Abstract
Stemless anatomic total shoulder arthroplasty (aTSA) is a promising option for the treatment of degenerative disease in patients. This novel technique avoids the stem-related complications associated with the traditional stemmed aTSA. Stemless aTSA offers additional benefits such as decreased operative time, preservation of bone stock, improved radiographic outcomes, and easier revision. Moreover, loading of the metaphyseal region rather than the diaphysial region with traditional stemmed implants can decrease stress shielding. When compared to stemmed-implants, stemless aTSA has demonstrated similar outcomes and complication rates. The purpose of this article is to analyze published outcomes and complications following the utilization of stemless aTSA. Additionally, key aspects of the surgical technique that may promote optimal results in stemless aTSA implantation are presented.
Keywords: Total shoulder arthroplasty, Anatomic total shoulder arthroplasty, Stemless, Stemmed, Proximal humerus, Literature review
Introduced by Péan in 1893 as a platinum-rubber implant, total shoulder arthroplasty has evolved into a common procedure to address a number of glenohumeral joint pathologies.27 Multiple iterations of the implant have been developed to improve the function of the components and better accommodate for anatomic variability of patients. Traditionally, anatomic total shoulder arthroplasty (aTSA) has been composed of 3 components: a glenoid component, prosthetic head, and humeral stem.10 This configuration, often referred to as a stemmed aTSA, has been extensively investigated and has demonstrated good functional outcomes with minimal complications.24 Additionally, newer, fourth-generation convertible stemmed implants offer the possibility of revising a stemmed aTSA humeral component to a hemiarthroplasty (HA) or reverse total shoulder arthroplasty, without the need to explant the humeral stem.9 Although successful, these traditional stemmed implants continue to face challenges relating to the humeral component such as loosening, intraoperative fractures, and periprosthetic fractures.6
Developed by Levy and Copeland in the 1980s, humeral resurfacing with the utilization of stemmed components served as a valuable means to combat stem-related TSA complications.8 Subsequently in 2004, stemless or canal-sparing aTSA implants were introduced in Europe.8 These implant designs are based on metaphyseal fixation and provide a method that does not violate the humeral canal.17 Despite being a recent advancement in aTSA, stemless implants have exhibited accurate positioning of the humeral head independent of the location of the humeral shaft.3,4,7,24 In contrast to stemmed implants, stemless humeral implants offer several potential benefits including but not limited to: reduced risk of intraoperative periprosthetic humeral shaft fractures, decreased operative time and subsequent blood loss, preservation of humeral bone stock, and easier explantation.34 Early studies on 2-year outcomes have demonstrated that the safety and effectiveness of stemless aTSAs may be similar to stemmed aTSA for patients with osteoarthritis.34
However, restoration of proximal humerus anatomy (RPHA) in stemless aTSA has been identified as a potential limitation of the stemless components.14,18,31 As stemless implants depend on metaphyseal anchorage, RPHA relies heavily on the orientation of the humeral head cut as well as appropriate sizing of the humeral head.2,14,18 In 2014, Alolabi et al2 observed poor RPHA in two-thirds of cases, with 89% of these cases resulting from excessive medialization of the humeral head cut leading to overstuffing and insufficient RPHA. Since then, additional studies also raised the concern of RPHA with stemless components, with the orientation of the humeral head cut as the primary cause.14,18 Recently, Grubhofer et al14 demonstrated that even with the addition of a preoperative computed tomography (CT)-based 3-dimensional plan, RPHA occurred in only 35% of cases. As expected, the most common cause for imprecise RPHA was the level of the humeral head cut (80% of cases), which was set too high subsequently medializing the humerus and leading to overstuffing in 71% of cases.14
As multiple prior biomechanical and short-term clinical investigations have demonstrated an association of worse clinical outcomes in HA and aTSA with poor RPHA, attention must be paid to this aspect of the procedure in order to promote optimal outcomes.11,12,15,16,18,26,29,36 Furthermore, as the use of stemless shoulder implants are becoming more available, it remains important to evaluate the currently available evidence on advantages and disadvantages of these novel components. As such, this article describes implantation of an anatomic TSA utilizing a stemless humeral component and provides a current review of the literature as it pertains to clinical outcomes of this implant design.
