Abstract
Herpes simplex virus (HSV) has often been suggested for development as a vector, particularly for the nervous system. Considerable evidence has shown that for use of HSV as a vector, immediate-early (IE) gene expression must be minimized or abolished, otherwise such vectors are likely to be highly cytotoxic. Mutations of vmw65 which abolish IE promoter transactivating activity may also be included to reduce IE gene expression generally. However, when vmw65 mutations are combined with an IE gene deletion, such viruses are hard to propagate, even on cells which otherwise complement the IE gene deletion effectively. We have found that vmw65 mutants can be effectively grown on cell lines expressing equine herpesvirus 1 gene 12, a non-HSV homologue of vmw65 with little sequence similarity to its HSV counterpart. This prevents repair by homologous recombination of vmw65 mutations in the virus, which would occur if mutations were complemented by vmw65 itself. The gene 12 protein is not packaged into HSV virions, which is important if viruses grown on such cells are to be used as vectors. These results not only further strengthen the evidence for direct functional homology between and similar modes of action of the two proteins but have allowed the generation of gene 12-containing cell lines in which ICP4 and ICP27 expression is induced by virus infection (probably by ICP0) and which give efficient growth of viruses deficient in ICP27, ICP4, and vmw65 (the viruses also have ICP34.5/ORFP deleted). Efficient growth of such viruses has not previously been possible. As these viruses are highly deficient in IE gene expression generally, such virus-cell line combinations may provide an alternative to HSV vectors with deletions of all four of the regulatory IE genes which, for optimal growth, require cell lines containing all four IE genes but which are hard to generate due to the intrinsic cytotoxicity of each of the proteins.
Herpes simplex virus (HSV) type 1 (HSV-1) has often been suggested as a vector for gene delivery to the nervous system and also other cell types (for reviews, see references 4 and 13). As a vector, HSV has a number of potential advantages in that it naturally enters latency in neurons, providing the possibility of long-term gene expression; does not integrate into the host genome, preventing insertional mutagenesis (for example, the activation of oncogenes or inactivation of tumor suppressor genes); can accept very large DNA insertions, allowing the delivery of multiple genes; is easy to propagate; and can infect a wide variety of other cell types, as well as neurons. Some of these cell types are hard to transduce by other means. In our studies, for example, this has allowed highly efficient gene delivery to dendritic cells (>70%) and less efficient delivery to CD34+ hemopoietic cells (≈10%; 6), cell types which are otherwise both generally recalcitrant to gene transfer.
However, while HSV-1 is highly prevalent in the human population, in the vast majority of cases giving no obvious signs of disease, for use as a vector, the virus must be disabled for safety and to minimize toxicity to target cells. Various strategies for disablement have been reported, including the removal of genes which are unnecessary for growth in vitro but necessary for pathogenesis in vivo. Such genes include those encoding thymidine kinase (18, 26), ribonucleotide reductase (15), and ICP34.5 (5). However, for minimal toxicity, it has become apparent that expression of the regulatory immediate-early (IE) genes ICP0, ICP4, ICP22, and ICP27, which are themselves cytotoxic, must be minimized (20–22, 35, 43). Such reductions in IE gene expression minimize transcription from the vast majority of the 80 or so other genes in the HSV genome. Removal of ICP4 or ICP27 from the HSV genome completely prevents virus growth, and so, such deletions must be complemented in the cells used for virus propagation (9, 31). Deletion of ICP22 and/or ICP0, while these genes are not absolutely essential for virus growth (28, 32, 37, 42), reduces the virus titer. Thus, for the growth of HSV mutants with multiple IE gene deficiencies, cell lines must be produced which effectively complement deletions from the virus, and for effective growth of viruses with deletions in ICP4, ICP27, ICP22, and ICP0, all of these deficiencies would optimally need to be complemented. However, as the IE proteins are highly cytotoxic (20), IE gene expression in cell lines must be tightly regulated. This is usually achieved by the use of the homologous IE gene promoters, which are relatively inactive in the absence of virus infection (e.g., E5 cells [ICP4], B130/2 cells [ICP27], E26 cells [ICP4 and ICP27], and FO6 cells [ICP4, ICP27, and ICP0]; 10, 19, 34, 36). This reduces the problem of IE protein cytotoxicity but still leaves an inherent problem in the generation of cells which are highly effective at complementing multiple IE gene deficiencies.
A second strategy to reduce IE gene expression, rather than deletion of the IE genes themselves, is to include mutations in the gene encoding vmw65. vmw65 is a virion protein which transactivates IE promoters after virus infection (2, 27), and while it is an essential structural protein, specific mutations abolish the transactivating capability of the protein without affecting the structural integrity of the virus (1, 39). These mutations vastly reduce IE gene expression although at high multiplicity or, with the inclusion of hexamethylene bisacetamide (HMBA) in the medium, still allow efficient virus growth in culture (25).
For the construction of vector viruses, we have thus taken the approach of combining mutations in vmw65, which should reduce the expression of all IE genes, with deletion of ICP27 and/or ICP4, the two essential IE genes, giving viruses as described above, in which overall IE gene expression is minimized. However, we have found that in combination with deletion of ICP27 and/or ICP4, growth of vmw65 mutants is vastly reduced, even with HMBA and even in cells which otherwise effectively complement the deficiencies in ICP27 and/or ICP4. As in vmw65-deficient viruses the gene encoding vmw65 has not been deleted, with in our case only a small insertion in the protein (the in1814 mutation; 1), unaltered vmw65 cannot be included in the cells for virus growth. Here, homologous recombination between virus DNA and the vmw65 gene in the cell line would repair the vmw65 defect, preventing the stable propagation of viruses with the mutation. Moreover, such viruses would then package fully functional vmw65 derived from the cell line, reducing the effects of the mutation when the viruses were used as vectors in noncomplementing cells.
To solve this problem, we have tested the novel approach of using a non-HSV homologue of vmw65 (from equine herpesvirus 1 [EHV-1]) for complementation of vmw65-mediated defects in virus growth. The EHV-1 vmw65 homologue (gene 12) (29) has previously been shown by cotransfection experiments to be capable of transactivating HSV IE promoters (29), suggesting that the approach is possible. We have found that while there is minimal DNA similarity between EHV-1 gene 12 and the HSV gene encoding vmw65 (46% identity overall), minimizing the likelihood of repair of vmw65 defects by homologous recombination, when EHV-1 gene 12 is constitutively expressed in the cells used for virus propagation, growth defects associated with vmw65 mutation are abolished. Importantly, EHV-1 gene 12 protein is not packaged into HSV virions grown on such cells, and so, when viruses are used on noncomplementing cells, IE gene deficiencies associated with vmw65 mutation will be retained. When EHV-1 gene 12 is included in cells together with ICP4 and/or ICP27, viruses with ICP4 and/or ICP27 deleted and with mutations in vmw65 can be effectively propagated, which was not previously possible, although here the choice of the promoter driving ICP4 and/or ICP27 is important.
MATERIALS AND METHODS
Cell culture and virus propagation.
