Abstract
Interaction between the heterodimeric form of protein phosphatase 2A (PP2A) and polyomavirus middle T antigen (MT) is required for the subsequent assembly of a transformation-competent MT complex. To investigate the role of PP2A catalytic activity in MT complex formation, we undertook a mutational analysis of the PP2A 36-kDa catalytic C subunit. Several residues likely to be involved in the dephosphorylation mechanism were identified and mutated. The resultant catalytically inactive C subunit mutants were then analyzed for their ability to associate with a cellular (B subunit) or a viral (MT) B-type subunit. Strikingly, while all of the inactive mutants were severely impaired in their interaction with B subunit, most of these mutants formed complexes with polyomavirus MT. These findings indicate a potential role for these catalytically important residues in complex formation with cellular B subunit, but not in complex formation with MT. Transformation-competent MT is known to associate with, and modulate the activity of, several cellular proteins, including pp60c-src family kinases. To determine whether association of MT with an active PP2A A-C heterodimer is necessary for subsequent association with pp60c-src, catalytically inactive C subunits were examined for their ability to form complexes containing pp60c-src in MT-expressing cells. Two catalytically inactive C subunit mutants that efficiently formed complexes with MT also formed complexes that included an active pp60c-src kinase, demonstrating that PP2A activity is not essential in cis in MT complexes for subsequent pp60c-src association.
The early region of polyomavirus encodes three tumor (T) antigens. Of these, only the middle T antigen (MT) is necessary and sufficient to transform rodent fibroblasts. MT achieves its oncogenic potential by binding cellular proteins and modulating their activities (25). The known cellular binding partners of MT are the 36-kDa catalytic (C) and the 63-kDa constant regulatory (A) subunits of the serine/threonine protein phosphatase, PP2A (54, 69); the Src family tyrosine kinases pp60c-src (21), pp62c-yes (37), and pp59c-fyn (15, 33); the 85-kDa regulatory subunit of phosphatidylinositol (PI) 3-kinase (20, 35, 49); Shc (10, 26), Grb2 (indirectly via Shc) (10, 26); 14-3-3 proteins (51); phospholipase Cγ1 (PLCγ1) (65), and the 70-kDa heat shock proteins (Hsp70s) (52).
MT sequences involved in binding associated proteins have been identified through detailed mutational analysis. A portion of the N-terminal region of MT, for example, is homologous to the J domain of DnaJ-Hsp40 molecular chaperones and mediates the interaction with Hsp70s (7a, 9). Hsp70s only bind MT that is not bound by PP2A, probably due to overlapping binding sites. The role, if any, of Hsp70s in MT function is unknown. The regions of MT necessary for association of MT with the heterodimeric A-C core of PP2A include the extreme amino terminus of MT, two cysteine motifs located downstream of the J domain, and another region proximal to residue 179 (defined by the MT mutant, NG59) (references 8, 11, 19, 30, and 50 and references therein). PP2A binding to MT is essential for MT’s transformation competence. More recently, a region important for the binding of Src family tyrosine kinases has been mapped to a more distal region between amino acids 185 and 210 (7). Part of the functional consequence of MT complex formation with pp60c-src is the constitutive activation of this kinase and the phosphorylation of certain residues on MT, tyrosines 250, 315, and 322, which are located in specific recognition motifs. These phosphorylated motifs serve as docking sites for the signaling molecules, Shc (10, 26), PI 3-kinase (66), and PLCγ1 (65), respectively, which bind via their phosphotyrosine binding (PTB; Shc) or Src homology 2 (SH2, PI 3-kinase and PLCγ1) domains. Abrogation of Shc or PI 3-kinase binding to MT greatly impairs MT’s transformation ability, while the binding of PLCγ1 to MT seems to be of less importance (59, 65). The interaction of 14-3-3 with MT requires phosphorylation of a serine at position 257 in MT, which resembles the putative RSXSXP binding motif of 14-3-3 proteins (22, 45, 60). Its association with MT is independent of pp60c-src activation (50, 51). The functional consequences of the MT–14-3-3 interaction are not yet fully understood, but loss of 14-3-3 binding does not abrogate MT-mediated transformation (22).
Although several MT-associated proteins (PP2A, 14-3-3 proteins, and Hsp70s) appear to bind to MT independently, others, such as pp60c-src, Shc, PI 3-kinase, and PLCγ1, bind in a manner dependent on the complex formation of MT with another associated protein. For example, loss of PP2A binding always results in the loss of pp60c-src binding, although pp60c-src binding to MT can be disrupted without loss of PP2A (7). Loss of pp60c-src binding to MT prevents the association of Shc (and Grb2), PI 3-kinase, and PLCγ1 even though MT mutants defective in Shc (10, 26), PI 3-kinase (66), and PLCγ1 (65) exist that have wild-type (wt) levels of pp60c-src bound and activated. To explain these data, we previously proposed a model for the ordered assembly of certain components of the MT complex (8). In this model, PP2A association with MT is required for pp60c-src association to occur. Subsequent phosphorylation of MT on tyrosines 250, 315, and 322 by an activated Src kinase then provides binding sites for Shc, the PI 3-kinase 85-kDa subunit, and PLCγ1, respectively. In addition, once bound to MT, PI 3-kinase, Shc, and PLCγ1 become phosphorylated on tyrosine, presumably by an activated Src kinase, activating signaling through these molecules. Although the dependency of Shc, PI 3-kinase, and PLCγ1 binding to MT on pp60c-src binding is readily understandable, the molecular basis for association of PP2A with MT being critical for further MT complex assembly is less so. In particular, it is not known whether PP2A plays a structural, nonenzymatic role in complex assembly or whether there is an undefined role for PP2A activity in this assembly.
Some data suggest that PP2A may play a role in regulating the phosphorylation of MT in vivo. Incubation of MT-expressing cells with okadaic acid, which preferentially inhibits PP2A in vivo (28), enhanced the phosphorylation status of wild-type MT and, to an even greater extent, that of an MT mutant, NG59, which is unable to bind PP2A (68). MT is known to be phosphorylated on serine 257 (22) and on other unidentified serine(s) and threonine(s). A particular 58-kDa phosphorylated form of MT seems to preferentially interact with pp60c-src (43). Thus, the possibility exists that PP2A activity regulates pp60c-src association via modulation of the phosphorylation status of MT.
