Abstract
Hypothesis
The reverse shoulder arthroplasty, as introduced by Grammont, has had many modifications over time. One of these modifications was reducing the neck-shaft angle (NSA) from 155 degrees to 135 degrees. Biomechanical studies indicated that lowering the NSA increases external rotation and reduces abduction and the incidence of scapular notching. The purpose of this study was to compare range of motion, functional outcome measures, and complications in patients undergoing reverse shoulder arthroplasty, depending on the NSA, through a systematic review and meta-analysis.
Methods
A literature search was conducted (articles published from January 1985 to January 2020) in the PubMed/MEDLINE, Embase, and CINAHL databases and the Cochrane library. All studies reporting outcomes after primary reverse shoulder arthroplasty for osteoarthritis and rotator cuff–related disease were included. Patients were divided into 2 groups: a medialized design (MD) with an NSA of 150-155 degrees and a lateralized design (LD) with an NSA of less than 150 degrees. Pooled effects were calculated in the form of mean differences and 95% confidence intervals (CIs). Risk of bias was assessed using the Risk Of Bias In Non-Randomized Studies - of Interventions tool for non-Randomized Controlled Trials and the Risk Of Bias 2 tool for Randomized Controlled Trials.
Results
A total of 21 studies and 3134 arthroplasties were included: 1366 with an MD and 1678 with an LD. The mean age was 73.0 years (MD 74.0 and LD 72.5). A direct comparative meta-analysis was not feasible, and therefore, all data were compared using the minimal clinically important difference. The MD group demonstrated a larger improvement in abduction (56.76°, 95% CI 37.03-76.49) than the LD group (48.52°, 95% CI 28.27-68.78), however the LD group demonstrated a larger improvement in external rotation with the arm at the side (MD: 7.69°, 95% CI 0.01-15.37; LD: 16.14° 95% CI 7.18-25.09). When looking at the postoperative range of motion, the MD group had more abduction than the LD group (MD: 136.28°, 95% CI 127.36-145.20; LD: 127.77° 95% CI 117.02-138.52). Both designs had a comparable improvement in the Constant Murley score (MD 42.04 points, LD 41.14 points). Lowering the NSA was accompanied by a decrease in dislocation rate (MD: 4.6%; LD: 1.4%; P value .037) and notching rate (MD: 40.3%; LD: 17.3%; P value <.0001).
Conclusion
In our analysis, lowering the NSA decreases the amount of abduction but increases the amount of external rotation. This change in range of motion is accompanied by less scapular notching and dislocations. There is no clear impact on functional outcome measures.
Keywords: Reverse shoulder arthroplasty, meta-analysis, neck-shaft angle, rotator cuff arthropathy, scapular notching, dislocation, biomechanical, range of motion
Since the introduction of the reverse shoulder arthroplasty (RSA) by Neer in the seventies, indications for RSA have continued to expand.17 Nowadays, RSA is a common treatment option for numerous conditions including rotator cuff tear arthropathy (CTA), proximal humeral fractures, and rheumatoid arthritis. The original design by Neer comprised a type of arthroplasty with a center of rotation positioned more medially and distally compared with other designs.1 These early implant designs were unsuccessful owing to excessive constraint, leading to high rates of loosening and poor functional outcomes.55 In 1985, Grammont et al23 introduced a new concept with the center of rotation residing within the glenoid neck. In accordance with the design by Neer, the center of rotation was moved more medially and distally by using a hemispherical glenosphere and placing it more inferiorly on the glenoid. In later alterations, the glenosphere was changed from a two-thirds radius to a hemisphere to medialize the center of rotation even further.17 Therefore, this design is also referred to as a medialized design (MD).7
Nowadays, many surgeons consider the design by Grammont et al as being the gold standard. Nevertheless, this design has certain drawbacks, such as scapular notching.49 To address this problem, manufacturers reduced the neck-shaft angle (NSA) from 155 degrees in the original design to as low as 135 degrees. These newer designs are also referred to as lateralized designs (LD).7 Biomechanical studies indicated that lowering the NSA improves range of motion (ROM) and reduces the incidence of scapular notching.7,19,34,59 These biomechanical studies suggest an increase in forward flexion, external rotation and adduction, as well as a decrease in abduction.7,32 On the other hand, it is postulated by some that the dislocation rate increases.2,16,45 However, this has not been confirmed in cadaveric or cohort studies.
Despite the widespread use of the original Grammont design as well as implants with a smaller NSA, there remains a paucity of comparative data on postoperative ROM, functional outcome measures, and complication rates. Consequently, the influence of the NSA on these outcomes is still debatable. To our knowledge, no meta-analyses have been published comparing the standard design of Grammont et al to a design with a lowered NSA.14,48
We hypothesized that the original design by Grammont et al results in reduced ROM and comparable functional outcome measures and complications, compared with a design with a smaller NSA. In this review, we want to provide a meta-analytical comparison of both designs.
Materials and methods
Protocol and registration
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses guidelines.40 The review was registered at our institutional review board.
Search strategy
We conducted a literature search in following databases: PubMed/MEDLINE, Embase, CINAHL, and the Cochrane library. “Humeral head inclination” and “reverse total shoulder arthroplasty” were used as key words. The search included all articles published before the first of January 2020. Only articles published after 1985 were included because this was the year the design by Grammont et al was first introduced.17 The reference lists of included articles were screened for studies of interest missed by the initial search.
Inclusion and exclusion criteria
Type of subjects
Studies were included if the patients had had a primary RSA for 1 of the following indications: CTA, rheumatoid arthritis and other forms of arthritis, primary glenohumeral osteoarthritis, and massive rotator cuff tear with or without pseudoparalysis.
Studies were excluded if the arthroplasty had 1 or more of the following indications: tumors, revision arthroplasties, acute proximal humeral fractures, postfracture sequelae, infections, locked anterior dislocation and chronic dislocation, and osteochondrodysplasia. Studies with a mixed study population, containing the aforementioned exclusion criteria, were excluded. Revision, fractures, and fracture sequelae were excluded based on available literature reporting worse functional outcomes and higher complication rates.28,47
Type of interventions
All studies using a design derived from the original design by Grammont et al were included. Short-stem arthroplasty and other designs (eg, Kessel arthroplasty) were therefore excluded.10,39 Patients with previous rotator cuff repair or shoulder-stabilizing surgery (both open and arthroscopic) were included. Arthroplasty with glenoid grafting for glenoid bone defect and arthroplasty with bony increased offset (BIO) were not seen as a confounder and were therefore included.8
Tendon transfers (eg, latissimus dorsi transfer) were seen as a confounder and excluded from the analysis. Studies were excluded if the NSA of the used arthroplasty was blinded.
Study characteristics
We included studies in English, German, and Dutch language. Studies were included if the follow-up had a minimal duration of 24 months. In case of longer follow up, the data with the largest amount of arthroplasties were included. Level I up to level IV studies were eligible for inclusion.
Meta-analyses, systematic reviews, clinical practice guidelines, medical conference abstracts, letters to the editor, cadaveric studies, biomechanical studies, surgical technique descriptions, and case reports were excluded.
Outcome measures
The primary outcome measure is the ROM. ROM, except for internal rotation with the arm at the side, had to be reported as degrees of active motion. Internal rotation with the arm at the side had to be reported either in degrees or as the highest vertebral level that could be reached by the thumb. ROM had to be measured by a physician or independent researcher. The following planes of motion were recorded: forward flexion (FF), external rotation with the arm at the side (ER), external rotation with the arm in 90 degrees of abduction (ERA), internal rotation with the arm at the side (IR), internal rotation with the arm in 90 degrees of abduction (IRA), and abduction (AB).
