Abstract
INTRODUCTION
Use of ambulatory holmium laser enucleation of the prostate (HoLEP) is uncommon among Canadian urologists. Our objectives were to determine the feasibility (ambulatory success rate) and safety (early complication rate) of ambulatory HoLEP in a Canadian population.
METHODS
We prospectively evaluated consecutive patients from June 2020 to May 2022 presenting for ambulatory HoLEP using Moses™ technology at our institution (MoLEP). Ambulatory success was defined as no hospital admission within 48 hours following the procedure. Thirty-day adverse events were also identified and graded according to the Clavien-Dindo (CD) classification. All procedures were planned to be ambulatory regardless of prostate size or anticoagulant treatment. We generated a logistic regression model to identify factors associated with ambulatory failure.
RESULTS
A total of 61 patients underwent MoLEP, 52 of whom met the eligibility criteria. The mean age was 71.0 years (standard deviation 6.2). Most patients (67%, 35/52) were catheter or self-catheterization-dependent. The ambulatory success rate was 87% (45/52); 6/52 (11.5%) required hospitalization following MoLEP and one patient (2%) was re-admitted within 48 hours of the procedure. Hematuria was the sole cause of ambulatory failure. Thirty-day major complication rate (CD ≥3) was 6% (3/52) and the minor complication rate (CD <3) was 37% (19/52). The identified adverse events included hematuria (10/52), urinary retention (6/52), and cystitis (4/52). Based on univariate analysis, we did not identify factors significantly associated with ambulatory failure.
CONCLUSIONS
The MoLEP ambulatory success rate is high, and the 30-day major adverse event rate is low. In this small, Canadian cohort, ambulatory MoLEP seems feasible and safe.
INTRODUCTION
Benign prostatic hyperplasia (BPH) affects up to 60% of the male population by the age of 60 years.1 Holmium laser enucleation of the prostate (HoLEP) is a minimally invasive surgical procedure for BPH. It can be performed on a prostate of any size.2–4 Although the technique is associated with a steep learning curve, its efficacy is well-proven.5–7 The efficacy and safety of HoLEP are comparable or superior to transurethral resection of prostate (TURP) independent of prostate size.8,9 With the development of a new generation of holmium laser (Moses Effect™), “MoLEP” has resulted in shorter operative time and reduction in blood loss, positioning HoLEP more favorably for ambulatory surgery.10,11
Given the benefits of laser enucleation, some experts have assessed the feasibility and safety of this minimally invasive procedure in an ambulatory setting.12–19 Ambulatory surgery (or day-case surgery) was initially strictly described by Comat et al in a prospective study, as a hospital stay of less than 12 hours and no medical assistance for the first 48 hours.15 According to a meta-analysis by Salciccia et al, the pooled ambulatory failure rate of HoLEP is 11.8% (95% confidence interval [CI] 7–16.7) and the complication rate is similar to inpatient HoLEP.20
Since the start of COVID-19 pandemic, many BPH procedures requiring hospitalization have been postponed. As suggested by Medina-Polo et al, BPH procedures with the lowest complication rate and the shortest hospital stay should be encouraged during the COVID-19 crisis.21 Ambulatory HoLEP does seem to meet these criteria and endoscopic BPH laser procedures have been gaining in popularity among Canadian urologists.22,23 Recently, a group of Canadian urologists showed that, for selected patients, same-day catheter removal is a safe option;12 however, more data are needed in Canada regarding the possible management of laser enucleation procedures, particularly in an ambulatory setting. Therefore, this study aimed to confirm the feasibility (ambulatory success rate) and safety (early complication rate) of ambulatory MoLEP in a prospective Canadian cohort. The secondary objectives of this study were to identify potential clinical factors associated with MoLEP ambulatory failure and to assess functional outcomes.
METHODS
Study design and population
We performed a prospective, observational study on all consecutive patients who presented for MoLEP procedure from June 2020 to May 2022 at our university-affiliated hospital center. All procedures were planned to be ambulatory regardless of prostate size or anticoagulant treatment, unless the preoperative internal medicine team’s evaluation suggested unstable medical comorbidities requiring hospitalization, if patients were unaccompanied the night of the operation, or if they lived more than a one-hour drive away from any hospital center. Patients were excluded from the study if they had had a past prostate surgery affecting the prostate capsular dissection plan (i.e., transurethral needle ablation), if they had sole median lobe enucleation, or if they lacked the capacity to give informed consent. The study protocol was approved by our institutional review board (databank 2021–3909).
Ambulatory success was defined as an hospital stay of less than 12 hours and no hospital admission within 48 hours following the procedure.15 In our institution, MoLEP is essentially performed on prostates greater than 80 grams (patients referred from colleagues and from non-university hospitals in the region). We also included patients with smaller prostates enlisted by the surgeon.
Data collection
Prior to MoLEP, patients were assessed with a full history and physical exam, including digital rectal exam, the International Prostate Symptom Score (IPSS), uroflow studies, postvoid residual (PVR), blood count, kidney function, and prostate-specific antigen (PSA). Preoperative prostate volume was assessed via transrectal ultrasound. Perioperative data were also collected. In the month following the procedure, any emergency consultations or hospitalizations were documented. All adverse events were graded according to the Clavien-Dindo (CD) classification and counter-verified with the patient at the one-month followup.24 Uroflow studies, blood work, and IPSS questionnaire were repeated at the three-month followup. All data were prospectively collected in our databank.
