Abstract
The humeral head is considered the second most common site for osteonecrosis to occur after the femoral head. As seen in the femoral head, the circulatory implications characteristic of this condition are attributable to the interaction between a genetic predisposition and the exposure to certain risk factors. There is no consensus regarding the pathogenesis of osteonecrosis, yet the final common pathway results in disrupted blood supply, increased intraosseous pressure, and bone death. Disease staging using radiography and magnetic resonance imaging is predictive of disease progression and can help the orthopedic surgeon to guide treatment. Although there is a myriad of treatment modalities, there is a lack of high-quality evidence to conclude what is the most appropriate treatment option for each stage of humeral head osteonecrosis. Nonoperative treatment is the preferred option in early-stage disease, and it may prevent disease progression. Nonetheless, in some cases, disease progression occurs despite nonoperative measures, and surgical treatment is required. The purpose of this article is to provide an updated review of the available evidence on risk factors, diagnosis, and treatment of atraumatic humeral head osteonecrosis.
Keywords: Shoulder, Osteonecrosis, Humeral head, Natural history, Pathogenesis, Treatment, Risk factors
Osteonecrosis is a relatively common condition characterized by a reduction in subchondral blood supply leading to necrosis of the cells, subchondral collapse, and ultimately joint degeneration. The etiology of the blood supply disruption can be either traumatic or atraumatic.67 The humeral head is the second most common location for atraumatic osteonecrosis to occur after the femoral head; therefore, atraumatic osteonecrosis and its sequelae are not infrequently seen in the shoulder.33
Atraumatic humeral head osteonecrosis (HHO) can lead to substantial morbidity, with the vast majority of untreated patients progressing to articular collapse, especially those who remain symptomatic.26,47 There is a lack of complete understanding in the body of knowledge regarding the pathogenesis and prognostic factors for osteonecrosis. In addition, osteonecrosis usually presents in young active patients in whom arthroplasty is not ideal, posing a great challenge for orthopedic surgeons when deciding a treatment option. The purpose of this review is to summarize the current knowledge on the pathophysiology, risk factors, diagnosis, and treatment strategies for atraumatic osteonecrosis of the humeral head (AOHH).
Vascular supply of proximal humerus
The humeral head is directly supplied by the anterior and posterior circumflex arteries along with several anastomotic contributions of the suprascapular, thoracoacromial, and subscapular arteries. Traditionally, it was considered that the anterolateral branch of the anterior circumflex artery provided the main blood supply to the humeral head. This finding was supported by Gerber et al14 in 1990 in a study of 29 cadavers using a radiopaque contrast technique. However, this notion was later questioned, given the fact that the anterior circumflex artery is disrupted in association with 80% of proximal humeral fractures, and the resultant osteonecrosis is infrequent. To clarify this inconsistency, in 2010, Hettrich et al performed a quantitative assessment of the vascularity of the proximal part of the humerus using magnetic resonance imaging (MRI) with gadolinium contrast in 24 cadavers.29 These authors found that the posterior circumflex artery provided two-thirds of the blood supply to the humeral head overall and provided significantly more of the blood supply in 3 of the 4 quadrants of the humeral head. This finding changed the notion that the anterior circumflex artery provided the main blood supply to the humeral head and postulated a possible explanation for the relatively low rates of osteonecrosis seen in association with displaced fractures of the proximal part of the humerus.
Risk factors and pathogenesis
Osteonecrosis is a multifactorial condition that develops in individuals with a genetic predisposition who are exposed to one or several risk factors (Table I).
Table I.
Factors associated with osteonecrosis of the humeral head.
Although different mechanisms of pathogenesis have been described depending on the etiology, all of them share a common final pathway, resulting in disrupted blood supply, increased intraosseous pressure, and bone death.67 Different studies have demonstrated that osteocyte apoptosis seen in osteonecrosis produces a release of paracrine factors that trigger a bone remodeling cycle.4,41 Regardless of the osteonecrosis etiology, this bone remodeling cycle is poorly regulated with abnormal osteoblast and osteoclast activity leading to microfractures, subchondral fracture, articular surface collapse, and finally, glenohumeral arthritis.
Corticosteroid use and osteonecrosis
Corticosteroid (CS) use is the most common cause of atraumatic osteonecrosis in any anatomical region, including the shoulder.23,47,63 There are 2 accepted theories on the pathogenesis of CS-induced osteonecrosis. First, Jones et al35 hypothesized that cortisone generates fatty liver and hypertriglyceridemia, resulting in systemic fat embolism that may affect the subchondral bone circulation. A second theory proposes that instead of fat embolism, glucocorticoids induce local changes within the bone that result in increased differentiation of mesenchymal stem cells (MSCs) into adipocytes, fat cell hypertrophy, reduced osteoclast and osteoblast differentiation, and osteocyte apoptosis.69,78,79,82 Whether due to fat embolism or local changes within the bone, fat cell hypertrophy and hyperplasia result in increased intramedullary pressure and decreased blood flow.
CS-induced osteonecrosis may be multifocal, and MRI has been suggested as a screening method for multifocal osteonecrosis. Nawata et al demonstrated that hips and knees are first affected shoulders in CS-induced osteonecrosis. Therefore, they suggested that any osteonecrosis observed in the hip and/or the knee joints warrants a second MRI screening of the shoulders.58 A population with a particularly high risk for CS-induced osteonecrosis are children with acute lymphoblastic lymphoma and leukemia.70 HHO is an underappreciated adverse late effect of therapy in these patients, which can limit the quality of life and functionality. Two different studies have reported the severity and functional impairment of HHO among children with acute lymphoblastic lymphoma and leukemia.31,38 In these studies, despite a large proportion of the patients were being asymptomatic, 35%-63% of the shoulders had necrotic lesions involving >30% of the humeral head.31
CS usage has recently increased worldwide because of its role in the treatment of patients with severe coronavirus disease 2019 (COVID-19).30 Li et al51 reported that the incidence of osteonecrosis among patients with severe acute respiratory syndrome who received high cumulative doses and long treatment durations of glucocorticoids was 32%. Considering the similarity between severe acute respiratory syndrome and COVID-19 and the fact that osteonecrosis has already been reported after COVID-19 and CS treatment,51 clinicians must be aware of this condition in convalescent COVID-19 patients with shoulder symptoms who received CS treatment.