Complications and functional outcome measures were recorded as secondary outcome measures. Complications included the following: scapular notching, infection, dislocation, aseptic loosening, radiological lucencies around the glenoid component, radiological lucencies around the humeral component, neuropraxia, and periprosthetic fractures (both traumatic and stress type). Functional outcome measures included the following: the Constant-Murley score, the American Shoulder and Elbow Surgeons scoring system, the Simple Shoulder Test, the visual analogue scale for pain, the Disabilities of the Arm Shoulder and Hand score, the Subjective Shoulder Value, the Western Ontario Osteoarthritis of the Shoulder index, the Oxford Shoulder Score, and the University of California Los Angeles shoulder score.4,13,21,31,35,37
Study selection
The online search was performed independently by 2 reviewers (H.G. and V.G.). In case of doubt about inclusion, authors 5 and 6 (V.B. and O. LH.) were consulted. During primary identification, title and abstract were checked for reverse-type arthroplasty, presence of primary and secondary outcome measures, and type of study. These articles were then cross-referenced for duplicates. After primary identification, author one (L.H.) screened title and abstract for inclusion and exclusion criteria. If the abstract was not clear, the entire text would be screened for the outcome of interest. The results of the screening and reasons for exclusion were registered and checked by author 2 (N.S.). In case of doubt, authors 5 and 6 would be consulted. If the study did not mention the primary outcome of interest (ROM in any plane), it was excluded from further analysis. After screening, full-text screening was conducted by authors one and 2 to check for eligibility. In case of disagreement, authors 5 and 6 would be consulted. Articles with overlapping study populations were checked for eligibility, and only the best suitable article would be included. In case of missing data, the author would be contacted for more information. If neither the article nor author could provide the necessary data, then the article would be excluded from further analysis.
Risk of bias
For determination of risk of bias, the Risk Of Bias In Non-Randomized Studies - of Interventions tool by Cochrane was used for non-Randomized Controlled Trials (RCTs) and the Risk Of Bias 2 tool for RCTs.51,52 Both authors one and 2 assessed risk of bias, and in case of disagreement, author 5 and 6 were consulted. A more intense rehabilitation protocol was defined as a confounder and BIO and glenoid grafting as cointerventions. Risk of bias was classified as low, moderate, serious, or critical. Studies classified as critical would be excluded from the analysis. Those classified as serious were discussed by all the authors whether to include it in the final analysis.
Data collection and statistical methods
Data collection
All data were registered in an Excel database (Microsoft Excel, 2010) and exported for statistical analysis in Stata (StataCorp. 2015. Stata Statistical Software: Release 14; StataCorp LP, College Station, TX, USA) or SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, version 24.0.; IBM Corp., Armonk, NY, USA) as needed. For study eligibility, a data collection form was used based on the data collection form for RCTs and non-RCTs by Cochrane and adapted to our necessities.27
The following information was extracted from each study when available (1) overall eligibility and study characteristics (level of evidence, type of study, study design, location of the study, inclusion and exclusion criteria used, amount and types of patient groups, distribution of age, distribution of sex, and patients lost to follow-up), (2) intervention (amount of patients, amount of arthroplasties, type and manufacturer of the arthroplasty, goal of the study, inclusion period, duration of follow-up, amount of patients and/or arthroplasties per patient group, follow-up per patient group, and confounders), (3) outcomes (ROM as previously defined, type and amount of complications, type and score of functional outcome measures).
Statistical methods
We divided patients into 2 groups as per the type of arthroplasty: the MD group was defined as NSA of 150 degrees or more, and the LD group was defined as an NSA lower than 150 degrees. For primary and secondary effect measures, we conducted a subgroup analysis based on preoperative indication. A subgroup analysis was not performed if there was insufficient data or if the sample size was too small (less than 2 studies and/or forty patients).
A meta-analysis was performed using the mean difference using a random effects model for ROM and functional outcome measures. For ROM, the difference was calculated between both groups (ΔROM) to determine if the threshold for minimal clinically important difference (MCID) was reached.50 Cutoff values for the MCID are as follows: abduction 7 degrees, forward flexion twelve degrees, and external rotation 3 degrees. Complications were compared using a Chi-square of Fisher exact test as needed. Alpha level was set at 0.05. Continuous outcomes are presented as mean difference and 95% confidence intervals (CIs). Dichotomous outcomes are presented as percentage of patients and amount of patients compared with each respective group.
Heterogeneity was assessed using I2. An I2 less than 60% was used as a cutoff for homogeneity.27 Standard deviation was calculated from the CI if available. We did not impute standard deviation if no other estimate of variance was available besides confidence interval.
Results
Literature search
PubMed/MEDLINE, Embase, CINAHL, and the Cochrane library each yielded 1657, 19, 2, and 0 studies, respectively (Fig. 1).40 We identified 58 supplementary studies through screening of references, for a total of 1736 studies. Identification through screening of title and abstract excluded 1343 studies and another 232 studies after more thoroughly screening. One hundred sixty-one articles were assessed for eligibility, after which 125 more were excluded. Sixteen studies were excluded because of “other reasons,” for example, articles that dealt with anatomic shoulder arthroplasty. Twenty-two authors were contacted for further clarification, and 2 of them were able to provide the necessary data. Twelve articles were discussed between the first and second authors until they reached an agreement on inclusion or exclusion. Fifteen studies did not provide necessary data (eg, CI interval) for a quantitative analysis or was the author able to provide them and were therefore excluded. Eventually, 21 studies were included in the meta-analysis.
Figure 1.
Flowchart based on the PRISMA statement. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Study characteristics
The total amount of included arthroplasties was 3134: 1366 in the MD group and 1678 in the LD group (Table I). Mean follow-up was 43.8 months (range 24-110.4). Most data were collected approximately 24 months after surgery, with an exception of the study by Favard et al,15 where data were collected at least 5 years after surgery. The mean age was 73.0 years (range 66.2-77). Of the remaining 21 studies, 30.0% of the population was men. Mean follow-up was 46.0 months (range 24-110.4) in the MD group and 41.4 months (range 24-45) in the LD group, and mean age was 74.0 (range 66.2-75.8) in the MD group and 72.5 (range 71-74.7) in the LD group. In the respective groups, 34.5% and 30.2% of the patients were men.
Table I.
Study characteristics.
