Abstract
The lateral ulnar collateral ligament (LUCL) is a primary lateral stabilizer of the elbow that originates from the isometric center of the capitulum and inserts into the supinator crest of the ulna. LUCL injury may be due to trauma, chronic strain, or iatrogenic lesion. In patients with symptomatic LUCL insufficiency and recurrent posterolateral rotatory instability, surgical reconstruction can restore elbow stability. In primary acute treatment, the injured LUCL is reattached to the lateral epicondyle with transosseous sutures and anchors placed at the isometric origin of the ligament. If the ligament quality is poor, patients with chronic elbow instability may require reconstruction with a tendon autograft or allograft. Alternatively, the LUCL can be reconstructed by transposition of the local extensor fascia septum, a local flap that exploits the common extensor fascia connected to a thin strip of extensor digitorum quinti or the extensor digitorum communis intermuscular septum. We describe a new LUCL reconstruction technique based on the transposition of the local extensor fascia septum and report the preliminary result in a series of patients aged 50 years or less.
Keywords: Lateral ulnar collateral ligament, Soft tissue lesions, Trauma, Chronic strain, Iatrogenic lesion, Transposition of the local extensor fascia septum
The lateral ulnar collateral ligament (LUCL) is a primary lateral stabilizer of the elbow. The ulnar band of the lateral collateral ligament (LCL) complex originates from the isometric center of the capitulum and inserts into the supinator crest of the ulna. LUCL injury is usually due to trauma, chronic strain, or iatrogenic lesion. Acute severe injury of the LCL complex can lead to posterolateral rotatory instability (PLRI).14 The lesion is commonly the result of trauma, such as a fall on an outstretched arm with the forearm supinated and the elbow slightly flexed; the humeroulnar joint rotates posterolaterally to dislocate or subluxate the elbow posteriorly. Chronic LUCL strain may arise from long-standing cubitus varus deformity resulting, for instance, from malunion of childhood supracondylar fractures, long-term crutch use,15 or iatrogenic injury due to aggressive surgical release for lateral epicondylitis or to surgical exposure of radial head or capitulum fractures. In primary acute treatment, the injured LUCL is reattached to the lateral epicondyle with transosseous sutures and anchors placed at the isometric origin of the ligament. Patients with chronic elbow instability and poor-quality ligament tissue may require reconstruction with a tendon autograft or allograft. Transposition of the local extensor fascia septum uses a local flap of common extensor fascia connected to a thin strip of extensor digitorum quinti (EDQ) or extensor digitorum communis (EDC) intermuscular septum. We describe a new LUCL reconstruction technique involving the transposition of the local extensor fascia septum and report the preliminary result obtained in patients aged 50 years or less.
Open surgical reconstruction
Surgical reconstruction is recommended in patients with symptomatic LUCL insufficiency and recurrent PLRI to restore elbow stability. In primary acute treatment, the injured LUCL is reattached to the lateral epicondyle with transosseous sutures and anchors placed at the isometric origin of the ligament. In patients with an attenuated ligament but good-quality tissue, an advanced or imbricated suture can be performed to achieve structural stability.