Patient selection
The patient selection process begins with a standard comprehensive workup of patients presenting with shoulder pain. This involves a complete history and physical examination, focusing on the patient’s functional limitations, and status of the rotator cuff. Radiographic evaluation begins with anteroposterior and axillary radiographs of the shoulder, assessing for humeral head or glenoid deformity, osteophytes, and glenohumeral joint space narrowing. Advanced imaging through CT is preferred to aid in characterizing the glenoid bone wear, especially in scenarios where operative intervention is indicated as it is utilized for presurgical planning.
A stemless TSA is offered to patients who present with recalcitrant symptoms and failure of a comprehensive course of nonoperative treatments. Furthermore, patients have glenohumeral arthritis, an intact rotator cuff, and no evidence of humeral bone deficiency.
Indications
-
•
Patients with an intact rotator cuff and sufficient humeral and glenoid bone stock who suffer from symptomatic, end-stage glenohumeral arthritis.
-
•
Symptoms, including pain and disability, that are refractory to comprehensive nonoperative therapies, such as nonsteroidal anti-inflammatory drugs, glenohumeral joint injections, and physical therapy.
Contraindications
-
•
Full thickness rotator cuff tear not amenable to fixation
-
•
Rotator cuff arthropathy
-
•
Substantial glenoid bone loss
-
•
Insufficient humeral bone stock
-
•
Proximal humerus fracture
-
•
Glenoid retroversion >25 degrees
-
•
Active infection
-
•
Large proximal humeral cysts
-
•
Poor bone quality as evidenced by rapid bone destruction in serial radiographs and/or bone resorption
Surgical technique
Patient positioning
The procedure begins with general anesthesia induction with or without an interscalene block. Subsequently, the patient is positioned in the standard beach chair position, with the medial border of the scapula on the edge of the bed. As needed, a rolled-up towel can be placed in between the patient’s scapula for further stabilization during glenoid implantation. Last, a pneumatic arm positioner or a Mayo stand is utilized throughout the procedure in order to facilitate extremity positioning and exposure.
Approach and initial exposure
A standard deltopectoral approach is employed for shoulder exposure. Typically, this approach should provide excellent exposure, allowing for complete visualization of the entire glenoid and proximal humerus. Key landmarks consisting of the anterior clavicle, coracoid process, and midpoint of the arm at the level of the axilla are utilized. Next, a vertical incision beginning on the lateral aspect of the coracoid process and reaching 10 cm inferiorly to a point medial to the mid-aspect of the arm is made. Once identified proximally, the deltopectoral interval is developed distally. At this point, the cephalic vein is preserved and retracted medially or laterally, according to the surgeon's preference. The subdeltoid space is then accessed above the insertion of the deltoid. This space is subsequently carried proximally up to the subacromial space, releasing adhesions between the lateral humerus and the deltoid. Lastly, the plane between the conjoint tendon and subscapularis is developed, followed by the isolation and ligation of the anterior circumflex humeral artery and accompanying veins.
Subscapularis release
Using two #2 nonabsorbable sutures, the long head of the biceps tendon is tenodesed high in the bicipital groove. This will allow the bicipital sheet and biceps to help reinforce the subscapularis repair laterally. During the tenotomy, a retention stitch is placed in the superolateral corner of the subscapularis and another in the lateral midportion. Alternatively, the subscapularis can be managed through lesser tuberosity osteotomy (LTO) or a peel technique. The humerus is then exposed up to the inferomedial neck through direct release of the glenohumeral capsule. At this time, some osteophytes of the proximal humerus can be removed (Fig. 1).
Figure 1.
Proximal humerus osteophyte removal.