Preoperative evaluation
During the preoperative consultation, patients were briefed about the ambulatory MoLEP procedure and received documentation describing the surgery and possible complications. Urine cultures were collected one week prior to MoLEP. Patients were treated with a 72-hour course of adapted antibiotics (48 hours pre-MoLEP and 24 hours post-MoLEP) if the urine culture was positive and/or if they were catheter-dependent. Antiplatelet and anticoagulant therapies were suspended before surgery and re-started 48 hours postoperatively.
Perioperative care and technique
The day of the surgery, HoLEP was performed using an “en bloc” technique with a holmium Lumenis® Moses pulse 120 W laser.25 All procedures were performed by the same surgeon (experience of 100–150 cases after a dedicated fellowship) during the morning operative period. The type of anesthesia was determined by the attending anesthesiologist. Single-dose intravenous cefazoline was given to all patients at induction. Tobramycin or vancomycin was given if patients had a known penicillin allergy. A 550 μm laser fiber was used with a 26F Storz® endoscope for all cases. The laser settings were 2 J at 45 Hz for enucleation and 1 J at 20 Hz for coagulation. A Storz® morcellator was used for prostate tissue morcellation. Meticulous hemostasis was obtained with the holmium laser before insertion of a 20 Fr three-way catheter inflated to 40 mL. Continuous bladder irrigation was administered for 3–4 hours after the operation, followed by a single 20 mg intravenous furosemide dose. If hematuria was minimal (hematuria score of ≤4 without continuous bladder irrigation13), the patient was discharged with the catheter. All patients were advised to properly self-hydrate and to consult our institution’s emergency room in case of any adverse events. The day after MoLEP, the urinary catheter was removed in the morning, and voiding trials were overseen by local community care centers.
Statistical analysis
We performed a descriptive analysis of the pre- and postoperative patient characteristics, as well as ambulatory success and complication rates. We substratified the data into two categories: MoLEP ambulatory success patients and MoLEP ambulatory failure patients. We compared the groups using Fisher’s exact test and the Mann-Whitney U test. We used a penalized logistic regression model to assess potential risk factors for failure. We performed univariate analysis for each of the variables collected and present the results as odds ratios (ORs) with 95% CIs. We set the threshold of significance at p <0.05. We used SPSS Statistics version 28 (IBM Corp., Armonk, NY, U.S.) and R version 4.0.2 (R Core Team, Vienna, Austria) for analyses.
RESULTS
A total of 61 patients underwent MoLEP. As shown in Figure 1, 52 patients met the eligibility criteria and were included in our analysis. The median age was 71.0 years (standard deviation [SD] 6.2). Thirty-five (67.3%) patients were catheter- or self-catheterization-dependant for an average of 9.9 months (SD 5.9) prior to the operation. In total, four patients had undergone prior TURP, 12 were on antiplatelet therapy, and five were on anticoagulant therapy. The type of anesthesia was variable: 21/52 (40%) patients had spinal anesthesia, 16/52 (31%) patients had laryngeal mask airway anesthesia, and 15/52 (29%) patients were intubated. The mean postoperative time to discharge was 9.8 hours (SD 11.7) and the mean time to postoperative catheter removal was 1.1 days (SD 0.3).
Figure 1.
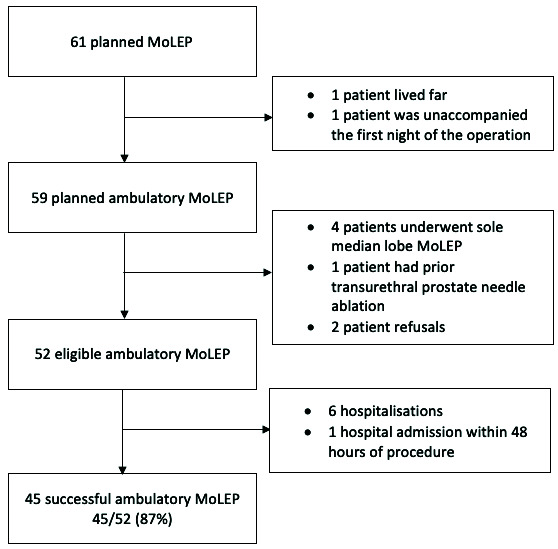
Study flow chart. MoLEP: Moses™ technology for ambulatory holmium laser enucleation of the prostate.
The ambulatory success rate was 87% (45/52). As shown in Figure 1, six patients were hospitalized following MoLEP and one patient was re-admitted after consulting the emergency room within 48 hours of the operation. Hematuria was the sole cause of ambulatory failure.