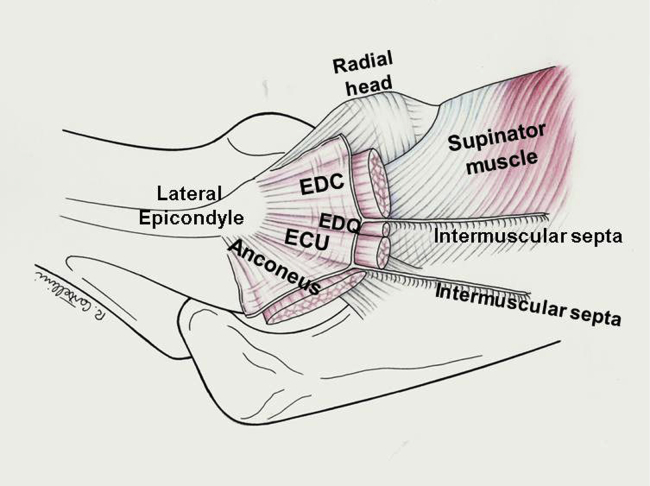
Patients with chronic elbow instability and inadequate ligament quality may require reconstruction with a tendon autograft/allograft. The graft selection options include an allograft (eg, plantaris tendon, semitendinosus or gracilis tendon) or an autograft (palmaris longus free strip of triceps tendon or fascia lata, gracilis tendon).3,13,16,18 We describe the transposition of the local extensor fascia septum as an LUCL reconstruction technique. This is a local flap of common extensor fascia connected to a thin strip of EDQ or EDC intermuscular septum (Fig. 1). The superficial fascia of the common extensor tendons is raised from its bed with the fascial septum, preserving the epicondyle insertion, moved laterally, and reinserted into a drill hole made in the supinator crest. The common extensor fascia is a substantial band that is easily identified and detached from the muscle fibers. In an anatomical study,4 Cohen and Hastings described a distinct band of extensor carpi ulnaris (ECU) fascia, about 6 cm in length and 0.8 cm in width, running from the lateral epicondyle to the ulna. Its fibers were fused with the remaining fascia on the ECU surface, and in some cases, they merged proximally with the annular ligament and the LCL complex. Interestingly, “…a stout four-to-six millimeters intermuscular septum was noted to separate the extensor digitorum communis and extensor digiti quinti muscle compartments.”4 Proximal to the radiocapitellar joint, this septal band inserts into the lateral epicondyle, where its fibers merge with the underlying LCL complex and the extensor tendon insertion.
Figure 1.
The superficial antebrachial fascia is a dense membranous layer covering the extensor muscles and tendons. It originates from the supracondylar ridge of the lateral epicondyle and is attached posteriorly to the olecranon and the dorsal border of the ulna. Numerous intermuscular septa that enclose and separate the muscles and provide insertions for muscle fibers originate from its depth. The EDQ is a slender muscle that together with the ECU lies lateral to the EDC. A well-developed, thicker deep septum divides the two muscles and inserts proximally into the lateral epicondyle, where its fibers merge with the underlying LCL. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis; LCL, lateral collateral ligament.
Local extensor fascia septum transposition: surgical technique
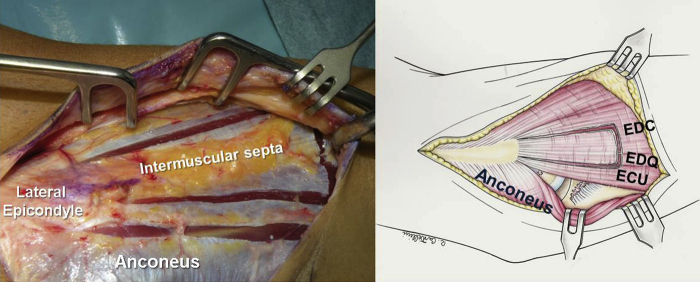
The patient lies in the supine position, and a sterile tourniquet is applied to the upper arm. The distal limited Kocher approach is performed (Fig. 2). The interval between the ECU and the anconeus is developed (Fig. 3), carefully preserving the ECU fascial band with the remaining fibers of the LCL epicondyle insertion. The fascial band usually merges with the insertions of the extensor tendons and the ECU.
Figure 2.
Development of the Kocher interval between the anconeus and the ECU allows visualization of the remainder of the ruptured LCL. ECU, extensor carpi ulnaris; LCL, lateral collateral ligament.
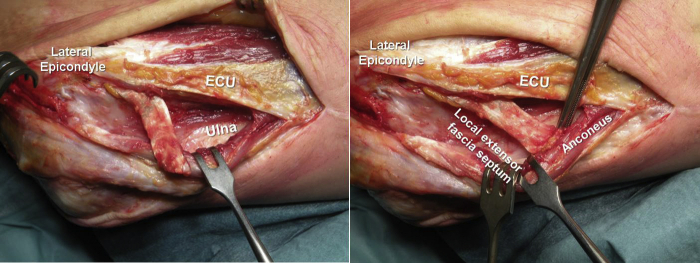
Figure 3.