In addition to playing a role in the assembly of the MT signaling complex, PP2A bound to MT may have other functions in the MT complex. A long-standing question concerns whether MT-bound PP2A phosphotyrosyl phosphatase activity (2, 14) contributes to the activation of the MT-associated kinase, pp60c-src, which, in the MT complex, lacks an inhibitory phosphorylation on tyrosine 527 (13). In addition, although in vitro experiments have shown that incubation with PP2A can reactivate serine 608-autophosphorylated PI 3-kinase p85 subunit (24), there is no evidence yet that this activating dephosphorylation takes place in the MT complex. Therefore, the role of the PP2A catalytic activity in the MT complex has yet to be defined.
wt PP2A consists of a heterodimer between the C subunit and the A subunit, which can further associate with one of several variable regulatory B-type subunits (17). Association with the regulatory subunits seems to confer substrate specificity on the catalytic subunit. For example, the 55-kDa B subunit increases the activity of the A-C heterodimer up to 100-fold towards cdc2-phosphorylated histone H1 (1, 29, 44, 62). In cells stably expressing MT, an estimated 10% of total cellular PP2A is found complexed to MT (32a, 68). Although MT appears to substitute for the B subunit in a portion of heterotrimeric PP2A complexes and is thus considered a viral B-type subunit, MT-associated PP2A has altered enzymatic properties compared to the wt holoenzyme (14, 47). Nevertheless, the consequences of the MT-induced changes in PP2A on a biochemical level are poorly understood, in part because relevant in vivo substrates need to be identified.
To investigate the role of PP2A activity in the assembly and functioning of the MT signaling complex, we undertook a mutational analysis of the PP2A C subunit, focusing on residues potentially involved in catalysis. The effects of these mutations on PP2A activity and subunit composition were examined. Individual mutation of certain invariant amino acids abolished the catalytic activity of the PP2A C subunit without disrupting complex formation with the A subunit. Interestingly, PP2A A-C heterodimers containing these mutant C subunits showed a severe reduction in B subunit binding, while association with polyomavirus MT was either unaffected or less affected. Similarly, an active PP2A in complex with MT did not appear to be essential for subsequent pp60c-src association and activation because two of the catalytically inactive C subunit mutants were found in MT complexes containing activated pp60c-src.
MATERIALS AND METHODS
Plasmids and mutagenesis.
Sequence comparison was performed with Lasergene software (DNASTAR). The generation of pGRE5-2 vector constructs expressing the H59Q and H118Q mutants is described elsewhere (46). The construction of a pcDNA I Amp vector expressing hemagglutinin (HA)-tagged wt PP2A C subunit has been previously described (47). Site-directed mutagenesis was performed on C subunit cDNA cloned in pcDNA I Amp vector according to the manufacturer’s instructions with the Muta-Gene phagemid in vitro-mutagenesis kit (Bio-Rad). The following single-stranded oligonucleotides containing the desired nucleotide change were used in the mutagenesis reactions: D57N, 5′ GTCTGTGGAAATGTGCATG 3′; D85N, 5′ TTTATGGGAAATTATGTTG 3′; and R89A, 5′ TTATGTTGACGCAGGATATTAT 3′. The entire cDNA of every mutant was sequenced to confirm successful mutagenesis and to ensure that no additional mutation had occurred. Mutant and wt C subunit cDNAs, including the epitope tag sequence, were cloned into the dexamethasone-inducible vector pGRE 5-2 (41). An inducible vector was chosen in order to minimize the potential deleterious effects of wt and mutant C subunits (if any) while lines were being carried in culture and to provide an uninduced control in analyses of their effects.
Cells and cell culture.
NIH 3T3 lines expressing wt polyomavirus MT and a geneticin resistance gene (16) were transfected by the calcium phosphate precipitation method (58). Twenty micrograms of pGRE5-2 vector only or vector containing wt or mutant C subunits was cotransfected together with 2 μg of a plasmid conferring resistance to hygromycin B. The selection medium contained 200 to 300 μg of hygromycin B/ml and 400 μg of geneticin/ml. After 14 days, cell clones were pooled and expanded to mass cultures. After 24 h of induction with 25 mM dexamethasone, the pooled clones were tested for expression of epitope-tagged protein by immunoblotting with 12CA5 antibody. Cell lines producing the recombinant protein were maintained at 37°C in Dulbecco’s modified Eagle’s medium–10% calf serum containing hygromycin B and geneticin. All C subunits were expressed at 10 to 50% of the level of endogenous wt C subunit. Of note, basal levels were substantial, with very little induction in the presence of dexamethasone. Therefore all experiments presented in this paper were carried out without the addition of dexamethasone.
Immunoprecipitations, immunoblotting, and in vitro kinase assays.
The details of preparation of cell lysates and immunoprecipitation of C subunits with 12CA5 antibody cross-linked to protein A-Sepharose beads have been described previously (47). Control, vector-only cell lysate was used in an amount equal to that required for the C subunit cell line expressing the lowest level of C subunit. After being washed, the immune complexes were in most cases split into three parts. One part was analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) (38), while the others were tested for phosphatase activity towards two different PP2A substrates (phosphorylase a and Histone H1). However, in cases where immune complexes were also analyzed for associated kinase activity, immune complexes were divided into four parts. Immunoblotting (67) was performed with mouse monoclonal anti-HA tag antibody (16B12; 1:5,000; BAbCo), rabbit anti-B subunit (no. 16) and anti-A subunit (R39) antibodies from our laboratory (1:5,000), mouse monoclonal anti-MT antibody (F4; supernatant diluted 1:40) (53), mouse monoclonal anti-phosphotyrosine antibody (4G10), mouse monoclonal anti-pp60c-src antibody 327 (ascites diluted 1:6,000) (39), rabbit anti-Shc antibody (1:2,500; Upstate Biotechnology Inc.), and anti-PI 3-kinase antibody (1:2,000; Upstate Biotechnology Inc.). Immunoblots were developed with enhanced chemiluminescence (NEN). Kinase activity present in the anti-epitope tag immunoprecipitates was assayed by incubating the immunoprecipitates in 30 μl of kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MnCl2) containing 10 μCi of [γ-32P]ATP. The assay mixtures were incubated for 20 min at room temperature with occasional shaking. After the supernatant was removed, the immune complexes were washed once with kinase buffer and then heated for 3 min at 95°C in sample buffer and analyzed by SDS-10% PAGE.
Phosphatase assays.
Phosphatase activity present in anti-epitope tag immunoprecipitates was assayed with phosphorylase a and histone H1. γ-32P-labeled phosphorylase a substrate was prepared from phosphorylase b (Gibco-BRL) according to the manufacturer’s instructions. Histone H1 was phosphorylated by mitotic p34cdc2 purified from nocodazole-arrested HeLa cells as described previously (44). The amounts of lysates used for immunoprecipitation were normalized according to epitope-tagged C subunit expression levels. Assays were performed at a linear range and with subsaturating amounts of each substrate.
RESULTS
Identification of catalytically important residues in the PP2A C subunit.