The cell lines used were all based on BHK cells and cultured in Dulbecco’s modified Eagle medium plus 10% fetal calf serum. Cell lines were generated by using standard methods of calcium phosphate transfection with the plasmids indicated, antibiotic selection, and cloning out of resistant colonies under constant selection. Neomycin was used at a concentration of 800 μg/ml, and phleomycin D1 (Zeocin; Cayla, Toulouse, France) was used at a concentration of 750 μg/ml. Where indicated, HMBA was included in the medium at a concentration of 3 mM and dexamethasone was used at a concentration of 1 μM. Growth curves were determined in duplicate by using 24-well plates at a multiplicity of infection (MOI) of 0.01. The yields presented are average values per well. All tissue culture reagents were from GIBCO unless otherwise stated. Chloramphenicol acetyltransferase (CAT) assays were performed by standard methods (16) using HSV IE promoter constructs pIGA102, pIGA95, and pIGA65 encoding the ICP4, ICP27, and ICP0 promoters driving cat (14; gift from S. Silverstein, Columbia University, New York, N.Y.) and pCMV-VP16 containing HSV vmw65 under cytomegalovirus (CMV) promoter control (6).
Viruses.
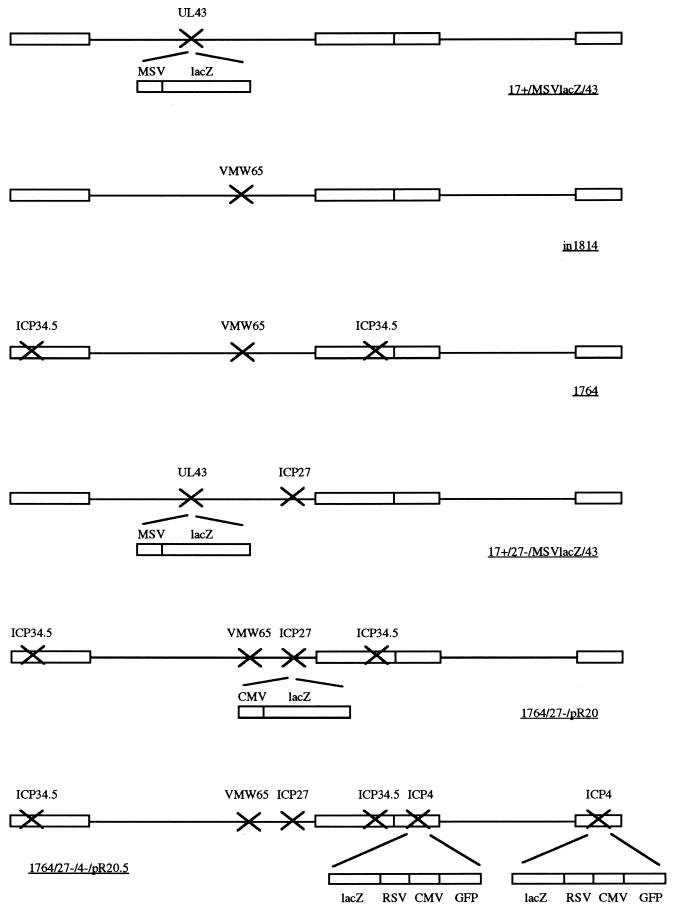
The viruses used in this study are shown in Fig. 1. Virus strains in1814 and 1764 have been previously reported (1, 5). Virus strains 17+/MSVlacZ/43 and 17+/27−/MSVlacZ/43 contain a Moloney murine sarcoma virus long terminal repeat (11) promoter/lacZ (Hind-BamHI from pCH110; Pharmacia) cassette inserted into the unique NsiI site of the nonessential UL43 gene (24) of HSV-1 strains 17+ (wild type) and 17+/27-w, respectively. HSV strains 17+/27-w and 1764/27-w were prepared by removing the lacZ insertion from the ICP27 gene in virus strains 17+/27−/pR20 and 1764/27−/pR20 (see below), respectively, by recombination with empty ICP27 flanking regions and selection of non-5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)-staining recombinant plaques using B130/2 cells which complement ICP27 (19). Virus strains 17+/27−/pR20 and 1764/27−/pR20 contain a latency-associated transcript (LAT) (nucleotides [nt] 118,866 to 120,219 [PstI-BstXI])/CMV/lacZ cassette inserted so as to delete the entire ICP27 coding sequence, UL55, UL56 (both nonessential genes; 30), and part of the LAT region in virus strains 17+ and 1764, respectively, using flanking regions (nt 110,095 to 113,229 [EcoRI-NdeI] and 120,468 to 125,068 [HpaI-SacI] separated by a unique BglII site in plasmid pΔ27LAT) and the selection and purification of X-Gal-staining plaques on B130/2 cells. Virus strain 1764/27−/4−/pR20.5 was constructed by insertion of a cassette consisting of the gene for green fluorescent protein (E-GFP; Clontech) and lacZ driven by the CMV and Rous sarcoma virus promoters, respectively, in a back-to-back orientation and separated by LAT sequences (PstI-BstXI as described above) into ICP4 flanking regions (nt 123,459 to 126,774 [Sau3aI-Sau3aI] and 131,730 to 134,792 [SphI-KpnI], with nt 124,945 to 125,723 [NotI-NotI; encodes ICP34.5] deleted, separated by unique XbaI and SalI sites in plasmid pΔICP4) and recombination into virus strain 1764/27-w by using B4/27 cells, which complement both ICP27 and ICP4. X-Gal-staining, green fluorescent plaques were selected and further purified. Cell line B4/27 was prepared by cotransfection of pSG130BS [containing the ICP27 promoter, coding sequence, and poly(A); 38]; p4/2, which contains the ICP4 promoter, coding region, and poly(A) (nt 126,764 to 131,730 [DdeI-SphI]) inserted into pSP72 (Promega); and pMAMneo (Invitrogen) into BHK cells. Neomycin-resistant clones were then selected. Virus structures were checked by Southern blotting and also by Western blotting with anti-ICP27 and anti-ICP4 antibodies (see below) of the viruses on noncomplementing cells, showing ICP27 and/or ICP4 not to be expressed from the corresponding viruses (data not shown). A full characterization of the more disabled of the viruses will be presented elsewhere. All nucleotide numbers refer to the HSV-1 strain 17+ genomic sequence (GenBank file he1cg).
FIG. 1.
Viruses used in the study. Further details are given in Materials and Methods. RSV, Rous sarcoma virus; GFP, green fluorescent protein; MSV, Moloney murine sarcoma virus.
Plasmids.