The septum between the EDQ and the EDC is identified by palpation anterior to the ECU insertion, whereas the EDQ is identified by passive movement of the small finger with the wrist blocked. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
A triangular fragment of common extensor fascia (ca. 2 × 6 cm wide and 8 cm long), centered on the intermuscular septum, is marked (Fig. 4). The septum can be identified by palpation 3-4 mm medial to the ECU humeral head. The EDQ and the fascial septum are easily identified anterior to the ECU insertion by passively moving the little finger with direct palpation.
Figure 4.
A triangular fragment of common extensor fascia (ca. 2 × 6 cm wide and 8 cm long) is marked and collected. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
The fascia can be elevated from the common extensor muscles, sparing the epicondyle insertion of the fascia and septum. The fascial flap should incorporate the superficial portion of the intermuscular septum (2-3 mm), preserving its continuity (Fig. 5, A–C) and keeping the deep portion intact.
Figure 5.
(A, B, and C) The superficial fascial flap is mobilized off the underlying muscles, preserving its continuity with the deep intermuscular septum. The muscle fiber insertions on the septa are raised and divided longitudinally, leaving 2-3 mm in continuity with the fascial flap. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
The common extensor fascial band is folded around the split septum as a rotation flap and mobilized, sparing the epicondyle insertion. Imbricated Krackow locking sutures are placed along the anterior and posterior aspects of the rotation flap, which provides a new ligament (Figs. 6 and 7).
Figure 6.
The fascial flap with the split septum is mobilized, preserving its epicondylar insertion. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
Figure 7.

The two long sides of the flap are folded around the split of the septum, and an imbricated Krackow locking suture is placed along the anterior and posterior aspects of the new ligament.
The interval between the EDC tendon insertion and the lateral insertions of the EDQ and the ECU is identified and split longitudinally, to expose the underlying oblique fibers of the supinator muscle (Figs. 8 and 9).
Figure 8.
The EDC is divided; the EDQ and the ECU tendons are split as a unit from the underlying supinator muscle, capsule, and annular ligament using blunt scissors. The oblique fibers of the supinator muscle allow identifying the deep plane of dissection. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
Figure 9.
The graft is rotated posterior to the ulnar insertion under the ECU-EDQ tendon unit and above the supinator muscle. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris.
If exposure of the lateral aspect of the joint is required, as in radial head replacement, the distal deep splitting approach may be extended superiorly, as in the column approach. The insertions of the common extensor tendons (EDC, extensor carpi radialis brevis/longus) are released from the lateral column and the lateral aspect of the epicondyle, carefully preserving the insertion of the new ligament. At the end of the surgical procedure, an incision is made in the anterior capsule, and the annular ligament is divided longitudinally with stay sutures placed on both sides of the incision for anatomical repair.
A plane under the ECU and EDQ tendons and above the supinator muscle fibers is carefully developed using blunt scissors so that the graft can be rotated posterior to the ulnar insertion and passed through it.
To ensure alignment with the native ligament, the new ligament is fixed with a suture anchor to the isometric point identified at the center of the lateral epicondyle (Fig. 10).
Figure 10.
To ensure its alignment with the native ligament at the isometric point, the new ligament is fixed with a suture anchor at the center of the lateral aspect of the capitulum.
A groove for the rotation flap (1-1.5 × 0.5 cm) is excavated with a high-speed burr in the supinator crest, perpendicular to the intended direction of the LCL. Three holes are then drilled into its bottom on the ulnar border (Fig. 11), and the graft sutures are passed through them. The new ligament is laid in the groove, and the sutures are stretched to the correct length after repeated flexion-extension movements. Next, the sutures are tied with the forearm in full pronation and the elbow at 40° of flexion. The new ligament is sutured proximal to the remains of the native ligament, and the annular ligament incision is repaired (Fig. 12). The extensor fascia is closed, and the Kocher interval is reapproximated with absorbable sutures (Figs. 13 and 14).
Figure 11.
The supinator crest is excavated with a high-speed burr to receive the rotational flap (about 1-1.5 cm × 0.5 cm), and three holes are drilled into the ulnar border of the excavated site. EDQ, extensor digitorum quinti; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis.
Figure 12.
