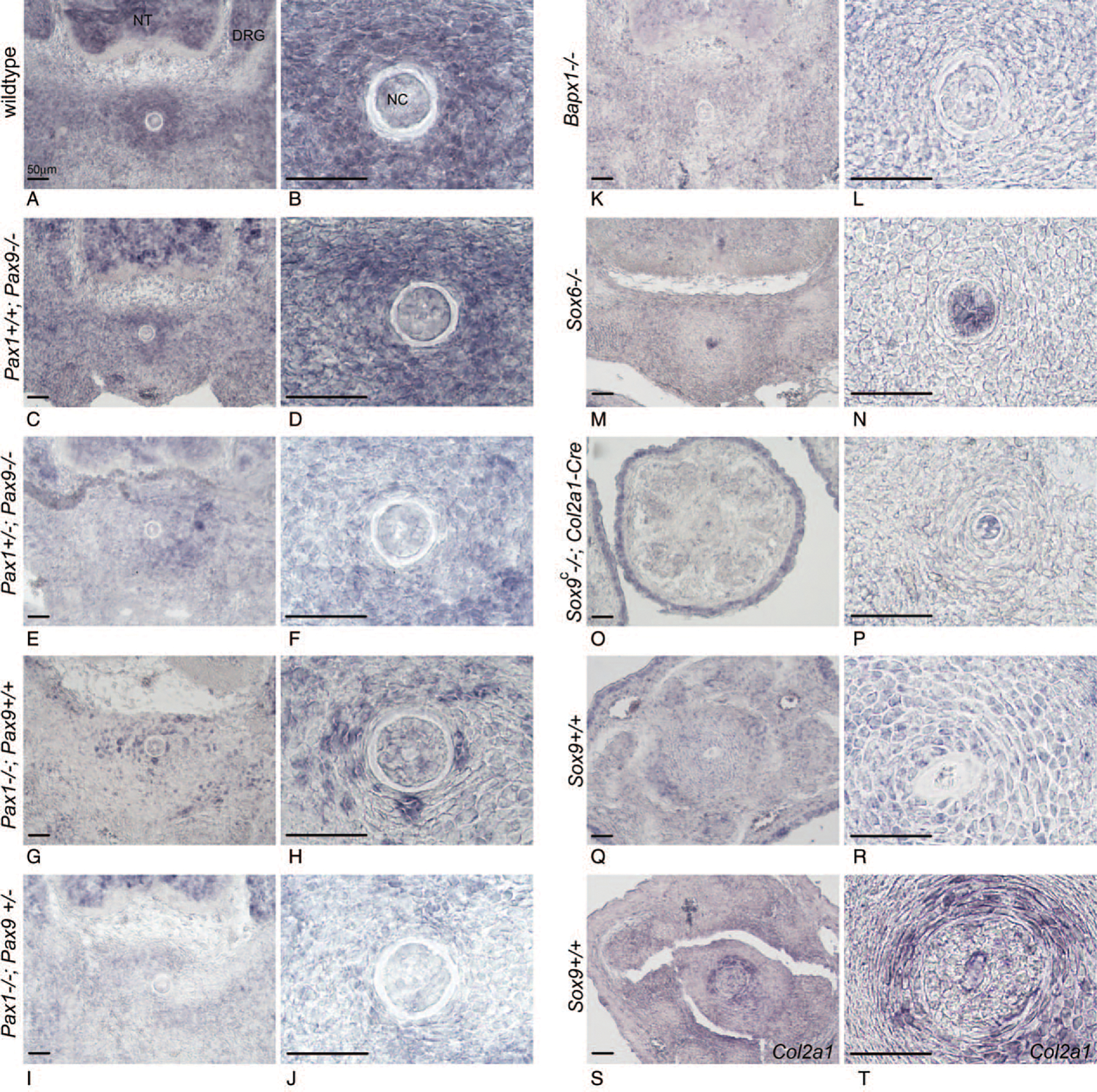
Expression of Klhl14as transcripts represented by probe 2 shown by RISH in Pax1/Pax9, Sox6, Sox9c, and Bapx1 mutant embryos in the developing IVD at 10x and 40x magnification in dorsoventral sections. Scale bar reflects 50μm. DRG indicates dorsal root ganglia; NC, notochord.