For the ICP27- and EHV-1 gene 12-containing cells generated in this study, EHV-1 gene 12 was inserted into pcDNA3 (Invitrogen) between the EcoRV and XbaI sites following excision of the EHV-1 gene 12 coding sequence from plasmid pcDNA/AmpETIF (supplied by M. Grapes, Marie Curie Institute) with EcoRI and XbaI. The ICP27 coding sequence promoter and poly(A) (excised from pSG130BS with SacI and SphI) were inserted between the EcoRI and SalI sites in pPGKneo (40), thus putting each of the genes into a plasmid encoding neomycin selection. For the generation of cell lines containing ICP4, the following plasmids were constructed. For ICP4 under ICP4 promoter control, a phleomycin resistance gene cassette was excised from plasmid pVgRxR (Invitrogen) as a BamHI fragment and inserted into the unique BglII site of plasmid p4/2 (see above), giving plasmid p4/2zeo. For ICP4 under ICP27 promoter and poly(A) control, the ICP4 promoter in plasmid p4/2zeo (upstream of the BstEII site [HSV nt 131,187]) was replaced with a BamHI-DrdI (HSV nt 113,322 to 113,728) promoter fragment from pSG130BS. The ICP4 poly(A) sequence was replaced by removal of sequences after the MseI site (HSV nt 127,167), which were replaced with an EcoNI-SacI (HSV nt 115,267 to 115,743) fragment from pSG130BS encoding the ICP27 poly(A) sequence. For mouse mammary tumor virus (MMTV) promoter control, the neomycin resistance gene (excised as a BamHI fragment) in plasmid pMAMneo (Invitrogen) was replaced with the phleomycin resistance gene as described above, again as a BamHI fragment. The ICP4 coding region (nt 127,167 to 131,187 [MseI-BstEII]) was then inserted after the MMTV promoter at the XhoI site, giving plasmid pMAMzeo/ICP4. All plasmids were linearized for generation of cell lines.
Western blotting.
Western blotting was performed on cell extracts or virus stocks by standard methods using ECL (Amersham) and antibodies to either HSV-1 ICP27, ICP4, ICP0 (all from ABI) or vmw65 (POS1; supplied by Matt Grapes) or EHV-1 gene 12 (23). Equal loading of lanes was checked by Coomassie blue staining of duplicate gels, in most cases using extract from ≈105 cells per lane or 105 PFU of virus (HSV or EHV) per lane.
RESULTS
EHV-1 gene 12 can transactivate HSV IE promoters.
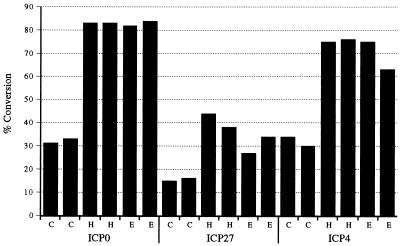
Previous work has shown that a non-HSV homologue of vmw65, EHV-1 gene 12, can transactivate at least some HSV IE promoters (29). Other herpesviruses encode similar homologous proteins. For example, the genomes of varicella-zoster virus, bovine herpesvirus 1, and EHV-4 also encode homologues of vmw65 (3, 8, 29). Of these proteins, the EHV-1 gene 12-encoded protein has been shown to have the greatest transactivating effect on HSV IE promoters, at least on the promoters for the ICP4- and ICP0-encoding genes (29). In an initial experiment using the HSV ICP0, ICP4, and ICP27 promoters driving a cat reporter gene in cotransfection experiments, we sought to confirm and further extend this work to the ICP27 promoter, which had not previously been tested. These results showed efficient transactivation of ICP0 and ICP4 (similar to the transactivation provided by HSV vmw65), as before, but minimal effects on the ICP27 promoter (Fig. 2). Thus, EHV-1 gene 12 may be able to complement the growth of HSV deficient in vmw65 transactivating activity, as well as to function in cotransfection experiments as reported previously and here, although it might be expected that some deficiency in ICP27 would still be apparent. However, after activation of ICP0, it might also be expected that any ICP27 deficiency would be minimal due to the likely potential of ICP0 to transactivate the ICP27 promoter (see later).
FIG. 2.
EHV-1 gene 12-induced transactivation of HSV IE promoters. Duplicate CAT assays were performed in which plasmids encoding the HSV-1 ICP0, ICP27, and ICP4 promoters driving cat (see Materials and Methods) were cotransfected into BHK cells together with either a vector control plasmid (C), a plasmid with HSV-1 vmw65 under CMV promoter control (H), or a similar plasmid containing EHV-1 gene 12 (E). Percent conversion of the substrate to the acetylated form is shown.
EHV-1 gene 12 complements growth deficiencies in HSV-1 vmw65 mutants.
HSV vmw65 mutants deficient in transactivating activity but not packaging, such as in1814 or V422 (1, 39), can be grown in noncomplementing cells at a high MOI or with inclusion in the medium of HMBA (25), which has a generalized promoter-activating effect. However, we have found that when such mutants have further disabling mutations, for example, in ICP27, even in cells which effectively complement such deficiencies, only limited growth occurs. This is the case even at a high MOI or with HMBA. To test whether EHV-1 gene 12 could overcome such growth deficiencies, EHV-1 gene 12 was subcloned into pcDNA3 (Promega) containing a neomycin resistance gene and driving gene 12 from a CMV promoter. This was transfected into BHK cells either alone or at an equal molar ratio with a second plasmid containing the HSV ICP27 promoter, coding sequence, and poly(A) region. After neomycin selection, the contents of the wells were trypsinized and reseeded into new six-well plates and the ability to support the replication of viruses (as estimated by total virus yield) with an inactivating mutation in vmw65 (in1814) or with an inactivating mutation in vmw65 together with deletion of ICP34.5 (1764) or also together with ICP27 (1764/27−/pR20) was tested. The viruses used for the work described in this paper are shown in Fig. 1.
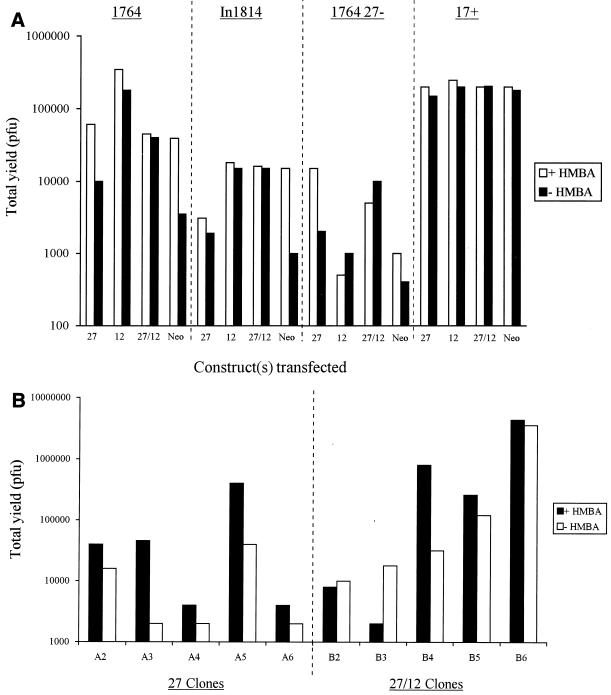
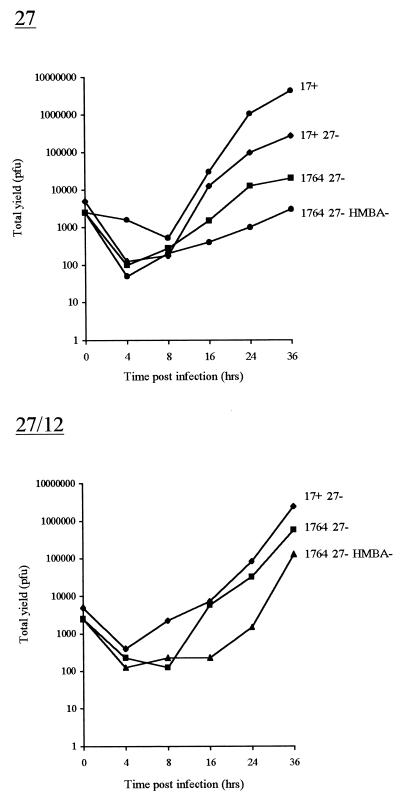
The experiments described above were performed both in the presence and in the absence of HMBA, giving, in these uncloned, neomycin-resistant cells, the average effect of EHV-1 gene 12 expression without the clonal variation which could result from picking of individual colonies. The experiments were performed at a relatively low MOI, as growth deficiencies associated with mutations in vmw65 are more evident under these conditions. Figure 3A shows that in each case, gene 12 considerably improved virus growth and minimized the difference in virus yield obtained when HMBA was included in the medium (similar to the case of the wild-type virus, where the effect of HMBA is minimal). This shows that EHV-1 gene 12 can functionally compensate for deficiencies in virus growth caused by vmw65 inactivation and allow considerable improvement in the growth of vmw65/ICP27 double mutants.