The graft sutures are passed through the ulnar holes, the new ligament is introduced into the groove, and the sutures are tied with correct tension with the elbow in 40° of flexion and the forearm fully pronated. The remaining native ligament and the annular ligament are repaired in continuity with the LUCL. LUCL, lateral ulnar collateral ligament.
Figure 13.
The extensor fascia is closed and the Kocher interval is reapproximated with absorbable sutures. ECU, extensor carpi ulnaris.
Figure 14.
X-ray scans (case #2).
Postoperative care
-
•
0-4 weeks: Elbow immobilization in a static posterior splint.
-
•
4-8 weeks: Hinged elbow brace enabling active and passive movement but limiting supination.
-
•
8 weeks: Active and passive movement without the brace, avoiding full supination and varus stress, and use of upper limb for normal daily activities.
Statistical analysis
Data are reported as mean (± standard deviation). The normal distribution of data was tested with the Shapiro-Wilk test, and homoscedasticity was tested with the F test for homogeneity of variances. The Wilcoxon signed-rank test was used to compare variables between preoperative and postoperative assessments. A P value < .05 (2-tailed) was considered significant. Analyses were performed using the STATA software package (2009, release 11; Stata Corp, College Station, TX, USA).
Preliminary clinical experience
From 2017 to 2019, 10 consecutive patients with chronic PLRI of the elbow—7 men and 3 women—underwent LUCL reconstruction with transposition of the local extensor fascia septum at our institution. All procedures were performed by the same surgeon. Patients’ mean age at the time of surgery was 33 years (range, 25-50), and the mean interval from injury to surgery was 15 months (range, 10-20). The right elbow was involved in 9 cases (Table I). PLRI had developed after a documented traumatic dislocation of the elbow. Eight patients suffered from recurrent elbow instability (dislocation or subluxation). Two patients described snapping sensations or elbow locking symptoms. Moderate pain was reported by 7 patients, and severe pain was reported by one. Physical examination demonstrated severe limitations in daily living and sports activities in all cases. Anteroposterior dynamic examination under fluoroscopy documented varus instability in all cases. Before surgery, all patients had a positive lateral pivot shift test and a varus/valgus stability test under anesthesia. In one patient (#3), the recurrent PLRI had induced early degenerative changes to the capitulum surface; however, no additional surgical steps were performed on his elbow.
Table I.
Preoperative clinical assessment with the Mayo Elbow Performance Score (MEPS), the QuickDash questionnaire, and the visual analog scale (VAS) for pain.
| Case # | Gender M/F | Side L/R | Dominant arm L/R | Age (yr) | Mechanism of injury | Symptoms | Symptom duration (mo) | ROM extension | ROM flexion | MEPS | QuickDASH | VAS | Pivot shift test under anesthesia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | R | R | 48 | Dislocation | Recurrent subluxation | 14 | 0 | 130 | 75 | 20.5 | 4 | Positive |
| 2 | F | R | R | 35 | Dislocation | Snapping-locking | 18 | 20 | 140 | 80 | 11.4 | 5 | Positive |
| 3 | M | R | R | 50 | Subluxation | Recurrent subluxation | 20 | 0 | 130 | 80 | 9.1 | 2 | Positive |
| 4 | M | R | R | 30 | Dislocation | Recurrent subluxation | 12 | 0 | 140 | 95 | 11.4 | 0 | Positive |
| 5 | M | R | L | 33 | Subluxation | Recurrent subluxation | 18 | 10 | 140 | 75 | 13.6 | 3 | Positive |
| 6 | M | L | R | 25 | Dislocation | Snapping-locking | 16 | 10 | 130 | 90 | 11.4 | 0 | Positive |
| 7 | F | R | R | 28 | Subluxation | Recurrent subluxation | 13 | 0 | 130 | 75 | 15.9 | 5 | Positive |
| 8 | F | R | R | 35 | Dislocation | Recurrent subluxation | 15 | 0 | 140 | 80 | 20.5 | 5 | Positive |
| 9 | M | R | R | 25 | Subluxation | Recurrent subluxation | 10 | 0 | 140 | 65 | 22.7 | 7 | Positive |
| 10 | M | R | R | 27 | Dislocation | Recurrent subluxation | 14 | 0 | 140 | 80 | 18.2 | 5 | Positive |
ROM, range of motion.