DISCUSSION
The IVD is a focus of regenerative medicine with many clinical trials ongoing (clinicaltrials.gov). Considered anatomically a rather simple organ of only two major tissue types: the AF, which encapsulates the central NP, identities of the heterogeneous cell populations present in the AF and NP of mature IVDs and the signaling cascades resulting in these different cell types remain to be determined in most animals including humans.21,26,27 The mouse has long served as a model organism to study human development and disease.28 Data achieved through microarray transcriptome analysis of loss-of-function mutations in Pax1/Pax9,8 encoding key TFs in murine vertebral column development, suggested the Klhl14as and sense transcripts as downstream targets of the Pax1 and Pax9 TFs. Probes spotted on the Illumina microarray used for generating the transcriptome data were located in exon9 of the Klhl14 and exon4 of the Klhl14as transcript, the latter was included in the sequence of our probe1 and probe3, both showing an identical expression pattern (data not shown). Expression of these probes was similar but not as strong as probe2. On the basis of the location of the Illumina microarray probe in exon4 of Klhl14as, the transcriptome data most likely reflect transcript variant 4930426D05Rik-202. All currently known Klhl14as transcripts fulfill the requirements to be classified as lnc NATs as all appear noncoding and exceed 200nt in length.2 Of the probes designed to detect the Klhl14as transcripts, the strongest signal was observed with probe2 detecting exon3, likely, because it is shared by all four reported transcripts. For comparison, the RISH probe reported in the study by Credendino et al15 using adult mouse tissue corresponded to position 331 to 603 and the qRT-PCR product to position 501 to 560. Probe4 was designed to cover the overlap between the Klhl14 sense and Klhl14as transcripts, located in exon2 and exon1, respectively. Our data provide a qualitative analysis of Klhl14as expression through RISH to validate and complement our previous quantitative analysis obtained through microarray expression profiling.8,12 A biological function of Klhl14 sense and antisense in higher eukaryotes has not been described and is presently under investigation.
Owing to the overlapping expression of Klhl14as with that of key skeletogenic TFs, especially Pax1, we further analyzed the Klhl14as transcripts as represented by probe2 in relevant mouse mutant embryos. A decrease in functional Pax alleles correlated with a reduction in Klhl14as signal in the iAF. Notochordal Klhl14as seemed unaffected in these Pax mutants, possibly because Pax1 or Pax9 are not expressed in the notochord at this point. Hence, cells sorted for the Pax1/Pax9 lineage used in the transcriptome analysis should be of AF origin.8 As especially the caudal axial skeleton of Pax1 and Pax9 mutant mice is malformed, one could argue that cell loss is causative to reduced Klhl14as expression. However, as downregulation of Klhl14as in the Pax1 and Pax9 cell lineage was first identified after functional ablation of these TFs, Pax1/Pax9 cell-lineage sorting8 (Figure 1A), a role of Pax1 and Pax9 in maintaining Klhl14as transcription would be supported (Figure 4B). Pax1 and Pax9 have previously been linked with the Sox trio in their implication in IVD development and a negative feedback-loop between Pax1/Pax9 and Sox5/Sox6 was suggested.11 Interestingly, a nearly two-fold upregulation of 4930426D05Rik (Klhl14as) was identified in Sox5/Sox6 double mutant versus Sox6 heterozygous cells when mining the transcriptome data.12 Intriguingly, 4930426D05Rik (Klhl14as) and Pax1 transcripts were identified as upregulated in these mutant cells. 6330403N15Rik (Klhl14) was not identified as differentially regulated in the Sox5/Sox6 mutants. Transcriptome analysis for Sox9 and Bapx1 mutants was conducted with an earlier version of the Illumina microarray, which did not contain probes for 4930426D05Rik or 6330403N15Rik at the time. Both transcripts likely resulted from a genome-wide lncRNA screen in mouse conducted by the RIKEN FANTOM consortium.29,30 RISH suggests that Klhl14as expression is dependent on both Sox9 and Bapx1 expression in the iAF and on Bapx1 expression in the notochord. This suggests that, while Klhl14as is present throughout the developing IVD, it is differently regulated in iAF cells and those of notochordal origin. Sox6 and Sox9 unlike Bapx1 do not seem to be required for Klhl14as expression in the notochord, which in mouse will give rise to the NP. Whether these Sox TFs partially restrict Klhl14as expression in the notochord in the wildtype would require detailed single cell expression profiling of notochordal cells.
Figure 4.