FIG. 3.
EHV-1 gene 12 complements growth deficiencies in HSV vmw65 mutants. (A) Total yields of the indicated viruses when grown on uncloned BHK cells stably transfected with either a control plasmid (Neo), a plasmid encoding ICP27 under ICP27 promoter control (columns 27), a plasmid encoding EHV-1 gene 12 under CMV promoter control (columns 12), or both the ICP27- and EHV-1 gene 12-encoding plasmids together (columns 27/12). (B) Growth of an HSV-1 mutant deficient in both ICP27 and vmw65 (1764/27−/pR20) on individual clones of BHK cells stably transfected with either the ICP27 plasmid or both the ICP27- and EHV-1 gene 12-encoding plasmids as for panel A.
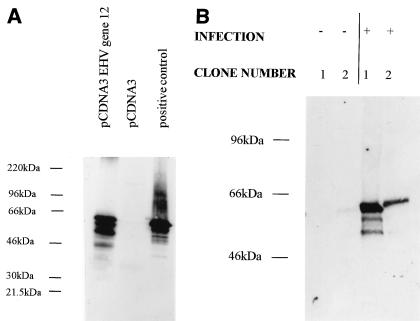
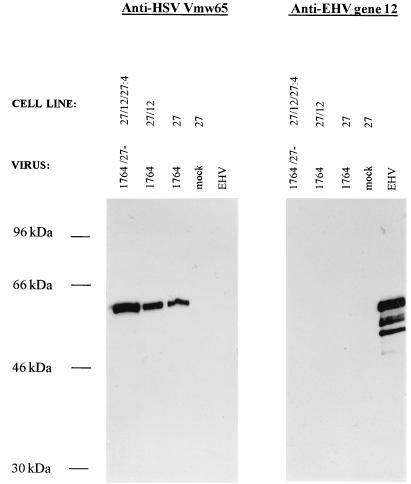
Next, cell lines were cloned out after transfection with only the ICP27 gene-containing plasmid or the ICP27 gene-containing plasmid together with the EHV-1 gene 12-containing plasmid. This again showed that in most cases, better growth of viruses deficient in both ICP27 and vmw65 could be obtained by using clones resulting from the dual transfection (Fig. 3B shows five representative clones in each case). Clones A5 and B6 were used in further experiments in comparison to untransfected BHK control cells. These experiments showed considerably larger plaques when viruses with vmw65 inactivated, with or without deletion of ICP27, were grown on cells containing EHV-1 gene 12. EHV-1 gene 12 expression was confirmed in both the uncloned cells (Fig. 4A) and the cloned cells, where it was found that significant EHV-1 gene 12 could only be detected after virus infection (longer exposures showed expression also without infection; Fig. 4B). This suggests that EHV-1 gene 12 is toxic to cells and selected against such that expression is induced by, e.g., ICP0 after infection. The anti-EHV-1 gene 12 antibody does not cross-react with HSV vmw65 (see later). One-step growth curves further confirmed the increased permissivity of EHV-1 gene 12-containing cells for the growth of HSV-1 vmw65 mutants (Fig. 5). For both of these experiments (except as noted otherwise), HMBA was included, which only slightly increased virus growth in EHV-1 gene 12-containing cells, further demonstrating the relatively poor growth characteristics of ICP27/vmw65 double mutants, even in the presence of HMBA and when ICP27 is otherwise effectively complemented. The above results show that these growth defects can be minimized by the inclusion of EHV-1 gene 12 in the cells used for virus growth. This is, surprisingly, expressed at relatively low constitutive levels in cloned cells, but expression is induced after virus infection.
FIG. 4.
Western blots showing EHV-1 gene 12 protein expression in EHV-1 gene 12-containing cells. (A) EHV-1 gene 12 expression in uninfected, uncloned-out, neomycin-resistant cells transfected with either pDNA3 EHV-1 gene 12 (see Materials and Methods) or pcDNA3. The positive control was purified EHV-1. (B) EHV-1 gene 12 expression in two representative cloned cell lines containing EHV-1 gene 12 and ICP27 with (+) and without (−) infection with 1764/27−/pR20.
FIG. 5.
Growth of HSV mutants on EHV-1 gene 12- and ICP27 gene-containing cell lines. Growth curves of the indicated viruses on cell lines containing either the gene for ICP27 or the gene for ICP27 and EHV-1 gene 12 are shown.
EHV-1 gene 12 is not packaged into HSV virions.
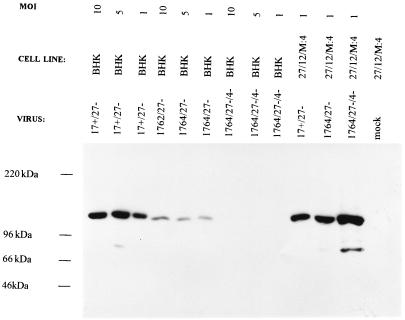
For EHV-1 gene 12 to be useful in cell lines for the growth of vmw65-deficient HSV for vector purposes, it is important that EHV-1 gene 12 cannot be packaged into HSV virions, since if this were the case, the advantages of reduced HSV IE gene expression in target cells in potentially reducing the cytotoxicity of such vectors would be minimal. To test whether EHV-1 gene 12 could be packaged into HSV virions, two experiments were performed. First, one-step growth curves were determined. HSV vmw65 mutants were plated at a low MOI onto nonengineered BHK cells. Growth was identical whether or not virus stocks had previously been prepared on EHV-1 gene 12-containing cells or nonengineered BHK cells (data not shown). If EHV-1 gene 12 was packaged, a growth advantage would have been expected here. Second, Western blotting of virus samples was performed in which vmw65 and vmw65/ICP27 mutants were grown on either BHK cells or cells containing EHV-1 gene 12. These were probed with either an anti-HSV vmw65 antibody or an anti-EHV-1 gene 12 antibody in comparison to a purified EHV-positive control. This showed a strong anti-vmw65 signal in all cases with HSV or the various HSV vmw65 mutants but no signal for EHV-1 gene 12, other than in the EHV-1 positive-control lane, independently of the cell line on which the viruses were prepared (Fig. 6). Thus, EHV-1 gene 12 is not packaged into HSV virions even though it can functionally compensate for growth deficiencies caused by mutations affecting the transactivating activity of vmw65. vmw65 is detectable in these blots, as the in1814 mutation used is a linker insertion mutation which produces a protein that is capable of fulfilling its essential structural role yet is incapable of transactivating IE gene expression.