The natural progression of HHO related to CS treatment was described by Hernigou et al in a cohort of 125 patients (215 shoulders).26 The delay between the beginning of the CS treatment and the diagnosis of HHO averaged 15 months. Of the patients who were initially asymptomatic, 74% developed pain, and collapse occurred in 54% during the follow-up. Staging at initial visit, occurrence of pain, and continuation of peak doses of CS therapy predicted progression of disease in asymptomatic shoulders, whereas in the symptomatic shoulders, extent and location of the lesion were the main risk factors for progression.26
Although CS treatment is a risk factor for osteonecrosis, not every patient receiving CS treatment develops osteonecrosis. Thus, there is an increased body of evidence supporting the role of genetic factors in the development of CS-induced osteonecrosis. To date, at least 7 different gene polymorphisms have been proposed as risk factors for the development of CS-induced osteonecrosis.48 One of these polymorphisms occurs in the ACP1 gene, a key regulator of osteoblast differentiation.84 Polymorphism in the ACP1 gene has been associated with increased risk for osteonecrosis (ON) and increased cholesterol levels in patients receiving high-dose CS therapy.40 Polymorphisms in the GRIN3A and GRIK1 genes have been associated with an increased osteonecrosis risk in independent cohorts of children with acute lymphoblastic leukemia undergoing high-dose CS therapy.37,85 These genes encode glutamate receptor subunits, suggesting a role of the glutamate receptor pathway in the pathogenesis of CS-induced osteonecrosis.37 In addition, Sherief et al70 identified that factors strongly associated with CS-induced osteonecrosis, such as older age (>10 years) and lower levels of 25-hydroxy vitamin D, were associated with polymorphisms of the plasminogen activator inhibitor 1 and vitamin D receptor genes. Given that most findings on genetic polymorphisms have not been replicated in independent cohorts, pharmacogenetic testing in clinical practice is not currently indicated. However, this is a promising area of research that could help stratify osteonecrosis risk in patients requiring long-term high-dose CS therapy.
Hemoglobinopathies
Sickle cell disease (SCD), thalassemia, and G6PD deficiency are recognized hematological conditions that can induce osteonecrosis. SCD is the most common hemoglobinopathy related to ON. Osteonecrosis pathophysiology in SCD consists of deformed blood cells that obstruct the microcirculation of the humeral head leading to microinfarcts.8 Milner et al evaluated the prevalence and incidence of HHO in a cohort of 2524 patients with SCD who were entered into a prospective study and followed for an average of 5.6 years.54 The prevalence of radiographic evident HHO was 5.6%, with little difference in age-adjusted prevalence among genotypes. However, specifically at ages ranging from 5 to 24 years, there were striking differences in HHO rates among genotypes, with the highest prevalence (3.25%) seen in patients homozygous for hemoglobin S (S/S genotype). Patients with hemoglobin S/hemoglobin C (S/C) had a prevalence of HHO of 1.1%, and no S/beta+ thalassemia patients aged <25 years had HHO on entry. The natural course of symptomatic HHO in adults with SCD was described by Poignard et al demonstrating that untreated symptomatic HHO related to SCD has a high likelihood of progressing to humeral head collapse (86%), and the natural evolution in the long term requires surgical treatment for 61% of these patients.60 Furthermore, these authors found a higher risk of disease progression in patients with S/S hemoglobin genotype, large lesion size (≥36% of humeral head volume), medial or posterior location of the lesion, and Stage II or greater.60
Alcohol
Alcohol triggers a pathophysiological mechanism similar to that proposed for CS-induced osteonecrosis in which hyperlipidemia results in subchondral fat embolization.34 Studies on osteonecrosis of the hip have found a dose–response relationship between alcohol intake and osteonecrosis. Consumers of 400-1000 mL and ≥1000 mL of alcohol per week have a greater risk of ON (Relative risk = 9.8 and 17.9, respectively, P < .001) compared with nondrinkers.53
In an in vitro study, Yu et al evaluated the role of adalimumab, a tumoral necrosis factor alpha (TNF-alpha) inhibitor, in alcohol-induced ON. These authors found an enhancement of the osteogenic activity with the use of adalimumab, thereby suggesting a role of inflammatory cytokines (TNF-alpha and miR-31) in alcohol-induced osteonecrosis.83 Nevertheless, further research is needed before considering TNF-alpha inhibitors as a therapeutic option in the clinical practice. The current recommended treatment in these patients aims to reduce hyperlipidemia through dietary recommendations and lipid-lowering drugs.33
Dysbarism (Caisson disease)
Dysbaric osteonecrosis (DON) appears typically in divers who suffer a sudden change in pressure, which may result in intravascular air embolism that can occlude smaller vessels.8 Prevalence of DON among professional divers is highly variable, being higher in countries such as Hawaii, Turkey, Korea, and Japan.76
A study conducted by Miyanishi et al found that plasminogen activator inhibitor concentrations >38 ng/mL and a diving depth >35 m were independent predictors for DON.55 Another study by Gempp et al demonstrated that factors influencing the occurrence of DON were age ≥40 years, body mass index >25.5 kg/m2, total dive ≥40 minutes, delay to hyperbaric treatment >6 hours, and residual symptoms over several days after discharge, sometimes preceded by worsening of pain during recompression.
Gaucher’s disease
Type 1 Gaucher’s disease, an autosomal recessive disease caused by a deficient activity of B-glucocerebrosidase, induces accumulation of glucocerebroside in macrophage lysosomes. The pathophysiology leading to osteonecrosis in Gaucher’s disease is not completely understood, but there is a compromise of the bone marrow with obstruction of small vessels that finally leads to ischemia. The presence of anemia (odds ratio, 1.59; confidence interval 95%, 1.06-2.38; P < .05)43 and splenectomy (odds ratio, 10; confidence interval 95%, 1.7-58.4; P < .01) have been described as risk factors for osteonecrosis in this patient population.65
Idiopathic osteonecrosis and other etiologies
In some cases, the direct cause of osteonecrosis cannot be determined and is classified as idiopathic. A significant association has been reported between idiopathic osteonecrosis of the femoral head in adults and the presence of either factor V Leiden or prothrombin 20210A mutations.3 However, no study has evaluated this association in patients with idiopathic osteonecrosis of the humeral head. Postoperative HHO has been reported after arthroscopic débridement and rotator cuff repair in patients with no known risk factors for osteonecrosis.9,17 Other uncommon etiologies of atraumatic HHO reported in the literature include fat embolism secondary to pancreatitis,36 hypofibrinolysis and thrombophilic state induced by estrogen excess in pregnant women,2,15 smoking,53 chemotherapy (bevacizumab),45 and radiation exposure.52 Patients with systemic lupus erythematosus (SLE) are at greater risk for developing osteonecrosis, and SLE has been considered an independent risk factor for osteonecrosis.71
In conclusion, although several risk factors and etiologies have been described for osteonecrosis, several aspects of the pathophysiology of this condition remain unclear. CS use is by far the most common cause of atraumatic HHO, and an active surveillance for HHO in patients under high-dose CS regimes is warranted, especially in those patients who develop hip or knee osteonecrosis. Children with acute lymphoblastic leukemia or lymphoma and adult patients with SLE under CS treatment are populations at higher risk of osteonecrosis, and thus, a higher index of suspicion is recommended in patients with these conditions and shoulder symptoms. Progression of CS-induced HHO is almost inevitable, and a high proportion of patients develop symptoms and collapse, requiring surgical treatment. Research on the role of gene polymorphisms and inflammatory cytokines in the development of osteonecrosis is promising and holds exciting potential as targets for pharmacological interventions to prevent disease presentation and progression.
