Abstract
Background
Historically, scapulothoracic fusion (STF) is performed using steel wire and plate construct fixation. The purpose of this study is to report a recent fusion achieved through ultra-high-molecular-weight polyethylene-reinforced suture fixation as well as to perform a systematic literature review of techniques, fusion rates, complications, and reoperation.
Methods
Patient data were gathered from chart review and clinical encounters. For the review, MEDLINE, Embase, and Ovid databases were queried for STF cases. Thirty articles reporting on 386 fusion procedures were included.
Results
Including this patient, 5 of 387 (1.3%) STFs have been attempted with fiber suture. Fusion rates of metal-only constructs is 90.8% (346 of 381) with 11.3% (43 of 381) requiring wire removal or trimming because of symptomatic hardware and 7% (27 of 381) causing a postoperative pneumothorax. Although a small sample size, all fiber-suture constructs have achieved union without implant removal and without pneumothorax development. In this patient, fusion was determined radiographically at 6 months with substantial improvement in pain level and function.
Conclusion
Scapulothoracic fusion has benefit to patients to have failed other management options for winged scapula, most commonly those with neurologic trauma or facioscapulohumeral muscular dystrophy. With advancements in surgical options, fiber-suture offers an alternative to steel wire to achieve fusion. Further cases with longer term follow-up are needed to determine if significant differences in outcomes exist between constructs.
Keywords: Scapulothoracic fusion, Scapulothoracic arthrodesis, Thoracoscapular fusion, Letournel, FiberTape, FiberWire, Winged Scapula
Scapular winging, instability, or dyskinesia can have a multitude of etiologies; these most commonly are due to direct neurologic injury to key nerves4,16,19,22,26,27,29 or muscular wasting, such as in facioscapulohumeral muscular dystrophy.1,2,16, 17, 18,20,22, 23, 24, 25,28,29,3,31,33,5,8, 9, 10, 11, 12,14 Bony deformities including rib or scapular osteochondromas in multiple hereditary exostoses, glenoid instability, or scapular dislocations have also been indicated as causes.13,15,16,19,22,29 If initial management of physical therapy and bracing fail to adequately resolve symptoms and prevent scapular winging, different surgical options can be considered.23 In cases where conservative measures and other surgical options, such as muscle transfers, have failed, scapulothoracic fusion (STF) or arthrodesis should be considered.
As first described by Letournel et al,23 fusion of the scapula to the dorsum of three to five ribs provides stability, relieves pain, and improves function. Historically, this has been performed with steel cables, and then later with the addition of plate constructs with or without bone autograft and costotomies.23 By decorticating the dorsal ribs and using the reconstruction plate as a scapular buttress, cabling steel wire in singles or pairs around the ribs provides for a stable construct. The addition of iliac crest autograft or bone chip allograft contributes to successful fusion.1,2,13,15, 16, 17, 18, 19, 20,22, 23, 24,3,25,27, 28, 29,31,33,4,5,7, 8, 9,11,12 More recently, the introduction of ultra-high-molecular-weight polyethylene (UHMWPE)–reinforced suture has offered an alternative to traditional metal cables, having been shown to have greater strength than stainless steel as fracture tension bands.6,32 Their use for the purposes of achieving a STF, however, is scarce, with only 3 reports describing 4 cases.10,13,26
Therefore, the purposes of this study were to (1) report a case and surgical technique for STF using UHMWPE-reinforced suture fixation and (2) perform systematic literature review of scapulothoracic arthrodesis methods evaluating technique, fusion, and reoperation.
Methods
For the case report, we collected date from chart review and clinical follow-up encounters. The systematic review was completed by searching the MEDLINE, Embase, and Ovid databases from their inceptions to November 1, 2020. The following search terms were used: “(scapulothoracic and fusion) OR (scapulothoracic and arthrodesis) OR (scapula and arthrodesis) OR (scapula and fusion) OR (thoracoscapular and fusion) OR (thoracoscapular and arthrodesis) OR (Letournel and fusion) OR (Letournel and arthrodesis).” The initial query yielded 1397 reports. After removing duplicates, there were 1208 individual articles. Restricting the search to English-only and human-only reports resulted in 1112 studies. Book sections were excluded, limiting the amount to 931 articles. Abstracts were reviewed for reports of patients who underwent STF procedures, yielding 42 potential articles. Studies describing scapulopexy procedures, biomechanical experiments, and scapular reconstructions for oncologic cases were removed. Treatment guide articles not reporting specific patients were also excluded. Three articles were also excluded for using the same patient pool as more recent articles. Thirty studies reporting 386 fusion procedures were ultimately included. A study flow diagram is provided in Figure 1.1,2,12, 13, 14, 15, 16, 17, 18, 19, 20,22,3,23, 24, 25, 26, 27, 28, 29,31,33,4,5,7, 8, 9, 10, 11
Figure 1.