| Source | Level of evidence | Type of study | Study design | No. of arthroplasties (n) | No. of patients (n) | Follow-up (mo) | Age (yr) | Amount male (n) | Type of prosthesis |
|---|---|---|---|---|---|---|---|---|---|
| Favard (2011)15 | Level IV | Treatment study | Case series with no comparison group | 484 | 464 | 54 | 76,1 | 123 | Delta III Reverse Shoulder Prosthesis and Aequalis Tornier Reversed |
| Young (2011)57 | Level IV | Treatment study | Case series with no comparison group | 18 | 16 | 45,6 | 70,1 | 2 | Aequalis Tornier Reversed |
| Hattrup (2012)25 | Level IV | Treatment study | Case series with no comparison group | 19 | 16 | 37 | 70,0 | 5 | Delta III Reverse Shoulder Prosthesis and Trabecular Metal Reverse Shoulder System and Aequalis Tornier Reversed and Encore Reverse |
| Castricini (2013)11 | Level IV | Treatment study | Case series with no comparison group | 80 | 80 | 60 | 72,5 | 21 | Delta III Reverse Shoulder Prosthesis |
| Wiater (2014)54 | Level III | Treatment study | Retrospective comparative study | 101 | 101 | 24 | 71,9572,47∗ | 66 | Delta III Reverse Shoulder Prosthesis and Trabecular Metal Reverse Shoulder System and Aequalis Tornier Reversed |
| Morris (2015)41 | Level III | Treatment study | Retrospective comparative study | 68 | 68 | 37,68 | 71,3 68,5§ | 29 | Aequalis Tornier Reversed |
| Kiet (2015)30 | Level III | Treatment study | Retrospective comparative study | 53 | 53 | 25,2 | n/a | n/a | Trabecular Metal Reverse Shoulder System |
| Athwal (2015)5 | Level III | Treatment study | Case series with no comparison group | 40 | 40 | 34 | 74,0 | 17 | Aequalis Tornier Reversed |
| Rhee (2015)46 | Level III | Treatment study | Retrospective comparative study | 62 | 62 | 24 | 66,2 68,9† | 12 | Aequalis Tornier Reversed |
| Katz (2016)29 | Level IV | Treatment study | Case series with no comparison group | 140 | 134 | 45 | 72,0 | 34 | Arrow Prime Reverse |
| McFarland (2016)36 | Level IV | Treatment study | Case series with no comparison group | 42 | 42 | 36 | 71,0 | 19 | Encore Reverse |
| Bacle (2017)6 | Level IV | Treatment study | Case series with no comparison group | 191 | 186 | 39,9 | 75,0 | 41 | Delta III Reverse Shoulder Prosthesis and Aequalis Tornier Reversed |
| Müller (2018)42 | Level III | Treatment study | Retrospective comparative study | 89 | 86 | 60 | 73,8 71,5‖ | 6 | SMR Reverse Shoulder System |
| Merolla (2018)38 | Level III | Treatment study | Retrospective comparative study | 74 | 74 | 24 | 75,8 74,7‡ | 10 | Aequalis Reversed II and Aequalis Ascend Flex Convertible Shoulder System |
| Boutsiadis (2018)9 | Level II | Prognosis study | Retrospective study | 46 | 46 | 39 | 77,0 | 9 | Aequalis Tornier Reversed and Aequalis Ascend Flex Convertible Shoulder System |
| Alentorn-Geli (2018)3 | Level III | Treatment study | Retrospective comparative study | 16 | 16 | 35,1 | 72,5 | 11 | Comprehensive Reverse Shoulder System |
| Friedman (2019)20 | Level II | Prognosis study | Retrospective study | 1332 | 1332 | 44,5 | 72,2 | 475 | Equinoxe Reverse System |
| Oh (2019)44 | Level III | Treatment study | Retrospective comparative study | 80 | 80 | 31,4 | 72,1 | 15 | Comprehensive Reverse Shoulder System |
| Franceschetti (2019)18 | Level III | Treatment study | Retrospective comparative study | 59 | 59 | 24,9 | 69,9 | 22 | Aequalis Ascend Flex Convertible Shoulder System |
| Van Ochten (2019)53 | Level IV | Treatment study | Case series with no comparison group | 27 | 27 | 110,4 | 73 | 1 | Delta III Reverse Shoulder Prosthesis |
| Gobezie (2019)22 | Level I | Treatment study | Randomized controlled trial | 68 | 68 | 38 | 73 | 23 | Univers Revers |
Cemented vs. uncemented cohort.
Cohort with 20 degrees of retroversion vs. zero degrees of retroversion.
Aequalis Reversed II cohort vs. Aequalis Ascend Flex cohort.
Cohort with no previous opioid abuse vs. with previous opioid abuse.
Cohort with 36-mm glenosphere vs. 44-mm glenosphere.
The studies by Boutsiadis et al9 and Friedman et al20 were Level II prognosis studies (Table II). We included 1 level I treatment study by Gobezie et al.22 All other studies were treatment studies based on retrospective cohort analyses of prospectively collected databases. The overall level of evidence was III or IV with an exception of those by Boutsiadis et al, Friedman et al, and Gobezie et al.
Table II.
Type of arthroplasty. Type, manufacturer, and inclination of included arthroplasties.
| Group MD | NSA (°) | No. of arthroplasties (N = 1366) | Group LD | NSA (°) | No. of arthroplasties (N = 1768) |
|---|---|---|---|---|---|
| Delta III Reverse Shoulder Prosthesis (DePuy) | 155 | 750 | Comprehensive Reverse Shoulder System (Zimmer-Biomet) | 147 | 96 |
| Aequalis Tornier Reversed (Wright Medical) | 155 | 336 | Encore Reverse (DJO Surgical) | 135 | 44 |
| Aequalis Reversed II (Wright Medical) | 155 | 36 | Arrow Prime Reverse (FH Ortho) | 135 | 140 |
| Trabecular Metal Reverse Shoulder System (Zimmer-Biomet) | 150 | 124 | Aequalis Ascend Flex Convertible Shoulder System (Wright Medical) | 145 | 119 |
| SMR Reverse Shoulder System (LimaCorporate) | 150 | 89 | Equinoxe Reverse System (Exactech) | 145 | 1332 |
| Univers Reverse (Arthrex) | 155 | 31 | Univers Revers (Arthrex) | 135 | 37 |
Risk of bias
The authors had a discrepancy in risk of bias in 6 studies. After discussion, a consensus was reached for all articles. Eight articles were classified as low risk of bias, eleven as moderate risk of bias, and 2 as a serious risk of bias. Most articles with a moderate risk of bias had either a risk of bias in outcome measurement or in the description of indications. The study by Katz et al29 had a serious risk of bias primarily because the design of the arthroplasty changed within the course of inclusion (bias due to deviations of intended interventions). The study did have an impact on complication rate, increasing the amount of glenoid loosening but was still included in the final analysis because there was no impact on our primary outcome (ROM). The study by Morris et al41 had a serious risk of bias because of irregularities causing a risk of bias in all fields. It was not excluded because it did not cause skewing of the outcome measures.
Primary and secondary outcome measures
There was a lack of studies with a direct comparison of both designs. Hence, a direct comparison of both primary and secondary outcome measures was not possible. Results were therefore calculated separately per design.
Range of motion
Individual outcomes for ROM are reported in Table III. In the MD group we found a statistically significant improvement of AB (56.76°), FF (71.30°), and ER (7.69°). No significant improvement of IRA (5.20°) and ERA (16.62°) was found (Table IV). In the LD group, we found a statistically significant progression in all reported planes of motion (FF 67.41°; AB 48.52°; ER 16.14°).
Table III.
Individual results – range of motion.