Clinical assessment
The clinical evaluation should begin with a thorough history to identify risk factors for osteonecrosis that facilitate the diagnosis and to clarify the presence and severity of symptoms, including pain and limitations in activities of daily living. HHO may occur at almost any age, with an average age at diagnosis of 46 years.47 Patients with HHO usually present with poorly localized intermitting deep shoulder pain that radiates down to the elbow. Range of motion (ROM) is not severely compromised until final stages of HHO when collapse and articular incongruity are present. Other findings in the physical examination may include mild to moderate pain at 60 degrees of abduction or 90 degrees of forward flexion as the diseased part of the humeral head comes into contact with the glenoid20 and a painful click due to joint irregularity or intra-articular loose bodies.18 Bicipital tendinitis because of loose bodies inside the bicipital tendon sheath has been reported as an infrequent form of presentation of HHO.80
A high index of suspicion is required, as there are no specific clinical findings for HHO. In addition, a good proportion of patients may be clinically asymptomatic despite advanced staging of the disease.
Radiographic staging and prognosis
Radiographic assessment of the shoulder is essential to diagnose HHO and to stage disease progression. Staging is critical in HHO, as treatment is mostly dictated by disease progression and severity. Radiographs should include anteroposterior, true anteroposterior, Y-scapular, and axillary views of the affected shoulder. A 40° posterior oblique view in internal and external rotation may be useful as well.20,22 The Cruess classification system7 is the most commonly used staging system for HHO. This classification system is based on progression to collapse and categorizes disease progression from Stage 1 in which no radiographic changes are observed to Stage V, in which humeral head collapse and glenoid involvement are present (Fig. 1). While widely used, there are some concerns with the Cruess classification, including the fact it was described more than 40 years ago with few studies assessing its validity and that it was not intended to guide treatment.6,7 As a result, a more recent simple radiological staging system of HHO based on radiological findings and the Association of Research Circulation Osseous principles was proposed.5 This system divides the disease into stages 0 to 4: Stage 0 (all images normal), Stage 1 (radiograph and computed tomography normal, MRI abnormal), Stage 2 (radiographic evidence of sclerosis, osteolysis, and focal porosis but spherical outline of head intact), Stage 3 (subchondral collapse with preservation of joint space), and Stage 4 (advancing joint destruction due to secondary osteoarthritis and loss of joint space). Despite the potential advantages of this simplified system, no further studies have incorporated its use, and the Cruess classification remains to be used as the preferred classification system in HHO studies.
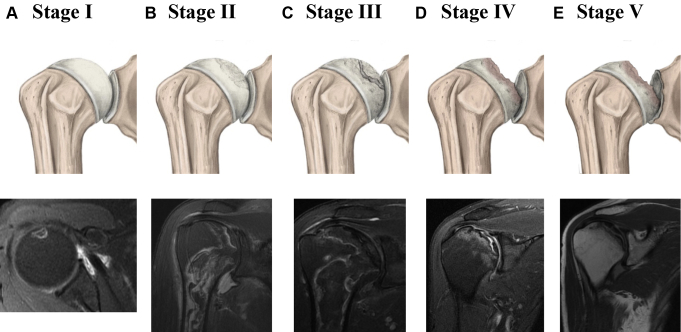
Figure 1.
Illustration and MRI findings of the stages of progression of osteonecrosis of the humeral head according to Cruess. (A) Stage I, preradiological stage characterized by a total absence of radiological changes. MRI findings include small focal lesion with a hyperintense signal abnormality in T2 weighted sequences. (B) Stage II, the curvature of the humeral head is maintained with radiologic signs of repair, including diffuse osteoporosis and mottled sclerosis. MRI findings include bone marrow signal abnormality and the double-line sign: a bright line (granulation tissue) and dark line (necrosis and sclerosis). (C) Stage III is defined by the presence of classical radiographic sign of osteonecrosis “the crescent sign.” This stage represents collapse of the subchondral bone. MRI findings include the crescent sign and associated bone marrow signal abnormality. (D) Stage IV is characterized for extensive collapse of the subchondral bone and severe deformity of the humeral head because of superior flattening. MRI findings include flattening of the humeral head and narrowing of the articular space. (E) Stage V shows pathologic changes in the glenoid in addition to the changes seen in Stage IV. MRI findings include severe humeral head collapse, glenoid cartilage wear, and osteoarthritic changes.
Although radiographs are the initial imaging study and are the primary tool used to stage disease, MRI is the most sensitive tool for diagnosing osteonecrosis especially among early-stage cases with normal radiographs. Typical MRI findings of osteonecrosis include a “band-like” sign (seen as a low-intensity lesion in T1-weighted images and high intensity in T2), humeral head and bone marrow signal abnormalities, double-line sign, and subchondral fracture.11,28,49 Bone scintigraphy has poor sensitivity and is not recommended for the diagnosis of HHO.57
MRI is not only useful for the diagnosis of HHO, but it may be useful to establish prognosis. Lesion extent and location as evaluated on MRI have been found as good predictors of osteonecrosis progression in symptomatic patients.26,60 Different methods have been described to measure lesion extent on MRI. Hernigou et al26 proposed to measure the percentage of the humeral head involved in the lesion by calculating the ratio of the volume of the lesion in relation to the volume of the humeral head considered as 1/2 of a sphere. The extent of involvement is graded as A indicating mild (<15%), B as moderate, and C as severe (>30%; as described by Steinberg et al75 for ON of the hip) according to the percentage of extent of the lesion in the humeral head. Using this method, Hernigou et al found that lesions B or C and posterior and medial lesions were at significantly higher risk of progression.26 Another technique to quantify lesion extent on MRI was described by Sakai et al66 using the necrotic angle. The necrotic angle was measured both in mid-oblique-coronal and mid-oblique-sagittal planes as the angle formed between the center of the humeral head and the 2 endpoints of the necrotic lesion on the joint surface. The lesions with initial necrotic angles of more than 90° on midoblique-coronal images and midoblique- sagittal images more often progressed to collapse than those with smaller angles.66
In conclusion, radiographic and MRI evaluations are pivotal for diagnosis, treatment selection, and prognosis. Radiographs are the most useful tool for disease staging and guiding treatment, whereas MRI is the most sensitive test for diagnosing early-stage disease, screening high-risk patients, and establishing prognosis in terms of disease progression in patients in whom clinical observation and conservative treatment are chosen.