Humeral preparation
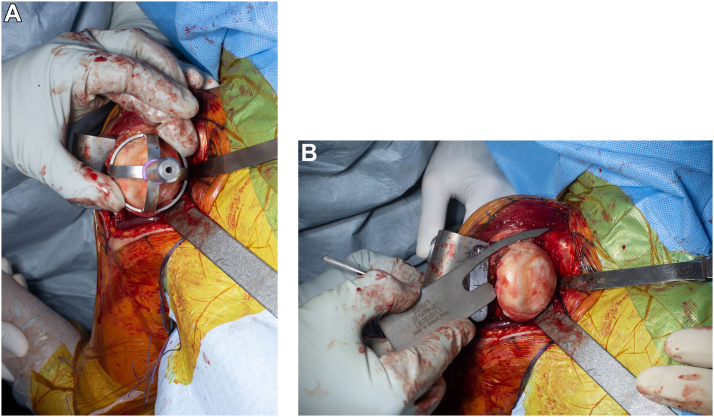
At this time, the humeral head is dislocated through a gentle adduction, extension, and external rotation motion. The bursal and articular sides of the rotator cuff are inspected for any major tears. In the case of irreparable rotator cuff tendon tear, the procedure would be converted to a reverse shoulder arthroplasty. Small, amenable tears that correspond to physical examination and imaging findings can be repaired accordingly. Once all humeral releases have been completed, the surgeon can use a cutting guide that fits over the humeral head (Fig. 2A) or use an extramedullary guide that provides a reference for inclination and version. The authors prefer to use an extramedullary reference to perform a humeral head osteotomy at 30° of retroversion with a cut exiting at the rotator cuff insertion (Fig. 2B). At this point, the bony integrity of the proximal humerus is tested by visual inspection and gentle manual palpation. If there is an excessive cavitary defect within the cut metaphyseal surface of the proximal humerus or manual palpation results in cancellous bone resorption, then a stemmed humeral component is preferred. In the authors practices, over 90% of anatomic shoulder replacements are candidates for stemless shoulder arthroplasty. A sizing guide is then used to determine the placement and a central guidewire is placed to ensure that the cut surface is smooth (Figs. 3 and 4A,B). A planer may be used to ensure a flush surface. Next, a central reamer is used to prepare the humerus metaphysis for the stemless implant (Fig. 4C). The trial broach is impacted into place (Fig. 5A–C).
Figure 2.
(A) Humeral resection utilizing an extramedullary humeral cutting guide that fits over the humeral head, (B) an extramedullary guide that includes a bar that goes down the forearm indicating 30 degrees of retroversion and an inclination of 135 degrees. An angel wing is used to ensure the osteotomy is performed right below at the level of the rotator cuff insertion.
Figure 3.
Templating of the stemless humeral anchor size.
Figure 4.
(A) Preparation of the humeral head beginning with pin placement. (B) Subsequent planing of the humeral surface. (C) Central reamer in the proximal humerus.
Figure 5.
(A, B) Sequential impaction of the trial broach over a pin. (C) Trial broach placed in the center of the humerus without violation of the proximal humerus.
Glenoid preparation
Regarding the glenoid, the patient is first positioned in additional reverse Trendelenburg with the arm in 70°-90° of abduction and slight flexion. The reverse Trendelenburg position can improve the view of the glenoid. The glenoid is exposed with 3 retractors placed posteriorly, anteriorly, and superiorly. Preoperative imaging is then reviewed and correlated with the clinical pattern of glenoid wear, inclination, and version. The anatomic center of the glenoid is marked and prepared to restore native version to neutral while minimizing bone removal. If needed, augmented glenoid components are utilized to maximize bony apposition while minimizing bone loss. The algorithm utilized by the authors is that with 0° to 10° of retroversion, the anterior glenoid is reamed to neutral. In 11° to 24° of retroversion, an augmented anatomic glenoid component is used. In the setting of greater than 25° of retroversion, a reverse arthroplasty with consideration of an augmented glenoid component is used. Surgeons prefer greater than 90% of glenoid component seating if possible, preoperative planning software is commonly utilized to create constructs with up to 100% seating. A hybrid glenoid component composed of a central porous titanium post and 3 peripheral interference fit pegs is then implanted using third-generation cementation technique (Fig. 6). Once impacted, the extravagated cement is removed and the component stability is checked.
Figure 6.
Implanted hybrid glenoid component.