| Study | Sample size (n) | ER pre | ER post | FF pre | FF post | AB pre | AB post | ERA pre | ERA post | IRA pre | IRA post |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group MD | |||||||||||
| Favard et al. (2011)15 | 484 | 4.9 ± 17.6 | 10.6 ± 18.8 | 69.3 ± 34 | 128.6 ± 32.6 | N/A | N/A | 23.5 ± 23.3 | 42.1 ± 30.2 | N/A | N/A |
| Young et al. (2011)57 | 18 | 15 ± 26 | 19.7 ± 18.3 | 77.5 ± 30.7 | 138.6 ± 32.5 | N/A | N/A | 16.9 ± 23.3 | 46.1 ± 28.1 | N/A | N/A |
| Hattrup et al. (2012)25 | 17 | 23.8 ± 23.2 | 53.4 ± 28.2 | 72.4 ± 45.6 | 137.6 ± 33.7 | 63.8 ± 48.9 | 134.7 ± 40.7 | N/A | N/A | N/A | N/A |
| Castricini et al. (2013)11 | 80 | 17 ± 11 | 37 ± 37 | 100 ± 20 | 150 ± 29 | 81 ± 19 | 133 ± 35 | N/A | N/A | N/A | N/A |
| Wiater et al. (2014)54 (1 – Cemented) | 37 | 7.3 ± 21.3 | 19.7 ± 19.3 | 52.4 ± 25.1 | 119.3 ± 22 | N/A | N/A | N/A | N/A | N/A | N/A |
| Wiater et al. (2014)54 (2 – Uncemented) | 64 | 22.1 ± 23.5 | 26.5 ± 21 | 73.3 ± 33.2 | 131 ± 18.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Morris et al. (2015)41 (1 – No opioid abuse) | 36 | 9 ± 13 | 27 ± 16 | 43 ± 51 | 147 ± 29 | 42 ± 49 | 145 ± 30 | N/A | N/A | N/A | N/A |
| Morris et al. (2015)41 (2 – Opioid abuse) | 32 | 8 ± 16 | 32 ± 16 | 40 ± 37 | 142 ± 30 | 38 ± 36 | 126 ± 39 | N/A | N/A | N/A | N/A |
| Kiet et al. (2015)30 | 53 | N/A | 38 ± 23 | N/A | 136 ± 31 | N/A | 129 ± 34 | N/A | N/A | N/A | N/A |
| Athwal et al. (2015)5 | 40 | N/A | 23 ± 16 | N/A | 141 ± 14 | N/A | N/A | N/A | N/A | N/A | N/A |
| Rhee et al. (2015)46 (1 – 20 degrees of retroversion) | 30 | 46 ± 24.5 | 47.2 ± 8.4 | 65.3 ± 35.5 | 127.5 ± 22.8 | N/A | N/A | 58.5 ± 30.1 | 74.3 ± 14.3 | N/A | N/A |
| Rhee et al. (2015)46 (2 – 0 degrees of retroversion) | 32 | 44.7 ± 24.5 | 43.9 ± 7 | 71.1 ± 34.6 | 120.6 ± 29.6 | N/A | N/A | 58.8 ± 27.1 | 70.3 ± 18.7 | N/A | N/A |
| Bacle et al. (2017)6 | 191 | 9 ± 14 | 10 ± 16 | 81 ± 43 | 138 ± 26 | N/A | N/A | 39 ± 21 | 44 ± 25 | N/A | N/A |
| Müller et al. (2018)42 (1 – 36-mm glenosphere) | 33 | 24 ± 19 | 19 ± 17 | 83 ± 39 | 125 ± 30 | N/A | N/A | 41 ± 21 | 51 ± 25 | 31 ± 23 | 39 ± 17 |
| Müller et al. (2018)42 (2 – 44-mm glenosphere) | 35 | 29 ± 19 | 19 ± 24 | 87 ± 36 | 130 ± 18 | N/A | N/A | 29 ± 20 | 56 ± 21 | 25 ± 18 | 33 ± 14 |
| Merolla et al. (2018)38 (1 – Aequalis II Reversed) | 36 | 15 ± 13.3 | 30 ± 9.9 | 65 ± 31.9 | 142 ± 21.1 | 57 ± 29.4 | 131 ± 19.9 | N/A | N/A | N/A | N/A |
| Boutsiadis et al. (2018)9 (1 – Aequalis Tornier) | 13 | 14 ± 20 | 14 ± 13 | 63 ± 21 | 148 ± 7 | N/A | 134 ± 8.5 | N/A | N/A | N/A | N/A |
| Boutsiadis et al. (2018)9 (3 – Aequalis Tornier-BIO) | 11 | 5 ± 20 | 24 ± 12 | 74 ± 35 | 158 ± 4 | N/A | 145 ± 7 | N/A | N/A | N/A | N/A |
| Gobezie et al. (2019)22 (2 – 155 degrees NSA) | 31 | 29 ± 15 | 30 ± 14 | 69.3 ± 27.6 | 111.9 ± 28.6 | 76 ± 50 | 135 ± 17 | N/A | N/A | N/A | N/A |
| Group LD | |||||||||||
| Hattrup et al. (2012)25 | 2 | 20 ± 28.3 | 40 ± 14.1 | 80 ± 84.9 | 140 ± 28.3 | 80 ± 84.9 | 130 (42.4) | N/A | N/A | N/A | N/A |
| Katz et al. (2016)29 | 140 | 20 ± 25.8 | 29 ± 16.7 | 73 ± 31.6 | 132 ± 26.1 | 61 ± 26.8 | 108 (24.7) | 30 ± 25.8 | 54 ± 23.6 | N/A | N/A |
| McFarland et al. (2016)36 | 42 | 16.8 ± 11.6 | 19.2 ± 12.6 | 88.6 ± 36.3 | 116.6 ± 26.2 | 91.9 ± 33.8 | 116.2 (26.7) | 41.8 ± 23.6 | 63.1 ± 21.5 | 21.1 ± 28.9 | 23.9 ± 28.5 |
| Alentorn-Geli et al. (2018)2 | 16 | 10 ± 13.1 | 53.7 ± 34.4 | 86.8 ± 18.9 | 160 ± 22.5 | N/A | n/a | N/A | N/A | N/A | N/A |
| Merolla et al. (2018)38 (2 – Ascend Flex Reversed) | 38 | 0 ± 21.3 | 32 ± 23.1 | 83 ± 29.1 | 142 ±17.2 | 74 ± 26.5 | 131 (16.7) | N/A | N/A | N/A | N/A |
| Boutsiadis et al. (2018)9 (2 – Ascend Flex Reversed) | 10 | −8 ± 21 | 31 ± 13 | 53 ± 22 | 149 ± 8 | N/A | 134 (9) | N/A | N/A | N/A | N/A |
| Boutsiadis et al. (2018)9 (4 – Ascend Flex Reversed-BIO) | 12 | 14 ± 20 | 30 ± 16 | 80 ± 35 | 152 ± 8 | N/A | 129 (11) | N/A | N/A | N/A | N/A |
| Alentorn-Geli et al. (2018)3 | 16 | 10 ± 13.1 | 53.7 ± 34.4 | 86.8 ± 18.9 | 160 ± 22.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Friedman et al. (2019)20 | 1332 | 18 ± 21.8 | 36.1 ± 17.2 | 85.6 ± 38.1 | 141.7 ± 23.6 | 72.3 ± 34.6 | 118.5 ± 28.8 | N/A | N/A | N/A | N/A |
| Oh et al. (2019)44 (1 – individualized retroversion) | 52 | 37.3 ± 21.1 | 52.3 ± 15.9 | 97.5 ± 49.8 | 141.9 ± 14.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Oh et al. (2019)44 (2 – fixed retroversion) | 28 | 27.5 ± 22.3 | 38.9 ± 16 | 96.4 ± 43.2 | 128.9 ± 28.2 | N/A | N/A | N/A | N/A | N/A | N/A |
| Franceschetti et al. (2019)18 (1 – BIO-RSA) | 30 | 16 ± 11 | 40 ± 18 | 81 ± 29 | 135 ± 25 | 65 ± 29 | 119 ± 26 | N/A | 64 ± 26 | N/A | N/A |
| Franceschetti et al. (2019)18 (2 – RSA) | 29 | 15 ± 11 | 32 ± 20 | 78 ± 31 | 136 ± 21 | 67 ± 28 | 118 ± 19 | N/A | 61 ± 20 | N/A | N/A |
| Gobezie et al. (2019)22 (1 – 135 degrees NSA) | 37 | 28 ± 14 | 29 ± 10 | 78 ± 47 | 132 ± 19 | N/A | N/A | N/A | N/A | N/A | N/A |
AB, abduction; ER, external rotation with the arm at the side; ERA, external rotation with the arm in 90 degrees of abduction; FF, forward flexion; IRA, internal rotation with the arm in 90 degrees of abduction; N/A, not available; post, postoperative value; pre, preoperative value.