FIG. 6.
The EHV-1 gene 12 protein is not packaged into HSV virions. Western blots resulting from polyacrylamide gel electrophoresis of partially purified preparations of each of the viruses indicated grown on the cell lines indicated and probed with either an anti-HSV-1 vmw65 or an anti-EHV-1 gene 12 antibody are shown. Purified EHV-1 was used as a positive control. Viruses were partially purified by freeze-thaw treatment, followed by low-speed centrifugation to remove cell debris and high-speed centrifugation of the resulting supernatants. Electrophoresis was performed on these resuspended high-speed pellets at ≈105 PFU of virus per lane.
Promoter choice for IE gene expression is important for cell lines containing EHV-1 gene 12 which also complement multiple HSV IE gene deficiencies.
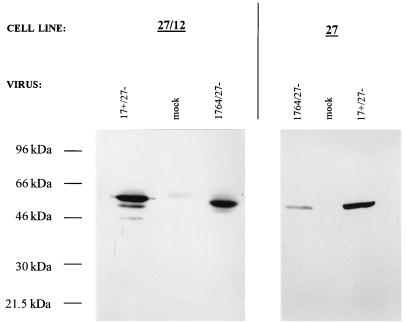
We were interested in generating cell lines capable of allowing the effective growth of viruses with vmw65 deficiencies and in which the genes for both ICP27 and ICP4 were also deleted, as such viruses might be expected to be minimally toxic in noncomplementing cells due to anticipated minimal IE gene expression (see introduction). We have found as described above, that the ICP27 promoter driving ICP27 provides effective cell lines complementing viruses with the gene for ICP27 deleted whether or not the cells contain EHV-1 gene 12. Thus, low-level gene 12 expression does not appear to significantly induce the expression of ICP27, which would be expected to be toxic, thus preventing the stable production of such cells. This could be because EHV-1 gene 12 does not significantly transactivate the ICP27 gene promoter (see earlier), but after virus infection, ICP27 expression, like EHV-1 gene 12 expression, is induced, allowing virus growth. This was confirmed by Western blotting of ICP27 and EHV-1 gene 12-containing cells before and after infection with ICP27- and ICP27/vmw65-deficient viruses (Fig. 7). ICP27 induction on cells containing ICP27 alone was also tested. The gene for ICP27 is completely deleted from these viruses, so no ICP27 can be expressed and detected from the incoming virus. After probing with an anti-ICP27 antibody, only minimal ICP27 levels could be detected before virus infection in both cases. These levels were greatly increased 24 h after infection with both ICP27- and ICP27/vmw65-deficient viruses in ICP27- and EHV-1 gene 12-containing cells and with ICP27-deficient virus on ICP27-containing cells but with a considerably smaller increase in ICP27-containing cells infected with the ICP27/vmw65-deficient virus. Thus, both ICP27 gene expression and higher-level EHV-1 gene 12 expression (Fig. 4) are induced by virus infection of ICP27 gene- and EHV-1 gene 12-containing cells, possibly following transactivation of ICP0 expression from the virus by the initial low-level expression of EHV-1 gene 12. Moreover, a deficiency in the induction of ICP27 in non-EHV-1 gene 12-containing cells with the ICP27/vmw65-deficient virus, as would be expected, is evident.
FIG. 7.
Virus infection induces expression from the ICP27 promoter in ICP27-containing cells. Shown is a Western blot in which extracts 24 h postinfection of either ICP27-containing or ICP27- and EHV-1 gene 12-containing cells, either mock infected or infected with the indicated viruses (MOI = 5), were probed with an anti-ICP27 antibody.
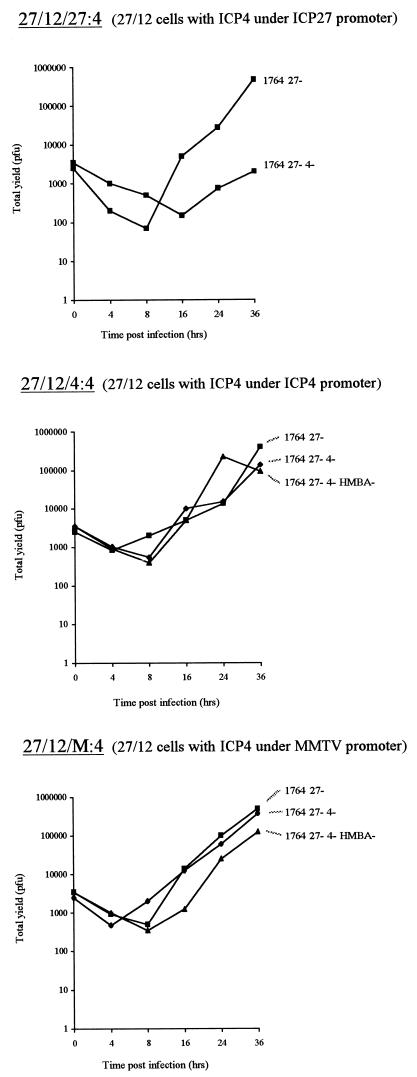
Thus, from the above-described results we anticipated that the ICP27 promoter might also provide optimal regulation of ICP4 in cells complementing vmw65, ICP27, and ICP4, the ICP27 promoter not being responsive to the EHV-1 gene 12 also present at low levels in the cell but apparently being responsive to virus infection. Hence, cell lines were produced in which ICP4 under ICP27 promoter and poly(A) control in a plasmid encoding phleomycin resistance was transfected into cells which already effectively allowed the propagation of viruses which lacked ICP27 and were deficient in vmw65 (cell line B5 described above). Phleomycin- and neomycin-resistant colonies were picked and cloned out. However, these were generally found to give only very poor growth of HSV mutants deficient in vmw65, ICP27, and ICP4 (see Table 1), with only 5 of the 140 colonies picked giving significant growth, and even this growth was limited (Fig. 8 shows virus growth on the best of these cell lines, called 27/12/27:4 cells).
TABLE 1.
Percentages of BHK clones capable of complementing ICP4 deficiencies in ICP27/ICP4/vmw65-deficient viruses when ICP4 was driven by various promoters
| Promoter driving ICP4 | No. of clones picked | No. giving significant growth | % Giving significant growth |
|---|---|---|---|
| ICP27 | 140 | 5 | 3.5 |
| ICP4 | 138 | 2 | 1.4 |
| MMTV | 88 | 60 | 68 |
FIG. 8.
Growth of HSV mutants on cell lines containing EHV-1 gene 12, ICP27, and ICP4. Shown are growth curves of the indicated viruses grown on cell lines containing ICP27 and EHV-1 gene 12 with ICP4 under either ICP27, ICP4, or MMTV promoter control. HMBA was included in the medium during virus growth, except where indicated otherwise.