Treatment
The goals of HHO treatment are to improve shoulder function, decrease pain, and avoid progression of osteonecrosis. Conservative treatment can be effective in Stages I and II, especially when symptoms are mild. When clinical and radiographic progression are evident, joint preserving procedures such as arthroscopic débridement and core decompression (CD) are preferred.67 More invasive surgical techniques such as resurfacing, hemiarthroplasty (HA), and total shoulder arthroplasty (TSA) are reserved for more advanced stages (III, IV, or V). Nevertheless, in elderly patients with end-stage ON and low functional demands, conservative treatment remains a feasible option.
Conservative treatment
High-level evidence on pharmacological treatment of HHO is lacking. Conservative interventions include physical therapy, nonsteroidal anti-inflammatory drugs, and activity modification to decrease the risk of collapse. In addition, in patients with CS-induced HHO, a careful evaluation of indication, dose, and duration of CS treatment should be performed to decrease the dose and duration to the greatest possible extent. In cases where high-dose CS therapy is imperative, the use of statin therapy has been suggested to decrease the risk of osteonecrosis.61 Early-stage osteonecrosis promptly treated with conservative treatment may reduce the risk of disease progression.
Mesenchmal stromal cell therapy is a novel conservative treatment that may have a role in the treatment of patients with osteonecrosis. Favorable outcomes in terms of decreased pain and prevention of articular collapse after MSC therapy have been demonstrated in patients with hip osteonecrosis16 and patients with posttraumatic shoulder osteonecrosis when used at the time of CD.27 Despite these good results, the body of evidence for this intervention is very weak, and thus, its routine use for patients with HHO may not be recommended. Similarly, the evidence on the effectiveness of hyaluronic acid injections in shoulder osteonecrosis is merely anecdotal,81 and therefore, there is a lack of evidence to recommend its use in this patient population. Further research is needed before recommending the routine use of these therapies.
In conclusion, conservative treatment is the first line of treatment for most patients with atraumatic HHO. However, conservative interventions are limited, and their effectiveness to decrease the risk of disease progression is limited. Future research should focus on a better understanding of osteonecrosis pathogenesis to identify potential pharmacological targets that may prevent disease presentation and progression.
Surgical treatment
Surgical treatment strategies are chosen based on the integrity of the joint surface and the subchondral bone. Traditionally, HHO is managed with joint preserving procedures in Cruess stages I and II, whereas Stages III through V require more invasive treatment such as shoulder arthroplasty.
Preoperative clinical conditions such as mild to moderate pain, preserved ROM, and minimal delay to surgery are associated with favorable postoperative outcomes. In contrast, patients with postradiation osteonecrosis demonstrated worse postoperative results.52
CD and bone grafting
Core decompression aims to reduce intraosseous pressure and restore normal circulation to the humeral head. The goal of this procedure is to delay arthroplasty in young patients with HHO, improve ROM, and reduce pain. The success rate of CD decreases as disease staging increases, and therefore, CD is recommended in Stages I, II, and III with minimal subchondral fracture.13,21,46,56 La Porte et al reported the results of CD in 63 shoulders with a follow-up of 10 years and documented successful outcomes in 94%, 88%, 70%, and 14% in Stages I, II, III, and IV, respectively.46,56 Harreld et al first reported the use of a small pin diameter (3.2 mm) technique for Stages I and II HHO with an improvement in the functional UCLA score from 14 to 27 points and a significant clinical improvement in terms of function and pain.21 In a case series of 25 shoulders with atraumatic HHO, Kennon et al reported the results of CD in 8 patients with Stage I or II.42 Of these, 7 patients (88%) progressed to Stage III and required additional surgical treatment. Furthermore, L’Insalata et al concluded that CD was not effective in preventing progression in Stage III ON.47 Kawamura et al39 and Inoue et al32 reported the use of vascularized scapular grafts after drilling in a 27-year-old man with HHO Stage II and a 17-year old woman with HHO Stage III, respectively. At the final follow-up, both patients were pain free and had full ROM. Although these cases demonstrated satisfactory results, the use of these techniques is limited to case reports, and the reproducibility of the surgical technique and clinical outcomes should be demonstrated in further studies, including a larger population of patients. Furthermore, it remains unclear what is the ideal candidate for this procedure and what patients benefit from vascularized grafts in addition to drilling.
Arthroscopic treatment
The first arthroscopic procedure for AOHH was reported by Hayes in 1989.24 Arthroscopy is a joint preserving procedure that delays more invasive methods, limits soft tissue damage and surgical time, and leads to a faster recovery. Although arthroscopic management was initially used to perform débridement, synovectomy, and loose bodies removal, currently, it is also used to assist with drilling (CD) and bone grafting.
In 2000, Hardy et al19 performed arthroscopic débridement, synovectomy, and removal of loose bodies in a 37-year-old woman with bilateral AOHH (right shoulder Stage III and left shoulder Stage IV). At the final follow-up, the Stage III shoulder was asymptomatic and had better ROM than the Stage IV shoulder, which remained symptomatic.
CD assisted by arthroscopy was reported by Dines et al10 in 3 patients and by Kircher et al44 in 1 patient; all these subjects had Stage II AOHH. At the final follow-up, 3 of the 4 patients were pain free, whereas one patient in the study by Dines et al remained symptomatic. Heid et al25 treated a young male with Stage III AOHH with arthroscopic assisted drilling and bone grafting. The authors covered the humeral head defect with an autologous bone graft (iliac crest) plus a bone morphogenetic protein (BMP 7). At 2-year follow-up, the patient had full integration of the bone graft, remained pain free, and had complete ROM.
Resurfacing arthroplasty
Resurfacing of the humeral head has gained increased attention because of its capacity to conserve native anatomy parameters associated with good functional results. In 2009, Uribe et al77 published a prospective study of patients with traumatic (n = 6) and atraumatic (n = 6) ON of the humeral head Stages III, IV, or V treated with partial humeral head resurfacing, showing significant improvements in ROM and pain scores (P < .001). In addition, Raiss et al compared the results of cementless resurfacing arthroplasty between atraumatic (n = 9) and traumatic (n = 8) ON of the humeral head and observed significantly higher clinical results in the atraumatic group at 3-year follow-up.62
Resurfacing arthroplasty in AOHH has shown better functional results and patient-reported outcomes when compared with other etiologies.50,73 Levy et al50 performed 54 resurfacing arthroplasties in patients aged <50 years with glenohumeral arthritis, of which 16 were AOHH cases. After 14-year follow-up, AOHH patients improved the mean age- and sex-adjusted Constant score from 12 points preoperatively to 75 points after surgery; also, AOHH was the group with the highest patient-reported outcomes when compared with other etiologies. Allen et al1 performed 9 inlay hemiarthroplasties (partial resurfacing) in patients with a mean age of 47 years and AOHH Stage II-IV. Significant improvement was observed in patient-reported outcomes (ASES, P = .01; VAS pain, P = .009), forward elevation (P = .012), and external rotation (P = .007) at 7.2 years follow-up.