Study selection flow diagram.
Case report
Initial presentation
The patient was a 44-year-old right-hand-dominant woman with a past medical history of bipolar 1 disorder, beta thalassemia, and rheumatoid arthritis. She underwent a radical right neck dissection in February of 2018 to remove a mucoepidermoid carcinoma from her salivary gland at an outside hospital. On initial presentation to our system, she reported that after her surgery, her right shoulder had become progressively unstable and painful with worsening scapular prominence despite physical therapy. She rated her pain as an 8/10 with shoulder movement. On examination, she had obvious asymmetry of her shoulders with the right glenohumeral joint held in a forward flexed position. She had atrophy of her trapezius and lateral displacement of her right scapula (Fig. 2). She was tender over her suprascapular fossa. She was able to abduct and forward flex actively to 90 degrees with prominent winging. Her active external rotation was 40 degrees and internal rotation was to her lumbar spine. Shoulder radiographs demonstrated no fractures or dislocations (Fig. 3). Electromyography was performed, demonstrating a complete injury to the right spinal accessory nerve and resulting trapezial denervation. The long thoracic nerve was intact. She underwent a spinal accessory nerve exploration in September 2018, where extensive neurolysis was performed for surrounding compressive scar tissue. The trapezius was contractile with neural stimulation intraoperatively. Unfortunately, her pain and dysfunction recurred after initial improvements. A repeat electromyography showed improved spinal accessory nerve motor–evoked response, but similar trapezial reinnervation as the prior electromyography; rhomboid weakness was also demonstrated, limiting tissue transfer options. As a salvage procedure, she elected to pursue a STF.
Figure 2.
Lateral scapular winging on clinic exam.
Figure 3.
Preoperative shoulder radiographs.
Surgical technique
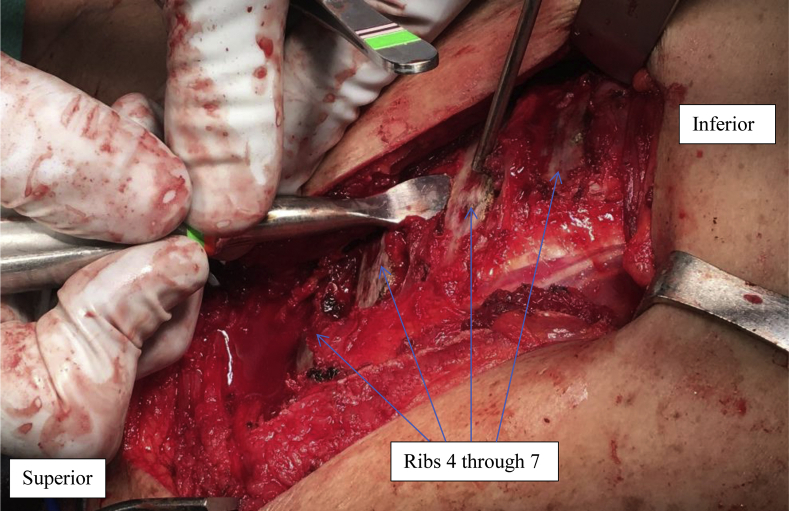
After general anesthesia with endotracheal intubation was achieved, she was positioned prone, and arm tables were attached to the operating room table to allow the operative arm to be manipulated and placed in external or internal rotation, as well as to allow for adduction and abduction to and from the torso as needed. The resting position was considered as the arm against the side of the torso in neutral rotation. The ipsilateral back, as well as some of the contralateral back just beyond the spinous processes were draped into the field to allow for anatomic referencing for rib selection (Fig. 4). Furthermore, the lower back was also draped in to allow for obtaining autologous iliac crest bone graft from the posterior iliac crest. A 14-cm incision was made medial and parallel to the medial border of the scapula (Fig. 4). The medial border of the scapula was exposed by removing the levator scapulae, rhomboids, serratus anterior, and the medial portions of the supraspinatus and infraspinatus (Fig. 5). The subscapularis was elevated off the medial-ventral scapula, and the medial 2 cm of the subscapularis muscular footprint was excised along the craniocaudal axis. The posterior surfaces of the 5 ribs deep to the scapula were then exposed using electrocautery. By counting the bony landmark of C7 vertebrae prominens, then carefully counting and marking the spinous process (by subcutaneous palpation) and corresponding ribs (by direct visualization and palpation) in sequential manner, it was determined that these were ribs 3 through 7. This was confirmed with an intraoperative chest radiograph. The pleura was identified and with a combination of electrocautery, freer elevator, and Matson rib stripper/elevator was peeled gently off the ribs’ ventral portions to allow for eventual passage of fixation (Fig. 6). The medial edge scapula was positioned approximately 2 inches from the midline to allow it to “sit” in its anatomic position. The arm was abducted to 90° and externally rotated to 90°; this fusion position was chosen as to best facilitate functional movement away