Data are shown as a mean ± standard deviation.
For Merolla et al, standard deviation was calculated using the confidence interval.45
Table IV.
Summary of range of motion.
| Plane of motion | No. of studies | ROM (degrees [95% CI]) | Heterogeneity |
No. of studies | ROM (degrees [95% CI]) | Heterogeneity |
ΔROM | ||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P value | I2 (%) | P value | ||||||
| Group MD | Group LD | ||||||||
| FF post | 14 | 149.79 (143.95, 155.64) | 0.0 | .755 | 10 | 145.21 (138.86, 153.55) | 0.0 | .981 | 4,58 |
| FF dif | 12 | 71.30 (60.25, 82.34) | 0.0 | .905 | 10 | 67.41 (54.66, 80.16) | 0.0 | .691 | 3,89 |
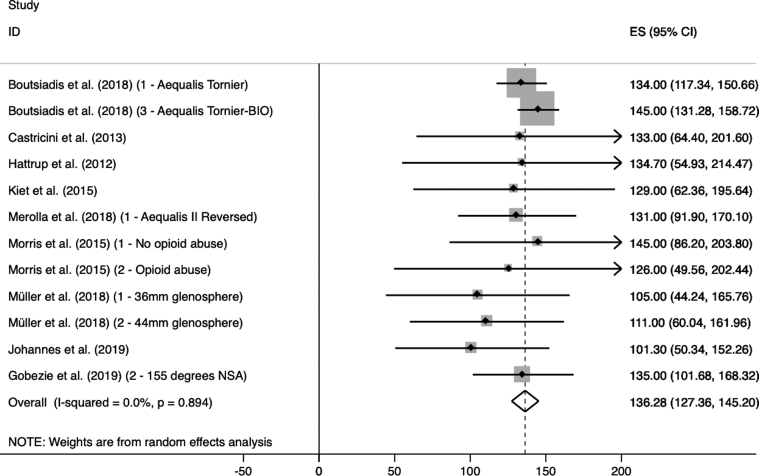
| AB post | 9 | 136.28 (127.36, 145.20) | 0.0 | .894 | 7 | 127.77 (117.02, 138.52) | 0.0 | .985 | 8,51∗ |
| AB dif | 7 | 56.76 (37.03, 76.49) | 0.0 | .773 | 6 | 48.52 (28.27, 68.78) | 0.0 | .990 | 8,24∗ |
| ERA post | 5 | 60.84 (45.14, 76.54) | 0.0 | .872 | 3 | 60.55 (38.55, 82.55) | 0.0 | .991 | 0,29 |
| ERA dif | 5 | 16.62 (−0.29, 33.53) | 0.0 | .989 | N/A | N/A | N/A | N/A | N/A |
| ER post | 14 | 31.20 (25.09, 37.32) | 0.0 | .664 | 10 | 33.28 (24.86, 41.69) | 0.0 | .986 | −2,08 |
| ER dif | 12 | 7.69 (0.01, 15.37) | 0.0 | .984 | 10 | 16.14 (7.18, 25.09) | 0.0 | .816 | −8,45∗ |
| IRA dif | 4 | 5.20 (−12.98, 23.38) | 0.0 | .988 | N/A | N/A | N/A | N/A | N/A |
| Group MD-CTA | Group LD-CTA | ||||||||
| FF post | 11 | 152.43 (146.30, 158.56) | 0.0 | .752 | 3 | 148.26 (138.27, 158.24) | 0.0 | .913 | 4,17 |
| FF dif | 8 | 79.31 (65.55, 93.06) | 0.0 | .679 | 3 | 76.33 (59.66, 93.00) | 1.7 | .397 | 2,98 |
| AB post | 8 | 137.71 (128.13, 147.28) | 0.0 | .806 | 3 | 129.83 (118.21, 141.45) | 0.0 | .940 | 7,88∗ |
| AB dif | 5 | 61.31 (34.61, 88.01) | 0.6 | .403 | 2 | 54.21 (27.70, 80.72) | 0.0 | .982 | 7,1∗ |
| ERA post | 3 | 51.32 (23.50, 79.14) | 0.0 | .931 | N/A | N/A | N/A | N/A | N/A |
| ERA dif | 3 | 13.91 (4.51, 23.21) | 0.0 | .858 | N/A | N/A | N/A | N/A | N/A |
| ER post | 10 | 24.05 (15.22, 32.87) | 0.0 | .985 | 3 | 32.63 (17.73, 47,54) | 0.0 | .995 | −8,58∗ |
| ER dif | 8 | 10.01 (−0.51, 20.54) | 0.0 | .791 | 3 | 24.78 (10.34, 39.22) | 0.0 | .850 | N/A |
| IRA dif | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
AB, abduction; CTA, cuff tear arthropathy; dif, difference; ER, external rotation with the arm at the side; ERA, external rotation with the arm in 90 degrees of abduction; FF, forward flexion; IRA, internal rotation with the arm in 90 degrees of abduction; LD, lateralized design; MD, medialized design; post, postoperative value; ROM, range of motion.
Range of motion (ROM) is shown as mean (95% confidence interval). ΔROM: difference in range of motion.
Value greater than the minimal clinical important difference.
In the CTA subgroups, the overall results are similar to those of the entire group. In the MD-CTA subgroup, however, there was no significant improvement in ER (10.01°, 95% CI -0.51 to 20.54) anymore.
The threshold for MCID was reached for both postoperative AB (ΔROM 8.51°) and improvement in AB (ΔROM 8.24°), where the MD group had more AB. The threshold was also reached for improvement in ER (ΔROM 8.45°) with the LD group having more ER. In the CTA subgroup, the MD group has, similarly, more postoperative AB (ΔROM 7.88°) and a larger improvement in AB (ΔROM 7.1°) compared with the LD group. However, the LD group has more postoperative ER (ΔROM 8.58°). The MCID for postoperative ER was not calculated because the MD group did not have a significant progression.
Functional outcome measures
In both groups, there was a statistically significant improvement in the American Shoulder and Elbow Surgeons scoring system, Constant-Murley score, visual analogue scale for pain, and Simple Shoulder Test: 44.11, 42.04, 5.60, and 3.93 points in the MD group, respectively, and 43.47, 41.14, 6.08, and 4.98 points in the LD group, respectively (Table V). For the CTA subgroups, only the Constant-Murley score could be calculated, and we found a similar improvement in both groups (MD: 43.38; LD: 41.49).
Table V.
Summary of functional outcome measures.
| Functional outcome measure | No. of studies | ROM (degrees [95% CI]) | Heterogeneity |
|
|---|---|---|---|---|
| I2 (%) | P value | |||
| Group MD | ||||
| ASES – difference | 4 | 44.11 (29.54, 58.68) | 0.0 | .987 |
| CMS – difference | 10 | 42.04 (38.75, 45.32) | 0.0 | .922 |
| VASP – difference | 6 | −5.60 (−6.92, −4.27) | 0.0 | .612 |
| SST – difference | 3 | 3.93 (−0.43, 8.30) | 65.9 | .053 |
| Group MD – CTA | ||||
| CMS – difference | 5 | 43.38 (39.68, 47.09) | 0.0 | .697 |
| Group LD | ||||
| ASES – difference | 5 | 43.47 (30.09, 56.84) | 0.0 | .999 |
| CMS – difference | 4 | 41.14 (35.35, 46.92) | 32.1 | .195 |
| VASP – difference | 7 | −6.08 (−7.99, −4.17) | 56.6 | .018 |
| SST – difference | 4 | 4.98 (2.95, 7.00) | 0.0 | .915 |
| Group LD – CTA | ||||
| CMS – difference | 3 | 41.49 (34.93, 48.05) | 45.6 | .118 |
ASES, American Shoulder and Elbow Surgeons scoring system; CMS, Constant Murley score; CTA, cuff tear arthropathy; LD, lateralized design; MD, medialized design; VASP, VAS score for pain.