The mean follow-up was 26 months (range, 24-30). All patients were assessed for pain, range of motion, and subjective and objective stability. The Mayo Elbow Performance Score (MEPS),12 the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire score,6 and the visual analog scale (VAS) score for pain8 were calculated before surgery and at the last follow-up. At the last follow-up, 9 patients (90%) reported a completely stable elbow. The tenth (#3) described a marked improvement compared with his preoperative condition, but was unable to return to his previous level of sport activity because he felt that his elbow could not lift weights as before the trauma. The range of motion improved or was preserved in all patients (extension, from 4° ± 6.99° to 2° ± 4.22°; flexion, from 136° ± 5.16° to 135° ± 5.27°; both P ˃ .05).
All clinical measures improved significantly (P < .05): the mean MEPS from 79 (79.5 ± 8.32) to 98 (98.5 ± 4.74); the mean QuickDASH score from 15.4 (15.47 ± 4.76) to 0.9 (0.91 ± 1.58), and the mean VAS score from 3.6 (3.6 ± 2.32) to 0.2 (0.2 ± 0.63) (Tables I and II).
Table II.
Postoperative clinical assessment with the Mayo Elbow Performance Score (MEPS), the QuickDash questionnaire, the visual analog scale (VAS) for pain and the pivot shift test.
| Case | Follow-up (mo) | ROM extension | ROM flexion | MEPS | QuickDASH | VAS | X-ray assessment: ectopic bone formation | X-ray assessment: degenerative joint changes | Pivot shift test | Return to previous sports activities | Sports activities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 0 | 130 | 100 | 2.3 | 0 | Lateral epicondyle | No | Negative | Yes | Volleyball |
| 2 | 30 | 10 | 140 | 100 | 0 | 0 | No | No | Negative | Yes | Volleyball |
| 3 | 25 | 0 | 130 | 85 | 4.5 | 2 | No | Capitulum | Negative | No | Weightlifting |
| 4 | 28 | 0 | 140 | 100 | 0 | 0 | No | No | Negative | Yes | Basketball |
| 5 | 24 | 10 | 130 | 100 | 2.3 | 0 | No | No | Negative | Yes | Volleyball |
| 6 | 24 | 0 | 130 | 100 | 0 | 0 | No | No | Negative | Yes | Free climbing |
| 7 | 27 | 0 | 130 | 100 | 0 | 0 | Lateral epicondyle | No | Negative | Yes | Weightlifting |
| 8 | 24 | 0 | 140 | 100 | 0 | 0 | No | No | Negative | Yes | Karate |
| 9 | 29 | 0 | 140 | 100 | 0 | 0 | No | No | Negative | Yes | Weightlifting |
| 10 | 24 | 0 | 140 | 100 | 0 | 0 | No | No | Negative | Yes | Weightlifting |
All patients returned to their sports. X-ray assessments: degenerative joint changes and ectopic bone formation.
ROM, range of motion.
At the last clinical evaluation, 9 of 10 patients (90%) were completely without pain (Table II), whereas the lateral pivot shift test was negative for all elbows. All patients were satisfied with the outcome of their procedure, and 90% returned to their sports to the preinjury level. The X-rays showed a small ectopic bone formation without clinical relevance on the lateral epicondyle in 2 patients (#1 and 7). Early degenerative changes, detected in one patient (#3), did not progress during the study period.
Discussion
LUCL insufficiency is the most common cause of recurrent elbow instability. The ulnar portion of the LCL complex is attenuated, and symptoms vary from recurrent posterolateral subluxation to subtle pain and discomfort.17
Patients with recurrent subluxation describe the elbow as slipping in and out of the joint and often report popping, snapping, clicking, or locking when the forearm is supinated and the elbow slightly flexed. Eight patients of our series had recurrent subluxation, and two reported snapping or locking during normal activities.