Final component placement
Attention is returned to the humerus, where the humerus is exposed, and the humeral head is sized accordingly. The authors prefer to trial a humeral head that is selected to match the diameter of the resected humeral head, excluding osteophytes (Fig. 7). Special attention is placed on the level of the humeral head as it pertains to the greater tuberosity with a goal of avoiding overstuffing and increased tension to the rotator cuff. Final trialing is performed to optimize the soft tissue tension of the construct. In general, the humeral head should cover the osteotomy positioned 2 to 3 mm above the greater tuberosity without impinging on the deep surface of the rotator cuff. The authors prefer to use a humeral head that allows the head to be dialed from 0.5 mm to 4.5 mm increments in any direction. The head can then be dialed in any direction according to the treating surgeon preference. This is key because rotator cuff failure with a fixed eccentric head can be a problem. The humeral head should face opposite of the glenoid with the arm in the neutral position, and posterior stress should lead to translation of the humeral head to 40 to 50% of the diameter with a spontaneous reduction back to a centered position. Forward elevation, internal and external rotation is then checked to determine full passive motion without any impingement. The trial broach is then exchanged for the final anchor component (Fig. 8). Multiple interrupted sutures are then placed in the rotator interval with the arm in external rotation. The final humeral head is dialed to match the desired position determined by the trial component in both amount and direction (Fig. 9). The final humeral head is then impacted into place and the shoulder is subsequently reduced (Fig. 10).
Figure 7.
Eccentric humeral head trial dialed at the desired position to optimize soft tissue tension and prevent rotator cuff impingement.
Figure 8.
(A) Final stemless humeral anchor with porous plasma spray coating. (B) Implanted anchor in the central portion of the proximal humerus.
Figure 9.
Eccentric final humeral head component with offset options demonstrated.
Figure 10.
(A) Final humeral head placed appropriately covering the osteotomy with rotator interval sutures placed. (B) Humeral head position demonstrating desired head position just above the greater tuberosity.
Closure and rehabilitation
Closure begins with tying the previously placed rotator interval sutures with the arm in external rotation in order to avoid over tightening the rotator interval. The subscapularis tenotomy is then repaired tendon-to-tendon utilizing multiple interrupted sutures. Furthermore, the deltopectoral interval is closed using number-2 Vicryl suture. Last, the subcutaneous tissue and skin are closed in layers, achieving a watertight closure.
Postoperative rehabilitation begins by immediately placing patients in a shoulder immobilizer with an abduction pillow and beginning passive range of motion (ROM) on day 1. At this time, a supervised physical therapy program is also initiated. Sling immobilization lasts for around 6 weeks. By week 6, the patient can begin active assisted ROM exercises. Once complete active assisted ROM is achieved, the patient can start active ROM. Isometric strengthening can begin by week 8, followed by elastic band strengthening by week 12 (Fig. 11).
Figure 11.
Anterior-posterior shoulder radiograph demonstrating a stemless anatomic TSA at 5 years of follow-up.
Discussion
Though less common, traditional stemmed aTSA has been associated with stress shielding resulting from proximal humeral bone resorption, implant loosening, and intraoperative humeral periprosthetic fractures.28,35 Due to recent advancements, stemless aTSA has become a promising option for the treatment of degenerative shoulder disease in patients with an intact rotator cuff and sufficient humeral bone stock. At the present moment, current investigations propose significant improvement in functional outcomes with similar complication and revision rates when compared with stemmed-implants. Key technical points include careful patient selection, proximal humerus exposure, accurate head cut at the desired version and inclination, interrogation of bony integrity, efficient humeral preparation with a central guidewire and reamer, and the flexibility in humeral head position with eccentric components and ability to dial position based on soft tissue tension.
Akin to stemmed aTSA, stemless components have also demonstrated major clinical improvements in patients with glenohumeral osteoarthritis. In 2017, Uschok et al32 performed a randomized control trial (RCT) of 20 aTSA treated with a stemless component (Eclipse; Arthrex, Freiham, Germany) compared to 20 stemmed aTSA (Univers II; Arthrex, Freiham, Germany). Both groups demonstrated significant improvements in Constant scores (CS), with no notable differences between components at a minimum of 5-year follow-up. Notably, stemless implants did not show any increased signs of prosthetic loosening and had higher bone density values when compared to stemmed components. Subsequently, Wiater et al34 performed a multicentered prospective RCT, comparing 2-year outcomes of 116 patients treated with stemless aTSA (Comprehensive Nano humeral stem; Zimmer Biomet, Warsaw, IN, USA) and 123 treated with stemmed implants (Comprehensive Mini [83 mm] humeral stems; Zimmer Biomet, Warsaw, IN, USA). At 2 years of follow-up, all primary outcomes (American Shoulder and Elbow Surgeon (ASES) score, single assessment numeric evaluation (SANE) score, and adjusted Constant scores) demonstrated noninferiority of the stemless component with no differences in secondary outcomes between stemless and stemmed cohorts. Device-related complications were similar between stemless (8%) and stemmed components (7%), and there were no radiographic signs of loosening in either cohort.