ROM is shown as mean (95% confidence interval).
Complications
More radiological lucencies around the glenoid (4.4% vs. 2.0%) and humeral component (12.3% vs. 1.5%) were found in the MD group vs. the LD group (Table VI). This difference was only statistically significant for the humeral component (P value <.00001). There seems to be a higher incidence of aseptic loosening of the glenoid in the LD group (5.8%) than that of the MD group (2.9%), but this difference was not statistically significant. The incidence of scapular notching is significantly higher in the MD group (40.3%) than in the LD group (17.3%; P value <.00001). The total amount of fractures is comparable in both designs. There were more dislocations in the MD group (4.6%) compared with the LD group (1.4%; P value .037).
Table VI.
Summary of complications.
| Complication | Group MD | Group LD | P value | Group MD – CTA | Group LD – CTA | P value |
|---|---|---|---|---|---|---|
| Radiological lucencies around glenoid component | 4.4 (15/341) | 2.0 (4/196) | .2246 | 13.9 (5/36) | 10.5 (4/38) | .7325 |
| Radiological lucencies around humeral component | 12.3 (42/341) | 1.5 (3/196) | <.00001∗ | 0 (0/36) | 0 (0/38) | 1 |
| Aseptic loosening of glenoid component | 2.9 (13/450) | 5.8 (15/259) | .05603 | 1.9 (2/104) | 0 (0/38) | 1 |
| Neupraxia | 2.1 (7/338) | 2.8 (5/184) | .7612 | 1.5 (1/68) | N/A | N/A |
| Scapular notching | 40.3 (220/546) | 17.3 (69/398) | <.00001∗ | 44.4 (64/144) | 11.3 (11/97) | <.00001∗ |
| Infections | 2.5 (13/513) | 2.1 (7/339) | .8182 | 2.2 (2/89) | 7.9 (3/38) | .1578 |
| Perioperative fractures | 0.1 (1/687) | 1.4 (3/217) | .045∗ | 0 (0/157) | 0 (0/38) | 1 |
| Traumatic fractures | 2.0 (13/648) | 3.2 (7/217) | .302 | 2.5 (4/157) | 5.3 (2/38) | .3319 |
| Acromial/scapular stress fractures | 1.7 (12/718) | 0.9 (2/217) | .5405 | 3.2 (5/157) | 2.6 (1/38) | 1 |
| Dislocations | 4.6 (29/636) | 1.4 (3/216) | .037∗ | 3.4 (3/89) | 2.1 (2/97) | .6714 |
CTA, cuff tear arthropathy; LD, lateralized design; MD, medialized design.
Data are shown as % (n/N).
Statistically significant.
In the CTA subgroups, most results are comparable to the entire group, except for radiological lucencies around the glenoid (MD: 13.9%; LD: 10.5%) and humerus (MD: 0%; LD 0%), which were not significantly different anymore. There were not enough patients in other subgroups to perform a statistical comparison.
Discussion
Different NSAs are used in different implant designs for RSA. The primary goal of this study was to perform a meta-analysis on ROM after RSA with a different NSA. The most important findings of this study are an increased abduction in the MD (Fig. 2) but, on the contrary, an increased external rotation in the LD (Fig. 3). In patients with CTA, no significant improvement in external rotation is to be expected with an MD RSA compared with the preoperative status.
Figure 2.
MD postoperative abduction. MD, medialized design.
Figure 3.
LD postoperative external rotation with the arm at the side. LD, lateralized design.
Because of a lack of direct comparative clinical studies between the different designs, a direct statistical comparison was not feasible. Therefore, we compared both designs using the MCID (Table IV). MCID has been determined as 3 degrees for ER, twelve degrees for FF, and 7 degrees for AB.50
Both the improvement and postoperative AB in the MD group exceed the MCID compared with the LD group (ΔROM 8.24° and 8.51°, respectively). In an MD with an NSA larger than 150 degrees, the humeral component is placed more inferior and medially. As a consequence, the acromiohumeral distance increases, and the amount of abduction increases.32 This is confirmed by biomechanical studies and suggested by the systematic review by Samitier et al48 where the MD had 15.8° more postoperative AB compared with the LD. The increase in AB in these studies is accompanied by a decrease in adduction. As adduction was not reported in the included studies, we could not confirm this.
The LD group had a bigger improvement in ER (ΔROM 8.45°) and more postoperative ER (ΔROM 2.08°) compared with the MD group. The postoperative ER exceeds the MCID in favor of the LD group. Both Samitier et al48 and Erickson et al14 support this with the LD having 17° and 9.9° more postoperative ER, respectively. This is can be explained by biomechanical studies suggesting a larger impingement-free excursion in external rotation, and both the posterior deltoid and remaining rotator cuff having a more optimal moment arm.7,24,26 The increased moment arm of the remaining rotator cuff is partly caused by an increased origin-to-insertion distance, increasing the muscle tension of the remaining rotator cuff. However, this distance, and therefore the moment arm, decreases with increasing abduction. These findings are also confirmed in the CTA subgroup analysis where the MD group did not significantly improve in ER, and the LD group exceeded the MCID for postoperative ER (ΔROM 8.58°) compared with the MD group. We believe this subgroup to be the best representation of the biomechanical impact of implant design because rotational function mostly originates from the design and deltoid muscle lever arm. We would therefore suggest using techniques such as a latissimus dorsi transfer or lateralization of the glenoid (BIO or other techniques) in patients with a preoperatively impaired external rotation in combination with an MD RSA .
Both improvement in FF (ΔROM 2.98°) and postoperative FF (ΔROM 4.17°) did not reach the threshold of a MCID. Regardless of significance we concluded that the NSA does not clinically influence FF. Therefore, we could not confirm findings by other reviews suggesting an increased FF in the MD.14,48
In biomechanical studies, a substantial improvement in IR is described for the LD.7,32 In our study, this could not be confirmed because the reported data were too heterogeneous to perform an analysis. Because IR is just as important for clinical function as ER, we would therefore strongly recommend the adaptation of a more uniform and validated method of measurement. Furthermore, we believe that measuring the ROM in a single plane of motion does not provide the best translation into clinical function. Movement of the upper limb takes place in multiple planes of motion at the same time and is often combined with a rotational movement. The adaptation of techniques measuring the functional workspace would therefore provide a more realistic and more reproducible result.43
In our analysis, we found an improvement of all summarized functional outcome measures in both groups. The scores between both groups did not reach an MCID.50 Overall results were comparable with those described by Samitier et al.48 In the CTA subgroup, where we would expect the largest impact of implant design, we could not confirm an MCID between designs. Functional outcome measures are not only influenced by ROM but also by pain and other parameters. We therefore believe this discrepancy with ROM is because the analyzed functional outcome measures do not offer the best representation of actual clinical function.