Patients with chronic LUCL insufficiency experience lateral pain with a sensation of instability in certain positions when the elbow is loaded. This was the case in 80% of our patients and was the primary factor influencing the MEPS, QuickDash scores, and VAS scores. The PLRI was assessed by tests involving humeroradial joint subluxation or the typical apprehension response. Radial subluxation is easily demonstrated under anesthesia.1,14,15,17,21
Standard X-rays (anteroposterior and lateral views) are often normal. After ligament injury, small fragments of avulsed bone may be detected under the lateral epicondyle on anteroposterior radiographs. This was the case of patients #3 and 9. Posterior subluxation of the radial head with widening of the humeroulnar joint space can be detected in lateral views, whereas LUCL insufficiency is best demonstrated using radiographic or fluoroscopic stress views during the pivot shift test under anesthesia. Both conditions were detected in all our patients.
Although standard magnetic resonance imaging plays a limited role, magnetic resonance arthrography may disclose LCL complex injury. Magnetic resonance imaging may depict chondral damage to the capitulum or intra-articular loose bodies, as in one of our patients (#3).
Surgical management of recurrent PLRI involves direct LUCL repair or its reconstruction with a free tendon or fascia lata graft or with transposition of the local extensor fascia septum.
Direct repair should be performed only in patients with good LUCL tissue quality; in the other cases, the ulnar portion of the LCL complex should be reconstructed using a free tendon or fascia graft.
In patients with chronic ligament insufficiency, LUCL reconstruction with autologous graft tissue is recommended when reinsertion and plication do not appear to stabilize the humeroradial joint.
An autologous free tendon graft is usually preferred, using the palmaris longus tendon (present in 85% of individuals),18 the plantaris tendon (found in 80% of lower limbs), a strip of triceps tendon,3,16 or the gracilis or semitendinosus tendon. Different fixation techniques can be used. The traditional approaches include the figure-of-8 yoke technique,13 the docking technique,11 and the circumferential graft or box loop technique.5,7 Our ten patients with chronic LUCL insufficiency were managed with local transposition of the extensor fascia septum. The approach restored adequate lateral elbow stability and enabled return to the preinjury level of sport performance in 90% of patients. These outcomes are comparable with those obtained by other researchers with different types of autografts/allografts.2,9,10,19,20 The chief advantage of this novel technique lies in its simplicity since it uses donor tissue from the same surgical area and preserves the epicondyle insertion of the transposed local extensor fascia septum. For correct alignment with the native ligament, identification of the isometric point at the center of the lateral epicondyle allows fixation of the new ligament with a suture anchor, sparing the insertion in the transposed soft tissue. The new ligament is accommodated into a specially prepared ulnar groove, and after repeated flexion-extension movements, the sutures are tied with the forearm in full pronation and the elbow at 40° of flexion. There was no donor site morbidity, nor were there any complications related to soft tissue transposition. No adverse functional effects on hand or wrist movement were seen at the last follow-up. We attribute this outcome to the preservation of the muscle insertions and of the deep portion of the intermuscular septum. It would be interesting to compare prospectively patients treated with this technique and with other approaches using a tendon autograft/allograft.
Conclusion
To the best of our knowledge, there are no studies describing local extensor fascia septum transposition as a reconstruction technique for chronic LUCL lesions. If ligament quality is poor, this is a useful alternative to other approaches using tendon autografts/allografts. Our experience with a mean follow-up of two years showed that the approach can stabilize the lateral compartment. Comparison of larger patient groups managed by this and other autograft/allograft reconstruction techniques is clearly needed to establish the respective medium- and long-term outcomes.
Disclaimers:
Funding: No funding was disclosed by the authors.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this technical note.