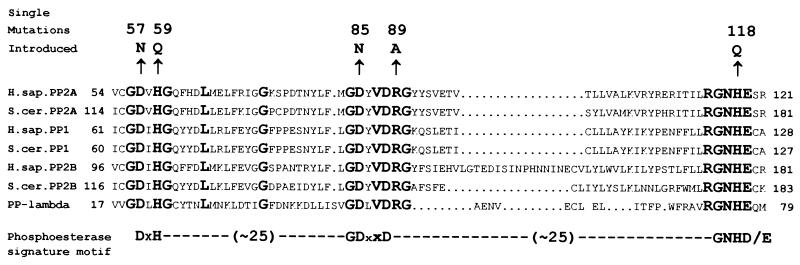
To study the role of PP2A catalytic activity in the MT complex, C subunit residues potentially involved in catalysis first had to be identified. For this purpose, we aligned PP2A C subunit sequences with sequences of related serine/threonine phosphatases. This alignment (Fig. 1) revealed a limited number of invariant or highly conserved residues (5), suggesting a common catalytic mechanism. Based on this alignment, we targeted two invariant histidines, H59 and H118, for mutagenesis (46). During the course of our study, Zhuo and coworkers defined a motif which appears to be necessary for the catalytic mechanism of a variety of enzymes involved in the hydrolysis of phosphate esters (71). The crystal structures of PP1 and PP2B, published shortly thereafter, confirmed the existence of this conserved phosphoesterase signature motif in these enzymes and, in addition, identified it as a metallophosphoesterase motif (27, 31, 36). The consensus sequence of the motif is shown in Fig. 1. The residue after the second D in the GDXXD is an arginine (R) in all serine/threonine phosphatases cloned to date. These results, taken together with data from the mutational analysis of bacteriophage λ phosphatase and PP1, suggested that many of the invariant residues were located at the active site, participating in metal ion binding, substrate binding, and catalysis (70). Based on this information, we chose to mutate three of the other absolutely conserved residues, D57, D85, and R89.
FIG. 1.
Sequence alignment of the catalytic domains of representative PPP family members to identify conserved residues. Sequences of three examples of the major PPP family members from two different species (Homo sapiens [H.sap.] and Saccharomyces cerevisiae [S.cer.]) and, in addition, the sequence of a related phosphatase from bacteriophage λ (PP-lambda) were aligned with DNASTAR Lasergene sofware. Shown is a portion of the alignment containing the phosphatase domains (sequences containing the phosphoesterase signature motif), with amino acids displayed in the single-letter code. The numbers to the left and right of each sequence indicate the position within the full-length sequence. H.sap.PP2A is the human PP2A α catalytic subunit (SwissProt accession no., P05323 and P13197) (3, 64). S.cer.PP2A is encoded by the S. cerevisiae gene PPH21 (SwissProt accession no., P23594) (55, 61). H.sap.PP1 is the human protein phosphatase 1 alpha1 catalytic subunit (SwissProt accession no., P08129, P22802, and P20653) (4, 63). S.cer.PP1 is the protein phosphatase 1 catalytic subunit encoded by the S. cerevisiae gene GLC7 (SwissProt accession no., P32598) (48). H.sap.PP2B is the human protein phosphatase 2B catalytic subunit 1 (SwissProt accession no., P16298) (32). S.cer.PP2B is the protein phosphatase 2B catalytic subunit A1 encoded by the S. cerevisiae gene CNA1 (SwissProt accession no., P23287) (23, 40). PP-lambda is the gene product of the bacteriophage λ orf 221 (SwissProt accession no., P03772) (18). Invariant residues are shown in boldface with an increased font size. The phosphoesterase signature motif as it was defined by Zhuo et al. (71) is shown below the alignments. Single-amino-acid substitutions present in the human PP2A α catalytic subunit mutants used for this study are shown above the corresponding amino acids of the wt sequence (arrows). The position of each substitution is indicated by the number above the substituted amino acid.
Using site-directed mutagenesis, we individually substituted these amino acids in the predicted catalytic core of the PP2A C subunit (Fig. 1). “Safe” substitutions least likely to disturb the overall structure of the active site were chosen (6). Two of these mutants, H59Q and H118Q, were used in a separate study and therefore are described elsewhere (46). However, activity and subunit binding data for these mutants are also presented in this study to allow comparison with the additional mutants reported here.
To test the mutated C subunits for stability and catalytic activity, HA epitope-tagged wt and mutant C subunits were cloned into the eukaryotic expression vector pGRE5-2 and then stably introduced into MT-expressing NIH 3T3 cells. Epitope tagging allows the recombinant C subunits to be distinguished from the intracellular enzyme. In addition, as we have shown previously, trimeric cellular and viral PP2A complexes can be coimmunoprecipitated via the epitope tag (47). Western blots of whole-cell lysates from the transfected cells, probed with the anti-epitope tag antibody, showed that pooled cell clones of wt and mutant C subunits expressed stable proteins (data not shown). The mutant R89A consistently migrated more slowly in SDS-PAGE than the other mutants or wt C subunit. Sequencing of the full-length cDNA confirmed that the observed mobility shift was not caused by any mutation other than the one which was intentionally introduced.
Phosphatase assays were performed on epitope tag immunoprecipitates of recombinant wt and mutant C subunits with phosphorylase a and histone H1 as substrates. Similar amounts of immunoprecipitated wt and mutant C subunits (D57N, H59Q, D85N, R89A, and H118Q) were compared (Fig. 2, bottom). All mutants displayed activity levels similar to that seen with immunoprecipitates from vector control cells which did not express any recombinant C subunits (Fig. 2, top). Interestingly, mutation of several amino acids conserved in all PPP family members but not in λ phosphatase (Ser93 and Ser171), or of even less conserved residues (His63, Ser75, and Cys165), resulted in only a partial loss or no loss of catalytic activity (data not shown). Thus, the loss of activity seemed to correlate well with the predicted roles and the degree of conservation of the mutated residues.
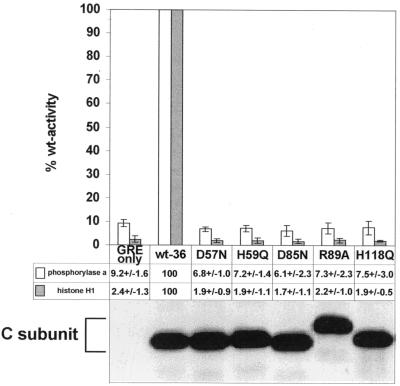
FIG. 2.
Analysis of the phosphatase activity in immunoprecipitates of epitope-tagged wild-type and mutant C subunits. Lysates of cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits were immunoprecipitated with 12CA5 antibody. After being washed, the immunoprecipitates were split into three parts and analyzed by SDS-PAGE and immunoblotting or tested for catalytic activity towards phosphorylase a and cdc2-phosphorylated histone H1 as substrates. The C subunit mutants are referred to by the mutated position preceded by the wt residue and followed by the introduced amino acid. The immunoblot below the graph shows that comparable amounts of immunoprecipitated wt and mutant C subunits were tested for catalytic activities. This anti-epitope tag antibody immunoblot of anti-epitope tag C subunit immunoprecipitates was generated from one of the four experiments used for the determination of the phosphatase activities and is representative of the other blots. Phosphatase activities of the vector control and mutant C subunit immunoprecipitates are presented below the graph as percentages of the activity obtained for immunoprecipitated wt C subunit. The mean values ± standard deviations of four independent experiments are depicted in column graphs. The data for H59Q and H118Q are from Ogris et al. (46). The values obtained for the vector control, GREonly, are most likely due to nonspecific binding of endogenous phosphatases from the cell lysates.