Decreased operative time and subsequent blood loss are other proposed advantages of stemless TSA.25 While the prospective RCT by Wiater et al34 was unable to demonstrate a difference in operative time, other studies report significantly shorter operative time and decreased intraoperative blood loss when compared to stemmed aTSA.5,21 A systematic review and meta-analysis by Liu et al24 included 22 studies with 962 patients undergoing stemless aTSA. Pooled analysis indicated that while there were no significant differences in postoperative Constant scores (1.3 vs. 5.8) or complication rates (8.3% vs 10.6%), there was a significant shorter operative time (mean difference, -15.03 minutes) and decreased blood loss (mean difference, -96.95 mL) among patients treated with stemless aTSA compared to the stemmed cohort. No transfusions were reported among the stemless cohorts, whereas stemmed aTSA transfusions rates were 14%. However, future studies are needed to determine whether the decreased blood loss in stemmed TSA patients reaches a clinically significant threshold for blood transfusion.
In a later systematic review and meta-analysis of 31 studies, Willems et al35 reported clinical and radiographic outcomes, as well as complications and revisions of 1944 stemless aTSA, HA, or RSA implants. The Constant-Murley score (CMS), ASES, and ROM of the stemless aTSA/HA were recorded and compared to published references criteria in shoulder arthroplasty for minimal clinically importance difference (MCID) and substantial clinical benefit (SCB). CMS, ASES, and ROM exceeded the MCID and SCB at both short- and medium-term follow-up. In this study, authors observed a 9.8% complication rate and 5.1% revision rate, which were similar to previously reported stemmed aTSA cohorts. The most common cause for revision was rotator cuff failure (2.5%), which was similar to the study by Liu et al (2.2%).21 Rotator cuff insufficiency or secondary tears were higher (3.7%) than stemmed aTSA (0.9% or 2.7%) which may suggest an increased sensitivity of the rotator cuff in the setting of stemless aTSA. This may arise from the increased variability in RPHA and/or the potential limitation of utilizing a LTO in a metaphyseal-based implant.1,14,18,31 As expected, there were less intraoperative humeral fractures in the stemless cohorts (0.5%), when compared to stemmed implants as reported in other studies (1.9%-2.3%), which supports this additional potential benefit offered by stemless implants.35 Radiographic outcomes focused on radiolucent lines (RLL) and osteolysis, which may be signs of component loosening and bone loss (stress shielding), respectively. Among the stemless TSA/HA cohorts, these findings were reported in 7.1% and 7.7% of cases. Osteolysis rates in stemmed TSAs ranged from 23% to 63%.35 Radiolucency in stemmed cohorts ranged between 7% and 59% of cases.19,30,33
Substantial bone loss, proximal humerus fractures, insufficient humeral bone stock, and poor bone quality are contraindications for stemless aTSA. Given the necessity for adequate metaphyseal bone quality, there is concern for use in elderly patients who are more likely to have poor bone quality. Most studies in the United States include mainly younger patients. However, a recent study by Goldberg et al13 evaluates whether stemless humeral implant (CoCr ellipsoid humerus) can be safely employed in patients of more advanced age. This series included 57 patients with a mean age of 73 years and a minimum follow-up of 2 years. Clinical outcomes such as ROM, ASES, SANE, visual analog scale, and Patient-Reported Outcomes Measurement Information System were recorded and compared to published references criteria in shoulder arthroplasty for MCID and SCB. ROM and patient-reported outcomes showed statistically significant and clinically meaningful improvements. ASES exceeded the threshold for MCID and SCB. SANE, visual analog scale, and Patient-Reported Outcomes Measurement Information System exceeded MCID. There were no implant failures and a low rate (5%) of complications. Only 2 humeral implants demonstrated low-grade radiolucency (grade 1) around the fixation pegs, according to the modified Lazarus grading system.20 These short-term outcomes are encouraging and suggest that stemless design may be a safe option even among older patients.