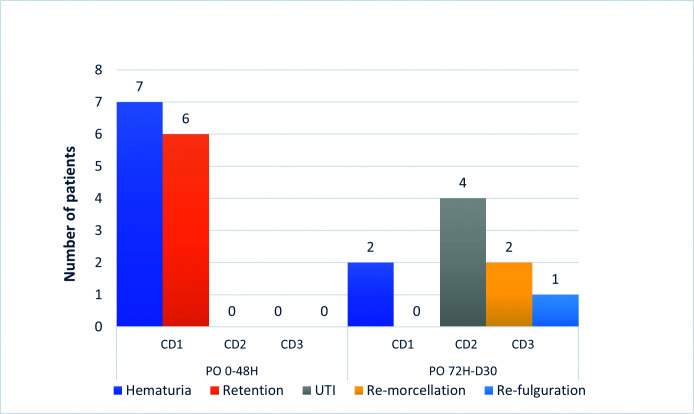
Hospitalizations occurring between postoperative days 3 and 30 included two patients requiring re-morcellation and one patient requiring re-operation for hemostasis. All other patients that presented with a postoperative complication were handled by general practitioners (outpatient care). Complications classified according to their time of occurrence within or after 48 hours are depicted in Figure 2.
Figure 2.
Thirty-day postoperative complications’ timeline according to the Clavien-Dindo score (CD). Hematuria required continuous bladder irrigations. Re-fulguration and re-morcellation were performed under general anesthesia. UTI: urinary tract infection.
The overall 30-day complication rate was 42% (22/52); major complications (CD ≥3) accounted for 6% (3/52). A total of 10 patients had hematuria, nine requiring continuous bladder irrigation (CD 1) and one requiring re-operation for hemostasis on postoperative day 16 (CD 3A). No patient required blood transfusions. Four patients presented symptoms of cystitis and had a positive urine culture that required oral antibiotics (CD 2). There was no urinary sepsis. Six patients had urinary retentions (CD 1). Of these urinary retentions, two were caused by blood clots, two were later diagnosed with acontractile bladder, and two were caused by residual intravesical prostate adenoma obstructing the bladder outlet. The last two patients required re-morcellation of the remaining intravesical prostate adenoma tissue (CD 3B).
Demographic and pre-, peri-, and postoperative data are summarized and substratified into two groups in Tables 1 and 2. There were few significant differences in the variables between the MoLEP ambulatory success and the MoLEP ambulatory failure groups. The operative time was longer in the failure group compared with the success group (126.1±47.5 vs. 97.4±30.4 minutes, p=0.046). Postoperative hemoglobin levels were lower in the failure group (120.5±16.6 vs. 142.8±12.2 g/L, p=0.002). Patients in the ambulatory failure group had higher morcellated prostate volumes (72.1±35.7 vs. 55.3± 2.4 cm3, p=0.3) and catheter dependence (85.7% vs. 64.4%, p=0.6) than in the ambulatory success group, although these differences were not significant.
Table 1.
Demographic and preoperative data
| Ambulatory success (n=45) | Ambulatory failure (n=7) | Total (n=52) | p | |
|---|---|---|---|---|
|
| ||||
| Age, years | 0.355 | |||
| Mean (SD) | 70.6 (5.8) | 73.5 (8.3) | 71.0 (6.2) | |
| Median | 70.8 | 74.6 | 71.0 | |
| Range | (54.8–83.8) | (61.2–83.7) | (54.8–83.8) | |
|
| ||||
| Prior TURP surgery, n (%) | 3 (6.7) | 1 (14.3) | 4 (7.7) | 0.450 |
|
| ||||
| Prior prostate embolization, n (%) | 4 (8.9) | 0 (0) | 4 (7.7) | 1.000 |
|
| ||||
| Prior abdominal surgery, n (%) | 14 (31.1) | 1 (14.3) | 15 (28.8) | 0.658 |
|
| ||||
| Diabetes, n (%) | 6 (13.3) | 0 (0) | 6 (11.5) | 0.580 |
|
| ||||
| Known prostate cancer under active surveillance protocol, n (%) | 5 (11.1) | 0 (0) | 5 (9.6) | 1.000 |
|
| ||||
| Antiplatelet therapy, n (%) | 10 (22.2) | 2 (28.6) | 12 (23.1) | 0.656 |
|
| ||||
| Anticoagulant therapy, n (%) | 5 (11.1) | 0 (0) | 5 (9.6) | 1.000 |
|
| ||||
| α-blocker use, n (%) | 38 (84.4) | 6 (85.7) | 44 (84.6) | 1.000 |
|
| ||||
| 5-α reductase inhibitor use, n (%) | 26 (57.8) | 5 (71.4) | 31 (59.6) | 0.687 |
|
| ||||
| Urinary retention total, n (%) | 29 (64.4) | 6 (85.7) | 35 (67.3) | 0.601 |
| Catheter-dependent retention, n (%) | 16 (35.6) | 3 (42.9) | 19 (36.5) | |
| Self-catheterization-dependent retention, n (%) | 13 (28.9) | 3 (42.9) | 16 (30.8) | |
|
| ||||
| Time of catheter or self-catheterization dependence (months) | 0.442 | |||
| Mean (SD) | 10.7 (6.3) | 8.0 (1.8) | 10.2 (5.9) | |
| Median | 9.0 | 7.5 | 9.0 | |
| Range | (2–26) | (6–11) | (2–26) | |
|
| ||||
| Hemoglobin (g/L) | 0.324 | |||
| Mean (SD) | 143.6 (16.4) | 139.6 (11.4) | 143.0 (15.8) | |
| Median | 144.0 | 140.0 | 144.0 | |
| Range | (62.0–176.0) | (118.0–151.0) | (62.0–176.0) | |
|
| ||||
| Prostate-specific antigen (ng/mL) | 0.820 | |||
| Mean (SD) | 6.6 (5.4) | 7.0 (7.4) | 6.6 (5.7) | |
| Median | 5.1 | 4.2 | 4.9 | |
| Range | (0.9–26.0) | (0.8–22.9) | (0.8–26.0) | |
|
| ||||
| Serum creatinine (micromole/L) | 0.494 | |||
| Mean (SD) | 92.4 (24.8) | 85.0 (20.1) | 91.4 (24.2) | |
| Median | 84.0 | 79.0 | 84.0 | |
| Range | (63.0–183.0) | (56.0–117.0) | (56.0–183.0) | |
|
| ||||
| Prostate volume (cm 3 ) | 0.108 | |||
| Mean (SD) | 106.9 (43.3) | 138.0 (44.5) | 111.1 (44.3) | |
| Median | 104.0 | 131.0 | 107.5 | |
| Range | (41.8–200.0) | (92.3–221.0) | (41.8–221.0) | |
SD: standard deviation; TURP: transurethral resection of the prostate.