As the humeral component is placed more inferiorly in an MD, the acromiohumeral distance increases. One consequence is more AB compared with an LD, but this comes at the expense of more scapular notching. Both in the overall group (MD 40.3%; LD 17.3%; P value <.00001) and in the CTA subgroups (MD 44.4%; LD 11.3%; P value <.00001), the MD group had significantly more notching. This is a confirmation of results published by others with a notching rate of about 50% in an MD and 0-5% in an LD.2,19,59 In the CTA subgroup, the amount of notching increases in the MD group (44.4%) but decreases in the LD group (11.3%). Other reviews have confirmed that patients with CTA have a shortened scapular neck length, which increases the risk of notching.19 Early results suggested that scapular notching had no impact on outcome; however, a more recent review found a deterioration in functional outcomes and increased glenoid loosening in a midterm follow-up.19
In some studies, it was suggested that an LD would be more prone to dislocation because of decreased deltoid muscle tension.2,12,16,33,45 However, in our study, there were significantly more dislocations in the MD group (MD: 4.6%; LD: 1.4%; P value .037).
Some studies have suggested the MD to be more at risk for acromial stress fractures because of an increased tension of the deltoid muscle, whereas others suggested this position to reduce the same tension.56,58 We could not confirm either hypothesis. However, in both our study and in other reviews, the LD seemed to be less at risk for acromial stress fractures (MD 1.7%; LD 0.9%; P value .5405). For example, in the review by Alentorn-Geli et al,2 an incidence of acromial fractures of 2.3% was reported in the MD vs. 0.6% in the LD.
Strengths and limitations
To our knowledge, this is the first meta-analysis on functional outcomes after RSA with a different NSA. In comparison with other reviews, we have a larger sample size in the LD group (1678 arthroplasties) and an equal sample size in both designs and as a consequence, a larger comparative value.2,14,48,49,59 However, the overall risk of bias of the included articles is low to moderate and there was a low heterogeneity. We acknowledge that there is a difference in experience between both implants, where there the MD has advantage. In addition, more recent studies with an MD could potentially have better results because of the learning curve, and the entire group can therefore be skewed because of the older studies.
The studies were grouped based on NSA into a medialized or lateralized design. However, the implant can also be lateralized using a glenoid bone graft (BIO), lateralized glenosphere, or other means of lateralization. This was taken into account for risk of bias as a cointervention but not for the statistical analysis. We did not distinguish between inlay or onlay humeral implant design. Further research is necessary to determine the impact of each of these parameters.
Conclusion
This analysis confirms that lowering the NSA decreases the amount of abduction and increases the amount of external rotation with the arm at the side. There is no effect on forward flexion. This alteration is accompanied by a decrease in the rate of dislocations and scapular notching. In patients with a rotator cuff tear arthropathy, no improvement in external rotation is to be expected. To determine the clinical benefit of these alterations, it is necessary to measure ROM as the amount of functional workspace rather than a single plane of motion.
Conflict of interest
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Funding
No funding was disclosed by the author(s).
Acknowledgments
The authors thank F. van Osch, epidemiologist and statistician at VieCuri hospitals, for running all calculations.
Footnotes
Institutional review board approval was not required for this review article.
References
- 1.Ahir S.P., Walker P.S., Squire-Taylor C.J., Blunn G.W., Bayley J.I.L. Analysis of glenoid fixation for a reversed anatomy fixed-fulcrum shoulder replacement. J Biomech. 2004;37:1699–1708. doi: 10.1016/j.jbiomech.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Alentorn-Geli E., Samitier G., Torrens C., Wright T.W. Reverse shoulder arthroplasty. Part 2: Systematic review of reoperations, revisions, problems, and complications. Int J Shoulder Surg. 2015;9:60–67. doi: 10.4103/0973-6042.154771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alentorn-Geli E., Wanderman N.R., Assenmacher A.T., Sperling J.W., Cofield R.H., Sánchez-Sotelo J. Anatomic total shoulder arthroplasty with posterior capsular plication versus reverse shoulder arthroplasty in patients with biconcave glenoids: A matched cohort study. J Orthop Surg. 2018;26:1–8. doi: 10.1177/2309499018768570. [DOI] [PubMed] [Google Scholar]
- 4.Angst F., Schwyzer H.K., Aeschlimann A., Simmen B.R., Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and Its Short Version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society Standardized Shoulder. Arthritis Care Res. 2011;63(Suppl 11):S174–S188. doi: 10.1002/acr.20630. [DOI] [PubMed] [Google Scholar]
- 5.Athwal G.S., MacDermid J.C., Reddy K.M., Marsh J.P., Faber K.J., Drosdowech D. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg. 2015;24:468–473. doi: 10.1016/j.jse.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Bacle G., Nove-Josserand L., Garaud P., Walch G. Long-term outcomes of reverse total shoulder arthroplasty: A follow-up of a previous study. J Bone Joint Surg - Am. 2017;99:454–461. doi: 10.2106/JBJS.16.00223. [DOI] [PubMed] [Google Scholar]
- 7.Berliner J.L., Regalado-Magdos A., Ma C.B., Feeley B.T. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:150–160. doi: 10.1016/j.jse.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P., Moineau G., Roussanne Y., O’Shea K. Bony increased-offset reversed shoulder arthroplasty minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469:2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutsiadis A., Lenoir H., Denard P.J., Panisset J.C., Brossard P., Delsol P., et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:1226–1234. doi: 10.1016/j.jse.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Brostrom L.A., Wallensten R., Olsson E., Anderson D. The Kessel prosthesis in total shoulder arthroplasty: A five-year experience. Clin Orthop Relat Res. 1992;277:155–160. [PubMed] [Google Scholar]
- 11.Castricini R., Gasparini G., Di Luggo F., De Benedetto M., De Gori M., Galasso O. Health-related quality of life and functionality after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1639–1649. doi: 10.1016/j.jse.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Chae J., Siljander M., Michael Wiater J. Instability in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2018;26:587–596. doi: 10.5435/JAAOS-D-16-00-408. [DOI] [PubMed] [Google Scholar]
- 13.Constant C.R., Murley A.H.G. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 14.Erickson B.J., Frank R.M., Harris J.D., Mall N., Romeo A.A. The influence of humeral head inclination in reverse total shoulder arthroplasty: A systematic review. J Shoulder Elbow Surg. 2015;24:988–993. doi: 10.1016/j.jse.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Favard L., Levigne C., Nerot C., Gerber C., De Wilde L., Mole D. Reverse prostheses in arthropathies with cuff tear are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469:2469–2475. doi: 10.1007/s11999-011-1833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferle M., Pastor M.F., Hagenah J., Hurschler C., Smith T. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg. 2019;28:966–973. doi: 10.1016/j.jse.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Flatow E.L., Harrison A.K. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469:2432–2439. doi: 10.1007/s11999-010-1733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franceschetti E., Ranieri R., Giovanetti de Sanctis E., Palumbo A., Franceschi F. Clinical results of bony increased-offset reverse shoulder arthroplasty (BIO-RSA) associated with an onlay 145° curved stem in patients with cuff tear arthropathy: a comparative study. J Shoulder Elbow Surg. 2020;29:58–67. doi: 10.1016/j.jse.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Friedman R.J., Barcel D.A., Eichinger J.K. Scapular Notching in Reverse Total Shoulder Arthroplasty. J Am Acad Orthop Surg. 2019;27:200–209. doi: 10.5435/JAAOS-D-17-00026. [DOI] [PubMed] [Google Scholar]
- 20.Friedman R.J., Eichinger J., Schoch B., Wright T., Zuckerman J., Flurin P.-H., et al. Preoperative parameters that predict postoperative patient-reported outcome measures and range of motion with anatomic and reverse total shoulder arthroplasty. JSES Open Access. 2019;3:266–272. doi: 10.1016/j.jses.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbart M.K., Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 22.Gobezie R., Shishani Y., Lederman E., Denard P.J. Can a functional difference be detected in reverse arthroplasty with 135° versus 155° prosthesis for the treatment of rotator cuff arthropathy: a prospective randomized study. J Shoulder Elbow Surg. 2019;28:813–818. doi: 10.1016/j.jse.2018.11.064. [DOI] [PubMed] [Google Scholar]
- 23.Grammont P., Trouilloud P., Laffay J.D.X. Concept study and realization of a new total shoulder prosthesis. Rhumatologie. 1987;39:407–418. [Google Scholar]
- 24.Greiner S., Schmidt C., König C., Perka C., Herrmann S. Lateralized reverse shoulder arthroplasty maintains rotational function of the remaining rotator cuff shoulder. Clin Orthop Relat Res. 2013;471:940–946. doi: 10.1007/s11999-012-2692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattrup S.J., Sanchez-Sotelo J., Sperling J.W., Cofield R.H. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37:1888–1894. doi: 10.1016/j.jhsa.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann S., König C., Heller M., Perka C., Greiner S. Reverse shoulder arthroplasty leads to significant biomechanical changes in the remaining rotator cuff. J Orthop Surg Res. 2011;6:1–7. doi: 10.1186/1749-799X-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J.W.V. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane. 2019. www.training.cochrane.org/handbook Available at: Accessed September 1, 2020.