Revision rates after resurfacing arthroplasty for AOHH vary between 11% and 37.5%.1,73 Preoperative factors associated with failure were glenoid erosion (P = .001) and rotator cuff tear (P = .0017).73 A better understanding of the optimal candidates for resurfacing arthroplasty can decrease the revision rates currently reported. Based on the current evidence, resurfacing arthroplasty can be considered in young patients with advanced stages of AOHH, acceptable bone stock (>60%), minimal glenoid erosion, and no or minimal rotator cuff involvement.
Hemiarthroplasty and total shoulder replacement arthroplasty
There are no standardized criteria to choose between HA and TSA in patients with advanced AOHH. Decision-making depends on several factors, such as patient age, activity demands, radiological stage, and intraoperative findings. Nonetheless, the orthopedic surgeon’s expertise and judgment should determine treatment selection.
Most of the published studies have not found significant differences in clinical outcomes between HA and TSA for AOHH.12,13,64,74 Both TSA and HA have demonstrated satisfaction rates over 80% in mid-term and long-term follow-up studies.52,68 In a case series by Schoch et al,68 the authors reported on 67 HA and 71 TSA for AOHH, documenting significant improvement in ROM (P < .001) and pain (P < .001) in both groups. However, there was a higher active elevation in the HA than in the TSA group (P < .04). In this study, the authors chose between HA and TSA based on intraoperative findings; when four-fifths or more of the glenoid area were covered with cartilage, HA was performed. Meanwhile, TSA was considered in patients with severe glenoid wear or humeral head subluxation into the deficient glenoid area. In general, hemiarthroplasty is recommended when glenoid wear is minimal or nonexistent; total shoulder arthroplasty is generally recommended when both humerus and glenoid are involved.
Ristow et al64 reported the mid-term results (3.9-year mean follow-up) of 29 shoulders with osteonecrosis of the humeral head treated either with HA or TSA (19 HA and 10 TSA) and reported statistically significant improvement in all 4 functionality measures (P < .01). There were no significant differences in patient-reported outcomes, ROM, or revision rates between treatment methods.64 Smith et al reported the results of 31 patients with CS-induced AOHH who were treated with HA. Of the 31 patients, 14 reported unsatisfactory results because of persistent pain and lack of abduction (<90°).72 Interestingly, of these 14 failed cases, 7 had thinned glenoid cartilage (>50%) in preoperative radiographs, and this may have influenced the unsatisfactory results.
A caveat in patients with high-stage CS-induced AOHH undergoing either HA or TSA is the poor bone stock in the calcar region that may increase the risk of intraoperative fracture.59,64 Although press-fit stems may be used, caution is advised when reaming the humeral canal. In some studies, cemented stems were preferred to avoid this potential complication.59,64,68
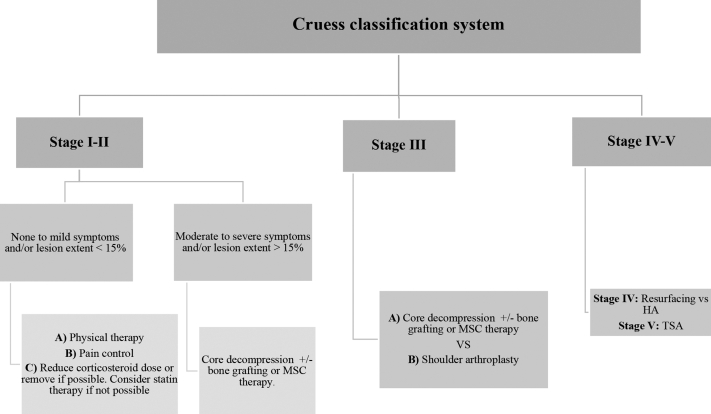
In conclusion, although high complication rates remain a primary concern, the different surgical treatments for the management of AOHH have an overall success rate >70%.13 These surgical procedures allow patients to perform basic daily activities with minimal pain, thereby improving their quality of life. Surgical treatment is mainly guided by disease staging and treatment, and based on the current available data, a treatment algorithm is presented in Figure 2.
Figure 2.
Proposed treatment algorithm for atraumatic osteonecrosis of the humeral head (AOHH). HA, hemiarthroplasty; TSA, total shoulder arthroplasty; MSC, mesenchymal stem cells.
Conclusion
Patients with AOHH are usually asymptomatic, and therefore, early-stage diagnosis is rare. Thus, patients with risk factors may benefit from screening methods, such as MRI of the most commonly affected joints. When AOHH is diagnosed, patient symptoms, age, functional demands, and radiographic staging are crucial to decide between conservative treatment and surgery. If conservative treatment fails to limit AOHH progression, more aggressive methods may be necessary. CD guided by fluoroscopy with or without bone grafting is a reasonable option for patients in the early stages. Shoulder arthroplasty should be reserved for patients with advanced AOHH.
Disclaimers:
Funding: No funding was disclosed by the authors.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Acknowledgments
The authors would like to express their sincere gratitude to Mercedes Salas, MD, for her valuable contributions to the editing of this article.
Footnotes
Institutional review board approval was not required for this review article.