Mutations that inactivate C subunit affect its ability to form B subunit-containing, but not MT-containing, complexes in vivo.
To exclude the possibility that the loss of catalytic activity was due to severe structural changes of the mutant C subunits, we analyzed the abilities of these mutants to form a heterodimer with the A subunit. Anti-epitope tag immunoprecipitates from MT-transformed NIH 3T3 cells expressing epitope-tagged wt and inactive C subunits were probed by immunoblotting for the presence of A subunit (Fig. 3). Three of the five inactive mutants, H59Q, D85N, and R89A, were able to complex with substantial amounts of A subunit, while two, D57N and H118Q, were impaired in binding, although small amounts of A subunit could be repeatedly immunoprecipitated with these mutants (data not shown). In addition, H118Q, for unknown reasons, demonstrated substantial variability in binding A subunit, at times associating at higher levels (data not shown). The fact that H59Q, D85N, and R89A bind substantial amounts of A subunit suggests that these mutants fold properly. Therefore, the loss of catalytic activity caused by these mutations indicates a direct role for these residues in catalysis. Given that D57N and H118Q also bind some A subunit yet show no activity, these residues likewise probably play more than a structural role in PP2A activity.
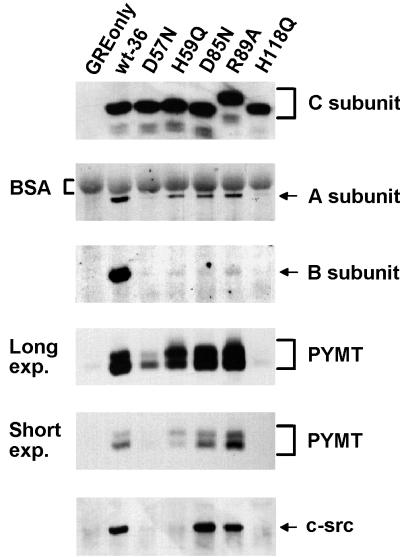
FIG. 3.
Complex formation of mutant PP2A C subunits with cellular and viral PP2A regulatory subunits and with pp60c-src. Lysates of cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits (see Fig. 2 for nomenclature) were immunoprecipitated with 12CA5 antibody and analyzed by SDS-PAGE and immunoblotting. The Western blot was sequentially probed with antibodies recognizing the epitope-tagged C subunit, A subunit, B subunit, polyomavirus MT (PYMT), and pp60c-src (c-src). Two different exposures (exp.) of the MT panel are shown. MT in complex with wt and inactive C subunits migrates as a doublet in SDS-PAGE, although the relative abundance of the two bands varies depending on the particular C subunit. The altered migration of the R89A mutant C subunit in SDS-PAGE was constantly seen and seems to be due to the substitution of the R, since several other amino acid changes at this position caused the same effect (data not shown). In the A subunit panel, background signal from bovine serum albumin (BSA), which was used for preblocking of the protein A-Sepharose beads, is indicated with brackets. The data shown are representative of results obtained from four independent experiments.
MT-expressing NIH 3T3 cells allow the simultaneous study of the ability of the A-C heterodimer to complex with cellular B subunit or with MT (47). MT is produced at a low level (∼10%) relative to PP2A in these cells, and therefore, trimeric PP2A complexes containing B subunit and MT exist within the same cell (47). To test if the catalytically inactive C subunit mutants are able to form PP2A complexes containing MT or B subunit, anti-epitope tag C subunit immunoprecipitates were also probed for the presence of B subunit and MT (Fig. 3). While all of the catalytically inactive mutants that still efficiently formed a complex with the A subunit retained the ability to complex with polyomavirus MT, they were all severely impaired in binding to the B subunit. The mutants D57N and H118Q were impaired in binding of both the cellular B subunit and MT, most likely due to their decreased ability to form the heterodimeric A-C core. However, like the other three mutants that bind A subunit well, D57N shows a more severe loss of B subunit binding than of MT binding (Fig. 3 and data not shown). H118Q, on the other hand, shows similar defects in binding both B subunit and MT. These results suggest that PP2A catalytic activity or, at a minimum, these particular active-site residues are important for complex formation with a cellular B-type subunit but not for complex formation with the viral B-type subunit, MT.
Interaction with some inactive C subunit mutants leads to a shift in the ratio of the 56- and 58-kDa forms of MT.
MT predominantly migrates in SDS-PAGE as two major bands, a more abundant 56-kDa band and a less abundant 58-kDa band. The 58-kDa form appears to be due to additional serine phosphorylation (43). Interestingly, MT in complex with certain inactive C subunit mutants showed a relative increase in the 58-kDa species compared to MT in complex with wt C subunit (Fig. 3). The change in the ratio of the two MT bands was most pronounced for MT in complex with H59Q. In addition, a smaller change was seen for MT bound to D85N, while MT bound to R89A demonstrated even less change. Finally, D57N-associated MT showed no reproducible difference from wt C subunit-associated MT. The observed differences among these three mutants was probably not due to residual PP2A activity, since we were unable to detect activity for any of the three mutants in multiple experiments.
Inactive C subunit mutants allow assembly of MT-pp60c-src complexes.
Association of MT with pp60c-src family tyrosine kinases and activation of the associated kinase is a necessary step in MT-transformation. While PP2A interaction with MT is required for pp60c-src binding, the role of PP2A phosphatase activity in this process is unclear. We therefore tested the ability of the inactive PP2A mutants to support assembly of an MT-pp60c-src complex by probing epitope tag immunoprecipitates of each mutant for the presence of pp60c-src. For these experiments, the same blots that were probed for the presence of C, A, and B subunits and MT were further probed with antibodies against pp60c-src (Fig. 3). As expected, D57N and H118Q, the mutants that bound little MT in this experiment, did not coimmunoprecipitate much pp60c-src. On the other hand, the inactive mutants D85N and R89A, which bound wt levels of MT, bound levels of pp60c-src which were at least comparable with that bound by wt C subunit (Fig. 3 and data not shown). In fact, D85N consistently bound pp60c-src at a level higher than the wt level (data not shown). These results clearly indicate that PP2A catalytic activity is not essential for the assembly of an MT-pp60c-src complex. Surprisingly, H59Q, which is competent to interact with A subunit and MT, bound very little pp60c-src. Although the reason for this is not known, it is interesting to note that this mutant induced the most pronounced shift in the MT with which it complexed.
PP2A activity does not appear to be necessary in cis for MT-associated pp60c-src to be functional.