Table 2.
Perioperative and postoperative data
| Ambulatory success (n=45) | Ambulatory failure (n=7) | Total (n=52) | p | |
|---|---|---|---|---|
|
| ||||
| ASA score ≥ 3, n (%) | 10 (22.2) | 0 (0) | 10 (19.2) | 0.322 |
|
| ||||
| Anesthetic type, n (%) | 0.613 | |||
| Spinal | 17 (37.8) | 4 (57.1) | 21 (40.4) | |
| Laryngeal mask airway | 14 (31.1) | 2 (28.6) | 16 (30.8) | |
| Intubation | 14 (31.1) | 1 (14.3) | 15 (28.8) | |
|
| ||||
| Surgery time (min) | 0.046 | |||
| Mean (SD) | 97.4 (30.4) | 126.1 (47.5) | 101.3 (34.1) | |
| Median | 90.0 | 109.0 | 91.0 | |
| Range | (56.0–202.0) | (88.0–225.0) | (53.0–225.0) | |
|
| ||||
| Delivered energy (J) | 0.604 | |||
| Mean (SD) | 93.1 (26.4) | 83.0 (10.4) | 92.2 (25.4) | |
| Median | 86.6 | 80.9 | 84.6 | |
| Range | (38.9–140.2) | (72.6–97.4) | (38.9–140.2) | |
|
| ||||
| Morcellated prostate volume (g) | 0.302 | |||
| Mean (SD) | 55.3 (22.4) | 72.1 (35.7) | 57.5 (24.9) | |
| Median | 53.0 | 72.0 | 53.5 | |
| Range | (20.0–111.5) | (31.5–127.5) | (20.0–127.5) | |
|
| ||||
| Benign pathology, n (%) | 37 (82.2) | 7 (100.0) | 44 (84.6) | 0.578 |
|
| ||||
| Hemoglobin (g/L) | 0.002 | |||
| Mean (SD) | 142.8 (12.2) | 120.5 (16.6) | 137.2 (16.3) | |
| Median | 143.0 | 126.5 | 138.5 | |
| Range | (118.0–170.0) | (92.0–136.0) | (92.0–170.0) | |
| Change from baseline (SD) | −6.9 (8.3) | −19.2 (17.4) | −10.4 (12.5) | |
|
| ||||
| Prostate-specific antigen(ng/mL) | 0.543 | |||
| Mean (SD) | 0.8 (0.8) | 1.1 (1.8) | 0.8 (0.9) | |
| Median | 0.6 | 0.5 | 0.6 | |
| Range | (0.1–2.9) | (<0.1–4.7) | (<0.1–4.7) | |
| Change from baseline (SD) | −6.3 (5.5) | −3.3 (2.1) | −5.7 (5.3) | |
|
| ||||
| Serum creatinine (micromole/L) | 0.346 | |||
| Mean (SD) | 91.3 (20.4) | 81.0 (22.5) | 89.8 (20.8) | |
| Median | 88.5 | 78.0 | 88.0 | |
| Range | (65.0–155.0) | (46.0–115.0) | (46.0–155.0) | |
| Change from baseline (SD) | 0.7 (13.3) | −4.0 (10.0) | 0.0 (12.9) | |
ASA: American Society of Anesthesiologists; SD: standard deviation.
The factors associated with MoLEP ambulatory failure (univariate analysis) are listed in Table 3. Although operative time was significantly longer in the ambulatory failure group (p=0.046), it was not significantly associated with ambulatory failure (OR 1.02, 95% CI 0.99–1.05, p=0.07). The prostate volume and morcellated volume also tended to be associated with ambulatory failure, although not significantly (OR 1.02, 95% CI 1.00–1.04, p=0.11 and OR 1.02, 95% CI 0.99–1.06, p=0.12, respectively). Antiplatelet and anticoagulant therapies were not associated with ambulatory failure (OR 1.54, 95% CI 0.18–7.66, p=0.62 and OR 0.49, 95% CI 0.0–5.20, p=0.67, respectively).
Table 3.