After obtaining these disappointing results, we decided to test other promoters driving ICP4 since the ICP27 promoter, for unexplained reasons, provided inappropriate regulation of ICP4. Thus, further phleomycin- and neomycin-resistant cell lines were produced in which ICP4 was driven either by the ICP4 promoter and poly(A) or by the dexamethasone-inducible MMTV promoter and a simian virus 40 poly(A). It was hoped that either correct regulation of ICP4 expression by the ICP4 promoter or dexamethasone-inducible ICP4 expression might provide cell lines capable of improved growth of viruses deficient in vmw65, ICP27, and ICP4. The MMTV promoter was used, as well as the ICP4 promoter, in these experiments, as the low levels of EHV-1 gene 12 already expressed in the cells might be expected to stimulate the ICP4 promoter (see earlier), generating toxic levels of ICP4. It was hoped that this would not be the case if the MMTV promoter was used.
We picked 138 and 88 clones by using the ICP4 and MMTV promoters, respectively, and analyzed the virus growth characteristics (Table 1). Of the ICP4 promoter-driven clones, the majority were of only limited permissivity for vmw65/ICP27/ICP4-deficient viruses, although two clones were capable of efficient growth. One of these was selected for further study (27/12/4:4 cells). It was thought that this variability probably reflected positional effects altering the regulation of the ICP4 promoter in the context of EHV gene 12-expressing cells, in some rare cases allowing efficient growth of vmw65/ICP27/ICP4-deficient viruses. However, of the 88 clones picked in which ICP4 expression was controlled by the MMTV promoter, 60 grew ICP4-deficient viruses efficiently (initially with the inclusion of dexamethasone in the medium at the time of inoculation—see below), at least as well as on the two ICP4 promoter-containing cell lines described above. This indicated that with the MMTV promoter, positional effects are of minimal importance for effective ICP4 regulation in the context of EHV gene 12-containing cell lines, unlike when the ICP4 promoter is used. Again, one clone was selected for further work (27/12/M:4 cells). Figure 8 also shows one-step growth curves resulting from the growth of vmw65/ICP27/ICP4-deficient viruses and vmw65/ICP27-deficient viruses on the best of each of these types of cell line at an MOI of 0.01 (i.e., also on 27/12/4:4 and 27/12/M:4 cells). At higher, more optimal MOIs, 106 to 107 PFU/ml can usually be harvested from the culture medium by using the cell lines shown, in which ICP4 expression is driven by either the ICP4 or the MMTV promoter.
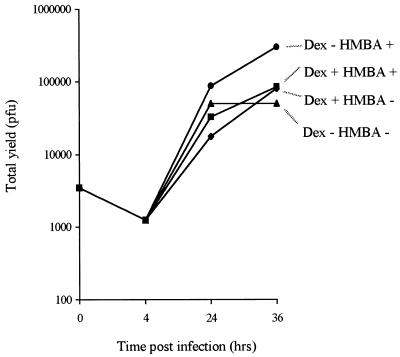
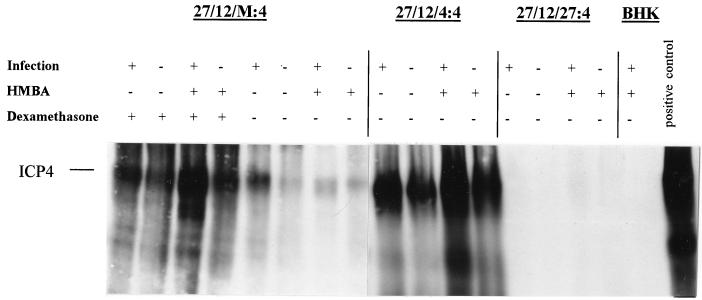
Interestingly, inclusion of dexamethasone in the medium at the time of inoculation using cells containing ICP4 under MMTV promoter control did not increase the yield of vmw65/ICP27/ICP4-deficient viruses, suggesting that the MMTV promoter, like the ICP27 promoter, is also responsive to virus infection, but here in a fashion appropriate to allow effective virus growth. The greatest yield of vmw65/ICP27/ICP4-deficient virus was obtained in the presence of HMBA but without dexamethasone (Fig. 9). To confirm and examine the responsiveness of ICP4 expression levels to virus infection, HMBA, and dexamethasone (for 27/12/M:4 cells), Western blotting was performed with each of the cell lines with and without virus infection and with and without HMBA and dexamethasone (for 27/12/M:4 cells; Fig. 10). This showed only very low levels of ICP4 in 27/12/27:4 cells (in which vmw65/ICP27/ICP4-deficient viruses grow poorly) and higher virus- and HMBA-inducible levels of ICP4 in 27/12/4:4 and 27/12/M:4 cells. Thus, in both of these cell lines, ICP4 levels are constitutively relatively low but expression is stimulated by virus infection and/or HMBA. Dexamethasone also induced ICP4 in 27/12/M:4 cells, but as discussed above, this did not further enhance virus growth. Thus, in the context of EHV-1 gene 12-containing cells, the MMTV promoter appears to be generally induced by virus infection in a position-independent manner (many clones capable of producing virus growth were obtained) and this induction provides optimal ICP4 regulation for virus growth. The ICP4 promoter, on the other hand, can provide virus-inducible expression appropriate for virus growth, but this is probably in a considerably more position-dependent fashion, as such clones effective at producing virus growth were only rarely obtained.
FIG. 9.
Effects of dexamethasone (Dex) and/or HMBA on the growth of an HSV-1 mutant deficient in ICP27, ICP4, and vmw65 (1764/27−/4−/pR20.5) in 27/12/M:4 cells.
FIG. 10.
Inducibility of ICP4 expression levels by virus infection in ICP4-containing cells. Shown are Western blots probed with an anti-ICP4 antibody following growth of an HSV-1 mutant deficient in ICP27, ICP4, and vmw65 (1764/27−/4−/pR20.5) in the cells indicated and under the conditions indicated at an MOI of 5, 48 h postinfection. The positive control was growth of an ICP4-containing virus strain (1764/27−/pR20) on BHK cells. After long exposure, low-level ICP4 could also be detected in the infection-positive or HMBA 27/12/27:4-positive lanes, although it is not evident in this exposure.
Western blot assays were also performed on BHK and 27/12/M:4 cells infected with either the ICP27-deficient virus, the ICP27/ICP34.5/vmw65-deficient virus, or the ICP27/ICP4/ICP34.5/vmw65-deficient virus and probed with an anti-ICP0 antibody (Fig. 11). This showed that while the ICP27-deficient virus gave significant levels of ICP0 expression in noncomplementing (BHK) cells, the virus deficient in ICP27, ICP34.5, and vmw65 gave only low-level ICP0 expression under such circumstances, while the virus with ICP4 also deleted gave detectable ICP0 expression only after long exposure of Western blots (and thus, ICP0 is not visible in Fig. 11), even at a high MOI. Thus, the vmw65 mutation appears to reduce ICP0 expression levels, at least for the most disabled virus, to minimal levels in noncomplementing cells. However, on 27/4/M:4 cells, which are highly permissive for the growth of the fully disabled virus and which contain EHV-1 gene 12, as well as ICP4 and ICP27, ICP0 is produced in high abundance, presumably following transactivation of the ICP0 promoter by EHV-1 gene 12.