- 28.Holton J., Yousri T., Arealis G., Levy O. The role of reverse shoulder arthroplasty in management of proximal humerus fractures with fracture sequelae: A systematic review of the literature. Orthop Rev (Pavia) 2017;9:27–31. doi: 10.4081/or.2017.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz D., Valenti P., Kany J., Elkholti K., Werthel J.D. Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int Orthop. 2016;40:99–108. doi: 10.1007/s00264-015-2976-3. [DOI] [PubMed] [Google Scholar]
- 30.Kiet T.K., Feeley B.T., Naimark M., Gajiu T., Hall S.L., Chung T.T., et al. Outcomes after shoulder replacement: Comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:179–185. doi: 10.1016/j.jse.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 31.King G.J.W., Richards R.R., Zuckerman J.D., Blasier R., Diliman C., Friedman R.J., et al. A standardized method for assessment of elbow function. J Shoulder Elbow Surg. 1999;8:351–354. doi: 10.1016/s1058-2746(99)90159-3. [DOI] [PubMed] [Google Scholar]
- 32.Lädermann A., Denard P.J., Boileau P., Farron A., Deransart P., Terrier A., et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39:2205–2213. doi: 10.1007/s00264-015-2984-3. [DOI] [PubMed] [Google Scholar]
- 33.Langohr G.D.G., Willing R., Medley J.B., Athwal G.S., Johnson J.A. Contact mechanics of reverse total shoulder arthroplasty during abduction: The effect of neck-shaft angle, humeral cup depth, and glenosphere diameter. J Shoulder Elbow Surg. 2016;25:589–597. doi: 10.1016/j.jse.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Lévigne C., Boileau P., Favard L., Garaud P., Molé D., Sirveaux F., et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Lo I.K.Y., Griffin S., Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthr Cartil. 2001;9:771–778. doi: 10.1053/joca.2001.0474. [DOI] [PubMed] [Google Scholar]
- 36.McFarland E.G., Huri G., Hyun Y.S., Petersen S.A., Srikumaran U. Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J Bone Joint Surg - Am. 2016;98:1801–1807. doi: 10.2106/JBJS.15.01181. [DOI] [PubMed] [Google Scholar]
- 37.McLean J.M., Awwad D., Lisle R., Besanko J., Shivakkumar D., Leith J. An international, multicenter cohort study comparing 6 shoulder clinical scores in an asymptomatic population. J Shoulder Elbow Surg. 2018;27:306–314. doi: 10.1016/j.jse.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Merolla G., Walch G., Ascione F., Paladini P., Fabbri E., Padolino A., et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27:701–710. doi: 10.1016/j.jse.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Middleton C., Uri O., Phillips S., Barmpagiannis K., Higgs D., Falworth M., et al. A reverse shoulder arthroplasty with increased offset for the treatment of cuffdeficient shoulders with glenohumeral arthritis. Bone Jt J. 2014;96-B:936–942. doi: 10.1302/030-620X.96B7.32946. [DOI] [PubMed] [Google Scholar]
- 40.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris B.J., Laughlin M.S., Elkousy H.A., Gartsman G.M., Edwards T.B. Preoperative opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:11–16. doi: 10.1016/j.jse.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Müller A.M., Born M., Jung C., Flury M., Kolling C., Schwyzer H.K., et al. Glenosphere size in reverse shoulder arthroplasty: is larger better for external rotation and abduction strength? J Shoulder Elbow Surg. 2018;27:44–52. doi: 10.1016/j.jse.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Ngan A., Xiao W., Curran P.F., Tseng W.J., Hung L.W., Nguyen C., et al. Functional workspace and patient-reported outcomes improve after reverse and total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28:2121–2127. doi: 10.1016/j.jse.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Oh J.H., Sharma N., Rhee S.M., Park J.H. Do individualized humeral retroversion and subscapularis repair affect the clinical outcomes of reverse total shoulder arthroplasty? J Shoulder Elbow Surg. 2020;29:821–829. doi: 10.1016/j.jse.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Oh J.H., Shin S.J., McGarry M.H., Scott J.H., Heckmann N., Lee T.Q. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1091–1098. doi: 10.1016/j.jse.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Rhee Y.G., Cho N.S., Moon S.C. Effects of humeral component retroversion on functional outcomes in reverse total shoulder arthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2015;24:1574–1581. doi: 10.1016/j.jse.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Saltzman B.M., Chalmers P.N., Gupta A.K., Romeo A.A., Nicholson G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647–1654. doi: 10.1016/j.jse.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Samitier G., Alentorn-Geli E., Torrens C., Wright T.W. Reverse shoulder arthroplasty. Part 1: Systematic review of clinical and functional outcomes. Int J Shoulder Surg. 2015;9:24–31. doi: 10.4103/0973-6042.150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scarlat M.M. Complications with reverse total shoulder arthroplasty and recent evolutions. Int Orthop. 2013;37:843–851. doi: 10.1007/s00264-013-1832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simovitch R., Flurin P.H., Wright T., Zuckerman J.D., Roche C.P. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg. 2018;27:298–305. doi: 10.1016/j.jse.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:I4898. doi: 10.1136/bmj.I4898. [DOI] [PubMed] [Google Scholar]
- 53.van Ochten J.H.M., van der Pluijm M., Pouw M., Felsch Q.T.M., Heesterbeek P., de Vos M.J. Long – Term survivorship and clinical and radiological follow – up of the primary uncemented Delta III reverse shoulder prosthesis. J Orthop. 2019;16:342–346. doi: 10.1016/j.jor.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiater J.M., Moravek J.E., Budge M.D., Koueiter D.M., Marcantonio D., Wiater B.P. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: A comparative study with 2 to 5years of follow-up. J Shoulder Elbow Surg. 2014;23:1208–1214. doi: 10.1016/j.jse.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 55.Wirth M.A., Rockwood C.A. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg - Series A. 1996;78:603–616. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Wong M.T., Langohr G.D.G., Athwal G.S., Johnson J.A. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg. 2016;25:1889–1895. doi: 10.1016/j.jse.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Young A.A., Smith M.M., Bacle G., Moraga C., Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg - Ser A. 2011;93:1915–1923. doi: 10.2106/JBJS.J.00300. [DOI] [PubMed] [Google Scholar]
- 58.Zmistowski B., Gutman M., Horvath Y., Abboud J.A., Williams G.R., Namdari S. Acromial stress fracture following reverse total shoulder arthroplasty: incidence and predictors. J Shoulder Elbow Surg. 2020;29:799–806. doi: 10.1016/j.jse.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: A systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]