Strength of association between risk factors and ambulatory failure according to univariable penalized logistic regression
| OR (95% confidence interval) | p | |
|---|---|---|
|
| ||
| Preoperative | ||
|
| ||
| Age | 1.08 (0.95–1.30) | 0.27 |
|
| ||
| Prior TURP surgery | 2.80 (0.11–22.05) | 0.38 |
|
| ||
| Prior prostate embolization | 0.61 (0.00–6.80) | 0.78 |
|
| ||
| Prior abdominal surgery | 0.50 (0.02–2.71) | 0.48 |
|
| ||
| Diabetes | 0.41 (0.00–4.10) | 0.58 |
|
| ||
| Known prostate cancer under active surveillance protocol | 0.49 (0.00–5.20) | 0.67 |
|
| ||
| Antiplatelet therapy | 1.54 (0.18–7.66) | 0.62 |
|
| ||
| Anticoagulant therapy | 0.49 (0.00–5.20) | 0.67 |
|
| ||
| α-blocker use | 0.84 (0.14–22.53) | 0.87 |
|
| ||
| 5-α reductase inhibitor use | 1.62 (0.35–13.70) | 0.56 |
|
| ||
| Urinary retention total | ||
| Catheter-dependent retention | 2.33 (0.34–64.27) | 0.43 |
| Self-catheterization-dependent retention | 2.85 (0.41–79.66) | 0.33 |
|
| ||
| Time of catheter or self-catheterization dependence | 0.93 (0.68–1.10) | 0.44 |
|
| ||
| Prostate-specific antigen | 1.03 (0.86–1.15) | 0.68 |
|
| ||
| Prostate volume | 1.02 (1.00–1.04) | 0.11 |
|
| ||
| ASA score ≥ 3 | 0.23 (0.00–2.13) | 0.34 |
|
| ||
| Perioperative | ||
|
| ||
| Anesthetic type | ||
| Laryngeal mask airway | 0.67 (0.08–3.60) | 0.65 |
| Intubation | 0.40 (0.02–2.49) | 0.38 |
|
| ||
| Surgery time | 1.02 (0.99–1.05) | 0.07 |
|
| ||
| Delivered energy | 0.99 (0.93–1.00) | 0.51 |
|
| ||
| Morcellated prostate volume | 1.02 (0.99–1.06) | 0.12 |
|
| ||
| Postoperative | ||
|
| ||
| Benign pathology | 3.40 (0.35–inf.) | 0.44 |
|
| ||
| Prostate-specific antigen | 1.41 (0.54–2.98) | 0.37 |
Table 4 presents data regarding the functional outcomes at three months. There were clinically significant changes from baseline in IPSS (−11.3, SD 8.2), IPSS quality of life (−2.7, SD 2.3), PVR volume (−177.4, SD 147.5), and peak urinary flow (QMax; +16.0, SD 12.5) at the three-month post-MoLEP followup. Most importantly, the number of catheter- or self-catheterization-dependent patients dropped from 67.3% (35/52) to 3.8% (2/52) after MoLEP.
Table 4.
Three-month postoperative functional outcomes
| Preoperative (n=45) | 3 months postoperative (n=45) | |
|---|---|---|
|
| ||
| IPSS * | ||
| Mean (SD) | 18.1/35 (7.6/35) | 6.7/35 (4.3/35) |
| Median | 19.0/35 | 6.0/35 |
| Range | (0/35–30/35) | (0/35–18/35) |
| Change from baseline (SD) | – | −11.3 (8.2) |
|
| ||
| IPSS quality of life * | ||
| Mean (SD) | 4.5/6 (1.6/6) | 1.7/6 (1.8/6) |
| Median | 4.5/6 | 1.0/6 |
| Range | (0/6–6/6) | (0/6–6/6) |
| Change from baseline (SD) | – | −2.7 (2.3) |
|
| ||
| Postvoid residual volume (ml) * | ||
| Mean (SD) | 219.1 (148.9) | 75.0 (71.3) |
| Median | 167.0 | 44.0 |
| Range | (60.0–600.0) | (0–259.0) |
| Change from baseline (SD)* | – | −177.4 (147.5) |
|
| ||
| Qmax (mL/second) ** | ||
| Mean (SD) | 4.1 (6.5) | 19.8 (11.2) |
| Median | 0.0 | 17.0 |
| Range | (0.0–36.0) | (3.5–49.0) |
| Change from baseline (SD)* | – | +16.0 (12.5) |
|
| ||
| Catheter or self-catheterization-dependent, n (%) | 35 (67.3) | 2 (3.8) |
Only applies to patients non-catheter or self-catheterization-dependent.
Patients who were catheter or self-catheterization-dependent were considered to have a Qmax of 0.
IPSS: International Prostate Symptom Score; SD: standard deviation; Qmax: maximum urinary flow rate.