from the torso. The posterior surfaces of the ribs, as well as the ventral medial scapula were then decorticated using a high-speed burr until punctate bony bleeding was encountered on both surfaces (Fig. 7). Adequate contact of both the burred areas of the ribs and scapula was ensured with the arm against the side of the torso. Autologous corticocancellous bone graft was then obtained from the ipsilateral iliac crest. Once the graft was obtained and the harvest site was closed, we turned out attention back to the scapula. A 7-hole 3.5-mm reconstruction plate was laid on the medial scapula in the medial infraspinatus fossa, and a cut 4-hole 1/3rd tubular plate was laid on the medial scapula in the medial supraspinatus fossa (Fig. 8). Two-millimeter holes were drilled through the scapula in the corresponding positions of plate holes. UHMWPE-reinforced suture (Arthrex FiberTape, Arthrex Inc., Naples, FL, USA) tapes were passed in cerclage fashion around each of the 5 ribs twice using the cerclage wire passer (Fig. 9). Rib 3 was connected through the 4 hole plate, rib 4 in-between the 4 hole and 7 hole plate, and ribs 5, 6, and 7 through the 7 hole plate. A Cobb elevator gently exposed the paths previously cleared from the pleura to facilitate this. Previously obtained autograft and cancellous chips were packed between the decorticated sections of the ribs and scapula. Each FiberTape loop was then fed into the Arthrex FiberTape Cerclage Tensioner system (AR-7800; Arthrex Inc., Naples, FL, USA). These were sequentially tightened, starting with the FiberTape loop around 4th rib near the scapular spine. They were each tightened initially to 30 pounds of pressure and then were increased cyclically to 40 pounds of pressure (Fig. 10). The FiberTapes were cut. At this point, a pneumothorax was ruled out clinically using saline pooling in the wound with simultaneous valsalva maneuver simulation by anesthesia, which did not demonstrate any bubbles. Radiographs were obtained before closure, and postoperative imaging was also performed in the recovery unit to ensure no pneumothorax was present (Fig. 11).
Figure 4.
Draping method as well as skin markings indicating incision. Spinous processes are marked and numbered for anatomic referencing.
Figure 5.
Exposed medial scapular border.
Figure 6.
Exposed rib surfaces with elevation of pleura off ventral rib.
Figure 7.
Burring of undersurface of scapular and dorsal surfaces of ribs.
Figure 8.
Diagram of scapular plate positioning.
Figure 9.
Ultra-high-molecular-weight polyethylene–reinforced sutures passed through scapular plates and ribs just before addition of bone graft and suture tensioning.
Figure 10.
Final scaulothoracic fusion construct before closure. Note non-elevation of scapular off chest wall with arm internally rotated.
Figure 11.
Immediate postoperative radiographs.
Postoperative management
She was non–weight-bearing in a shoulder immobilizer for the first four weeks postoperatively, able to use her arm below elbow level while restricting any shoulder range of motion. After the first five weeks, she was transitioned into a simple sling and allowed to begin right shoulder internal and external rotation exercises. In postoperative week 8, she began gradual forward flexion and abduction while weaning out of the sling. By week 10, she was bearing weight with her right arm. Radiographs were obtained after 1 month, demonstrating maintained hardware position (Fig. 12). She was gradually progressed through discontinuation of the sling and active abduction of the shoulder by month 3. A computed tomography scan demonstrated bony fusion at 6 months postoperatively (Fig. 13). She had painless range of motion in forward flexion to 90 degrees, abduction to 80, external rotation to 30, and internal rotation to her hip. There was no scapular winging (Fig. 14) or tenderness to palpation about the scapula and shoulder girdle. She was satisfied with her results. She has since been seen most recently 15 months postoperatively and remains very satisfied with her pain relief and functionality with her right arm without any hardware complications.