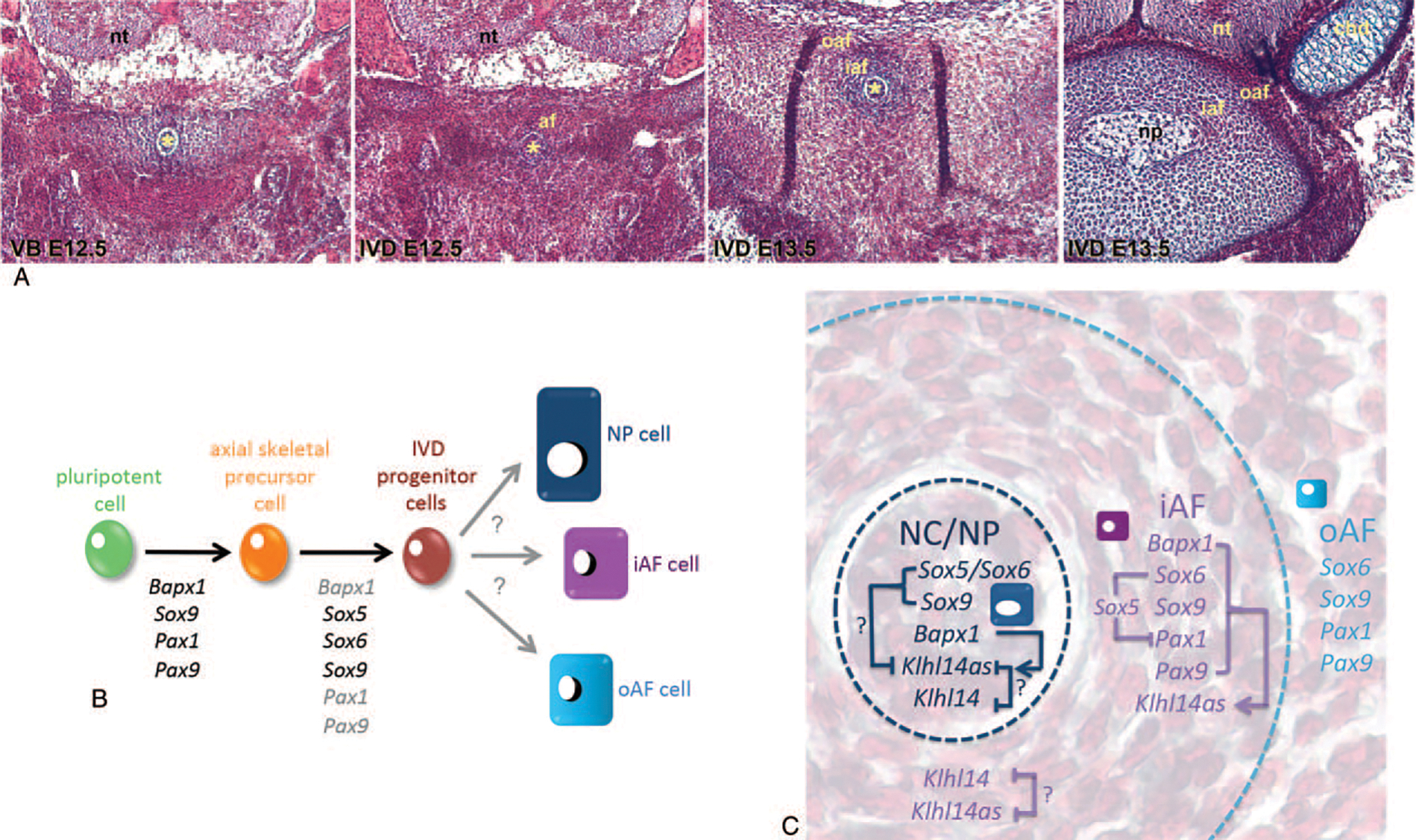
(A) Tetrachrome staining of sections through the developing murine vertebral body with loosely arranged cells surrounding the notochord (left) and more densely packed cells in the developing annulus fibrosus of the IVD, which clearly delineated into an inner and outer section by E15.5, when remnant notochordal cells form the nucleus pulposus. Chondrocytes as seen in the vertebral body protrusions are morphologically distinct from NP and AF cells. (B) Key orchestrating TFs of early cell lineage commitment have been identified through gene loss-of-function studies in mouse; however, less is known about how IVD progenitor cells differentiate toward the heterogeneous cell populations of a mature IVD. (C) Klh14as transcripts are differently impacted on in the notochord and inner annulus fibrosus. While a critical level of Pax transcripts seems necessary in the iAF, Sox6 and Sox9 are dispensable for Klhl14as expression in the NP, yet might keep Klhl14as transcript levels limited. Bapx1 expression is necessary in both iAF and notochordal cells to maintain Klhl14as. Klhl14as might regulate Klhl14 transcript availability in areas of coexpression. A role of Klhl14 in axial skeleton development has not been described so far. Arrows represent transcriptional and/or posttranscriptional regulation. Asterix: notochord; af indicates annulus fibrosus; cnd, chondrocytes; iaf, inner annulus fibrosus; oaf, outer annulus fibrosus; np, nucleous pulposus; nt, neural tube.