DISCUSSION
This study, aiming to assess the feasibility and safety of ambulatory MoLEP procedures, represents the second series in a Canadian population.12 Our ambulatory success rate was high (87%) and concordant with most of the literature. Salciccia et al reported, in a meta-analysis on outpatient management of BPH procedures, a variable HoLEP ambulatory failure rate ranging from 2.5–51.1%.20 The pooled failure rate was 11.8% (95% CI 7–16.7). This is also in line with a recent large prospective series by Agarwal et al (n=207), with a successful HoLEP same-day discharge of 87.4%.14 The ambulatory success rate may vary according to its definition. Compared with Comat and colleagues, who also used a strict 48-hour window to define ambulatory failure but used a 100 W holmium laser, the success rate was 80% in 201515 and improved to 87% in 2019,26 showing that the experience gained by the surgeon and also the team over time plays an important role in increasing ambulatory success. It also highlights that such ambulatory success rates (87%) are achievable with less experience (our institution) and that the laser technology might not be a determining factor, as shown in the meta-analysis by Gauhar et al.11 Indeed, in that review, the length of postoperative stay did not significantly favor MoLEP over HoLEP.
Regarding the specific advantages when using Moses™ technology, studies have found shorter operative time and reduction in blood loss.10,11 Day-case MoLEP has not been as well-documented as standard HoLEP. Nottingham and colleagues had a rate of same-day discharge of 69%;27 however, they had recently transitioned to an ambulatory setting three months prior, which may explain the lower rate of success. This may also reinforce the importance of ambulatory protocols and teamwork, considering the two surgeons were highly experienced (≥200 procedures). On a more promising note, Assmus et al showed that 86% of patients (32/37) undergoing MoLEP and using anticoagulation or antiplatelet medications could be discharge the same day, and that less than 2% were re-admitted within 90 days.28
Our inclusion criteria were liberal: we included all prostate volumes, patients with indwelling catheters, patients with past TURP or past prostate embolization, and patients on antiplatelet and/or anticoagulant therapy. This renders ambulatory HoLEP more accessible but is also at risk for increasing the ambulatory failure and complication rates. Mouton et al identified age, the American Society of Anaesthesiology (ASA) score, anticoagulant therapy, the surgeon’s experience, and operative time as factors associated with ambulatory HoLEP early complications.19 This overlaps with the findings of Comat et al, who identified age and the ASA score as risk factors for ambulatory failure in a multivariate analysis.15 On the other hand, Lee at al found that small prostates (≤40 g) and morning operations had a higher rate of successful day-case HoLEP.17
Our data partly correlate with the above findings; MoLEP ambulatory failure patients who were hospitalized had significantly longer operative times. Although not significant, the ambulatory failure group had larger preoperative prostate volumes and higher morcellated prostate volumes. Larger prostates led to longer operative times and hypothetically increased the chance of complications, therefore increasing the risk of day-case surgery failure. We also noted that patients who depended on catheters or on self-catheterization had higher ambulatory failure rates compared with the ambulatory success group, but this was not significant. We hypothesize that these indwelling catheters lead to inflammatory and well-vascularized prostates, making HoLEP operations technically challenging. Gross hematuria was the most common post-MoLEP complication and can solely explain the lower postoperative hemoglobin in the day-case surgery failure group. In our univariate analysis, we did not identify factors associated with MoLEP ambulatory failure, certainly due to our low rate of failure (7/52 patients).
The reported complication rates of ambulatory HoLEP can be highly variable, from 12.8–56.7%.20 Nonetheless, our 30-day major complications (CD ≥3) rate was low at 6% (3/52), and the overall complication rate was 42%. This is consistent with the findings reported by Comat et al, who reported a 37% rate of overall complications on 90 consecutive patients.15 Gross hematuria has been reported as the predominant factor leading to ambulatory failure (the cause of failure in 25–87% of cases), while urinary infections and acute urinary retentions seem to be more prevalent within the first postoperative month.13,15,17,29 Hematuria was the sole cause of ambulatory failure in our cohort, reinforcing our appropriate preoperative planning because no patients refused to leave for social/anxiety reasons. Abdul-Muhsin et al reported an 8.5% ambulatory failure rate for these reasons.13 Urinary retentions were attributable to blood clots, residual adenoma, or acontractile bladders. No patient experienced urinary retention due to a failure to alleviate prostatic urethra obstruction, which further reinforces the efficacy of HoLEP. Kim et al found that 35% of urinary retentions following HoLEP were not caused by a blood clot.30 In addition, two of our patients experienced urinary obstruction secondary to residual intravesical prostatic adenoma and required re-morcellation (CD 3). These findings further stress the importance of adequate perioperative hemostasis to allow proper intravesical visibility and therefore, to achieve complete prostatic adenoma morcellation.
The complication rate of ambulatory MoLEP we found is similar to that of inpatient HoLEP.20,31 Agarwal et al did not find any differences in the 90-day complication rate and the CD ≥3 complication rate between planned inpatient HoLEP, unplanned inpatient HoLEP, and ambulatory HoLEP, even though this was not their primary objective.14
Proper HoLEP technique, aggressive hemostasis with the holmium laser, and adequate antibiotic prophylaxis contributed to our relatively low major complication rates. By doing so, and with the support of local community care centers for catheter removal, we could perform day-case MoLEP during the COVID-19 pandemic. Since the start of this pandemic, many Canadian hospitals have been restricting operations requiring hospitalization, mostly because of the limited number of available hospital beds and human resources. The advantages of avoiding most hospitalizations with day-case MoLEP operations include increasing the availability of human resources, maintaining accessibility to BPH procedures in the COVID-19 era, and reducing medical expenses.13,18,32 Moreover, because our functional outcomes are comparable to that of inpatient procedures,5,6 accessible MoLEP during the COVID-19 crisis permitted our patients to attain better quality of life in a reasonable amount of time.