Figure 12.
One-month follow-up radiographs.
Figure 13.
Computer tomography scan demonstrating healed scapulothoracic fusion.
Figure 14.
No clinical scapular winging at 6-month follow-up.
Results and discussion
STF is a salvage procedure for recalcitrant scapular winging and persistent activity-limiting pain. The advent of UHMWPE-reinforced fiber sutures has provided high-strength alternative fixation methods to the classic steel wire construct options.6,10,13,21,23,32 In this article, we present the case of a patient who underwent successful right scapulothoracic arthrodesis using UHMWPE sutures and steel plates.
In the systematic review, the selected studies reported results of 386 attempted STFs (Table I). Of these, 381 (98.7%) were performed with various metal constructs. Metal cables with buttress plates were used in 211 cases; these achieved fusion in 89.1% of cases (Table II).2,3,22,23,27, 28, 29,33,5,8,11, 12, 13,15, 16, 17 Nineteen pneumothoraces have been reported with this construct type, 9% of all cases. Fifty-three reoperations were required, including 34 instances of painful or prominent hardware removal or trimming. The vast majority of wire and plate constructs used steel wires, while titanium wires were used in 3 cases in 1 study. Two of these 3 cases required revision for nonunion.27 In 119 cases, metal cables were used without a plate, with pneumothoraces being developed in 5% of cases.1,4,7,14,18,19,25,31 Fusion was achieved in 93.2% of these, and 20 reoperations occurred. Nine reoperations were for hardware removal. Another fusion technique, as originally described by Copeland et al,9 relies on screw fixation of the scapula to the dorsal ribs. Fifty-one cases of screw fixation have been described, with 92.2% achieving fusion and 2% having a pneumothorax identified postoperatively.9,20,24 Six reoperations were needed, with 2 for painful hardware removal. While this study cannot draw conclusions on union rate statistical differences, metal cables with or without a buttress plate and screw fixation appear to offer similar rates of bony union. Metal implants were a source of hardware irritation requiring revision in 11.3% (43 of 381) of cases; these were mostly attributed to either pain with range of motion or superficial skin irritation. While most included studies did not indicate length until hardware removal, 4 articles reporting on 10 cases requiring hardware removal did (23% of hardware-removal cases).13,16,22,27 These procedures were performed at an average of 12.5 months postoperatively (range 2.5 to 36 months). In total, reoperations occurred after 20.7% of fusion attempts, and 7% of cases had pneumothoraces. These underscore the substantial complication rate of metal hardware for this uncommon procedure.
Table I.
Included studies.
| Article | Cases | Fusion technique | Nonunion events | Pneumothoraces | Painful hardware removal or cable shortening | Total number of reoperations |
|---|---|---|---|---|---|---|
| Andrews et al., 19981 | 6 | Steel wires over ribs 4-6. Autograft | 0 cases | 1 case | 0 cases | 1 – Broken drain removal |
| Berne et al., 20032 | 49 | Steel wires over ribs 5 and 6, rib 4 osteotomized and inserted through scapular hole, fused with plate | 1 nonunion | 5 cases | 2 cases | 2 – wire removal |
| Bhatia et al., 20123 | 1 | Steel wires over ribs 3-6, 5-hole plate, autograft | 0 cases | 0 cases | 0 cases | 0 |
| Bizot et al., 20034 | 8 | Steel wires over 3 to 5 ribs, autograft | 3 nonunions | 0 cases | 0 cases | 3 2 – nonunion revisions 1 – traumatic scapular fracture |
| Boileau et al., 20205 | 10 | Steel wire, 7 to 8 hole plate, autograft | 1 nonunion | 0 cases | 0 cases | 0 |
| Bunch and Siegel, 19937 | 17 | Steel wires over 3 to 5 ribs, autograft | 0 nonunions | 0 cases | 0 cases | 0 |