While seemingly of less complexity than most organs, the mature IVD with its unique environment remains largely a mystery when it comes to its resident cells. In mouse, drastic morphological changes occur within the nascent IVD between E12.5 to E15.5 (Figure 4A), resulting in a notochord-derived NP and a less well-studied inner and outer AF, comprised of cells that clearly differ histologically from chondrocytes (Figure 4A). Although many of the early determinants that drive differentiation from pluripotent stem cells to skeletal precursor cells and IVD progenitor cells have been investigated through mutagenesis in mouse models,8,11–13 factors that ultimately lead to the differentiation into heterogeneous groups of mature AF and NP cells remain to be defined (Figure 4B). Involvement of lnc NATs in regulation of transcription and translation is adding yet another level of complexity to this goal. Whether Klhl14as will merely serve as a biomarker for IVD cells in the future or have some important biological function ultimately needs to be addressed through loss- and gain-of-function analysis. However, from our study it is evident, that expression of Klhl14as in the iAF is dependent on key skeletogenic TFs like Pax1, Pax9, Sox6, Sox9, and Bapx1, while the regulation appears more complex in the notochord (Figure 4C). The overlap of the entire exon1 of the Klhl14as transcript with the Klhl14 mRNA suggests an antisense-type mechanism for at least two of the transcripts of this lnc NAT. Implications of Klhl14as in IVD development are possible based on the overlap of Klhl14as expression with that of key skeletogenic TFs. Furthermore, although no role of Klhl14 in axial skeleton development has been reported so far, it is possible that the skeletogenic TFs exert control over Klhl14 by regulating Klhl14as availability through at least two of the currently identified Klhl14as transcript variants in the NP and/or iAF of the developing murine IVD (Figure 4C).
CONCLUSION
We are the first to describe the expression of Klhl14as and Klhl14 in the developing axial skeleton and, more specifically, the IVD. Furthermore, we are the first to describe the differential control of Klhl14as by key skeletogenic TFs in the developing NP and AF of the murine IVD. Our data suggest a possible involvement of Klhl14as with an extensive vertebral-IVD gene regulatory network established previously and contribute further evidence that lncRNAs participate in complex gene regulatory networks in eukaryotic cells.
Key Points.
First description of the long nonconding RNA Klhl14as in IVD development.
Signaling cascades leading to the differentiation into heterogeneous groups of mature AF and NP cells remain to be defined.
Klhl14as is part of a complex vertebral-IVD gene regulatory network.
Klhl14as is differentially regulated by key skeletogenic transcription factors.
Klhl14as is differentially regulated in cells giving rise to the future nucleus pulposus and annulus fibrosus.
Acknowledgment
We are grateful to Sook Peng Yap, Sumantra Chatterjee, and Wenqing Jean Lee for sharing data.
This work was supported by the Bayard and Virginia Clarkson Endowment to Professor Thomas Lufkin.