While the current literature reports good outcomes that are comparable to stemmed implants, there is a growing body of evidence regarding the longer-term outcomes of a stemless aTSA. Recently, Magosch et al22 reported on 75 stemless HA/aTSA with no evidence of clinical or radiographic loosening of the stemless humeral head component after a mean period of 11 years, which was comparable to the long-term results of standard stemmed aTSA. Similarly, Märtens et al23 demonstrated a similar 10-year survivorship between stemmed and stemless aTSA (95.3% vs. 91.5%; P = .251) and no evidence of radiographic loosening of any of the humeral components. With these similarities, utilization of a stemless component when indicated may also provide a future advantage during revision surgery due to preservation of humeral bone stock and potential for simpler explantation, leading to a higher likelihood of conventional components.
Of note, difficulty with achieving the appropriate RPHA remains a concern with the use of stemless components especially with a freehand humeral cut.14,18,31 Frequently identified reasons for poor anatomic restoration include (1) the level of the humeral head cut, (2) the choice of humeral head size, and (3) the head neck angle of the humeral head cut. Therefore, technical pearls to consider include performing an accurate head cut at the level of the rotator cuff insertion at the desired version and inclination utilizing a guide, and careful soft tissue balancing with the ability to appropriately size and position the humeral head in any direction with the use of an eccentric component. Additional considerations include commencing with appropriate patient selection that involves patients with glenohumeral arthritis and an intact rotator cuff. It is important to confirm sufficient glenoid bone and general bone quality, which could entail CT imaging. Intraoperatively, the rotator cuff tendon attachments must be protected. An additional challenge with this technique may involve the management of the subscapularis. While there are 3 subscapularis approach techniques (tenotomy, LTO, or peel technique), the choice is based on surgeon preference and experience. Though there is a concern for compromising implant fixation and periprosthetic fracture utilizing a lesser tuberosity osteotomy in conjunction with a metaphyseal-based stemless component, Aibinder et al1 demonstrated that all 3 techniques are safe and effective at 2 years of follow-up. Nevertheless, future studies are warranted to determine the long-term outcomes of the various subscapularis management techniques.
Conclusion
Anatomic TSA with the use of a stemless humeral component is becoming an increasingly utilized configuration. This article presents a review of the published literature and our preferred technique for stemless humeral component implantation. Future investigations are warranted to continue to understand the long-term clinical and radiographic outcomes of this promising humeral fixation method.
Disclaimers:
Funding: No funding was disclosed by the authors.
Conflicts of interest: Thomas R. Duquin: Biomet: Paid consultant; Paid presenter or speaker; Research support Integer: Paid consultant Journal of Orthopaedics and Traumatology: Editorial or governing board Zimmer: IP royalties; Paid consultant; Research support. John Sperling: Innomed: IP royalties Journal of Shoulder and Elbow Surgery: Editorial or governing board Responsive Arthroscopy: IP royalties; Stock or stock Options SLACK Incorporated: Editorial or governing board Zimmer: IP royalties; Paid consultant. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Given his role as Co-Editor-in-Chief of this publication, Dr. John Sperling had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dr. Ed Craig.
Footnotes
Mayo Clinic Institutional Review Board approved this study (IRB #12-007498).