Although catalytically inactive PP2A can support MT complex formation with pp60c-src, it was still possible that the lack of PP2A activity in these complexes might reduce the activity of the associated pp60c-src. As a first test of this possibility, we carried out in vitro kinase assays on immunoprecipitates of epitope-tagged wt and inactive mutant C subunits. MT, pp60c-src, and the 85-kDa subunit of PI 3-kinase, known substrates for the associated kinase, were examined. As seen in Fig. 4, these proteins were phosphorylated in the immunoprecipitates of wt C subunit and of those inactive C subunit mutants which retained the ability to form MT complexes containing pp60c-src. Thus, the lack of PP2A activity did not appear to dramatically reduce the activity of the associated pp60c-src.
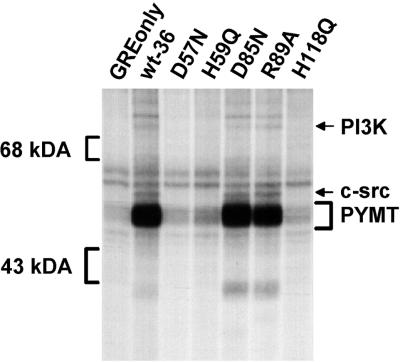
FIG. 4.
The pp60c-src complexed with the catalytically inactive C subunit mutants, D85N and R89A, is activated similarly to pp60c-src complexed with wt C subunit. In vitro kinase assays were performed on anti-epitope tag immunoprecipitates prepared from cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits (see Fig. 2 for nomenclature). After incubation of the immunoprecipitates with [γ-32P]ATP, proteins were separated by SDS-PAGE and phosphoproteins were visualized by autoradiography. The positions of the 43- and 68-kDa markers, pp60c-src (c-src), the 85-kDa subunit of PI 3-kinase (PI3K) and MT (PYMT) are indicated. The data shown are representative of results obtained from two independent experiments.
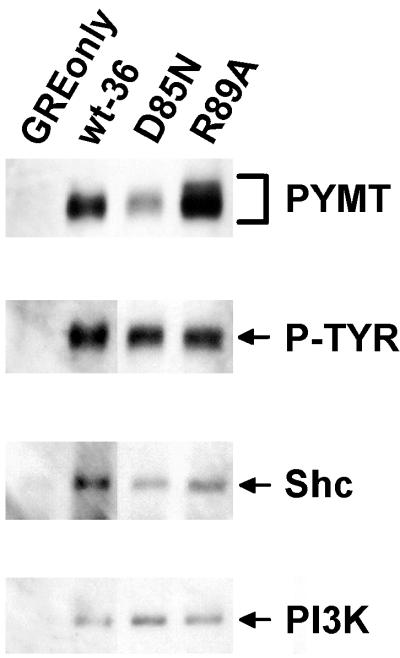
Activated, MT-associated pp60c-src is known to phosphorylate several tyrosine residues in MT which, when phosphorylated, serve as docking sites for SH2 or PTB domain-containing proteins like PI 3-kinase (66) or Shc (10, 26). To determine if pp60c-src in MT complexes containing inactive PP2A was functionally active, we investigated whether the MT in these complexes was tyrosine phosphorylated in vivo and associated with PI 3-kinase and Shc. Immunoprecipitates of wt C subunit and of inactive C subunits competent to form MT complexes containing pp60c-src (D85N and R89A) were sequentially probed with anti-phosphotyrosine antibodies, anti-Shc antibodies, and anti-p85 PI 3-kinase subunit antibodies. The results presented in Fig. 5 indicate that MT associated with the inactive mutants D85N and R89A is indeed phosphorylated on tyrosine in vivo and associates with both PI 3-kinase and Shc. Consistent with the increased amount of pp60c-src associated with D85N, this mutant showed a relative increase in phosphotyrosine content and Shc–PI 3-kinase binding in vivo. Thus, D85N- and R89A-associated MTs are most likely phosphorylated on Y250 and Y315, the known docking sites of Shc and PI 3-kinase. Consistent with the fact that H59Q binds almost no pp60c-src, little tyrosine phosphorylation could be detected in the MT coimmunoprecipitating with this mutant (data not shown).
FIG. 5.
The inactive C subunit mutants capable of forming complexes with MT and pp60c-src also associate with Shc and PI 3-kinase. Lysates of cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits (see Fig. 2 for nomenclature) were immunoprecipitated with 12CA5 antibody. Only those inactive C subunit mutants which retained the ability to bind substantial amounts of pp60c-src were analyzed. The Western blot was sequentially probed with antibodies recognizing the 85-kDa subunit of PI 3-kinase (PI3K), Shc, phosphotyrosine (the phosphotyrosine-containing MT band [P-TYR] is indicated), and MT (PYMT). All lanes shown are from the same experiment and gel, but they were not all originally adjacent. The data shown are representative of results obtained from three independent experiments.
DISCUSSION
In an MT-transformed cell, nearly all of the MT binds to the heterodimeric A-C form of PP2A (54), occupying an estimated 1/10 of the total PP2A in the cell (32a, 68). A fraction of these complexes further associate with pp60c-src family kinases, and a portion of these complexes in turn are phosphorylated to different extents on three tyrosines, facilitating the recruitment of PI 3-kinase, Shc, and PLCγ1, leading to the final MT signaling complex(es) known to be essential for MT-mediated transformation. In this study, we investigated the importance of PP2A activity for assembly of the MT signaling complex in vivo by performing an in vivo analysis of PP2A catalytic activity and subunit composition facilitated by efficient immunoprecipitation of PP2A complexes via epitope-tagged C subunits (47). The results are summarized in Table 1. Several PP2A C subunit residues essential for catalysis were identified. These residues, along with one other that was identified in a separate study (46), were shown to be important for complex formation with a cellular B-type subunit but not with the viral B-type subunit, MT. Most catalytically inactive PP2A C subunits capable of forming heterodimers with A subunit could also bind to MT, indicating that these residues, and thus PP2A catalytic activity, are not necessary for the formation of these complexes. Furthermore, two catalytically inactive C subunit mutants supported the subsequent association of pp60c-src, PI 3-kinase, and Shc to form what appeared to be fully functional MT complexes. The pp60c-src in these complexes appeared to be properly activated; the MT in them was phosphorylated on tyrosine in vivo, and PI 3-kinase and Shc were associated at wt levels. Thus, although the association of pp60c-src, PI 3-kinase, and Shc was previously shown to depend on PP2A binding, PP2A activity, at least in cis, is not required for these associations.
TABLE 1.
Summary of PP2A C subunit mutant activities and complexed proteins
| Namea | Conservation of mutated residueb | Activity (− background) (%wt)c | Coimmunoprecipitation of proteinsd
|
|||||
|---|---|---|---|---|---|---|---|---|
| A subunite | B subunit | MT | Srcf | Shc | p85(PI3K) | |||
| Wt36 | Not applicable | 100 | ++ | ++ | ++ | ++ | ++ | ++ |
| D57N | Invariant | 0 | +/− | +/− | +/− | − | NA | NA |
| H59Q | Invariant | 0 | ++ | +/− | ++ | +/− | NA | NA |
| D85N | Invariant | 0 | ++ | +/− | ++ | ++ | ++ | ++ |
| R89A | Invariant | 0 | ++ | +/− | ++ | ++ | ++ | ++ |
| H118Q | Invariant | 0 | +/− | +/− | +/− | − | NA | NA |
Wt36, wt C subunit. See the legend to Fig. 2 for nomenclature of the mutants.