References
- 1.Kraus P, Sivakamasundari V, Lim SL, et al. Making sense of Dlx1 antisense RNA. Dev Biol 2013;376:224–35. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Kraus WL. From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev 2015;36:25–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salviano-Silva A, Lobo-Alves SC, Almeida RC, et al. Besides pathology: long non-coding RNA in cell and tissue homeostasis. Noncoding RNA 2018;4:pii: E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen WK, Yu XH, Yang W, et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif 2017;50:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derrien T, Guigo R. [Long non-coding RNAs with enhancer-like function in human cells]. Med Sci (Paris) 2011;27:359–61. [DOI] [PubMed] [Google Scholar]
- 6.Wan ZY, Song F, Sun Z, et al. Aberrantly expressed long noncoding RNAs in human intervertebral disc degeneration: a microarray related study. Arthritis Res Ther 2014;16:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallin J, Mizutani Y, Imai K, et al. A new Pax gene, Pax-9, maps to mouse chromosome 12. Mamm Genome 1993;4:354–8. [DOI] [PubMed] [Google Scholar]
- 8.Sivakamasundari V, Kraus P, Sun W, et al. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol Open 2017;6:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters H, Neubuser A, Balling R. Pax genes and organogenesis: Pax9 meets tooth development. Eur J Oral Sci 1998;106 (suppl 1):38–43. [DOI] [PubMed] [Google Scholar]
- 10.Wilm B, Dahl E, Peters H, et al. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. Proc Natl Acad Sci U S A 1998;95:8692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivakamasundari V, Kraus P, Lufkin T. Regulatory functions of Pax1 and Pax9 in mammalian cells. In: Uchiumi F, editor. Gene Expression and Regulation in Mammalian Cells. Rijeka: InTech; 2018. pp. 181–207. [Google Scholar]
- 12.Lee WJ, Chatterjee S, Yap SP, et al. An integrative developmental genomics and systems biology approach to identify an in vivo sox trio-mediated gene regulatory network in murine embryos. Biomed Res Int 2017;2017:8932583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Sivakamasundari V, Yap SP, et al. In vivo genome-wide analysis of multiple tissues identifies gene regulatory networks, novel functions and downstream regulatory genes for Bapx1 and its co-regulation with Sox9 in the mammalian vertebral column. BMC Genomics 2014;15:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giles LM, Li L, Chin LS. Printor, a novel torsinA-interacting protein implicated in dystonia pathogenesis. J Biol Chem 2009;284:21765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Credendino SC, Lewin N, de Oliveira M, et al. Tissue- and cell type-specific expression of the long noncoding RNA Klhl14-AS in mouse. Int J Genomics 2017;2017:9769171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagman H, Amendola E, Parrillo L, et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev Biol 2011;359:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage 2001;9 (suppl A):S69–75. [DOI] [PubMed] [Google Scholar]
- 18.Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development 1999;126:5699–711. [DOI] [PubMed] [Google Scholar]
- 19.Sivakamasundari V, Kraus P, Jie S, et al. Pax1 (EGFP): new wildtype and mutant EGFP mouse lines for molecular and fate mapping studies. Genesis 2013;51:420–9. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee S, Kraus P, Sivakamasundari V, et al. A conditional mouse line for lineage tracing of Sox9 loss-of-function cells using enhanced green fluorescent protein. Biotechnol Lett 2013;35:1991–6. [DOI] [PubMed] [Google Scholar]
- 21.Kraus P, Yerden R, Kocsis V, et al. RNA in situ hybridization characterization of non-enzymatic derived bovine intervertebral disc cell lineages suggests progenitor cell potential. Acta Histochem 2017;119:150–60. [DOI] [PubMed] [Google Scholar]
- 22.Kraus P, Yerden R, Sipes D, et al. A quantitative and qualitative RNA expression profiling assay for cell culture with single cell resolution. Cytotechnology 2018;70:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus P, Lufkin T. Mammalian Dlx homeobox gene control of craniofacial and inner ear morphogenesis. J Cell Biochem 1999;suppl 32–33:133–40. [DOI] [PubMed] [Google Scholar]
- 24.Robledo RF, Lufkin T. Dlx5 and Dlx6 homeobox genes are required for specification of the mammalian vestibular apparatus. Genesis 2006;44:425–37. [DOI] [PubMed] [Google Scholar]
- 25.Choi KS, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev 2012;129:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutges J, Creemers LB, Dhert W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage 2010;18:416–23. [DOI] [PubMed] [Google Scholar]
- 27.Molinos M, Cunha C, Almeida CR, et al. Age-correlated phenotypic alterations in cells isolated from human degenerated intervertebral discs with contained hernias. Spine (Phila Pa 1976) 2018;43:E274–84. [DOI] [PubMed] [Google Scholar]
- 28.Kraus P, Sivakamasundari V, Xing X, et al. Generating mouse lines for lineage tracing and knockout studies. Methods Mol Biol 2014;1194:37–62. [DOI] [PubMed] [Google Scholar]
- 29.Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science 2005;309:1559–63. [DOI] [PubMed] [Google Scholar]
- 30.Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science 2005;309: 1564–6. [DOI] [PubMed] [Google Scholar]