References
- 1.Arvind C.H., Hargreaves D.G. Table top relocation test: New clinical test for postero-lateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2006;15:500–501. doi: 10.1016/j.jse.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Badhrinarayanan S., Desai A., Watson J.J., White C.H.R., Phadnis J. Indications, Outcomes, and Complications of Lateral Ulnar Collateral Ligament Reconstruction of the Elbow for Chronic Posterolateral Rotatory Instability: A Systematic Review. Am J Sports Med. 2021;49:830–837. doi: 10.1177/0363546520927412. [DOI] [PubMed] [Google Scholar]
- 3.Baumfeld J.A., Van Riet R.P., Zobitz M.E., Eygendaal D., Kai-Nan A.N., Steinmann S.P. Triceps tendon properties and its potential as an autograft. J Shoulder Elbow Surg. 2010;19:607–609. doi: 10.1016/j.jse.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M.S., Hastings H.H. Rotatory instability of the elbow, the anatomy and role of the lateral stabilizers. J Bone Joint Surg Am. 1997;79:225–233. [PubMed] [Google Scholar]
- 5.Finkbone P.R., O’Driscoll S.W. Box loop ligament reconstruction of the elbow for medial and lateral instability. J Shoulder Elbow Surg. 2015;24:647–654. doi: 10.1016/j.jse.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Gummesson C., Ward M., Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (Quick DASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:2–3. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hack L.M., Heinze N., Wegmann K., Lappen S., Leschinger T., Burkhart K.J., et al. The circumferential graft technique for treatment of multidirectional elbow instability: a comparative biomechanical evaluation. J Shoulder Elbow Surg. 2016;25:127–135. doi: 10.1016/j.jse.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Johnson E. Visual analog scale (VAS) Am J Phys Med Rehabil. 2001;80:717. doi: 10.1097/00002060-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lee B.P., Teo L.H. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12:476–479. doi: 10.1016/s1058-2746(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 10.Lin K., Shen P., Lee C., Pan R., Lin L., Shen H. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43:1657–1661. doi: 10.1016/j.injury.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Metha J.A., Bain G.I. Posterolateral rotatory instability of the elbow. J Am Acad Orthop Surg. 2004;12:405–415. doi: 10.5435/00124635-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Morrey B., An K. 3rd ed. WB Saunders; Philadelphia, PA: 2000. The Elbow and Its Disorders: Functional Evaluation of the Elbow. ISBN 0721677525. [Google Scholar]
- 13.Nestor B.J., O’Driscoll S.W., Morrey B.F. Ligamentous reconstruction for postero-lateral rotatory instability of the elbow. J Bone Joint Surg. (Am) 1992;74:1235–1241. [PubMed] [Google Scholar]
- 14.O’Driscoll S.W., Bell D.F., Morrey B.F. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73A:440–446. [PubMed] [Google Scholar]
- 15.O’Driscoll S.W., Spinner R.J., McKee M.D., Kibler W.B., Hastings H., Morrey B.F., et al. Tardy postero-lateral rotatory instability of the elbow due to cubitus varus. J Bone Joint Surg Am. 2001;83A:1358. doi: 10.2106/00004623-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Olsen B.S., Sojberg J.O. The treatment of recurrent postero-lateral instability of the elbow. J Bone Joint Surg Br. 2003;85:342–346. doi: 10.1302/0301-620x.85b3.13669. [DOI] [PubMed] [Google Scholar]
- 17.Regan W., Lapnes P.C. Prospective evaluation of two diagnostic apprehension signs for posterolateral instability of the elbow. J Shoulder Elbow Surg. 2006;15:344–346. doi: 10.1016/j.jse.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Reimann A.F., Daseler E.H., Anson B.J., Beaton L.E. – The palmaris longus muscle and tendon: A study of 1600 extremities – Anat. Rec. 1944;89:495–505. [Google Scholar]
- 19.Rodriguez M.J., Kusnezov N.A., Dunn J.C., Waterman B.R., Kilcoyne K.G. Functional outcomes following lateral ulnar collateral ligament reconstruction for symptomatic posterolateral rotatory instability of the elbow in an athletic population. J Shoulder Elbow Surg. 2018;27:112–117. doi: 10.1016/j.jse.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Sotelo J., Morrey B.F., O’Driscoll S.W. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg. (Br) 2005;87:54–61. doi: 10.1302/0301-620X.87B1.15096. [DOI] [PubMed] [Google Scholar]
- 21.Smith J.P., III, Savoie F.H., III, Field L.D. Postero-lateral rotatory instability of the elbow. Clin Orthop. 2001;20:47–58. doi: 10.1016/s0278-5919(05)70246-5. [DOI] [PubMed] [Google Scholar]