| Cooney et al., 20138 | 14 | Braided steel cable over 4 to 5 ribs, two 3.5 reconstruction plates as washers, autograft and allograft | 1 nonunion | 0 cases | 5 cases | 6 1 – nonunion revision 5 – wire removal |
| Copeland et al., 19999 | 14 | Screw fixation to ribs 4-6, autograft, tibial cortical struts | 2 nonunions | 1 case | 2 cases | 4 2 – screw removal 1 – nonunion revision 1 – screw pullout revision |
| Davey et al., 202010 | 2 | FiberTape, 6 hole tubular plate, autograft and allograft | 0 cases | 0 cases | 0 cases | 0 |
| Diab et al., 200511 | 11 | Steel wire for ribs 2-6 or 3-7, compression or semitubular plate as washer, autograft | 0 nonunions | 0 cases | 2 cases | 2 – wire trimming |
| Elhassan et al., 200812 | 2 | Steel wires over ribs 3-6, 4- or 5- hole compression plate, autograft | 0 cases | 0 cases | 0 cases | 0 |
| Endrizzi et al., 201513 | 2 | Case A: Steel wires, semitubular plates, autograft Case B: FiberWire suture and autograft |
0 cases | 0 cases | 1 case (case A) | 1 – steel wire and plate removal |
| Eren, et al., 202014 | 64 | Braided steel cable from ribs 2 through 7, autograft and allograft | 3 nonunions | 4 cases | 3 cases | 7 3 – wire trimming 2 – nonunion revisions 2 – cable revisions due to rib fractures |
| Faisal et al., 201215 | 1 | Braided steel cables from ribs 2-5 with 8-hole plate, autograft | 0 cases | 0 cases | 0 cases | 0 |
| Goel et al. 201416 | 12 | Stainless steel wire from ribs 3 through 6, 5-hole compression plate, autograft, and allograft | 2 nonunions | 1 case | 6 cases | 7 1 – nonunion revision 6 – hardware removal |
| Jakab and Gledhill, 199318 | 4 | Steel wires, autograft | 0 cases | 0 cases | 0 cases | 0 |
| Jeon et al., 200519 | 6 | Steel wires stabilized by a rush rod, autograft | 1 nonunion | 0 cases | 6 cases | 7 1 – nonunion revision 6 – hardware removals |
| Kocialkowski et al., 199120 | 2 | Screw fixation, allograft, and autograft | 0 cases | 0 cases | 0 cases | 0 |
| Krishnan et al. 200522 | 24 | Braided steel cable, plate, autograft, and allograft | 9 nonunions | 6 cases | 1 case | 10 9 – nonunion revisions 1 – hardware removal |
| Le Hanneur and Saint-Cast, 201717 | 8 | Steel wire for ribs 4-6 or 3-5, 2-hole tubular plates, superior rib osteotomized and inserted through scapular hole, fused with 6-hole plate | 1 nonunion | 0 cases | 2 cases | 3 1 – nonunion revision 2 – hardware removal |
| Letournel et al., 199023 | 16 | Steel wire for ribs 4-6 or 5-7, 2-hole tubular plates, superior rib osteotomized and inserted through scapular hole, fused with a 6-hole plate | 0 nonunions | 3 cases | 0 cases | 0 |
| Levy, 201424 | 35 | 4.5-mm cortical screw fixation to 3 to 5 ribs with washers, autograft and allograft | 2 cases | 1 case | 0 cases | 2 – nonunion revision |
| Mackenzie et al., 200325 | 2 | Steel wires for ribs 4-6, autograft | 0 nonunions | 0 cases | 0 cases | 1 – immediate revision of fixation due to loss of brachial pulse |
| Ozturk et al., 201326 | 1 | Polymer cable, semitubular plate, allograft and BMP-2 | 0 cases | 0 cases | 0 cases | 0 |
| Pahys et al., 200927 | 3 | Titanium wires over ribs 4-7 with semitubular plate | 2 nonunions | 0 cases | 2 cases | 2- hardware removal |
| Rhee and Ha, 200628 | 9 | Steel wires for ribs 3-6, 5- or 6-hole plate, autograft | 0 nonunions | 0 cases | 0 cases | 0 |
| Sewell et al., 201229 | 42 | Braided steel cables over ribs 3-7, 9- or 10-hole semitubular plate, autograft | 6 nonunions | 3 cases | 13 cases | 20 6 – nonunion revision 13 – hardware removal 1 – excision of superomedial scapula |
| Szomor et al., 200030 | 1 | Achilles tendon allograft looped around ribs through scapular drill holes secured with Ethibond suture | 0 cases | 0 cases | 0 cases | 0 |
| Twyman et al., 199631 | 12 | Steel wires, autograft | 1 nonunion | 1 case | 0 cases | 1 – nonunion revision |
| Ziaee et al., 200633 | 8 | Steel wires, plate | 0 cases | 1 case | 0 cases | 0 |
Table II.