FIG. 11.
Induction of ICP0 expression from vmw65-deficient viruses during growth on MMTV:4 cells. Shown is a Western blot of 27/12/M:4 cell extracts (24 h postinfection) probed with an anti-ICP0 antibody following growth of the indicated viruses at an MOI of 1 in comparison to extracts of BHK cells (in which virus growth is not possible) inoculated with the various viruses at a range of MOIs. ICP0 is not detectable in BHK cells inoculated with the 1764/27−/4− virus but is abundantly expressed in 27/12/M:4 cells.
Thus, taking Fig. 7, 10, and 11 together, a model of IE gene regulation following virus infection of 27/12/M:4 cells would envisage that in the absence of virus infection, EHV-1 gene 12 is expressed weakly from the CMV promoter and that only low levels of ICP27 and ICP4 are produced in the cell. Following infection with an ICP4/ICP27/vmw65 mutant virus, EHV-1 gene 12 transactivates ICP0 expression from the virus which, in turn, activates further EHV-1 gene 12 expression and the ICP27 and MMTV promoters.
DISCUSSION
Herpesviruses other than HSV have been shown in a number of studies to encode homologues of vmw65 (e.g., in EHV-1, varicella-zoster virus, and bovine herpesvirus 1) (3, 8, 29) which, as in HSV, are virion proteins that, in complex with host factors, transactivate viral IE gene promoters soon after virus infection. It has also been shown that for EHV-1 and -4 at least, such proteins can effectively transactivate IE gene promoters from heterologous herpesviruses even though consensus binding sites (TAATGARAT-like motifs) are somewhat different between the different viruses and the level of sequence similarity between the proteins from the different viruses is not particularly high (3, 12, 23). These were thus somewhat surprising findings at the time.
We have extended this work and found that in the case of EHV-1 gene 12, not only can the transactivating activity on HSV IE promoters be demonstrated in cotransfection experiments but the EHV-1 gene 12 protein can also fully substitute for the vmw65 transactivating activity during virus growth. This provides a convincing demonstration that even though the EHV-1 gene 12 and vmw65 proteins have considerably divergent amino acid sequences, they can still perform a fully homologous transactivating function in the virus life cycle which extends to the ability to substitute for the heterologous protein in virus growth. Thus, even though the protein and consensus binding sequences have diverged considerably during evolution, the necessity to retain the ability to interact with very similar host factors in each case has probably led to the generation of proteins that are very different yet can still functionally substitute for one another in virus growth. As well as the lack of sequence similarity between the proteins (34% identical at the amino acid level, 46% identical at the DNA level; University of Wisconsin Genetics Computer Group GAP program), the large differences between the proteins is further demonstrated by the fact that they differ completely in functional layout (17). Thus, while the transactivating and DNA binding activities of vmw65 can be localized to specific C- and N-terminal domains, respectively (33, 41), such activities cannot be separated in EHV-1 gene 12, where the whole protein is required in each case (17).
However, while we have found that EHV-1 gene 12 can substitute for transactivation by vmw65 during the growth of HSV, gene 12 does not appear to be packaged into HSV particles and thus cannot perform the essential structural role of vmw65 in HSV. This result also confirms that growth deficiencies in HSV-1 containing the in1814 mutation are indeed due to the deficiency in transactivation associated with the in1814 mutation rather than due to an effect on the structural function of vmw65, which was otherwise formally possible (30). Thus, if a structural effect were the case, growth deficiencies associated with the in1814 mutation could not be overcome by EHV-1 gene 12, as it is not packaged and thus cannot structurally substitute for vmw65.
All of the above information, while of interest in the functional comparison of herpesvirus proteins, also provides a novel means by which the propagation of HSV vector viruses can be improved. Thus, for use as a vector, for which HSV has a number of potential advantages, particularly in the nervous system, the virus has to be disabled so that it is no longer pathogenic and, moreover, is minimally cytotoxic. A number of studies have shown that minimal cytotoxicity can probably only be obtained when IE gene expression is minimized (20–22, 35, 43), preventing toxicity from these highly cytotoxic proteins themselves, and also preventing the expression of most of the ≈80 other genes in the HSV genome. Mutations of vmw65 can greatly reduce IE gene expression (1, 39), and these mutations may be particularly attractive in vectors when one or more essential IE genes have also been deleted (see introduction). However, such viruses are highly compromised for growth, even when the essential IE gene defect is otherwise effectively complemented. Moreover, vmw65 cannot be expressed from the cell line for virus growth, as this would (i) be packaged and (ii) quickly repair the defect in the virus by homologous recombination. Thus, while HMBA can improve growth characteristics to some extent (25), complementation of vmw65 provides a problem.
We have shown that EHV-1 gene 12 can compensate for vmw65 transactivation deficiencies when vmw65 is mutated alone or together with the deletion of ICP27 and/or ICP4, the two essential IE genes. EHV-1 gene 12 is not packaged into HSV virions, and recombinational repair of the vmw65 mutation in vector viruses is not possible by homologous means due to the only minimal sequence similarity between EHV-1 gene 12 and vmw65 generally and as EHV-1 gene 12 does not encode any sequence similar to the region altered in vmw65.
We have also defined the parameters necessary for the efficient production of EHV-1 gene 12-containing cell lines containing ICP4 and/or ICP27 such that these proteins are only significantly expressed in response to virus infection. ICP4 and ICP27 would otherwise be expected to be cytotoxic, preventing the generation of such cell lines in a stable fashion. Here the virus-cell line combinations used have only minimal DNA sequence overlap (≈30 bp at one end of ICP4), and thus, recombinational repair of any of the deficiencies in the vector viruses is again minimized. Efficient growth of HSV with essential IE gene deletions and incorporation of vmw65 transactivation deficiencies was not previously possible and potentially provides advantages over deletion or inactivation of ICP27, ICP4, ICP0, and ICP22 individually. These are the four regulatory IE genes, and for optimally efficient growth of such disabled viruses, cell lines containing all of the genes would need to be produced, although ICP0 and ICP22 are not absolutely essential for virus growth. As each of these four IE genes is highly cytotoxic, such cell lines are hard to generate, and while cells containing ICP27, ICP4, and ICP0 have been produced (36), no cell line containing all four of these IE genes has been reported.
The virus-cell line combinations reported here may thus provide an alternative to the deletion or inactivation of all of the IE genes for the production of nontoxic HSV vectors and the concurrent problem of the generation of cell lines allowing their efficient growth. Other non-HSV vmw65 homologues may also be used in a similar way. Particularly when combined with deletions in other nonessential genes which are either virion proteins or would be expected to be expressed in the absence of IE gene expression (e.g., the virion host shutoff protein [vhs], ICP34.5, ORFP, or ICP6), such viruses might be anticipated to be minimally toxic and yet still be capable of efficient growth for vector stock production in vitro.
ACKNOWLEDGMENTS
Suzanne Thomas and Caroline Lilley contributed equally to this work.