References
- 1.Aibinder W.R., Bicknell R.T., Bartsch S., Scheibel M., Athwal G.S. Subscapularis management in stemless total shoulder arthroplasty: tenotomy versus peel versus lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2019;28:1942–1947. doi: 10.1016/j.jse.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Alolabi B., Youderian A.R., Napolitano L., Szerlip B.W., Evans P.J., Nowinski R.J., et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1740–1746. doi: 10.1016/j.jse.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Baranek E.S., Trofa D.P., Levine W.N., Goldberg S.S. Accuracy of humeral implant positioning using a canal-sparing total shoulder arthroplasty system. J Shoulder Elbow Arthroplasty. 2019 doi: 10.1177/2471549219844837. 247154921984483. [DOI] [Google Scholar]
- 4.Beck S., Beck V., Wegner A., Dudda M., Patsalis T., Jäger M. Long-term survivorship of stemless anatomical shoulder replacement. Int Orthop. 2018;42:1327–1330. doi: 10.1007/s00264-018-3779-0. [DOI] [PubMed] [Google Scholar]
- 5.Berth A., Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: a comparison of the functional outcome after a minimum of two years follow-up. J Orthop Trauma. 2013;14:31–37. doi: 10.1007/s10195-012-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohsali K.I., Wirth M.A., Rockwood C.A. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279–2292. doi: 10.2106/jbjs.f.00125. [DOI] [PubMed] [Google Scholar]
- 7.Brabston E.W., Fehringer E.V., Owen M.T., Ponce B.A. Stemless Humeral Implants in Total Shoulder Arthroplasty. J Am Acad Orthop Surg. 2020;28:e277–e287. doi: 10.5435/JAAOS-D-16-00747. [DOI] [PubMed] [Google Scholar]
- 8.Copeland S. The continuing development of shoulder replacement: “reaching the surface”. J Bone Joint Surg Am. 2006;88:900–905. doi: 10.2106/JBJS.F.00024. [DOI] [PubMed] [Google Scholar]
- 9.Crosby L.A., Wright T.W., Yu S., Zuckerman J.D. Conversion to reverse total shoulder arthroplasty with and without Humeral Stem Retention: The Role of a Convertible-Platform Stem. J Bone Joint Surg Am. 2017;99:736–742. doi: 10.2106/JBJS.16.00683. [DOI] [PubMed] [Google Scholar]
- 10.De Wilde L., Van Tongel A. The history of shoulder arthroplasty. Joint Replace Technology. Elsevier. 2014:pp 571–pp 601. doi: 10.1533/9780857098474.4.571. [DOI] [Google Scholar]
- 11.von Engelhardt L.V., Manzke M., Breil-Wirth A., Filler T.J., Jerosch J. Restoration of the joint geometry and outcome after stemless TESS shoulder arthroplasty. World J Orthop. 2017;8:790–797. doi: 10.5312/wjo.v8.i10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franta A.K., Lenters T.R., Mounce D., Neradilek B., Matsen F.A., 3rd The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16:555–562. doi: 10.1016/j.jse.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg S.S., Baranek E.S., Korbel K.C., Blaine T.A., Levine W.N. Anatomic total shoulder arthroplasty using a stem-free ellipsoid humeral implant in patients of all ages. J Shoulder Elbow Surg. 2021;30:e572–e582. doi: 10.1016/j.jse.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Grubhofer F., Muniz Martinez A.R., Haberli J., Selig M.E., Ernstbrunner L., Price M.D., et al. Does computerized CT-based 3D planning of the humeral head cut help to restore the anatomy of the proximal humerus after stemless total shoulder arthroplasty? J Shoulder Elbow Surg. 2021;30:e309–e316. doi: 10.1016/j.jse.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Harryman D.T., Sidles J.A., Harris S.L., Lippitt S.B., Matsen F.A., 3rd The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1995;77:555–563. doi: 10.2106/00004623-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hasan S.S., Leith J.M., Campbell B., Kapil R., Smith K.L., Matsen F.A., 3rd Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11:431–441. doi: 10.1067/mse.2002.125806. [DOI] [PubMed] [Google Scholar]
- 17.Hawi N., Tauber M., Messina M.J., Habermeyer P., Martetschläger F. Anat stemless Shoulder arthroplasty Relat Outcomes a Syst Rev BMC Musculoskelet. Disord. 2016;17:1–10. doi: 10.1186/s12891-016-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadum B., Hassany H., Wadsten M., Sayed-Noor A., Sjödén G. Geometrical analysis of stemless shoulder arthroplasty: a radiological study of seventy TESS total shoulder prostheses. Int Orthop. 2016;40:751–758. doi: 10.1007/s00264-015-2935-z. [DOI] [PubMed] [Google Scholar]