Limitations
This series represents a prospective study in a Canadian population in a healthcare system strained by the COVID-19 pandemic, further supporting the feasibility of an ambulatory HoLEP setting. This study does have a few limitations.
The cohort is relatively small and limited to a single center, with a surgeon who had performed 100–150 procedures after a dedicated fellowship; however, these results are consistent with studies from more experienced surgeons (>250 patients12,26), showing that great success rates in ambulatory HoLEP can be achieved before reaching such experience. The improvement in the ambulatory success rate over time has recently been shown by Klein et al, who reported 70% ambulatory success for the first 88 patients and 87% after more than 178 patients.26 There is also a possibility of inherent selection bias. Peaks of COVID-19 outbreaks restrained our access to the operating room for urological functional surgeries and limited patient recruitment for this study. Therefore, our statistical analysis was limited to univariate penalized logistic regressions. In addition, the low rate of failure (only 7/52 patients) certainly explains the lack of independent variables associated with ambulatory failure. Finally, patient discharge was subjective according to the attending urologist. Even though all MoLEP surgeries were performed, and patients were evaluated by the same urologist, discharge criteria could have been variable.
CONCLUSIONS
Ambulatory MoLEP is both safe and feasible. The successful same-day discharge rate was high (87%) and the 30-day major complication rate was low (6%) when performed by an experienced surgeon and nursing team and in collaboration with internists to assess and manage the bleeding risk. Adverse events can, for the most, be managed on an outpatient basis. We did not identify factors associated with ambulatory failure; however, the operative time and hemoglobin drop were significantly higher in the ambulatory failure group. Given its many benefits, widespread adoption of ambulatory HoLEP should be considered across Canada.
KEY MESSAGES.
■ Moses™ technology for ambulatory holmium laser enucleation of the prostate has high success (87%) and low major complication rates (Clavien-Dindo classification score ≥3) (6%).
■ Hematuria was the sole cause of ambulatory failure.
ACKNOWLEDGMENTS
The authors would like to thank Samuel Lemaire-Paquette and Catherine Allard, statisticians from the Centre de recherche du CHUS, for their support in the development of statistics and contribution to the elaboration of the statistical analysis subsection of the article. The authors would also like to express gratitude to the nurses of our surgical department, Mathieu Simard and Diane Rodrigue, for helping out and making these HoLEP procedures possible in our institution.
Footnotes
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline part I-Initial work-up and medical management. J Urol. 2021;206:806–17. doi: 10.1097/JU.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 2.Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline part II-Surgical evaluation and treatment. J Urol. 2021;206:818–26. doi: 10.1097/JU.0000000000002184. [DOI] [PubMed] [Google Scholar]
- 3.Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate vs. open prostatectomy for prostates greater than 100 grams: 5-year followup results of a randomized clinical trial. Eur Urol. 2008;53:160–6. doi: 10.1016/j.eururo.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Krambeck AE, Handa SE, Lingeman JE. Holmium laser enucleation of the prostate for prostates larger than 175 g. J Endourol. 2010;24:433–7. doi: 10.1089/end.2009.0147. [DOI] [PubMed] [Google Scholar]
- 5.Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): Long-term results, reoperation rate, and possible impact of the learning curve. Eur Urol. 2007;52:1465–71. doi: 10.1016/j.eururo.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 6.Fallara G, Capogrosso P, Schifano N, et al. Ten-year followup results after holmium laser enucleation of the prostate. Eur Urol Focus. 2021;7:612–7. doi: 10.1016/j.euf.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Otero J, García-Gómez B, García-González L, et al. Critical analysis of a multicentric experience with holmium laser enucleation of the prostate for benign prostatic hyperplasia: Outcomes and complications of 10 years of routine clinical practice. BJU Int. 2020;126:177–82. doi: 10.1111/bju.15028. [DOI] [PubMed] [Google Scholar]
- 8.Das AK, Han TM, Hardacker TJ. Holmium laser enucleation of the prostate (HoLEP): Size-independent gold standard for surgical management of benign prostatic hyperplasia. Can J Urol. 2020;27:44–50. [PubMed] [Google Scholar]