Invariant, absolutely conserved among all PPP family members.
Background activity from immunoprecipitates prepared from control cells containing only empty vector (see Fig. 2) was subtracted, and values of ≤0 were reported as 0.
++, substantial amount binds; +/−, small amount binds; −, not detectable; NA, not assayed. All values are relative to C subunit. PI3K, PI 3-kinase.
Long exposures of all experiments, such as the one shown in Fig. 3, clearly show that a small amount of A subunit associates with D57N and H118Q (data not shown).
In some experiments, H59Q binds a small amount of Src (data not shown).
The functional significance of the dependence of MT-pp60c-src association on PP2A binding is not clear. One possibility is that this dependence ensures that every MT complex containing pp60c-src, PI 3-kinase, Shc, or PLCγ1 will have a molecule of PP2A bound as well. If this were the case, it would be consistent with the possibility that PP2A regulates other MT-associated proteins once the complex is formed, something PP2A is known to do in vitro, at least for PI 3-kinase (12, 24). Our initial efforts to probe for differences in associated PI 3-kinase activity between wt C subunit and the inactive mutants competent to form a complete MT complex have been unsuccessful, in large part, perhaps, because only a very small amount of PI 3-kinase is present in immunoprecipitates of epitope-tagged wt and mutant C subunits.
Another possibility is that PP2A binding is important for correct localization of the MT complex (7). MT mutants that do not bind PP2A but retain the membrane binding sequence display a dramatically altered subcellular distribution (7). Improper localization of MT not associated with PP2A might even be the explanation for the lack of pp60c-src binding seen for MTs that do not bind PP2A. However, PP2A association alone is not sufficient for proper localization of MT and subsequent pp60c-src association. An intact MT membrane anchor site is required for membrane localization (42) and for pp60c-src binding. Thus, PP2A binding, in combination with an intact membrane binding sequence, may be important for proper localization of MT and subsequent association of pp60c-src.
Several differences between MT-bound PP2A and B subunit-bound PP2A have been found in substrate specificity and in the determinants in the A-C heterodimer necessary for the stable association of the respective B-type subunit (47, 56, 57). For example, the C terminus of the PP2A C subunit is required for binding the B subunit but is dispensable for polyomavirus MT binding (47). Our present study provides yet another example of a difference between MT and B subunit binding to the A-C heterodimer. All of the inactive C subunit mutants were severely impaired in their interaction with B subunit, while MT binding was not affected in four of the five inactive mutants. Therefore these active-site residues, and perhaps PP2A activity, appear to be important for complex formation with the cellular B subunit but not for complex formation with MT. It is tempting to speculate that B subunit’s requirement for these active site residues as well as for an intact C terminus, a known site of covalent modifications, could be part of a potential mechanism which normally regulates PP2A complex formation with B subunit. MT binding to the A-C heterodimer would be predicted to be immune to such regulation.
Another possibility consistent with our current findings is that PP2A serves an essential structural, noncatalytic role in the assembly of a transformation-competent MT complex. A structural role for PP2A might involve alterations in the conformation of MT upon PP2A binding, thereby affecting MT’s affinity for pp60c-src. Alternatively, although there is no evidence at present for a direct interaction between PP2A and pp60c-src, PP2A might interact directly with pp60c-src, helping to stabilize its interaction with the MT complex.
Our data do not rule out the possibility that MT-associated PP2A activity may act in trans in the assembly of the MT complex because endogenous, wt PP2A complexed with MT might be providing this activity for the MTs complexed with inactive C subunits in the same cells. We have found that low levels of okadaic acid, a cell-permeable PP2A inhibitor, have no detectable effect on the activation of MT-associated pp60c-src (unpublished data). This suggests that PP2A activity may not be necessary, even in trans, for MT complex assembly, but it is possible that some residual PP2A activity was occurring in vivo under the conditions used.
Four of the five inactive C subunit mutants analyzed in this study showed an increase in a slower-migrating form(s) of the associated MT, suggesting that catalytic activity of the MT-associated PP2A might be important for the proper posttranslational modification (most likely phosphorylation) of MT. However, the fact that the MT found associated with the inactive mutant D57N appeared unchanged raises the possibility that all the observed shifts might be due to other effects of the inactivating C subunit mutations on the final MT complex. One possibility is that inactivating mutations might alter the conformation of the mutant C subunit (or its associated A subunit) so that, once bound in an MT complex, it interferes with the normal dephosphorylation of MT by a phosphatase. A similar scenario could be envisioned whereby the mutations alter the ability of the mutant C subunits to induce conformational changes in MT that affect the accessibility of certain sites on MT to kinases or phosphatases. The exact location(s) of the modification(s) in the MTs associated with the mutant C subunits have not been determined; therefore, it is unclear at this point whether the differences in the ratios of the MT bands are primarily due to changes in the extent of phosphorylation of one particular site or to differential phosphorylation at multiple sites. It will be of interest in the future to determine the nature and location of the modification(s) responsible for the dramatic shift seen with the mutant H59Q. Because this mutant is unable to bind pp60c-src, it is possible that the modification(s) responsible for the shift affects pp60c-src association.
Mutational analysis of active-site residues in PP2A has lagged behind similar analyses of related phosphatases, probably due to the fact that bacterially expressed wt PP2A C subunit is predominantly produced as an insoluble, inactive protein. To overcome this problem, we have performed analyses on epitope-tagged C subunits expressed in mammalian cells. The PP2A C subunit residues targeted for mutation in this study (Asp57, Asp85, and Arg89) are among the first reported amino acids in PP2A shown to be catalytically important by a genetic and biochemical analysis. The only other putative active-site mutations described to date for PP2A are those in the H59Q and H118Q mutants that were used in this study. Data for these two mutants were reported originally in a separate study describing a novel cellular protein that associates with them (46).
The identity of the metallophosphoesterase motif residues (27, 31, 36) among almost all of the protein-serine-threonine phosphatases (PSTPases) (5) suggests a common mechanism for all PSTPases, including PP2A. From crystallographic and mutational studies of related phosphatases, one could predict that PP2A residues His59, Asp57, and Asp85, along with other residues, would help coordinate two divalent metal ions present in the active site. A similar prediction would suggest that Arg89 in PP2A might help several other residues and the two active-site metal ions bind the phosphate oxygens of the substrate and play a role in catalysis (34, 70). A metal-activated water molecule most likely makes a nucleophilic attack on the substrate phosphorus atom in an Sn2 reaction. His118 in PP2A would be predicted to act as a general acid, protonating the leaving-group oxygen of the substrate serine or threonine residue. Our data are in good agreement with the predicted role of the signature motif residues for PP2A catalysis, since single-substitution mutations of these putative PP2A active-site residues (Asp57, His59, Asp85, Arg89, and His118) abolished the catalytic activity of PP2A. Thus, our results not only provide some of the first experimental data on PP2A catalytic-site residues but also support the model of an absolutely conserved catalytic core among the PPP family of serine/threonine phosphatases. Additional mutational studies using the approach described in this study should continue to extend our understanding of PP2A catalysis, regulation, and function in normal and MT-transformed cells.