We are grateful to Matt Grapes (Marie Curie Institute) for providing the EHV-1 gene 12-encoding plasmid and anti-vmw65 antibody; Chris Preston (MRC Institute of Virology), David Meredith (Leeds University), and Gretchen Caughman (Medical College of Georgia) for providing HSV mutant in1814, purified EHV-1, and the anti-EHV-1 gene 12 antibody, respectively; and to Keith Howard, Jill Smith, and Barry Gibb for preparation of some of the viruses and cell lines used.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of α genes. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter D E, Misra V. Sequences of the bovine herpesvirus 1 homologue of herpes-simplex virus α-trans-inducing factor (UL48) Gene. 1992;119:259–263. doi: 10.1016/0378-1119(92)90280-3. [DOI] [PubMed] [Google Scholar]
- 4.Coffin R S, Latchman D S. Herpes simplex virus-based vectors. In: Latchman D S, editor. Genetic manipulation of the nervous system. London, England: Academic Press; 1995. pp. 99–112. [Google Scholar]
- 5.Coffin R S, Maclean A R, Latchman D S, Brown S M. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 1996;3:886–891. [PubMed] [Google Scholar]
- 6.Coffin R S, Thomas S K, Thomas D P, Latchman D S. The herpes simplex virus 2Kb latency associated transcript (LAT) leader sequence allows efficient expression of downstream proteins which is enhanced in neuronal cells: possible functions of LAT ORFs. J Gen Virol. 1998;79:3019–3026. doi: 10.1099/0022-1317-79-12-3019. [DOI] [PubMed] [Google Scholar]
- 7.Coffin R S, Thomas S K, Thomas N S B, Lilley C E, Pizzey A R, Griffiths C H, Gibb B J, Wagstaff M J D, Inges S J, Binks M H, Chain B M, Thrasher A J, Rutault K, Latchman D S. Pure populations of transduced primary human cells can be produced using GFP expressing herpes virus vectors and flow cytometry. Gene Ther. 1998;5:718–722. doi: 10.1038/sj.gt.3300634. [DOI] [PubMed] [Google Scholar]
- 8.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 9.Deluca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deluca N A, Schaffer P A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987;15:4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar R, McClements W L, Enquist L W, Vande Woude G F. Nucleotide sequence of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci USA. 1980;77:3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot G, O’Hare P. Equine herpesvirus 1 gene 12, the functional homologue of herpes simplex virus VP16, transactivates via octamer sequences in the equine herpesvirus IE gene promoter. Virology. 1995;213:258–262. doi: 10.1006/viro.1995.1568. [DOI] [PubMed] [Google Scholar]
- 13.Fink D J, Deluca N A, Goins W F, Glorioso J C. Gene transfer to neurons using herpes simplex virus vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 14.Gelman I H, Silverstein S. Herpes simplex virus immediate-early promoters are responsive to virus and cell trans-acting factors. J Virol. 1987;61:2286–2296. doi: 10.1128/jvi.61.7.2286-2296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein D J, Weller S K. Herpes simplex virus type-1-induced ribonucleotide reductase-activity is dispensable for virus growth and DNA-synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman C M. High efficiency gene transfer into mammalian cells. In: Glover D M, editor. DNA cloning, a practical approach. Oxford, England: IRL Press; 1985. pp. 143–190. [Google Scholar]
- 17.Grapes M, O’Hare P. Abstracts of the 22nd International Herpesvirus Workshop 1998. 1998. Interaction between the N- and C-terminal domains of the HSV-1 regulatory protein VP16-H suggested by studies of VP16-H and its EHV-1 homologues, abstr. 67; p. 93. [Google Scholar]
- 18.Ho D Y, Mocarski E S. β-Galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988;167:279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 19.Howard M K, Kershaw T, Gibb B, Storey N, Maclean A R, Zeng B-Y, Tel B C, Jenner P, Brown S M, Woolf C J, Anderson P N, Coffin R S, Latchman D S. High efficiency gene transfer to the central nervous system of rodents and primates using herpes virus vectors lacking functional ICP27 and ICP34.5. Gene Ther. 1998;5:1137–1147. doi: 10.1038/sj.gt.3300700. [DOI] [PubMed] [Google Scholar]
- 20.Johnson P A, Wang M J, Friedman T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson P A, Miyanohara A, Levine F, Cahill T, Friedman T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisky D M, Wolfe D, Goins W F, Marconi P C, Ramakrishnan R, Mata M, Rouse R J D, Fink D J, Glorioso J C. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 23.Lewis J B, Thompson Y G, Feng X, Holden V R, O’Callaghan D, Caughman G B. Structural and antigenic identification of the ORF12 protein (αTIF) of equine herpesvirus 1. Virology. 1997;230:369–375. doi: 10.1006/viro.1997.8477. [DOI] [PubMed] [Google Scholar]
- 24.MacLean C A, Efstathiou S, Elliott M L, Jamieson F E, McGeoch D J. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J Gen Virol. 1991;72:897–906. doi: 10.1099/0022-1317-72-4-897. [DOI] [PubMed] [Google Scholar]
- 25.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes-simplex virus immediate-early gene expression in the absence of trans-induction by vmw65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 26.Palella T D, Silverman L J, Schroll C T, Homa F L, Levine M, Kelley W M. Herpes simplex virus-mediated human hypoxanthine-guanine phosphoribosyltransferase gene transfer into neuronal cells. Mol Cell Biol. 1988;8:457–460. doi: 10.1128/mcb.8.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellet P E, McKnight J L C, Jenkins F J, Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing α genes. Proc Natl Acad Sci USA. 1985;82:5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Post L E, Roizman B. A generalised technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus type 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 29.Purewal A S, Allsopp R, Riggio M, Telford E A R, Azam S, Davison A J, Eddington N. Equid herpesvirus-1 and herpesvirus-4 encode functional homologs of herpes-simplex virus type-1 virion transactivator protein, VP16. Virology. 1994;198:385–389. doi: 10.1006/viro.1994.1047. [DOI] [PubMed] [Google Scholar]
- 30.Roizman R, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 31.Sacks W R, Greene C C, Aschman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadowski I, Ma J, Treizenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 34.Samaniego L A, Webb A L, Deluca N A. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samaniego L A, Neiderhiser L, Deluca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samaniego L A, Wu N, Deluca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears A E, Halliburton I W, Meigner B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekulovich R E, Leary K, Sandri-Goldin R M. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smiley J R, Duncan J. 1814 linker insertion mutation. J. Virol. 71:6191–6193. 1997. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 produces a phenotype similar to that of the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leeds to osteopretosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 41.Stern S, Herr W. The herpes simplex virus transactivator VP16 recognises the Oct-1 homeo domain: evidence for a homeodomain recognition subdomain. Genes Dev. 1991;5:2555–2566. doi: 10.1101/gad.5.12b.2555. [DOI] [PubMed] [Google Scholar]
- 42.Stow N D, Stow E C. Isolation and characterisation of a herpes simplex type 1 mutant containing a deletion in the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 43.Wu N, Watkins S C, Schaffer P A, Deluca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]