References
- 1.Allen D.A., Merrill C., Cote M.P., Mazzocca A.D. Inlay hemiarthroplasty of the humeral head for nontraumatic osteonecrosis. Semin Arthroplasty. 2020;30:117–122. doi: 10.1053/j.sart.2020.06.001. [DOI] [Google Scholar]
- 2.Benavente Valdepeñas A.M., Berruezo M.I.M., Gutiérrez P.A., García R.P. Osteonecrosis of the shoulder after pregnancy | Osteonecrosis de hombro tras embarazo. Reumatol Clin. 2007;3:137–138. doi: 10.1016/S1699-258X(07)73680-X. [DOI] [PubMed] [Google Scholar]
- 3.Björkman A., Svensson P.J., Hillarp A., Burtscher I.M., Rünow A., Benoni G. Factor V leiden and prothrombin gene mutation: risk factors for osteonecrosis of the femoral head in adults. Clin Orthop Relat Res. 2004;425:168–172. [PubMed] [Google Scholar]
- 4.Chen H., Senda T., Kubo K. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol. 2015;48:61–68. doi: 10.1007/s00795-015-0099-y. [DOI] [PubMed] [Google Scholar]
- 5.Colegate-Stone T.J., Aggarwal S., Karuppaiah K., Tavakkolizadeh A., Sinha J., Reichert I.L. The staged management of gleno-humeral joint osteonecrosis in patients with haematological-induced disease—a cohort review. Int Orthop. 2018;42:1651–1659. doi: 10.1007/s00264-018-3957-0. [DOI] [PubMed] [Google Scholar]
- 6.Cruess R.L. Experience with steroid-induced avascular necrosis of the shoulder and etiologic considerations regarding osteonecrosis of the hip. Clin Orthop Relat Res. 1978:86–93. [PubMed] [Google Scholar]
- 7.Cruess R.L. Steroid induced avascular necrosis of the head of the humerus. Natural history and management. J Bone Joint Surg Br. 1976;58:313–317. doi: 10.1302/0301-620X.58B3.956247. [DOI] [PubMed] [Google Scholar]
- 8.Cushner M.A., Friedman R.J. Osteonecrosis of the humeral head. J Am Acad Orthop Surg. 1997;5:339–346. doi: 10.5435/00124635-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Dilisio M.F., Noble J.S., Bell R.H., Noel C.R. Postarthroscopic humeral head osteonecrosis treated with reverse total shoulder arthroplasty. Orthopedics. 2013;36:377–380. doi: 10.3928/01477447-20130222-30. [DOI] [PubMed] [Google Scholar]
- 10.Dines J.S., Strauss E.J., Fealy S., Craig E.V. Arthroscopic-assisted core decompression of the humeral head. Arthroscopy. 2007;23:103.e1–103.e4. doi: 10.1016/j.arthro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Douka M., Vassalou E.E., Plagou A.P., Karantanas A.H. Imaging of shoulder arthropathies. Hell J Radiol. 2021;6:35–55. doi: 10.36162/hjr.v6i1.377. [DOI] [Google Scholar]
- 12.Feeley B.T., Fealy S., Dines D.M., Warren R.F., Craig E.V. Hemiarthroplasty and total shoulder arthroplasty for avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2008;17:689–694. doi: 10.1016/j.jse.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi F., Franceschetti E., Paciotti M., Torre G., Samuelsson K., Papalia R., et al. Surgical management of osteonecrosis of the humeral head: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25:3270–3278. doi: 10.1007/s00167-016-4169-z. [DOI] [PubMed] [Google Scholar]
- 14.Gerber C., Schneeberger A.G., Vinh T.S. The arterial vascularization of the humeral head. An anatomical study. J Bone Joint Surg Am. 1990;72:1486–1494. [PubMed] [Google Scholar]
- 15.Glueck C.J., Freiberg R.A., Fontaine R.N., Tracy T., Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Barrena E., Padilla-Eguiluz N.G., Rosset P., Hernigou P., Baldini N., Ciapetti G., et al. Osteonecrosis of the femoral head safely healed with autologous, expanded, bone marrow-derived mesenchymal stromal cells in a multicentric trial with minimum 5 years follow-up. J Clin Med. 2021;10:1–14. doi: 10.3390/jcm10030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto M., Gotoh M., Mitsui Y., Okawa T., Higuchi F., Nagata K. Rapid collapse of the humeral head after arthroscopic rotator cuff repair. Knee Surgery, Sport. Traumatol Arthrosc. 2015;23:514–516. doi: 10.1007/s00167-013-2790-7. [DOI] [PubMed] [Google Scholar]
- 18.Gruson K.I., Kwon Y.W. Atraumatic osteonecrosis of the humeral head. Bull NYU Hosp Jt Dis. 2009;67:6–14. [PubMed] [Google Scholar]
- 19.Hardy P., Decrette E., Jeanrot C., Colom A., Lortat-Jacob A., Benoit J. Arthroscopic treatment of bilateral humeral head osteonecrosis. Arthroscopy. 2000;16:332–335. doi: 10.1016/s0749-8063(00)90059-8. [DOI] [PubMed] [Google Scholar]
- 20.Harreld K.L., Marker D.R., Wiesler E.R., Shafiq B., Mont M.A. Osteonecrosis of the humeral head. J Am Acad Orthop Surg. 2009;17:345–355. doi: 10.5435/00124635-200906000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Harreld K.L., Marulanda G.A., Ulrich S.D., Marker D.R., Seyler T.M., Mont M.A. Small-diameter percutaneous decompression for osteonecrosis of the shoulder. Am J Orthop (Belle Mead NJ) 2009;38:348–354. [PubMed] [Google Scholar]
- 22.Hattrup S.J. Indications, technique, and results of shoulder arthroplasty in osteonecrosis. Orthop Clin North Am. 1998;29:445–451. doi: 10.1016/s0030-5898(05)70020-1. [DOI] [PubMed] [Google Scholar]
- 23.Hattrup S.J., Cofield R.H. Osteonecrosis of the humeral head: Relationship of disease stage, extent, and cause to natural history. J Shoulder Elbow Surg. 1999;8:559–564. doi: 10.1016/s1058-2746(99)90089-7. [DOI] [PubMed] [Google Scholar]
- 24.Hayes J.M. Arthroscopic treatment of steroid-induced osteonecrosis of the humeral head. Arthroscopy. 1989;5:218–221. doi: 10.1016/0749-8063(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 25.Heid A., Dickschas J., Schoeffl V. Cortison-induzierte Humeruskopfnekrose bei akuter myeloischer Leukämie. Unfallchirurg. 2013;116:180–184. doi: 10.1007/s00113-012-2243-7. [DOI] [PubMed] [Google Scholar]
- 26.Hernigou P., Flouzat-Lachaniette C.H., Roussignol X., Poignard A. The natural progression of shoulder osteonecrosis related to corticosteroid treatment. Clin Orthop Relat Res. 2010;468:1809–1816. doi: 10.1007/s11999-009-1094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernigou P., Hernigou J., Scarlat M. Mesenchymal stem cell therapy improved outcome of early post-traumatic shoulder osteonecrosis: a prospective randomized clinical study of fifty patients with over ten year follow-up. Int Orthop. 2021;45:2643–2652. doi: 10.1007/s00264-021-05160-9. [DOI] [PubMed] [Google Scholar]