Study outcomes.
| UHMWPE-reinforced suture fixation∗ | Metal only constructs | Screw fixation | Metal cables with plate | Metal cables without plate | |
|---|---|---|---|---|---|
| Total N (%) | 5 (1) | 381 (98) | 51 (13) | 211 (55) | 119 (31) |
| Fusion N (%) | 5 (100) | 346 (91) | 47 (92) | 188 (89) | 111 (93) |
| Pneumothorax N (%) | 0 (0) | 27 (7) | 2 (4) | 19 (9) | 6 (5) |
| Reoperation N (%) | 0 (0) | 79 (21) | 6 (12) | 53 (25) | 20 (17) |
UHMWPE, ultra-high-molecular-weight polyethylene.
Numbers rounded to nearest whole number.
Includes the present study.
Constructs relying on nonmetal wires are rarely reported. One author reported looping Achilles tendon allograft through scapular drill holes around the dorsal ribs as a fusion technique.30 This patient achieved fusion without a reoperation or pneumothorax development. More recently, and including this study, high-strength suture has been used in 5 patients for STF.10,13,26 Previous suture types used by Ozturket al26 include polymer cerclage cables (SuperCable Isoelastic Polymer Cerclage; Kinamed Inc., Camarillo, CA, USA) with additional polyester tapes (Mersiline, Ethicon Inc., Somerville, NJ, USA) if bone quality was too poor for the polymer cerclage cables. Number 5 FiberWire (Arthrex FiberWire, Arthrex Inc., Naples, FL, USA) was used in 1 article with good results.13 This case used 2-mm FiberTape (Arthrex FiberTape, Arthrex), which is similar in structure to FiberWire but may provide broader compression owing to its wider surface area. The specific tensioner used (AR-7800, Arthrex) also allows for more precise and uniform tension across the construct. One previous study used the fixation method used in this case with similar results.10 From these 5 total cases, there are no reported pneumothoraces, no reoperation events, and all patients have achieved union. No patients reported irritation from the implants. It is possible that the suture construct may be less irritable to surrounding soft tissue and carries lower risk of penetrating injury to the pleura; this potentially may decrease the rates of subsequent complications and operations for removal of hardware. It is possible that hardware irritation develops in these patients in the future; however, as average length to hardware removal among the metal constructs was 12.5 months postoperatively and as the suture-construct studies had an average follow-up lengths of 15 months (range 6 to 24 months), it appears that there is at least a lower rate of early irritation necessitating removal. Further study comparing steel wire against UHMWPE-reinforced suture for bony fusion is warranted for analysis. Additional studies usig definitive functional scores would also offer benefit in comparing construct outcomes.
Limitations
This review has limitations. With now only 5 reported cases of STF for heterogenous etiologies using fiber-suture constructs, it is difficult to draw generalizable conclusions on the success rate of the procedure, as well as its potential for reoperation. No included studies in the literature review were higher than level IV evidence. As such, with no prospective studies or comparison groups available, statistical significance could not be calculated for fusion rate differences or incidences of painful hardware between metal and fiber-suture construct groups.
Conclusion
For patients with painful, activity-limiting scapular winging, STF represents a salvage procedure. Previous fusion techniques with steel cables with or without reconstruction plates, or screw fixation have been shown to have substantial rates of painful hardware, pneumothoraces, and reoperations. The introduction of UHMWPE-suture offers another option to achieve fusion, and while cases using this are limited, reports to this point have demonstrated successful fusion without implant irritation, pneumothorax development, or reoperation need. Further study with larger cohorts, longer-term follow-up is needed to fully assess this technique for STF.
Conflicts of interest
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Funding
No funding was disclosed by the author(s).
Footnotes
Institutional review board approval was not required for this study.