- 9.Magistro G, Schott M, Keller P, et al. Enucleation vs. resection: A matched-pair analysis of TURP, HoLEP, and bipolar TUEP in medium-sized prostates. Urology. 2021;154:221–6. doi: 10.1016/j.urology.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Kavoussi NL, Nimmagadda N, Robles J, et al. MOSES™ technology for holmium laser enucleation of the prostate: A prospective, double-blind, randomized controlled trial. J Urol. 2021;206:104–8. doi: 10.1097/JU.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 11.Gauhar V, Gilling P, Pirola GM, et al. Does MOSES technology enhance the efficiency and outcomes of standard holmium laser enucleation of the prostate? Results of a systematic review and meta-analysis of comparative studies. Eur Urol Focus. 2022;8:1362–9. doi: 10.1016/j.euf.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Noureldin Y, Gupta A, Hodhod A, et al. Same-day trial of void and discharge following standard vs. MOSES™ holmium laser enucleation of the prostate: A single-center experience. Can Urol Assoc J. 2023;17:E23–8. doi: 10.5489/cuaj.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul-Muhsin H, Critchlow W, Navaratnam A, et al. Feasibility of holmium laser enucleation of the prostate as a 1-day surgery. World J Urol. 2020;38:1017–25. doi: 10.1007/s00345-019-02831-6. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal DK, Large T, Tong Y, et al. Same-day discharge is a successful approach for the majority of patients undergoing holmium laser enucleation of the prostate. Eur Urol Focu. 2022;8:228–34. doi: 10.1016/j.euf.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Comat V, Marquette T, Sutter W, et al. Day-case holmium laser enucleation of the prostate: Prospective evaluation of 90 consecutive cases. J Endourol. 2017;31:1056–61. doi: 10.1089/end.2017.0196. [DOI] [PubMed] [Google Scholar]
- 16.Gabbay G, Bernhard J-C, Renard O, et al. [Holmium laser enucleation of the prostate as a day case surgery: Prospective evaluation of the first 30 patients]. Prog Urol. 2015;25:34–9. doi: 10.1016/j.purol.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 17.Lee S-M, Gordon K, McMillan R, et al. Day-case holmium laser enucleation of the prostate: Feasibility, safety, and predictive factors. Ann R Coll Surg Engl. 2018;100:475–9. doi: 10.1308/rcsann.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lwin AA, Zeng J, Evans P, et al. Holmium laser enucleation of the prostate is safe and feasible as a same-day surgery. Urology. 2020;138:119–24. doi: 10.1016/j.urology.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Mouton M, Michel C, Bourgi A, et al. [Holmium laser enucleation of the prostate: Analysis of early complications. Patient selection for day-case surgery]. Prog Urol. 2020;30:89–96. doi: 10.1016/j.purol.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Salciccia S, Del Giudice F, Maggi M, et al. Safety and feasibility of outpatient surgery in benign prostatic hyperplasia: A systematic review and meta-analysis. J Endourol. 2021;35:395–408. doi: 10.1089/end.2020.0538. [DOI] [PubMed] [Google Scholar]
- 21.Medina-Polo J, Téigell Tobar J, Romero-Otero J, et al. Benign prostatic hyperplasia management during COVID-19 pandemic. Arch Esp Urol. 2020:73405–412. [PubMed] [Google Scholar]
- 22.Hueber P-A, Zorn KC. Canadian trend in surgical management of benign prostatic hyperplasia and laser therapy from 2007–2008 to 2011–2012. Can Urol Assoc J. 2013;7:E582–6. doi: 10.5489/cuaj.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaBossiere JR, Wallis CJD, Herschorn S, et al. Surgical management of benign prostatic obstruction: 20-year population-level trends. Can Urol Assoc J. 2020;14:252–7. doi: 10.5489/cuaj.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitropoulos D, Artibani W, Biyani CS, et al. Validation of the Clavien-Dindo grading system in urology by the European Association of Urology Guidelines Ad Hoc Panel. Eur Urol Focus. 2018;4:608–13. doi: 10.1016/j.euf.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Saitta G, Becerra JEA, Del Álamo JF, et al. World J Urol. 2019;37:2451–8. doi: 10.1007/s00345-019-02671-4. [DOI] [PubMed] [Google Scholar]
- 26.Klein C, Marquette T, Comat V, et al. Evolution of day-case holmium laser enucleation of the prostate success rate over time. J Endourol. 2021;35:342–8. doi: 10.1089/end.2020.0337. [DOI] [PubMed] [Google Scholar]
- 27.Nottingham CU, Large T, Agarwal DK, et al. Comparison of newly optimized MOSES technology vs. standard holmium:YAG for endoscopic laser enucleation of the prostate. J Endourol. 2021;35:1393–9. doi: 10.1089/end.2020.0996. [DOI] [PubMed] [Google Scholar]
- 28.Assmus MA, Lee MS, Krambeck AE. MOSES laser enucleation of the prostate (MoLEP) Urology Video Journal. 2022;13:100123. doi: 10.1016/j.urolvj.2021.100123. [DOI] [Google Scholar]
- 29.Cynk M, Georgiadis G, Moore E, et al. Day-case holmium laser enucleation of the prostate. J Clin Urol. 2015;8:268–73. doi: 10.1177/2051415814560188. [DOI] [Google Scholar]
- 30.Kim SH, Yoo C, Choo M, et al. Factors affecting de novo urinary retention after holmium laser enucleation of the prostate. PLoS One. 2014;9:e84938. doi: 10.1371/journal.pone.0084938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kampantais S, Dimopoulos P, Tasleem A, et al. Assessing the learning curve of holmium laser enucleation of prostate (HoLEP): A systematic review. Urology. 2018;120:9–22. doi: 10.1016/j.urology.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Larner TRG, Agarwal D, Costello AJ. Day-case holmium laser enucleation of the prostate for gland volumes of <60 mL: Early experience. BJU Int. 2003;91:61–4. doi: 10.1046/j.1464-410X.2003.03086.x. [DOI] [PubMed] [Google Scholar]