- 28.Hernigou P., Hernigou J., Scarlat M. Shoulder Osteonecrosis: Pathogenesis, Causes, Clinical Evaluation, Imaging, and Classification. Orthop Surg. 2020;12:1340–1349. doi: 10.1111/os.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettrich C.M., Boraiah S., Dyke J.P., Neviaser A., Helfet D.L., Lorich D.G. Quantitative assessment of the vascularity of the proximal part of the humerus. J Bone Joint Surg Am. 2010;92:943–948. doi: 10.2106/JBJS.H.01144. [DOI] [PubMed] [Google Scholar]
- 30.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inaba H., Varechtchouk O., Neel M.D., Ehrhardt M.J., Metzger M.L., Karol S.E., et al. Whole-joint magnetic resonance imaging to assess osteonecrosis in pediatric patients with acute lymphoblastic lymphoma. Pediatr Blood Cancer. 2020;67:1–9. doi: 10.1002/pbc.28336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue K., Suenaga N., Oizumi N., Tanaka Y., Minami A. A vascularized scapular graft for juvenile osteonecrosis of the humeral head. J Shoulder Elbow Surg. 2012;21:e9–e13. doi: 10.1016/j.jse.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs B. Alcoholism-induced bone necrosis. N Y State J Med. 1992;92:334–338. [PubMed] [Google Scholar]
- 34.Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res. 1978;130:51–67. [PubMed] [Google Scholar]
- 35.Jones J.P., Engleman E.P., Najarian J.S. Systemic Fat Embolism after Renal Homotransplantation and Treatment with Corticosteroids. N Engl J Med. 1965;273:1453–1458. doi: 10.1056/NEJM196512302732703. [DOI] [PubMed] [Google Scholar]
- 36.Karasick D., Schweitzer M.E. Case 4: intraosseous fat necrosis associated with pancreatitis. Radiology. 1998;209:521–524. doi: 10.1148/radiology.209.2.9807583. [DOI] [PubMed] [Google Scholar]
- 37.Karol S.E., Yang W., Van Driest S.L., Chang T.Y., Kaste S., Bowton E., et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126:1770–1776. doi: 10.1182/blood-2015-05-643601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaste S.C., DeFeo B.M., Neel M.D., Weiss K.S., Fernandez-Pineda I., Ness K.K. Osteonecrosis of the Shoulders in Pediatric Patients Treated for Leukemia or Lymphoma: Single-Institutional Experience. J Pediatr Orthop. 2019;39:104–110. doi: 10.1097/BPO.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamura K., Kawate K., Yajima H., Kobata Y., Takakura Y. Vascularized scapular grafting for treatment of osteonecrosis of the humeral head. J Reconstr Microsurg. 2008;24:559–564. doi: 10.1055/s-0028-1090621. [DOI] [PubMed] [Google Scholar]
- 40.Kawedia J.D., Kaste S.C., Pei D., Panetta J.C., Cai X., Cheng C., et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenkre J.S., Bassett J.H.D. The bone remodelling cycle. Ann Clin Biochem. 2018;55:308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 42.Kennon J.C., Smith J.P., Crosby L.A. Core decompression and arthroplasty outcomes for atraumatic osteonecrosis of the humeral head. J Shoulder Elbow Surg. 2016;25:1442–1448. doi: 10.1016/j.jse.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Khan A., Hangartner T., Weinreb N.J., Taylor J.S., Mistry P.K. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Miner Res. 2012;27:1839–1848. doi: 10.1002/jbmr.1680. [DOI] [PubMed] [Google Scholar]
- 44.Kircher J., Patzer T., Ziskoven C., Bittersohl B., Hedtmann A., Krauspe R. Arthroscopically assisted retrograde drilling of the humeral head with a guiding device. Knee Surg Sports Traumatol Arthrosc. 2015;23:1442–1446. doi: 10.1007/s00167-013-2783-6. [DOI] [PubMed] [Google Scholar]
- 45.Koczywas M., Cristea M.C. Osteonecrosis of the humeral head in a patient with non-small cell lung cancer receiving Bevacizumab. J Thorac Oncol. 2011;6:1960–1961. doi: 10.1097/JTO.0b013e31822e726f. [DOI] [PubMed] [Google Scholar]
- 46.LaPorte D.M., Mont M.A., Mohan V., Pierre-Jacques H., Jones L.C., Hungerford D.S. Osteonecrosis of the humeral head treated by core decompression. Clin Orthop Relat Res. 1998:254–260. doi: 10.1097/00003086-199810000-00027. [DOI] [PubMed] [Google Scholar]
- 47.L’Insalata J.C., Pagnani M.J., Warren R.F., Dines D.M. Humeral head osteonecrosis: clinical course and radiographic predictors of outcome. J Shoulder Elbow Surg. 1996;5:355–361. doi: 10.1016/s1058-2746(96)80066-8. [DOI] [PubMed] [Google Scholar]
- 48.Lauschke V.M., Zhou Y., Ingelman-Sundberg M. Novel genetic and epigenetic factors of importance for inter-individual differences in drug disposition, response and toxicity. Pharmacol Ther. 2019;197:122. doi: 10.1016/j.pharmthera.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J.A., Farooki S., Ashman C.J., Yu J.S. MR patterns of involvement of humeral head osteonecrosis. J Comput Assist Tomogr. 2002;26:839–842. doi: 10.1097/00004728-200209000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Levy O., Tsvieli O., Merchant J., Young L., Trimarchi A., Dattani R., et al. Surface replacement arthroplasty for glenohumeral arthropathy in patients aged younger than fiftyyears: Results after a minimum ten-year follow-up. J Shoulder Elbow Surg. 2015;24:1049–1060. doi: 10.1016/j.jse.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 51.Li W., Huang Z., Tan B., Chen G., Li X., Xiong K., et al. General recommendation for assessment and management on the risk of glucocorticoid-induced osteonecrosis in patients with COVID-19. J Orthop Translat. 2021;31:1–9. doi: 10.1016/j.jot.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansat P., Huser L., Mansat M., Bellumore Y., Rongières M., Bonnevialle P. Shoulder arthroplasty for atraumatic avascular necrosis of the humeral head: Nineteen shoulders followed up for a mean of seven years. J Shoulder Elbow Surg. 2005;14:114–120. doi: 10.1016/j.jse.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo K., Hirohata T., Sugioka Y., Ikeda M., Fukuda A. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988:115–123. [PubMed] [Google Scholar]
- 54.Milner P.F., Kraus A.P., Sebes J.I., Sleeper L.A., Dukes K.A., Embury S.H., et al. Osteonecrosis of the humeral head in sickle cell disease. Clin Orthop Relat Res. 1993;289:136–143. [PubMed] [Google Scholar]
- 55.Miyanishi K., Kamo Y., Ihara H., Naka T., Hirakawa M., Sugioka Y. Risk factors for dysbaric osteonecrosis. Rheumatology. 2006;45:855–858. doi: 10.1093/rheumatology/kel013. [DOI] [PubMed] [Google Scholar]
- 56.Mont M.A., Maar D.C., Urquhart M.W., Lennox D., Hungerford D.S. Avascular necrosis of the humeral head treated by core decompression. A retrospective review. J Bone Joint Surg Br. 1993;75:785–788. doi: 10.1302/0301-620X.75B5.8376440. [DOI] [PubMed] [Google Scholar]