References
- 1.Andrews C.T., Taylor T.C., Patterson V.H. Scapulothoracic arthrodesis for patients with facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 1998;8:580–584. doi: 10.1016/s0960-8966(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 2.Berne D., Laude F., Laporte C., Fardeau M., Saillant G. Scapulothoracic arthrodesis in facioscapulohumeral muscular dystrophy. Clin Orthop Relat Res. 2003:106–113. doi: 10.1097/01.blo.0000057790.10364.35. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S., Hsu A.R., Harwood D., Toleikis J.R., Mather R.C., Romeo A.A. The value of somatosensory evoked potential monitoring during scapulothoracic arthrodesis: Case report and review of literature. J Shoulder Elbow Surg. 2012;21:e14–e18. doi: 10.1016/j.jse.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Bizot P., Teboul F., Nizard R., Sedel L. Scapulothoracic fusion for serratus anterior paralysis. J Shoulder Elbow Surg. 2003;12:561–565. doi: 10.1016/S1058-2746(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P., Pison A., Wilson A., van der Meijden O., Sacconi S., Trojani C., et al. Bilateral scapulothoracic arthrodesis for facioscapulohumeral muscular dystrophy: function, fusion, and respiratory consequences. J Shoulder Elbow Surg. 2020;29:931–940. doi: 10.1016/j.jse.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Bryant T.L., Anderson C.L., Stevens C.G., Conrad B.P., Vincent H.K., Sadasivan K.K. Comparison of cannulated screws with fiberwire or stainless steel wire for patella fracture fixation: Apilot study. J Orthop. 2015;12:92–96. doi: 10.1016/j.jor.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunch W.H., Siegel I.M. Scapulothoracic arthrodesis in facioscapulohumeral muscular dystrophy. Review of seventeen procedures with three to twenty-one-year follow-up. J Bone Joint Surg Am. 1993;75:372–376. doi: 10.2106/00004623-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cooney A.D., Gill I., Stuart P.R. The outcome of scapulothoracic arthrodesis using cerclage wires, plates, and allograft for facioscapulohumeral dystrophy. J Shoulder Elbow Surg. 2014;23:e8–e13. doi: 10.1016/j.jse.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Copeland S.A., Levy O., Warner G.C., Dodenhoff R.M. Clinical Orthopaedics and Related Research. Lippincott Williams and Wilkins; 1999. The shoulder in patients with muscular dystrophy; pp. 80–91. [PubMed] [Google Scholar]
- 10.Davey M.S., Kenyon R., Pauzenberger L., Grant Freemantle M.C., Mullett H. Bilateral scapulothoracic fusions fixed with high-strength suture tapes for facioscapulohumeral dystrophy. JBJS Case Connect. 2020;10 doi: 10.2106/JBJS.CC.20.00042. [DOI] [PubMed] [Google Scholar]
- 11.Diab M., Darras B.T., Shapiro F. Scapulothoracic fusion for facioscapulohumeral muscular dystrophy. J Bone Joint Surg Am. 2005;87:2267–2275. doi: 10.2106/JBJS.D.02952. [DOI] [PubMed] [Google Scholar]
- 12.Elhassan B., Chung S.T., Ozbaydar M., Diller D., Warner J.J.P. Scapulothoracic fusion for clavicular insufficiency: A report of two cases. J Bone Joint Surg Am. 2008;90:874–880. doi: 10.2106/JBJS.G.00986. [DOI] [PubMed] [Google Scholar]
- 13.Endrizzi D.P., Shubert D.J., White R.R. Scapulothoracic fusion for low-energy intrathoracic scapula dislocation: A report of two cases. J Shoulder Elbow Surg. 2015;24:e91–e95. doi: 10.1016/j.jse.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Eren İ., Erşen A., Birsel O., Atalar A.C., Oflazer P., Demirhan M. Functional Outcomes and Complications Following Scapulothoracic Arthrodesis in Patients with Facioscapulohumeral Dystrophy. J Bone Joint Surg Am. 2020;102:237–244. doi: 10.2106/JBJS.19.00571. [DOI] [PubMed] [Google Scholar]