ACKNOWLEDGMENTS
Mouse monoclonal antibodies 327 and 4G10 and anti-PI 3-kinase antibodies were kindly provided by J. Brugge, T. Roberts, and Upstate Biotechnology, Inc., respectively. We thank Brian Hemmings for the C subunit cDNA, John White for the pGRE vector, Robert Liddington for advice on safe substitutions, and Carlos Moreno, Cori Beychok, and Richard Green for critical reading of the manuscript.
This work was supported by a National Institutes of Health grant to D.C.P. (CA57327). E.O. was supported by an Erwin Schrödinger Fellowship from Austrian Fonds zur Förderung der Wissenschaftlichen Forschung and by grants from the Austrian Science Foundation (FWF, MOB-12523) and from the Herzfelder Familienstiftung.
REFERENCES
- 1.Agostinis P, Derua R, Sarno S, Goris J, Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP,Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by P34cdc2 kinase. Eur J Biochem. 1992;205:241–248. doi: 10.1111/j.1432-1033.1992.tb16774.x. [DOI] [PubMed] [Google Scholar]
- 2.Agostinis P, Donella-Deana A, Van Hoof C, Cesaro L, Brunati A M, Ruzzene M, Merlevede W, Pinna L A, Goris J. A comparative study of the phosphotyrosyl phosphatase specificity of protein phosphatase type 2A and phosphotyrosyl phosphatase type 1B using phosphopeptides and the phosphoproteins p50/HS1, c-Fgr and Lyn. Eur J Biochem. 1996;236:548–557. doi: 10.1111/j.1432-1033.1996.00548.x. [DOI] [PubMed] [Google Scholar]
- 3.Arino J, Woon C W, Brautigan D L, Miller T, Jr, Johnson G L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc Natl Acad Sci USA. 1988;85:4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker H M, Jones T A, da Cruz e Silva E F, Spurr N K, Sheer D, Cohen P T. Localization of the gene encoding a type I protein phosphatase catalytic subunit to human chromosome band 11q13. Genomics. 1990;7:159–166. doi: 10.1016/0888-7543(90)90536-4. [DOI] [PubMed] [Google Scholar]
- 5.Barton G J, Cohen P T, Barford D. Conservation analysis and structure prediction of the protein serine/threonine phosphatases. Sequence similarity with diadenosine tetraphosphatase from Escherichia coli suggests homology to the protein phosphatases. Eur J Biochem. 1994;220:225–237. doi: 10.1111/j.1432-1033.1994.tb18618.x. [DOI] [PubMed] [Google Scholar]
- 6.Bordo D, Argos P. Suggestions for “safe” residue substitutions in site-directed mutagenesis. J Mol Biol. 1991;217:721–729. doi: 10.1016/0022-2836(91)90528-e. [DOI] [PubMed] [Google Scholar]
- 7.Brewster C E, Glover H R, Dilworth S M. pp60c-src binding to polyomavirus middle T-antigen (MT) requires residues 185 to 210 of the MT sequence. J Virol. 1997;71:5512–5520. doi: 10.1128/jvi.71.7.5512-5520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Campbell, K. S., and T. M. Roberts. Unpublished data.
- 8.Campbell K S, Auger K R, Hemmings B A, Roberts T M, Pallas D C. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B S, DeCaprio J A. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K S, Ogris E, Burke B, Su W, Auger K R, Druker B J, Schaffhausen B S, Roberts T M, Pallas D C. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci USA. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael G G, Benjamin T L. Identification of DNA sequence changes leading to loss of transforming ability in polyoma virus. J Biol Chem. 1980;255:230–235. [PubMed] [Google Scholar]
- 12.Carpenter C L, Auger K R, Duckworth B C, Hou W M, Schaffhausen B, Cantley L C. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993;13:1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright C A, Kaplan P L, Cooper J A, Hunter T, Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986;6:1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayla X, Ballmer-Hofer K, Merlevede W, Goris J. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur J Biochem. 1993;214:281–286. doi: 10.1111/j.1432-1033.1993.tb17922.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S H, Harvey R, Espino P C, Semba K, Yamamoto T, Toyoshima K, Smith A E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988;7:3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherington V, Morgan B, Spiegelman B M, Roberts T M. Recombinant retroviruses that transduce individual polyoma tumor antigens: effects on growth and differentiation. Proc Natl Acad Sci USA. 1986;83:4307–4311. doi: 10.1073/pnas.83.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P T, Cohen P. Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem J. 1989;260:931–934. doi: 10.1042/bj2600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook D N, Hassell J A. The amino terminus of polyomavirus middle T antigen is required for transformation. J Virol. 1990;64:1879–1887. doi: 10.1128/jvi.64.5.1879-1887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtneidge S A, Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987;50:1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- 21.Courtneidge S A, Smith A E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature (London) 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 22.Cullere X, Rose P, Thathamangalam U, Chatterjee A, Mullane K P, Pallas D C, Benjamin T L, Roberts T M, Schaffhausen B S. Serine 257 phosphorylation regulates association of polyomavirus middle T antigen with 14-3-3 proteins. J Virol. 1998;72:558–563. doi: 10.1128/jvi.72.1.558-563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cyert M S, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci USA. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. . (Erratum, 89:4220, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhand R, Hiles I, Panayotou G, Roche S, Fry M J, Gout I, Totty N F, Truong O, Vicendo P, Yonezawa K, et al. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilworth S M. Polyoma virus middle T antigen: meddler or mimic? Trends Microbiol. 1995;3:31–35. doi: 10.1016/s0966-842x(00)88866-6. [DOI] [PubMed] [Google Scholar]
- 26.Dilworth S M, Brewster C E, Jones M D, Lanfrancone L, Pelicci G, Pelicci P G. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature. 1994;367:87–90. doi: 10.1038/367087a0. [DOI] [PubMed] [Google Scholar]
- 27.Egloff M P, Cohen P T, Reinemer P, Barford D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J Mol Biol. 1995;254:942–959. doi: 10.1006/jmbi.1995.0667. [DOI] [PubMed] [Google Scholar]
- 28.Favre B, Turowski P, Hemmings B A. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- 29.Ferrigno P, Langan T A, Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993;4:669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glenn G M, Eckhart W. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J Virol. 1995;69:3729–3736. doi: 10.1128/jvi.69.6.3729-3736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg J, Huang H B, Kwon Y G, Greengard P, Nairn A C, Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995;376:745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- 32.Guerini D, Klee C B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci USA. 1989;86:9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Haehnel, L., and D. C. Pallas. Unpublished data.