- 57.Mont M.A., Ulrich S.D., Seyler T.M., Smith J.M., Marker D.R., McGrath M.S., et al. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008;35:1629–1634. [PubMed] [Google Scholar]
- 58.Nawata K., Nakamura J., Hagiwara S., Wako Y., Miura M., Kawarai Y., et al. Predictive value of magnetic resonance imaging for multifocal osteonecrosis screening associated with glucocorticoid therapy. Mod Rheumatol. 2020;30:586–591. doi: 10.1080/14397595.2019.1623363. [DOI] [PubMed] [Google Scholar]
- 59.Orfaly R.M., Rockwood C.A., Esenyel C.Z., Wirth M.A. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16:S27–S32. doi: 10.1016/j.jse.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Poignard A., Flouzat-Lachaniette C.H., Amzallag J., Galacteros F., Hernigou P. The natural progression of symptomatic humeral head osteonecrosis in adults with sickle cell disease. J Bone Joint Surg Am. 2012;94:156–162. doi: 10.2106/JBJS.J.00919. [DOI] [PubMed] [Google Scholar]
- 61.Pritchett J.W. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 62.Raiss P., Kasten P., Baumann F., Moser M., Rickert M., Loew M. Treatment of osteonecrosis of the humeral head with cementless surface replacement arthroplasty. J Bone Joint Surg Am. 2009;91:340–349. doi: 10.2106/JBJS.H.00560. [DOI] [PubMed] [Google Scholar]
- 63.Ranalletta M., Bertona A., Tanoira I., Rossi L.A., Bongiovanni S., Maignón G.D. Results of partial resurfacing of humeral head in patients with avascular necrosis. Rev Esp Cir Ortop Traumatol (Engl Ed) 2019;63:29–34. doi: 10.1016/j.recot.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Ristow J.J., Ellison C.M., Mickschl D.J., Berg K.C., Haidet K.C., Gray J.R., et al. Outcomes of shoulder replacement in humeral head avascular necrosis. J Shoulder Elbow Surg. 2019;28:9–14. doi: 10.1016/j.jse.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Rodrigue S.W., Rosenthal D.I., Barton N.W., Zurakowski D., Mankin H.J. Risk factors for osteonecrosis in patients with type 1 Gaucher’s disease. Clin Orthop Relat Res. 1999:201–207. [PubMed] [Google Scholar]
- 66.Sakai T., Sugano N., Nishii T., Hananouchi T., Yoshikawa H. Extent of osteonecrosis on MRI predicts humeral head collapse. Clin Orthop Relat Res. 2008:1074–1080. doi: 10.1007/s11999-008-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarris I., Weiser R., Sotereanos D.G. Pathogenesis and treatment of osteonecrosis of the shoulder. Orthop Clin North Am. 2004;35:397–404. doi: 10.1016/j.ocl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Schoch B.S., Barlow J.D., Schleck C., Cofield R.H., Sperling J.W. Shoulder arthroplasty for atraumatic osteonecrosis of the humeral head. J Shoulder Elbow Surg. 2016;25:238–245. doi: 10.1016/j.jse.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 69.Seamon J., Keller T., Saleh J., Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:1–11. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sherief L.M., Beshir M., Raafat N., Abdelkhalek E.R., Mokhtar W.A., Elgerby K.M., et al. Genetic polymorphism of vitamin D receptors and plasminogen activator inhibitor-1 and osteonecrosis risk in childhood acute lymphoblastic leukemia. Mol Genet Genomic Med. 2021;9:1–9. doi: 10.1002/mgg3.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shigemura T., Nakamura J., Kishida S., Harada Y., Ohtori S., Kamikawa K., et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford) 2011;50:2023–2028. doi: 10.1093/rheumatology/ker277. [DOI] [PubMed] [Google Scholar]
- 72.Smith R.G., Sperling J.W., Cofield R.H., Hattrup S.J., Schleck C.D. Shoulder hemiarthroplasty for steroid-associated osteonecrosis. J Shoulder Elbow Surg. 2008;17:685–688. doi: 10.1016/j.jse.2008.01.149. [DOI] [PubMed] [Google Scholar]
- 73.Soudy K., Szymanski C., Lalanne C., Bourgault C., Thiounn A., Cotten A., et al. Results and limitations of humeral head resurfacing: 105 cases at a mean follow-up of 5 years. Orthop Traumatol Surg Res. 2017;103:415–420. doi: 10.1016/j.otsr.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 74.Sperling J.W., Cofield R.H., Rowland C.M. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg. 1998;80:464–473. doi: 10.2106/00004623-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Joint Surg. Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 76.Uguen M., Pougnet R., Uguen A., Loddé B., Dewitte J.D. Dysbaric osteonecrosis among professional divers: a literature review. Undersea Hyperb Med. 2014;41:579–587. [PubMed] [Google Scholar]
- 77.Uribe J.W., Botto-van Bemden A. Partial humeral head resurfacing for osteonecrosis. J Shoulder Elbow Surg. 2009;18:711–716. doi: 10.1016/j.jse.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinstein R.S., Nicholas R.W., Manolagas S.C. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 80.Wiesler E.R., Sarlikiotis T., Mavrogenis A.F., Kokkalis Z.T. Attrition tendinitis of long head of biceps brachii in relation to humeral head osteonecrosis: Case report. J Surg Orthop Adv. 2013;22:245–248. doi: 10.3113/JSOA.2013.0245. [DOI] [PubMed] [Google Scholar]
- 81.Wong J.Y.-A., Le S., Lo C., Costandi A., Chernishof V., Kim E. Hyaluronic acid injections for treatment of pediatric sickle cell avascular necrosis of the humeral head. Reg Anesth Pain Med. 2022;47:136–138. doi: 10.1136/rapm-2021-102842. [DOI] [PubMed] [Google Scholar]
- 82.Yin L., Li Y., Wang Y. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–588. [PubMed] [Google Scholar]
- 83.Yu L., Xu Y., Qu H., Yu Y., Li W., Zhao Y., et al. Decrease of MiR-31 induced by TNF-α inhibitor activates SATB2/RUNX2 pathway and promotes osteogenic differentiation in ethanol-induced osteonecrosis. J Cell Physiol. 2019;234:4314–4326. doi: 10.1002/jcp.27210. [DOI] [PubMed] [Google Scholar]
- 84.Zambuzzi W.F., Granjeiro J.M., Parikh K., Yuvaraj S., Peppelenbosch M.P., Ferreira C.V. Modulation of Src activity by low molecular weight protein tyrosine phosphatase during osteoblast differentiation. Cell Physiol Biochem. 2008;22:497–506. doi: 10.1159/000185506. [DOI] [PubMed] [Google Scholar]
- 85.Zgheib N.K., El-Khoury H., Maamari D., Basbous M., Saab R., Muwakkit S.A. A GRIN3A polymorphism may be associated with glucocorticoid-induced symptomatic osteonecrosis in children with acute lymphoblastic leukemia. Per Med. 2021;18:431–439. doi: 10.2217/pme-2020-0167. [DOI] [PubMed] [Google Scholar]