- 15.Faisal S.A., Campbell P.T., Skirving A.P. Sequential ipsilateral glenohumeral arthrodesis and scapulothoracic fusion: a case report. J Shoulder Elbow Surg. 2012;21:e18–e20. doi: 10.1016/j.jse.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 16.Goel D.P., Romanowski J.R., Shi L.L., Warner J.J.P. Scapulothoracic fusion: Outcomes and complications. J Shoulder Elbow Surg. 2014;23:542–547. doi: 10.1016/j.jse.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Le Hanneur M., Saint-Cast Y. Long-term results of Letournel scapulothoracic fusion in facioscapulohumeral muscular dystrophy: A retrospective study of eight cases. Orthop Traumatol Surg Res. 2017;103:421–425. doi: 10.1016/j.otsr.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Jakab E., Gledhill R.B. Simplified technique for scapulocostal fusion in facioscapulohumeral dystrophy. J Pediatr Orthop. 1993;13:749–751. doi: 10.1097/01241398-199311000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Jeon I.H., Neumann L., Wallace W.A. Scapulothoracic fusion for painful winging of the scapula in nondystrophic patients. J Shoulder Elbow Surg. 2005;14:400–406. doi: 10.1016/j.jse.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Kocialkowski A., Frostick S.P., Wallace W.A. One-stage bilateral thoracoscapular fusion using allografts: A case report. Clin Orthop Relat Res. 1991:264–267. [PubMed] [Google Scholar]
- 21.Kord D., Liu E., Horner N.S., Athwal G.S., Khan M., Alolabi B. Outcomes of scapulothoracic fusion in facioscapulohumeral muscular dystrophy: A systematic review. Shoulder Elbow. 2020;12(2):75–90. doi: 10.1177/1758573219866195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan S.G., Hawkins R.J., Michelotti J.D., Litchfield R., Willis R.B., Kim Y.K. Scapulothoracic arthrodesis: Indications, technique, and results. Clin Orthop Relat Res. 2005;435:126–133. doi: 10.1097/01.blo.0000156659.11078.80. [DOI] [PubMed] [Google Scholar]
- 23.Letournel E., Fardeau M., Lytle J.O., Serrault M., Gosselin R.A. Scapulothoracic arthrodesis for patients who have fascioscapulohumeral muscular dystrophy. J Bone Joint Surg Am. 1990;72:78–84. [PubMed] [Google Scholar]
- 24.Levy O. Thoracoscapular fusion for winging of the scapula with screw fixation for fascioscapulohumeral dystrophy (Modified Copeland-Howard Procedure) JBJS Essent Surg Tech. 2014;4:e12. doi: 10.2106/jbjs.st.m.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie W.G., Riddle E.C., Earley J.L., Sawatzky B.J. A neurovascular complication after scapulothoracic arthrodesis. Clin Orthop Relat Res. 2003:157–161. doi: 10.1097/00003086-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Ozturk B.Y., Burns T.C., Warren R.F. Scapulothoracic fusion with nonmetallic cables. Tech Shoulder Elbow Surg. 2013;14:42–46. doi: 10.1097/BTE.0b013e31828905bc. [DOI] [Google Scholar]
- 27.Pahys J.M., Mulcahey M.J., Hutchinson D., Betz R.R. Scapular stabilization in patients with spinal cord injury. J Spinal Cord Med. 2009;32(4):389–397. doi: 10.1080/10790268.2009.11754408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee Y.G., Ha J.H. Long-term results of scapulothoracic arthrodesis of facioscapulohumeral muscular dystrophy. J Shoulder Elbow Surg. 2006;15:445–450. doi: 10.1016/j.jse.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Sewell M.D., Higgs D.S., Al-Hadithy N., Falworth M., Bayley I., Lambert S.M. The outcome of scapulothoracic fusion for painful winging of the scapula in dystrophic and non-dystrophic conditions. J Bone Joint Surg Br. 2012;94 B:1253–1259. doi: 10.1302/0301-620X.94B9.29402. [DOI] [PubMed] [Google Scholar]
- 30.Szomor Z.L., Fermanis G., Murrell G.A.C. Scapulothoracic fusion for a stroke patient with Achilles tendon allograft. J Shoulder Elbow Surg. 2000;9:342–343. doi: 10.1067/mse.2000.105127. [DOI] [PubMed] [Google Scholar]
- 31.Twyman R.S., Harper G.D., Edgar M.A. Thoracoscapular fusion in facioscapulohumeral dystrophy: clinical review of a new surgical method. J Shoulder Elbow Surg. 1996;5:201–205. doi: 10.1016/s1058-2746(05)80006-0. [DOI] [PubMed] [Google Scholar]
- 32.Wright P.B., Kosmopoulos V., Coté R.E., Tayag T.J., Nana A.D. FiberWire® is superior in strength to stainless steel wire for tension band fixation of transverse patellar fractures. Injury. 2009;40:1200–1203. doi: 10.1016/j.injury.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Ziaee M., Abolghasemian M., Majd M. Scapulothoracic arthrodesis for winged scapula due to facioscapulohumeral dystrophy (a new technique) Am J Orthop (Belle Mead NJ) 2006;35:311–315. [PubMed] [Google Scholar]