- 33.Horak I D, Kawakami T, Gregory F, Robbins K C, Bolen J B. Association of p60fyn with middle tumor antigen in murine polyomavirus-transformed rat cells. J Virol. 1989;63:2343–2347. doi: 10.1128/jvi.63.5.2343-2347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H B, Horiuchi A, Goldberg J, Greengard P, Nairn A C. Site-directed mutagenesis of amino acid residues of protein phosphatase 1 involved in catalysis and inhibitor binding. Proc Natl Acad Sci USA. 1997;94:3530–3535. doi: 10.1073/pnas.94.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan D R, Whitman M, Schaffhausen B, Pallas D C, White M, Cantley L, Roberts T M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 36.Kissinger C R, Parge H E, Knighton D R, Lewis C T, Pelletier L A, Tempczyk A, Kalish V J, Tucker K D, Showalter R E, Moomaw E W, Gastinel L N, Habuka N, Chen X, Maldonado F, Barker J E, Bacquet R, Villafranca J E. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995;378:641–644. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- 37.Kornbluth S, Sudol M, Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987;325:171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Lipsich L A, Lewis A J, Brugge J S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Ishii S, Tokai M, Tsutsumi H, Ohki O, Akada R, Tanaka K, Tsuchiya E, Fukui S, Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991;227:52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- 41.Mader S, White J H. A steroid-inducible promoter for the controlled overexpression of cloned genes in eukaryotic cells. Proc Natl Acad Sci USA. 1993;90:5603–5607. doi: 10.1073/pnas.90.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markland W, Oostra B A, Harvey R, Markham A F, Colledge W H, Smith A E. Site-directed mutagenesis of polyomavirus middle-T antigen sequences encoding tyrosine 315 and tyrosine 250. J Virol. 1986;59:384–391. doi: 10.1128/jvi.59.2.384-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews J T, Benjamin T L. 12-O-tetradecanoylphorbol-13-acetate stimulates phosphorylation of the 58,000-Mr form of polyomavirus middle T antigen in vivo: implications for a possible role of protein kinase C in middle T function. J Virol. 1986;58:239–246. doi: 10.1128/jvi.58.2.239-246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer-Jaekel R E, Ohkura H, Ferrigno P, Andjelkovic N, Shiomi K, Uemura T, Glover D M, Hemmings B A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J Cell Sci. 1994;107:2609–2618. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- 45.Muslin A J, Tanner J W, Allen P M, Shaw A S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 46.Ogris E, Du X, Nelson K C, Mak E K, Yu X X, Lane W S, Pallas D C. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogris E, Gibson D M, Pallas D C. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–917. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 48.Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- 49.Otsu M, Hiles I, Gout I, Fry M J, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N, et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65:91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- 50.Pallas D C, Cherington V, Morgan W, DeAnda J, Kaplan D, Schaffhausen B, Roberts T M. Cellular proteins that associate with the middle and small T antigens of polyomavirus. J Virol. 1988;62:3934–3940. doi: 10.1128/jvi.62.11.3934-3940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallas D C, Fu H, Haehnel L C, Weller W, Collier R J, Roberts T M. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science. 1994;265:535–537. doi: 10.1126/science.8036498. [DOI] [PubMed] [Google Scholar]
- 52.Pallas D C, Morgan W, Roberts T M. The cellular proteins which can associate specifically with polyomavirus middle T antigen in human 293 cells include the major human 70-kilodalton heat shock proteins. J Virol. 1989;63:4533–4539. doi: 10.1128/jvi.63.11.4533-4539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pallas D C, Schley C, Mahoney M, Harlow E, Schaffhausen B S, Roberts T M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986;60:1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 55.Ronne H, Carlberg M, Hu G Z, Nehlin J O. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991;11:4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruediger R, Fields K, Walter G. Binding specificity of protein phosphatase 2A core enzyme for regulatory B subunits and T antigens. J Virol. 1999;73:839–842. doi: 10.1128/jvi.73.1.839-842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruediger R, Roeckel D, Fait J, Bergqvist A, Magnusson G, Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992;12:4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.Schaffhausen B S, Liang T J, Carmichael G G, Benjamin T L. Residual transforming activity of PY1178T, a mutant lacking the principal in vitro tyrosine phosphorylation site, is not affected by removal of the secondary tyrosine phosphorylation site at residue 322. Virology. 1985;143:671–675. doi: 10.1016/0042-6822(85)90410-6. [DOI] [PubMed] [Google Scholar]
- 60.Senften M, Dilworth S, Ballmer-Hofer K. Multimerization of polyomavirus middle-T antigen. J Virol. 1997;71:6990–6995. doi: 10.1128/jvi.71.9.6990-6995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sneddon A A, Cohen P T, Stark M J. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990;9:4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sola M M, Langan T, Cohen P. p34cdc2 phosphorylation sites in histone H1 are dephosphorylated by protein phosphatase 2A1. Biochim Biophys Acta. 1991;1094:211–216. doi: 10.1016/0167-4889(91)90011-l. [DOI] [PubMed] [Google Scholar]
- 63.Song Q, Khanna K K, Lu H, Lavin M F. Cloning and characterization of a human protein phosphatase 1-encoding cDNA. Gene. 1993;129:291–295. doi: 10.1016/0378-1119(93)90282-8. [DOI] [PubMed] [Google Scholar]
- 64.Stone S R, Mayer R, Wernet W, Maurer F, Hofsteenge J, Hemmings B A. The nucleotide sequence of the cDNA encoding the human lung protein phosphatase 2A alpha catalytic subunit. Nucleic Acids Res. 1988;16:11365. doi: 10.1093/nar/16.23.11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su W, Liu W, Schaffhausen B S, Roberts T M. Association of polyomavirus middle tumor antigen with phospholipase C-gamma 1. J Biol Chem. 1995;270:12331–12334. doi: 10.1074/jbc.270.21.12331. [DOI] [PubMed] [Google Scholar]
- 66.Talmage D A, Freund R, Young A T, Dahl J, Dawe C J, Benjamin T L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989;59:55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- 67.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulug E T, Cartwright A J, Courtneidge S A. Characterization of the interaction of polyomavirus middle T antigen with type 2A protein phosphatase. J Virol. 1992;66:1458–1467. doi: 10.1128/jvi.66.3.1458-1467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walter G, Ruediger R, Slaughter C, Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci USA. 1990;87:2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Zhang Z, Brew K, Lee E Y. Mutational analysis of the catalytic subunit of muscle protein phosphatase-1. Biochemistry. 1996;35:6276–6282. doi: 10.1021/bi952954l. [DOI] [PubMed] [Google Scholar]
- 71.Zhuo S, Clemens J C, Stone R L, Dixon J E. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J Biol Chem. 1994;269:26234–26238. [PubMed] [Google Scholar]