- 19.LaChaud G.Y., Schoch B.S., Wright T.W., Roche C., Flurin P.H., Zuckerman J.D., et al. Humeral stem lucencies correlate with clinical outcomes in anatomic total shoulder arthroplasty. JSES Int. 2020;4:669–674. doi: 10.1016/j.jseint.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus M.D., Jensen K.L., Southworth C., Matsen F.A., 3rd The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84:1174–1182. doi: 10.2106/00004623-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Liu E.Y., Kord D., Horner N.S., Leroux T., Alolabi B., Khan M. Stemless anatomic total shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2020;29:1928–1937. doi: 10.1016/j.jse.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Magosch P., Lichtenberg S., Habermeyer P. Survival of stemless humeral head replacement in anatomic shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2021;30:e343–e355. doi: 10.1016/j.jse.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Märtens N., Heinze M., Awiszus F., Bertrand J., Lohmann C.H., Berth A. Long-term survival and failure analysis of anatomical stemmed and stemless shoulder arthroplasties. Bone Joint J. 2021;103-B:1292–1300. doi: 10.1302/0301-620X.103B7.BJJ-2020-0915.R3. [DOI] [PubMed] [Google Scholar]
- 24.Nash H.M., Trang G., Bryant S.A., Mirvish A.B., Gardner B.B., Chakrabarti M.O., et al. Stemless Total Shoulder Arthroplasty With Orthobiologic Augmentation. Arthrosc Tech. 2021;10:e531–e538. doi: 10.1016/j.eats.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nixon R.A., Dang K.H., Haberli J.E., O’Donnell E.A. Surgical time and outcomes of stemmed versus stemless total shoulder arthroplasty. J Shoulder Elbow Surg. 2022;31:S83–S89. doi: 10.1016/j.jse.2022.01.129. [DOI] [PubMed] [Google Scholar]
- 26.Nyffeler R.W., Sheikh R., Jacob H.A.C., Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86:575–580. doi: 10.2106/00004623-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Pean J.E. Des moyens prothetiques destines a obtenir la reparation des parties osseuses. Gaz Hop Paris. 1894;67:291. [Google Scholar]
- 28.Shin Y.-S., Lee W.-S., Won J.-S. Comparison of stemless and conventional stemmed shoulder arthroplasties in shoulder arthropathy: A meta-analysis. Medicine. 2021;100:e23989. doi: 10.1097/MD.0000000000023989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrier A., Ramondetti S., Merlini F., Pioletti D.D., Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1184–1190. doi: 10.1016/j.jse.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Throckmorton T.W., Zarkadas P.C., Sperling J.W., Cofield R.H. Radiographic stability of ingrowth humeral stems in total shoulder arthroplasty. Clin Orthop Relat Res. 2010;468:2122–2128. doi: 10.1007/s11999-010-1299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upfill-Brown A., Satariano N., Feeley B. Stemless shoulder arthroplasty: review of short and medium-term results. JSES Open Access. 2019;3:154–161. doi: 10.1016/j.jses.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uschok S., Magosch P., Moe M., Lichtenberg S., Habermeyer P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J. Shoulder Elbow Surg. 2017;26:225–232. doi: 10.1016/j.jse.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Verborgt O., El-Abiad R., Gazielly D.F. Long-term results of uncemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16:S13–S18. doi: 10.1016/j.jse.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Wiater J.M., Levy J.C., Wright S.A., Brockmeier S.F., Duquin T.R., Wright J.O., et al. Prospective, Blinded, Randomized Controlled Trial of Stemless Versus Stemmed Humeral Components in Anatomic Total Shoulder Arthroplasty: Results at Short-Term Follow-up. J Bone Joint Surg Am. 2020;102:1974–1984. doi: 10.2106/JBJS.19.01478. [DOI] [PubMed] [Google Scholar]
- 35.Willems J.I.P., Hoffmann J., Sierevelt I.N., van den Bekerom M.P.J., Alta T.D.W., van Noort A. Results of stemless shoulder arthroplasty: a systematic review and meta-analysis. EFORT Open Rev. 2021;6:35. doi: 10.1302/2058-5241.6.200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams G.R., Jr., Wong K.L., Pepe M.D., Tan V., Silverberg D., Ramsey M.L., et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10:399–409. doi: 10.1067/mse.2001.116871. [DOI] [PubMed] [Google Scholar]